Abstract
Cardiopulmonary exercise testing (CPET) was limited to peak oxygen consumption analysis (VO2peak), and now the ventilation/carbon dioxide production (VE/VCO2) slope is recognized as having independent prognostic value. Unlike VO2peak, the VE/VCO2 slope does not require maximal effort, making it more feasible. There is no consensus on how to measure the VE/VCO2 slope; therefore, we assessed whether different methods affect its value. This is a retrospective study assessing sociodemographic data, left ventricular ejection fraction, CPET parameters, and indications of patients referred for CPET. The VE/VCO2 slope was measured to the first ventilatory threshold (VT1-slope), secondary threshold (VT2-slope), and included all test data (full-slope). Of the 697 CPETs analyzed, 308 reached VT2. All VE/VCO2 slopes increased with age, regardless of test indications. In patients not reaching VT2, the VT1-slope was 32 vs. 36 (p < 0.001) for the full-slope; in those surpassing VT2, the VT1-slope was 29 vs. 33 (p < 0.001) for the VT2-slope and 37 (all p < 0.001) for the full-slope. The mean difference between the submaximal and full-slopes was ±4 units, sufficient to reclassify patients from low to high risk for heart failure or pulmonary hypertension. We conclude that the method used for determining the VE/VCO2 slope greatly influences the result, the significant variations limiting its prognostic value. The calculation method must be standardized to improve its prognostic value.
Keywords: CPET, VE/VCO2 slope, VO2peak
1. Introduction
The maximal cardiopulmonary exercise test (CPET) consists of exercise with a gradually increasing intensity until exhaustion or the appearance of limiting symptoms [1]. The main parameters measured are ventilation (VE), oxygen consumption (VO2), and carbon dioxide production (VCO2). The CPET is extremely useful for the evaluation of cardiovascular, respiratory, muscular, and metabolic systems during exercise and is considered the gold standard for evaluating cardiopulmonary function [1,2]. It is used, notably, for the prognosis or follow-up assessment of patients with cardiac or respiratory diseases in the pre-operative period, or the prescription of physical exercises for athletes or patients [3,4].
Early prognostic studies about the CPET were limited to the analysis of maximal aerobic power by a measure of the peak oxygen consumption (VO2peak). This parameter is used to characterize the functional severity of heart failure (HF) and as a criterion for listing heart transplantations and preoperative risk stratification [5,6]. A drawback to the use of the VO2peak is that factors unrelated to either cardiac or pulmonary pathology can falsely reduce its value. For example, if a patient is exercise-intolerant or lacks motivation to reach the maximal effort, the VO2peak interpretation could be limited [6]. In addition, the VO2peak resulting from a submaximal test produces a false, poorly predictive score compared with that from a maximal test [7]. Due to these potential difficulties in estimating the VO2peak, along with confounding factors such as age, sex or obesity, experts have sought other primary CPET factors to improve its predictive value [5,6,7,8]. The relationship between VE and VCO2, expressed as the VE/VCO2 slope, has been identified and documented [9]. A normal ventilatory response of the VE/VCO2 slope should be under 30.0 [2]. Patients with cardiopulmonary disease often present an abnormal ventilation response, characterized by an increase in the VE/VCO2 slope; for any given VCO2 level, VE is greater than normal.
The VE/VCO2 slope, VO2peak and presence of exercise oscillatory ventilation (EOV) are considered primary CPET variables and are strong predictors of adverse events in heart failure (Class 1A) [2]. In addition, the VE/VCO2 slope is responsive to pharmacological, surgical, and exercise interventions in HF patients and can be used to assess therapeutic efficacy (Class 1A) [2]. In patients with chronic HF, a Kaplan–Meier analysis revealed a 1-year cardiac-related mortality of 75% in patients with a VE/VCO2 slope >35.6 and 25% in those with a VE/VCO2 slope <35.6; 1-year cardiac-related hospitalization was 77% in patients with a VE/VCO2 slope >32.5 and 23% in those with a VE/VCO2 slope <32.5 [6].
From a physiological point of view, the relationship of VE and VCO2 is conserved during submaximal exercise, characterizing the ventilatory exercise response of the patient. The VE/VCO2 slope from rest to VT2 is linear; therefore, an accurate VE/VCO2 slope should be obtained even if the exercise test is submaximal, making it more feasible [10].
To date, there are several methods of calculating the VE/VCO2 slope, varying from one study to another, and expert recommendations are not that clear. Some authors calculate the slope only using the data from the start of exercise to the aerobic threshold (i.e., first ventilatory threshold (VT1)) or the ventilatory compensation point (i.e., secondary ventilatory threshold (VT2)), while others use all the data from the start to the maximum effort (full-slope) [10]. Thus, during a single CPET, several VE/VCO2 slope measurements can be obtained depending on the method used. This measurement heterogeneity certainly limits the clinical relevance and prognostic value of the VE/VCO2 slope when the test is maximal. This work aims to evaluate and compare the different methods of measuring the VE/VCO2 slope, considering values from rest up to VT1 (VT1-slope), to VT2 (VT2-slope), and including all the data from the entire test (full-slope). We formulated the primary hypothesis that the gradient of the VE/VCO2 slope is greater for all time intervals (i.e., up to VT1, VT2, and over the entire test). In addition, we hypothesized that the VE/VCO2 slope is influenced by age and cardiopulmonary disease.
2. Materials and Methods
2.1. Study Setting
We carried out a retrospective cross-sectional study at the cardiology unit of the Erasme Hospital, Brussels, Belgium, between May and December 2019. Data from 697 successive CPETs carried out on a cycloergometer were included in our database. We used a Strobe checklist for retrospective study.
2.2. Participants
All patients referred for CPET as part of their medical work-up were included in the study: Cardiac patients were referred for HF or pulmonary hypertension follow-up or the beginning of cardiac rehabilitation after ischemic or valvular surgery; pulmonary populations were addressed for pulmonary fibrosis, chronic obstructive pulmonary disease (COPD) follow up or pre-operative thoracic surgery; the dyspnea group was composed of patients addressed for exercise testing after a normal cardiac and pulmonary screening; and the last group (other) was composed of oncology, obesity (but normal cardiac screening) or rheumatoid arthritis patients. Patients excluded were those with Gold III and IV (COPD), those who did not achieve VT1, those who performed a submaximal effort (i.e., peak respiratory exchange ratio (RERpeak) < 1.10), and those whose VT1 and VT2 could not be accurately determined.
2.3. Procedure
CPET was performed on a cycloergometer (Ergoselect II 1200; Ergoline, Bitz, Germany) with a step-by-step increase in load mode. CPET consisted of a three-minute warm-up followed by increments of 10–30 W per minute in a standing position. The increments were chosen according to the individual’s estimated capacity, such that the duration of the effort was between 8 and 12 min. Patients were required to maintain a cycling cadence of 60 rpm. VO2 (oxygen uptake), VCO2 (carbon dioxide output), and ventilation (VE) were collected breath by breath via a facial mask and analyzed every 8 s using a metabolic system (Exp’Air®, Medisoft, Dinant, Belgium). The flow in the pitot tube was calibrated with a 3 L syringe, which was annually calibrated, and verified by the manufacturer. The O2 and CO2 fractions were measured by laser cells calibrated with two different gas mixtures (room air (FiO2: 20.85%; FiCO2: 0.10%) and standardized gas in a different high-pressure bottle (FiO2: 16.00%; FiCO2: 4.00%)). Arterial oxygen saturation (SpO2) was continuously measured via a finger pulse oximeter (SenSmart 8100S Serie; Nonin, MN, USA). Heart rate (HR) was obtained with a 12-lead electrocardiogram (ECG) (Strässle & Co. DT 100, Albstadt, Germany), and blood pressure (BP) (Medisoft Ergoline 4M, Dinant, Belgium) was measured at the end of each stage.
The aim was to obtain a maximal test, and different criteria of hemodynamic, respiratory, metabolic or clinical limitations were considered. Limiting factors included ischemic electrocardiogram abnormalities, severe rhythm or conduction changes, or inadequate blood pressure (BP) response (systolic BP > 250 mmHg or systolic BP reduction during exercise for the two following steps), as well as clinical criteria such as pallor or profuse sweating. Metabolic limitation was defined as RER > 1.15 with an oxygen respiratory equivalent >40 or a VO2 plateau (an increase of less than 100 mL/min while workload increased further). Respiratory limitation was expressed as partial or complete consumption of the ventilatory reserve (VR) (VE max at the end of effort/maximal voluntary ventilation (MVV) > 70%), as well as desaturation indicated by an SpO2 < 75% at the end of the effort. Finally, muscular fatigue in the legs or respiratory discomfort indicated that the test was maximal but did not constitute a reason to stop. CPET was conducted to exhaustion if no criteria for stopping were present following the European and American guidelines [8,11].
2.4. Data Collection
The data collected for each patient were sociodemographic, including age and gender, body mass index (BMI), CPET indication, left ventricular ejection fraction (LVEF), and CPET parameters (VO2, VCO2,VE, and RER on a breath-by-breath file extracted from the device). VE/VCO2 slope calculations were determined using linear regression analysis of VE and VCO2 obtained up to VT1 (VT1-slope), VT2 (VT2-slope), and over the entire test (full-slope). To determine VT1 (i.e., aerobic threshold), the V-slope method established by Beaver et al. was used [12]. This consists of plotting the point cloud of VCO2 versus VO2 to detect the break that reflects a sudden increase in VCO2. VT2 (i.e., ventilatory compensation point) was determined via the Wasserman method using respiratory CO2 equivalents [12]; a sudden rise in the VE/VCO2 ratio, marked by a break in the curve, indicated VT2. Ventilatory thresholds were reviewed by two independent blinded exercise physiologists and the VO2 peak was expressed as % of predicted value (PV) according to the literature [13]. Patients were divided according to whether they surpassed VT2 (VT2 group) or not (VT1 group). Within both groups, we performed sub-analyses according to age and CPET indication (i.e., cardiac disease, lung disease or others, and dyspnea work-up).
2.5. Data Analysis
The Kolmogorov–Smirnov test confirmed that the continuous data distribution was non-gaussian. These data are expressed as the median and interquartile range [P25–P75]. We used the Mann–Whitney test to compare variables between patients who achieved VT2 (VT2 group) and those who did not (VT1 group). The Wilcoxon signed-rank test was used to compare variables within the two groups. The Spearman nonparametric correlation coefficient was used for correlation analysis. All analyses were performed using SPSS software version 22 (Chicago, IL, USA). A p-value < 0.05 was considered significant.
3. Results
3.1. General Characteristics of the Study Population
Among the 697 CPETs analyzed in this study, 389 patients did not reach VT2 (VT1 group) and 308 surpassed VT2 (VT2 group). Patients’ characteristics for each group are presented in Table 1. Patients of the VT1 group were older, had a higher BMI, drew less on their ventilatory reserve (VR), and achieved a lower VO2peak and RERpeak. Regarding the VE/VCO2 slopes, the VT1-slope was higher in the VT1 group than in the VT2 group. In contrast, there was no difference in the full-slope between the two groups.
Table 1.
General characteristics of the study population.
| Population (n = 697) |
Group VT1 VT2 Not Reached (n = 389) |
Group VT2 VT2 Surpassed (n = 308) |
p-Value | |
|---|---|---|---|---|
| Age (years) | 57 [47–66] | 59 [49–67] | 54 [44–63] | <0.001 |
| BMI (kg/m2) | 27 [23–30] | 27 [24–31] | 26 [23–30] | <0.006 |
| VO2 peak (% PV) | 69 [57–83] | 64 [53–78] | 76 [64–88] | <0.001 |
| RER peak | 1.19 [1.13–1.26] | 1.16 [1.1–1.2] | 1.20 [1.17–1.28] | <0.001 |
| VT1-slope | 31 [27–35] | 32 [29–37] | 29 [25–33] | <0.001 |
| VT2-slope | - | - | 33 [29–37] | - |
| Full-slope | 37 [33–42] | 36 [33–42] | 37 [33–41] | <0.870 |
| VR (% MVV) | 59 [49–72] | 55 [47–67] | 64 [54–74] | <0.001 |
| Sex ratio M/F | 1.27 | 1.08 | 1.56 | 0.031 |
| LVEF (%) | 60 [60, 60] | 60 [58–60] | 60 [60, 60] | 0.55 |
3.2. Association between VE/VCO2 Slope and Age
Table 2 shows the VE/VCO2 slope values according to age. In the VT1 group, an increase in the VT1-slope and full-slope with age was observed. In the VT2 group, the same relationship between age and increases in VE/VCO2 slopes was observed. Considering all patients, we found a positive correlation between age and the VT1-slope (Spearman’s ρ = 0.201, n = 697, p < 0.0001), the VT2-slope (Spearman’s ρ = 0.191, n = 308, p < 0.001), and the full-slope (Spearman’s ρ = 0.137, n = 697, p < 0.0001).
Table 2.
Median VE/VCO2 slope values according to age for patients in the VT1 and VT2 groups.
| VT1 Group, n = 389 | VT2 Group, n = 308 | ||||||
|---|---|---|---|---|---|---|---|
| Age (Years) | VT1-Slope | Full-Slope | % Difference VT1 vs. Full | VT1-Slope | VT2-Slope | Full-Slope | % Difference VT1 vs. Full/VT2 vs. Full |
| <30 | 31 [28–34] | 34 [32–40] | 9.67% | 28 [24–34] | 31 [27–38] | 34 [31–42] | 21.43/9.68 |
| 31–40 | 32 [29–35] | 36 [31–40] | 12.50% | 28 [25–32] | 31 [27–36] | 35 [32–40] | 25.00/12.90 |
| 41–50 | 30 [26–36] | 36 [31–40] | 20.00% | 28 [25–31] | 32 [29–34] | 37 [33–41] | 32.14/15.63 |
| 51–60 | 32 [30–36] | 37 [33–41] | 15.63% | 28 [25–33] | 32 [29–37] | 36 [33–41] | 28.57/12.50 |
| 61–70 | 33 [30–37] | 37 [33–43] | 12.12% | 29 [27–36] | 34 [30–39] | 38 [35–43] | 31.03/11.76 |
| >70 | 34 [29–42] | 38 [33–47] | 11.76% | 30 [26–34] | 35 [30–38] | 38 [34–41] | 26.67/8.57 |
3.3. Patient Characteristics According to Stress Test Indications
Table 3 shows the patient characteristics according to their indications for the stress test. Regardless of the stress-test indication, patients of the VT1 group were older, had a higher BMI, and reached a lower RERpeak and VO2peak. For VE, except for lung disease patients, the VT2 group drew more vigorously on their VR than the VT1 group. Regardless of the stress-test indication, the VT1-slope was higher in the VT1 than in the VT2 group. In contrast, there was no difference in the full-slope between the two groups.
Table 3.
Patient characteristics according to stress-test indications.
| Cardiac Diseases | Lung Diseases | |||||
|
VT1 Group
(n = 191) |
VT2 Group
(n = 186) |
p-Value |
VT1 Group
(n = 70) |
VT2 Group
(n = 32) |
p-Value | |
| Age (years) | 63 [51–69] | 57 [47–66] | <0.001 | 62 [53–69] | 55 [41–63] | 0.006 |
| BMI (kg/m2) | 27 [24–31] | 26 [23–29] | <0.001 | 25 [21–30] | 26 [21–29] | 0.003 |
| VO2peak (% PV) |
60 [51–76] | 75 [64–89] | <0.001 | 65 [52–79] | 74 [53–80] | 0.034 |
| RERpeak | 1.17 [1.12–1.23] | 1.22 [1.16–1.28] | <0.001 | 1.16 [1.1–1.21] | 1.22 [1.2–1.26] | <0.001 |
| VR (% VMM) |
54 [46–65] | 64 [54–74] | <0.001 | 59 [48–73] | 62 [53–71] | 0.785 |
| VT1-slope | 33 [29–38] | 29 [26–37] | <0.001 | 34 [30–37] | 28 [25–35] | 0.04 |
| VT2-slope | - | 33 [30–34] | - | - | 32 [29–40] | |
| Full-slope | 37 [33–43] | 37 [33–42] | 0.899 | 38 [33–44] | 37 [33–46] | 0.562 |
| Others | Dyspnea Work-Up | |||||
|
VT1 Group
(n = 81) |
VT2 Group
(n = 64) |
p-Value |
VT1 Group
(n = 47) |
VT2 Group
(n = 26) |
p-Value | |
| Age (years) | 53 [45–63] | 52 [44–60] | <0.001 | 53 [46–64] | 43 [34–52] | <0.001 |
| BMI (Kg/m2) | 29 [24–33] | 27 [25–31] | 0.003 | 27 [24–34] | 26 [21–31] | 0.035 |
| VO2peak (% VP) |
68 [57–83] | 78 [64–88] | 0.005 | 60 [56–80] | 76 [66–100] | 0.022 |
| RERpeak | 1.16 [1.09–1.21] | 1.23 [1.17–1.33] | <0.001 | 1.15 [1.07–1.23] | 1.23 [1.17–1.28] | 0.001 |
| VR (% VMM) |
55 [48–66] | 67 [57–79] | 0.01 | 56 [49–67] | 63 [52–76] | 0.02 |
| VT1-slope | 31 [27–36] | 27 [25–31] | 0.014 | 31 [28–35] | 29 [24–32] | 0.014 |
| VT2-slope | - | 31 [29–35] | - | - | 32 [28–35] | |
| Full-slope | 36 [31–40] | 36 [32–40] | 0.105 | 36 [32–40] | 36 [31–39] | 0.347 |
3.4. Comparison of Slopes According to Stress Test Indications
For each stress-test indication in the VT1 group, the full-slope was higher than the VT1-slope (Table 4). Considering all patients in the VT1 group, the median difference between the VE/VCO2 full-slope and the VT1-slope was + 4 [2–6] units (p < 0.001).
Table 4.
Comparison of VE/VCO2 slopes according to test indications in VT1 and VT2 groups.
| VT1 Group, n = 389 | VT2 Group, n = 308 | ||||||
|---|---|---|---|---|---|---|---|
| VT1-Slope | Full-Slope | VT1 vs. Full | VT1-Slope | VT2-Slope | Full-Slope | VT1 vs. VT2/ VT2 vs. Full |
|
| Lung | 34 [30–37] | 38 [33–45] | <0.001 | 28 [25–31] | 31 [29–37] | 36 [32–41] | <0.001/<0.001 |
| Others | 31 [27–36] | 36 [31–40] | <0.001 | 27 [25–31] | 31 [29–35] | 36 [32–40] | <0.001/<0.001 |
| Dyspnea work up | 31 [28–35] | 36 [32–40] | <0.001 | 29 [24–32] | 32 [28–35] | 36 [31–39] | <0.001/<0.001 |
| Cardiac | 33 [29–38] | 37 [33–47] | <0.001 | 29 [26–34] | 33 [30–37] | 38 [33–42] | <0.001/<0.001 |
| All | 32 [29–37] | 36 [33–42] | <0.001 | 29 [25–33] | 33 [29–37] | 37 [33–41] | <0.001/<0.001 |
For each stress-test indication in the VT2 group, the full-slope was higher than the VT1-slope and VT2-slope, and the VT2-slope was higher than the VT1-slope (Table 4). Considering all patients in the VT2 group, the VE/VCO2 full-slope was higher than the VT1-slope (+ 7 [5–11] units; p < 0.001) and the VT2-slope (+ 4 [2–5] units; p < 0.0001). In this group, the VT2-slope was higher than the VT1-slope (+ 3 [2–5] units; p < 0.0001).
3.5. Relationship between VO2 and VE/VCO2 Slope
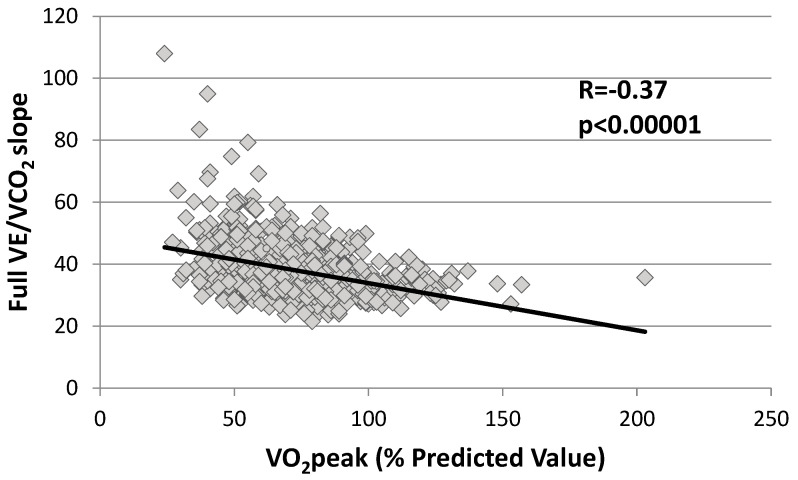
Figure 1 shows a negative correlation between the full-slope and VO2peak in the whole population (Spearman’s ρ = 0.37, n = 697, p < 0.0001). This negative correlation was observed irrespective of whether patients achieved VT2 or not (Table 5).
Figure 1.
Correlation between VO2peak (% PV) and full VE/VCO2 slope in all patients (grey square). VE/VCO2 slopes was significantly correlated to VO2max. The black line represents the linear correlation curve.
Table 5.
Correlations between VO2peak (% Predicted Value) and VT1-VT2 and full VE/VCO2 slope according to the level of effort achieved.
| VO2peak(% PV) | ||||
|---|---|---|---|---|
| n | r | p-Value | ||
| All | VT1-slope | 697 | −0.41 | <0.001 |
| full-slope | 697 | −0.37 | <0.001 | |
| VT1 group | VT1-slope | 389 | −0.4 | <0.001 |
| full-slope | 389 | −0.42 | <0.001 | |
| VT2 group | VT1-slope | 308 | −0.32 | <0.001 |
| VT2-slope | 308 | −0.32 | <0.001 | |
| full- slope | 308 | −0.33 | <0.001 | |
4. Discussion
This cross-sectional study was carried out to assess and compare different methods of measuring the VE/VCO2 slope at the following time intervals: from rest (1) to VT1 (VT1-slope); (2) to VT2 when achieved (VT2-slope); and (3) over the entire test (full-slope). Regardless of the CPET test indication, the gradient of the VE/VCO2 slope was greater for all time intervals in both patients who surpassed VT2 as well as those who did not. Importantly, while the VT1 group presented a lower VO2 peak than the VT2 group, there was no difference regarding the full-slope in either group. As expected, there was a positive correlation between age and the VE/VCO2 slopes [14]. Finally, a negative correlation between the VE/VCO2 slope and VO2peak was also observed.
The steepness of the increase in VE as regards VCO2 (VE/VCO2 slope) is indicative of ventilatory efficiency and can identify an abnormal ventilatory response to exercise or increased physiological dead space. Measurement of the VE/VCO2 slope is a noninvasive, reproducible, prognostic marker in several chronic cardiopulmonary diseases, along with other exercise-related variables such as VO2peak [11]. We found a highly significant negative correlation between all the VE/VCO2 slopes and the VO2peak. This is in line with other studies supporting that the VE/VCO2 slope is an extremely useful prognostic tool in the VO2peak grey area (i.e., between 10 and 18 mL/min/kg) [15]. It is also thought that the VE/VCO2 slope calculated from data acquired below the ventilatory compensation point (i.e., VT2), determined from submaximal exercise, carries the same prognostic information as the VE/VCO2 slope calculated from a maximal test. Clinicians have been interested in obtaining the same prognostic information from submaximal exercise as currently obtained from maximal exercise to make testing more applicable to activities of daily living for patients who are unable or unwilling to perform a maximal exercise test [16].
Based on the alveolar ventilation equation, there are three variables that determine VE/VCO2: the physiological dead space ventilation relative to tidal volume (VD/VT), the amount of CO2 produced, and the arterial PaCO2. Many cardiopulmonary diseases present an altered PaCO2 set-point and chemosensitivity, which then influence VE/VCO2. Inefficient gas exchange caused by increased ventilation–perfusion heterogeneity increases VD/VT, thus contributing to the abnormal VE/VCO2 slope seen in cardiopulmonary diseases. Both the PaCO2 and VD/VT variables evolve continuously throughout exercise and are largely dependent on the level of effort achieved, which directly affects the value of the VE/VCO2 slope [17]. Our results show an increase in the VE/VCO2 slope between VT1 and VT2, which supports the fact that maximal and submaximal VE/VCO2 slopes are not equivalent and it is important to evaluate correctly the VT2 and VT2 points.
However, in many cardiorespiratory disease patients, maximal metabolic exercise testing is not feasible, and a final respiratory exchange ratio of <1.10 is common [7]. All our CPETs were performed with the objective of being maximal; nevertheless, more than half of our patients did not reach VT2. We still found that these patients had a median RERpeak of 1.16, confirming that our CPETs showed a consequent level of effort [18]. To compare the different VE/VCO2 slope measurement methods, we divided our sample into two groups depending on whether they had surpassed VT2 or not. Regardless of the CPET test indication, the gradient of the VE/VCO2 slope was greater for all time intervals in both patients who surpassed VT2 and those who did not. The increase observed between the VT1-slope and VT2-slope suggests that the relationship between VE and VCO2 is not fully linear up to VT2 [17]. While the mechanisms underlying this observation are not completely understood, the causes of the increase in the VE/VCO2 slope before and after VT2 are well characterized. VE is driven by acidosis and CO2 after VT2, signifying that the VE/VCO2 slope becomes much steeper (greater VE increase per unit of VCO2) [17]. Therefore, using the entire CPET (i.e., full-slope) to calculate the VE/VCO2 slope will produce a steeper slope than by using just the data from rest to VT1 or VT2 [16,17]. In both patient groups (VT1 and VT2), the mean difference between submaximal slopes (i.e., the VT1-slope and VT2-slope) and the full-slope was about 4 units, which is sufficient to reclassify a patient from a low-risk into a high-risk category for HF or pulmonary hypertension [19].
No standard definition has been established as to how the VE/VCO2 slope should be measured. Some researchers do not include data after identifying VT2 when reached. Here, subjectivity is introduced as data are only omitted from tests that identify VT2; thus, slopes could be calculated differently depending on the patient. In our evaluation of CPETs, the VE/VCO2 slope is a more significant prognostic tool; therefore, how it is measured must be standardized. The American Heart Association (AHA) stated that: “… calculation of the VE/VCO2 slope with all exercise data obtained from a progressive exercise test (initiation to peak effort) appears to provide additional clinical information compared with submaximal calculations (i.e., those that use linear data points before the steepening associated with ventilatory compensation for metabolic acidosis)” [20].
Like others, we found a strong correlation between the full-slope and submaximal slopes (VT1 vs. full slope (R = 0.84), VT2 vs. full slope (0.92) and VT1 vs. VT2 (0.88); p value < 0.001 for the three values). As the full-slope calculation considers all points used to calculate the submaximal slope, this correlation was expected. However, an important part of the “prognostic signal” appears to arise after VT2. It is not fully clear why; it might be because higher exercise levels in patients with poorer cardiac function produce greater acidosis with a steeper VE/VCO2 slope after VT2. Ingle et al. suggested that a significant predictor of outcome derives from the difference between the submaximal VE/VCO2 slope and the VE/VCO2 slope after VT2; the greater this difference, the worse the prognosis is. They concluded that all data points (i.e., full-slope) should be used to measure the VE/VCO2 slope and thus obtain its highest prognostic value [21]. When calculated over the entire test, we found that the VE/VCO2 slope (i.e., full-slope) was identical between patients who surpassed VT2 and those who did not. This is particularly relevant since our data show that less than half of patients achieved VT2 despite metabolically maximal exercise. This suggests that, even without reaching VT2, the full-slope is likely better fitted than the submaximal slope to assess prognosis in cardiopulmonary disease patients.
In patients not achieving VT2, the VE/VCO2 slope data acquired below VT2 provide a prognostic substitute; however, some prognostic sensitivity is lost. Not being able to reach VT2 yet having a high VE/VCO2 slope could be related to advanced illness and poor prognosis [21,22]. Patients who did not achieve VT2, compared with those who did, had a higher BMI, were on average 4.5 years older, and had a lower VO2peak of 12% and a lower RERpeak. This lower VO2peak cannot be explained just by their older age as a decrease in VO2max of 0.5 to 1% per year is generally admitted [23]. As suggested by the lower RERpeak obtained, the difference in VO2peak between the two groups could be partly explained by a less metabolically intense effort [2,11]. These patients were also probably unable to exercise to VT2 because of advanced illness, as suggested by a higher VT1-slope, likely associated with metabolic deconditioning. Like others, we observed a slight increase in the VE/VCO2 slope values with age, regardless of the measurement method and the test indication. This supports the use of relative VE/VCO2 slope values rather than absolute values [14]. A VE/VCO2 slope measured at 35 may be normal at 70 years but abnormal at 20 years. Increased pulmonary artery pressure, reduced cardiac output, increased dead space/tidal volume ratio, hypersensitivity to chemoreceptor activation, deconditioning, and muscle weakness are some of the many processes involved in the mechanisms underlying the VE/VCO2 slope increase with age [17,24].
Strengths and Limitations
To our knowledge, this is the first study to assess the differences in VE/VCO2 slopes up to VT1, up to VT2, and over the entire test in different cardiopulmonary diseases. Our results clearly show that the VE/VCO2 slope measurement method used changes its value and should be homogenized to improve its prognostic power.
There are, however, several limitations of the present study that could affect the results or conclusions. First, this is a single-center retrospective study with a relatively small sample size; however, it offers the advantage of all parameters being measured with the same device and the same operators. Second, the VT1 and VT2 could be sometimes quite subjective. The thresholds have been reviewed by two independent physiologists to reduce the subjectivity part of the threshold’s determination. The two physiologists have determined threshold independently. When the thresholds were not located at the same level of workload, the two physiologists reviewed the thresholds together to agree on the same level of workload. Third, not all CPET centers use the same ramp protocol as studied herein [11]; therefore, the use of other testing modalities or protocols may affect the generalizability of our findings. Another issue involves calculating the VE/VCO2 slope via linear regression, which likely fits to a submaximal VE/VCO2 slope, yet the VE/VCO2 slope from an entire CPET is not linear [25,26]. However, the recommendations are to use the angular coefficient of the VE vs. VCO2 relationship and consider it as a linear regression. Therefore, there are no references for a nonlinear equation.
5. Conclusions
In conclusion, our data demonstrate the existence of great differences depending on the method used to calculate VE/VCO2 slopes. The significant variations observed certainly limit the prognostic value of the VE/VCO2 slope and homogenization of the calculation method seems critical to improve its prognostic value. The difference we observed between the full-slope and submaximal slopes was sufficient to reclassify HF patients from low-risk to high-risk categories. This supports the use of the data from the entire test (full-slope) to calculate the VE/VCO2 slope.
Acknowledgments
Editorial assistance, in the form of language editing and correction, was provided by XpertScientific Editing and Consulting Services.
Author Contributions
M.C., K.F., A.G., D.T.N. and M.L. contributed to the conception and design of the work; M.C., K.F., A.G., D.T.N. and M.L. contributed to the acquisition, analysis, and interpretation of data for the work; M.C., D.T.N. and M.L. drafted the manuscript; M.C., K.F., A.G., D.T.N. and M.L. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the ULB-Erasme ethics committee (2021/406). The data collected were anonymous and the study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon demand from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the “Fonds Erasme pour la Recherche Médicale”, Belgium (to M. Chaumont and A. Gillet); the “Fonds Simone et Désiré Drieghe-Miller”, Belgium (to M. Chaumont); the “Fondation pour la Chirurgie Cardiaque”, Belgium (to M. Chaumont); the “Fondation Emile Saucez-René Van Poucke”, Belgium (to M. Chaumont); the “Prix Docteur & Mrs Rene Tagnon”, Belgium (to M. Chaumont); the “Fondation IRIS”, Belgium (to M. Chaumont); and the “Prix de l’Association André Vésale”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Adachi H. Cardiopulmonary Exercise Test. Int. Heart J. 2017;58:654–665. doi: 10.1536/ihj.17-264. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M., Bandera F., Ozemek C., Systrom D., Arena R. Cardiopulmonary Exercise Testing: What Is its Value? J. Am. Coll. Cardiol. 2017;70:1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Levett D.Z.H., Jack S., Swart M., Carlisle J., Wilson J., Snowden C., Riley M., Danjoux G., Ward S., Older P., et al. Perioperative cardiopulmonary exercise testing (CPET): Consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br. J. Anaesth. 2018;120:484–500. doi: 10.1016/j.bja.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Löllgen H., Leyk D. Exercise Testing in Sports Medicine. Dtsch. Ärztebl. Int. 2018;115:409–416. doi: 10.3238/arztebl.2018.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmariah S., Goldberg L.R., Allen M.T., Kao A. Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J. Am. Coll. Cardiol. 2006;47:2237–2242. doi: 10.1016/j.jacc.2005.11.089. [DOI] [PubMed] [Google Scholar]
- 6.Sarullo F.M., Fazio G., Brusca I., Fasullo S., Paterna S., Licata P., Novo G., Novo S., Di Pasquale P. Cardiopulmonary Exercise Testing in Patients with Chronic Heart Failure: Prognostic Comparison from Peak VO2 and VE/VCO2 Slope. Open Cardiovasc. Med. J. 2010;4:127–134. doi: 10.2174/1874192401004010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keteyian S.J., Patel M., Kraus W.E., Brawner C.A., McConnell T.R., Piña I.L., Leifer E.S., Fleg J.L., Blackburn G., Fonarow G., et al. Variables Measured During Cardiopulmonary Exercise Testing as Predictors of Mortality in Chronic Systolic Heart Failure. J. Am. Coll. Cardiol. 2016;67:780–789. doi: 10.1016/j.jacc.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra R., Bakken K., D’Elia E., Lewis G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016;4:607–616. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Poggio R., Arazi H.C., Giorgi M., Miriuka S.G. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: A meta-analysis of the published literature. Am. Heart J. 2010;160:1004–1014. doi: 10.1016/j.ahj.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Arena R., Myers J., Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: An evidence-based review. Heart Fail. Rev. 2008;13:245–269. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 11.Glaab T., Taube C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022;23:9. doi: 10.1186/s12931-021-01895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaver W.L., Wasserman K., Whipp B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 13.Hansen J.E., Sue D.Y., Wasserman K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 14.Salvioni E., Corrà U., Piepoli M., Rovai S., Correale M., Paolillo S., Pasquali M., Magrì D., Vitale G., Fusini L., et al. Gender and age normalization and ventilation efficiency during exercise in heart failure with reduced ejection fraction. ESC Heart Fail. 2020;7:368–377. doi: 10.1002/ehf2.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrà U., Agostoni P.G., Anker S.D., Coats A.J.S., Crespo Leiro M.G., de Boer R.A., Harjola V.-P., Hill L., Lainscak M., Lund L.H., et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018;20:3–15. doi: 10.1002/ejhf.979. [DOI] [PubMed] [Google Scholar]
- 16.Arena R., Humphrey R., Peberdy M.A. Prognostic ability of VE/VCO2 slope calculations using different exercise test time intervals in subjects with heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2003;10:463–468. doi: 10.1097/01.hjr.0000102817.74402.5b. [DOI] [PubMed] [Google Scholar]
- 17.Weatherald J., Sattler C., Garcia G., Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: The role of chemosensitivity and dead space. Eur. Respir. J. 2018;51:1700860. doi: 10.1183/13993003.00860-2017. [DOI] [PubMed] [Google Scholar]
- 18.Mezzani A. Cardiopulmonary Exercise Testing: Basics of Methodology and Measurements. Ann. Am. Thorac. Soc. 2017;14 (Supp. 1):S3–S11. doi: 10.1513/AnnalsATS.201612-997FR. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M., Arena R., Halle M., Piepoli M.F., Myers J., Lavie C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018;39:1144–1161. doi: 10.1093/eurheartj/ehw180. [DOI] [PubMed] [Google Scholar]
- 20.Balady G.J., Arena R., Sietsema K., Myers J., Coke L., Fletcher G.F., Forman D., Franklin B., Guazzi M., Gulati M., et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 21.Ingle L., Goode K., Carroll S., Sloan R., Boyes C., Cleland J.G.F., Clark A.L. Prognostic values of the Ve/VCO2 slope calculated from different time intervals in patients with suspected heart failure. Int. J. Cardiol. 2007;118:350–355. doi: 10.1016/j.ijcard.2006.07.105. [DOI] [PubMed] [Google Scholar]
- 22.Bard R.L., Gillespie B.W., Clarke N.S., Egan T.G., Nicklas J.M. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J. Heart Lung. Transplant. 2006;25:589–595. doi: 10.1016/j.healun.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 23.Kim C.H., Wheatley C.M., Behnia M., Johnson B.D. The Effect of Aging on Relationships between Lean Body Mass and VO2max in Rowers. PLoS ONE. 2016;11:e0160275. doi: 10.1371/journal.pone.0160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harridge S.D., Lazarus N.R. Physical Activity, Aging, and Physiological Function. Physiology. 2017;32:152–161. doi: 10.1152/physiol.00029.2016. [DOI] [PubMed] [Google Scholar]
- 25.Sun X.G., Hansen J.E., Garatachea N., Storer T.W., Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am. J. Respir. Crit. Care Med. 2002;166:1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 26.Nayor M., Xanthakis V., Tanguay M., Blodgett J.B., Shah R.V., Schoenike M., Sbarbaro J., Farrell R., Malhotra R., Houstis N.E., et al. Clinical and Hemodynamic Associations and Prognostic Implications of Ventilatory Efficiency in Patients With Preserved Left Ventricular Systolic Function. Circ. Heart Fail. 2020;13:e006729. doi: 10.1161/CIRCHEARTFAILURE.119.006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon demand from the authors.