Abstract
Bacillus cereus causes a highly fulminant endophthalmitis which usually results in blindness. We previously concluded that hemolysin BL (HBL), a tripartite necrotizing pore-forming toxin, is a probable endophthalmitis virulence factor because it is highly toxic to retinal tissue in vitro and in vivo. We also determined that B. cereus produces additional retinal toxins that might contribute to virulence. Here we fractionated crude B. cereus culture supernatant by anion-exchange chromatography and found that in vitro retinal toxicity was also associated with phosphatidylcholine-preferring phospholipase C (PC-PLC). The pure enzyme also caused retinal necrosis in vivo. We showed that phosphatidylinositol-specific PLC and sphingomyelinase were nontoxic and that two hemolysins, cereolysin O and a novel hemolysin designated hemolysin IV, were marginally toxic in vitro. The histopathology of experimental septic endophthalmitis in rabbits mimicked the pathology produced by pure HBL, and both HBL and PC-PLC were detected at toxic concentrations in infected vitreous fluid. Bacterial cells were first seen associated with the posterior margin of the lens and eventually were located throughout the lens cortex. Detection of collagenase in the vitreous humor suggested that infiltration was facilitated by the breakdown of the protective collagen lens capsule by that enzyme. This work supports our conclusion that HBL contributes to B. cereus virulence and implicates PC-PLC and collagenase as additional virulence factors.
Bacillus cereus is one of the most common causes of posttraumatic and metastatic bacterial endophthalmitis. Among organisms that infect the eye, B. cereus has one of the most rapidly evolving courses of infection (11) and is one of the most destructive (25–28). The infection usually progresses from injury to enucleation in 24 to 48 h. It is extremely refractory, and blindness often occurs even in cases in which aggressive and appropriate antimicrobial therapy is instituted before the loss of visual acuity (15, 31).
It is generally believed that the activities of bacterial toxins directly influence the severity and final visual outcome of these infections. We have suggested that the expression of exotoxins by B. cereus may account in large part for the ineffectiveness of antibiotics (6). Once the toxins are expressed, killing the bacteria does not prevent damage by toxins. There is some evidence to support the idea that toxins play key roles in the pathology of fulminant endophthalmitis, but there have been few studies to directly address the issue, and little is known about the contributions of specific toxins.
We have shown that purified hemolysin BL (HBL), a tripartite B. cereus pore-forming toxin, is highly necrotic to rabbit retinal tissue in vitro and that intravitreal injection of HBL into rabbits produces symptoms that mimic the severity and course of B. cereus endophthalmitis (6). Upon intravitreal injection, HBL produced a distinctive folding pattern in the retinal outer nuclear layer and the attached layer of rods and cones. Intravitreal injection of crude exotoxin (culture supernatant fluid) from the endophthalmitis-associated isolate B. cereus MGBC 145 produced an identical folding pattern, suggesting that HBL had a major influence on the overall ocular toxicity of the crude preparations. However, neutralization of HBL in the crude toxin reduced in vitro retinal toxicity by only 50%, indicating that other factors significantly contribute to overall B. cereus retinal toxicity.
The present paper describes efforts to identify secreted B. cereus endophthalmitis virulence factors other than HBL. This organism secretes a wide variety of proteins which might be considered virulence factors a priori. Many of these, including HBL, nonhemolytic enterotoxin (NHE), and the three phospholipases discussed below, appear to be positively regulated by a pleiotropic regulator called PlcR (1). We fractionated crude B. cereus MGBC 145 culture supernatant by anion-exchange chromatography and monitored the eluete for retinal toxicity by an in vitro assay. Toxicity was associated with a single peak that corresponded with the elution of the B. cereus phosphatidylcholine-preferring phospholipase C (PC-PLC). Pure PC-PLC was also toxic to intact retinal tissue in vitro and caused retinal necrosis in vivo. Both PC-PLC and HBL were detected in the vitreous fluid of infected eyes, suggesting a role for these factors in vivo. We also tested several other potential virulence factors for in vitro retinal toxicity. Cereolysin O (CLO), phosphatidylinositol-specific phospholipase C (PI-PLC), sphingomyelinase (SMase), and a hemolysin provisionally designated hemolysin IV (Hly-IV) were all less toxic than PC-PLC.
Histopathological studies of experimental B. cereus endophthalmitis showed outer retinal layer folding identical to that produced by pure HBL. There was also a strong propensity for bacterial colonization and degradation of the lens cortex. Collagenase was detected in vitreous fluid of infected eyes, suggesting that the protective collagen lens capsule was compromised by this enzyme, permitting entry of the bacteria into the lens.
MATERIALS AND METHODS
Toxins and antibodies.
Crude exotoxin consisted of culture supernatant from a culture of B. cereus MGBC 145 grown in brain heart infusion broth supplemented with 0.1% glucose (BHIG) for 6 h at 32°C. It was prepared and, when necessary, concentrated as described previously (6, 7).
The following B. cereus enzymes were purchased: PI-PLC (EC 3.1.4.10; Boehringer Mannheim [catalog no. 1 743 069]), SMase (EC 3.1.4.12; Sigma Chemical Co., St. Louis, Mo. [catalog no. S 7651]), grade I PC-PLC (EC 3.1.4.3; Boehringer Mannheim [catalog no. 691 950]) for toxicity studies, and grade II PC-PLC (Boehringer Mannheim [catalog no. 108 502]) for antibody production.
Components of HBL from B. cereus F837/76 were purified as described previously (7). The following proteins were all isolated as by-products of HBL purification: SMase (used for antibody production; N-terminal amino acid sequence, D T S T D Q N N T L K V M T H N V Y M L) and CLO (N terminus, E T Q A G N A T D A I K N A S D I N T G I A N L X Y D S R D I L K V N G) from B. cereus F837/76 and Hly-IV from B. cereus MGBC 145 (N terminus, Q T T S Q V V T D I G Q N A K T H T S Y N T F N X D Q A D N). The hemolysin designated as CLO was inhibited by cholesterol and contained residues (underlined) conserved among six known sulfhydryl-activated hemolysins, confirming it as CLO. It was stored in 10 mM dithiothreitol to maintain reducing conditions. The N terminus of SMase is identical to that reported in reference 24. The N terminus of Hly-IV did not match known sequences in several sequence databases.
The proteins that we isolated were all greater than 95% pure as determined by densitometry of denaturing electrophoresis gels stained with Coomassie brilliant blue. The commercial PC-PLC was composed of intact PC-PLC (28 kDa; ca. 80%) and a 25-kDa PC-PLC degradation product (ca. 20%), which reacted with antibodies specific to the enzyme. No other bands were evident, and the preparation did not react with antibodies to SMase. The commercial SMase consisted of intact SMase (ca. 90%) and a 30-kDa SMase degradation product (10%) which reacted with SMase antibodies. A minor band that did not react with the antibodies ran with the dye front (<14 kDa). The enzyme preparation contained no PC-PLC activity. Both enzymes used here exhibited some degradation but were sufficiently pure to rule out interference from nonspecific proteins.
Polyclonal antibodies were produced by injecting electrophoresis gel slices containing the antigen of interest into rabbits (6, 21). The specific antisera to all three HBL components were described earlier (6). Antisera to all antigens reacted with a single major band from B. cereus culture supernatants on Western blots. Immunoglobulin G (IgG) fractions were purified by affinity chromatography on HiTrap Protein G columns (Pharmacia, Piscataway, N.J.). Mouse monoclonal antibodies specific for HBL component B were produced in ascites fluid at Harlan Bioproducts for Science, Inc. (Indianapolis, Ind.).
Chromatography.
Anion-exchange chromatography on Whatman DE-52 and hydroxyapatite (HA) chromatography were performed as described previously for B. cereus F837/76 (7) with the following exceptions. The anion exchanger was equilibrated with 25 mM Tris-HCl, pH 7.5, rather than the pH 5.9 bis-Tris–HCl, for isolation of proteins from B. cereus MGBC 145. HA chromatography was performed with Macro-Prep ceramic hydroxyapatite (Bio-Rad, Richmond, Calif.) rather than Bio-Gel HTP.
In chromatography fractions, PC-PLC, SMase, and components of HBL and HBLa, a homolog of HBL (8), were detected with dot blots. Dot intensities were quantitated densitometrically with a digital imaging system equipped with spot densitometry analysis software (Alpha Innotech Corp., San Leandro, Calif.). Hemolysis activity of fractions was measured at room temperature or 37°C in a turbidometric microplate assay using washed swine, sheep, and hamster erythrocytes as previously described (8).
In vitro methods for retinal toxicity assay.
Retinal toxicity was determined by measuring the release of lactate dehydrogenase (LDH) from isolated rabbit retinal tissue. The retinal tissue was prepared either as buttons or as dispersed suspensions of retinal cells. The retinal button assay was described previously (6). In this method, 3-mm-diameter buttons are cut from the retinas of dissected rabbit eyes. These buttons are placed in wells of a microtiter plate under serum-free culture medium in the presence or absence of toxin samples. Toxicity is determined by measuring the release of LDH and comparing it with that caused by a concentrated B. cereus culture supernatant. The retinal cell suspension assay was identical to the retinal button assay except that rather than cutting buttons, the entire retina was gently homogenized in a 4-ml suspension in a glass tissue grinder with a Teflon pestle. Homogenized retinal tissue was washed four times by centrifugation (12,000 × g, 10 s), and the washed cells were suspended to an approximate optical density at 630 nm of 2.0. Each assay mixture contained 100 μl of this working retinal suspension and 10 μl of sample in wells of a 96-well microtiter plate. A single rabbit provided enough retinal suspension to fill a 96-well microtiter plate. In both assays, samples were incubated 90 min at 37°C, the plates were centrifuged, and the supernatants were assayed for LDH activity. Concentrated (100×) B. cereus MGBC 145 culture supernatant was used as a positive control for both assay methods.
Chromatography fractions were screened with the suspension assay because it conveniently permitted testing of numerous samples simultaneously. However, the retinal button assay maintains normal retinal architecture and therefore was used as our standard method for determining retinal toxicity. The relative toxicities of the various purified toxins and enzymes were determined with the button assay, and the toxicities of all chromatography fractions screened with the suspension assay were confirmed with retinal buttons.
LDH assays.
The continuous assay for LDH described in reference 6 was used for the retinal button assay but was replaced by a stop-time assay for the retinal suspension assay to accommodate a large number of samples. The LDH reaction was carried out in microtiter plate wells containing 30 μl of retinal cell supernatant and 135 μl of substrate (0.88 mM NADH and 4 mM pyruvate in 0.2 M Tris-Cl, pH 7.3). The reaction was stopped after 10 min at 22 to 25°C by the addition of 135 μl of 25 mM oxamic acid (32), and 250 μl of each sample was transferred to a cuvette containing 0.45 ml of 25 mM oxamic acid. Residual NADH was measured at 340 nm. LDH activity was estimated as the difference between the NADH contents (A340) of the samples and the negative controls (consisting of retinal suspension and buffer).
In vivo endophthalmitis models.
Our in vivo sterile endophthalmitis model, in which cell-free toxin samples are injected intravitreally, was described previously (6). Injections of B. cereus cells in our septic endophthalmitis model were performed essentially the same as for the sterile endophthalmitis model and as described by Callegan et al. (13), except that paracentesis of the aqueous chamber was not performed. This did not affect the ocular architecture of negative controls (i.e., eyes into which only buffer was injected). The rabbits used in this study were primarily Dutch belted, but several New Zealand white rabbits were also employed. The latter possess thicker lens capsules, making it possible to identify bacterial cells in the process of penetrating the capsule when eyes were harvested 12 h after infection. Anesthesia prior to intravitreal injection and prior to all clinical examinations was delivered by intramuscular injection of ketamine (35 mg kg−1), xylazine (5 mg kg−1), and acepromazine (0.75 mg kg−1). After injection, eyes were examined for intraoperative complications by indirect ophthalmoscopy and then every 4 h for clinical signs until the rabbits were killed. Atropine was applied topically at 6 h to induce long-term mydriasis. All injected samples were in 0.1-ml volumes. B. cereus inocula were grown overnight in BHIG at 32°C to a density of ca. 109 CFU ml−1 and diluted in sterile saline to 103 to 104 CFU ml−1 prior to injection (102 to 103 CFU eye−1). Either enucleated eyes were fixed and sent for histopathology preparation or the liquid vitreous was collected for bacterial growth estimation and enzyme and toxin analysis. Fixed eyes were processed at the Eye Pathology Laboratory at the University of Wisconsin—Madison. Eyes were cut in the horizontal plane, and central sections were embedded in paraffin in a Fisher automatic tissue processor. Sections were cut 6 μm thick and were stained with hematoxylin-eosin or with the Gram stain.
Gel diffusion assays for hemolysis and PC-PLC.
Ocular fluids and B. cereus culture supernatants were assayed for hemolysis activity by a sheep blood gel diffusion method (5, 7). PC-PLC was quantitated by a nearly identical method in which blood was replaced by 1.5 mg of l-α-phosphatidylcholine (Sigma type II-S from soybean) ml−1. The gels were incubated 16 h at 25°C. Zone areas for both assays were measured by using a digital imaging system equipped with analysis software (Alpha Innotech Corp.). Hemolysis activity in ocular fluids is reported relative to the activity in a BHIG culture supernatant of B. cereus MGBC 145 as determined by comparison to a dilution series of the supernatant. PC-PLC activity was quantitated by comparison of zone areas to those in a standard curve for pure PC-PLC.
Collagenase assay.
For determination of collagenase activity, 5 μl of sample was added to 45 μl of 10 mg-ml−1 gelatin or 10 mg-ml−1 type IV collagen (Sigma type IV) in a solution containing 50 mM Tris-Cl, 145 mM NaCl, and 5 mM CaCl2 and incubated at 37°C for 18 h. Intact collagen was precipitated with an equal volume of 50% trichloroacetic acid, and the levels of soluble amino acids and peptides were estimated by a standard ninhydrin method (16). Activity was measured as the difference in acid-soluble amino acids and peptides between the 18-h samples and zero time controls in which 5 μl of sample was added to substrate after it had been incubated at 37°C for 18 h and immediately prior to acid precipitation. Activity was estimated by comparison with a standard curve of clostridiopeptidase A (EC 3.4.24.3; Sigma) assuming a preparation activity of 1.8 U mg−1 as labeled.
Estimation of HBL component B in vitreous humor.
Some components of normal rabbit vitreous fluid cross-reacted in dot blot analyses employing monoclonal antibodies to the HBL components. Therefore, HBL component B was estimated by performing spot densitometry of Western blots. Vitreous samples from infected eyes were electrophoresed and blotted next to samples of normal vitreous fluid and BHIG culture supernatant of B. cereus MGBC 145. Specific bands were identified by comparison with nonspecific bands present in the normal vitreous humor. Band intensities were quantitated densitometrically as described above for dot blots. The bands were quantitated as integrated density values divided by 1,000. An integrated density value is the product of the area of a band and its average pixel density (above background density).
Electrophoresis and protein blotting were performed on prepoured minigels (Bio-Rad) as described previously (7). The blots were probed with mouse monoclonal antibodies to the B component of HBL, which were subsequently detected with peroxidase-labeled goat anti-mouse antibodies.
RESULTS
Identification of PC-PLC as a primary retinal toxin.
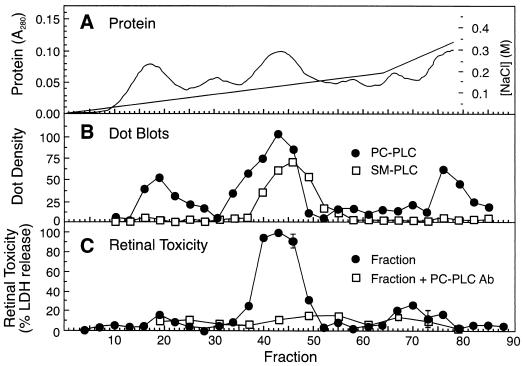
Figure 1 shows that only one major retinal toxicity peak eluted from an anion-exchange column loaded with crude B. cereus culture supernatant. The fractions under this peak contained PC-PLC and SMase (Fig. 1) as well as HBL components L1 and L2 (not shown), but toxicity was largely eliminated by specific antibody to PC-PLC. Fraction 43 from the toxicity peak was also tested by the retinal button assay in the presence or absence of EDTA and neutralizing antibodies (purified IgG) to PC-PLC, SMase, and HBL components L1 and L2. A quantity of fraction 43 (2.5 μl) that caused ca. 83% of maximal LDH release was inhibited 87% by EDTA and 92% by antibody to PC-PLC but was not affected by the other antibodies. Therefore, the main toxic component under this peak was PC-PLC, a metalloenzyme containing up to three tightly bound zinc atoms in its active site (20, 23). EDTA presumably inhibited retinal toxicity by chelating the zinc.
FIG. 1.
Elution profile of protein, phospholipases, and retinal toxicity from a DE-52 anion-exchange column loaded with crude B. cereus culture supernatant. (A) NaCl gradient (straight lines) and elution of protein (curve) as determined by measuring the A280. (B) Elution of PC-PLC and SMase (SM-PLC) as detected by dot blot analysis. Dot densities, expressed as integrated density values (103), were measured with a digital imaging system as described in Materials and Methods. (C) Retinal toxicity of chromatography fractions and inhibition of toxicity by polyclonal antibodies (Ab; purified IgG) to PC-PLC. The nonbound fraction (not shown) was hemolytic but not significantly toxic to retinal tissue.
As suggested by the three peaks in Fig. 1, PC-PLC exhibits multiple ionic forms, which differ in zinc content and enzyme conformation (10). Two of the PC-PLC peaks exhibited minor retinal toxicity. It is possible that the toxicity of this enzyme varies with its zinc content.
The anion-exchange column associated with Fig. 1 produced four peaks of hemolysis activity, including the nonbound fraction and fractions 17 to 22, 24 to 28, and 65 to 73. The first two peaks (nonbound and fractions 17 to 22) were not inhibited by cholesterol. The second peak (fractions 17 to 22) corresponded with the first minor retinal toxicity peak as well as the first PC-PLC peak. Anti-PC-PLC antibodies only marginally reduced the retinal toxicity of these fractions, indicating that PC-PLC was not responsible for all of the toxicity. The hemolysin under the third peak (fractions 24 to 28) was inhibited by cholesterol, indicating that it was CLO. The last peak of hemolysis corresponded to the second minor retinal toxicity peak and occurred where the HBL B component eluted and was inhibited by anti-HLB antibodies. Hemolysis was produced under the B peak because L1 and L2 tail into that peak (7).
In vitro analysis of potential B. cereus virulence factors.
We examined the following potential virulence factors for retinal toxicity in the retinal button assay: PC-PLC, SMase, PI-PLC, HBL, CLO, and Hly-IV. Hly-IV was isolated from the second hemolysis peak described above (fractions 17 to 22). The hemolytic activity from fractions 17 to 22 (Fig. 1) appeared in the nonbound fraction of an HA column (PC-PLC was bound to the column). That fraction contained one major protein of ca. 35 kDa (as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) with an isoelectric point of 5 to 5.5 (PhastGel IEF), and the N-terminal sequence provided in Materials and Methods. This novel toxin was named Hly-IV for consistency with the nomenclature used for other B. cereus hemolysins, Hly-I (CLO) (14), Hly-II (3), and Hly-III (4).
Each potential virulence factor was tested in the retinal button assay at several concentrations (n = 3 per concentration). HBL produced a dose response nearly identical to that seen previously (6), with a rapid increase in toxicity up to a concentration of about 4 nM (ca. 150 ng ml−1 per component) and no increase at higher concentrations. The maximum LDH release caused by HBL and by PC-PLC was approximately 50% of that of the positive control (concentrated B. cereus culture supernatant). LDH release by both CLO and Hly-IV plateaued at about 20% of maximal release. For comparison, the concentrations needed for each potential virulence factor to produce 20 and 50% maximal LDH release are shown in Table 1. PI-PLC did not induce LDH release at levels above that of negative controls, and SMase produced a maximum 5% release at 140 nM (5 μg ml−1). The presence of 5 mM CaCl2 and 5 mM MgCl2 did not enhance SMase toxicity as it does hemolysis (30).
TABLE 1.
Approximate dose required to reach 20 or 50% of maximal retinal toxicitya
| Sample | Concentration (nM) required for toxicity ofb:
|
|
|---|---|---|
| 20% maximal | 50% maximal | |
| HBL | 1–1.5 | 3–4 |
| PC-PLC | 25–28 | 180 |
| CLO | 90 | NA |
| Hly-IV | 180 | NA |
| SMase | NA | NA |
| PI-PLC | NA | NA |
Toxicity (ΔA340 min−1 ± standard deviation) for the B. cereus culture supernatant was 0.79 ± 0.010, and that for the negative control was 0.013 ± 0.006 (n = 5).
NA, not achieved. Maximum toxicities were as follows: CLO, 20%; Hly-IV, 20%; SMase, 4 to 5%; and PI-PLC, 0 to 2%.
PC-PLC and SMase are sometimes considered a single toxic unit because they act synergistically to lyse certain erythrocytes (18). We tested the enzymes individually and in combination in the retinal button assay. Each enzyme was added to retinal buttons at a concentration of 30 nM (ca. 1 μg ml−1), either individually or in combination, in the presence of 5 mM CaCl2 and 5 mM MgCl2. The released LDH activities (mean ΔA340 min−1 ± standard deviation) were as follows: PC-PLC, 0.098 ± 0.0157; SMase, 0.023 ± 0.0045; and PC-PLC plus SMase, 0.116 ± 0.0122 (n = 3; positive control, 0.408 ± 0.0213; negative control, 0.003 ± 0.0036). Similar results occurred at 15 nM enzyme concentrations. Since the activity of the PC-PLC and SMase combination (0.116) was less than the additive value of each (0.121), there was clearly no synergy between these two enzymes in causing retinal toxicity in vitro.
Neutralization of in vitro retinal toxicity in crude B. cereus culture supernatants.
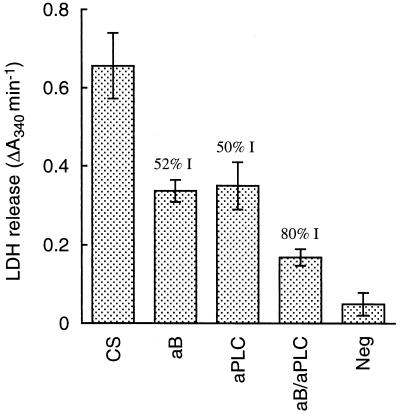
Figure 2 shows the effects of neutralizing HBL and PC-PLC in crude B. cereus culture supernatant in the retinal button assay. Antibody to each protein inhibited toxicity by about 50%, and the combined antibodies inhibited toxicity by 80%. Although this assay was not strictly quantitative, it is clear that HBL and PC-PLC each made major contributions to the retinal toxicity of culture supernatant and that an additional factor(s) also contributed, but to a lesser extent.
FIG. 2.
Effect on retinal toxicity of neutralizing HBL and PC-PLC in crude B. cereus culture supernatant. Crude BHIG culture supernatant was added to retinal buttons (n = 5) in the presence or absence of polyclonal antibodies (purified IgG) to HBL component B and PC-PLC. CS, culture supernatant with no antibody; aB, CS with antibody to B; aPLC, CS with antibody to PC-PLC; aB/aPLC, CS with antibodies to PC-PLC and component B; Neg, negative control (BHIG). The retinal button toxicity assay was performed as described in Materials and Methods. Numbers above bars show percent inhibition (% I) relative to the antibody-free CS and negative control.
In vivo retinal toxicity of purified PC-PLC.
For determination of its in vivo toxicity, PC-PLC was injected intravitreally. Damage induced by PC-PLC involved primarily the retina, with occasional mild vitritis or minor hemorrhage of the vessels at the optic disk. Retinal damage appeared clinically as isolated oblong white spots of full-thickness retinal necrosis ranging from about 3 to 12 disk diameters in size. Necrosis was generally localized to an area just inferior to the optic disk and medullary rays. In two eyes the necrotic retinal tissue was torn. Necrosis appeared as early as 4 h after injection of 5 μg of PC-PLC, while vitritis, hemorrhage, and retinal tearing appeared after 12 to 18 h.
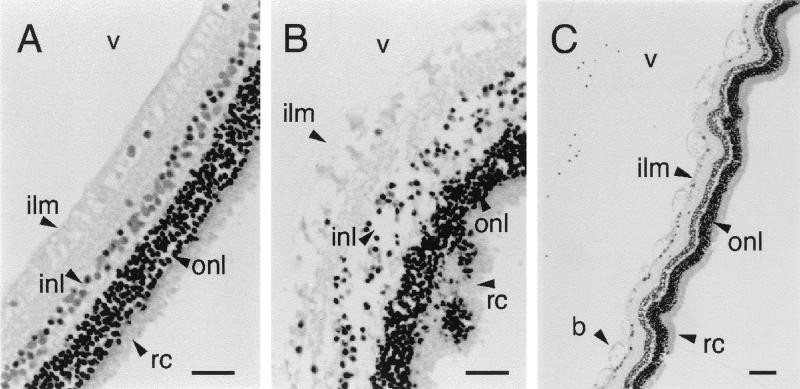
Figure 3 shows histopathological changes 4 h after injection of 5 μg of PC-PLC and 18 h after injection of 0.5 μg of the enzyme. The most noticeable damage occurred in the inner layers of the retina, including disruption of the inner limiting membrane, inner plexiform layer, and inner nuclear layer. Compared to normal retina, the nuclei of the inner nuclear layer exhibited hyperchromatism typical of pyknosis while the nuclei of the rods and cones remained relatively unchanged (Fig. 3B). When 0.5 μg of PC-PLC was injected, retinal disruption was not as pronounced and changes appeared histopathologically as localized blebbing of the inner limiting membrane (Fig. 3C). The choroid always appeared normal, with no edema or polymorphonuclear leukocyte (PMN) infiltration. PMNs occasionally appeared in the vitreous humor, particularly after 18 h, and surrounding the optic disk (the primary vascular contact with the vitreous fluid in the rabbit eye).
FIG. 3.
Retinal changes caused by intraocular injection of PC-PLC. (A) Control retina from eye injected with 0.1 ml of sterile saline. Bar, 50 μm. (B) Necrotic section of retina obtained 4 h after injection of 5 μg (10 U) of pure PC-PLC, showing disintegration of inner layers and pyknosis in the inner nuclear layer. Bar, 50 μm. (C) Blebbing (b) of the inner limiting membrane 18 h after injection of 0.5 μg of PC-PLC. Bar, 100 μm. All sections were stained with hematoxylin-eosin. ilm, inner limiting membrane; inl, inner nuclear layer; onl, outer nuclear layer; rc, layer of rods and cones; v, vitreous humor.
Histopathology of septic B. cereus endophthalmitis.
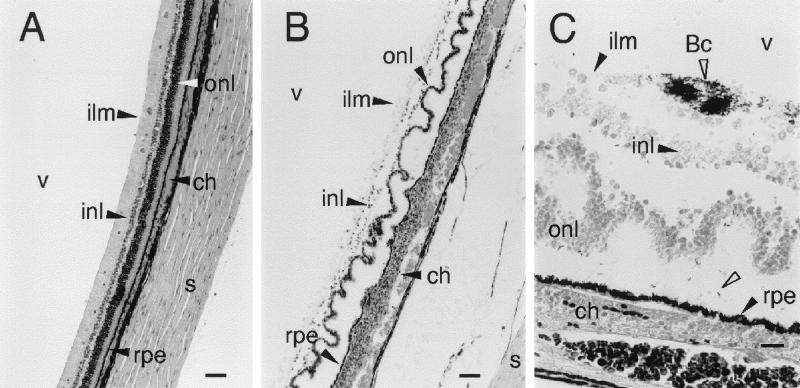
Intravitreal injection of 102 to 103 B. cereus cells resulted in highly fulminant endophthalmitis. Within 12 to 18 h, retinal necrosis, choroidal edema, and inflammation appeared, remarkably similar to the histopathological changes produced by sterile B. cereus culture supernatants and pure HBL (6) (Fig. 4B). At progressive stages of infection, bacterial cells associated with the retina appeared initially at the inner limiting membrane but eventually penetrated the full thickness of the retina (Fig. 4C). We did not see penetration of bacteria beyond the retinal pigment epithelium within the 18-h limit of the infections.
FIG. 4.
Retinal histopathology of experimental B. cereus endophthalmitis. (A) Control retina from eye injected with 0.1 ml of sterile saline. Stained with hematoxylin-eosin; bar, 100 μm. (B) Retinal and choroidal changes produced during B. cereus endophthalmitis. The outer nuclear layer shows extensive folding, and the choroid (ch) is engorged and heavily infiltrated with PMNs. PMNs can be seen crossing the retinal pigment epithelium (rpe) at local sites. Stained with hematoxylin-eosin; bar, 100 μm. (C) Magnified view of infected retina showing B. cereus (Bc; open arrowheads) aggregation at the surface of the retina and near the retinal pigment epithelium. Gram stain; bar, 20 μm. Abbreviations are defined in the legend to Fig. 3.
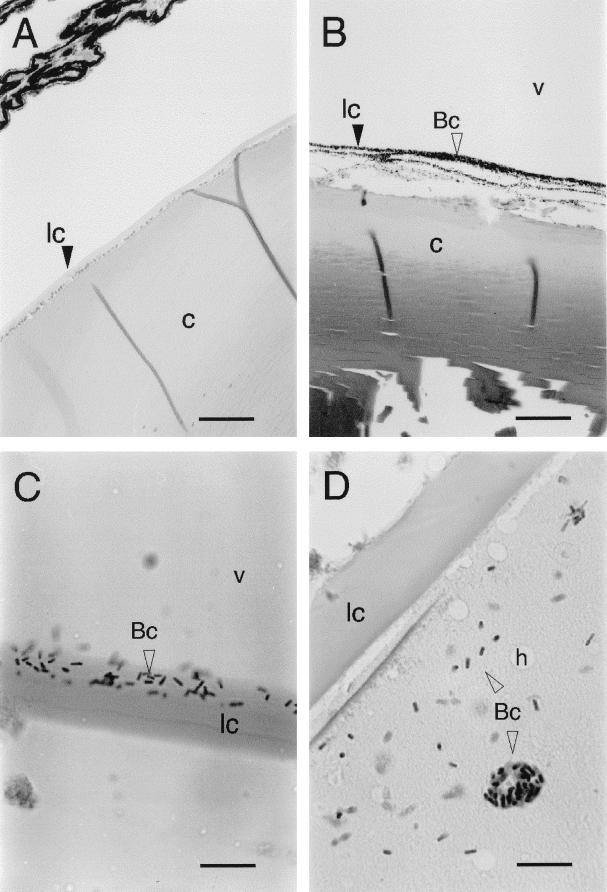
The initial appearance of bacteria consistently occurred at the posterior margin of the lens, where the cells could be seen before they appeared at the retina and before retinal necrosis was evident. Progression of infection was accompanied by infiltration of bacteria throughout the lens cortex but not the lens nucleus (Fig. 5). The lens cortex sometimes exhibited a “Swiss cheese” appearance because of the presence of “holes” 5 to 15 μm in diameter (Fig. 5D). The cause of these holes is not known, but they appeared only in advanced infections. They may be artifacts of fixation or mounting caused by significant alteration of the lens texture during infection.
FIG. 5.
Progressive stages of B. cereus infiltration of rabbit lens during experimental endophthalmitis. (A) Control lens from eye injected with 0.1 ml of sterile saline. Bar, 200 μm. (B) Accumulation of B. cereus (Bc; open arrowhead) along the posterior margin of the lens 12 h postinfection. Bar, 100 μm. (C) B. cereus partially penetrating the posterior lens capsule. Bar, 20 μm. (D) Complete infiltration of lens cortex by B. cereus. Bar, 20 μm. Abbreviations: lc, lens capsule; c, lens cortex; v, vitreous fluid; h, hole. Open arrowheads point to B. cereus cells. All panels were stained with hematoxylin-eosin.
Intraocular expression of PC-PLC, collagenase, and hemolysis activities.
Vitreous fluid specimens from three eyes infected for 12 h were analyzed for bacterial cell counts and PC-PLC activity. Eye 1 contained 2.5 × 109 CFU of B. cereus ml−1 and the equivalent of 10 U of PC-PLC ml−1 (ca. 5 μg ml−1). Eye 2 contained 2.5 × 108 CFU ml−1 and 4 U of PC-PLC ml−1. The vitreous and aqueous fluids of eye 3 contained 7.6 × 108 CFU ml−1 and 5 U of PC-PLC ml−1. For comparison, a culture grown under standard conditions for toxin production, 5 h in BHIG broth at 32°C and 200 rpm, contained 1.6 × 108 CFU of bacteria ml−1 and 5 U of PC-PLC ml−1.
In a collagenase assay using gelatin as a substrate, the mean A620 values ± standard deviations (n = 6; ninhydrin assay) for samples of eye 1 vitreous fluid, normal vitreous humor, and strain MGBC 145 in BHIG, respectively, were 0.38 ± 0.063, −0.01 ± 0.012, and 0.73 ± 0.216, respectively (the respective negative-control values were 0.07 ± 0.014, 0.04 ± 0.009, and 0.57 ± 0.076). By comparison with a clostridiopeptidase A standard curve, vitreous humor from the infected eye 1 contained approximately 107 mU of collegenase activity ml−1, compared with none for normal vitreous fluid and 207 mU ml−1 for strain MGBC 145 in BHIG. Eyes 2 and 3 contained about 5 and 20 mU ml−1, respectively (raw data not shown). The vitreal and BHIG samples also hydrolyzed type IV collagen (Sigma type VI), though to a lesser extent than gelatin (data not shown).
Hemolysis activity in ocular fluids was measured by gel diffusion assay. The activities of vitreal samples, expressed as percentages relative to the hemolysis caused by supernatant from a 5-h BHIG culture (32°C, 200 rpm), were as follows: eye 1, 17 to 22%; eye 2, 9 to 10%; and eye 3, 7 to 8%. The activity in aqueous fluid from eye 3 was about 0.1 to 0.15 times that of the culture supernatant. The zone diameter produced by 10 μl of vitreous fluid from eye 1 was 8 mm. Based on a comparison to a dilution series of the pure toxin, this diameter corresponded to approximately 6 ng per component of HBL (600 ng ml−1 in 10 μl).
Detection of intravitreal HBL component B by Western blot analysis.
Eye 1 was analyzed by Western blotting with antibodies that detect the 38-kDa HBL component B and a cross-reactive 42-kDa protein called Ba, which is a component of a second tripartite toxin expressed by B. cereus MGBC 145, called HBLa (8). Normal vitreous humor from a control eye exhibited four cross-reactive bands at the bottom of the blot, between 18 kDa and the dye front, which were absent from both the vitreous sample from eye 1 and the BHIG culture supernatant. The B and Ba components were present in both the vitreous fluid from eye 1 and the BHIG culture supernatant. The vitreous humor from eye 1 also contained two degradation products, of 33 and 27 kDa. The total density values (arbitrary units) for specific bands were 83.2 in the BHIG culture supernatant and 6.2 for the eye 1 vitreous sample. The culture supernatant contained 2.5 to 5 μg of B ml−1 as estimated by dot blot analysis, suggesting that the vitreous fluid contained 0.25 to 0.5 μg of component B ml−1 (a range similar to that estimated from the hemolysis above). This may be an underestimate if degradation prior to the Western blotting was significant.
DISCUSSION
The progression of B. cereus endophthalmitis is remarkably rapid in humans and in animal models (11, 12). The primary cause of blindness in this infection is rapid necrosis of retinal tissue, which appears to be mediated directly by secreted B. cereus toxins (6, 12). We previously determined that secreted B. cereus toxins are directly necrotic to rabbit retinal tissue in vitro and in vivo (6). In particular, we found that HBL is remarkably toxic. Maximal activity occurs with doses between 1.3 and 4 nM (ca. 50 to 150 ng ml−1). We also found that neutralization of HBL in crude culture supernatant only partially inhibited retinal toxicity in vitro, indicating that other secreted factors are important.
Our present results strongly suggest that among the numerous toxins and enzymes secreted by B. cereus, PC-PLC is a second major contributor to retinal toxicity. It was responsible for the single major peak of in vitro retinal toxicity produced upon chromatographic fractionation of the proteins secreted into a complex culture medium (Fig. 1). Significant activity due to HBL toxins was not detected in column fractions because under the conditions used here, their components were largely separated and therefore inactive (data not shown). Any additional single-component toxins in the supernatant were significantly less toxic than PC-PLC. PC-PLC was also toxic in vivo (Fig. 2) and was produced in surprisingly large amounts during experimental septic B. cereus endophthalmitis.
The toxicity of PC-PLC can presumably be explained by the unusual proportions of phospholipids in retinal tissue. PC-PLC preferentially hydrolyzes phosphatidylcholine (PC) and is also active against phosphatidylethanolamine (PE) and phosphatidylserine (PS) (22). These three phospholipids comprise about 86% of the mole percentage of phospholipids in the rabbit retina (43.9% PC, 34.7% PE, and 7.4% PS) (9). In addition, the presence of sphingomyelin (SM) in membranes inhibits the activity of PC-PLC (29); therefore, the exceptionally low concentration of SM in the rabbit retina (3.4%) most likely fosters the ability of PC-PLC to compromise retinal cell integrity. These factors are likely to be relevant to the human infection as well, since the phospholipid content of the human retina is 47.8% PC, 31.7% PE, 8.6% PS, and 4.3% SM (9).
The histological changes produced by PC-PLC in vivo (Fig. 2) were in distinct contrast to those produced by intraocular injection of purified HBL or crude B. cereus culture supernatant (6). PC-PLC did not produce the distinct retinal folding, choroidal edema, and vigorous inflammation that is typical of intravitreal injection of pure HBL or B. cereus culture supernatant and of B. cereus infection. We previously suggested that the vascular permeability activity of HBL breaches the blood-retina barrier and engenders an immune response from outside of the immune-privileged inner eye (6). PC-PLC may be unable to efficiently breach that barrier.
We detected HBL directly within infected eyes, and the histopathology of septic B. cereus endophthalmitis strongly suggested a major role for HBL because the retinal and inflammatory changes occurring during the infection were essentially identical to those produced by pure HBL (6). The tissue destruction caused by HBL likely masks the effects of PC-PLC in toxin mixtures and during infection.
HBL is a member of a family of tripartite toxins that currently comprises three members. Two of these, HBL and HBLa, were produced here intravitreally by MGBC 145. We did not test for the third member, NHE (19), but strain MGBC 145 does produce at least one component (NHEA) in vitro (unpublished observations). In a recent study, HBL operon knockout in B. cereus MGBC 145 retarded the progression of experimental endophthalmitis but did not prevent a full-blown infection comparable to that caused by the wild-type strain (13). It will be interesting to see the effects of knocking out the entire HBL family.
We could not be certain that our approach of screening chromatographic fractions in vitro would identify all of the major B. cereus retinal toxins. Therefore, we tested a variety of B. cereus exoproteins that might be expected to act as virulence factors either because they possess documented toxic activities or, a priori, because they are enzymatically active against cell membrane constituents. In addition to pure HBL and PC-PLC, we tested two other phospholipases, SMase and PI-PLC, and two hemolytic toxins, CLO (Hly-I) and Hly-IV. None approached the toxic potencies of HBL and PC-PLC.
Neither SMase nor PI-PLC caused significant release of LDH from retinal buttons, which was not surprising considering that the rabbit retina contains only 4.3% SM and 4.4% phosphatidylinositol (9). The SM content also explains why SMase and PC-PLC did not synergistically damage retinal tissue as they do human (28.9% PC and 26.9% SM) and swine (23.2% PC and 26.5% SM) erythrocytes (17, 18; D. J. Beecher and A. C. L. Wong, Abstr. Gen. Meet. Am. Soc. Microbiol., abstr. 24, 1996).
While CLO and Hly-IV were not very potent, they were toxic, nevertheless, and probably account in part for the residual toxicity in supernatants neutralized of HBL and PC-PLC activities (Fig. 2). CLO is a pore-forming toxin that is a member of a large class of cytolysins exemplified by streptolysin O (2). All of these toxins bind to cholesterol on the cell surface and subsequently form large transmembrane pores. Cholesterol accounts for about 40% of the total neutral lipids in the retina (9), so it was surprising that CLO was not more toxic.
Hly-IV is not fully characterized yet, but based on the rates at which hemolysis zones appear on blood agar overlays of isoelectric-focusing gels, it appears to be either one of the most rapidly acting or one of the most abundant hemolysins in crude culture supernatants from many B. cereus strains, including the one used here (data not shown). Nanomolar concentrations very rapidly lyse sheep, swine, and guinea pig erythrocytes in suspension. This potent activity against membranes of diverse phospholipid content makes Hly-IV a possible virulence factor a priori.
Bacterial invasion of the lens is a common feature of B. cereus endophthalmitis in animal models (12, 13; D. M. O'Day et al., Abstr. Assoc. Res. Vis. Ophthalmol. Annu. Spring Meet., abstr. 4, 1980) and in human infection (26). The histological analysis of infected eyes in this study showed that bacterial cells were initially evident in association with the posterior lens capsule, which was progressively degraded, and that bacteria eventually colonized the entire lens cortex (Fig. 5B and D). We also saw bacteria apparently in the act of penetrating the posterior lens capsule (Fig. 5C) and detected hemolysis and PC-PLC activities in aqueous fluid, indicating a breach of the barrier between the aqueous and vitreous chambers. The lens capsule is composed primarily of type IV collagen (9) and acts as an important permeability barrier. It therefore appears that this barrier was breached by the action of bacterial collagenase, which was detected in the vitreous humor of infected eyes.
In addition, the architecture of the lens cortex appeared to be compromised by bacterial infiltration (“holes” in Fig. 5D). The expression of B. cereus phospholipases may contribute to this effect, considering that the combined PC-SM content in lens fiber cell membranes is 37 to 67% in rabbits and 49 to 58% in humans (9). Bacterial infiltration of an ocular lens may not be consequential per se, because it can be treated effectively. However, during the course of the infection, the lens may provide a haven in which B. cereus can grow, protected from inflammatory cells, while continuing to produce diffusable toxins (inflammatory cells did not enter the lens [Fig. 5D]).
As noted above, the distribution of bacterial cell densities within infected eyes was heterogeneous. High densities of bacteria occurred at the posterior lens capsule (Fig. 5), as did aggregations of cells at the retinal surface (Fig. 4C). High-density growth associated with surfaces may exhibit distinct metabolic and expression characteristics compared with the more dispersed free-swimming bacteria that presumably grow in vitreous fluid. The concentrations of secreted proteins are likely much higher around the high-density aggregates than in the vitreous liquid that we sampled.
This study demonstrated for the first time that suspected virulence factors are actually produced during experimental septic B. cereus endophthalmitis. This work supports our previous conclusion that HBL contributes to the virulence of B. cereus endophthalmitis. The recent identification of HBL homologs has revealed that HBL is the prototype of a family of related toxins, perhaps with additional members. This will further complicate attempts to decipher the specific roles of individual toxins. PC-PLC and collagenase each emerged as a likely virulence factor, the former based on in vitro toxicity and the latter because of the behavior of B. cereus during infections. The B. cereus taxonomic group (B. cereus, B. thuringiensis, B. mycoides, and B. anthracis) is highly diverse, and expression of individual proteins differs greatly from strain to strain. An exception is PC-PLC, which is expressed by the great majority of isolates. Thus, almost all strains in this group can be expected to secrete at least one retinal toxin during endophthalmitis.
ACKNOWLEDGMENTS
This research was supported by funds from the National Eye Institute, National Institutes of Health (grant no. EY10308-02), and the College of Agricultural and Life Sciences, University of Wisconsin—Madison.
REFERENCES
- 1.Agaisse H, Gominet M, Økstad O A, Kolstø A B, Lereclus D. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol Microbiol. 1999;32:1043–1053. doi: 10.1046/j.1365-2958.1999.01419.x. [DOI] [PubMed] [Google Scholar]
- 2.Alouf J E. Introduction to the family of the structurally related cholesterol-binding cytolysins (‘sulfhydryl-activated’ toxins) In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1999. pp. 443–456. [Google Scholar]
- 3.Baida G, Budarina Z I, Kuzmin N P, Solonin A S. Complete nucleotide sequence and molecular characterization of hemolysin II gene from Bacillus cereus. FEMS Microbiol Lett. 1999;180:7–14. doi: 10.1111/j.1574-6968.1999.tb08771.x. [DOI] [PubMed] [Google Scholar]
- 4.Baida G E, Kuzmin N P. Cloning and primary sequence of a new hemolysin gene from Bacillus cereus. Biochim Biophys Acta. 1995;1246:151–154. doi: 10.1016/0167-4781(95)00150-f. [DOI] [PubMed] [Google Scholar]
- 5.Beecher D J, Macmillan J D. Characterization of the components of hemolysin BL from Bacillus cereus. Infect Immun. 1991;59:1778–1784. doi: 10.1128/iai.59.5.1778-1784.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beecher D J, Pulido J S, Barney N P, Wong A C L. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect Immun. 1995;63:632–639. doi: 10.1128/iai.63.2.632-639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beecher D J, Wong A C L. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect Immun. 1994;62:980–986. doi: 10.1128/iai.62.3.980-986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beecher D J, Wong A C L. Tripartite haemolysin BL: isolation and characterization of two distinct homologous sets of components from a single Bacillus cereus isolate. Microbiology. 2000;146:1371–1380. doi: 10.1099/00221287-146-6-1371. [DOI] [PubMed] [Google Scholar]
- 9.Berman E R. Biochemistry of the eye. New York, N.Y: Plenum Press; 1991. [Google Scholar]
- 10.Bjørklid E, Little C. The isoelectric point of phospholipase C from Bacillus cereus. FEBS Lett. 1980;113:161–163. doi: 10.1016/0014-5793(80)80582-5. [DOI] [PubMed] [Google Scholar]
- 11.Brinton G S, Topping T M, Hyndiuk R A, Aaberg T M, Reeser F H, Abrams G W. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102:547–550. doi: 10.1001/archopht.1984.01040030425016. [DOI] [PubMed] [Google Scholar]
- 12.Callegan M C, Booth M C, Jett B D, Gilmore M S. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun. 1999;67:3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callegan M C, Jett B D, Hancock L E, Gilmore M S. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect Immun. 1999;67:3357–3366. doi: 10.1128/iai.67.7.3357-3366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coolbaugh J C, Williams R P. Production and characterization of two hemolysins of Bacillus cereus. Can J Microbiol. 1978;24:1289–1295. doi: 10.1139/m78-209. [DOI] [PubMed] [Google Scholar]
- 15.Davey R T, Jr, Tauber W B. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- 16.Doi E, Shibata D, Matoba T. Modified colorimetric ninhydrin methods for peptidase assay. Anal Biochem. 1981;118:173–184. doi: 10.1016/0003-2697(81)90175-5. [DOI] [PubMed] [Google Scholar]
- 17.Fehrenbach F J, Jürgens D. Cooperative membrane-active (lytic) processes. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1991. pp. 187–213. [Google Scholar]
- 18.Gilmore M S, Cruz-Rodz A L, Leimeister-Wächter M, Kreft J, Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989;171:744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granum P E, O'Sullivan K, Lund T. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol Lett. 1999;177:225–229. doi: 10.1111/j.1574-6968.1999.tb13736.x. [DOI] [PubMed] [Google Scholar]
- 20.Hansen S, Hough E, Svensson L A, Wong Y L, Martin S F. Crystal structure of phospholipase C from Bacillus cereus complexed with a substrate analog. J Mol Biol. 1993;234:179–187. doi: 10.1006/jmbi.1993.1572. [DOI] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 22.Hergenrother P J, Martin S F. Determination of the kinetic parameters for phospholipase C (Bacillus cereus) on different phospholipid substrates using a chromogenic assay based on the quantitation of inorganic phosphate. Anal Biochem. 1997;251:45–49. doi: 10.1006/abio.1997.2251. [DOI] [PubMed] [Google Scholar]
- 23.Hough E, Hansen L K, Birknes B, Jynge K, Hansen S, Hordvik A, Little C, Dodson E, Derewenda Z. High-resolution (1.5 Å) crystal structure of phospholipase C from Bacillus cereus. Nature. 1989;338:357–360. doi: 10.1038/338357a0. [DOI] [PubMed] [Google Scholar]
- 24.Johansen T, Holm T, Guddal P H, Sletten K, Haugli F B, Little C. Cloning and sequencing of the gene encoding the phosphatidylcholine-preferring phospholipase C of Bacillus cereus. Gene. 1988;65:293–304. doi: 10.1016/0378-1119(88)90466-0. [DOI] [PubMed] [Google Scholar]
- 25.Levin M R, D'Amico D J. Traumatic endophthalmitis. In: Shingleton B J, Hersh P S, Kenyon K R, editors. Eye trauma. St. Louis, Mo: Mosby Year Book; 1991. pp. 242–252. [Google Scholar]
- 26.O'Day D M, Smith R S, Gregg C R, Turnbull P C B, Head W S, Ives J A, Ho P C. The problem of Bacillus species infections with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 27.Parke D W, Brinton G S. Endophthalmitis. In: Tabbara K F, Hyndiuk R A, editors. Infections of the eye. Boston, Mass: Little, Brown & Co.; 1986. pp. 563–585. [Google Scholar]
- 28.Pflugfelder S C, Flynn H W., Jr Infectious endophthalmitis. Infect Dis Clin N Am. 1992;6:859–873. [PubMed] [Google Scholar]
- 29.Ruiz-Arguello M B, Goni F M, Alonso A. Phospholipase C hydrolysis of phospholipids in bilayers of mixed lipid compositions. Biochemistry. 1998;37:11621–11628. doi: 10.1021/bi980615x. [DOI] [PubMed] [Google Scholar]
- 30.Tomita M, Taguchi R, Ikezawa H. Sphingomyelinase of Bacillus cereus as a bacterial hemolysin. J Toxicol-Tox Rev. 1991;10:169–207. [Google Scholar]
- 31.Vahey J B, Flynn H W., Jr Results in the management of Bacillus endophthalmitis. Ophthalmic Surg. 1991;22:681–686. [PubMed] [Google Scholar]
- 32.Wilkinson J H, Walter S J. Oxamate as a differential inhibitor of lactate dehydrogenase isoenzymes. Enzyme. 1972;13:170–176. doi: 10.1159/000459658. [DOI] [PubMed] [Google Scholar]