Abstract
Introduction
The pathogenesis between oxidative stress and periodontitis was correlated. The Oxidative Balance Score (OBS) is a systematic tool to assess the effects of diet and lifestyle in relation to oxidative stress. However, the association between OBS and periodontitis has not been reported previously.
Methods
Sixteen dietary factors and four lifestyle factors were selected to score the OBS. Multivariate logistic regression and sensitivity analysis were used to investigate the relationship between OBS and periodontitis based on data from the National Health and Nutrition Examination Survey (NHANES) 1999–2018. Subgroup analysis and interaction tests were used to investigate whether this association was stable across populations.
Results
This study included 3,706 participants. There was a negative linear association between OBS and periodontitis in all participants [0.89 (0.80, 0.97)], and after converting OBS to a quartile variable, participants with OBS in the highest quartile had a 29% lower risk of periodontitis than those with OBS in the lowest quartile [0.71 (0.42, 0.98)]. This negative association differed with respect to age and diabetes.
Conclusion
There is a negative association between OBS and periodontitis in US adults. Our results suggest that OBS may be used as a biomarker for measuring periodontitis.
Keywords: Oxidative Balance Score (OBS), periodontitis, oxidative stress, NHANES, antioxidants
1. Introduction
Periodontitis is a common inflammatory disease of the oral cavity (1, 2), with the majority of cases occurring in people aged 55–59 years old (3), which is an important cause of tooth loss in adults (4). Plaque biofilm initiates the process, influencing the host’s immunological function and inflammatory response (5, 6). Bacterial and their metabolite-produced inflammatory mediators cause immunological dysfunction and periodontal tissue damage (7). Several previous epidemiological studies have demonstrated that periodontitis is a risk factor for various systemic illnesses (8, 9), with low-grade inflammation in the peripheral circulation being linked to the genesis and development of several diseases (10). Periodontitis has been linked to depression (11), Alzheimer’s disease (12), metabolic syndrome (13), and cardiovascular disease in various studies (14, 15).
An increasing body of research shows that the inflammatory response to periodontitis is linked to elevated local and systemic oxidative stress, as well as decreased antioxidant capacity (16, 17). Reactive oxygen species, which are primarily created in excess by hyperactive neutrophils as periodontitis develops, are not counterbalanced by the antioxidant defense system and cause tissue damage (18), increased metabolites of protein damage (19), DNA damage (20), and lipid peroxidation are characteristics of the process (21). Periodontitis may potentially have an impact on the antioxidants’ local and systemic actions (19).
The relationship between oxidative stress and periodontitis has attracted the interest of researchers. Over the past 40 years, studies have been reported on the association between several antioxidants and the prevalence of periodontitis (22–24). However, there are differences in data collection methods for dietary intake in many studies, which may explain the conflicting results of some studies (25). More importantly, antioxidants work systematically in concert, so measuring individual species in isolation can present limitations (26). For example, in addition to dietary factors, a number of lifestyle factors, including smoking (27), alcohol consumption (28), physical activity, and obesity (29, 30), also have an impact on organismal inflammation and oxidative stress.
The Oxidative Balance Score (OBS) is a composite indicator that assesses the oxidative balance of an individual (31). Generally, a higher OBS indicates a preference for antioxidants over pro-oxidants (32). The negative associations between OBS and a number of inflammation-related diseases has been found in several epidemiological studies, including cardiovascular disease (33), type 2 diabetes (34), chronic kidney disease (35), and osteoarthritis (36). However, no studies have assessed the association between OBS and periodontitis. Therefore, I conducted a cross-sectional study to examine the association between dietary and lifestyle integrated OBS and periodontitis according to the National Health and Nutrition Examination Survey (NHANES) 1999–2018.
2. Materials and methods
2.1. Study population
The NHANES is a continuous nationwide survey that investigates the nutrition and health condition of adults and children in the United States (37, 38). The National Center for Health Statistics (NCHS) Research Ethics Review Board authorized the study protocol. At the time of recruiting, all participants provided written consent at the time of recruitment. This study utilizes the most recent five survey cycles of data from the last decade to conduct the survey. We excluded 69,441 participants without complete OBS data, 43,516 participants with missing periodontal examination data, and 215 extreme dietary intakes. The study eventually included 3,706 participants (Figure 1).
FIGURE 1.
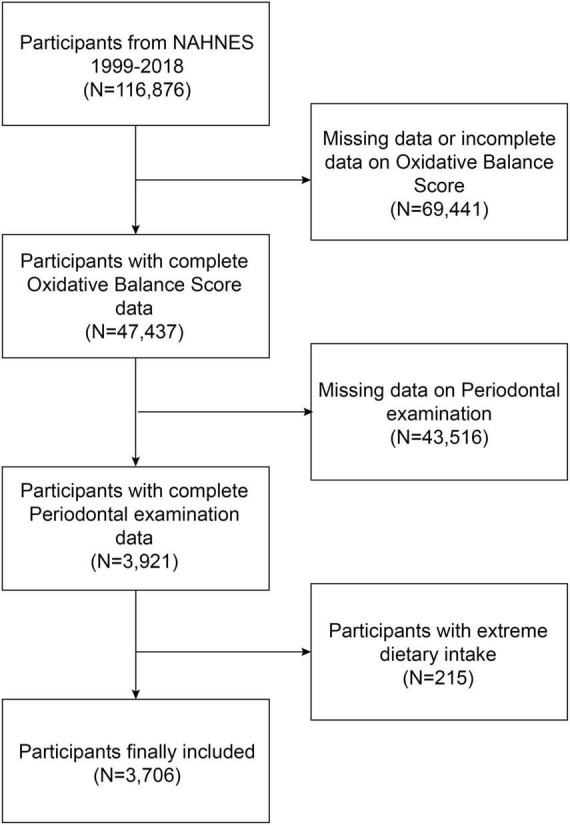
Flow chart of participants selection. NHANES, National Health and Nutrition Examination Survey.
2.2. Oxidative Balance Score
Based on past research and experience, the OBS calculation combines the contributions of 16 dietary factors and 4 lifestyle factors, including 15 antioxidants and 5 pro-oxidants (39–42). Table 1 demonstrates the detailed scoring scheme of the OBS, with the first through third quartiles assigned a score of 0–2 for dietary antioxidants and 0 for pro-oxidants in the highest tertile and 2 in the lowest tertile. For lifestyle factors including physical activity (0 points for <400 MET-minute/week; 1 point for 400–1,000 MET-minute/week; 2 points for >1,000 MET-minute/week), alcohol intake (0 points for >30 g/d; 1 point for 0–30 g/d; 2 points for None), BMI (0 points for obesity. 1 for overweight; 2 for normal weight) and serum cotinine level (0 points for >0.038 ng/mL; 1 point for 0.038–1.13 ng/mL; 2 points for <1.13 ng/mL) (43, 44).
TABLE 1.
Oxidative Balance Score assignment scheme.
| OBS components | Property | Scoring assignment | ||
| 0 | 1 | 2 | ||
| Dietary components | ||||
| Dietary fiber (g/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Carotene (RE/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Riboflavin (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Niacin (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Vitamin B6 (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Total folate (mcg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Vitamin B12 (mcg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Vitamin C (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Vitamin E (ATE) (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Calcium (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Magnesium (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Zinc (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Copper (mg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Selenium (mcg/d) | Antioxidant | Tertile 1 | Tertile 2 | Tertile 3 |
| Total fat (g/d) | Prooxidant | Tertile 3 | Tertile 2 | Tertile 1 |
| Iron (mg/d) | Prooxidant | Tertile 3 | Tertile 2 | Tertile 1 |
| Lifestyle components | ||||
| Physical activity (MET-minute/week) | Antioxidant | <400 | 400–1,000 | >1,000 |
| Alcohol (g/d) | Prooxidant | >30 | 0–30 | None |
| Body mass index (kg/m2) | Prooxidant | >30 | 25–30 | <25 |
| Cotinine (ng/mL) | Prooxidant | >0.038 | 0.038–1.13 | <1.13 |
OBS, Oxidative Balance Score; RE, retinol equivalent; ATE, alpha-tocopherol equivalent; MET, metabolic equivalent.
2.3. Periodontitis
All participants in this study were examined by experienced dentists, and the specific examination procedures are described in the operating manual available on the NHANES website (45–47). For the classification of periodontal disease, we used the 2012 CDC/American Academy of Periodontology (AAP) case definition of periodontitis. Mild periodontitis was classified as two interproximal sites with attachment loss (AL) of three millimeters and two interproximal sites with pocket depth (PD) of four millimeters (not on the same tooth) or one site with PD of five millimeters. Moderate periodontitis was defined as two interproximal sites with AL 4 mm (not on the same tooth) or two interproximal sites with PD 5 mm (not on the same tooth). A total of 2 interproximal sites with AL 6 mm (not on the same tooth) and 1 interproximal site with PD 5 mm were categorized as severe periodontitis. The final number of periodontitis cases was the sum of mild, moderate, and severe cases (48).
2.4. Covariables
Covariates included age, gender, LDL-C (low-density lipoprotein cholesterol), race, diabetes, family income-to-poverty ratio (PIR), cancer, waist circumference, triglycerides, education level, and serum klotho levels. Detailed information on variable collection methods can be found in the NHANES Survey Methods and Analysis Guide.1
2.5. Statistical analysis
All analyses were performed with R (version 4.2) or Empowerstats (version 4.1). The chi-square test and t-test were used to assess the demographic characteristics of the participants by OBS quartile. Weighted multivariate logistic regression analyses were used to investigate the linear associations between OBS and periodontitis. After transforming OBS from a continuous variable to a categorical variable (quartile) a trend test was used to investigate the trend of linear association between OBS and periodontitis. Subgroup analysis was used to investigate the association between OBS and periodontitis in people of different gender, race, education, and diabetes status, and interaction tests were used to investigate whether the associations were consistent across subgroups. Statistical significance defined as two-sided p < 0.05.
3. Results
3.1. Baseline characteristics
At the time of assessment, the mean (SD) age of the 3,706 participants was 53.37 (14.89) years, 53.13% participants were females, and a total of 2,598 participants (70.08%) were diagnosed with periodontitis. In comparison to the bottom OBS quartile, participants in the top OBS quartile are more likely to be males, non-Hispanic white people and younger; In terms of socioeconomic status, higher OBS participants were more likely to have higher educational attainment and higher income; in terms of lifestyle, a lower proportion of higher OBS participants drank alcohol. In addition, participants in the lowest OBS quartile were more likely to with cancer and diabetes; to have lower BMI and waist circumference in terms of body size, and lower serum cotinine levels (Table 2). Supplementary Table 1 depicts the differences in clinical characteristics of participants with and without periodontitis.
TABLE 2.
Basic characteristics of participants by Oxidative Balance Score quartile.
| Characteristics | Oxidative Balance Score | P-value | |||
|
Q1 N = 927 |
Q2 N = 926 |
Q3 N = 926 |
Q4 N = 927 |
||
| Age (years) | 54.80 ± 15.29 | 53.43 ± 14.49 | 52.97 ± 14.39 | 52.61 ± 14.24 | 0.018 |
| Periodontitis, (%) | <0.001 | ||||
| Yes | 75.13 | 70.58 | 63.44 | 52.67 | |
| No | 24.87 | 29.42 | 36.56 | 47.33 | |
| Sex, (%) | <0.001 | ||||
| Male | 36.57 | 47.08 | 55.83 | 62.89 | |
| Female | 63.43 | 52.92 | 44.17 | 37.11 | |
| Race/Ethnicity, (%) | <0.001 | ||||
| Non-Hispanic white | 47.03 | 48.16 | 51.51 | 54.37 | |
| Non-Hispanic black | 21.47 | 17.93 | 15.01 | 11.87 | |
| Mexican American | 15.53 | 17.49 | 19.33 | 19.20 | |
| Other race/Multiracial | 15.97 | 16.42 | 14.15 | 14.56 | |
| Education level, n (%) | <0.001 | ||||
| Less than high school | 37.22 | 30.67 | 27.11 | 22.33 | |
| High school | 25.89 | 21.60 | 23.33 | 18.55 | |
| More than high school | 36.89 | 47.73 | 49.56 | 59.12 | |
| Smoking, (%) | 0.206 | ||||
| Ever | 50.05 | 46.44 | 47.30 | 45.31 | |
| Never | 49.95 | 53.56 | 52.70 | 54.69 | |
| Drinking alcohol, (%) | 0.006 | ||||
| Ever | 71.81 | 61.12 | 62.35 | 58.37 | |
| Never | 28.19 | 38.88 | 37.65 | 41.63 | |
| Cancer, (%) | 0.341 | ||||
| Yes | 10.79 | 9.83 | 9.18 | 12.51 | |
| No | 89.21 | 90.17 | 90.82 | 87.49 | |
| Diabetes, (%) | 0.001 | ||||
| Yes | 15.43 | 15.77 | 10.04 | 10.25 | |
| No | 82.31 | 81.97 | 87.58 | 86.95 | |
| Borderline | 2.26 | 2.26 | 2.38 | 2.80 | |
| BMI (kg/m2) | 30.02 ± 6.99 | 29.98 ± 6.84 | 29.29 ± 6.21 | 28.69 ± 6.06 | <0.001 |
| Waist circumference (cm) | 101.30 ± 15.91 | 101.69 ± 16.10 | 100.37 ± 14.98 | 98.99 ± 14.52 | 0.002 |
| PIR | 2.18 ± 1.51 | 2.46 ± 1.58 | 2.70 ± 1.63 | 3.00 ± 1.66 | <0.001 |
| Triglycerides (mg/dL) | 141.84 ± 129.14 | 134.99 ± 106.87 | 139.92 ± 107.54 | 132.09 ± 143.62 | 0.514 |
| Klotho (pg/mL) | 813.23 ± 303.24 | 817.82 ± 277.08 | 831.34 ± 271.82 | 815.73 ± 313.40 | 0.823 |
| LDL-C (mg/dL) | 119.76 ± 34.45 | 120.93 ± 35.04 | 121.05 ± 35.01 | 116.19 ± 33.77 | 0.184 |
| Serum cotinine (ng/ml) | 98.92 ± 153.72 | 51.32 ± 122.73 | 36.25 ± 101.03 | 29.92 ± 95.40 | <0.001 |
Mean ± SD for continuous variables: the P-value was calculated by the weighted linear regression model. (%) for categorical variables: the P-value was calculated by the weighted chi-square test. Q, quartile; PIR, ratio of family income to poverty; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol.
3.2. Association between Oxidative Balance Score and periodontitis
Table 3 shows the association between OBS and periodontitis. We found higher OBS was negatively correlated with periodontitis both in crude model [0.79 (0.65, 0.92)] and adjusted model [0.82 (0.70, 0.93)]. After adjusted all covariables, each one-unit increase in the OBS score was found to be associated with an 11% decrease in the risk of periodontitis [0.89 (0.80, 0.97)]. After changing the OBS from a continuous to a categorical variable, sensitivity analyses were carried out. In the fully adjusted model, participants in the highest quartile had a 29% lower risk of periodontitis compared to those in the lowest quartile of OBS [0.71 (0.42, 0.98)]. Supplementary Tables 2, 3 demonstrate the association between OBS and periodontitis-related variables, with results showing that higher OBS scores are associated with lower C-reactive protein levels and with higher grades of self-reported oral health.
TABLE 3.
The associations between Oxidative Balance Score and periodontitis.
| Exposure | Model 1 [OR (95% CI)] | Model 2 [OR (95% CI)] | Model 3 [OR (95% CI)] |
| Oxidative Balance Score (continuous) | 0.79 (0.65, 0.92) | 0.82 (0.70, 0.93) | 0.89 (0.80, 0.97) |
| Oxidative Balance Score (quartile) | |||
| Quartile 1 | reference | reference | reference |
| Quartile 2 | 0.91 (0.79, 1.03) | 0.90 (0.81, 0.99) | 0.93 (0.88, 0.98) |
| Quartile 3 | 0.83 (0.72, 0.94) | 0.81 (0.72, 0.91) | 0.84 (0.70, 1.00) |
| Quartile 4 | 0.53 (0.35, 0.72) | 0.62 (0.39, 0.85) | 0.71 (0.42, 0.98) |
| P for trend | <0.001 | <0.001 | <0.001 |
Model 1: No covariates were adjusted. Model 2: Age, gender, and race were adjusted. Model 3: Age, gender, race, diabetes, cancer, PIR, triglycerides, klotho, and LDL-C were adjusted. PIR, ratio of family income to poverty; LDL-C, low-density lipoprotein cholesterol.
3.3. Subgroup analyses
Subgroup analyses and interaction tests stratified by age, sex, race, BMI, and diabetes were performed to assess whether the relationship between OBS and periodontitis was consistent in the general population and to identify any potentially different population settings. Our findings showed that the associations were inconsistent. There was no statistical significance for gender, race, or BMI, as shown in Table 4, but we did find a significant interaction between age and diabetes (P for interaction <0.05). The negative association effect of OBS with periodontitis was significantly greater in older adults older than 60 years [0.83 (0.76, 0.91)] than in those younger than 60 years [0.91 (0.84, 0.98)]. In addition, this negative association effect was significantly greater in participants with diabetes [0.75 (0.56, 0.94)] than in those without diabetes [0.98 (0.95, 1.02)]. Although there were inconsistent effect values for the association between OBS and periodontitis in some subgroups, our results suggest that a negative association between OBS and periodontitis was maintained in all subgroups.
TABLE 4.
Subgroup analysis of the association between Oxidative Balance Score and periodontitis.
| Subgroup | Oxidative Balance Score [OR (95%CI)] | P for interaction |
| Sex | 0.182 | |
| Male | 0.85 (0.75, 0.95) | |
| Female | 0.93 (0.90, 0.96) | |
| Age | 0.046 | |
| <60 years | 0.91 (0.84, 0.98) | |
| ≥60 years | 0.83 (0.76, 0.91) | |
| Race/Ethnicity | 0.089 | |
| Non-Hispanic white | 0.88 (0.81, 0.95) | |
| Non-Hispanic black | 0.79 (0.63, 0.95) | |
| Mexican American | 0.94 (0.85, 1.03) | |
| Other race/Multiracial | 0.92 (0.89, 0.96) | |
| Education level, n (%) | 0.579 | |
| Less than high school | 0.85 (0.72, 0.98) | |
| High school | 0.91 (0.82, 1.00) | |
| More than high school | 0.81 (0.70, 0.92) | |
| Diabetes, (%) | 0.028 | |
| Yes | 0.75 (0.56, 0.94) | |
| No | 0.98 (0.95, 1.02) | |
| Borderline | 0.93 (0.91, 0.95) |
Age, gender, race, diabetes, cancer, PIR, triglycerides, klotho, and LDL-C were adjusted. PIR, ratio of family income to poverty; LDL-C, low-density lipoprotein cholesterol.
4. Discussion
In the cross-sectional study that enrolled 3,706 representative participants, we observed a negative association between the OBS and periodontitis, and there was significant dependence of age and diabetes on this association, indicating that an increased OBS may contribute to a decreased risk of periodontitis. Our results suggest that the management of OBS in dietary intake and lifestyle may alleviate the occurrence of periodontitis.
To our knowledge, this is the first study to assess the relationship between OBS and periodontitis, and it highlights the negative association between OBS levels derived from dietary intake and lifestyle and the risk of periodontitis. Previous studies have found that oxidative stress has a negative impact on periodontitis risk and oral health (49). Tamaki et al. (50) investigated the association between serum oxidative stress levels and periodontitis in 200 adult participants from the community with periodontitis. The results of the age-adjusted logistic analysis showed a statistically significant association between high ROM levels and clinical attachment loss (50). In a cohort study that included 770 participants with chronic kidney disease (CKD), Sharma et al. (51) attempted to investigate the causal association between oxidative stress, periodontitis, and renal function using a mediator analysis model, and the authors found a bidirectional negative association between periodontitis and renal function, with oxidative stress providing the pathobiological basis for this relationship. Li et al. investigated the association between four serum antioxidant vitamins (vitamins A, C, D, and E) and periodontitis in a cross-sectional study that included 6,158 Americans. The results showed a significant negative association between vitamins C and D and periodontitis, and in addition, the authors concluded that periodontitis increased the level of systemic inflammation in the obese population. In our analysis, we detected a linear negative association between OBS and periodontitis (48). A trend test considering OBS as a quartile also demonstrated a dose-response relationship between OBS and periodontitis. It has been widely reported that OBS can be used as an indicator of inflammatory diseases (52), and the association between OBS and periodontitis was also recognized in our study.
The results of the subgroup analysis showed significant differences in the association between OBS and periodontitis with respect to age and diabetes, which is partially consistent with previous studies. Ebersole et al. (53) evaluated the association between five antioxidants (folate, vitamin D, vitamin E, cis-beta-carotene, and β-cryptoxanthin). The authors found an interaction between age and periodontitis-related levels of these nutrients, with reduced levels of these antioxidants increasing with age in moderate and severe periodontitis (53). Our interaction test showed that the negative association effect between OBS and periodontitis was more significant in the elderly. Furthermore, diabetes and periodontitis share a common pathogenesis associated with altered immune inflammatory responses at the systemic level (54). An animal study showed that periodontitis exacerbates oxidative stress levels in rats with diabetes (55). Our results also suggest that participants with diabetes are more prominent in the negative association between OBS and periodontitis.
The role of oxidative stress and inflammation in the pathogenesis of periodontitis has attracted the attention of researchers for decades (56, 57). Although many past observational cross-sectional studies have confirmed the association of oxidative stress with periodontitis, the low specificity of oxidative stress markers requires caution in interpreting the results, and a meta-analysis that included 16 observational studies outlined the substantial heterogeneity introduced by differences in patient populations and analytical tools (58). In fact, with the exception of vitamin C, which is considered a well-known strong antioxidant, the associations between other dietary components and inflammatory diseases are often conflicting (59, 60). Therefore, the introduction of a comprehensive scoring system reflecting dietary and non-dietary antioxidant and pro-oxidant exposures to assess the relationship between oxidative stress and periodontitis is warranted (61). OBS, which combines dietary and lifestyle factors, has been shown to be a useful marker for inflammatory diseases in studies in different countries and regions (43, 62, 63).
The strengths of our study include the simultaneous consideration of multiple dietary and lifestyle factors for the oxidative potential of periodontitis; secondly, the use of a complex multi-stage probability sampling design and appropriate covariate adjustment increased the reliability and representativeness of our study. Our study has some limitations. First, due to the design of the cross-sectional study, a causal relationship between OBS and periodontitis could not be inferred (64). In addition, database limitations prevented the inclusion of all covariates that have an impact on oxidative stress, such as environmental pollution, flavonoid intake, and Oxidative markers (65). Nevertheless, the correlation between periodontitis and current OBS was stable enough to be less likely to be significantly influenced by unincluded factors.
5. Conclusion
In conclusion, higher OBS indicates that dietary and lifestyle antioxidant exposure is superior to prooxidant exposure and is associated with a lower risk of periodontitis. Our results suggest that OBS may serve as a biomarker for periodontitis in adults. However, further studies are still needed to validate our findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving human participants were reviewed and approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HQ read and approved the final manuscript, performed the analysis, wrote a draft of this manuscript, conceived the study design, contributed to the interpretation of the results, and critically revised the manuscript for important intellectual content.
Acknowledgments
I would like to thank all participants in this study.
Abbreviations
OBS, Oxidative Balance Score; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; AAP, Academy of Periodontology; AL, attachment loss; PD, pocket depth; LDL-C, low-density lipoprotein cholesterol; PIR, income-to-poverty ratio; CKD, chronic kidney disease.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1138488/full#supplementary-material
References
- 1.Li W, Shang Q, Yang D, Peng J, Zhao H, Xu H, et al. Abnormal micronutrient intake is associated with the risk of periodontitis: a dose-response association study based on NHANES 2009-2014. Nutrients. (2022) 14:2466. 10.3390/nu14122466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zong G, Scott A, Griffiths H, Zock P, Dietrich T, Newson R. Serum α-tocopherol has a nonlinear inverse association with periodontitis among US adults. J Nutr. (2015) 145:893–9. 10.3945/jn.114.203703 [DOI] [PubMed] [Google Scholar]
- 3.Jin F, Song J, Luo Y, Wang B, Ding M, Hu J, et al. Association between skull bone mineral density and periodontitis: using the National Health and Nutrition Examination Survey (2011-2014). PLoS One. (2022) 17:e0271475. 10.1371/journal.pone.0271475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pihlstrom B, Michalowicz B, Johnson N. Periodontal diseases. Lancet. (2005) 366:1809–20. [DOI] [PubMed] [Google Scholar]
- 5.Page R, Kornman K. The pathogenesis of human periodontitis: an introduction. Periodontology. (2000) 1997:9–11. [DOI] [PubMed] [Google Scholar]
- 6.Chapple I, Matthews J. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology. (2000) 2007:160–232. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Li L, Zhou Y, Chen X, Xu Y. Association between triglyceride-glucose index and risk of periodontitis: a cross-sectional study. Int J Gen Med. (2021) 14:9807–16. 10.2147/IJGM.S339863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui F, Almeida-da-Silva C, Huynh B, Trinh A, Liu J, Woodward J, et al. Association between periodontal pathogens and systemic disease. Biomed J. (2019) 42:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jepsen S, Caton J, Albandar J, Bissada N, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. (2018) 89(Suppl. 1):S237–48. [DOI] [PubMed] [Google Scholar]
- 10.Chapple I, Milward M, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. (2007) 137:657–64. 10.1093/jn/137.3.657 [DOI] [PubMed] [Google Scholar]
- 11.Araújo M, Martins C, Costa L, Cota L, Faria R, Cunha F, et al. Association between depression and periodontitis: a systematic review and meta-analysis. J Clin Periodontol. (2016) 43:216–28. [DOI] [PubMed] [Google Scholar]
- 12.Liccardo D, Marzano F, Carraturo F, Guida M, Femminella G, Bencivenga L, et al. Potential bidirectional relationship between periodontitis and Alzheimer’s disease. Front Physiol. (2020) 11:683. 10.3389/fphys.2020.00683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirih F, Monajemzadeh S, Singh N, Sinacola R, Shin J, Chen T, et al. Association between metabolic syndrome and periodontitis: the role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontology. (2021) 87:50–75. 10.1111/prd.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey J, D’Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. (2020) 47:268–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. (2019) 20:1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sczepanik F, Grossi M, Casati M, Goldberg M, Glogauer M, Fine N, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontology. (2020) 84:45–68. [DOI] [PubMed] [Google Scholar]
- 17.Boesing F, Patiño J, da Silva V, Moreira E. The interface between obesity and periodontitis with emphasis on oxidative stress and inflammatory response. Obes Rev. (2009) 10:290–7. 10.1111/j.1467-789X.2008.00555.x [DOI] [PubMed] [Google Scholar]
- 18.White P, Chicca I, Cooper P, Milward M, Chapple I. Neutrophil extracellular traps in periodontitis: a web of intrigue. J Dent Res. (2016) 95:26–34. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. (2017) 8:910. 10.3389/fphys.2017.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aquino-Martinez R, Khosla S, Farr J, Monroe D. Periodontal disease and senescent cells: new players for an old oral health problem? Int J Mol Sci. (2020) 21:7441. 10.3390/ijms21207441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veljovic T, Djuric M, Mirnic J, Gusic I, Maletin A, Ramic B, et al. Lipid peroxidation levels in saliva and plasma of patients suffering from periodontitis. J Clin Med. (2022) 11:3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Jiao J, Qi Y, Yu W, Yang S, Zhang J, et al. Curcumin: a review of experimental studies and mechanisms related to periodontitis treatment. J Periodontal Res. (2021) 56:837–47. 10.1111/jre.12914 [DOI] [PubMed] [Google Scholar]
- 23.Linden G, McClean K, Woodside J, Patterson C, Evans A, Young I, et al. Antioxidants and periodontitis in 60-70-year-old men. J Clin Periodontol. (2009) 36:843–9. 10.1111/j.1600-051X.2009.01468.x [DOI] [PubMed] [Google Scholar]
- 24.Jacob R, Omaye S, Skala J, Leggott P, Rothman D, Murray P. Experimental vitamin C depletion and supplementation in young men. Nutrient interactions and dental health effects. Ann N Y Acad Sci. (1987) 498:333–46. 10.1111/j.1749-6632.1987.tb23772.x [DOI] [PubMed] [Google Scholar]
- 25.Xie R, Zhang Y, Yan T, Huang X, Xie S, Liu C, et al. Relationship between nonalcoholic fatty liver disease and bone mineral density in adolescents. Medicine. (2022) 101:e31164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell S, Dietrich T, Chapple I. Prediction of serum total antioxidant activity from the concentration of individual serum antioxidants. Clin Chim Acta. (2006) 372:188–94. [DOI] [PubMed] [Google Scholar]
- 27.Barreiro E, Peinado V, Galdiz J, Ferrer E, Marin-Corral J, Sánchez F, et al. Cigarette smoke-induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med. (2010) 182:477–88. [DOI] [PubMed] [Google Scholar]
- 28.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. (2006) 65:278–90. [DOI] [PubMed] [Google Scholar]
- 29.Arazi H, Eghbali E, Suzuki K. Creatine supplementation, physical exercise and oxidative stress markers: a review of the mechanisms and effectiveness. Nutrients. (2021) 13:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakubiak G, Osadnik K, Lejawa M, Kasperczyk S, Osadnik T, Pawlas N. Oxidative stress in association with metabolic health and obesity in young adults. Oxid Med Cell Longev. (2021) 2021:9987352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong S, Bostick R, Flanders W, McClellan W, Thyagarajan B, Gross M, et al. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol Biomarkers Prev. (2014) 23:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakkur S, Bostick R, Roblin D, Ndirangu M, Okosun I, Annor F, et al. Oxidative balance score and oxidative stress biomarkers in a study of Whites, African Americans, and African immigrants. Biomarkers. (2014) 19:471–80. 10.3109/1354750X.2014.937361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Son D, Kwon Y. Association between oxidative balance score and new-onset hypertension in adults: a community-based prospective cohort study. Front Nutr. (2022) 9:1066159. 10.3389/fnut.2022.1066159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demirer B, Yardımcı H, Erem Basmaz S. Inflammation level in type 2 diabetes is associated with dietary advanced glycation end products, Mediterranean diet adherence and oxidative balance score: a pathway analysis. J Diabetes Complications. (2022) 37:108354. 10.1016/j.jdiacomp.2022.108354 [DOI] [PubMed] [Google Scholar]
- 35.Ilori T, Sun Ro Y, Kong S, Gutierrez O, Ojo A, Judd S, et al. Oxidative balance score and chronic kidney disease. Am J Nephrol. (2015) 42:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Joo Y, Han M, Kwon S, Park W, Park K, et al. Relationship between oxidative balance score and quality of life in patients with osteoarthritis: data from the Korea National Health and Nutrition Examination Survey (2014-2015). Medicine. (2019) 98:e16355. 10.1097/MD.0000000000016355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie R, Huang X, Liu Q, Liu M. Positive association between high-density lipoprotein cholesterol and bone mineral density in U.S. adults: the NHANES 2011-2018. J Orthop Surg Res. (2022) 17:92. 10.1186/s13018-022-02986-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie R, Liu M. Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol. (2022) 13:857110. 10.3389/fendo.2022.857110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández E, Amiano P, Ruiz-Canela M, Molina-Montes E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. (2019) 11:774. 10.3390/nu11040774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashoori M, Saedisomeolia A. Riboflavin (vitamin B2) and oxidative stress: a review. Br J Nutr. (2014) 111:1985–91. [DOI] [PubMed] [Google Scholar]
- 41.Kaplon R, Gano L, Seals D. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J Appl Physiol. (2014) 116:156–63. 10.1152/japplphysiol.00969.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Lagemaat E, de Groot L, van den Heuvel E. Vitamin B in relation to oxidative stress: a systematic review. Nutrients. (2019) 11:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho A, Kwon Y, Lim H, Lee H, Kim S, Shim J, et al. Oxidative balance score and serum γ-glutamyltransferase level among Korean adults: a nationwide population-based study. Eur J Nutr. (2018) 57:1237–44. 10.1007/s00394-017-1407-1 [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Peng S, Chen L, Chen H, Cheng X, Tang Y. Association between the oxidative balance score and telomere length from the National Health and Nutrition Examination Survey 1999-2002. Oxid Med Cell Longev. (2022) 2022:1345071. 10.1155/2022/1345071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. 10.3389/fimmu.2022.925690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie R, Zhang Y. Is assessing the degree of hepatic steatosis and fibrosis based on index calculations the best choice for epidemiological studies? Environ Pollut. (2022) 317:120783. 10.1016/j.envpol.2022.120783 [DOI] [PubMed] [Google Scholar]
- 47.Xie R, Liu Y, Wang J, Zhang C, Xiao M, Liu M, et al. Race and gender differences in the associations between cadmium exposure and bone mineral density in US adults. Biol Trace Elem Res. (2022) 22:3521–25. 10.1007/s12011-022-03521-y [DOI] [PubMed] [Google Scholar]
- 48.Li A, Tang Z, Zhu P, van den Bosch F, Chen Y, Xu S, et al. Serum antioxidant vitamins mediate the association between periodontitis and metabolically unhealthy overweight/obesity. Nutrients. (2022) 14:4939. 10.3390/nu14224939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. (2019) 46:608–22. 10.1111/jcpe.13112 [DOI] [PubMed] [Google Scholar]
- 50.Tamaki N, Hayashida H, Fukui M, Kitamura M, Kawasaki K, Nakazato M, et al. Oxidative stress and antibody levels to periodontal bacteria in adults: the Nagasaki Islands Study. Oral Dis. (2014) 20:e49–56. 10.1111/odi.12127 [DOI] [PubMed] [Google Scholar]
- 51.Sharma P, Fenton A, Dias I, Heaton B, Brown C, Sidhu A, et al. Oxidative stress links periodontal inflammation and renal function. J Clin Periodontol. (2021) 48:357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahat R, Singh N, Rathore V, Arora M, Yadav T. Cross-sectional correlates of oxidative stress and inflammation with glucose intolerance in prediabetes. Diabetes Metab Syndr. (2019) 13:616–21. 10.1016/j.dsx.2018.11.045 [DOI] [PubMed] [Google Scholar]
- 53.Ebersole J, Lambert J, Bush H, Huja P, Basu A. Serum nutrient levels and aging effects on periodontitis. Nutrients. (2018) 10:1986. 10.3390/nu10121986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preshaw P, Alba A, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. (2012) 55:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choubaya C, Chahine R, Zalloua P, Salameh Z. Periodontitis and diabetes interrelationships in rats: biochemical and histopathological variables. J Diabetes Metab Disord. (2019) 18:163–72. 10.1007/s40200-019-00403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapira L, Borinski R, Sela M, Soskolne A. Superoxide formation and chemiluminescence of peripheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J Clin Periodontol. (1991) 18:44–8. 10.1111/j.1600-051x.1991.tb01118.x [DOI] [PubMed] [Google Scholar]
- 57.Chapple I. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. (1997) 24:287–96. [DOI] [PubMed] [Google Scholar]
- 58.Liu Z, Liu Y, Song Y, Zhang X, Wang S, Wang Z. Systemic oxidative stress biomarkers in chronic periodontitis: a meta-analysis. Dis Markers. (2014) 2014:931083. 10.1155/2014/931083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sen A, Marsche G, Freudenberger P, Schallert M, Toeglhofer A, Nagl C, et al. Association between higher plasma lutein, zeaxanthin, and vitamin C concentrations and longer telomere length: results of the Austrian Stroke Prevention Study. J Am Geriatr Soc. (2014) 62:222–9. 10.1111/jgs.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, He H, Wang J, Guo X, Lin H, Chen H, et al. Oxidative stress-dependent frataxin inhibition mediated alcoholic hepatocytotoxicity through ferroptosis. Toxicology. (2020) 445:152584. 10.1016/j.tox.2020.152584 [DOI] [PubMed] [Google Scholar]
- 61.Dash C, Goodman M, Flanders W, Mink P, McCullough M, Bostick R. Using pathway-specific comprehensive exposure scores in epidemiology: application to oxidative balance in a pooled case-control study of incident, sporadic colorectal adenomas. Am J Epidemiol. (2013) 178:610–24. 10.1093/aje/kwt007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dash C, Bostick R, Goodman M, Flanders W, Patel R, Shah R, et al. Oxidative balance scores and risk of incident colorectal cancer in a US prospective cohort study. Am J Epidemiol. (2015) 181:584–94. 10.1093/aje/kwu318 [DOI] [PubMed] [Google Scholar]
- 63.Agalliu I, Kirsh V, Kreiger N, Soskolne C, Rohan T. Oxidative balance score and risk of prostate cancer: results from a case-cohort study. Cancer Epidemiol. (2011) 35:353–61. [DOI] [PubMed] [Google Scholar]
- 64.Bullon P, Newman H, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontology. (2014) 64:139–53. 10.1111/j.1600-0757.2012.00455.x [DOI] [PubMed] [Google Scholar]
- 65.Baima G, Corana M, Iaderosa G, Romano F, Citterio F, Meoni G, et al. Metabolomics of gingival crevicular fluid to identify biomarkers for periodontitis: a systematic review with meta-analysis. J Periodontal Res. (2021) 56:633–45. 10.1111/jre.12872 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.


