Abstract
Purpose
Excessive necroptosis contributes to the pathogenesis of several inflammatory and neurodegenerative diseases. Here, using a high-throughput screening approach, we investigated the anti-necroptosis effects of piperlongumine, an alkaloid isolated from the long pepper plant, in vitro and in a mouse model of systemic inflammatory response syndrome (SIRS).
Methods
A natural compound library was screened for anti-necroptosis effects in cellular. The underlying mechanism of action of the top candidate piperlongumine was explored by quantifying the necroptosis marker phosphorylated receptor-interacting protein kinase 1 (p-RIPK1) by Western blotting. The anti-inflammatory effect of piperlongumine was assessed in a tumor necrosis factor α (TNFα)-induced SIRS model in mice.
Results
Among the compounds investigated, piperlongumine significantly rescued cell viability. The half maximal effective concentration (EC50) of piperlongumine for inhibiting necroptosis was 0.47 μM in HT-29 cells, 6.41 μM in FADD-deficient Jurkat cells, and 2.33 µM in CCRF-CEM cells, while the half maximal inhibitory concentration (IC50) was 95.4 µM in HT-29 cells, 93.02 µM in FADD-deficient Jurkat cells, and 161.1 µM in CCRF-CEM cells. Piperlongumine also significantly inhibited TNFα-induced intracellular RIPK1 Ser166 phosphorylation in cell lines and significantly prevented decreases in body temperature and improved survival in SIRS mice.
Conclusion
As a potent necroptosis inhibitor, piperlongumine prevents phosphorylation of RIPK1 at its activation residue Ser166. Piperlongumine thus potently inhibits necroptosis at concentrations safe enough for human cells in vitro and inhibits TNFα-induced SIRS in mice. Piperlongumine has potential clinical translational value for the treatment of the spectrum of diseases associated with necroptosis, including SIRS.
Keywords: piperlongumine, natural product, drug repositioning, necroptosis, RIPK1
Introduction
Piperlongumine is a natural alkaloid compound found primarily in the long pepper and other Piper (pepper) plants.1,2 Piperlongumine and its derivatives have many biological and pharmacological effects including neuroprotective, anti-inflammatory, anti-platelet aggregation, immune, anti-aging, anti-tumor, and anti-diabetes actions. It is safe and has minimal toxicity, and it has long been used as a traditional Chinese medicine. Of these biological actions, the anti-tumor activities of piperlongumine and its derivatives have been most extensively studied.2–5
Necroptosis is a form of cell death initiated by activation of receptor-interacting protein kinase 1 (RIPK1), receptor-interacting protein kinase 3 (RIPK3), and mixed lineage kinase domain (MLKL) to form the “programmed necrosome-necrosome”.6 MLKL-related pore formation and membrane damage caused by osmotic shock release damage-associated molecular patterns (DAMPs), which trigger a cascade of inflammation.7 Necroptosis has now been implicated in several pathologies including neurodegenerative disease, cardiovascular abnormalities, and inflammatory diseases such as atherosclerosis, infectious disease, inflammatory bowel disease, ischemia-reperfusion injury, and hepatitis.8,9 Thus, necroptosis inhibitors are an exciting new class of agents for the treatment of many important acute and chronic diseases. Many drugs have been developed from natural products and/or their active derivatives, inspiring modern drug research and development efforts;10,11 indeed, natural products account for over 60% of all new small molecule drugs.12 Therefore, here we conducted high-throughput screening of a natural compound library and in doing so identify piperlongumine as an effective inhibitor of necroptosis.
Materials and Methods
Biological Reagents
Natural Product Library (Selleck Chemicals, Houston, TX; L1400), piperlongumine (Selleck Chemicals, S7551, CAS: 20069-09-4), Necrostatin-1 (Nec-1, Selleck Chemicals, S8037), Z-VAD-fmk (Selleck Chemicals, S7023); recombinant murine/human TNF-α (Sino Biological, Beijing, China; 50349-MNAE); SMAC mimetic (SM-164, Beyotime Biotechnology, Jiangsu, China; C0114-10 mM); dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). Anti-RIPK1 and anti-human phosphorylated RIPK1 antibodies for Western blotting were purchased from BD Biosciences (Franklin Lakes, NJ) and Cell Signaling Technology (Danvers, MA), respectively.
Cells and Cell Culture
Minimal essential medium (MEM) was supplemented with 100 μg/mL antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) (Gibco, Thermo Fisher Scientific, Waltham, MA), 10% fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology, China), and 1x non-essential amino acids (NEAA, Gibco). HT-29 cells and FADD-deficient Jurkat cells purchased from the ATCC were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco). CCRF-CEM cells were purchased from the ATCC and cultured in RPMI-1640 medium (HyClone Laboratories, Cytiva, Marlborough, MA) supplemented with 10% FBS and glutamine. All cell lines were cultured at 37°C in a humidified atmosphere with 5% CO2.
Necroptosis Induction and Cell Viability Analysis
Human TNFα (hTNFα) (50 ng/mL) solution was used to induce necroptosis in FADD-deficient Jurkat cells. HT-29 and CCRF-CEM cells were pretreated with z-VAD-fmk (20 µM) and smac164 (200 nM) for 30 min before adding hTNFα (20 ng/mL) to induce necroptosis. Compounds were added to cells at the indicated concentrations and incubated for 1 day. The Cell Titer-Glo Luminescent ATP Assay kit (Promega, Madison, WI) was used to measure cell viability. Absorbance or luminescence was determined using a BioTek 312e microplate reader.
Animal Experiments
All animal care and experimental protocols were approved by the China Three Gorges University (CTGU) Laboratory Animal Center’s Ethical Committee, and experiments were performed according to National Institutes of Health guidelines (NIH Publication No. 85-23, revised 1996). Mice were housed in the Laboratory Animal Center of the CTGU in a specific pathogen-free (SPF) animal facility (55 ± 5% humidity and 23 ± 2°C) with a light/dark cycle of 12:12 h. C57BL/6 J male mice used in the TNFα-induced systemic inflammatory response syndrome (SIRS) model were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). In addition, mice were administered 200 µL of compound (piperlongumine or Nec-1) in solvent or an equal volume of solvent (2.5% DMSO + 45% PEG 300 + 52.5% ddH2O) by gavage followed by tail vein injection of TNFα (2 µg per mouse) to induce SIRS. The rectal temperature of mice was then measured using an electronic thermometer. Mouse body temperature and signs of death were observed every 2 h for the first 12 h and then every 12 h until 72 h unless they died.
Western Blotting Analysis
Human FADD-deficient Jurkat cells and HT-29 cells were stimulated with TNFα and pre-incubated with 10 µM piperlongumine in the presence or absence of SM-164 + z-VAD for 30 min before induction of necroptosis. On microscopic analysis, the cells in the control group underwent obvious cell death, but cells in the treatment group did not. At this time, expression of the necrosis marker p-RIPK1 Ser166 was assessed by Western blot analysis. Cell samples were completely lysed in 1% NP-40 buffer, NaCl, Tris-base (pH 7.5), Pierce protease inhibitor tablets (Thermo Fisher Scientific), phenylmethylsulfonyl fluoride (PMSF), and NaF/Na3VO4 and the supernatant collected after centrifugation at 12,000× g for 15 min at 4°C. A 95°C water bath was then used to heat the supernatant and loading buffer for 10 min. Proteins were separated on SDS-PAGE gels and transferred to PVDF membranes (Millipore, Sigma, Burlington, MA). Membranes were washed three times in TBS containing Tween 20 and blocked with 5% skimmed milk before incubation with specific primary antibodies overnight at 4°C. Primary antibodies were all diluted to 1:1000. After three washes, membranes were incubated with HRP-conjugated secondary antibodies (Servicebio) at room temperature for 2 h. Blots were developed using a Tanon-4800 345 instrument (Tanon Science & Technology Co., Ltd., Shanghai, China).
The ADP-Glo™ Kinase Assay
To test the inhibition of RIPK1 kinase activity for piperlongumine, we followed the protocols for the RIPK1 kinase system (Promega, Cat# VA7591) and ADP-Glo™ Kinase Assay (Promega, Cat# V6930).
Statistical Analysis
One-way ANOVA and Student’s t-tests were used for comparisons of more than two or two groups, respectively. Results are expressed as means and standard deviations (SD). The Mantel Cox Log rank test was used to analyze differences in survival curves using GraphPad Prism 8.0 (GraphPad Prism, La Jolla, CA), and a P-value < 0.05 was considered statistically significant.
Results
In Cellular Drug Screening Identifies Piperlongumine as a Necroptosis Inhibitor
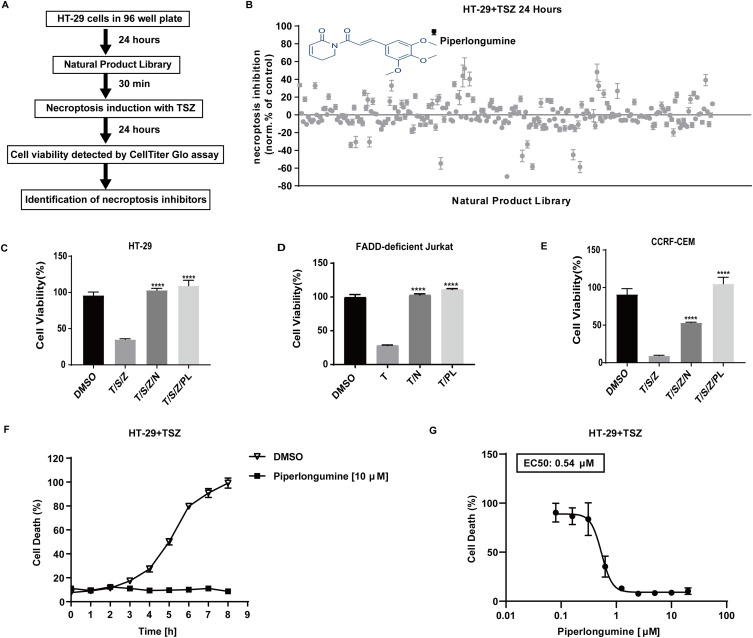
To search for novel necroptosis inhibitors, we screened a library consisting of 256 chemicals derived from natural products. Cultured HT-29 cells were pre-treated with each chemical prior to TSZ treatment (Figure 1A). Of the compounds investigated, piperlongumine most significantly rescued cell viability (Figure 1B and Supplementary Table 1). Necrostatin-1 (Nec-1) is a well-established specific inhibitor of the necroptosis-related RIPK1 kinase activity, so it was used here as a positive control. As expected, both Nec-1 and piperlongumine blocked necroptosis in HT-29 (Figure 1C), FADD-deficient Jurkat T cells (Figure 1D), and T-lineage leukemia CCRF–CEM cells (Figure 1E). Also, piperlongumine could both time-dependently (Figure 1F) and dose-dependently (Figure 1G) inhibit necroptosis induced by TSZ using SYTOX Green to detect cell death directly. Therefore, piperlongumine could be a potent and effective inhibitor of necroptosis.
Figure 1.
In cellular drug screening identifies piperlongumine as a necroptosis inhibitor. (A) Workflow diagram for a drug screen. (B) Inhibition values were calculated for different drugs on HT-29 cells (DMSO=0, Nec-1 (25 µM) =100) treated with SM164 (200 nM) and z-VAD-fmk (20 µM) followed by hTNFα (20 ng/mL). Values represent mean value± S.D. for 256 drugs assayed at 10 μM in duplicates, respectively. See Supplementary Table 1 for raw cell viability data of drug screen. For viability tests, (C) HT-29 cells (D) FADD-deficient Jurkat cells (E) CCRF-CEM cells were pretreated with piperlongumine (10 µM) plus SZ for 30 min, and then stimulated with hTNFα for 24 h to induce necroptosis (DMSO = 100). ATP (Cell Titer Glo) was used as a luminescence-based cell viability assay throughout the experiment. ****P < 0.0001, a significant difference was shown between the TSZ-induced cells and the drug pretreated cells. (F) HT-29 cells were pretreated with PL (10 μM) in the presence of SM-164 (200 nM) and z-VAD-fmk (20 μM) for 30 min followed by human TNF-α (20 ng/mL) for 8h to induce necroptosis. Dead cells were monitored in the presence of the cell membrane impermeable SYTOX Green (2.5 μM). The fluorescence was evaluated every 1 h from each cell. Triton X 100 (0.1%) for 3 h as maximal fluorescence intensities were taken as 100% dead cells. Data are represented as a percentage of dead cells after normalization to total cell number for each group. (G) Cell death was assessed using sytox green by dose-dependent protection of piperlongumine (0.1 µM-20 µM) against TSZ-induced necroptosis in HT-29 cells for 24 h.
Piperlongumine Efficiently Blocks Necroptosis
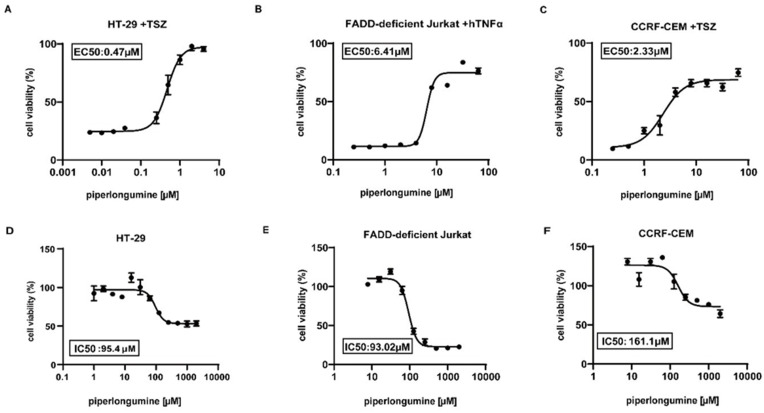
We next performed dose–response assays to obtain the effective concentration producing 50% inhibition of necroptotic cell death for piperlongumine. The half maximal effective concentration (EC50) of piperlongumine for the inhibition of necroptosis was 0.47 μM in HT-29 cells (Figure 2A), 6.41 μM in FADD-deficient Jurkat cells (Figure 2B), and 2.33 μM in CCRF-CEM cells (Figure 2C). To assess piperlongumine toxicity, the half maximal inhibitory concentration (IC50) was determined, which was 95.4 µM in HT-29 cells (Figure 2D), 93.02 µM in FADD-deficient Jurkat cells (Figure 2E), and 161.1 µM in CCRF-CEM cells (Figure 2F). Overall, the EC50 was much lower than the IC50, characterizing a therapeutic window for necroptosis inhibition. Thus, piperlongumine efficiently inhibited necroptosis at a concentration safe enough for human cells.
Figure 2.
Piperlongumine efficiently blocks necroptosis. The viability of EC50 in HT-29 cells (A), FADD-deficient Jurkat cells (B), and CCRF-CEM cells (C) was determined 24 h after the stimulation of hTNFα to induce necroptosis followed by pretreating with various concentrations of piperlongumine plus SZ for 30 min. The viability of IC50 was measured 24 h following incubation with different concentrations of piperlongumine in (D) HT-29 cells (E) FADD-deficient Jurkat cells and (F) CCRF-CEM cells.
Piperlongumine Inhibits Phosphorylation of RIPK1 at Ser166
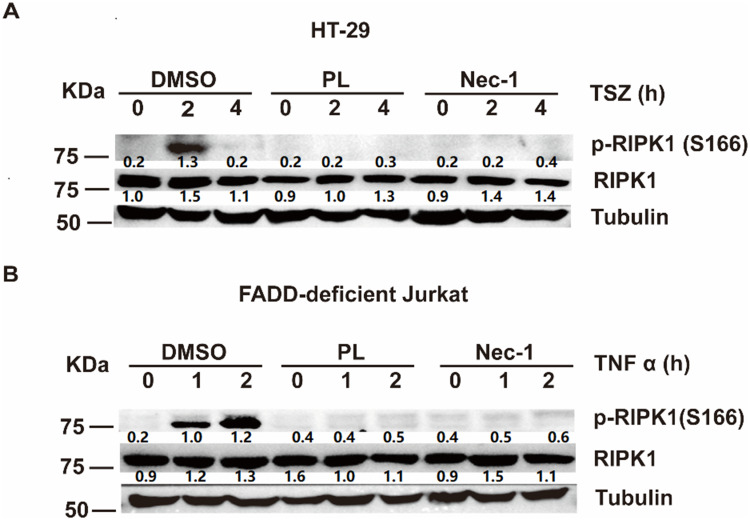
We next explored the molecular mechanism underlying piperlongumine-mediated inhibition of necroptosis. Necroptotic signaling begins with TNFα-induced phosphorylation of RIPK1 at Ser166, which further promotes RIPK3 and MLKL activation to induce cell death.13,14 Phosphorylation of RIPK1 at Ser166 has been identified as a biomarker of RIPK1 activation,15,16 so RIPK1 activation was analyzed by immunoblotting using anti-p-RIPK1 Ser166 antibodies. Piperlongumine, like Nec-1, significantly inhibited TNFα-induced intracellular p-RIPK1 Ser166 expression in HT-29 cells (Figure 3A) and FADD-deficient Jurkat cells (Figure 3B). Accordingly, piperlongumine likely inhibits necroptosis by inhibiting RIPK1 phosphorylation at Ser166.
Figure 3.
Piperlongumine inhibits phosphorylation of RIPK1 at Ser166. (A) HT-29 cells were pretreated 30 min using 10 µM piperlongumine, 25 µM Nec-1, or 0.2% DMSO incubating with SM164 (200 nM) and z-VAD-fmk (20 µM), and then stimulated by hTNF-α (20 ng/mL) to induce necroptosis for the specified time. (B) FADD-deficient Jurkat cells were pretreated with 10 µM piperlongumine, 25 µM Nec-1, or 0.2% DMSO for 30 min and incubated with hTNF-α (50 ng/mL) for the indicated times. The levels of p-RIPK1S166 were determined via Western blotting. The RIPK1 inhibitor Nec-1 was used as a positive control. Each lane of protein band density was normalized with the corresponding tubulin protein density. Scan gray scale of the bands and calculate relative expression level. The Image-processing software (Image J) was used as the image quantification software.
Piperlongumine Protects Mice from TNF-α-Induced SIRS
TNF-α-induced systemic inflammatory response syndrome (SIRS) is an animal model of sepsis, and its pathogenic mechanism involves cell death.17 SIRS was induced in mice by intravenous injection of murine TNF-α, and mice developed cell death in lung epithelial cells, hepatocytes, and vascular endothelial cells.18 As an inhibitor of cell death, Nec-1 effectively prevents reductions in body temperature and death in SIRS mice,19 confirming that TNF-α-induced SIRS mice are a suitable animal model for preclinical studies of cell death inhibitors.20
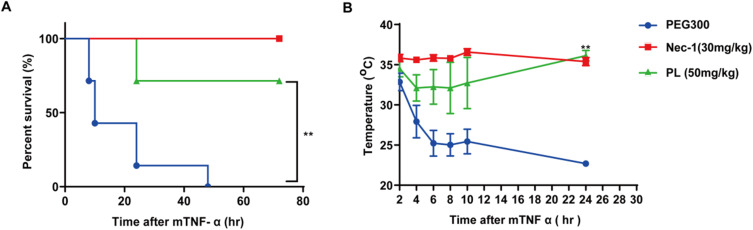
We examined whether piperlongumine could prevent the death of SIRS mice following oral gavage with piperlongumine compared with controls without piperlongumine treatment. Piperlongumine, like Nec-1, significantly improved survival (Figure 4A) and prevented decreases in the body temperature (Figure 4B) in SIRS mice. These results demonstrate that piperlongumine effectively protects against TNF-α-induced SIRS in vivo.
Figure 4.
Piperlongumine protects mice from TNF-α-induced SIRS. Piperlongumine (50 mg/kg) or Nec-1 (30 mg/kg) was pretreated via gavage for 30 min, followed by mTNF-α injections (2 µg per mouse) to induce SIRS in 10-week-old male C57BL/6J mice. These images demonstrate (A) the survival curves and (B) the body temperature changes for the vehicle (PEG300), piperlongumine and Nec-1 treated mice (n = 9 for each group). **P < 0.05, a significant difference was shown between the vehicle control group and the experimental group.
Discussion
Here we discovered piperlongumine as a novel anti-necroptosis agent using a cell-based high-throughput screening approach. We found that piperlongumine efficiently inhibits necroptosis, which has been implicated in the development of inflammatory and neurodegenerative diseases. Our findings suggest that piperlongumine may be a highly potent inhibitor of RIPK1 and therefore a new drug candidate for the treatment of RIPK1-related diseases.
Piperlongumine was a significantly more efficacious inhibitor of necroptosis than the other 2658 natural compounds assessed. The EC50 of piperlongumine for inhibiting necroptosis was also far less than its IC50 values. Piperlongumine therefore shows the critical advantages of high safety and high efficacy in inhibiting necroptosis in human-derived cell lines. These data suggest that piperlongumine may have translational value as a drug for the spectrum of diseases associated with necroptosis, including SIRS.
Septic shock and death can be rapidly induced by systemic over production of TNFα and other proinflammatory cytokines.21 Intravenous injection of TNFα in mice results in shock and death accompanied by necroptosis in multiple organs and an inflammatory factor storm. Our study confirmed that piperlongumine could rescue SIRS mice from death while improving their hypothermia status. Sepsis and SIRS remain major causes of death in intensive care unit patients,22–24 and our findings now suggest that piperlongumine has potential as a therapeutic agent for sepsis and SIRS.
In the necroptosis pathway, RIPK1 plays a central role in inducing cell death. RIPK1 activity can be regulated by its tyrosine phosphorylation, and preventing autophosphorylation of RIPK1 at Ser166 inhibits RIPK1-mediated cell death.15,16 We found that piperlongumine inhibits phosphorylation of RIPK1 Ser166, suggesting that piperlongumine inhibits RIPK1 kinase activity-dependent cell death, providing a potential anti-inflammatory mechanism of action.
A previous study suggesting that piperlongumine can inhibit apoptosis and block NF-κB signaling in ischemic cerebral injury models.25 It has been also demonstrated that piperlongumine inhibits cancer growth by inhibiting NF‑κB activation in vitro.26,27 Our results presented some conflicts with other findings that piperlongumine activates RIPK1 to trigger necroptosis in bladder cancer cells.28
There have also been reports that piperlongumine can pass across the blood-brain barrier into the central nervous system (CNS).29 Therefore, piperlongumine, as a novel necroptosis inhibitor, holds promise not only for the treatment of systemic diseases such as sepsis, psoriasis, rheumatoid arthritis, inflammatory bowel disease, and vitiligo but also CNS and neurodegenerative diseases such as stroke, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), and multiple sclerosis.
This study has limitations. First, TNF-α triggers several signaling pathways leading to NF-κB activation, RIPK1-independent apoptosis, RIPK1-dependent apoptosis, and necroptosis. Our findings showed that piperlongumine specifically inhibited TSZ-induced necroptosis (Supplementary Figure 1A), but did not protect against TS-induced apoptosis in HT-29 (Supplementary Figure 1B). Furthermore, piperlongumine did not inhibit RIPK1 kinase activity directly in vitro ADP-Glo kinase assays (Supplementary Figure 1C). Thus, the exact mechanism and pathway by which piperlongumine inhibits necroptosis requires further clarification. More studies on the effects of piperlongumine on TNF-α-induced TNF receptor 1 (TNFR1) complex formation, NF-κB activation, and caspase activation are warranted. Second, given its favorable pharmacological activity and unique chemical structure, piperlongumine and its derivatives are promising lead clinical drug candidates, and further structural studies need to be performed to pave the way for its clinical application for the targeted treatment of diseases characterized by abnormal activation of necroptosis.
Acknowledgments
We sincerely thank Hongbing Zhang, Professor of Peking Union Medical College, for his helpful instruction and reviewing the manuscript.
Funding Statement
This research was supported by grants from Local Science and Technology Development projects guided by the central government (ZYYD2020000202), the General Project of Hubei Province Health Committee (WJ2021M257), Yichang Famous Doctor Studio, Yichang Training Talents of Innovation Entrepreneurship and Excellence-creating project (JY201701), Hubei Province’s Outstanding Medical Academic Leader program (EWT201947), Project of Yichang City Medical and Health Research (A22-2-031), Science and Technology Research Project of Hubei Provincial Department of Education (No.Q20221214) and PhD Research Project Start-up Fund of the First Hospital of Yichang (No.Q2021003).
Ethical Approval
The animal study was under the reviewing and approval of the Medical Animal Care & Welfare Committee of CTGU (China Three Gorges University) (No.20210B0801A).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report a patent “new application of piperlongumine in inhibiting necroptosis” (ZL202111429558.8) issued to the First Hospital of Yichang. The authors have no other conflicts of interest to declare.
References
- 1.Chen SY, Liu GH, Chao WY, et al. Piperlongumine suppresses proliferation of human oral squamous cell carcinoma through cell cycle arrest, apoptosis and senescence. Int J Mol Sci. 2016;17(4). doi: 10.3390/ijms17040616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JA, Na HH, Jin HO, Kim KC. Increased expression of FosB through reactive oxygen species accumulation functions as pro-apoptotic protein in piperlongumine treated MCF7 breast cancer cells. Mol Cells. 2019;42(12):884–892. doi: 10.14348/molcells.2019.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi DG, Venkatesan J, Shim MS. Selective anticancer therapy using pro-oxidant drug-loaded chitosan-fucoidan nanoparticles. Int J Mol Sci. 2019;20:13. doi: 10.3390/ijms20133220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makhov P, Golovine K, Teper E, et al. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br J Cancer. 2014;110(4):899–907. doi: 10.1038/bjc.2013.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu P, Qian J, Xu Z, et al. Overview of piperlongumine analogues and their therapeutic potential. Eur J Med Chem. 2021;220:113471. doi: 10.1016/j.ejmech.2021.113471 [DOI] [PubMed] [Google Scholar]
- 6.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci U S A. 2019;116(20):9714–9722. doi: 10.1073/pnas.1901179116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20(1):19–33. doi: 10.1038/s41583-018-0093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18(2):127–136. doi: 10.1038/nrm.2016.149 [DOI] [PubMed] [Google Scholar]
- 9.Shan B, Pan H, Najafov A, Yuan J. Necroptosis in development and diseases. Genes Dev. 2018;32(5–6):327–340. doi: 10.1101/gad.312561.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouprina N, Noskov VN, Larionov V. Selective isolation of large segments from individual microbial genomes and environmental DNA samples using transformation-associated recombination cloning in yeast. Nat Protoc. 2020;15(3):734–749. doi: 10.1038/s41596-019-0280-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musalov M, Potapov VA, Yakimov VA, et al. A regioselective synthesis of novel functionalized organochalcogen compounds by chalcogenocyclofunctionalization reactions based on chalcogen halides and natural products. Molecules. 2021;26(12):3729. doi: 10.3390/molecules26123729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Shu C, Lian X, Zhang Z. New antibacterial bagremycins F and G from the marine-derived streptomyces sp. ZZ745. Mar Drugs. 2018;16(9):330. doi: 10.3390/md16090330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YH, Chou TF, Young L, et al. Tumor suppressor death-associated protein kinase 1 inhibits necroptosis by p38 MAPK activation. Cell Death Dis. 2020;11(5):305. doi: 10.1038/s41419-020-2534-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng J, Ito Y, Shi L, et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8(1):359. doi: 10.1038/s41467-017-00406-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurien L, Nagata M, Schünke H, et al. Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat Commun. 2020;11(1):1747. doi: 10.1038/s41467-020-15466-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada T, Kudoh Y, Noguchi T, et al. The E3 ubiquitin-protein ligase RNF4 promotes TNF-α-induced cell death triggered by RIPK1. Int J Mol Sci. 2021;22(11):5796. doi: 10.3390/ijms22115796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng H, Liu Z, Li X, et al. Death-domain dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc Natl Acad Sci U S A. 2018;115(9):E2001–E2009. doi: 10.1073/pnas.1722013115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dannappel M, Vlantis K, Kumari S, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513(7516):90–94. doi: 10.1038/nature13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi N, Duprez L, Grootjans S, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Liang Y, Zhao S, et al. ZBP1 mediates interferon-induced necroptosis. Cell Mol Immunol. 2020;17(4):356–368. doi: 10.1038/s41423-019-0237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murapa P, Ward MR, Gandhapudi SK, Woodward JG, D’Orazio SEF. Heat shock factor 1 protects mice from rapid death during Listeria monocytogenes infection by regulating expression of tumor necrosis factor alpha during fever. Infect Immun. 2011;79(1):177–184. doi: 10.1128/IAI.00742-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heffernan DS, Monaghan SF, Ayala A. Lymphocyte integrin expression differences between SIRS and sepsis patients. Ir J Med Sci. 2017;186(4):981–987. doi: 10.1007/s11845-016-1525-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med. 2015;41(9):1620–1628. doi: 10.1007/s00134-015-3906-y [DOI] [PubMed] [Google Scholar]
- 25.Yang T, Sun S, Wang T, et al. Piperlonguminine is neuroprotective in experimental rat stroke. Int Immunopharmacol. 2014;23(2):447–451. doi: 10.1016/j.intimp.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 26.Awasthee N, Shekher A, Rai V, et al. Piperlongumine, a piper alkaloid, enhances the efficacy of doxorubicin in breast cancer: involvement of glucose import, ROS, NF-κB and lncRNAs. Apoptosis. 2022;27(3–4):261–282. doi: 10.1007/s10495-022-01711-6 [DOI] [PubMed] [Google Scholar]
- 27.Lu C, Zhang B, Xu T, et al. Piperlongumine reduces ovalbumin-induced asthma and airway inflammation by regulating nuclear factor-κB activation. Int J Mol Med. 2019;44(5):1855–1865. doi: 10.3892/ijmm.2019.4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X, Chen G, Hu W. Piperlongumine increases the sensitivity of bladder cancer to cisplatin by mitochondrial ROS. J Clin Lab Anal. 2022;36(6):e24452. doi: 10.1002/jcla.24452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TH, Song J, Kim SH, et al. Piperlongumine treatment inactivates peroxiredoxin 4, exacerbates endoplasmic reticulum stress, and preferentially kills high-grade glioma cells. Neuro Oncol. 2014;16(10):1354–1364. doi: 10.1093/neuonc/nou088 [DOI] [PMC free article] [PubMed] [Google Scholar]