Abstract
Background
In a changing health care environment where patient outcomes will be more closely scrutinized, the ability to predict surgical complications is becoming increasingly important. The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) online risk calculator is a popular tool to predict surgical risk. This paper aims to assess the applicability of the ACS NSQIP calculator to patients undergoing surgery for pancreatic neuroendocrine tumors (PNETs).
Methods
Using the US Neuroendocrine Tumor Study Group (USNET-SG), 890 patients who underwent pancreatic procedures between 1/1/2000–12/31/2016 were evaluated. Predicted and actual outcomes were compared using C-statistics and Brier scores.
Results
The most commonly performed procedure was distal pancreatectomy, followed by standard and pylorus-preserving pancreaticoduodenectomy. For the entire group of patients studied, C-statistics were highest for discharge destination (0.79) and cardiac complications (0.71), and less than 0.7 for all other complications. The Brier scores for surgical site infection (0.1441) and discharge to nursing/rehabilitation facility (0.0279) were below the Brier score cut-off, while the rest were equal to or above and therefore not useful for interpretation.
Conclusion
This work indicates that the ACS NSQIP risk calculator is a valuable tool that should be used with caution and in coordination with clinical assessment for PNET clinical decision-making.
Keywords: ACS NSQIP risk calculator, Pancreatic neuroendocrine tumor, PNET, Pan-NET
Introduction
Pancreatic neuroendocrine tumors (PNETs) are rare, highly variable lesions with approximately 1000 new cases diagnosed per year in the USA.1 Studies show the actual prevalence of these lesions may be higher, with up to 0.5–1.5% of individuals having PNETs on autopsy. PNETs are generally malignant with variable presentation—early due to secretory symptoms from functional tumors or late due to mass effect from nonfunctional tumors.2 Five-year survival varies from 95% in well-differentiated pancreatic neuroendocrine tumors, to 44% in well-differentiated carcinomas, and 0% in poorly differentiated carcinomas.3
Surgical options for treatment of PNET include enucleation, distal pancreatectomy, pancreaticoduodenectomy (Whipple), and total pancreatectomy depending on PNET type and location within the pancreas. Morbidity and mortality rates vary significantly by procedure as well as by hospital volume; in one large-scale study, distal pancreatectomy, pancreaticoduodenectomy, and total pancreatectomy had in-hospital mortality rates of 3.5%, 6.6%, and 8.3% respectively.4 With the high mortality and variability in outcomes, perioperative surgical risk estimation is especially valuable for risk stratification, operative planning, and to inform discussions with patients and families.
Various published risk calculators have become available to address this growing need. The first risk model to evaluate cardiac risk in noncardiac surgery was published in 1977 and led to the revised cardiac risk index, published in 1999.5,6 To address noncardiac morbidity and mortality, multiple disease-specific risk calculators were created. For pancreatic cancer patients, the Nationwide Inpatient Sample (NIS) was used to develop predictive models for pancreatic cancer.7,8 The American College of Surgeons National Surgical Quality Improvement Project (ACS NSQIP) database was used to develop a pancreatectomy risk calculator in 2010.9 Then, in 2013, the ACS NSQIP universal risk calculator was developed to provide risk of 30-day morbidity and mortality using 21 patient-specific risk factors for any surgical procedure; this was made publically available online and is now widely used in clinical practice.10
The applicability of the ACS NSQIP risk calculator has been examined in a variety of procedures and diseases from head and neck cancer to cystectomy.11–15 Use of the calculator has also been studied in other hepato-pancreato-biliary procedures and diseases including extrahepatic biliary malignancies and liver resection.16,17 However, the calculator’s ability to predict complications after procedures for PNETs has not been studied. The objective of this study was to examine the ability of the ACS NSQIP calculator to estimate perioperative morbidity and mortality in patients undergoing surgery for PNETs.
Methods
ACS NSQIP Calculator
The ACS NSQIP risk calculator is a widely used tool originally developed in 2013 using data collected from 393 hospitals between 2009 and 2012. It allows surgeons and patients to estimate risk of 8 postoperative complications using 21 preoperative factors (spanning procedure, demographics, and comorbidities).10 The risk calculator has now evolved to include data from 740 hospitals and provides estimated risk of 12 postoperative complications along with a predicted length of stay. Open, online access to the risk calculator is available at the following web address: https://riskcalculator.facs.org/RiskCalculator/.
Patient Selection
The US Neuroendocrine Tumor Study Group (USNET-SG) is a multi-institutional collaborative including data on neuroendocrine tumors from the Ohio State University (Columbus, OH), Emory University (Atlanta, GA), Stanford University (Palo Alto, CA), Virginia Mason Medical Center (Seattle, WA), University of Wisconsin (Madison, WI), Washington University (St. Louis, Mo), Vanderbilt University (Nashville, TN), and the University of Michigan (Ann Arbor, MI). The institutional review board of each institution approved the study. The USNET-SG was used to identify patients who had undergone resection for PNET between January 1, 2000 and December 31, 2016. This study only included adult patients undergoing first-time resection for pancreatic neuroendocrine tumor. Each institution independently performed chart review to obtain patient information, which was entered into a standardized data collection form.
Patient data was entered in to the ACS NSQIP risk calculator to perform risk assessment on August 25th, 2017.10 At the time that the analysis was performed, the calculator included entry of the following factors: procedure type (CPT code), age, sex, functional status, emergency case, ASA classification, steroid use, ascites, sepsis, ventilator status, disseminated cancer, insulin and non-insulin dependent diabetes mellitus, hypertension, congestive heart failure, dyspnea, current smoker, severe COPD, dialysis dependence, acute renal failure, and BMI.
The following outcomes were included in the study: any complication; serious complication (cardiac arrest, myocardial infarction, pneumonia, progressive renal insufficiency, acute renal failure, PE, DVT, return to the operating room, deep incisional SSI, organ space SSI, systemic sepsis, unplanned intubation, UTI, wound disruption); pneumonia; cardiac complication; surgical site infection; urinary tract infection; venous thromboembolism; renal failure; return to the operating room; readmission; discharge to nursing/rehab; length of stay; and death.
Statistical Analysis
Patient demographics and clinical characteristics were summarized using descriptive statistics including median and interquartile range for continuous variables and frequency and percentage for categorical variables. Logistic regression models were used to determine the association between predicted and actual risk. The predicted rates of complications in these patients were then compared with actual patient outcome.
The performance of the ACS NSQIP calculator in predicting risk was evaluated using two metrics: C-statistic and Brier score. The C-statistic is the area under the curve (AUC) of a receiver operating characteristic (ROC) curve. It is a measure of discrimination and graphs the sensitivity (true positive rate) versus 1-specificity (false positive rate). If the variable under study, in this case, the ACS NSQIP calculated risk, perfectly predicts patients who will have complication versus those who will not, the AUC will be 1. If the variable, ACS NSQIP calculated risk, completely fails to distinguish between those who will have a complication and those who will not, the AUC will be 0.5. Generally, AUC > 0.7 is considered “fair” and AUC > 0.8 is considered “good”.18
The Brier score is a simultaneous measure of calibration and discrimination. The Brier score is reported as a score between 0 and 1—and is calculated as the mean squared difference between a patient’s predicted probability and observed outcome. A score of 0 indicates no difference between predicted and actual outcome, and thus indicates the best possible test. A score of 1 indicates that the test did not predict the outcome. The Brier score is compared to a Brier score cutoff, which is partially based on incidence in the sample and above which it is no longer useful.10,19
All statistical analysis was completed using Stata MP 14.2 (StataCorp, College Station, TX).
Results
There were 890 patients who met inclusion criteria. Demographic and clinical data for these patients is shown in Table 1. The most commonly performed procedure was distal subtotal pancreatectomy (N = 559, 63%), followed by standard (N = 135; 16%) or pylorus-preserving pancreaticoduodenectomy (N = 110; 12%). The majority of included patients were under 65 years of age (N = 650; 73%), female (N = 458; 51%), were independent (N = 852, 96%), underwent elective surgery (N = 846; 99%), and were ASA class III with severe systemic disease (N = 507; 57%).
Table 1.
Demographic characteristics (n = 890)
| Calculator input | Type | Frequency (%) |
|---|---|---|
| Procedure type | Distal subtotal pancreatectomy | 559 (63) |
| Pancreaticoduodenectomy | 135 (16) | |
| Pylorus-sparing pancreaticoduodenectomy | 110 (12) | |
| Other | 83 (9) | |
| Age | Under 65 years | 650 (73) |
| 65–74 years | 168 (19) | |
| 75–84 years | 64 (7) | |
| 85 years or older | 8 (1) | |
| Body mass index, median (interquartile range) | 28 (24–32) | |
| Gender | Male | 432 (49) |
| Female | 458 (51) | |
| Functional status | Independent | 852 (96) |
| Partially dependent | 37 (4) | |
| Dependent | 1 (0.1) | |
| Elective surgery | 846 (99) | |
| ASA class | Healthy patient | 76 (8) |
| Mild systemic disease | 284 (32) | |
| Severe systemic disease | 507 (57) | |
| Severe systemic disease/life threat | 23 (3) | |
| Steroids | 21 (2) | |
| Ascites | 3 (0.3) | |
| Systemic sepsis | 1 (0.1) | |
| Ventilator dependent | 0 (0) | |
| Disseminated cancer | 70 (8) | |
| Diabetes | Yes-no medication | 19 (2) |
| Oral medication | 99 (11) | |
| Insulin | 68 (8) | |
| Hypertension | 396 (45) | |
| Congestive heart failure | 10 (1) | |
| Dyspnea | At rest | 16 (2) |
| With exertion | 3 (0.3) | |
| Smoking history | 143 (16) | |
| Severe COPD | 17 (2) | |
| Dialysis | 2 (0.2) | |
| Acute renal failure | 1 (0.1) |
Actual event rate among this patient cohort was then displayed alongside median predicted risk (Table 2). These figures are shown for demonstration purposes only and should not be directly compared using a statistical test as one (actual event rate) is a proportion and the other is a median (predicted risk). It is, however, noteworthy that the actual event rate was higher than the median predicted risk for any complication, serious complication, pneumonia, cardiac complication, surgical site infection, urinary tract infection, venous thromboembolism, return to the operating room, readmission, and death. The figures are comparable for discharge to nursing facility/rehabilitation (actual event rate N = 26; 3%; median predicted risk 2.9%; range, 0.5–52.4%). And, the actual event rate for renal failure (N = 9; 1%) is much lower than the median predicted risk of 7% (range, 1.0–50.0%).
Table 2.
Comparison of actual events versus predicted risk
| Actual events | Predicted risk (%) | |||
|---|---|---|---|---|
|
|
|
|||
| Outcome | N (%) | Median risk | Minimum | Maximum |
| Any complication | 225 (25%) | 22.0 | 10.2 | 51.9 |
| Serious complication | 215 (24%) | 19.3 | 8.6 | 45.2 |
| Pneumonia | 25 (3%) | 2.5 | 0.3 | 13.9 |
| Cardiac complication | 9 (1%) | 0.7 | 0.0 | 11.7 |
| Surgical site infection | 156 (18%) | 13.9 | 5.5 | 35.3 |
| Urinary tract infection | 39 (5%) | 2.5 | 0.7 | 7.1 |
| Venous thromboembolism | 28 (3%) | 2.7 | 1.0 | 7.8 |
| Renal failure | 9 (1%) | 7.0 | 1.0 | 50.0 |
| Return to the OR | 41 (5%) | 3.3 | 1.5 | 10.9 |
| Readmission | 201 (23%) | 14.7 | 7.8 | 29.0 |
| Discharge to nursing/rehab | 26 (3%) | 2.9 | 0.5 | 52.4 |
| Length of stay (median days, range) | 7 (5–10) | 7.0 | 4.0 | 27.5 |
| Death | 12 (1%) | 0.4 | 0.0 | 18.9 |
The median predicted risk for those who did and did not have an event were then compared (Table 3). For all outcomes, median predicted risk was higher among those who did have an event than among those who did not. ROC analysis was then performed (Fig. 1; Table 3). The C-statistics were highest and fair for discharge destination (0.79) and cardiac complications (0.71). The C-statistics were poor (< 0.7) for all other complications including any complication (0.54), serious complication (0.55), pneumonia (0.50), surgical site infection (0.57), urinary tract infection (0.55), renal failure (0.50), return to the OR (0.59), readmission (0.55), and death within 30 days of operation (0.63). Brier scores and Brier score cut-offs were then calculated and are listed in the final columns of Table 3. The Brier scores for surgical site infection (0.1441) and discharge to nursing/rehabilitation facility (0.0279) were below the Brier score cut-off, while the rest were equal to or above and therefore not useful for interpretation.
Table 3.
Risk calculator outcomes
| Outcome | Did not have event | Had event | AUC | Brier score | Brier score cut-off | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| N (%) | Median risk % (range) | N (%) | Median risk % (range) | ||||
| Any complication | 665 (75%) | 21.7 (10.2–51.9) | 225 (25%) | 23.6 (10.2–46.7) | 0.5424 | 0.1913 | 0.1889 |
| Serious complication | 675 (76%) | 19.1(8.6–45.2) | 215 (24%) | 21.1 (8.7–43.2) | 0.5518 | 0.1849 | 0.1832 |
| Pneumonia | 820 (97%) | 2.6 (0.3–13.9) | 25 (3%) | 2.8 (0.4–6.1) | 0.4969 | 0.0291 | 0.0287 |
| Cardiac complication | 836 (99%) | 0.7 (0.0–11.7) | 9 (1%) | 1.7 (0.3–6.0) | 0.7129 | 0.0105 | 0.0105 |
| Surgical site infection | 734 (82%) | 13.7 (6.0–35.3) | 156 (18%) | 15.1 (5.5–32.7) | 0.5707 | 0.1441 | 0.1446 |
| Urinary tract infection | 802 (95%) | 2.7 (0.7–7.1) | 39 (5%) | 2.8 (0.7–4.9) | 0.5491 | 0.0446 | 0.0442 |
| Venous thromboembolism | 862 (97%) | 2.7 (1.0–7.8) | 28 (3%) | 3.2 (1.2–5.3) | 0.5539 | 0.0305 | 0.0305 |
| Renal failure | 835 (99%) | 0.6 (0.0–5.9) | 9 (1%) | 1.1 (0.1–3.7) | 0.5000 | 0.0105 | 0.0105 |
| Return to the OR | 807 (95%) | 3.3 (1.6–10.9) | 41 (5%) | 3.9 (1.9–10.1) | 0.5889 | 0.0460 | 0.0460 |
| Readmission | 684 (77%) | 14.6 (7.8–29) | 201 (23%) | 15.1 (7.8–23.3) | 0.5459 | 0.1820 | 0.1818 |
| Discharge to nursing/rehab | 864 (97%) | 2.8 (0.5–50.8) | 26 (3%) | 13.4 (0.5–52.4) | 0.7912 | 0.0279 | 0.0284 |
| Death | 878 (99%) | 0.4 (0.0–18.9) | 12 (1%) | 0.9 (0.1–9.7) | 0.6328 | 0.0134 | 0.0133 |
Fig. 1.
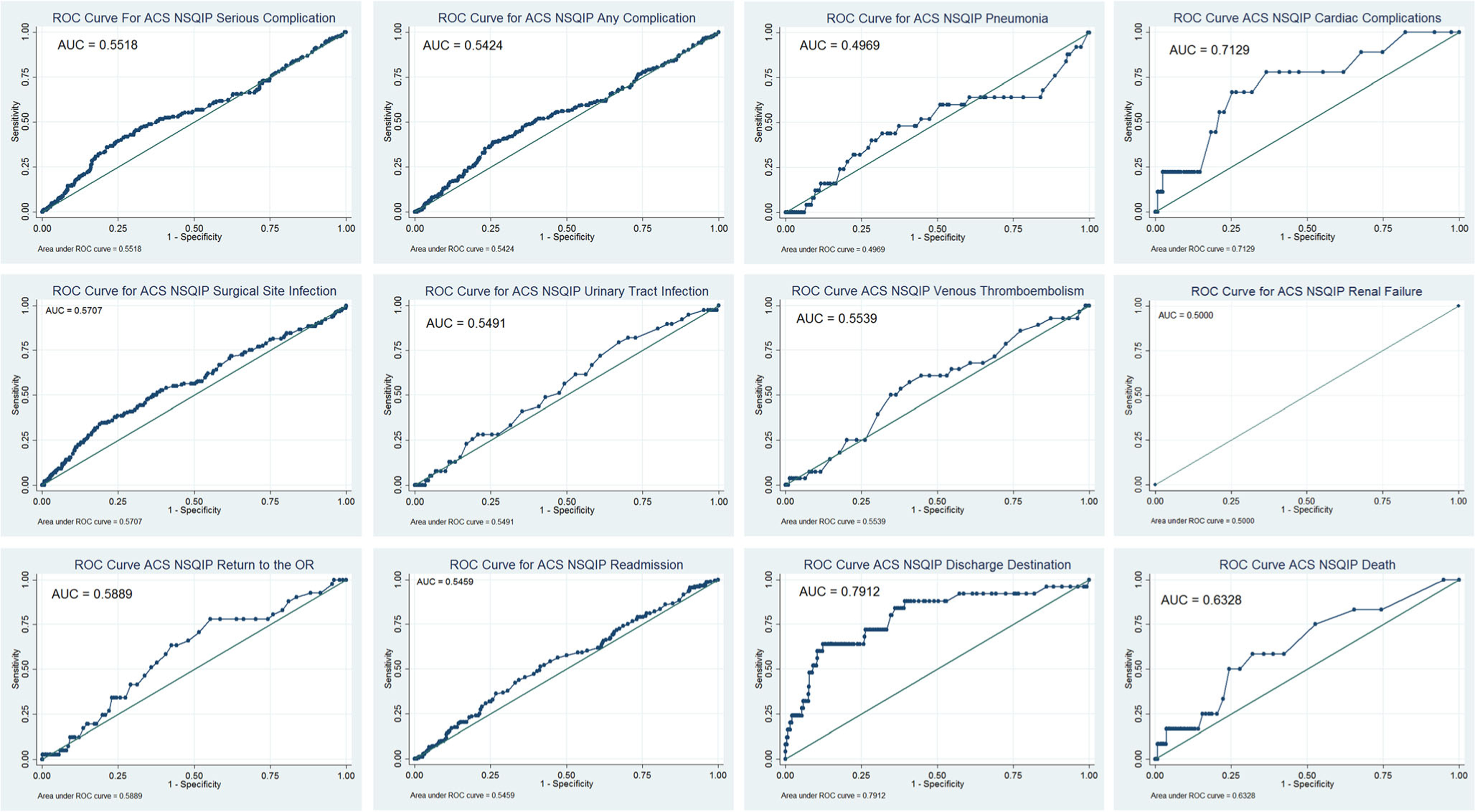
Receiver operator characteristic curves comparing predicted to actual events for any complication, serious complication, pneumonia, cardiac complication, surgical site infection, urinary tract infection, venous thromboembolism, renal failure, return to the operating room, readmission, discharge destination, and death
Discussion
This study compares actual event rate and predicted risk for surgical complications within 30 days of operation for 890 patients with pancreatic neuroendocrine tumor from the USNET-SG. The ACS NSQIP calculator is a popular, convenient, easy-to-use tool to allow physicians to assess surgical risk. We evaluated the prediction capabilities of the ACS NSQIP risk calculator in patients undergoing pancreatic resection for PNET. In comparing actual event rate and median predicted risk, we found that actual event rate for all events except renal failure was higher than median predicted risk. We also showed that, based on ROC analysis with AUC > 0.7, the calculator was able to reasonably predict discharge destination and cardiac complication after resection for PNET, but that it failed to accurately predict other complications. Calculated Brier scores were also only useful for surgical site infection and discharge destination. These are not only quality benchmarks for hospitals and physicians, but also of significant importance to patients and families.
Previous work has demonstrated that in case mix restricted populations, the C-statistic may decrease with a decrease in model performance.20 The Brier score, however, reflects both calibration and discrimination and may be a more useful statistical tool to evaluate model quality in homogenous populations.19,21 In this study, we found that C-statistic was only considered “fair” for cardiac complication and discharge destination, while Brier score was only a useful measure for surgical site infection and discharge destination. Additionally, as our group has discussed previously, one important complication of pancreatic surgery not addressed by the ACS NSQIP risk calculator is postoperative pancreatic fistula.17 Others have developed models for predicting postoperative pancreatic fistula after pancreaticoduodenectomy. One such model includes body mass index and pancreatic duct width and has a C-statistic of 0.832, while another includes main pancreatic duct index < 0.25, away from portal vein on computed tomography, disease other pancreatic cancer, male gender and intra-abdominal thickness and reported a C-statistic of 0.834.22,23 The ACS NSQIP risk calculator, which is designed to be more universally applicable, does not include any specific risk factors for postoperative pancreatic fistula. This may somewhat limit its utility in predicting postoperative complications for patients undergoing pancreatic resection. We might therefore conclude that the ACS NSQIP risk calculator, while remaining a valuable tool, should be used with caution in patients undergoing pancreatic resection for PNET.
The benefit of the universal calculator is its ability to be used for any surgical procedure. However, our results indicate that the ACS NSQIP risk calculator should be used with caution in patients undergoing resection for PNET. This may be because the ACS NSQIP calculator is limited by a lack of diagnosis discrimination. While it has been demonstrated that the indication for pancreatectomy—malignant versus nonmalignant—does not appear to be significantly associated with the risk of postpancreatectomy complications including leak abscess and fistula,24 studies examining the use of the calculator in patients undergoing pancreatic resection for pancreatic adenocarcinoma and studies comparing the risks of complications and mortality between patients undergoing pancreatectomy for pancreatic neuroendocrine tumor versus pancreatic adenocarcinoma are lacking. This is an important area for future work.
Previous studies in a variety of fields including surgical oncology, urology, and gynecology have reported similar results using the ASC-NSQIP for risk prediction.11,13–15,17 Thus, an area of future research would be to compare the predictive capability of the original procedure-specific risk calculators developed by ACS with the universal risk calculator; perhaps, in certain niche areas, the procedure-specific calculator would yield higher predictive ability. Another area of work would be to include complications that are more specific to the procedure being performed. For example, in the case of PNETS, the ability to predict likelihood of pancreatic leak would be of immense value in surgical decision-making.
This study has many strengths and some limitations. The USNET-SG is a multi-institutional collaborative including data on neuroendocrine tumors from eight large academic medical centers. This allowed us to amass a large cohort of patients on which to perform this analysis. However, the use of patients undergoing resection at academic medical centers only does somewhat limit the generalizability of these results. Additionally, although we were able to include 890 patients, the sample size for each specific procedure is still too small to perform procedure-specific subgroup analysis. Furthermore, we were not able to separate benign and malignant or functional and nonfunctional PNETs—and it has been reported previously that the morbidity and mortality of the NSQIP population among patients undergoing hepato-pancreato-biliary surgery does vary based on disease etiology.25
In conclusion, we found that actual event rate was higher than predicted risk for most surgical complications for 890 patients with pancreatic neuroendocrine tumor from the USNET-SG. In comparing patients who had an event versus those who did not, median predicted risk was higher among patients who had an event for all complications. Based on ROC analysis, we showed that the calculator reasonably predicted risk of discharge destination and cardiac complication, but failed to accurately predict other complications. Additionally, Brier scores were only useful for surgical site infection and discharge destination. Although the ACS NSQIP risk calculator remains useful for predicting morbidity and mortality among the NSQIP population as a whole, it should be used with caution in patients undergoing pancreatic resection for PNET.
Footnotes
Authorship AD, EWB, AGL, GP, EM, FGR, ZK, SRK, VRR, RCF, BAK, KI, PMS, HN, MB, SKM, TMP, CRS, and MD contributed to the conception and design of this work and acquisition of data. EWB, AD, CRS, and MD performed data analysis and interpretation of the data and prepared the manuscript. AD, EWB, AGL, GP, EM, FGR, ZK, SRK, VRR, RCF, BAK, KI, PMS, HN, MB,SKM, TMP, CRS, and MD revised the manuscript critically for important intellectual content, approved the final version to be published, and agree to be accountable for all aspects of the work.
References
- 1.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-JD et al. Cancer Survival Among Adults: U.S. SEER Program, 1988–2001. Patient and Tumor Characteristics. 2007. [Google Scholar]
- 2.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135(5):1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95(5):627–35. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 4.McPhee JT et al. Perioperative mortality for pancreatectomy: A national perspective. Ann. Surg. 2007; 246:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297(16):845–50. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- 6.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9. [DOI] [PubMed] [Google Scholar]
- 7.Hill JS, Zhou Z, Simons JP, Ng SC, McDade TP, Whalen GF et al. A simple risk score to predict in-hospital mortality after pancreatic resection for cancer. Ann Surg Oncol. 2010;17(7):1802–7. doi: 10.1245/s10434-010-0947-x. [DOI] [PubMed] [Google Scholar]
- 8.Are C, Afuh C, Ravipati L, Sasson A, Ullrich F, Smith L. Preoperative nomogram to predict risk of perioperative mortality following pancreatic resections for malignancy. J Gastrointest Surg. 2009;13(12):2152–62. doi: 10.1007/s11605-009-1051-z. [DOI] [PubMed] [Google Scholar]
- 9.Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB (Oxford). 2010;12(7):488–97. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CYet al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–42.e1–3. doi: 10.1016/j.jamcollsurg.2013.07.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohut A, Orfanelli T, Poggio JL, Gibbon D, Buckley De Meritens A, Richard S. Morbidity and Mortality Risk Assessment in Gynecologic Oncology Surgery Using the American College of Surgeons National Surgical Quality Improvement Program Database. Int J Gynecol Cancer. 2018;28(4):840–7. doi: 10.1097/IGC.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 12.Vosler PS, Orsini M, Enepekides DJ, Higgins KM. Predicting complications of major head and neck oncological surgery: an evaluation of the ACS NSQIP surgical risk calculator. J Otolaryngol Head Neck Surg. 2018;47(1):21. doi: 10.1186/s40463-018-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beal EW, Saunders ND, Kearney JF, Lyon E, Wei L, Squires MH et al. Accuracy of the ACS NSQIP Online Risk Calculator Depends on How You Look at It: Results from the United States Gastric Cancer Collaborative. Am Surg. 2018;84(3):358–64. [PubMed] [Google Scholar]
- 14.Margolick J, Wiseman SM. Risk of major complications following thyroidectomy and parathyroidectomy: Utility of the NSQIP surgical risk calculator. Am J Surg. 2018;215(5):936–41. doi: 10.1016/j.amjsurg.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Golan S, Adamsky MA, Johnson SC, Barashi NS, Smith ZL, Rodriguez MV et al. National Surgical Quality Improvement Program surgical risk calculator poorly predicts complications in patients undergoing radical cystectomy with urinary diversion. Urol Oncol. 2018;36(2):77.e1-.e7. doi: 10.1016/j.urolonc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Jiang HY, Kohtakangas EL, Asai K & Shum JB. Predictive Power of the NSQIP Risk Calculator for Early Post-Operative Outcomes After Whipple: Experience from a Regional Center in Northern Ontario. J Gastrointest. Cancer. 2018;49:288–294. [DOI] [PubMed] [Google Scholar]
- 17.Beal EW, Lyon E, Kearney J, Wei L, Ethun CG, Black SM et al. Evaluating the American College of Surgeons National Surgical Quality Improvement project risk calculator: results from the U.S. Extrahepatic Biliary Malignancy Consortium. HPB (Oxford). 2017;19(12):1104–11. doi: 10.1016/j.hpb.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013:9, 280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen ME, Ko CY, Bilimoria KY, Zhou L, Huffman K, Wang X et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. doi: 10.1016/j.jamcollsurg.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Merkow RP, Hall BL, Cohen ME, Dimick JB, Wang E, Chow WB et al. Relevance of the c-statistic when evaluating risk-adjustment models in surgery. J Am Coll Surg. 2012;214(5):822–30. doi: 10.1016/j.jamcollsurg.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts KJ, Hodson J, Mehrzad H, Marudanayagam R, Sutcliffe RP, Muiesan P et al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB (Oxford). 2014;16(7):620–8. doi: 10.1111/hpb.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2011;35(12):2747–55. doi: 10.1007/s00268-011-1253-x. [DOI] [PubMed] [Google Scholar]
- 24.VinY Sima CS, GetrajdmanGI Brown KT, Covey A,Brennan MF et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207(4):490–8. doi: 10.1016/j.jamcollsurg.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Kneuertz PJ, Pitt HA, Bilimoria KY, Smiley JP, Cohen ME, Ko CY et al. Risk of morbidity and mortality following hepato-pancreatobiliary surgery. J Gastrointest Surg. 2012;16(9):1727–35. doi: 10.1007/s11605-012-1938-y. [DOI] [PubMed] [Google Scholar]


