Abstract
Gene therapy is a technique involving the modification of an individual’s genes for treating a particular disease. The key to effective gene therapy is an efficient carrier delivery system. Viral vectors that have been artificially modified to lose their pathogenicity are used widely as a delivery system, with the key advantages of their natural high transduction efficiency and stable expression. With decades of development, viral vector-based gene therapies have achieved promising clinical outcomes. Currently, the three key vector strategies are based on adeno-associated viruses, adenoviruses, and lentiviruses. However, certain challenges, such as immunotoxicity and “off-target”, continue to exist. In the present review, the above three viral vectors are discussed along with their respective therapeutic applications. In addition, the major translational challenges encountered in viral vector-based gene therapies are summarized, and the possible strategies to address these challenges are also discussed.
Keywords: gene therapy, adeno-associated viruses, adenoviruses, lentiviruses, challenges
1. Introduction
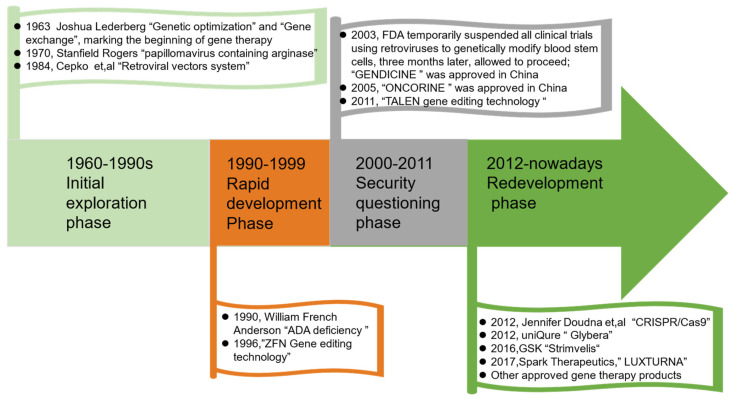
Human gene therapy seeks to modify or manipulate the expression of a gene or alter the biological properties of living cells for a therapeutic objective. This technology has the potential to cure diseases that are treatable and not entirely curable with conventional medications, thereby providing treatments for diseases previously classified as untreatable [1]. Therefore, human gene therapy is one of the research hotspots in the current century [2]. Recently, several gene therapies have been approved by regulators across the world for the treatment of various conditions, including cancer, blindness, and metabolic disorders [3]. For instance, Zolgensma (onasemnogene abeparvovec) has been approved as a treatment for spinal muscular atrophy, while Luxturna (voretigene neparvovec) has been approved for treating a form of retinal dystrophy capable of causing blindness. As early as 1970, Stanfield Rogers, an American doctor, attempted to treat argininemia by injecting a papillomavirus containing arginase. This was the first human gene therapy trial and is, therefore, an important event in scientific research history, even though it ended in failure [4]. In 1990, William French Anderson et al. conducted a trial for the correction of adenosine deaminase (ADA) deficiency by injecting the T cells transformed using a recombinant retrovirus carrying the ADA gene. This was the first gene therapy protocol that was federally approved [5]. Since then, it has been a long journey (Figure 1), with gene therapy currently producing novel treatment options in multiple fields of medicine. The current clinical trials and approved drugs for gene editing are listed in Table 1 and Table 2, respectively.
Figure 1.
Table 1.
Key viral vector-based gene therapy products in 2022.
| Drug | Development Stage | Mechanism of Action/Target Gene | Indication | Manufacture | Vector |
|---|---|---|---|---|---|
| ET140203 | Phase II Clinical Trial | Immuno-oncology therapy | Hepatocellular (liver) cancer (HCC) (including secondary metastases) | Eureka Therapeutics | Lentivirus |
| OTOF-GT | Preclinical | Otoferlin | Hearing loss general | Sensorion | Adeno-associated virus |
| AVR-RD-02 | Phase II Clinical Trial | Glucocerebrosidase beta | Gaucher’s disease | Avrobio | lentivirus |
| LX1004 | Phase II Clinical Trial | CLN2 | Neuronal ceroid lipofuscinosis (NCL) | Lexeo Therapeutics | Adeno-associated virus |
| SRP-9001 | Pre-registration | Micro-dystrophin | Duchenne muscular dystrophy (DMD) | Sarepta Therapeutics | Adeno-associated virus |
| Vyjuvek | Pre-registration | COL7A1 | Epidermolysis bullosa | Krystal Biotech | Herpes simplex virus |
| OCU400 | Phase II Clinical Trial | NR2E3 | Retinitis pigmentosa (RP) (ophthalmology) | Ocugen | Adeno-associated virus |
| Lumevoq | Pre-registration | ND4 | Leber’s hereditary optic neuropathy (LHON) | Genethon and GenSight Biologics | Adeno-associated virus |
| AVB-PGRN | Preclinical | Progranulin | Dementia, frontotemporal | AviadoBio | Adeno-associated virus |
| ADVM-022 | Phase II Clinical Trial | Aflibercept | wet age-related macular degeneration; diabetic retinopathy and other retinal conditions | Adverum Biotechnologies | Adeno-associated virus |
| ADVM-062 | Preclinical | L-opsin | Achromatopsia | Adverum Biotechnologies | Adeno-associated virus |
| 4D-125 | Phase II Clinical Trial | Retinitis pigmentosa GTPase regulator | Retinitis pigmentosa | 4D Molecular Therapeutics Roche | Adeno-associated virus |
| NR-082 | Phase III Clinical Trial | NADH dehydrogenase subunit 4 | Leber’s hereditary optic neuropathy | Wuhan Neurophth Biotechnology | Adeno-associated virus |
| ATA-100 | Phase II Clinical Trial | Fukutin related protein | Dystrophy, limb-girdle muscular, type 2I | Atamyo Therapeutics | Adeno-associated virus |
| SBT101 | Phase II Clinical Trial | ATP binding cassette subfamily D member 1 | Adrenoleukodystrophy | SwanBio Therapeutics | Adeno-associated virus |
| ASC-618 | Phase II Clinical Trial | B domain deleted liver-codon optimized factor VIII | Hemophilia A | ASC Therapeutics | Adeno-associated virus |
Table 2.
The currently approved viral vector-based gene therapy products.
| Trade Name | Generic Name | Locations Approved | Price * | Indication | Manufacturer | Vector |
|---|---|---|---|---|---|---|
| ABECMA | Idecabtagene vicleucel | US, EU, JP, CAN | $419,500 | Multiple myeloma. | Celgene Corporation, a Bristol Myers Squibb Company. | Lentiviral |
| ADSTILADRIN | Nadofaragene firadenovec | US | $158,600–$262,000 | High-risk Bacillus Calmette-Guérin (BCG)—unresponsive. Non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) and with or without papillary tumors | Ferring Pharmaceuticals A/S. | Adenoviral |
| BREYANZI | Lisocabtagene maraleucel | US, EU, CAN, JP, UK, CH | $470,939.53 | Large B-cell lymphoma (LBCL). | Juno Therapeutics, Inc., a Bristol Myers Squibb Company. | Lentiviral |
| CARVYKTI | Ciltacabtagene autoleucel | US, EU, UK, JP, CAN | $465,000 | Relapsed or refractory multiple myeloma. | Janssen Biotech, Inc. | Lentiviral |
| HEMGENIX | Etranacogene dezaparvovec | US | $3,500,000 | Hemophilia B (congenital Factor IX deficiency). | CSL Behring LLC. | Adeno-associated virus |
| IMLYGIC | Talimogene laherparepvec | US, EU, CHN, UK, AUS | $65,000 | Unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after the initial surgery. | BioVex Inc., a wholly owned subsidiary of Amgen, Inc. | Herpes simplex virus –1 |
| KYMRIAH | Tisagenlecleucel | US, EU, UK, CAN, JP, AUS, KR, CH | $475,000 | Relapsed or refractory follicular lymphoma. | Novartis Pharmaceuticals Corporation. | Lentiviral |
| LUXTURNA | Voretigene neparvovec | US, EU, UK, AUS, CAN, KR | $850,000 | Confirmed biallelic RPE65 mutation-associated retinal dystrophy | Spark Therapeutics, Inc. | Adeno-associated virus |
| SKYSONA | Elivaldogene autotemcel | US, EU, UK # | $3,000,000 | Neurological dysfunction in boys aged 4–17 years with early, active cerebral adrenoleukodystrophy (CALD). | Bluebird Bio, Inc. | Lentiviral |
| TECARTUS | Brexucabtagene autoleucel | US, EU, UK, CAN | $373,000 | Relapsed or refractory mantle cell lymphoma (MCL) and B-cell precursor acute lymphoblastic leukemia (ALL). | Kite Pharma, Inc. | Retroviral |
| YESCARTA | Axicabtagene ciloleucel | US, EU, UK, JP, CHN, CAN | $373,000 | Large B-cell lymphoma. | Kite Pharma Inc. | Retroviral |
| ZYNTEGLO | Betibeglogene autotemcel | US, CAN, EU # | $2,800,000 | Adult and pediatric patients with ß-thalassemia who require regular red blood cell (RBC) transfusions. | Bluebird bio Inc. | Lentiviral |
| ZOLGENSMA | Onasemnogene abeparvovec | US, EU, JP, AUS, CAN, BRA, TWN, KR, ISL, NO, LIE, UK | $2,125,000 | Spinal muscular atrophy (Type I) | Novartis Gene Therapies, Inc. | Adeno-associated virus |
| GLYBERA | Alipogene tiparvovec | EU # | $1,000,000 | Lipoprotein lipase deficiency. | uniQure biopharma. | Adeno-associated virus |
| LIBMELDY | Atidarsagene autotemcel | EU, UK, ISL, NO | $3,780,000 | Metachromatic leukodystrophy (MLD). | Orchard Therapeutics (Netherlands). | Lentiviral |
| ROCTAVIAN | Valoctocogene roxaparvovec | EU, UK | Around $2,500,000 | Hemophilia A (congenital factor VIII [FVIII] deficiency). | BioMarin International Limited. | Adeno associated virus |
| STRIMVELIS | autologous CD34+ enriched cells | EU, UK | $665,000 | Severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID). | Orchard Therapeutics (Netherlands). | Retroviral |
| UPSTAZA | Eladocagene exuparvovec | EU, UK | more than $3,600,000 |
Severe deficiency of aromatic L-amino acid decarboxylase (AADC). | PTC Therapeutics International Limited. | adeno associated virus |
| GENDICINE | Recombinant p53 gene | CHN | N/A | Head and neck cancer. | Shenzhen SiBiono GeneTech Co. Ltd. | Adenoviral |
| ONCORINE | E1B/E3 deficient adenovirus | CHN | N/A | Head and neck cancer; Nasopharyngeal cancer. | Shanghai Sunway Biotech Co. Ltd. | Adenoviral |
| REXIN | G mutant cyclin-G1 gene | PHL | N/A | Solid tumors. | Epeius Biotechnologies. | Retroviral |
| DELYTACT | Teserpaturev | JP | $12,500 | Malignant glioma. | Daiichi Sankyo. | Herpes simplex virus –1 |
| RELMA-CEL | Relmacabtagene autoleucel | CHN | N/A | Diffuse large B-cell lymphoma | JW Therapeutics. | Lentiviral |
| ZALMOXIS | N/A | EU # | $263,000 | Haploidentical hematopoietic stem cell transplantation (HSCT) of adult patients at a high risk of hematological malignancies. | MolMed S.p.A. | Retroviral |
| INVOSSA-K | N/A | KR # | N/A | Osteoarthritis. | Kolon Life Science. | Retroviral |
| RIGVIR | N/A | LV, EE, PL, AM, BLR | N/A | Local treatment of skin and subcutaneous metastases of melanoma. | SIA Latima. | Echovirus 7 |
Data statistics as of 2023/01. #: Withdrawal of the marketing authorization. *: Pricing varies depending on the country. N/A: Not available.
In comparison to traditional treatment options, such as proteins or small molecules, which may require repeated infusion, gene therapy delivered to long-lived cells could provide sustained production of endogenous proteins [8], i.e., gene therapy offers “one-shot” curative benefits following the introduction of correct genetic material into the patients in principle [9]. Moreover, no “impossible target medicine” situation exists, and first-time potentially curative options would be offered to all patients [10].
Four basic gene therapy approaches exist, which are as follows: gene supplementation [11,12], gene silencing [13], gene replacement [14], and gene editing [15]. The initial gene therapy focused on the delivery of transgenes, i.e., the repairment or replacement of defective genes [16]. Later, with the rapid development of functional genomics and the iterative diversification of nucleases related to gene editing, an unprecedented revolution occurred in gene therapy approaches. The advent of clustered regularly-interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) technology has rendered gene editing much simpler and effective and has, therefore, led to a breakthrough in the field of gene editing after meganucleases, the first generation of gene editing technology based on the zinc finger nucleases (ZFNs) technology, and the second generation of gene editing technology based on the transcription activation-like effector nuclease (TALEN) technology [17,18]. In particular, engineering the Chimeric Antigen Receptor-T (CAR-T) cells using CRISPR/Cas9 gene editing holds huge potential to improve the efficacy and safety of T cells-based cancer therapy. The ease of use and high efficiency offered by CRISPR/Cas9 gene editing has enabled efficient gene knockout, site-specific knock-in, and genome-wide screening in T cells [19,20]. In addition, CRISPRs are applicable in several other fields, such as natural antivirals, anti-infective systems, antimicrobials, modification of the epigenetic state of DNA/RNA, and rewriting of histone epigenetic marks [21,22].
Long-term gene therapy involves the administration of a specific genetic material (i.e., DNA or RNA) via a carrier, referred to as a “delivery vector,” which facilitates the entry of the foreign genetic material into target cells [23,24]. The delivery vectors are of two types: viral vectors and non-viral vectors. The present review focuses only on viral vectors. The commonly used viral vectors are adeno-associated viruses (AAVs), adenoviruses (Ads), or lentiviruses (LVs).
The present review highlights the key developments and challenges in the field of viral gene therapy, followed by a discussion of the possible strategies to address these challenges.
2. AAV Vectors
AAV-mediated gene transfer has great potential as a therapeutic approach [8]. Most of the currently developed AAV vectors are directed toward monogenic diseases, which belong to the category of rare diseases [25]. The FDA has approved the gene therapy products based on two viral vectors, which are both AAV vectors: LUXTURNA (Spark Therapeutics, Inc.) for the treatment of patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy and ZOLGENSMA for the treatment of pediatric patients below two years of age having spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene. The use of recombinant AAV serotypes with unique tropisms to deliver cytotoxic therapy may also be considered a local antitumor therapy approach. EBV+ B-cells exhibit increased susceptibility to rAAV6.2 infection. Therefore, the introduction of a functional suicide gene into the rAAV6.2 genome could serve as a candidate vector for the development of rAAV-based oncolytic therapy targeting focal EBV-bearing B-lymphoproliferative disorders [26]. Intracranial interferon-beta (IFN-β) gene therapy based on the local administration of AAV vectors was reported to have successfully treated non-invasive orthotopic glioblastoma models and was also effective against migrating tumors [27]. The AAV vectors have several critical properties that could be exploited for gene delivery in cancer therapy [28].
AAV was initially discovered as a contaminant in adenovirus preparations [29]. The AAV genome comprises a single-stranded DNA approximately 4.8 kilobases (kb) in length. In addition, the AAV has a small (~25 nm) icosahedral capsid composed of three types of structural proteins, namely, VP1, VP2, and VP3 [30]. AAVs are replication-deficient parvoviruses, which have traditionally required co-infection with a helper adenovirus or herpes virus to achieve efficient infection [31]. Currently, the AAV-Helper-free system is used mostly in clinical research. On their own, AAVs are thought to be non-pathogenic and are yet to be concretely linked to any of the known human diseases. AAVs have at least 12 natural serotypes, each of which exhibits different tissue tropisms (Table 3). This is mainly because of the different affinities of these serotypes to an array of primary cell surface glycoprotein receptors and secondary receptors or coreceptors. For instance, heparan sulfate proteoglycan is thought to act as a primary receptor for AAV-2. The reported co-receptors for AAV include alpha V beta5 integrin, fibroblast growth factor receptor 1 (FGFR-1), and hepatocyte growth factor receptor (c-Met). The attachment of AAV-3 strain H relies on heparin, heparan sulfate, and FGFR-1 [32].
Table 3.
The tissue tropisms of different AAV serotypes and the representative clinical trials.
| AAV Serotype | Tissue-Specific Tropisms | Key Pipeline | Disease | The Delivered Gene | Sponsor (s) | The Clinical Trial Stage |
|---|---|---|---|---|---|---|
| AAV1 | Muscle, heart, skeletal muscle (including cardiac muscle), nerve tissue | Glybera | Lipoprotein lipase deficiency | Lipoprotein lipase | UniQure | Approved # |
| AAV2 | Central nervous system, muscle, liver, brain tissue, eye | BIIB111 | Hereditary ophthalmopathy | Rab escort protein 1 | NightstaRx Ltd., a Biogen Company | Phase III completed, suspended |
| AAV3 | Muscles, liver, lung, eye | N/A | N/A | N/A | N/A | N/A |
| AAV4 | Central nervous system, muscle, eye, brain | N/A | N/A | N/A | N/A | N/A |
| AAV5 | Lung, eye, central nerve, joint synovium, pancreas | BMN 270 | Hemophilia type-A | Coagulation factor VIII | BioMarin Pharmaceutical | Approved |
| AAV6 | Lung, heart | SB-525 | Hemophilia type-A | Coagulation factor VIII | Pfizer | Phase III |
| AAV7 | Muscle, liver | N/A | N/A | N/A | N/A | N/A |
| AAV8 | Liver, eye, central nerve, muscle | BIIB112 | X-linked retinoschisis | Pigmentosa GTPase regulator | NightstaRx Ltd., a Biogen Company | Phase III, suspended |
| AAV9 | Heart, muscle, lung(alveolar), liver, central nervous system | PF-06939926 | Duchenne muscular dystrophy | Truncated dystrophin | Pfizer | Phase III |
| AAV-DJ | Liver, retina, lung, kidney | N/A | N/A | N/A | N/A | N/A |
| AAV-DJ/8 | Liver, eye, central nervous system, muscle | N/A | N/A | N/A | N/A | N/A |
| AAV-Rh10 | Lung, heart, muscle, central nervous system, liver | LYS-GM101 | GM1 gangliosidosis | beta-galactosidase | Lysogene | Phase II |
| AAV11 | Unknown | N/A | N/A | N/A | N/A | N/A |
| AAV12 | Nasal | N/A | N/A | N/A | N/A | N/A |
| AAV13 | Unknown | N/A | N/A | N/A | N/A | N/A |
N/A: Not available. #: Withdrawal of the marketing authorization.
AAV is a non-enveloped virus that may be engineered to deliver DNA to target cells. The virus genome is not integrated into the host cell but rather forms episomal concatemers in the host cell nucleus. These head-to-tail circular concatemers remain intact in non-dividing cells, such as neurons and cardiomyocytes, and are, therefore, capable of expressing transgenes over several months [33]. AAV vectors provide a relatively stable expression in dividing cells as well. The frequency of integration events may increase if an extremely high multiplicity infection is used or if the cell is infected in the presence of an adenoviral replicase. Recently, Dhwanil A et al. indicated that chromosomal integrations occurred at a surprisingly high frequency of 1–3% both in vitro and in vivo [34]. Moreover, according to recent research, high copy numbers of the AAV9 vector led to severe toxicity in animal models [35].
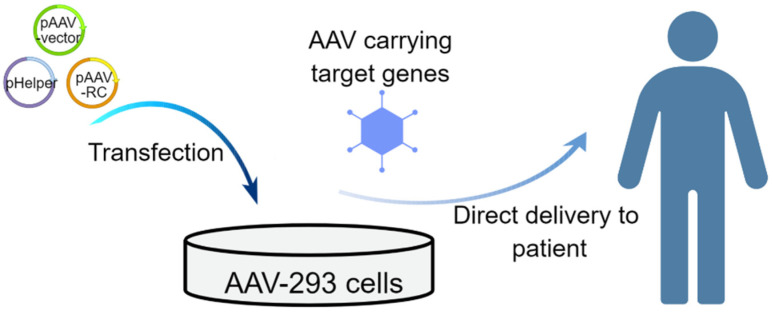
The AAV vectors are usually preferred for in vivo gene therapy due to several advantages, including the ability to transduce both dividing and quiescent cells, robust in vivo transduction efficiency, long-term transgene expression in quiescent cells, tropism for specific tissues and cell types, relatively low immunogenicity, non-pathogenicity, and a history of clinical safety (Figure 2).
Figure 2.
Schematic of the in vivo strategies that use AAV vectors for treating genetic diseases. The AAV Helper-Free System allows the production of infectious recombinant AAV virions without requiring the use of a helper virus. Most of the adenovirus gene products are supplied on the plasmid pHelper (i.e., E2A, E4, and VA RNA genes) that is co-transfected into AAV-293 cells. The wild-type AAV-2 genome comprises the viral rep and cap genes (for encoding replication and capsid genes, respectively), which are flanked by inverted terminal repeats (ITRs) that contain all the cis-acting elements necessary for replication and packaging. The rep and cap genes are removed from the viral vector and are supplied in "trans" on plasmid pAAV-RC. The AAV vectors have a natural ability to deliver genetic material into cells and may, therefore, be administered directly to the patient.
However, certain major obstacles limit the widespread application of AAV vectors, such as:
-
(1)
Insert size: The recommended maximum insert size for cloning into AAV vector is limited. In order to counter this problem, the AAV genome is combined, via homologous recombination, to the same inverted terminal repeat (ITR) sequences. Large volumes of gene expression cassettes are divided into two or more vectors and then transported to the same cells. Two or three separate AAV vectors have been delivered successfully in animals and enabled the successful expression of functional dystrophin. The other approach involves gene fragment cutting, which is aimed at larger gene fragments. Only the functional regions are intercepted, such as delivering the B-domain-deleted factor VIII gene [36]. In addition, essentially 96% of the AAV genome may be removed to permit the engineering of the AAV vector for gene therapy [37].
-
(2)
Targeted tissue specificity: In off-target tissues, cell gene expression may cause toxicity or induce unwanted immune responses. Christos Kiourtis et al. reported that AAV8-TBG vectors serve as reliable and efficient tools for hepatocyte-specific genetic manipulation with minimal off-target effects [38]. Moreover, Reifler Aaron et al. demonstrated that compared to Opn4Cre mice, a recombinant serotype-2 adeno-associated virus (rAAV2-Opn4-Cre)-mediated Cre recombinase expression in melanopsin ganglion cells occurred without leaky expression in rod/cone photoreceptors [39]. Improving the targeted tissue specificity in gene therapy would assist in enhancing the efficacy of the therapy. The tissue specificity of AAV vector-based tissue targeting is determined by the capsid proteins of AVV. Previous studies have used the capsid and viral machinery derived from the AAV serotype 2 (AAV2), which continues to be the basis for most AAV systems, although engineered capsids, such as DJ and DJ8, which exhibit tissue-specific tropisms or higher infectivity, have become available now [40]. Different AAV serotypes exhibit different tissue tropisms and are usually applied to different clinical studies (Table 3). In addition, selecting appropriate tissue-specific promoters is important [41]. Trials have reported the use of tissue-specific strong promoters, such as albumin and synapsin, to achieve an expression specific to a particular tissue [42,43]. Systemic diseases usually require high doses of particles to be administered for clinical trials [44]. While high doses are necessary to achieve sufficient transgene expression in the target cell populations, they may lead to severe adverse effects due to off-target expression, such as hepatotoxicity [45], neurotoxicity [46], atypical hemolytic uremic syndrome (aHUS) [47], and even death in critical cases [35,48,49,50]. Increased target specificity of rAAVs would reduce the necessary viral dose as well as the off-target adverse effects. Therefore, it is imperative to develop AAV gene delivery vectors that are optimized for cell-type-specific delivery.
-
(3)
Inefficient transduction: AAV vectors may be engineered at the transgene level, for example, to optimize codons, promoters, and cis-elements, which may have the greatest potential to positively impact all AAV vectors used in the clinic [37].
-
(4)
Immune response: According to published studies, >90% of humans have been infected with AAV, while ~50% of humans may have neutralizing antibodies (Nabs) [37,51,52]. These antibodies could stimulate the production of inflammatory molecules, activate cell-death pathways, and induce the development of killer T cells capable of targeting the AAV-containing cells for destruction. Further studies revealed a set of memory CD8+ T cells against AAV capsid in humans (who have been naturally infected with AAV), which could eliminate transduced hepatocytes [53]. In addition, a considerable prevalence of neutralizing antibodies against AAV (particularly against serotype 2) has been reported in the human population, which could block the gene transfer to the liver above a certain titer. While using AAV vectors alone does not elicit a strong immune response similar to that elicited upon using other viruses such as adenovirus, the above findings highlight that the immune system remains an obstacle in the in vivo gene transfer. Anastasia Conti reported that a drug named Anakinra reduces the inflammation induced by gene editing.
-
(5)
Impractical production strategies and low viral quantities [54]: Industries and research institutions should explore how to reduce the number of plasmids required for transient transfection and improve transfection efficiency. In addition, how to increase cell culture density, expand the production capacity, and remove the empty virus should be explored.
-
(6)
High cost: The high cost of gene therapy research (such as the high cost of plasmids) and development has led to extremely high terminal commercial pricing. For instance, Zolgensma costs $2.125 million. This high cost, combined with the lack of an insurance payment system, is an issue for patients unable to afford such high costs on their own. Evidently, the traditional payment mechanisms are not adequate for gene therapies, which raises the necessity of adopting novel and efficient payment mechanisms.
-
(7)
Disease case narrow: The analysis data demonstrate that the proportion of trials for agents targeting regions other than the eye, liver, muscle, and CNS is low. Major organs, such as the heart, the kidney, and the lung, continue to be almost inaccessible to AAV-based gene therapies [55]. Several ongoing AAV gene therapy trials could translate into novel products being approved for clinical use in the future [56].
Despite certain disadvantages, AAV vectors nonetheless hold great potential to revolutionize the clinical management of human diseases.
3. Ad Vectors
Ad is a large and complex, non-enveloped, double-stranded DNA (dsDNA), icosahedral virus, which is 70 to 90 nm in size. Ad possesses an icosahedral protein capsid that accommodates a 26–45-kb linear, double-stranded DNA genome. Over 100 serologically different types of adenoviruses exist, among which 49 types infect humans [57,58]. According to their specific type, these viruses may bind to various cell surface proteins to facilitate their entry into the target cells [59]. As gene therapy tools, the high efficiency of Ads has resulted in over 450 protocols being approved so far for clinical trials [60].
Similar to AAV, Ad does not integrate into the host genome. Ad is the most efficient gene delivery system for a broad range of cell and tissue types. This is because most human cells express the primary adenovirus receptor and the secondary integrin receptors, such as Coxsackie and Adenovirus Receptor (CAR), CD46, and desmoglein-2 (DSG-2), as well as the glycans GD1a and polysialic acid [61,62]. Ad was the first DNA virus to enter rigorous therapeutic development, largely because of its well-defined biology, genetic stability, large transgene capacity (up to 36 kb), and ease of large-scale production. In addition, Ad leads to side effects that are considerably milder compared to chemotherapy [63,64,65]. Adenoviral vectors were initially used for brain cell transduction in the early 1990s [66]. The non-human canine adenovirus type 2 (CAV-2)-based vectors are capable of directing a gene to the neurons in the brain, spinal cord, and peripheral nervous system [67]. Adenovirus vectors may be divided into two groups: (1) replication-deficient viruses and (2) replication-competent, oncolytic viruses (OVs) [64]. The most commonly used adenoviral vector is the human Ad serotype 5, which is a common cold virus that circulates in humans with a seropositivity rate of 40–60% [68]. This virus has been rendered replication-defective through the deletion of the E1 and E3 genes [69]. The other contemporary Ad vectors have been derived from human adenovirus serotype 2 (HAd2) [70].
So far, three generations of adenoviral vectors have been developed. The first generation of Ad vectors was engineered by replacing the E1A/E1B region with transgene cassettes that could be up to 4.5 kb in length. These Ad viral vectors could induce high-level innate inflammatory responses within the first 24 h of transduction [71]. In the second generation of adenoviral vectors, the transgene capacity was enhanced further by additionally deleting the E2/E4 site, although the overall production yield remained low due to the decreased replication ability in producer cell lines [72]. The third-generation adenovirus vectors, also referred to as the helper-dependent or gutless adenovirus, have all of their viral sequences deleted, except for the ITRs and the packaging signal. The associated in vivo immune response in these viral vectors is highly reduced compared to the first- and second-generation adenovirus vectors, although high transduction efficiency and tropism are maintained [73].
Ad vectors are the most commonly used vectors in cancer gene therapy. Ad vectors are also used in vaccines to express foreign antigens [74]. Among all diseases, cancer remains the leading cause of death worldwide and accounted for nearly 10 million deaths in 2020 [75]. The overall risk accumulation is combined with the tendency for cellular repair mechanisms to become less effective as the individual grows older [76]. Cancer may be treated with surgery, radiation therapy, and/or systemic therapy (chemotherapy, hormone therapy, and targeted biological therapy). However, traditional treatments, such as surgery, may lead to side effects, including the inhibition of cellular immunity, reduction in the activity of natural killer cells, and reduction in the levels of anti-angiogenic factors [77,78,79]. Recently, viral vector gene therapy has received much attention as a novel treatment modality for cancer because of the flexibility and effectiveness it offers [80,81].
Most cases of cancer, when detected at an advanced stage, cannot be cured with traditional therapeutic modalities. Therefore, to improve tumor penetration and local amplification of the antitumor effect, oncolytic agents were developed, such as the conditionally replicating adenoviruses (CRAds). Viral infection in tumor cells results in the replication, oncolysis, and subsequent release of the virus progeny. Importantly, this replication cycle allows for a dramatic local amplification of the input dose. In theory, CRAds would replicate until all cancer cells are lysed [82]. On the other hand, similar to the other types of oncolytic virotherapy, oncolytic adenoviruses may, in addition to de-bulking the tumor, elicit powerful antiviral and antitumor immune responses. These viruses may transform a cold immunosuppressive tumor into one which is inflamed [83,84]. In other words, antitumor immunity is more important than direct oncolysis, as the former allows for the generation of tumor-specific memory T cells [65,85]. Consistent with this, the 2018 Nobel Prize in Physiology or Medicine was awarded for the discovery of cancer therapy based on the inhibition of negative immune regulation. Immune checkpoints (ICPs), in addition to controlling autoimmunity, play a key role in host defenses aimed at eradicating pathogenic microbes and microbial strategies, while also regulating the balance among tolerance, autoimmunity, infection, and immunopathology [86]. The antibodies targeting the T cell inhibitory checkpoint proteins, namely, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD1) protein, and the PD1 ligand (PDL1), have been approved for the treatment of a variety of cancers, including melanoma, non-small-cell lung cancer (NSCLC), head and neck cancer, bladder cancer, renal cell carcinoma (RCC), hepatocellular carcinoma, and several types of tumors [87].
In addition, adenoviral vectors may be used in therapeutic cancer vaccines. These vaccine adenoviral vectors are capable of inducing both innate and adaptive immune responses in mammalian hosts [88]. An example is ETBX-011, which has been developed to treat patients with cancers that express the carcinoembryonic antigen [89]. Another example would be Ad-E6E7, which generates an enhanced immune response against HPV-positive tumors [90]. In particular, great progress has been achieved recently in utilizing the Ad-based vectors as a vaccine platform for HIV and cancer immunotherapy approaches as well as in the vaccination for other infections. The recent pandemic of coronavirus disease 2019 (COVID-19), which was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to an unprecedented development of multiple vaccines. Among these vaccines, Ad-vectored vaccines are also playing important roles in the global vaccine efforts against COVID-19. Certain examples include Ad26.COV2.S, ChAdOx1 nCov-19, Ad5 nCoV, and Gam-COVID-Vac vaccines, all of which have demonstrated efficacy in protecting against symptomatic COVID-19 disease in humans [91]. Despite these successes, the innate and pre-existing immunity against Ad vectors remains a serious challenge in the development and application of these vectors [92]. Moreover, according to clinical records, the administration of an adenovirus serotype 5 (Ad5) vector in a gene therapy trial led to lethal systemic inflammation in the subject [93]. One approach that could be used to overcome this obstacle is to sequentially administer two or more antigenically distinct viruses. This approach would ensure that the specific immunity that arises after the administration of the first virus does not inhibit the therapeutic effects of the second virus [94]. In addition, various non-human Ad vectors have been considered for development. Anurag Sharma et al. reported that the non-human adenovirus (Ad) vectors derived from bovine Ad serotype 3 (BAd3) or porcine Ad serotype 3 (PAd3) could circumvent the pre-existing immunity against human Ad (HAd) [95].
Although adenoviruses are tissue-specific and flexible, an intravenous administration of these viruses may induce acute liver injury, as has been reported in animal models [96]. In comparison to AAV, Ad has a short duration of expression in vivo [97]. Ad vectors have been studied in rodents, primates, and humans, and variable results have been achieved, which highlights the necessity for further detailed investigations on the natural history of Ad infection in humans and for questioning the value of animal models in determining the safety of virus vectors [96]. However, developing an ideal model that mimics the human infection remains the key focus of biomedical research.
4. LV Vectors
LVs belong to the orthoretroviridae subfamily of the genus retroviruses [98]. LVs may be divided into two major classes—primate and non-primate LVs [99]. The morphology and genome organization of all LVs are similar in several aspects: all LVs are pleomorphic spherical-shaped particles with diameters of approximately 100 nm [100], containing a diploid genome comprising two single-stranded positive-sense RNA molecules. LV vectors typically included the following required elements: 5′ long terminal repeat (LTR) through the Ψ packaging signal, central polypurine tract/chain termination sequence (cPPT/CTS), Rev responsive element (RRE), and 3′ LTR, including the poly (A) signal [101]. The classification and the specific structures of different LVs have been detailed in a previous report [9].
Currently, four generations of lentiviral vectors have been developed. The first-generation lentiviral vectors contained a significant portion of the HIV genome and exhibited a high frequency of transfer of genetic material into the host cell [102]. The lentiviral accessory genes vif, vpr, vpu, and nef, and LV regulatory genes tat and rev, were included in the first-generation LV vectors [102]. In order to achieve further safety, the second-generation LV vectors were developed by removing vif, vpr, vpu, and nef, which used to be present in the first-generation of LV vectors, as these are not necessary for the transfer of genetic material to the host cell [103]. The third-generation LV vectors are considered to be replication-incompetent and self-inactivating vectors. In this generation, the viral tat gene, which is essential for the replication of the wild-type human immunodeficiency virus type 1 (HIV-1), has been deleted. In addition, the vector packaging functions have been separated into three separate plasmids rather than two plasmids to reduce the risk of recombination during plasmid amplification and viral vector manufacture. An altered 3′ LTR renders the vector “self-inactivated”, which prevents the integrated genes from being repackaged. A heterologous coat protein [e.g., vesicular stomatitis virus G protein (VSV-G)] is used in place of the native HIV-1 envelope protein, and such vectors allow for the infection of a broad range of host cell types. These reasons render the third generation of LV vectors safer than the second generation LV vectors, which has allowed for the widespread application of the former [104]. The third-generation LV vectors generate virus particles using four plasmids and a producer cell line. The rationale behind including four plasmids is to enhance safety, as separating genetic components reduces the chances of recombination [105]. However, homologous recombination between the constructs remains possible nevertheless since the RRE sequence and a part of the packaging sequence in the gag gene are present in both transfer and structural packaging constructs. In order to resolve these concerns, the RRE sequences were replaced with heterologous sequences that have a similar function and do not require the REV protein. Another approach to resolving the above-stated issue is based on codon optimization. These described solutions led to the emergence of the fourth generation of LV vectors. However, the titers had been affected in the fourth-generation LV vectors, which has limited their extensive application [106].
LVs offer several potentially unique advantages over traditional gene delivery systems. Unlike adenoviral or adeno-associated vectors, neutralizing antibodies are rarely generated against lentiviral vectors [107]. The most important advantage of LV vectors is their ability to provide long-term and stable gene expression, which is crucial for adolescents or pediatric patients; LV vectors are capable of infecting dividing/non-dividing cells, such as neurons [108] and osteocytes [109], and due to their relative low-immunogenic characteristics [110], LV vectors may incorporate constructs up to 9~10 kB in size [107,111].
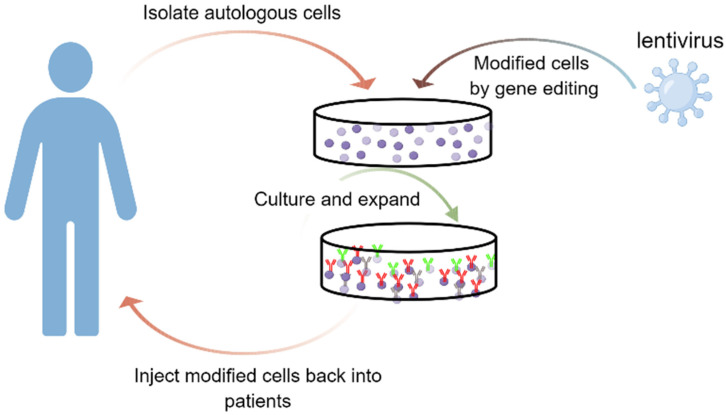
LV vectors are mainly used in ex vivo gene therapies (Figure 3), such as the one for B-cell acute lymphoblastic leukemia (B-ALL) [112]. B-ALL is a clonal malignant disease that originates in a single cell. B-ALL is characterized by the accumulation of blast cells that are phenotypically reminiscent of the normal stages of B-cell differentiation [113]. B-ALL remains a leading cause of non-traumatic death in children, and most adults diagnosed with it also succumb to the disease [114]. CAR-T therapy has successfully achieved extraordinary clinical outcomes in the treatment of B-ALL [115]. In order to develop CAR T-cells ex vivo, LVs appear particularly appealing due to their ability to stably integrate relatively large DNA inserts [116]. In the CAR T-cell therapy, the patient’s T-cells, either CD4+ or CD8+, are isolated and activated prior to transduction. The CAR transgene is then delivered into the activated T-cells via LV vectors and then expanded. Finally, the produced CAR T-cells are formulated in an adjusted buffer in a defined ratio of CD4+:CD8+ CAR T-cell [116,117]. A complete explanation of engineered CAR-T cells in cancer immunotherapy would not be provided in the present report and one could refer to other reports for the same [118,119,120]. The emergence of CAR-T cell therapy has paved a new way for cancer treatment. In 2017, Novartis received its first FDA approval for a CAR-T cell therapy, Kymriah (TM) (CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice. In 2019, EMA approved Zynteglo, a medicine used for the treatment of patients aged 12 years and older with transfusion-dependent β-thalassemia (TDT) who do not have a β0/β0 genotype and for whom hematopoietic stem cell (HSC) transplantation is appropriate although a human leukocyte antigen (HLA)-matched related HSC donor is not available. Zynteglo (betibeglogene autotemcel) is a genetically modified autologous CD34+ cell-enriched population that contains HSCs transduced with the LV vector encoding the βA-T87Q-globin gene. LV vectors have also been demonstrated as efficient gene transfer vehicles for human solid tumor cells, such as ovarian cancer cells [121], prostate cancer [122], and hepatocellular carcinoma [123].
Figure 3.
Schematic for the ex vivo strategies using LV vectors for treating genetic diseases. Cells are removed from the patient, genetically modified using LV vectors, and then returned to the patient.
The major concerns associated with LV vectors-based gene therapy include the possible generation of replication-competent LVs during vector production, mobilization of the vector by endogenous retroviruses in the genomes of patients, insertional mutagenesis that may lead to cancer, germline alteration resulting in trans-generational effects, and dissemination of new viruses from the gene therapy patients [108]. LV vectors typically insert into the host DNA as a single non-rearranged copy, and while these vectors exhibit improved stability and durability, the random insertion method nonetheless has the risk of activating the cancerous gene in the genome. Several products of Bluebird that are based on lentivirus vectors have led to such events in the clinical stage. For instance, a patient was detected with myelodysplastic syndrome [124], while another patient had developed acute myeloid leukemia after treatment with LentiGlobin gene therapy [125]. In CAR T-cells therapy for ALL patients, serious although manageable adverse events, including B-cell aplasia, tumor lysis syndrome, and cytokine release syndrome, have been reported. Basically, the use of non-integrating LVs (NILVs) reduces insertional mutagenesis and the risk of malignant cell transformation due to the integration of the lentiviral vectors [126]. As stated earlier, the usage of VSV-G alters the native tropism of lentiviral vectors to allow for the infection of a broad range of host cell types [127,128,129], which implies targeting such viruses to particular cell types is challenging due to non-tissue-specificity [129,130].
However, the observed differences could have been due to the differences in vector design, final formulation, immunomodulatory regimens (transient around vector administration), and surgical approach, among other reasons. With extensive and detailed studies on LV vectors over the past few years, this platform has been used widely in both research and clinical trials. Although certain problems remain to be addressed, the safe and efficient LV vectors are nonetheless considered promising as a tool for human gene therapy.
5. Other Viral Vectors
Other unspecified viral vectors include vaccinia, measles, herpes simplex, alphavirus, vesicular stomatitis, influenza, baculoviruses, etc. The vaccinia virus is capable of selectively replicating and propagating productively in tumor cells, resulting in oncolysis. In addition, its rapid viral particle production, wide host range, large genome size (approximately 200 kb), and safe handling render the vaccinia virus a suitable vector for gene therapy [131,132,133]. The measles virus is a non-integrating RNA virus with a long-standing safety record in humans when used as a vector for gene therapy. The measles virus offers a novel reprogramming platform for genomic modification-free iPSCs amenable for clinical translation [134]. The herpes simplex virus (HSV) also offers numerous advantages as a vector for delivering specific genes to the nervous system, including its large size, wide host range, and its ability to establish long-lived asymptomatic infections in neuronal cells [135]. Alphavirus vectors represent an attractive approach for gene therapy applications as these offer rapid and simple recombinant virus particle production and a broad range of mammalian host cell transduction [136]. The vesicular stomatitis virus (VSV) represents an attractive oncolytic virotherapy platform owing to its potent tumor cell-killing and immune-stimulating properties. However, this vector also presents certain challenges, such as inefficient systemic delivery, which might cause severe side effects, including neurotoxicity [137]. The influenza virus is a respiratory pathogen with a negative-sense, segmented RNA genome. The construction of recombinant influenza viruses in the laboratory was first reported in the 1980s. Different gene modifications result in influenza viruses with attenuated pathogenicity, which increase the safety profile of the influenza virus vector for use in cancer gene therapy [138]. Baculovirus has been used widely for recombinant protein production in insect cells for several years. It has also been developed into safe and efficient vectors for gene delivery, which offer advantages such as broad entry tropism and replication deficiency in mammalian cells [139].
Different viral vectors offer different advantages. The different characteristics of these viral vectors allow for their application in various aspects of the gene therapy landscape. The currently approved viral vector-based gene therapy products are listed in Table 2. These viral vectors complement the therapeutics used in the treatments for diseases, thereby rendering such treatments further diverse and selective.
6. Conclusions
Exceptional advancements have occurred in the last few years in the biopharma sector in terms of technology, which has led to a true paradigm shift in this industry. The advent and progress of novel therapeutic approaches using virus vector-based gene therapies have undoubtedly been important milestones in this sector. Currently, products are being developed across a wide range of indications, including autoimmune, cancer, ophthalmic, neurological, alimentary/metabolic, and sensory diseases.
Viral vectors that have been artificially modified to lose their pathogenicity are used widely as delivery systems in the field of gene therapy, with the key advantages being their naturally high transduction efficiency and stable expression. However, certain challenges remain, and these challenges vary from one viral vector to another. For instance, in AAV vectors, small-scale manufacturing under cGMP is a limitation that affects the speed of development and manufacture of gene therapies. In the case of Ad vectors, deciphering how to cope with the unwanted immune responses to therapies, which leads to severe side effects, is critical. Therefore, the attempt is to develop treatments for novel conditions. In LV vectors, the focus is on developing strategies to limit the risk and improve safety [140]. The advent of CRISPR/Cas9 has shifted the direction of the field of gene therapy from gene supplementation to gene editing. As a rapid modification of the CRISPR/Cas9 system, including its delivery system, this technology has been applied extensively in preclinical and clinical treatments. However, significant challenges have to be dealt with before the CRISPR/Cas technology could be used routinely in the clinic, among which the off-target effect is one of the major concerns, and greater research should be focused on limiting its impact [141].
The clinical use of viral vector-based gene therapies has been increasing rapidly, although the development of novel clinical frameworks for adequately assessing and managing the potential delayed effects of these novel therapies must be ensured. Gene therapies, somatic cell therapies, tissue-engineering medicines, and advanced therapy medicinal products (ATMPs) together represent the four main ATMP product groups. Most gene therapies are designed to achieve permanent or long-lasting effects in the human body, which inherently increases the risk of delayed adverse events, such as the use of adenovirus vectors [142]. It is necessary to continue studying the adverse effects of gene therapy, especially long-term adverse effects. Advances in virology and an improved understanding of viral biology would contribute to the development of viral vector variants that are suitable for translational applications, while also leading to better disease treatment outcomes.
Abbreviations
| ADA | adenosine deaminase CRISPR/Cas9: clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| ZFNs | zinc finger nucleases |
| TALEN | transcription activation-like effector nuclease |
| CAR-T | chimeric antigen receptor– T |
| AAVs | adeno-associated viruses |
| Ads | adenoviruses |
| LVs | lentiviruses |
| SMA | spinal muscular atrophy |
| SMN1 | survival motor neuron 1 |
| aHUS | atypical hemolytic uremic syndrome |
| Kb | kilobases |
| FGFR-1 | fibroblast growth factor receptor 1 |
| c-Met | hepatocyte growth factor receptor |
| ITR | inverted terminal repeat |
| AAV2 | AAV serotype 2 |
| Nabs | neutralizing antibodies |
| dsDNA | double-stranded DNA |
| CAR | coxsackie and adenovirus receptor |
| DSG-2 | desmoglein-2 |
| CAV-2 | canine adenovirus type 2 |
| OVs | oncolytic viruses |
| HAd2 | human adenovirus serotypes 2 |
| CRAds | conditionally replicating adenoviruses |
| ICPs | immune checkpoints |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| PD1 | programmed cell death 1 |
| PD-L1 | programmed cell death ligand 1 |
| NSCLC | non-small-cell lung cancer |
| RCC | renal cell carcinoma |
| BAd3 | bovine Ad serotype 3 |
| PAd3 | porcine Ad serotype 3 |
| HAd | human Ad |
| Ad5 | adenovirus serotype 5 |
| LTR | long terminal repeat |
| cPPT/CTS | central polypurine tract/chain termination sequence |
| RRE | Rev responsive element |
| VSV-G | vesicular stomatitis virus G protein |
| B-ALL | B-cell acute lymphoblastic leukemia |
| TDT | transfusion-dependent β-thalassemia |
| HSC | hematopoietic stem cell |
| HLA | human leukocyte antigen |
| NILVs | non-integrating LVs |
| ATMPs | advanced therapy medicinal products |
| HSV | herpes simplex virus |
| BCG | bacillus calmette-guérin |
| NMIBC | non-muscle invasive bladder cancer |
| CIS | carcinoma in situ |
| LBCL | large B-cell lymphoma |
| CALD | active cerebral adrenoleukodystrophy |
| MCL | mantle cell lymphoma |
| RBC | red blood cell |
| MLD | metachromatic leukodystrophy |
| ADA-SCID | severe combined immunodeficiency due to adenosine deaminase deficiency |
| AADC | aromatic L-amino acid decarboxylase |
| HSCT | hematopoietic stem cell transplantation |
Author Contributions
X.L. conceived this review and wrote the manuscript; Y.L. collected the relevant information; Z.Z. and B.L. edited the figures using Figdraw. X.N. and X.Y. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kumar S.R., Markusic D.M., Biswas M., High K.A., Herzog R.W. Clinical development of gene therapy: Results and lessons from recent successes. Mol. Ther. Methods Clin. Dev. 2016;3:16034. doi: 10.1038/mtm.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patil S.R., Al-Zoubi I.A., Raghuram P.H., Misra N., Yadav N., Alam M.K. Gene Therapy: A Comprehensive Review. Int. Med. J. 2018;25:361–364. [Google Scholar]
- 3.Ledford H. Gene therapy’s comeback: How scientists are trying to make it safer. Nature. 2022;606:443–444. doi: 10.1038/d41586-022-01518-0. [DOI] [PubMed] [Google Scholar]
- 4.Friedmann T. Stanfield Rogers: Insights into Virus Vectors and Failure of an Early Gene Therapy Model. Mol. Ther. 2001;4:285–288. doi: 10.1006/mthe.2001.0454. [DOI] [PubMed] [Google Scholar]
- 5.Anderson W.F. Human Gene Therapy. Science. 1992;256:808–813. doi: 10.1126/science.256.5058.808. [DOI] [PubMed] [Google Scholar]
- 6.Cepko C.L., Roberts B.E., Mulligan R.C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984;37:1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- 7.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.High K.A., Anguela X.M. Adeno-associated viral vectors for the treatment of hemophilia. Hum. Mol. Genet. 2016;25:R36–R41. doi: 10.1093/hmg/ddv475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munis A.M. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses. 2020;12:1106. doi: 10.3390/v12101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gene therapies should be for all. Nat. Med. 2021;27:1311. doi: 10.1038/s41591-021-01481-9. [DOI] [PubMed] [Google Scholar]
- 11.Smith A.J., Bainbridge J.W.B., Ali R.R. Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther. 2012;19:154–161. doi: 10.1038/gt.2011.161. [DOI] [PubMed] [Google Scholar]
- 12.Occelli L.M., Schoen C., Seeliger M.W., Biel M., Michalakis S., Petersen-Jones S.M., Rd-Cure Consortium Gene Supplementation Rescues Rod Function and Preserves Photoreceptor and Retinal Morphology in Dogs, Leading the Way Toward Treating Human PDE6A-Retinitis Pigmentosa. Hum Gene Ther. 2017;28:1189–1201. doi: 10.1089/hum.2017.155. [DOI] [PubMed] [Google Scholar]
- 13.Nóbrega C., Mendonça L., Matos C.A. Gene Therapy Strategies: Gene Silencing. In: Nóbrega C., Mendonça L., Matos C.A., editors. A Handbook of Gene and Cell Therapy. Springer International Publishing; Cham, Switzerland: 2020. pp. 127–146. [Google Scholar]
- 14.Narayanaswami P., Živković S. 11-Molecular and Genetic Therapies. In: Bertorini T.E., editor. Neuromuscular Disorders, 2nd eds. Elsevier; St. Louis, MO, USA: 2022. pp. 225–246. [Google Scholar]
- 15.Maeder M.L., Gersbach C.A. Genome-editing Technologies for Gene and Cell Therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2016;24:430–446. doi: 10.1038/mt.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobelt D., Pahle J., Walther W. A Brief Introduction to Current Cancer Gene Therapy. Methods Mol. Biol. 2022;2521:346–358. doi: 10.1007/978-1-0716-2441-8_1. [DOI] [PubMed] [Google Scholar]
- 17.Li H., Yang Y., Hong W., Huang M., Wu M., Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: Mech-anisms, advances and prospects. Signal Transduct. Target. Ther. 2020;5:1. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tröder S.E., Zevnik B. History of genome editing: From meganucleases to CRISPR. Lab. Anim. 2022;56:60–68. doi: 10.1177/0023677221994613. [DOI] [PubMed] [Google Scholar]
- 19.Dimitri A., Herbst F., Fraietta J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer. 2022;21:78. doi: 10.1186/s12943-022-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren J., Zhao Y. Advancing chimeric antigen receptor T cell therapy with CRISPR/Cas9. Protein Cell. 2017;8:634–643. doi: 10.1007/s13238-017-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brezgin S., Kostyusheva A., Kostyushev D., Chulanov V. Dead Cas Systems: Types, Principles, and Applications. Int. J. Mol. Sci. 2019;20:6041. doi: 10.3390/ijms20236041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrangou R., Horvath P. A decade of discovery: CRISPR functions and applications. Nat. Microbiol. 2017;2:17092. doi: 10.1038/nmicrobiol.2017.92. [DOI] [PubMed] [Google Scholar]
- 23.Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borel F., Mueller C. Design of AAV Vectors for Delivery of RNAi. Adeno-Assoc. Virus Vectors Des. Deliv. 2019;1950:3–18. doi: 10.1007/978-1-4939-9139-6_1. [DOI] [PubMed] [Google Scholar]
- 25.Brommel C.M., Cooney A.L., Sinn P.L. Adeno-Associated Virus-Based Gene Therapy for Lifelong Correction of Genetic Disease. Hum. Gene Ther. 2020;31:985–995. doi: 10.1089/hum.2020.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi E., Ravanshad M., Xie J., Panigrahi R., Jubbal S.S., Guru S.K., Guangping G., Ziyaeyan M., Fingeroth J. Serotype-dependent recombinant adeno-associated vector (AAV) infection of Ep-stein-Barr virus-positive B-cells, toward recombinant AAV-based therapy of focal EBV + lymphoproliferative disorders. Virol. J. 2021;18:223. doi: 10.1186/s12985-021-01695-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GuhaSarkar D., Neiswender J., Su Q., Gao G., Sena-Esteves M. Intracranial AAV-IFN-β gene therapy eliminates invasive xenograft glio-blastoma and improves survival in orthotopic syngeneic murine model. Mol. Oncol. 2017;11:180–193. doi: 10.1002/1878-0261.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park K., Kim W.J., Cho Y.H., Lee Y.I., Lee H., Jeong S., Cho E.S., Chang S.I., Moon S.K., Kang B.S., et al. Cancer gene therapy using adeno-associated virus vectors. Front. Biosci. A J. Virtual Libr. 2008;13:2653–2659. doi: 10.2741/2872. [DOI] [PubMed] [Google Scholar]
- 29.Hastie E., Samulski R.J. Adeno-associated virus at 50: A golden anniversary of discovery, research, and gene therapy suc-cess--a personal perspective. Hum. Gene Ther. 2015;26:257–265. doi: 10.1089/hum.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh N.L., Berguig G.Y., Karim O.A., Cortesio C.L., De Angelis R., Khan A.A., Gold D., Maga J.A., Bhat V.S. Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci. Rep. 2021;11:3012. doi: 10.1038/s41598-021-82599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith R.H., Afione S.A., Kotin R.M. Transposase-mediated construction of an integrated adeno-associated virus type 5 helper plasmid. Bio Tech. 2002;33:204–211. doi: 10.2144/02331dd04. [DOI] [PubMed] [Google Scholar]
- 32.Blackburn S.D., Steadman R.A., Johnson F.B. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch. Virol. 2006;151:617–623. doi: 10.1007/s00705-005-0650-6. [DOI] [PubMed] [Google Scholar]
- 33.Kaplitt M.G., Xiao X., Samulski R.J., Li J., Ojamaa K., Klein I.L., Makimura H., Kaplitt M.J., Strumpf R.K., Diethrich E.B. Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector. Ann. Thorac. Surg. 1996;62:1669–1676. doi: 10.1016/S0003-4975(96)00946-0. [DOI] [PubMed] [Google Scholar]
- 34.Dalwadi D.A., Calabria A., Tiyaboonchai A., Posey J., Naugler W.E., Montini E., Grompe M. AAV integration in human hepatocytes. Mol. Ther. J. Am. Soc. Gene Ther. 2021;29:2898–2909. doi: 10.1016/j.ymthe.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P., Richman L.K., Wilson J.M., Duan D., Calcedo R., et al. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen S., Tiefenbacher S., Robinson M., Huang M., Srimani J., MacKenzie D., Christianson T., Pasi K.J., Rangarajan S., Symington E., et al. Activity of transgene-produced B-domain–deleted factor VIII in human plasma following AAV5 gene therapy. Blood. 2020;136:2524–2534. doi: 10.1182/blood.2020005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 38.Kiourtis C., Wilczynska A., Nixon C., Clark W., May S., Bird T.G. Specificity and off-target effects of AAV8-TBG viral vectors for the manipu-lation of hepatocellular gene expression in mice. Biol. Open. 2021;10:bio058678. doi: 10.1242/bio.058678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reifler A.N., Wong K.Y. Adeno-associated virus (AAV)-mediated Cre recombinase expression in melanopsin ganglion cells without leaky expression in rod/cone photoreceptors. J. Neurosci. Methods. 2023;384:109762. doi: 10.1016/j.jneumeth.2022.109762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimm D., Lee J.S., Wang L., Desai T., Akache B., Storm T.A., Kay M.A. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and re-targeting of adeno-associated viruses. J. Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sack B.K., Herzog R.W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr. Opin. Mol. Ther. 2009;11:493–503. [PMC free article] [PubMed] [Google Scholar]
- 42.Massaro G., Hughes M.P., Whaler S.M., Wallom K.L., Priestman D.A., Platt F.M., Waddington S.N., Rahim A.A. Systemic AAV9 gene therapy using the synapsin I promoter rescues a mouse model of neuronopathic Gaucher disease but with limited cross-correction potential to astrocytes. Hum. Mol. Genet. 2020;29:1933–1949. doi: 10.1093/hmg/ddz317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Q., Qiao C., Li J., Li J., Xiao X. An engineered serum albumin-binding AAV9 capsid achieves improved liver transduction after intravenous delivery in mice. Gene Ther. 2019;27:237–244. doi: 10.1038/s41434-019-0107-2. [DOI] [PubMed] [Google Scholar]
- 44.Kaemmerer W.F.J.B., Medicine T. How will the field of gene therapy survive its success? Bioeng. Transl. Med. 2018;3:166–177. doi: 10.1002/btm2.10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ertl H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022;13:975803. doi: 10.3389/fimmu.2022.975803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crunkhorn S. Reducing AAV vector associated neurotoxicity. Nat. Rev. Drug Discov. 2020;20:20. doi: 10.1038/d41573-019-00198-2. [DOI] [PubMed] [Google Scholar]
- 47.Kishimoto T.K., Samulski R.J. Addressing high dose AAV toxicity–‘one and done’ or ‘slower and lower’? Expert Opin. Biol. Ther. 2022;22:1067–1071. doi: 10.1080/14712598.2022.2060737. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava A. AAV Vectors: Are They Safe? Hum. Gene Ther. 2020;31:697–699. doi: 10.1089/hum.2020.187. [DOI] [PubMed] [Google Scholar]
- 49.Wilson J.M., Flotte T.R. Moving Forward After Two Deaths in a Gene Therapy Trial of Myotubular Myopathy. Hum. Gene Ther. 2020;31:695–696. doi: 10.1089/hum.2020.182. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal S. High-dose AAV gene therapy deaths. Nat. Biotechnol. 2020;38:910. doi: 10.1038/s41587-020-0642-9. [DOI] [PubMed] [Google Scholar]
- 51.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandamme C., Adjali O., Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hui D.J., Edmonson S.C., Podsakoff G.M., Pien G.C., Ivanciu L., Camire R.M., Ertl H., Mingozzi F., High K.A., Basner-Tschakarjan E. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol. Ther. Methods Clin. Dev. 2015;2:15029. doi: 10.1038/mtm.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura T., Ferran B., Tsukahara Y., Shang Q., Desai S., Fedoce A., Pimentel D.R., Luptak I., Adachi T., Ido Y., et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci. Rep. 2019;9:13601. doi: 10.1038/s41598-019-49624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuzmin D.A., Shutova M.V., Johnston N.R., Smith O.P., Fedorin V.V., Kukushkin Y.S., van der Loo J.C.M., Johnstone E.C. The clinical landscape for AAV gene therapies. Nat. Rev. Drug Discov. 2021;20:173–174. doi: 10.1038/d41573-021-00017-7. [DOI] [PubMed] [Google Scholar]
- 56.O’Neil K.M., Wang B.J. An Analysis Of The Gene Therapy Viral Vector Landscape; 2021. [(accessed on 19 April 2023)]. Available online: https://www.cellandgene.com/doc/an-analysis-of-the-gene-therapy-viral-vector-landscape-0001.
- 57.Gallardo J., Pérez-Illana M., Martín-González N., San Martín C. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021;22:5240. doi: 10.3390/ijms22105240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usman N., Suarez M. Adenoviruses. StatPearls; Treasure Island, FL, USA: 2022. [Google Scholar]
- 59.Waddington S.N., McVey J.H., Bhella D., Parker A.L., Barker K., Atoda H., Pink R., Buckley S.M., Greig J.A., Denby L., et al. Adenovirus Serotype 5 Hexon Mediates Liver Gene Transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Chillón M., Bosch A. Adenovirus: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2014. [Google Scholar]
- 61.Lee C.S., Bishop E.S., Zhang R., Yu X., Farina E.M., Yan S., Zhao C., Zeng Z., Shu Y., Wu X., et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stasiak A.C., Stehle T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2020;209:325–333. doi: 10.1007/s00430-019-00645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crystal R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014;25:3–11. doi: 10.1089/hum.2013.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato-Dahlman M., LaRocca C.J., Yanagiba C., Yamamoto M. Adenovirus and Immunotherapy: Advancing Cancer Treatment by Combination. Cancers. 2020;12:1295. doi: 10.3390/cancers12051295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw A.R., Suzuki M. Immunology of Adenoviral Vectors in Cancer Therapy. Mol. Ther. Methods Clin. Dev. 2019;15:418–429. doi: 10.1016/j.omtm.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.La Salle G.L.G., Robert J.J., Berrard S., Ridoux V., Stratford-Perricaudet L.D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 67.Del Rio D., Beucher B., Lavigne M., Wehbi A., Gonzalez Dopeso-Reyes I., Saggio I., Kremer E.J. CAV-2 Vector Development and Gene Transfer in the Central and Peripheral Nervous Systems. Front. Mol. Neurosci. 2019;12:71. doi: 10.3389/fnmol.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Zhou P., Wu M., Yang K., Guo J., Wang X., Li J., Fang Z., Wang G., Xing M., et al. Adenovirus delivery of encoded monoclonal antibody protects against different types of influenza virus infection. NPJ Vaccines. 2020;5:57. doi: 10.1038/s41541-020-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santra S., Seaman M.S., Xu L., Barouch D.H., Lord C.I., Lifton M.A., Gorgone D.A., Beaudry K.R., Svehla K., Welcher B., et al. Replication-Defective Adenovirus Serotype 5 Vectors Elicit Durable Cellular and Humoral Immune Responses in Nonhuman Primates. J. Virol. 2005;79:6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bulcha J.T., Wang Y., Ma H., Tai P.W., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaack J. Induction and Inhibition of Innate Inflammatory Responses by Adenovirus Early Region Proteins. Viral Immunol. 2005;18:79–88. doi: 10.1089/vim.2005.18.79. [DOI] [PubMed] [Google Scholar]
- 72.Wang Q., Finer M.H. Second-generation adenovirus vectors. Nat. Med. 1996;2:714–716. doi: 10.1038/nm0696-714. [DOI] [PubMed] [Google Scholar]
- 73.Alba R., Bosch A., Chillon M. Gutless adenovirus: Last-generation adenovirus for gene therapy. Gene Ther. 2005;12:S18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- 74.Wold W.S., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization . Cancer Prevention. World Health Organization; Geneva, Switzerland: 2022. [Google Scholar]
- 77.Hu S., Alimire A., Lai Y., Hu H., Chen Z., Li Y. Trends and Frontiers of Research on Cancer Gene Therapy From 2016 to 2020: A Bibliometric Analysis. Front. Med. 2021;8:740710. doi: 10.3389/fmed.2021.740710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bar-Yosef S., Melamed R., Page G.G., Shakhar G., Shakhar K., Ben-Eliyahu S. Attenuation of the Tumor-promoting Effect of Surgery by Spinal Blockade in Rats. Anesthesiology. 2001;94:1066–1073. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 79.Zetter B.R. Angiogenesis and tumor metastasis. Annu. Rev. Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 80.Mulligan R.C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 81.Cross D., Burmester J.K. Gene therapy for cancer treatment: Past, present and future. Clin. Med. Res. 2006;4:218–227. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanerva A., Hemminki A. Adenoviruses for treatment of cancer. Ann. Med. 2005;37:33–43. doi: 10.1080/07853890410018934. [DOI] [PubMed] [Google Scholar]
- 83.Garofalo M., Pancer K.W., Wieczorek M., Staniszewska M., Salmaso S., Caliceti P., Kuryk L. From Immunosuppression to Immunomodulation-Turning Cold Tu-mours into Hot. J. Cancer. 2022;13:2884–2892. doi: 10.7150/jca.71992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liikanen I., Basnet S., Quixabeira D.C.A., Taipale K., Hemminki O., Oksanen M., Kankainen M., Juhila J., Kanerva A., Joensuu T., et al. Oncolytic adenovirus decreases the proportion of TIM-3+ subset of tumor-infiltrating CD8+ T cells with correlation to improved survival in patients with cancer. J. Immunother. Cancer. 2022;10:e003490. doi: 10.1136/jitc-2021-003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gujar S., Pol J.G., Kim Y., Lee P.W., Kroemer G. Antitumor Benefits of Antiviral Immunity: An Underappreciated Aspect of Oncolytic Viro-therapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. The function of programmed cell death 1 and its ligands in regulating auto-immunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 87.Egen J.G., Ouyang W., Wu L.C. Human Antitumor Immunity: Insights from Immunotherapy Clinical Trials. Immunity. 2020;52:36–54. doi: 10.1016/j.immuni.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 88.Rollier C.S., Spencer A.J., Sogaard K.C., Honeycutt J., Furze J., Bregu M., Gilbert S.C., Wyllie D., Hill A.V. Modification of Adenovirus vaccine vector-induced immune responses by expression of a signalling molecule. Sci. Rep. 2020;10:5716. doi: 10.1038/s41598-020-61730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gatti-Mays M.E., Redman J.M., Donahue R.N., Palena C., Madan R.A., Karzai F., Bilusic M., Sater H.A., Marté J.L., Cordes L.M., et al. A Phase I Trial Using a Multitargeted Recombinant Adenovirus 5 (CEA/MUC1/Brachyury)-Based Immunotherapy Vaccine Regimen in Patients with Advanced Cancer. Oncologist. 2020;25:479-e899. doi: 10.1634/theoncologist.2019-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Bates T.M., Kim E., Concha-Benavente F., Trivedi S., Mailliard R.B., Gambotto A., Ferris R.L. Enhanced Cytotoxic CD8 T Cell Priming Using Dendritic Cell-Expressing Human Papillomavirus-16 E6/E7-p16INK4 Fusion Protein with Sequenced Anti-Programmed Death-1. J. Immunol. 2016;196:2870–2878. doi: 10.4049/jimmunol.1502027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacob-Dolan C., Barouch D.H. COVID-19 Vaccines: Adenoviral Vectors. Annu. Rev. Med. 2022;73:41–54. doi: 10.1146/annurev-med-012621-102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dharmapuri S., Peruzzi D., Aurisicchio L. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin. Biol. Ther. 2009;9:1279–1287. doi: 10.1517/14712590903187053. [DOI] [PubMed] [Google Scholar]
- 93.Somanathan S., Calcedo R., Wilson J.M. Adenovirus-Antibody Complexes Contributed to Lethal Systemic Inflamma-tion in a Gene Therapy Trial. Mol. Ther. J. Am. Soc. Gene Ther. 2020;28:784–793. doi: 10.1016/j.ymthe.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tysome J.R., Li X., Wang S., Wang P., Gao D., Du P., Chen D., Gangeswaran R., Chard L.S., Yuan M., et al. A Novel Therapeutic Regimen to Eradicate Established Solid Tumors with an Effective Induction of Tumor-Specific Immunity. Clin. Cancer Res. 2012;18:6679–6689. doi: 10.1158/1078-0432.CCR-12-0979. [DOI] [PubMed] [Google Scholar]
- 95.Sharma A., Bangari D.S., Tandon M., Hogen Esch H., Mittal S.K. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res. 2010;153:134–142. doi: 10.1016/j.virusres.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Young L.S., Mautner V. The promise and potential hazards of adenovirus gene therapy. Gut. 2001;48:733. doi: 10.1136/gut.48.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang A.Y., Peng P.D., Ehrhardt A., Storm T.A., Kay M.A. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum. Gene Ther. 2004;15:405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 98.Acheampong E., Rosario-Otero M., Dornburg R., Pomerantz R.J. Replication of lentiviruses. Front. Biosci. A J. Virtual Libr. 2003;8:s156–s174. doi: 10.2741/935. [DOI] [PubMed] [Google Scholar]
- 99.Gifford R.J. Viral evolution in deep time: Lentiviruses and mammals. Trends Genet. TIG. 2012;28:89–100. doi: 10.1016/j.tig.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Cavalieri V., Baiamonte E., Iacono M.L. Non-Primate Lentiviral Vectors and Their Applications in Gene Therapy for Ocular Disorders. Viruses. 2018;10:316. doi: 10.3390/v10060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson N.M., Alvarado A.F., Moffatt T.N., Edavettal J.M., Swaminathan T.A., Braun S.E. HIV-based lentiviral vectors: Origin and sequence differences. Mol. Ther. Methods Clin. Dev. 2021;21:451–465. doi: 10.1016/j.omtm.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Milone M.C., O’doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merten O.W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gándara C., Affleck V., Stoll E.A. Manufacture of Third-Generation Lentivirus for Preclinical Use, with Process De-velopment Considerations for Translation to Good Manufacturing Practice. Hum. Gene Ther. Methods. 2018;29:1–15. doi: 10.1089/hgtb.2017.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Custom N.R. The Revival of Lentiviral Vectors. Nature. [(accessed on 19 April 2023)]. Available online: https://www.nature.com/articles/d42473-019-00271-9.
- 106.Tomás H.A., Rodrigues A.F., Alves P.M., Coroadinha A.S. In: Lentiviral Gene Therapy Vectors: Challenges and Future Directions. Francisco Martin M., editor. IntechOpen; Rijeka, Italy: 2013. Gene Therapy. Chapter 12. [Google Scholar]
- 107.Kalidasan V., Ng W.H., Ishola O.A., Ravichantar N., Tan J.J., Das K.T. A guide in lentiviral vector production for hard-to-transfect cells, using cardi-ac-derived c-kit expressing cells as a model system. Sci. Rep. 2021;11:19265. doi: 10.1038/s41598-021-98657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Connolly J.B. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9:1730–1734. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- 109.Brown B.D. A shot in the bone corrects a genetic disease. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:614–615. doi: 10.1038/mt.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abordo-Adesida E., Follenzi A., Barcia C., Sciascia S., Castro M.G., Naldini L., Lowenstein P.R. Stability of Lentiviral Vector-Mediated Transgene Expression in the Brain in the Presence of Systemic Antivector Immune Responses. Hum. Gene Ther. 2005;16:741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In Vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Lentiviral Vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 112.Institute N.C. Tisagenlecleucel. [(accessed on 19 April 2023)];2020 Available online: https://www.cancer.gov/about-cancer/treatment/drugs/tisagenlecleucel.
- 113.Cobaleda C., Sánchez-García I. B-cell acute lymphoblastic leukaemia: Towards understanding its cellular origin. Bioessays. 2009;31:600–609. doi: 10.1002/bies.200800234. [DOI] [PubMed] [Google Scholar]
- 114.Yeung DT O., Osborn M.P., White D.L. B-cell acute lymphoblastic leukaemia: Recent discoveries in molecular pathology, their prognostic significance, and a review of the current classification. Br. J. Haematol. 2022;197:13–27. doi: 10.1111/bjh.17879. [DOI] [PubMed] [Google Scholar]
- 115.Safarzadeh Kozani P., Safarzadeh Kozani P., Rahbarizadeh F. Optimizing the Clinical Impact of CAR-T Cell Therapy in B-Cell Acute Lymphoblastic Leukemia: Looking Back While Moving Forward. Front. Immunol. 2021;12:4453. doi: 10.3389/fimmu.2021.765097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poorebrahim M., Sadeghi S., Fakhr E., Abazari M.F., Poortahmasebi V., Kheirollahi A., Askari H., Rajabzadeh A., Rastegarpanah M., Linē A., et al. Production of CAR T-cells by GMP-grade lentiviral vectors: Latest advances and future prospects. Crit. Rev. Clin. Lab. Sci. 2019;56:393–419. doi: 10.1080/10408363.2019.1633512. [DOI] [PubMed] [Google Scholar]
- 117.Olweus J. Manufacture of CAR-T cells in the body. Nat. Biotechnol. 2017;35:520–521. doi: 10.1038/nbt.3898. [DOI] [PubMed] [Google Scholar]
- 118.Miliotou A.N., Papadopoulou L.C. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr. Pharm. Biotechnol. 2018;19:5–18. doi: 10.2174/1389201019666180418095526. [DOI] [PubMed] [Google Scholar]
- 119.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 120.Feins S., Kong W., Williams E.F., Milone M.C., Fraietta J.A. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol. 2019;94:S3–S9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 121.Indraccolo S., Habeler W., Tisato V., Stievano L., Piovan E., Tosello V., Esposito G., Wagner R., Uberla K., Chieco-Bianchi L., et al. Gene transfer in ovarian cancer cells: A comparison between retroviral and lentiviral vectors. Cancer Res. 2002;62:6099–6107. [PubMed] [Google Scholar]
- 122.Zheng J.-Y., Chen D., Chan J., Yu D., Ko E., Pang S. Regression of prostate cancer xenografts by a lentiviral vector specifically expressing diphtheria toxin A. Cancer Gene Ther. 2003;10:764–770. doi: 10.1038/sj.cgt.7700629. [DOI] [PubMed] [Google Scholar]
- 123.Gerolami R., Uch R., Faivre J., Garcia S., Hardwigsen J., Cardoso J., Mathieu S., Bagnis C., Brechot C., Mannoni P. Herpes simplex virus thymidine kinase-mediated suicide gene therapy for hepatocel-lular carcinoma using HIV-1-derived lentiviral vectors. J. Hepatol. 2004;40:291–297. doi: 10.1016/j.jhep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 124.Gene therapy needs a long-term approach. Nat. Med. 2021;27:563. doi: 10.1038/s41591-021-01333-6. [DOI] [PubMed] [Google Scholar]
- 125.Goyal S., Tisdale J., Schmidt M., Kanter J., Jaroscak J., Whitney D., Bitter H., Gregory P.D., Parsons G., Foos M., et al. Acute Myeloid Leukemia Case after Gene Therapy for Sickle Cell Disease. N. Engl. J. Med. 2022;386:138–147. doi: 10.1056/NEJMoa2109167. [DOI] [PubMed] [Google Scholar]
- 126.Gurumoorthy N., Nordin F., Tye G.J., Zaman W.S.W.K., Ng M.H. Non-Integrating Lentiviral Vectors in Clinical Applications: A Glance Through. Biomedicines. 2022;10:107. doi: 10.3390/biomedicines10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burns J.C., Friedmann T., Driever W., Burrascano M., Yee J.K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Transfiguracion J., Jaalouk D.E., Ghani K., Galipeau J., Kamen A. Size-Exclusion Chromatography Purification of High-Titer Vesicular Stomatitis Virus G Glycoprotein-Pseudotyped Retrovectors for Cell and Gene Therapy Applications. Hum. Gene Ther. 2003;14:1139–1153. doi: 10.1089/104303403322167984. [DOI] [PubMed] [Google Scholar]
- 129.Sakuma T., Barry M.A., Ikeda Y. Lentiviral vectors: Basic to translational. Biochem. J. 2012;443:603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 130.Parker C.L., Jacobs T.M., Huckaby J.T., Harit D., Lai S.K. Efficient and Highly Specific Gene Transfer Using Mutated Lentiviral Vectors Redirected with Bispecific Antibodies. mBio. 2020;11:e02990-19. doi: 10.1128/mBio.02990-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang X., Huang B., Deng L., Hu Z. Progress in gene therapy using oncolytic vaccinia virus as vectors. J. Cancer Res. Clin. Oncol. 2018;144:2433–2440. doi: 10.1007/s00432-018-2762-x. [DOI] [PubMed] [Google Scholar]
- 132.Guo Z.S., Bartlett D.L. Vaccinia as a vector for gene delivery. Expert Opin. Biol. Ther. 2004;4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 133.Deng L., Yang X., Ding Y., Fan J., Peng Y., Xu D., Huang B., Hu Z. Oncolytic therapy with vaccinia virus carrying IL-24 for hepatocellular carcinoma. Virol. J. 2022;19:44. doi: 10.1186/s12985-022-01779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Q., Vossen A., Ikeda Y., Devaux P. Measles vector as a multigene delivery platform facilitating iPSC reprogramming. Gene Ther. 2019;26:151–164. doi: 10.1038/s41434-019-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Latchman D.S. Herpes simplex virus vectors for gene therapy. Mol. Biotechnol. 1994;2:179–195. doi: 10.1007/BF02824809. [DOI] [PubMed] [Google Scholar]
- 136.Lundstrom K. Alphaviruses in Gene Therapy. Viruses. 2015;7:2321–2333. doi: 10.3390/v7052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Melzer M.K., Zeitlinger L., Mall S., Steiger K., Schmid R.M., Ebert O., Krackhardt A., Altomonte J. Enhanced Safety and Efficacy of Oncolytic VSV Therapy by Combination with T Cell Receptor Transgenic T Cells as Carriers. Mol. Ther. Oncolytics. 2019;12:26–40. doi: 10.1016/j.omto.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J., Arévalo M.T., Zeng M. Engineering influenza viral vectors. Bioengineered. 2013;4:9–14. doi: 10.4161/bioe.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ono C., Okamoto T., Abe T., Matsuura Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses. 2018;10:510. doi: 10.3390/v10090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arsenijevic Y., Berger A., Udry F., Kostic C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics. 2022;14:1605. doi: 10.3390/pharmaceutics14081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang B. CRISPR/Cas gene therapy. J. Cell. Physiol. 2021;236:2459–2481. doi: 10.1002/jcp.30064. [DOI] [PubMed] [Google Scholar]
- 142.Montes-Galindo D.A., Espiritu-Mojarro A.C., Melnikov V., Moy-López N.A., Soriano-Hernandez A.D., Galvan-Salazar H.R., Guzman-Muñiz J., Guzman-Esquivel J., Martinez-Fierro M.L., Rodriguez-Sanchez I.P., et al. Adenovirus 5 produces obesity and adverse met-abolic, morphological, and functional changes in the long term in animals fed a balanced diet or a high-fat diet: A study on hamsters. Arch. Virol. 2019;164:775–786. doi: 10.1007/s00705-018-04132-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.