Abstract
Background:
The current study sought to define the impact of lymph node metastasis (LNM) relative to tumor size on tumor recurrence after curative resection for nonfunctional pancreatic neuroendocrine tumors (NF-pNETs) ≤2 cm.
Methods:
Patients who underwent curative resection for ≤2-cm NF-pNETs were identified from a multi-institutional database. Risk factors associated with tumor recurrence as well as LNM were identified. Recurrence-free survival (RFS) was compared among patients with or without LNM.
Results:
A total of 392 ≤2-cm NF-pNETs patients were identified. Among the 328 patients who had lymph node dissection and evaluation, 42 (12.8%) patients had LNM. LNM was associated with tumor recurrence (hazard ratio, 3.06; P = .026) after surgery. RFS was worse among LNM vs no LNM patients (5-year RFS, 81.7% vs 94.1%; P = .019). Patients with tumors measuring 1.5–2 cm had a two-fold increase in the incidence of LNM vs patients with tumors <1.5 cm (17.9% vs 8.7%, odds ratio, 2.59; P = .022), as well as a higher risk of advanced tumor grade and higher Ki-67 levels (both P < .01). After curative resection, a total of 14 (8.0%) patients with a tumor of 1.5–2 cm and 10 (4.5%) patients with tumor <1.5 cm developed tumor recurrence.
Conclusion:
Surgical resection with lymphadenectomy should be considered for patients with NF-pNETs ≥1.5–2.0 cm.
Keywords: lymph node metastasis, neuroendocrine tumor, pancreas, surgery, tumor size
1 |. INTRODUCTION
Pancreatic neuroendocrine tumors (pNETs) are a collection of rare neoplasms with a wide variety of biologic aggressiveness. The incidence of pNETs has increased over the past few decades with an almost doubling in the identification of these tumors concurrent with improvements in cross-sectional imaging.1–3 Based on symptoms and hormone secretion, pNETs are generally classified as functional (F-pNETs) vs nonfunctional pNETs (NF-pNETs) with the majority of tumors (65%−90%) being classified in the latter group.4,5 The lack of early symptoms among patients with NF-pNETs often leads to late discovery, more advanced stage at diagnosis, and worse long-term outcomes compared with patients who have F-pNETs.6–8
According to the European Neuroendocrine Tumor Society (ENETS) and American Joint Committee on Cancer (AJCC) 8th Edition Staging Manual Guidelines, surgical resection is recommended for NF-pNETs >2 cm.9,10 The management of patients with NF-pNETs ≤2 cm is, however, more controversial. Due to the relatively low tendency to progress as well as the potential morbidity associated with pancreatic resection, some investigators have advocated for observation and surveillance of NF-pNETs ≤2 cm as the preferred management strategy.11–13 Other experts, however, have highlighted the potential for malignant differentiation, lymph node metastasis (LNM), and distant metastasis even among patients with small NF-pNETs and therefore have argued for resection.14–16 The topic is particularly important given that the incidence of NF-pNETs ≤2 cm in the United States has increased dramatically over the past two decades with the proportion of patients with NF-pNETs ≤2 cm increasing from 12.3% in 1988 to 20.2% in 2009.17
Currently, both the ENETS and the National Comprehensive Cancer Network recommend an individualized treatment strategy for small NF-pNETs that may involve resection or observation as dictated by clinical judgment, as well as patient risk and preference.9,18 As such, the optimal treatment strategy of small NF-pNETs remains uncertain, and the guidelines are often unclear and not applicable to a “real life” setting.19 In addition, as minimally invasive techniques have expanded, more and more surgeons have adopted surgical resection for pNETs regardless of lesion size.17,20,21 Most previous data have focused on tumor size and LNM as risk factors associated with long-term outcomes following resection of pNETs.4,5,21,22 In contrast, the incidence of LNM relative to tumor size on prognosis among patients with NF-pNETs ≤2 cm has not been well defined. Therefore, the objective of the current study was to define the impact of tumor size on risk of LNM, as well as to characterize the association of tumor size and LNM among patients undergoing curative-intent resection for small NF-pNETs ≤2 cm.
2 |. MATERIALS AND METHODS
2.1 |. Study cohort
Patients who underwent surgical resection for pNETs between 1997 and 2016 were identified from the US Neuroendocrine Tumor Study Group.23 Inclusion criteria for the current study were patients with (a) nonfunctional tumor, (b) largest tumor diameter ≤2 cm, and (c) curative-intent resection (R0/R1). Exclusion criteria included (a) presence of distant metastasis, (b) death within 90 days after operation, and (c) cytoreductive or palliative (R2) resection. NF-pNETs were defined as asymptomatic if the tumor had no evidence of hormone overproduction; patients with no tumor-related hormone function who had symptoms related to tumor expansion and invasiveness, such as abdominal pain, jaundice, weight loss, etc were still categorized as NF-pNETs.10 The study was approved by the Institutional Review Boards at each participating institution.
2.2 |. Data collection
Demographic, clinical, and pathologic data at each institution were collected using a standardized datasheet. Tumor size, primary tumor location, the total number of LNs examined (TNLE), the number of LNM, Ki-67, tumor differentiation, perineural invasion, vascular invasion, and surgical margin status were determined based on the final pathological report. A minimum margin width of >1 mm was designated as an R0 margin; an R1 margin was defined as the microscopic presence of tumor at the margin or a minimum margin length of ≤1 mm.24
Following surgery, each patient was followed regularly with ultrasonography, computed tomography, and/or magnetic resonance imaging to monitor for recurrence. Recurrence of NF-pNETs was determined by suspicious imaging finding or biopsy-proven tumor. Recurrence patterns were classified as pancreas-only and distant recurrence. Recurrence-free survival (RFS) was defined as the time from surgical resection to tumor recurrence.
2.3 |. Statistical analysis
Categorical variables were reported as totals and percentages. The χ2 test or the Fisher exact test was used for comparison, as appropriated. Continuous variables were expressed as median with interquartile ranges (IQRs) and compared using the Mann-Whitney U test. Kaplan-Meier survival curves were plotted and compared using the log-rank test. Cox-proportional hazard regression models were used to identify risk factors associated with RFS on univariate and multivariable analyses; results were reported as hazard ratios (HRs) and 95% confidence intervals (95% CI). Logistic regression models were used to identify factors associated with LNM with results as odds ratio (OR) and 95% CI. A P value < .05 (two-tailed) was considered statistically significant for all analyses. Statistical analyses were performed using SPSS 22.0 (IBM, Chicago, IL).
3 |. RESULTS
3.1 |. Baseline characteristics
Among 989 patients who underwent curative-intent resection for NF-pNETs, a total of 392 (39.6%) patients had a primary tumor ≤2 cm and comprised the analytic cohort (Table 1). The median age was 59 (IQR 50–66) years, and roughly half of the cohort was female (n = 204, 52.0%). A majority of patients had no genetic syndrome (n = 349, 89.3%), and more than one-half of patients were diagnosed incidentally without any antecedent symptoms (n = 213, 54.3%). Given that most NF-pNETs were located in the pancreatic tail (n = 175, 44.6%), the most common procedure was a distal pancreatectomy (n = 237, 60.5%). Median operative time was 235 (IQR 190–315) minutes with a median estimated blood loss of 200 (IQR 50–300) mL. In the postoperative period, 227 (58.1%) patients had at least one complication; roughly one-third of these patients (n = 87, 38.5%) experienced a Clavien-Dindo III-IV complication. On final pathology, most tumors were well-differentiated (n = 325, 92.6%) and had a low ki-67 < 3% (n = 207, 73.4%). R0 resection was achieved in the overwhelming majority of patients (n = 354, 90.5%).
TABLE 1.
Clinical and pathological characteristics of the study cohort with NF-pNETs ≤2.0 cm
| Variables | Overall (n = 392) | LNM (n = 42) | No LNM (n = 286) | P |
|---|---|---|---|---|
| Age, y | 59 (50–66) | 55 (46–65) | 60 (50–66) | .288 |
| Sex | .841 | |||
| Female | 204 (52.0%) | 20 (47.6%) | 150 (52.4%) | |
| Male | 188 (48.0%) | 22 (52.4%) | 136 (47.6%) | |
| Genetic syndrome | .004 | |||
| None | 349 (89.3%) | 29 (69.0%) | 259 (90.9%) | |
| MEN-1 | 37 (9.5%) | 12 (28.6%) | 23 (8.1%) | |
| VHL | 4 (1.0%) | 1 (2.4%) | 3 (1.1%) | |
| Symptomatic | .079 | |||
| No | 213 (54.3%) | 16 (38.1%) | 162 (56.6%) | |
| Yes | 179 (45.7%) | 26 (61.9%) | 124 (43.4%) | |
| Primary location | .000 | |||
| Head | 95 (24.2%) | 26 (61.9%) | 61 (21.3%) | |
| Uncinated | 15 (3.8%) | 1 (2.4%) | 10 (3.5%) | |
| Neck | 28 (7.1%) | 3 (7.1%) | 21 (7.3%) | |
| Body | 79 (20.2%) | 1 (2.4%) | 60 (21.0%) | |
| Tail | 175 (44.6%) | 11 (26.2%) | 134 (46.9%) | |
| Ki-67 category | .022 | |||
| <3% | 207 (73.4%) | 17 (51.5%) | 155 (74.2%) | |
| ≥3% | 75 (26.6%) | 16 (48.5%) | 54 (25.8%) | |
| Tumor differentiation | .791 | |||
| Well | 325 (92.6%) | 34 (89.5%) | 236 (92.2%) | |
| Moderately | 26 (7.4%) | 4 (10.5%) | 20 (7.8%) | |
| Surgical technique | .006 | |||
| Open | 278 (70.9%) | 39 (92.9%) | 197 (68.9%) | |
| Laparoscopic/robotic | 114 (29.1%) | 3 (7.1%) | 89 (31.1%) | |
| Type of resection | .000 | |||
| Enucleation | 39 (9.9%) | 4 (9.5%) | 11 (3.8%) | |
| Classic PD | 36 (9.2%) | 10 (23.8%) | 25 (8.7%) | |
| Pylorus preserving PD | 52 (13.3%) | 14 (33.3%) | 38 (13.3%) | |
| Central pancreatectomy | 24 (6.1%) | 1 (2.4%) | 11 (3.8%) | |
| Distal pancreatectomy | 237 (60.5%) | 12 (28.6%) | 198 (69.2%) | |
| Total pancreatectomy | 4 (1.0%) | 1 (2.4%) | 3 (1.0%) | |
| Perineural invasion | 40 (13.3%) | 12 (37.5%) | 26 (11.9%) | .000 |
| Major vascular resection | 5(1.3%) | 2(4.9%) | 2(0.7%) | .083 |
| Operation time, min | 235 (190–315) | 256 (214–345) | 240 (195–320) | .286 |
| Blood loss, mL | 200 (50–300) | 300 (200–800) | 200 (50–300) | .012 |
| Margin status | .008 | |||
| R0 | 354 (90.5%) | 32 (78.0%) | 266 (93.0%) | |
| R1 | 37 (9.5%) | 9 (22.0%) | 20 (7.0%) | |
| Postoperative morbidity | 227 (58.1%) | 24 (57.1%) | 161 (56.5%) | .920 |
| Severe complication(III-IV) | 87 (38.5%) | 12 (50.0%) | 61 (37.9%) | .515 |
Abbreviations: LNM, lymph node metastasis; PD, pancreatoduodenectomy.
3.2 |. Lymph node metastasis and tumor recurrence
After a median follow-up of 33.7 (IQR 12.0–59.4) months, only 24 (6.1%) patients experienced tumor recurrence. The 3-, 5-, and 10-year RFS for the entire cohort was 95.1%, 91.9%, and 75.1%, respectively. On univariate analysis, only LNM was associated with tumor recurrence (HR 3.06, 95% CI, 1.15–8.17; P = .026) (Table 2). Among the 328 patients who had a lymph node dissection, the incidence of LNM was 12.8% (n = 42) with the vast majority of patients having node-negative disease (n = 286, 87.2%). Perhaps not surprisingly, patients with LNM were more likely to have an associated genetic syndrome, high Ki-67, as well as perineural invasion compared with patients who had node-negative disease (Table 1). RFS among patients with LNM was worse compared with patients who had node-negative disease (5-year RFS, LNM 81.7% vs node-negative 94.1%; P = .019) (Figure 1).
TABLE 2.
Risk factors of tumor recurrence after curative resection for NF-pNETs ≤2 cm
| Univariate analysis |
||
|---|---|---|
| Variable | HR (95% CI) | P |
| Gender (F/M) | 0.75 (0.33–1.71) | .487 |
| Age (<65/≥65) | 0.40 (0.12–1.35) | .140 |
| Symptomatic | 1.71 (0.76–3.85) | .198 |
| Ki-67 category | .538 | |
| <3% | Ref. | |
| ≥3% | 1.34 (0.53–3.43) | |
| WHO grade | .426 | |
| G1 | Ref. | |
| G2 | 1.52 (0.54–4.27) | |
| Tumor differentiation | .757 | |
| Well | Ref. | |
| Moderately | 1.26 (0.29–5.54) | |
| Tumor size, cm | .137 | |
| <1.5 | Ref. | |
| 1.5–2 | 1.85 (0.82–4.18) | |
| Lymph nodes metastasis | 3.06 (1.15–8.17) | .026 |
| Perineural invasion | 1.19 (0.34–4.17) | .783 |
| Lymphovascular invasion | 2.03 (0.73–5.61) | .175 |
| Final resection status R0 |
Ref. | .859 |
| R1 | 1.12 (0.33–3.78) | |
Abbreviation: CI, confidence interval; HR, hazard ratio.
FIGURE 1.
Recurrence-free survival of patients with or without lymph nodal metastasis (LNM) in the whole cohort (n = 392)
3.3 |. Tumor size and nodal metastasis
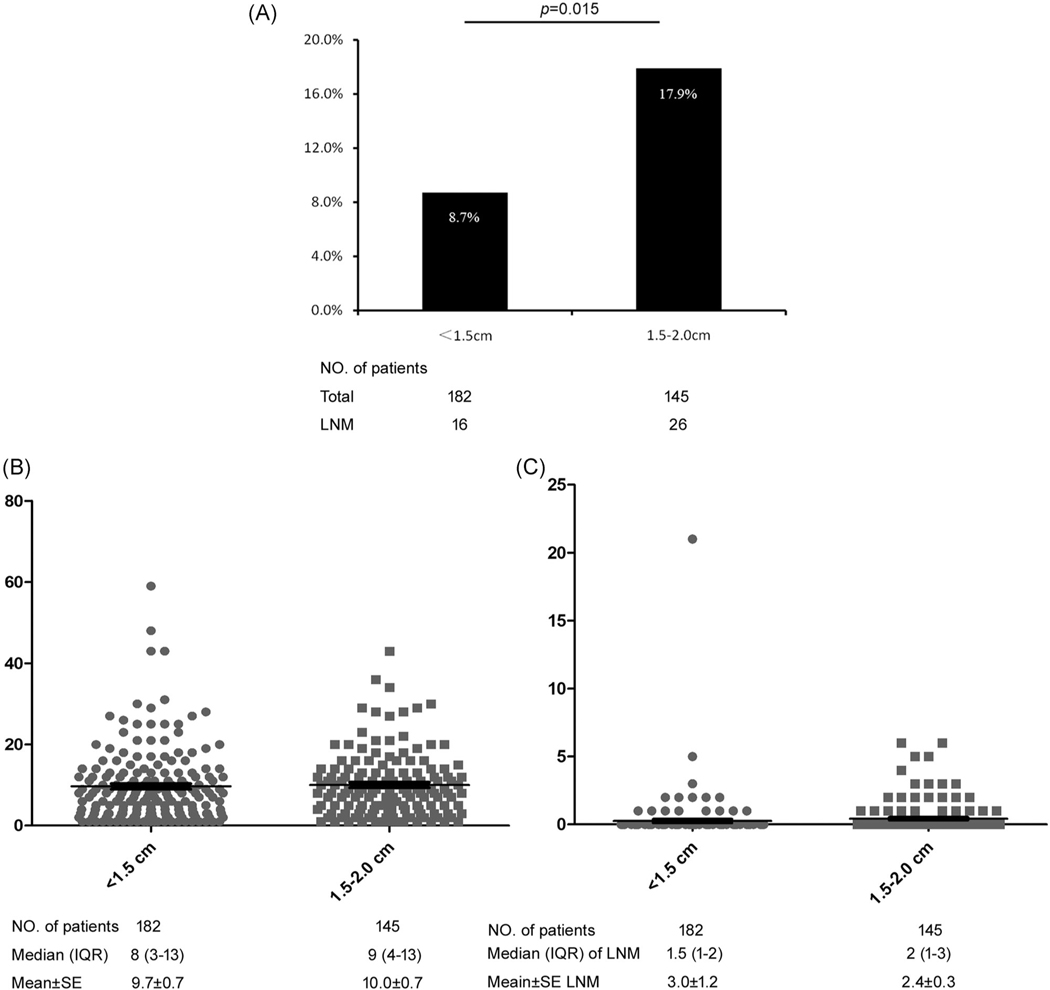
On multivariable analysis, tumor size (1.5–2.0 cm vs <1.5 cm, OR, 2.59, 95% CI, 1.15–5.83; P = .022) and Ki-67 category (≥3% vs <3%, OR, 2.20, 95% CI, 1.02–4.78; P = .045) were independently associated with risk of LNM (Table 3). Specifically, the incidence of LNM was almost two-fold higher among patients with 1.5–2 cm NF-pNETs (n = 145) vs <1.5 cm NF-pNETs (n = 183) (LNM, 1.5–2 cm 17.9% vs <1.5 cm 8.7%) (OR, 2.28, 95% CI, 1.17–4.44; P = .015) (Figure 2A), although TNLE and number of LNM were no different among patients with 1.5–2 cm NF-pNETs vs <1.5 cm NF-pNETs (TNLE, median 9 vs 8; P = .734; number of LNM, median 2 vs 1.5; P = .287) (Figure 2B,C).
TABLE 3.
Risk factors of lymph node metastasis for NF-pNETs ≤2 cm
| Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Variable | OR (95% CI) | P | OR (95% CI) | P |
| Gender (F/M) | 1.21 (0.63–2.32) |
.559 | ||
| Age (<65/≥65) | 0.74 (0.35–1.57) |
.432 | ||
| Functional status | 0.85 (0.10–6.91) |
.877 | ||
| Symptomatic | 2.12 (1.09–3.13) |
.027 | ||
| CgA (≤160/>160 ng/L) | 3.08 (0.91–10.37) |
.070 | ||
| Ki-67 category | ||||
| <3% | Ref. | .009 | Ref. | .045 |
| ≥3% | 2.70 (1.28–5.72) |
2.20 (1.02–4.78) |
||
| Tumor differentiation | ||||
| Well | Ref. | .570 | ||
| Moderately | 1.39 (0.45–4.31) |
|||
| WHO grade | ||||
| G1 | Ref. | .133 | ||
| G2 | 1.83 (0.83–4.03) |
|||
| Tumor size, cm | .015 | .022 | ||
| <1.5 | Ref. | Ref. | ||
| 1.5–2 | 2.28 (1.17–4.44) |
2.59 (1.15–5.83) |
||
Abbreviations: CI, confidence interval; OR, odds ratio.
FIGURE 2.
A, Incidence of lymph node metastasis (LNM) among patients with tumor <1.5 cm vs patients with a tumor of 1.5–2 cm. The total number of lymph nodes examined (B) and the number of LNM (C) of each patient in the two differently sized tumor groups
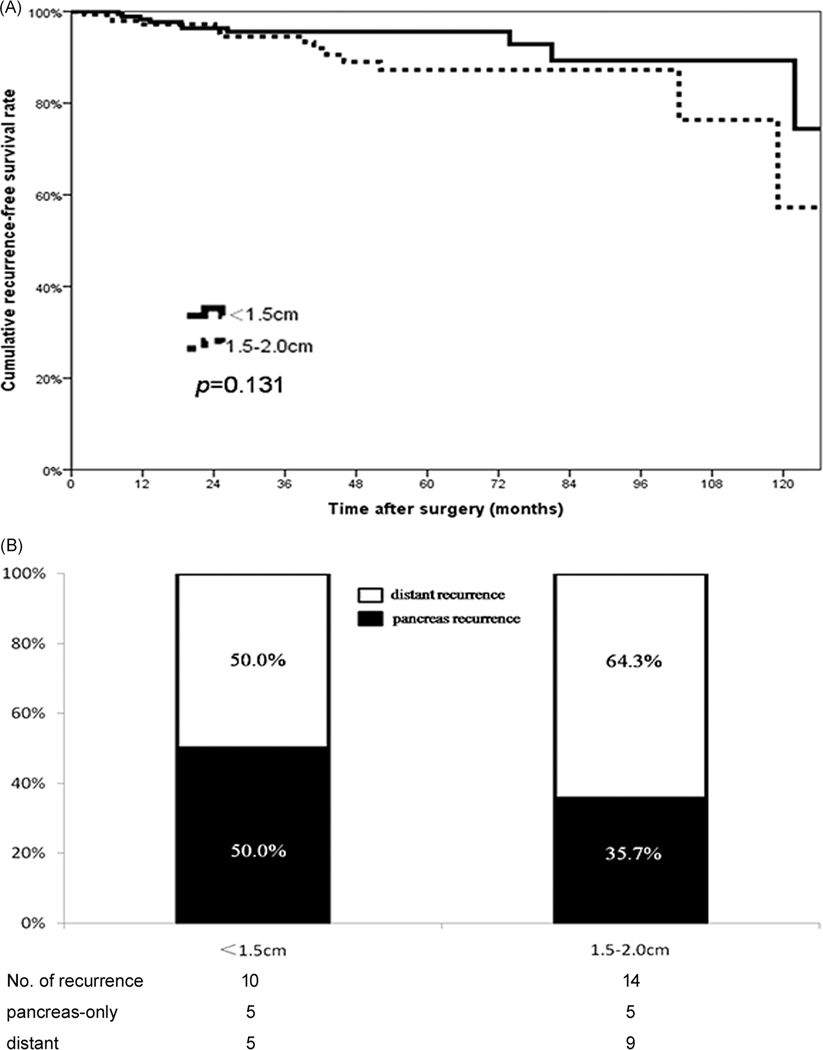
In addition to a higher incidence of LNM, 1.5–2 cm NF-pNETs were associated with more advanced disease including a Ki-67 ≥3% (1.5–2 cm 35.9% vs <1.5 cm 18.8%; P = .001), as well as worse WHO tumor grade (G2 grade, 1.5–2 cm 29.2% vs <1.5 cm 13.9%; P = .001) vs patients with a NF-pNETs <1.5 cm (Table 4). Of note, after curative resection, a total of 14 (8.0%) patients with tumors 1.5–2 cm and 10 (4.5%) patients with a tumor <1.5 cm developed tumor recurrence. RFS was no different among patients with NF-pNETs that measured 1.5–2 cm vs NF-pNETs <1.5 cm (5-year RFS, 1.5–2 cm 87.3% vs <1.5 cm 95.6%; P = .131) (Figure 3A). In addition, recurrence patterns were no different among patients with NF-pNETs of 1.5–2 cm and NF-pNETs <1.5 cm (distant recurrence, 64.3% vs 50.0%; P = .484) (Figure 3B).
TABLE 4.
Clinical and pathological characteristics of patients with NF-pNETs <1.5 cm and NF-pNETs of 1.5–2.0 cm
| Variables | <1.5 cm (n = 221) | 1.5–2.0 cm (n = 171) | P |
|---|---|---|---|
| Age, y | 59 (50–66) | 59 (50–67) | .119 |
| Sex | .416 | ||
| Female | 119 (53.8%) | 85 (49.7.%) | |
| Male | 102 (46.2%) | 86 (50.3%) | |
| Genetic syndrome | .323 | ||
| None | 201 (91.0%) | 148 (87.1%) | |
| MEN-1 | 19 (8.6%) | 18 (10.6%) | |
| VHL | 1 (0.5%) | 3 (1.8%) | |
| Symptomatic | .098 | ||
| No | 112 (50.7%) | 101 (59.1%) | |
| Yes | 109 (49.3%) | 70 (40.9%) | |
| Primary location | .072 | ||
| Head | 54 (24.4%) | 41 (24.0%) | |
| Uncinated | 9 (4.1%) | 6 (3.5%) | |
| Neck | 13 (5.9%) | 15 (8.8%) | |
| Body | 55 (24.9%) | 24 (14.0%) | |
| Tail | 90 (40.7%) | 85 (49.7%) | |
| Ki-67 category | .001 | ||
| <3% | 125 (81.2%) | 82 (64.1%) | |
| >3% | 29 (18.8%) | 46 (35.9%) | |
| Tumor differentiation | .064 | ||
| Well | 186 (94.9%) | 139 (89.7%) | |
| Moderately | 10 (5.1%) | 16 (10.3%) | |
| WHO grade | .001 | ||
| G1 | 149 (86.1%) | 97 (70.8%) | |
| G2 | 24 (13.9%) | 40 (29.2%) | |
| Lymph nodes metastasis | 16 (8.7%) | 26 (17.9%) | .013 |
| Surgical technique | .064 | ||
| Open | 165 (74.7%) | 113 (66.1%) | |
| Laparoscopic/robotic | 56 (25.3%) | 58 (33.9%) | |
| Type of resection | .571 | ||
| Enucleation | 21 (9.5%) | 18 (10.5%) | |
| Classic PD | 16 (7.2%) | 20 (11.7%) | |
| Pylorus preserving PD | 32 (14.5%) | 20 (11.7%) | |
| Central pancreatectomy | 16 (7.2%) | 8 (4.7%) | |
| Distal pancreatectomy | 134 (60.6%) | 103 (60.2%) | |
| Total pancreatectomy | 2 (0.9%) | 2 (1.2%) | |
| Perineural invasion | 24 (14.0%) | 16 (12.4%) | .681 |
| Major vascular resection | 3 (1.4%) | 2 (1.2%) | .877 |
| Lymphadenectomy | 183 (82.8%) | 145 (84.8%) | .584 |
| Operation time, min | 235 (184–311) | 220 (189–304) | .253 |
| Blood loss, mL | 200 (100–350) | 200 (100–300) | .004 |
| Margin status | .505 | ||
| R0 | 202 (91.4%) | 152 (89.4%) | |
| R1 | 19 (8.6%) | 18 (10.6%) | |
| Postoperative morbidity | 126 (57.3%) | 101 (59.1%) | .722 |
| Severe complication (III-V) | 53 (41.7%) | 34 (34.3%) | .257 |
Abbreviation: PD, pancreatoduodenectomy.
FIGURE 3.
A, Recurrence-free survival of patients with different tumor size (<1.5 cm, n = 221 vs 1.5–2 cm, n = 171). B, Recurrence patterns among patients with tumor <1.5 cm vs patients with tumor of 1.5–2 cm after curative resection
4 |. DISCUSSION
The treatment strategy for small NF-pNETs (≤2 cm) remains controversial, as both surgical resection and observation are recommended according to various guidelines.10,16 One of the main challenges in the management of small NF-pNETs is an accurate assessment of the natural history of the disease, as well as the ability to predict the risk of LNM and long-term outcomes. The clinical course of NF-pNETs ≤2 cm has not been well defined and, therefore, many surgeons often advocate for surveillance of these small tumors.17,20,21 Given that the general incidence of NF-pNETs is relatively low and most previous studies have been single-center series with small sample sizes, data on NF-pNETs <2 cm remain scarce. The current study was important because it demonstrated that roughly 40% of patients who underwent curative resection for NF-pNETs at one of several large HPB centers had a tumor size ≤2 cm. Of note, patients with a small NF-pNET had a two-fold increased incidence of LNM if the tumor measured 1.5–2 cm vs <1.5 cm (17.9% vs 8.7%; P = .015). Furthermore, LNM were present among 12.8% of patients with an NF-pNET ≤2 cm. In turn, LNM was associated with a three-fold increased risk of tumor recurrence after curative resection and a worse RFS vs patients who had no nodal disease after surgery (5-year RFS, nodal positive 81.7% vs nodal negative 94.1%; P = .019). Collectively, the data strongly suggest that surgery for NF-pNETs should be performed among patients with a tumor size ≥1.5 cm because of the relatively high incidence of LNM.
Some investigators have proposed that surveillance of NF-pNETs ≤2 cm is safe, as most of these tumors grow very slowly with no disease-related death among patients undergoing active surveillance.12,13 Data from two meta-analyses demonstrated that pNET tumor growth was observed in 50% of patients with small NF-pNET ≤2 cm; in addition, 9% of patients developed metastasis during surveillance.12,13 In a separate study of patients with NF-pNETs ≤2 cm derived from the National Cancer Data Base (NCDB), the authors reported a 5-year overall nondisease-specific survival of 27.6% among patients who did not undergo surgery compared with a 5-year survival of 82.2% among patients who underwent curativeintent resection.15 In the current study that examined surgical patients exclusively, RFS of 3- and 5-year RFS were 95.1% and 91.9%, respectively. Data from the current data were, therefore, more optimistic about the disease-specific prognosis for patients with small NF-pNETs ≤2 cm. Specifically, compared with the 5-year mortality of 18% reported in the NCDB study, we noted 5-year recurrence to be only about 8% of patients following resection of small pNETs. Our data were more consistent with the expected good prognosis of this patient population and likely reflected that the previous NCDB study captured all-cause mortality events, which included deaths not related to pNETs. In the current study, 5-year RFS among patients with NF-pNETs <1.5 (95.6%) tended to be better than the prognosis of patients with NF-pNETs measuring 1.5–2 cm (87.3%). In an earlier study by Zhang et al,25 the authors reported that surgical resection had the most long-term benefit among patients with pNETs ≥1.5 cm, while resection failed to demonstrate a difference in survival compared with surveillance among individuals with tumors <1.5 cm. Taken together, patients with NF-pNETs measuring 1.5–2 cm should be strongly considered for surgical resection, whereas patients with tumor <1.5 cm may be more appropriate candidates for surveillance.
While patients with small NF-pNET generally had a good prognosis, several factors were associated with a worse long-term survival, including tumor size, grade, and LNM. In particular, tumor size (1.5–2 cm vs <1.5 cm) was linked with a higher Ki-67 level, as well as more advanced WHO grade (Table 3). Jung et al26 had similarly reported that patients with a tumor measuring 1.5–2 cm had a higher likelihood to be WHO G2/G3 tumors vs tumors <1.5 cm. Tumor size, therefore, correlated with the potential for the presence of other adverse pathological features. In particular, the risk of recurrence was nearly two-fold higher among patients with tumors ≥1.5 cm (8.2%) compared with patients who had tumors <1.5 cm (4.5%)—suggesting a subset of patients with NF-pNETs ≤2 cm had a more aggressive natural history. Interestingly, among patients who did recur, the pattern of recurrence was no different comparing patients with tumor NF-pNET <1.5 cm vs ≥1.5 cm.
The presence of LNM has been particularly associated with an increased risk of tumor recurrence (Table 2). The need for routine performance of lymphadenectomy at the time of resection for NF-pNETs ≤2 cm is, however, somewhat controversial.5,15,17,21,27 National Cancer Center Network guidelines recommend regional lymph node evaluation at the time of resection for NF-pNETs, irrespective of tumor size.9,18 Despite this, some clinicians have suggested that enucleation of smaller pNETs without nodal evaluation may be acceptable.15,28 Our group previously reported that regional lymphadenectomy of at least eight lymph nodes was necessary to stage patients with pNETs accurately.23 In the current study, the median TNLE were eight and nine among patients with pNETs <1.5 cm and 1.5–2.0 cm, respectively (Figure 2B,C), suggesting that roughly half of the patients had an adequate number of LNs examined. Data on nodal disease is important as the presence of LNM renders the disease stage III regardless of tumor size both in the AJCC and ENETS staging systems.10,29–32 In the current study, LNM was noted in more than 1 in 10 patients (12.8%) who had an NF-pNET ≤2 cm. In particular, patients with an NF-pNET measuring 1.5 to 2 cm had a two-fold increased risk of LNM compared with patients who had an NF-pNET <1.5 cm (17.9% vs 8.7%). Several previous reports had similarly suggested a strong relationship between tumor size and risk of LNM.17,20,33 For example, Kuo et al17 reported an LNM incidence of 36% among patients with a pNET tumor measuring 16 to 20 mm vs 54% among patients with pNETs measuring and or >20 mm. Therefore, small pNET tumor size does not necessarily preclude the risk of metastasis to the regional nodal basin.15,17 In turn, patients with small NF-pNETs measuring >1.5 cm should undergo formal lymphadenectomy at the time of resection.
Several limitations should be considered when interpreting data in the current study. While the multi-institutional nature of the study undoubtedly increased the sample size, as well as the “real-world” application and generalizability of the data, there likely were some inconsistencies in patient selection for surgery, surgical techniques, as well as postoperative surveillance. There were also no patients with G3 ≤ 2 cm tumors, which was not surprising as these patients are generally not considered for surgical management and should be treated with systemic therapy.34 In addition, only patients undergoing curative-intent resection for pNETs were included in the analytic cohort. As such, there was no observation/surveillance group that consisted of patients with small NF-pNETs to serve as a comparator to assess the “true” benefit of surgery. The purpose of the study, however, was to determine the incidence and risk factors of LNM, as well as outcomes of patients with small NF-pNETs who underwent surgery.
In conclusion, assessing a large, US multi-institutional national cohort of patients with pNETs, roughly two out of every five patients who underwent curative-intent resection had a tumor ≤2 cm. Among patients undergoing surgical resection for small NF-pNETs, more than 1 in 10 patients had LNM. Patients with a tumor of 1.5–2 cm had a two-fold increase in the incidence of LNM vs patients with tumor <1.5 cm, as well as a higher risk of advanced tumor grade and higher Ki-67 levels. The presence of LNM was independently associated with a worse long-term RFS. While some previous studies have suggested that pNETs <2 cm are simply safe to follow,17,20,21 data from the current study strongly suggest that surgical resection with lymphadenectomy should be considered for patients with NF-pNETs ≥1.5–2.0 cm.
ACKNOWLEDGMENTS
Ding-Hui Dong and Xu-Feng Zhang were supported by the Clinical Research Award of the First Affiliated Hospital of the Xi’an Jiaotong University of China (No. XJTU1AF-CRF-2017-004). The authors appreciate the comprehensive work done by other members of the US Neuroendocrine Tumor Study Group: Dr. Alexandra G. Lopez-Aguiar MD, from Division of Surgical Oncology, Winship Cancer Institute, Emory University, Atlanta, Georgia; Dr. Eleftherios Makris MD from Department of Surgery, Stanford University, Palo Alto, California; Dr. Zaheer Kanji MD from Department of Surgery, Virginia Mason Medical Center, Seattle, Washington; Dr. Alexander Fisher MD from Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin; Dr. Bradley A. Krasnick MD from Department of Surgery, Washington University School of Medicine, St. Louis, Wisconsin; Dr. Paula M. Smith MD from Department of Surgery, Vanderbilt University, Nashville, Tennessee; Dr. Megan Beems MD from Department of Surgery, University of Michigan, Ann Arbor, Michigan; and Dr. Mary Dillhoff MD from The Ohio State University Wexner Medical Center and James Comprehensive Cancer Center, Columbus, Ohio.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
REFERENCES
- 1.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasumova GG, Tabatabaie O, Eskander MF, Tadikonda A, Ng SC, Tseng JF. National rise of primary pancreatic carcinoid tumors: comparison to functional and nonfunctional pancreatic neuroendocrine tumors. J Am Coll Surg. 2017;224:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Talamonti MS, Tomlinson JS, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490–500. [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Ji M, Chen J, et al. Surgery management for sporadic small (≤2 cm), non-functioning pancreatic neuroendocrine tumors: A consensus statement by the Chinese Study Group for Neuroendocrine Tumors (CSNET). Int J Oncol. 2017;50:567–574. [DOI] [PubMed] [Google Scholar]
- 6.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–548. [DOI] [PubMed] [Google Scholar]
- 7.Spolverato G, Bagante F, Aldrighetti L, et al. Neuroendocrine liver metastasis: prognostic implications of primary tumor site on patients undergoing curative intent liver surgery. J Gastrointest Surg. 2017;21:2039–2047. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XF, Beal EW, Chakedis J, et al. Early recurrence of neuroendocrine liver metastasis after curative hepatectomy: risk factors, prognosis, and treatment. J Gastrointest Surg. 2017;21: 1821–1830. [DOI] [PubMed] [Google Scholar]
- 9.Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MB. American Joint Committee on Cancer. New York: Springer; 2017. [Google Scholar]
- 11.Lee LC, Grant CS, Salomao DR, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965–974. [DOI] [PubMed] [Google Scholar]
- 12.Sallinen V, Le Large TYS, Galeev S, et al. Surveillance strategy for small asymptomatic non-functional pancreatic neuroendocrine tumors - a systematic review and meta-analysis. HPB. 2017;19:310–320. [DOI] [PubMed] [Google Scholar]
- 13.Partelli S, Cirocchi R, Crippa S, et al. Systematic review of active surveillance versus surgical management of asymptomatic small nonfunctioning pancreatic neuroendocrine neoplasms. Br J Surg. 2017;104:34–41. [DOI] [PubMed] [Google Scholar]
- 14.Haynes AB. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19: 117–123. [DOI] [PubMed] [Google Scholar]
- 17.Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;20:2815–2821. [DOI] [PubMed] [Google Scholar]
- 18.Clark OH, Benson AB 3rd, Berlin JD, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2009;7:712–747. [DOI] [PubMed] [Google Scholar]
- 19.Partelli S, Mazza M, Andreasi V, et al. Management of small asymptomatic nonfunctioning pancreatic neuroendocrine tumors: limitations to apply guidelines into real life. Surgery. 2019;166:157–163. [DOI] [PubMed] [Google Scholar]
- 20.Kishi Y, Shimada K, Nara S, Esaki M, Hiraoka N, Kosuge T. Basing treatment strategy for non-functional pancreatic neuroendocrine tumors on tumor size. Ann Surg Oncol. 2014;21:2882–2888. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Aguiar AG, Ethun CG, Zaidi MY, et al. The conundrum of <2-cm pancreatic neuroendocrine tumors: a preoperative risk score to predict lymph node metastases and guide surgical management. Surgery. 2019;166:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashim YM, Trinkaus KM, Linehan DC, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg. 2014;259:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XF, Xue F, Dong DH, et al. New nodal staging for primary pancreatic neuroendocrine tumors: a multi-institutional and national data analysis. Ann Surg. 2019. 10.1097/SLA.0000000000003478 [published online ahead of print July 26, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XF, Wu Z, Cloyd J, et al. Margin status and long-term prognosis of primary pancreatic neuroendocrine tumor after curative resection: results from the US neuroendocrine tumor study group. Surgery. 2019;165:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang IY, Zhao J, Fernandez-Del Castillo C, et al. Operative versus nonoperative management of nonfunctioning pancreatic neuroendocrine tumors. J Gastrointest Surg. 2016;20:277–283. [DOI] [PubMed] [Google Scholar]
- 26.Jung JG, Lee KT, Woo YS, et al. Behavior of small, asymptomatic, nonfunctioning pancreatic neuroendocrine tumors (NF-PNETs). Medicine. 2015;94:e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toste PA, Kadera BE, Tatishchev SF, et al. Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg. 2013;17:2105–2113. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Jin JB, Zhan Q, Deng XX, Shen BY. Impact and clinical predictors of lymph node metastases in nonfunctional pancreatic neuroendocrine tumors. Chin Med J. 2015;128:3335–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th Edition. Hoboken, NJ, USA: Wiley-Blackwell; 2017. [Google Scholar]
- 30.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. [DOI] [PubMed] [Google Scholar]
- 31.Rindi G, Klöppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–762. [DOI] [PubMed] [Google Scholar]
- 32.Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsutsumi K, Ohtsuka T, Mori Y, et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47:678–685. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016; 103:186–194. [DOI] [PubMed] [Google Scholar]