Abstract
Periodontal diseases are oral inflammatory diseases ranging from gingivitis to chronic periodontitis. Porphyromonas gingivalis is one of the major pathogens responsible for severe and chronic periodontitis. Plant extracts with antimicrobial activity could be considered possible alternatives to chlorhexidine, an antiseptic substance used in oral hygiene thatcan cause bacteria resistance. Here, two commercial extracts obtained from Cistus × incanus L. and Scutellaria lateriflora L. were chemically characterized usingUltra-High-Performance Liquid Chromatography (UHPLC) coupled with a Q-Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer. The extracts were studied for their bioaccessibility after simulated in vitro oral digestion, their antimicrobial activity against P. gingivalis, their protective effects against cellular invasion by P. gingivalis, and their antibiofilm activity. The extracts were found to contain very complex mixtures of polyphenols, which were quite stable after in vitro simulated oral digestion and demonstrated mild, dose-dependent inhibitory activity against P. gingivalis growth. This activity increased with the combination of the two extracts. Moreover, the combination of the extracts induced a reduction in P. gingivalis HaCaT invasiveness, and the reduction in biofilm came to around 80%. In conclusion, a combination of C. incanus and S. lateriflora showed promising effects useful in the treatment of gingivitis.
Keywords: Cistus × incanus L., Scutellarialateriflora L., oral health, gingivitis, Porphyromonas gingivalis
1. Introduction
Periodontal diseases include a range of chronic inflammatory conditions affecting the gingiva, bones, and tooth ligaments. They generally begin with plaque-induced gingivitis, initiated by bacteria embedded within the plaque near the gum line. Untreated gingivitis may progress to the loss of gingiva, bones, and ligaments, which may lead to chronic periodontitis, the ultimate result of which is the initiation of deep periodontal pockets (a hallmark of the disease) and tooth loss [1,2]. It is widely accepted that the etiology of periodontal disease is driven by several factors, including host immunity, environmental factors, and periodontal pathogens, i.e., Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, which form the so-called “red complex”. Accumulation of supragingival and subgingival polymicrobial biofilm communities induces a persistent host immune response within the periodontium [3]. The inflammation process can be reversed with the removal of the biofilm and, as a result, inflammation can be limited to the gingival epithelium and connective tissues. Nevertheless, if the persistent accumulation of biofilm leads to an irreversible inflammatory process, the disease progresses from gingivitis to periodontitis with the involvement of deeper periodontal tissues (i.e., the deepening of the gingival crevice, the destruction of periodontal ligaments, and alveolar bone loss) [4]. The outgrowth of periodontal pathogens further encourages this inflammatory cascade, and proteinaceous byproducts of tissue production (i.e., amino acids, collagen breakdown products, iron, and heme) reinforce the growth of periodontal pathogens, further increasing the inflammatory cascade [5,6,7].
Periodontal diseases are more prevalent in adults but may also occur in younger subjects (children and adolescents), where the amount of tissue destruction is usually commensurate with dental plaque levels, host defenses, and associated risk factors [8]. In the United States, a report by the Center for Disease Control and Prevention showed that 47.2% of adult people ≥30 years of age and 70.1% of the older people ≥65 years of age have some form of periodontal disease as their prevalence increases with age. Moreover, the condition is more common in men (56.4%) compared to women (38.4%) and is more prevalent in the population educated atbelow the high school level (66.9%) and in cigarette smokers (64.2%) [9].
Recent studies have recognized the emerging role of periodontitis in systemic inflammation and, in turn, in systemic disease states, such as oral cancer, Alzheimer’s disease, rheumatoid arthritis, diabetes, atherosclerosis, and inflammatory bowel disease [10]. Higher levels of systemic inflammation biomarkers, such as pro-inflammatory cytokines, i.e., tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6 and C-reactive protein, as a result of microbial translocation from periodontal lesions have been consistently observed in patients with periodontal disease [11,12]. However, despite considerable progress, the deeper relationships between the immune, inflammatory, infectious, and systemic features of periodontitis are not yet fully elucidated and are still under debate.
The greater impact that periodontal disease exerts on host health has drawn attention to its prevention and treatment at the initial stages of disease [13], as it is known that a patient with gingivitis can return to a state of full health, while a patient with periodontitis will remain as such for life, even following successful therapy. Treatment involves good oral hygiene, professional tooth cleaning, and the use of antibiotics and periodontal surgery [13]. Antiseptic mouthwash containing chlorhexidine has been developed to strengthen the effects of an oral hygiene routine. However, one of the major concerns with the use of chlorohexidine is microbial resistance, particularly in case of Acinetobacter baumannii, Escherichia coli, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae [14,15]. The search for alternative, safe, and promising treatments for gingivitis represents an urgent need for the prevention of periodontitis and its systemic complications. In this context, traditional herbal medicines and plant-based food supplements could be considered as an alternative approach aimed at avoiding the possible development of periodontitis and the adverse effects of strains resistant to chlorhexidine [16,17,18].
Cistus × incanus L. belongs to the family Cistaceae and is widespread along the Mediterranean coast of Europe. It was used as an effective anti-inflammatory and skin protective plant agent in Mediterranean folk medicines. Moreover, the use of C. incanus tea to rinse the mouth contributes to the degradation of biofilm, a well-known virulence factor, and the prevention of biofilm-induced diseases by decreasing the load of associated bacteria [19]. In addition to these properties, the extracts obtained from some Cistus species with compositions similar to C. incanus extracts are known for their antibacterial activity against oral cavity pathogens and have been suggested as alternative natural antibacterial and antibiofilm components against oral infections [20]. Scutellaria lateriflora L., also known as American skullcap, belongs to the family Lamiaceae and is one of most widely used nervine agents in North American and Western herbal medicine. Traditionally, it has been used to promote a healthy menstrual cycle and to treat hysteria, anxiety, insomnia, delirium tremens, epilepsy, withdrawal from barbiturates and tranquilizers, bronchitis, diarrhea, dysentery, jaundice, hepatitis, hypertension, thrombosis, and tumors [21]. Moreover, Scutellaria baicalensis shows synergistic antibacterial effects against oral bacterial biofilms in combination with chlorhexidine [22].
The increasing microbial resistance to chlorhexidine calls for adecrease in its use and the discovery of new combinations of plant extracts, which can act synergistically, with high antibacterial and antibiofilm properties. On the basis of the above information, the aim of this studywas to continue the researchon C. incanus and to evaluate the anti-gingivitis properties of S. lateriflora extracts, both alone and in combination with one another. This was done with the final aim of providing scientific evidence for the development of a new food supplement based on botanical extracts that is able to act at the oral-cavity level to prevent against periodontal diseases and improve the health of the oral cavity. Thus, two commercial extracts obtained from C. incanus and S. lateriflora were chemically characterized, and their oral bioaccessibility after in vitro simulated oral digestion was determined. Then, their in vitro antimicrobial activity against P. gingivalis, antibiofilm activity, and ability to enhance the barrier function of a gingival keratinocyte model system and exert a protective effect against invasion by P. gingivalis were evaluated.
2. Materials and Methods
2.1. Chemicals and Reagents
One batch of commercial, dry, powdered hydroalcoholic extract of C. incanus (standardized to contain ≥18% of total polyphenols, and arabic gum as a carrier agent), and one batch of commercial, dry, powdered hydroalcoholic extract of S. lateriflora (standardized to contain ≥10% of baicalin, and maize maltodextrin as carrier agent) obtained from the aerial parts of the plants, were provided by EPO S.R.L. (Milan, Italy). All the compounds used for the in vitro simulated oral digestion process were purchased from Carlo Erba (Milan, Italy): potassium chloride (KCl), dihydrogen potassium phosphate (KH2PO4), sodium carbonate (NaHCO3), magnesium chloride (MgCl2), ammonium carbonate (NH4)2CO3, calcium chloride (CaCl2), sodium chloride (NaCl), hydrochloric acid (HCl), and sodium hydroxide (NaOH). From Sigma–Aldrich, Merck KGaA (Milan, Italy), α-Amylase from Bacillus licheniformis, formic acid solution (1 M), acetic acid solution (1M), water, methanol, acetonitrile (ACN) LC–MS grade, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased. All media and reagents for the cell culture were purchased from Gibco (Milan, Italy).
2.2. Chemical Characterization of C. incanus and S. lateriflora Extracts Using Reversed-Phase, Ultra-High-Performance Liquid Chromatography (RP-UHPLC) Coupled with a Q-Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer
Stock solutions were prepared for the C. incanus and S. lateriflora extracts by accurately weighing 200 mg of extract and diluting them with a solution of 50:50 v/v acidified water (0.1% v/v formic acid) and methanol to a concentration of 10 mg/mL. From the stock solutions, 1 mL was taken and filtered prior to analysis (0.45 μm and 0.20 µm Minisart RC 4 membrane filters). The analysis wasperformed on a Thermo Ultimate RS 3000 paired online with a Q-Exactive hybrid quadrupole Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a heated electrospray ionization probe (HESI II). For the RP-UHPLC analysis, a Kinetex® EVOTM 150 mm × 2.1 mm, 2.6 µm (L × I.D, particle size, Phenomenex®, Bologna, Italy) column was employed at a flow rate of 0.4 mL/min. The mobile phases consisted of (A) 0.1% CH3COOH in H2O and (B) ACN plus 0.1% CH3COOH. The analysis was performed in a gradient as follows: 0–10.0 min, 2–35% B; 10–12 min, 35–70% B; 12–13 min, 70–98% B; hold for 2 min; and return to initial conditions after 0.1 min. The column oven was set to 40 °C and 5 µL of the extracts were injected. An HRMS analysis was performed with Full MS (m/z 100–850) and data-dependent acquisition (dd-MS2 top; N = 5). The resolution selected was 70,000 and 15,000 FWHM at m/z 200. Stepped normalized collision energy (NCE) was used with values of 15, 25, and 30. The negative ion mode (ESI-) was employed. Source parameters were as follows: sheath gas pressure, 50 arbitrary units; auxiliary gas flow, 13 arbitrary units; spray voltage, −2.50 kV; capillary temperature, 260 °C; auxiliary gas heater temperature, 300 °C; and S-lens RF value, 30 arbitrary units. Metabolite annotation was performed using Compound Discoverer (Thermo Scientific, V3.3, Waltham, MA, USA) in comparison with in silico natural product libraries, accurate mass, and theexisting literature as previously reported [23].
2.3. In Vitro Bioaccessibility of C. incanus and S. lateriflora Extracts Using Simulated Oral Digestion Processes and RP-UHPLC-Photodiode Array Detector (PDA) Analysis
The current study aims to evaluate the possibility of a new food supplement for acting locally in the oral cavity against the pathogens associated with periodontal diseases; thus, the impact of the oral digestion process was verified on the chemical composition of C. incanus and S. lateriflora extracts following a protocol set by Minekus et al. with some modifications [24]. In brief, 5 g of each extract were dissolved in 3.5 mL of previously prepared, simulated salivary fluid (SSF) comprising an electrolyte solution containing (K+), (Na+), (Cl−), (H2PO4−), (HCO3−, CO32−), (Mg2+), (NH4+), and (Ca2+). The same procedure was followed for the blank sample using 5 mL of water instead of the extracts. Then, 0.5 mL (1500 U/mL) of fresh α-amylase solution was added to both samples. In the end, water was added for the samples to reach a final volume of 10 mL, and the samples were incubated for 2 min at 37 °C. At the end of the oral digestion process, the samples were freeze dried and maintained at 4 °C prior to the RP-UHPLC-PDA analysis, which was performed on a Shimadzu Nexera LC30 (Shimadzu, Kyoto, Japan) with the same chromatographic conditions reported above; chromatograms were extracted at 280 and 330 nm.
2.4. Antimicrobial Activity of C. incanus and S. lateriflora Extracts against P. gingivalis
To evaluate the antimicrobial activity of C. incanus extract, S. lateriflora extract, their combinations in different ratios with the final concentrations of 60 mg/mL, and their carrier agents (i.e., maize maltodextrin and arabic gum), P. gingivalis (ATCC 33277), obtained from the LGC spa (ATCC distributor, Milan, Italy), was grown in a TSB-yeast extract medium supplemented with 0.05% cysteine hydrochloride, 0.02 μg/mL menadione, 5 μg/mL hemin, and 0.02% potassium nitrate in an anaerobic chamber (with 5% CO2) at 37 °C. In brief, serial dilutions of the samples were prepared at volumes of 100 μL/well in 96-well plates. The final concentrations of each of these were in the range of 60 to 5 mg/mL. To each well 20 μL of P. gingivalis, bacterial cell suspension was added at a final concentration of 1 × 106 colony-forming units (CFU)/mL. Amoxicillin (10 µg/mL) was used as a positive control. After incubation in an anaerobic chamber at 37 °C for 24 h, bacterial growth was then analyzed using a microplate reader (Tecan, Männedorf, Swiss) at 595 nm. Each test was performed in triplicate. The rate of growth inhibition was determined using the following formula:
| % Growth inhibition = 100 − [(100 × OD595 nm of the test sample)/OD595 nm of CTR] |
2.5. In Vitro Cell Model Systems
2.5.1. Human Keratinocyte Epithelial Cells (HaCaT)
Human immortalized keratinocytes (HaCaT) were grown as monolayers in a standard culture medium, Dulbecco’s Modified Eagle Medium (DMEM-10928_Gibco), and supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The medium was replaced every 48 h. The trypsinization process for HaCaT cells was always performed at 70% confluence.
2.5.2. Cytotoxic Activity of C. incanus and S. lateriflora Extracts on HaCaT Cells
To assess the cytotoxic activity of the C. incanus and S. lateriflora extracts on HaCaT, alone and in combination, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed. The MTT assay measures cellular metabolic activity as an indicator of vitality, proliferation, and cellular cytotoxicity [25]. Cells were cultured in DMEM and supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum at 37 °C with 5% CO2 in a humid environment, as previously described. A density of 5× 104 cells/well was seeded into 96-well plates and incubated for 24 h with (1) C. incanus extract at different concentrations ranging from 5 to 60 mg/mL, (2) S. lateriflora extract at different concentrations ranging from 5 to 60 mg/mL, or (3) their combinations in different ratios with a final concentration of 60 mg/mL. After 24 h of treatment, 100 μL of MTT solution (at a final concentration 0.5 mg/mL) was added to each well for 3 h at 37 °C. Then, the formazan crystals were solubilized by adding 100 μL of 100% DMSO to each well, and the viability rate was recorded at OD at 570 nm using a microplate reader (Tecan, Männedorf, Switzerland). Each test was performed in triplicate.
2.5.3. In Vitro Effects of C. incanus and S. lateriflora Extracts on Invasive Capacity of P. gingivalis Targeting HaCaT Cells
To investigate the effectiveness of the test extracts, alone and in combination, on the invasive capacity of P. gingivalis, an invasion assay was performed as described elsewhere [26]. HaCaT cells were seeded into 24-well plates (1 × 105 cells/well) and grown to ~70–80% confluence. The day after the pre-treatment experimental scheme, the cell monolayers were starved for 2 h in a DMEM-10928_Gibco medium without antibiotics and were treated with the samples at different concentrations ranging from 15 to 60 mg/mL for 1 at 37 °C. After preincubation with the samples, cells were infected with 1.5 × 108 CFU/mL P. gingivalis. In a parallel co-treatment experimental scheme, bacteria and samples at the same concentrations reported above were incubated for 1 h and then used for the cell monolayer treatment. For both experimental schemes, after 4 h of infection, cells were washed with PBS three times and then incubated with gentamicin (Sigma–Aldrich, 100 μg/mL) to kill all extracellular bacteria. After 2 h in the presence of gentamicin, cells were lysed with 0.1% Triton-X solution to evaluate the amountof intracellular bacteria. Serial dilutions of the cell lysates were made in PBS, plated on TB agar, and incubated at 37 °C overnight. Then, the CFU/mL was counted relative to the bacteria that invaded the cell monolayer after incubation for 24 h at 37 °C.
2.5.4. Effects of C. incanus and S. lateriflora Extracts on Pre-Formed Biofilm Mass Reduction
The ability for C. incanus and S. lateriflora extracts to degrade pre-formed biofilm was evaluated using acrystal violet (CV) assay [27]. Briefly, a bacterial inoculum was prepared at a density of 1 × 108 CFU/mL in TSB supplemented with 1% glucose. A volume of 100 µL of bacterial suspension was transferred to each well of a 96-well plate and incubated at 37 °C for 24 h under static conditions to allow biofilm formation. After incubation, non-adherent cells were removed through PBS washes, and C. incanus and S. lateriflora extracts at different concentrations ranging from 5 to 55 mg/mL were added to the mature biofilm. The untreated and EDTA-treated biofilms constituted negative and positive controls, respectively. After treatment, the growth medium was removed, and the biofilm was gently washed with PBS. The biofilm biomass was quantified by adding 100 µL of 0.1% CV to each well for 30 min at room temperature under shaken conditions. Excess CV was removed, washed with PBS, and then solubilized with 98% ethanol for 40 min at room temperature under shaking conditions. Absorbance values recorded at 570 nm using a microplate reader (Tecan, Männedorf, Swiss) and were proportional to the biofilm mass present, and the results were expressed by calculating the percentage of reduction of the biofilm mass compared to the control samples.
2.6. Statistical Analysis
Data are reported as mean ± standard deviation (SD). The bacterial growth percentage and the cell invasive capacity were compared with the control sample at each examination point using an independent samples t-test, setting the level of significance at p < 0.05. Moreover, a statistical comparison among groups was conducted with multiple t-tests for multiple comparison using the Holm–Sidak method to analyze the bacterial growth inhibition percentage or the biofilm mass reduction percentage induced by the plant extract combinations to determine significance, which was set to p < 0.05. For each concentration used in both the antimicrobial and antibiofilm activities, a biological replicate was obtained and averaged. The statistical analyses were performed using GraphPad Prism, version 8 (San Diego, CA, USA).
3. Results
3.1. Metabolic Profile of C. incanus and S. lateriflora Extracts
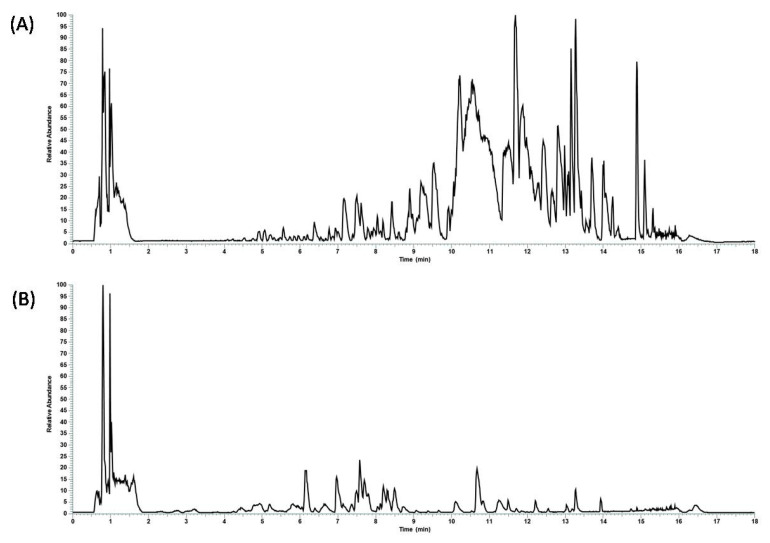
The first step was the chemical characterization of the commercial hydroalcoholic C. incanus and S. lateriflora extracts using RP-UHPLC coupled with a Q Exactive hybrid quadrupole-Orbitrap mass spectrometer. Through a comparison with in silico MS/MS spectra, accurate mass, and molecular formula, 101 and 117 compounds were tentatively annotated in C. incanus and S. lateriflora extracts, respectively, with confidence MSI lvl.2 [28] as reported in the Supplementary Tables. The base peak chromatograms are reported in Figure 1.
Figure 1.
Base peak chromatograms revealing the metabolic profile of undigested (A) S. lateriflora and (B) C. incanus extracts.
3.2. Bioaccessibility of C. incanus and S. lateriflora Extracts after In Vitro Simulated Oral Digestion
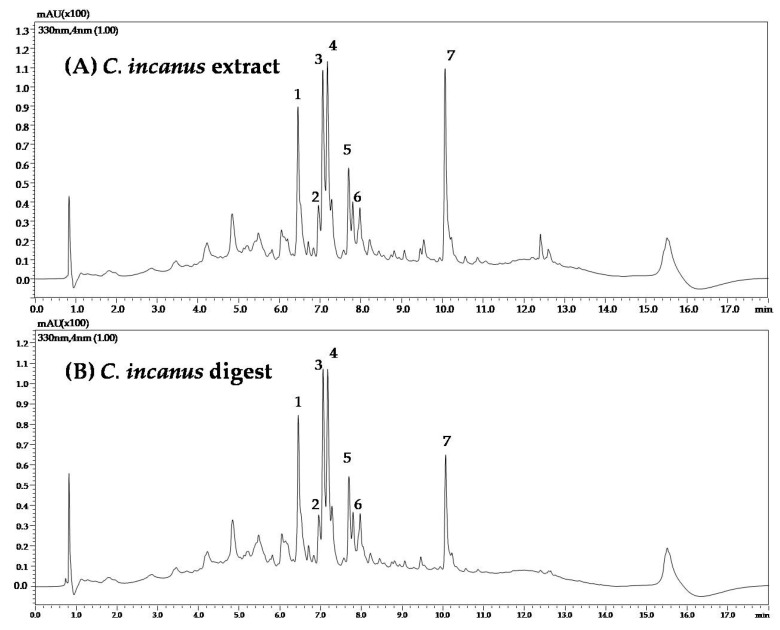
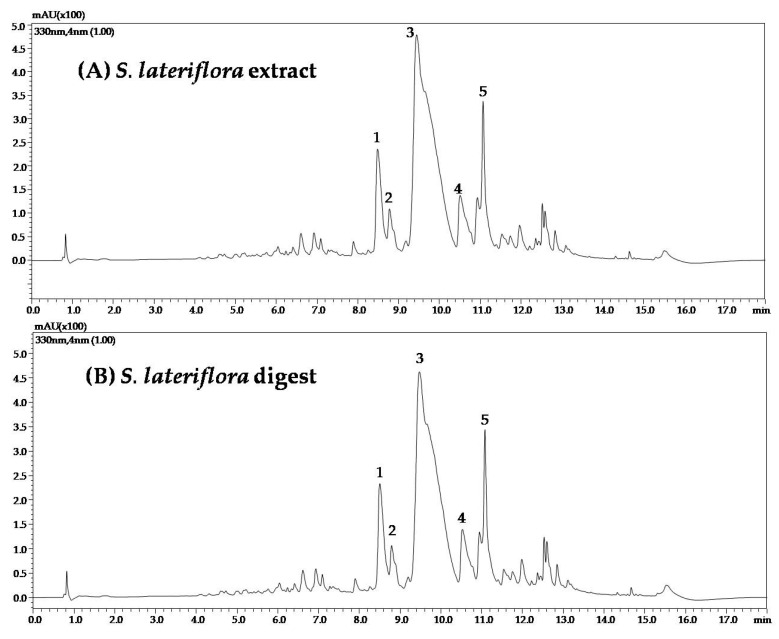
To evaluate the influence of the in vitro simulated oral digestion process on the compounds most represented in C. incanus and S. lateriflora extracts, they were analyzed using RP-UHPLC-PDA before and after oral digestion. A slight shift in retention times was observed for orally digested samples, probably due to a matrix effect reduction following in vitro simulated oral digestion. UV traces of C. incanus and S. lateriflora extracts before and after in vitro simulated oral digestion are reported in Figure 2 and Figure 3, respectively.
Figure 2.
UV traces of C. incanus extract (A) before and (B) after in vitro simulated oral digestion.
Figure 3.
UV traces of S. lateriflora extract (A) before and (B) after in vitro simulated oral digestion.
Table 1 shows the mean peak area reduction percentages, ranging from 6.4 to 11.6%, after oral digestion of C. incanus extract, with the exception of kaempferol 3-(3″-p-coumaoroylhexoside), which was degraded by 46% when compared with the peak area recorded before digestion, revealing a moderate degradation process. Table 2 shows the mean peak area reduction percentage after oral digestion of S. lateriflora extract main peaks, which was found to be lower than 10%, revealing a modest degradation process.
Table 1.
Mean peak area of the main eight peaks identified in C. incanus extract before and after oral digestion and area reduction percentage (%).
| C. incanus Compound | RT (min) | Mean Area before Digestion | Mean Area after Digestion |
Area Reduction Percentage (%) |
|---|---|---|---|---|
| Myricetin 3-hexoside | 6.45 | 3.66 × 105 | 3.42× 105 | 6.5 |
| Myricetin 3 alpha L-arabinofuranoisde | 6.95 | 1.05 × 105 | 9.25 × 104 | 11.6 |
| Quercetin-3-O-glucopyranoside | 7.06 | 3.93 × 105 | 3.76 × 105 | 4.3 |
| Quercetin-3-O-glucopyranoside isomer | 7.17 | 4.13 × 105 | 3.75 × 105 | 9.1 |
| Gujaverin | 7.69 | 1.54 × 105 | 1.45 × 105 | 6.4 |
| Gujaverin isomer | 7.79 | 7.40 × 104 | 6.61 × 104 | 10.6 |
| Kaempferol 3-(3″-p-coumaoroylhexoside) | 10.06 | 4.20 × 105 | 2.27 × 105 | 46.0 |
Table 2.
Mean peak area of the main peak present in S. lateriflora extract after oral digestion and area reduction percentage (%).
| S. lateriflora Compounds | RT (min) | Mean Area Phytcomplex | Mean Area Digest | Area Reduction Percentage (%) |
|---|---|---|---|---|
| Scutellarin | 8.48 | 1.15 × 106 | 1.05 × 106 | 8.9 |
| Isoscutellarin | 8.78 | 4.66 × 105 | 4.67 × 105 | 0.0 |
| Baicalein-6-glucuronide | 9.44 | 3.01 × 107 | 2.99 × 107 | 1.0 |
| Quercitrin | 10.51 | 1.00 × 106 | 9.32 × 105 | 7.1 |
| Oroxylin A-glucuronide | 11.07 | 3.39 × 106 | 3.39 × 106 | 0.0 |
3.3. Antibacterial Activity of C. incanus and S. lateriflora Extracts against P. gingivalis
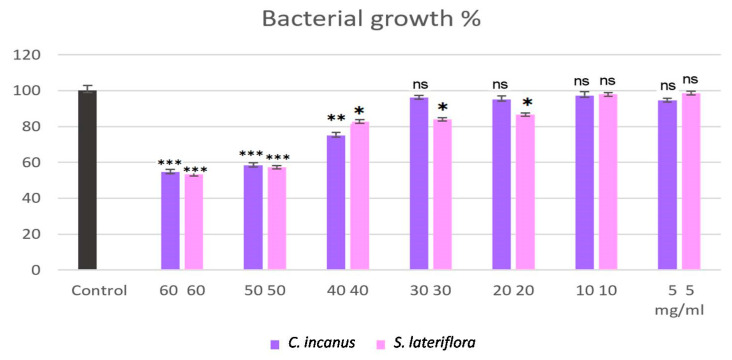
C. incanus and S. lateriflora extracts were tested for their antibacterial activity against P. gingivalis. The results showed mild dose-dependent bacterial growth inhibitory activity, which did not allow for the determination of the minimum inhibitory concentration (MIC) values. In fact, at the highest concentration (60 mg/mL) at the end of the treatment, the percentage of P. gingivalis growth was found to be reduced to 55% and 53% for C. incanus and S. lateriflora, respectively (Figure 4), in comparison to the control sample, in which P. gingivalis was grown without the extracts. In particular, compared to the control sample, the statistically significant differences in the inhibition of microbial growth by C. incanus extract and S. lateriflora extract treatments were recorded starting from the concentration of 40 mg/mL (p = 0.0025) and 20 mg/mL (p = 0.0198), respectively (Figure 4).
Figure 4.
Bacterial growth percentage of C. incanus and S. lateriflora extracts at different concentrations against P. gingivalis. Results are expressed as mean ± SD from three biological replicates (n = 3). ns: p > 0.05, *: p < 0.05, **: p < 0.01, ***: p < 0.001.
As expected, neither maltodextrin nor arabic gum exerted inhibitory effects on the bacterial growth.
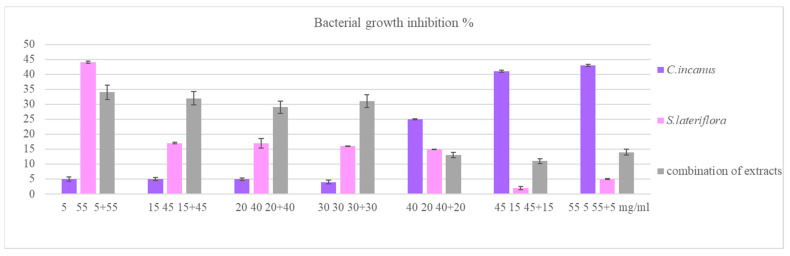
Considering the low recorded antibacterial activity, the extracts used alone (at the concentrations of 5, 15, 20, and 30 mg/mL) and their combinations in different ratios (i.e., 1:1, 1:2, 1:3, etc.) with the final concentrations of 60 mg/mL were subjected to the same test. The results, reported in Figure 5, are expressed as percentage of bacterial growth inhibition. As regards the antimicrobial activities of C. incanus and S. lateriflora used alone, the results of the statistical analysis have already been reported in Figure 4. As regards the antimicrobial activities of C. incanus and S. lateriflora combinations, a comparison between the bacterial growth percentage recorded following the treatment with C. incanus and S. lateriflora combinations and the bacterial growth percentage recorded in their absence (control sample) shows a significance in every combination (p < 0.05), including those combinations in which C. incanus used alone at concentrations lower than 40 mg/mL was not found to be effective.
Figure 5.
Bacterial growth inhibition percentage of C. incanus and S. lateriflora alone and in combinations at different concentrations against P. gingivalis.
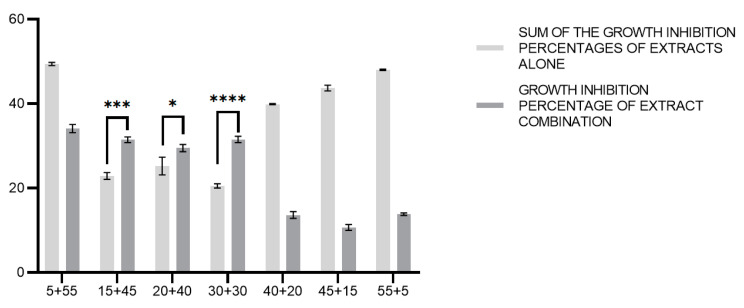
Interestingly, the results show that, for the combinations of C. incanus and S. lateriflora at concentration ratios of 1:3, 1:2, and 1:1, the percentages of bacterial growth inhibition are greater than the sum of the percentages of bacterial growth inhibition recorded for the individual C. incanus and S. lateriflora extracts at the same concentrations. More specifically, we compared the sums of the bacterial growth inhibitory activities induced by the treatments with C. incanus and S. lateriflora extracts used alone with the bacterial growth inhibitory activities induced by the combinations of these extracts using a multiple t-test analysis. As regards the combinations of C. incanus and S. lateriflora (at concentration ratios of 1:3, 1:2, and 1:1), the differences between the sum of the activities of the individual extracts and the activities of these combinations was found to be statistically significant (p < 0.001, p < 0.05, and p < 0.0001, respectively), with the combinations of extracts showing higher activities. In contrast, in the other cases, the sum of the activities of the individual extracts was greater when compared to the effects shown by the combinations of the two extracts (Figure 6).
Figure 6.
Comparison between the sums of the growth inhibition percentages of C. incanus and S. lateriflora alone and the growth inhibition percentages of C. incanus and S. lateriflora in combination. Results are expressed as mean ± SD from three biological replicated (n = 3). *: p < 0.05, ***: p < 0.001, ****: p < 0.0001.
3.4. Modulating Effects of C. incanus and S. lateriflora Extracts and Their Combinations on P. gingivalis Cell Invasive Capacity
To evaluate the activity of C. incanus and S. lateriflora extracts and their combinations on reducing the invasiveness of P. gingivalis in a HaCaT model system, HaCaT cells were treated with non-cytotoxic concentrations of C. incanus and S. lateriflora extracts (ranging from 10 to 60 mg/mL) before infection with P. gingivalis. The same was performed for the extract combinations at different ratios (i.e., 1:1, 1:2, 1:3, etc.) with final concentrations of 60 mg/mL (pre-treatment experimental condition). In addition, the co-treatment of HaCaT cells with P. gingivalis and the extracts used alone or in combination was also performed (co-treatment experimental condition). The results show that only the combination of C. incanus and S. lateriflora at the highest tested concentration (60 mg/mL), in the ratio 1:1, reduced the invasiveness of P. gingivalis, reflected as 6.4 × 105 ± 1.1 × 104 CFU/mL, compared to the control of 6.4 × 107 ± 2.0 × 105 CFU/mL (p = 0.0006). The other samples showed no modulation of the bacterial invasive capacity.
3.5. Effects of C. incanus and S. lateriflora Extracts and Their Combinations on the Degradation of Pre-Formed P. gingivalis Biofilm
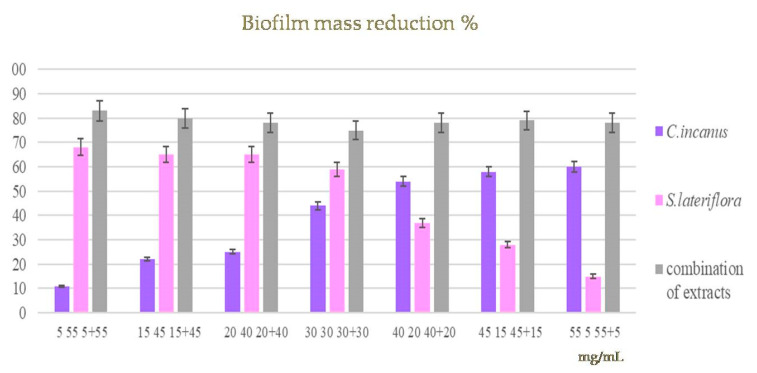
Biofilm formation is one of the main virulence mechanisms of P. gingivalis, contributing to an increase in the gingival tissue degradation process. The activity of C. incanus and S. lateriflora extracts and their combinations was evaluated on pre-formed biofilm. The biofilm biomass was quantified using CV in response to the treatments with the extracts used alone (at concentrations ranging from 5 to 55 mg/mL) and their combinations in different ratios (i.e., 1:1, 1:2, 1:3, etc.) with the final concentrations of 60 mg/mL, and then compared to measurements of the untreated mature biofilm. After 20 h of incubation, the samples induced a reduction in biofilm mass ranging from 11 to 56% and from 15 to 68% for the individual C. incanus and S. lateriflora, respectively. The combinations of the extracts in different ratios induced a biofilm mass reduction of about 80% (Figure 7). The statistical analysis shows that, by comparing the mass of biofilm produced by P. gingivalis following the treatment with C. incanus and S. lateriflora extracts used alone, in combination, and in their absence (control sample), we observed a statistically significant difference startingat the first tested concentration (5 mg/mL) (p < 0.05).
Figure 7.
A comparison between the mass of biofilm produced by P. gingivalis following treatment with C. incanus and S. lateriflora extracts alone, in combination, and in their absence (control) showed a significance starting at the first concentration tested (5 mg/mL).
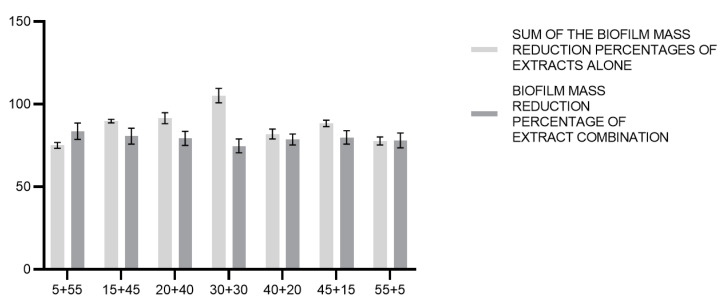
As regards the percentage of biofilm mass reduction inhibition, the sums of the activities exerted by C. incanus and S. lateriflora extracts used alone and those of the extract combinations were compared through a multiple t-test analysis. The results showed that the sum of the activities exerted by C. incanus and S. lateriflora used alone were greater compared to the effects exerted by the combinations of the two extracts (Figure 8). In this case, no significant difference was demonstrated (p < 0.05).
Figure 8.
Comparison between the theoretical sum of the biofilm mass reduction percentages of C. incanus and S. lateriflora in combinations and the biofilm mass reduction percentage of extract combination.
4. Discussion
P. gingivalis is one of the major pathogens responsible for severe and chronic manifestations of periodontal disease, producing a number of virulence factors that cause direct and indirect destruction to periodontal tissues through modulation of the host inflammatory response [29]. Although human gingival epithelium prevents intrusion by periodontal bacteria, P. gingivalis is able to invade gingival epithelial cells. Without any doubt, primary prevention (including the use of toothbrushes, dental floss, water picks, toothpicks, small interproximal brushes, rubber gum stimulators, and mouthwash with antimicrobial and antibiofilm activities) is not only the most effective but also the cheapest way of coping with periodontitis and its complications [30]. Considering microbial resistance to the antibacterial agents, scientific research draws its attention towards the assessment of plant extracts (rich in bioactive phytochemicals, i.e., flavonoids, alkaloids, tannins, and terpenoids) with antimicrobial activity that may counteract emerging microbial resistance, while exhibiting antimicrobial properties against P. gingivalis [31]. In this study, we demonstrate that acombination of two commercial extracts obtained from C. incanus and S. lateriflora, which consists of a complex mixture of bioactive compounds that is stable after in vitro simulated oral digestion, decreases the in vitro growth of P. gingivalis and enhances the barrier function of a gingival keratinocyte model system, exerting both protective effects against invasion by P. gingivalis and antibiofilm activity. In more detail, as far as the phytochemical profiles of C. incanus and S. lateriflora extracts are concerned, more than one hundred compounds were identified for each extract. The results obtained support previous reports on the phytochemical compositions of C. incanus and S. lateriflora extracts, although to date no study has determined the metabolic profile of these extracts in such detail [21,32,33,34,35,36,37,38,39]. Moreover, the bioaccessibility of C. incanus and S. lateriflora polyphenolic compounds, to which their antibacterial and antibiofilm activities against periodontal pathogens are ascribed, has a strong impact on their ability to exert their biological activities in the oral cavity and was assessed. To the best of our knowledge, no earlier investigation has been published indicating the bioaccessibility of C. incanus and S. lateriflora polyphenols after oral digestion, and we are reporting for the first time that the concentrations of the most represented polyphenols occurring in C. incanus and S. lateriflora extracts are stable under oral digestion conditions.
As far as the antibacterial activities of C. incanus and S. lateriflora extracts against P. gingivalis are concerned, our results show a mild inhibitory effecton bacterial growth for both extracts in a dose-dependent manner. Interestingly, the combinations of the extracts exerted a greater inhibition of bacterial growth, especially when S. lateriflora is present in the culture medium in higher concentrations than those of C. incanus. To the best of our knowledge, the antimicrobial activities of C. incanus and S. lateriflora extracts alone and in combination against P. gingivalis have never been studied. These results could be considered in agreement with data from the existing literature that demonstrates the antimicrobial properties of C. incanus against Gram-positive pathogens (i.e., S. aureus and S. epidermidis) [40] and Streptococcus mutans colonization on enamel samples exposed to oral fluids [34]. Moreover, S. lateriflora root extract showed antibacterial effects against Bacillus subtilis (NCIMB 3610) and E. coli (NCIMB 8879) with a minimum inhibition concentration of 5 mg/mL and 25 mg/mL, respectively [41]. Moreover, the combination of C. incanus and S. lateriflora yielded a slight reduction in the cellular invasiveness of P. gingivalis at the highest tested concentration in pre-treatment assay conditions. To the best of our knowledge, no earlier investigation has been published on the reduction in the cellular invasiveness of P. gingivalis in the presence of C. incanus and S. lateriflora extracts. The invasion of host cells is the first step for bacteria to establish pathogenic reservoirs and evade host defense mechanisms [42]. Furthermore, while the human gingival epithelium prevents intrusion by periodontal bacteria, P. gingivalis is able to invade gingival epithelial cells (primarily through the action of P. gingivalis proteases), breaking the oral epithelial barrier and spreading into periodontal tissues [42]. Thus, although the reduction in invasiveness recorded is moderate, it could contribute to a reduction in infection and periodontal tissue injury. Finally, as regards the capability of C. incanus and S. lateriflora to modulate mature P. gingivalis biofilm, known as subgingival plaque, the results show that the combination of these extracts is capable of the almost total degradation of biofilm. This result is all the more important considering that bacteria within a biofilm have shown 10–1000 times more antibiotic resistance than planktonic bacteria [43]. Oral biofilm protects bacteria from counteraction by the host’s immune system and antibacterial agents in vivo. Furthermore, cells embedded in biofilm are more resistant to antibiotic treatment as the virulence factors produced by P. gingivalis are contained within the biofilm, such as fimbriae, hemagglutinins, and proteinases [44]. The results obtained support previous investigations showing the antibiofilm activity of the extracts obtained from different species of the Cistus genus (i.e., C. creticus L., C. monspeliensis L., and C. laurifolius L.) and Scutellaria genus (i.e., S. baicalensis) against biofilm formation of Gram-positive bacteria (S. aureus, Bacillus subtilis, and S. mutans) and Gram-negative bacteria (E. coli, S. enterica, P. aeruginosa, and K. pneumonia) [20,22,45,46].
In conclusion, the combined effects of C. incanus and S. lateriflora in the inhibition of the growth of P. gingivalis and its invasiveness as well asthe reduction of pre-formed biofilm mass may open considerations of their use in the treatment of gingivitis and as adjunctive therapeutic agents to periodontitis. Further in vitro studies reflecting insights into the mechanism of action and clinical trials assessing the efficacy of these extracts in human subjects are currently in progress.
Acknowledgments
The authors wish to extend their gratitude to EPO S.R.L., Milan Italy for providing the samples tested in this investigation and to Eris Scott-Perring for English language support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12091826/s1, Table S1. Identified compounds in C. incanus extract according to molecular formula, m/z, and the retention time (RT); Table S2. Identified compounds in S. lateriflora extract according to molecular formula, m/z, and the retention time (RT).
Author Contributions
Conceptualization, M.D. and R.P.; methodology, A.D.M., A.D.F., E.S., D.G.B. and L.F.D.L.; software, A.D.M., A.D.F. and E.S.; validation, H.U. and D.G.B.; formal analysis, A.D.M. and L.F.D.L.; investigation, M.G. and H.R.E.-S.; resources, H.U.; data curation, H.U. and S.A.M.K.; writing—original draft preparation, H.U., A.D.M., A.D.F., E.S., D.G.B., L.F.D.L. and M.D.; writing—review and editing, H.U. and M.D.; visualization, P.C.; supervision, M.D. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partially supported by ‘DIREZIONE GENERALE UNIVERSITA’, RICERCA E OPEN INNOVATION of the Lombardy Region, Italy (DECRETO N. 9463 03/08/2020) that funded the project entitled: “EPO.MiO –Gli estrattivegetali per la salute del cavoorale con un impattofisiologicosistemico” within the call: “POR FESR 2014–2020—AZIONE I.1.B.1.1—BANDO INNODRIVER-S3—EDIZIONE 2019”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jeffcoat M.K., Hauth J.C., Geurs N.C., Reddy M.S., Cliver S.P., Hodgkins P.M., Goldenberg R.L. Periodontal disease and preterm birth: Results of a pilot intervention study. J. Periodontol. 2003;74:1214–1218. doi: 10.1902/jop.2003.74.8.1214. [DOI] [PubMed] [Google Scholar]
- 2.Gotsman I., Lotan C., Soskolne W.A., Rassovsky S., Pugatsch T., Lapidus L., Novikov Y., Masrawa S., Stabholz A. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J. Periodontol. 2007;78:849–858. doi: 10.1902/jop.2007.060301. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G., Chavakis T., Lambris J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000. 2020;84:14–34. doi: 10.1111/prd.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armitage G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosier B.T., Marsh P.D., Mira A. Resilience of the oral microbiota in health: Mechanisms that prevent dysbiosis. J. Dent. Res. 2018;97:371–380. doi: 10.1177/0022034517742139. [DOI] [PubMed] [Google Scholar]
- 6.Herrero E.R., Fernandes S., Verspecht T., Ugarte-Berzal E., Boon N., Proost P., Bernaerts K., Quirynen M., Teughels W. Dysbiotic biofilms deregulate the periodontal inflammatory response. J. Dent. Res. 2018;97:547–555. doi: 10.1177/0022034517752675. [DOI] [PubMed] [Google Scholar]
- 7.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, Periodontal Disease. [(accessed on 2 November 2022)];2013 Available online: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html.
- 10.Qasim S.S.B., Al-Otaibi D., Al-Jasser R., Gul S.S., Zafar M.S. An evidence-based update on the molecular mechanisms underlying periodontal diseases. Int. J. Mol. Sci. 2020;21:3829. doi: 10.3390/ijms21113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loos B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-García M., Hernández-Lemus E. Periodontal inflammation and systemic diseases: An overview. Front. Physiol. 2021;12:709438. doi: 10.3389/fphys.2021.709438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- 14.Bonez P.C., dos Santos Alves C.F., Dalmolin T.V., Agertt V.A., Mizdal C.R., da Costa Flores V., Marques J.B., Santos R.C.V., de Campos M.M.A. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control. 2013;41:e119–e122. doi: 10.1016/j.ajic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Buxser S. Has resistance to chlorhexidine increased among clinically-relevant bacteria? A systematic review of time course and subpopulation data. PLoS ONE. 2021;16:e0256336. doi: 10.1371/journal.pone.0256336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz Martínez C., Diaz Gómez M., Oh M.S. Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: A review. Pharm. Biol. 2017;55:1992–1998. doi: 10.1080/13880209.2017.1347188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar G., Jalaluddin M.D., Rout P., Mohanty R., Dileep C.L. Emerging trends of herbal care in dentistry. J. Clin. Diagn. Res. 2013;7:1827–1829. doi: 10.7860/JCDR/2013/6339.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irani S. Herbal medicine and oral health: A review. J. Int. Oral Health. 2016;8:989–994. [Google Scholar]
- 19.Hannig C., Spitzmüller B., Al-Ahmad A., Hannig M. Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J. Dent. 2008;36:540–545. doi: 10.1016/j.jdent.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Hickl J., Argyropoulou A., Sakavitsi M.E., Halabalaki M., Al-Ahmad A., Hellwig E., Aligiannis N., Skaltsounis A.L., Wittmer A., Vach K., et al. Mediterranean herb extracts inhibit microbial growth of representative oral microorganisms and biofilm formation of Streptococcus mutans. PLoS ONE. 2018;13:e0207574. doi: 10.1371/journal.pone.0207574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman S.H., Joshee N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Act. Plants. 2022;11:22–38. [Google Scholar]
- 22.Leung K.C.F., Seneviratne C.J., Li X., Leung P.C., Lau C.B.S., Wong C.H., Pang K.Y., Wong C.W., Wat E., Jin L. Synergistic antibacterial effects of nanoparticles encapsulated with Scutellaria baicalensis and pure chlorhexidine on oral bacterial biofilms. Nanomaterials. 2016;6:61. doi: 10.3390/nano6040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sommella E., Pagano F., Salviati E., Chieppa M., Bertamino A., Manfra M., Sala M., Novellino E., Campiglia P. Chemical profiling of bioactive constituents in hop cones and pellets extracts by online comprehensive two-dimensional liquid chromatography with tandem mass spectrometry and direct infusion Fourier transform ion cyclotron resonance mass spectrometry. J. Sep. Sci. 2018;41:1548–1557. doi: 10.1002/jssc.201701242. [DOI] [PubMed] [Google Scholar]
- 24.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carrière F., Boutrou R., Corredig M., Dupont D., et al. A standardized static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Schilling D., Pittelkow M.R., Kumar R. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene. 2001;20:7992–7997. doi: 10.1038/sj.onc.1204965. [DOI] [PubMed] [Google Scholar]
- 27.Misba L., Zaidi S., Khan A.U. A comparison of antibacterial and antibiofilm efficacy of phenothiazinium dyes between Gram positive and Gram negative bacterial biofilm. Photodiagn. Photodyn. Ther. 2017;18:24–33. doi: 10.1016/j.pdpdt.2017.01.177. [DOI] [PubMed] [Google Scholar]
- 28.Sumner L.W., Amberg A., Barrett D., Beale M.H., Beger R., Daykin C.A., Fan T.W.M., Fiehn O., Goodacre R., Griffin J.L., et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI) Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sao P., Vats S., Singh S. Porphyromonas gingivalis resistance and virulence: An integrated functional network analysis. Gene. 2022;839:146734. doi: 10.1016/j.gene.2022.146734. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Wang Y., Zhu X., Cao P., Wei S., Lu Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & LM Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017;113:396–402. doi: 10.1016/j.micpath.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Heupt L.K., Vierengel N., Groß J., Opatz T., Deschner J., von Loewenich F.D. Antimicrobial activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum extracts and rhein against Porphyromonas gingivalis. Antibiotics. 2022;11:186. doi: 10.3390/antibiotics11020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awad R., Arnason J.T., Trudeau V., Bergeron C., Budzinski J.W., Foster B.C., Merali Z. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties. Phytomedicine. 2003;10:640–649. doi: 10.1078/0944-7113-00374. [DOI] [PubMed] [Google Scholar]
- 33.Bernacka K., Bednarska K., Starzec A., Mazurek S., Fecka I. Antioxidant and antiglycation effects of Cistus × incanus water infusion, its phenolic components, and respective metabolites. Molecules. 2022;27:2432. doi: 10.3390/molecules27082432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittpahl G., Kölling-Speer I., Basche S., Herrmann E., Hannig M., Speer K., Hannig C. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and anti-adherent activity against Streptococcus mutans. Planta Med. 2015;81:1727–1735. doi: 10.1055/s-0035-1557822. [DOI] [PubMed] [Google Scholar]
- 35.Jeszka-Skowron M., Zgoła-Grześkowiak A., Frankowski R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC–MS/MS determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan Ngoc herbal tea. J. Food Compos. Anal. 2018;74:71–81. doi: 10.1016/j.jfca.2018.09.003. [DOI] [Google Scholar]
- 36.Riehle P., Vollmer M., Rohn S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013;53:891–899. doi: 10.1016/j.foodres.2012.09.020. [DOI] [Google Scholar]
- 37.Fecka I., Włodarczyk M., Starzec A. Isolation and structure elucidation of cistusin: A new ellagitannin from Cistus × incanus L. leaves. Ind. Crops Prod. 2020;158:112971. doi: 10.1016/j.indcrop.2020.112971. [DOI] [Google Scholar]
- 38.Li J., Wang Y.H., Smillie T.J., Khan I.A. Identification of phenolic compounds from Scutellaria lateriflora by liquid chromatography with ultraviolet photodiode array and electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012;63:120–127. doi: 10.1016/j.jpba.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda M., Iwabuchi K., Mimaki Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and their α-glucosidase inhibitory activities. Nat. Prod. Commun. 2012;7:1934578X1200700413. doi: 10.1177/1934578X1200700413. [DOI] [PubMed] [Google Scholar]
- 40.Kozłowska M., Ścibisz I., Przybył J.L., Laudy A.E., Majewska E., Tarnowska K., Małajowicz J., Ziarno M. Antioxidant and antibacterial activity of extracts from selected plant material. Appl. Sci. 2022;12:9871. doi: 10.3390/app12199871. [DOI] [Google Scholar]
- 41.Duffy C.F., Power R.F. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int. J. Antimicrob. Agents. 2001;17:527–529. doi: 10.1016/S0924-8579(01)00326-0. [DOI] [PubMed] [Google Scholar]
- 42.Hunstad D.A., Justice S.S. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 2010;64:203–221. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 43.Mah T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7:1061–1072. doi: 10.2217/fmb.12.76. [DOI] [PubMed] [Google Scholar]
- 44.Álvarez-Martínez F.J., Borrás-Rocher F., Micol V., Barrajón-Catalán E. Artificial intelligence applied to improve scientific reviews: The antibacterial activity of Cistus plants as proof of concept. Antibiotics. 2023;12:327. doi: 10.3390/antibiotics12020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nur Onal F., Ozturk I., Aydin Kose F., Der G., Kilinc E., Baykan S. Comparative evaluation of polyphenol contents and biological activities of five Cistus L. species native to Turkey. Chem. Biodivers. 2023;20:e202200915. doi: 10.1002/cbdv.202200915. [DOI] [PubMed] [Google Scholar]
- 46.Chen W., Li B., Li S., Ou Y.W., Ou Q. Effects of Scutellaria baicalensis on activity and biofilm formation of Klebsiella pneumoniae. Chin. Med. Sci. J. 2016;31:180–184. doi: 10.1016/S1001-9294(16)30048-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not available.