Abstract
Human granulocytic ehrlichiosis (HGE) is a potentially fatal, tick-borne disease caused by a bacterium related or identical to Ehrlichia phagocytophila. To identify and characterize E. phagocytophila group-specific protein antigen genes, we prepared and screened HGE agent and Ehrlichia equi genomic DNA expression libraries using polyclonal equine E. equi antibodies. Two clones, one each from HGE agent and E. equi, that were recognized specifically by antibodies to the E. phagocytophila group ehrlichiae had complete open reading frames of 3,693 and 3,615 nucleotides, respectively. The two clones were 96.6% identical and predicted a protein with at least 11 tandemly repeated ankyrin motifs. Thus, the gene was named ank (for ankyrin). When the encoded protein, named AnkA, was expressed in Escherichia coli, it was recognized by antibodies from rabbits and mice immunized with the HGE agent, sera from humans convalescent from HGE, and sera from horses convalescent from HGE and E. equi infection. Monospecific AnkA antibodies reacted with proteins in HGE agent immunoblots, and AnkA monoclonal antibodies detected cytoplasmic antigen in E. phagocytophila group bacteria and also detected antigen associated with chromatin in infected but not uninfected HL-60 cell cultures. These results suggest that this Ehrlichia protein may influence host cell gene expression.
Human granulocytic ehrlichiosis (HGE) is an emerging tick-borne infection with manifestations ranging from no symptoms to death (1, 6, 9). Most patients are mildly to moderately affected, with fever, headache, myalgias, leukopenia, thrombocytopenia, and elevations in serum hepatic transaminases. The causative microorganism, named the HGE agent, is a member of the Ehrlichia phagocytophila group, which also includes E. phagocytophila and Ehrlichia equi in a tight phylogenetic cluster, probably representing a single species (10, 25, 26).
The E. phagocytophila group has been characterized mainly through analysis of genes encoding the 16S rRNA and the groESL operon (2, 9, 25, 26). These genes are highly conserved and are therefore unlikely to reveal the phylogenetic and pathogenetic differences that could account for the diversity of clinical findings and the diversity of mammalian hosts in ehrlichial infection. The E. phagocytophila group has also been shown to possess specific genes that, unlike conserved genes, can be useful for phylogenetic comparisons among animal and human strains (4, 10). Moreover, studying the function of the proteins encoded by species-specific genes may provide insights into the pathogenetic potential of granulocytic ehrlichiae.
In recent months, several groups have cloned genes from the HGE agent (14, 19, 24, 28), including a 2,244-nucleotide (nt) gene encoding a 160-kDa protein antigen with multiple ankyrin motifs. However, to date no function has been attributed to any of the proteins encoded by these genes. The goals of the present work were to identify genes specific to E. phagocytophila group ehrlichiae and to begin elucidating their role in the intracellular infection caused by the E. phagocytophila group ehrlichiae.
(This work was presented in part at the 13th Sesqui-Annual Meeting of the American Society for Rickettsiology, Champion, Pa., 21 to 24 September 1997.)
MATERIALS AND METHODS
Construction and screening of HGE genomic libraries.
The HGE agent (BDS strain) and E. equi (MRK strain) were used to experimentally infect horses. Horse blood was obtained when ehrlichial morulae were shown by Wright staining in 50% or more of the neutrophils. Neutrophils were then purified by dextran sedimentation, washed in sterile phosphate-buffered saline (pH 7.4), and suspended in 0.1 M phosphate buffer, which contained 7% sucrose and 5 mM glutamine. Ehrlichiae were isolated from the neutrophils by Renografin (diatrizoate meglumine) density gradient centrifugation, as previously described (4, 10).
Genomic DNA from the HGE agent and E. equi was partially digested with Sau3A1 and electrophoresed to identify and purify fragments of between 4,000 and 9,000 bp from the agarose gel, using a genomic DNA purification kit from Qiagen (Valencia, Calif.). Gel-purified DNA was ligated to ZAP Express bacteriophage vector (Stratagene, La Jolla, Calif.), packaged, and plated according to the manufacturer's recommendations. Filters were finally screened by using as probe polyclonal antisera (diluted 1:80) derived from the horses utilized for the propagation and isolation of the HGE agent or E. equi. These polyclonal antisera were absorbed with washed Escherichia coli XL1-Blue MRF′ and self-ligated, purified pBK-CMV phagemids.
Positive bacteriophage clones were processed to excise the phagemids, which then were used to transform E. coli XLOLR and produce recombinant proteins. E. coli protein lysates were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis transferred to nitrocellulose filters, and tested against the following antisera: human, horse, dog, and mouse anti-HGE agent; horse anti-E. equi; dog anti-E. canis; human and rabbit anti-Ehrlichia chaffeensis; dog anti-Ehrlichia ewingii (courtesy of Sidney Ewing, Stillwater, Okla.), rabbit and mouse anti-Ehrlichia sennetsu; human anti-Rickettsia rickettsii; human anti-Orientia tsutsugamushi; mouse and rabbit anti-Rickettsia typhi; human anti-Coxiella burnetii; human anti-Borrelia burgdorferi (courtesy of Lou Magnarelli, New Haven, Conn.); human anti-Babesia microti (courtesy of Lou Magnarelli); mouse anti-Bartonella quintana and mouse anti-Bartonella henselae (courtesy of Philippe Brouqui, Marseille, France); and normal human, horse, dog, rabbit, and mouse sera. All antisera were also reacted with immunoblots prepared using E. coli XLOLR transformed with empty pBK-CMV phagemids, as a negative control. Antibodies bound to blotted antigens were finally detected as previously described (4, 10).
Characterization of two unique E. phagocytophila group-specific clones.
One clone from the HGE agent library (named hge-d), and one clone from the E. equi library (named ee-o) were sequenced on both strands. Partial open reading frames were identified in both, and complete gene sequences were then obtained by alignment of the sequences, PCR amplification of the flanking regions, and sequencing by gene walking. Southern blot analysis was performed using 5 μg of DNAs from the HGE agent (Webster strain), E. coli XLOLR, and HL-60 cells, which were digested with PstI, BamHI, and KpnI. In addition, HGE agent DNA was digested with EcoRI, HindIII, XhoI, and BglII. Digested DNAs were separated by overnight electrophoresis and then transferred onto Zeta-Probe GT (Bio-Rad, Hercules, Calif.). A 480-bp SalI-BamHI fragment (see Fig. 2) was used to make a 32P-labeled probe, using a DECAprime II labeling kit (Ambion, Austin, Tex.). Hybridization was carried out overnight at 60°C in 0.25 M phosphate buffer (pH 7.2)–7% SDS–5% dextran sulfate containing 1.5 × 106 trichloroacetic acid-precipitable cpm of the probe per ml of hybridization buffer. Blots were quickly washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and then washed for 1 h at 42°C with gentle shaking in 50 mM phosphate buffer (pH 7.2) with 5% SDS. The blots were wrapped in plastic and exposed overnight at −70°C to BioMax MS autoradiographic film (Eastman Kodak, Rochester, N.Y.).
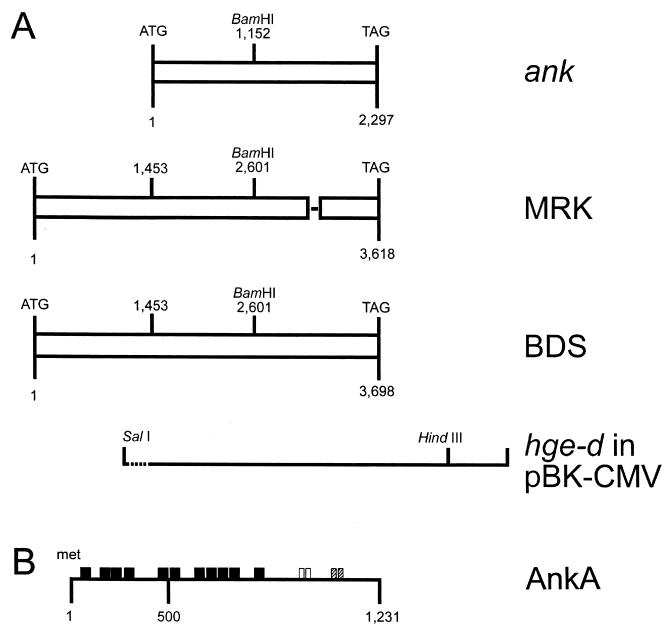
FIG. 2.
(A) Schematic representation and alignment of HGE agent (strain BDS) and E. equi (strain MRK) AnkA open reading frames. For comparison, the previously reported ank gene (24) is also shown at the top. hge-d in pBK-CMV refers to the initial clone, highlighting the SalI and HindIII sites used to subclone into the pMAL-c2 expression vector; the dotted line represents 15 nt of vector sequence. The SalI-BamHI fragment was the one used for probing Southern blots. The single line within the MRK sequences identifies the approximate position of the 81 nt coding for the second 27-amino-acid repeat, which is missing in AnkA from E. equi. (B) Schematic representation of AnkA. Closed boxes, 11 ankyrin repeats; open boxes, two contiguous 27-amino-acid repeats; hatched boxes, two 11-amino-acid repeats. Gene, clone, and protein designations are shown on the right.
Sequencing of a 444-bp ankA amplicon in various E. phagocytophila group strains.
A forward primer (5′-GAGAGATGCTTATGGTAAGAC-3′), and a reverse primer (5′-CGTTCAGCCATCATTGTGAC-3′) were designed to amplify a 444-bp fragment from hge-d and ee-o (see Fig. 2). PCR was performed on DNA prepared from the following samples: four human HGE agent strains (Webster, Spooner, and 96HE-97 from Wisconsin and NY-8 from New York) in HL-60 cells, one HGE agent strain (97E12 from a Minnesota dog) in HL-60 cells, one blood sample from another Minnesota dog infected with the HGE agent, one blood sample from a Spanish goat with tick-borne fever (courtesy of Philippe Brouqui), neutrophils isolated from a cow with tick-borne fever in Sweden (courtesy of Philippe Brouqui and Anneli Bjöersdorff) containing different local strains of E. phagocytophila, E. sennetsu Miyayama strain in P388D1 cells (courtesy C. of Pretzman, Ohio State Department of Health), E. risticii HRC-IL strain (ATCC VR-986) in P388D1 cells, E. chaffeensis Arkansas strain in DH82 cells (courtesy of J. Dawson, Centers for Disease Control and Prevention, Atlanta, Ga.), E. chaffeensis 91HE17 strain in DH82 cells, and E. canis Oklahoma strain in DH82 cells (courtesy J. Dawson). PCR was also performed on DNA from uninfected HL-60 cells. PCR products were evaluated for size and staining intensity by agarose gel electrophoresis, and then sequenced.
Immunologic features of AnkA.
A 2.5-kb SalI-HindIII fragment comprising the ankA partial open reading frame in hge-d (devoid of promoter sequences) was subcloned into pMAL-c2 (New England Biolabs, Beverly, Mass.) to allow the expression of a fusion protein comprised of 391 amino acids (43 kDa) from the maltose-binding protein (MBP) and 520 amino acids from the product of the hge-d ankA partial open reading frame (54-kDa predicted molecular mass) (see Fig. 2). The fusion protein, named MBP-AnkA, was purified on an amylose resin column, mixed with RIBI adjuvant system (RIBI Immunochem Research, Inc., Hamilton, Mont.), and used to immunize three BALB/c mice over a period of 45 days. Murine peripheral blood and splenocytes were used to produce polyclonal and monoclonal antibodies, respectively. Polyclonal antibodies were tested for reactivity to MBP-AnkA and whole HGE agent by Western blotting (10), using sera from the mice immunized with MBP-AnkA, from a mouse that was infected with the HGE agent (BDS strain), from a rabbit immunized with the HGE agent (Webster strain), from a rabbit immunized with MBP (GIBCO/BRL, Gaithersburg, Md.), or from a nonimmunized mouse and rabbit.
Monoclonal antibodies were produced by fusing splenocytes from MBP-AnkA-immunized mice to SP2/0 cells, using a ClonaCellTM-HY hybridoma cloning kit (Stem Cell Technologies, Inc., Vancouver, British Columbia, Canada). Hybridomas that secreted antibodies reactive with whole HGE agent, as determined by immunofluorescence, were selected for further screening by protein immunoblots. Hybridomas that secreted antibodies recognizing MBP-AnkA in protein immunoblots were subcloned, and the antibodies were purified on protein G-Sepharose columns (Pharmacia, Uppsala, Sweden).
Cellular localization of AnkA in the HGE agent and infected HL-60 cells.
Monoclonal antibodies to MBP-AnkA were used to define by electron microscopy the cellular distribution of AnkA within the HGE agent and in infected HL-60 cells. HL-60 cells were infected with HGE agent and propagated in vitro until at least 50% of them contained ehrlichial morulae as determined by Romanowsky staining. At that point, approximately 2.5 × 106 HL-60 cells were prepared for immunoelectron microscopy, as previously described (22, 23). Ultrathin sections were reacted with a monoclonal antibody directed against AnkA {IE3; immunoglobulin G1 kappa chain [IgG1(κ)]} and with a control antibody. Grids were then washed, dried, and reacted for 30 min at room temperature with protein A conjugated to 15-nm-diameter colloidal gold particles (AuroProbeTM EM Protein AG15; Amersham Life Science, Arlington Heights, Ill.), diluted 1:200 or 1:400 in 10 mM phosphate-buffered saline. Immunostained grids were finally stained with aqueous 2% uranyl acetate and 0.4% lead citrate and examined using a Philips 201 electron microscope.
Nucleotide sequence accession numbers.
The full-length gene sequences from the hge-d (BDS strain) and ee-o (MRK strain) clones have been deposited in GenBank under accession numbers AF047897 and AF153716, respectively.
RESULTS
Immunoreactivity of two E. phagocytophila group clones.
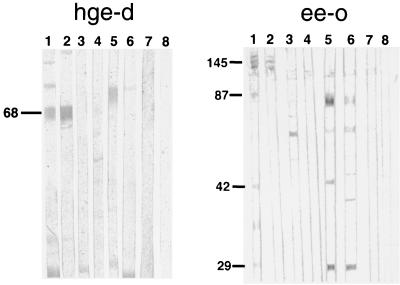
E. coli transformed with hge-d and ee-o expressed proteins of 68 and 145 kDa, respectively, that were recognized by sera from humans, horses, and dogs infected with the HGE agent, and from horses infected with E. equi, but not by the following sera: human and rabbit anti-E. chaffeensis, human anti-R. rickettsii, human anti-Borrelia burgdorferi, human anti-Babesia microti, antibodies to other Ehrlichia species or unrelated bacteria, and normal human, horse, or rabbit sera (Fig. 1). These results indicate that hge-d and ee-o expressed E. phagocytophila group-specific antigens.
FIG. 1.
E. coli transformed with hge-d (left panel) or ee-o (right panel) expresses a 68- or 145-kDa antigen, respectively, as determined by protein immunoblotting. The blots were reacted with the following primary antibodies: human anti-HGE agent (lanes 1), horse anti-HGE agent BDS strain (lanes 2), human anti-E. chaffeensis (lanes 3), dog anti-E. canis (lanes 4), human anti-Borrelia burgdorferi (lanes 5), and human anti-Babesia microti (lanes 6). Lanes 7 and 8 were reacted with normal human and horse sera, respectively. The location of the 68-kDa protein is indicated on the left.
Genomic features of ankA.
DNA sequencing revealed that the hge-d and ee-o clones contained similar partial open reading frames. A BLAST search revealed these to be 97% identical to a clone reported by Storey et al. (GenBank accession number AF020521) (24). The initial hge-d clone was 5′ truncated and lacked promoter sequences, whereas the ee-o clone had full 5′ promoter sequences but was truncated at the 3′ end. Subsequently, the sequencing done to complete the hge-d and ee-o partial open reading frames showed full-length genes of 3,693 and 3,615 nt, respectively, that were 96.6% identical. The diversity was primarily due to the presence in hge-d of two tandemly arranged 81-nt fragments, only one of which was present in ee-o. This analysis showed that the two genes were nearly identical, and thus they are named here ankA (for ankyrin). Both genes had identical promoter sequences 5′ to the ATG initiator codons, including the ribosomal binding sites and −10 (Pribnow box) and −35 regions. Translation of the two full-length open reading frames predicted proteins of 1,205 and 1,231 amino acids, named AnkA, with predicted molecular masses of 128 and 131 kDa, respectively. The most striking feature of AnkA was the presence of eight full ankyrin-like repeats of 33 amino acids and three partial ankyrin-like repeats (Fig. 2B) with a consensus sequence of -G-T-LH-AA--G-------L---G-------- (the signature mammalian ankyrin-like sequence is -G-TPLH-AA-GH---V/A-LL-GA-N/D----). Computer analyses showed that AnkA does not have transmembrane regions and that it is highly hydrophilic, suggesting the possibility that it is located within the ehrlichial cytoplasm. Computer analysis also showed the presence of the following motifs: one tyrosine kinase phosphorylation site (residues 367 to 374), one N-glycosylation site (residues 812 to 815), and several protein kinase C and casein kinase II phosphorylation sites. Four potential prokaryotic secretory signal cleavage sites were identified at positions 631 and 632, 682 and 683, 933 and 934, and 1136 and 1137; however, no N-terminal secretory signal sequence was identified by the method of Nakai and Kanehisa (20). Finally, computer analysis for internal repeats showed the presence of two identical repeats of 11 amino acids (ERPESIYADP) and two identical and consecutive repeats of 27 amino acids (GAEESIYEEIKDTAKGTTEVESTYTTVGA). The second 27-amino-acid repeat was missing in the predicted protein encoded by the full-length ankA gene in the MRK strain of E. equi. Digestion of the HGE agent genomic DNA with seven restriction endonucleases showed specific hybridization of the ankA probe predominantly to one locus, although less intense bands were identified at other loci in the HGE agent genome (Fig. 3).
FIG. 3.
Southern blot analysis of restriction enzyme-digested DNA from the HGE agent, E. coli XLOLR, and HL-60 cells. Lane 1, λDNA BstEII marker; lane 2, 50 pg of hge-d linearized with BamHI; lanes 3 to 6, HGE agent genomic DNA digested with EcoRI (lane 3), HindIII (lane 4), XhoI (lane 5), and BglII (lane 6); lanes 7 to 9, PstI-digested genomic DNA from the HGE agent (lane 7), E. coli XLOLR (lane 8), and HL-60 cells (lane 9); lanes 10 to 12: BamHI-digested genomic DNA from the HGE agent (lane 10), E. coli XLOLR (lane 11), and HL-60 cells (lane 12); lanes 13 to 15: KpnI-digested genomic DNA from the HGE agent (lane 13), E. coli XLOLR (lane 14), and HL-60 cells (lane 15). Molecular sizes are shown on the left.
Sequence variation of ankA in various E. phagocytophila group members.
We wished to assess whether the diversity observed between the ankA genes was also present among other E. phagocytophila group members from different geographic regions. Thus, we selected a 444-bp fragment from hge-d and ee-o and compared it to the same region amplified from the eight additional strains described in Materials and Methods. All of the HGE agent strains showed 100% identity, and the E. equi MRK strain showed 99.6% identity. The E. phagocytophila strains differed from each other (97.1% identity) and differed more substantially from the HGE agent and E. equi (Table 1).
TABLE 1.
Sequence analysis and comparison of a 444-bp amplicon from ankA genes in several E. phagocytophila group strains
| ankA | % Identity to ankA from:
|
|||
|---|---|---|---|---|
| HGE agent (BDS strain) | E. equi strain MRK |
E. phagocytophila
|
||
| Swedish strain | Spanish strain | |||
| HGE agenta | 100 | |||
| E. equi strain MRK | 99.6 | 100 | ||
| E. phagocytophila | ||||
| Swedish horse strain | 96.4 | 96.8 | 100 | |
| Spanish goat strain | 95.5 | 95.9 | 97.1 | 100 |
All strains of HGE agent tested have revealed identical nucleotide sequences; the BDS strain is shown as representative.
Immunologic features of AnkA.
The expression in E. coli of the original hge-d SalI-HindIII fragment yielded a fusion protein of approximately 110 kDa, of which 67 kDa was derived from AnkA. When it was used as an antigen for immunoblotting, the fusion protein was recognized by mouse polyclonal and monoclonal antibodies to MBP-AnkA and also by rabbit polyclonal antibodies to whole HGE agent (Fig. 4A, lane 2). These antibodies recognized two or three major bands, with the strongest being 110 kDa and the other migrating as a doublet at approximately 160 kDa (Fig. 4A, lanes 1, 2, and 6).
FIG. 4.
Protein immunoblots of recombinant MBP-AnkA (A), total lysate of E. coli transformed by ee-o (B), and whole HGE agent (C). (A) Recombinant MBP-AnkA probed with the following primary antibodies: mouse polyclonal anti-MBP-AnkA (lane 1), mouse polyclonal anti-HGE agent (BDS strain) (lane 2), nonimmune mouse serum (lane 3), rabbit anti-MBP (lane 4), nonimmune rabbit serum (lane 5), mouse monoclonal IE3 [IgG1(κ)] to MBP-AnkA (lane 6), and mouse monoclonal IgG1(κ) control antibody (lane 7). (B) Lysates of E. coli transformed by ee-o (lanes 1 and 3) or not transformed (lanes 2 and 4) were processed as described for panel A and probed with the following primary antibodies: mouse monoclonal IE3 [IgG1(κ)] to MBP-AnkA (lanes 1 and 2) and mouse polyclonal anti-HGE agent (lanes 3 and 4). (C) Whole HGE agent was processed as for panels A and B and probed with the following primary antibodies: rabbit anti-HGE agent (Webster strain) (lane 1), rabbit anti-MBP (lane 2), nonimmune rabbit serum (lane 3), mouse polyclonal anti-HGE agent (BDS strain) (lane 4), mouse polyclonal anti-MBP-AnkA (lane 5), and nonimmune mouse serum (lane 6). The numbers on the right represent the molecular masses (MWs) of HGE agent proteins that contain AnkA antigens (150, 90, 75, and 51 kDa) and the 42-kDa immunodominant HGE agent antigen that reacts only with polyclonal HGE agent antibodies.
When E. coli was transformed with ee-o, lysed, separated by SDS-polyacrylamide gel electrophoresis, and reacted with mouse polyclonal and monoclonal antibodies to MBP-AnkA, it produced a major band of approximately 160 kDa (Fig. 4B), suggesting that hge-d and ee-o encode proteins that share common epitopes. When whole HGE agent was used as the antigen for immunoblotting, it produced a major band of 153 kDa that was recognized not only by mouse polyclonal antibodies to whole HGE agent but also by monoclonal antibodies to MBP-AnkA (Fig. 4C, lanes 4 and 5). These results support the hypothesis that AnkA is a dominant HGE agent antigen.
AnkA is a cytoplasmic ehrlichial protein.
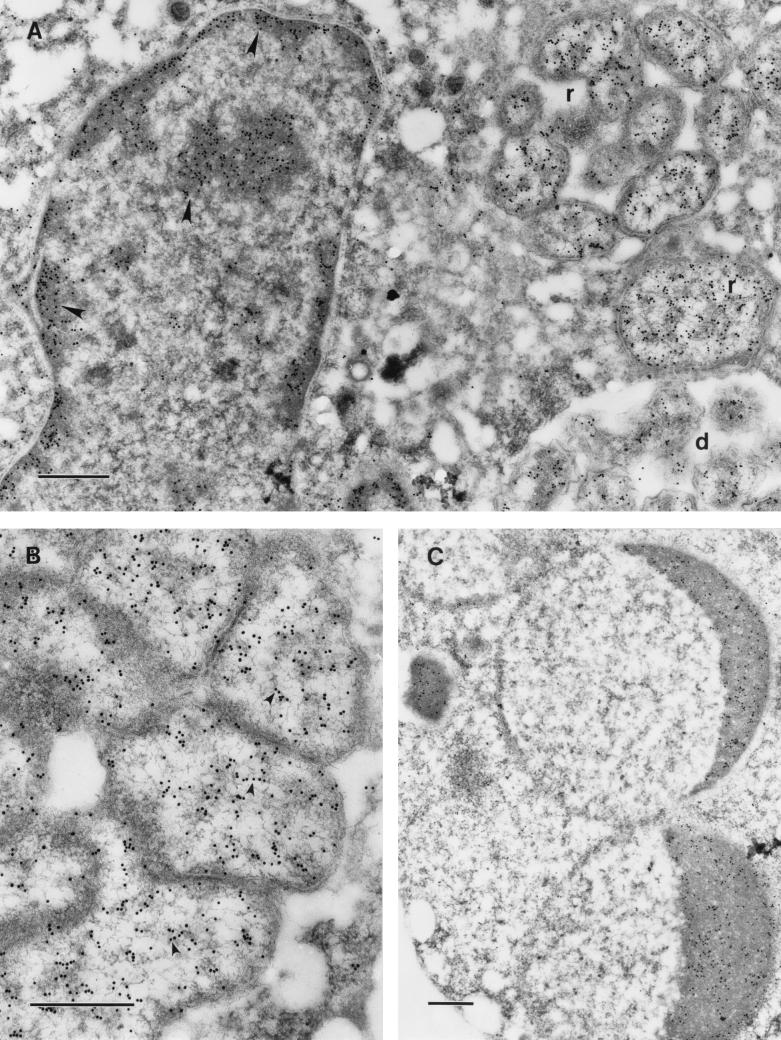
Murine monoclonal antibody to MBP-AnkA readily bound to the cytoplasm, but not to membrane and extracellular structures, of the HGE agent present within HL-60 vacuoles (Fig. 5A). Interestingly, the antibody also bound to the chromatin of HL-60 cells infected by the HGE agent (Fig. 5A and C) but not to that of uninfected HL-60 cells. Finally, the antibody bound to condensed chromatin of infected and apoptotic HL-60 cells (Fig. 5C). These results suggest that AnkA localizes in the cytoplasm of the HGE agent and that it is also secreted during infection of eukaryotic cells, associating with a yet-undefined nuclear component.
FIG. 5.
Immunoelectron microscopic visualization of AnkA with monoclonal antibody IE3 in HL-60 cells infected with HGE agent (Webster strain). Bars, 0.5 μm. (A) The label is localized in the cytoplasm of the ehrlichiae, both reticulate (r) and dense-cored (d) cells, and on condensed chromatin of the host cell nucleus (arrowheads). (B) In reticulate cells, the cytoplasm is labeled and many gold particles are aligned along the DNA fibrils of the nucleoid (arrowheads). (C) In an apoptotic cell, condensed chromatin of the apoptotic nucleus is heavily labeled.
DISCUSSION
Our hypothesis that E. phagocytophila group ehrlichiae contain species-specific protein antigens that might be critically involved in intracellular growth prompted a search in libraries of HGE agent and E. equi genomes. This resulted in the cloning from two distinct strains of a gene, called ankA that is similar to a gene previously reported (24). The analysis indicated that the gene originally reported by Storey et al. (24) lacked 1,453 nt at the 5′ end, partially explaining the discrepancy between the observed 160-kDa protein and the predicted 79-kDa protein.
AnkA is a protein rich in ankyrin motifs, of approximately 160 kDa, present in native HGE agent and E. equi. The occurrence of additional protein antigens of lower molecular mass and the presence of more than a single copy in whole-genome Southern blots suggest that the encoding gene is part of a multigene family in the HGE agent genome. The role of AnkA in ehrlichial function or in pathogenesis is not known. However, some of the adaptations that allow ehrlichiae to reside within vacuoles require communication through the endocytic membrane, a function facilitated by ankyrins (8, 12, 18, 21). However, the amino acid motif analyses and the ultrastructural localization studies do not support a role for AnkA in the trafficking of the ehrlichia-containing vacuole. Aside from the ankyrin repeats, BLAST analysis of protein databases has not revealed significant identity with other proteins of known function. We cannot exclude the possibility that a cross-reactive host protein was expressed during ehrlichia infection. However, the detection of an AnkA epitope associated with host cell chromatin in infected but not uninfected cell cultures suggests a potential role in regulation or modification of host cell gene transcription.
The potential interaction of AnkA with condensed host cell chromatin is a compelling finding, since proteins with ankyrin repeats are well described to affect proinflammatory cytokine expression by virtue of molecular similarities to cellular IκB and calcineurin proteins (5, 17). The related E. chaffeensis has been shown to inhibit proinflammatory cytokine production by preventing the dissociation of IκB from NF-κB, thus precluding NF-κB translocation to the nucleus (15). The identification of AnkA in association with condensed chromatin of host cells in infected cultures, including apoptotic cells, could suggest a role in down-regulated expression of the cell cycle regulators PCNA, pRB, and bcl-2 and inhibition of G1/S cell cycle transition, and induction of apoptosis and host cell death, as described for infected HL-60 cells by Hsieh et al. (13).
The 444-bp fragment, containing part of the region coding for the ankyrin repeats, could be detected by PCR in all North American E. phagocytophila laboratory strains and had nearly identical nucleic acid and predicted amino acid sequences; however, these differed modestly from those of E. phagocytophila strains present in geographically diverse parts of Europe, which in turn differed from each other by a similar degree. Such findings further confirm molecular, biological, and antigenic analyses indicating that E. equi, E. phagocytophila, and the HGE agent are a single, heterogeneous species (2, 7, 9, 16, 25, 26), as implied in previous phylogenetic studies (25).
Variation in the quantity of tandemly repeated units is an emerging hallmark of many bacteria, including Ehrlichia and Rickettsia spp. (3, 11, 27). The situation with AnkA is unusual in that it is a cytoplasmic protein, whereas most other tandemly repeated units in the Rickettsia and Ehrlichia genera appear to be cell surface expressed. Moreover, given the similar degrees of pathogenicity of E. equi and the HGE agent (7, 16), the 27-amino-acid repeat region is unlikely to be pathogenetically important in mammals.
In summary, we report the full-length sequence of AnkA from two geographically distinct strains. Sequence comparison showed that despite a high degree of conservation, geographic polymorphisms could be detected. The localization of AnkA with condensed chromatin of infected cells suggests a role in modulating host gene transcription.
ACKNOWLEDGMENTS
This work was supported by grant AI41213-01 from the National Institutes of Allergy and Infectious Diseases.
We thank Joan Valentine, Phil Richter, Jeff Barlough, and Elfriede DeRock for excellent technical assistance. Partial nucleotide sequencing was performed by Midland Laboratory (Midland, Tex.).
REFERENCES
- 1.Aguero-Rosenfeld M E, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Munoz J, Dumler J S. Human granulocytic ehrlichiosis (HGE): a case series from a single medical center in New York State. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson B E, McDonald G A, Jones D C, Regnery R L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990;58:2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asanovich K M, Bakken J S, Madigan J E, Aguero-Rosenfeld M, Wormser G P, Dumler J S. Antigenic diversity of granulocytic Ehrlichia species isolates from humans in Wisconsin, New York, and a California horse. J Infect Dis. 1997;176:1029–1034. doi: 10.1086/516529. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle P A, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 6.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 7.Barlough J E, Madigan J E, DeRock E, Dumler J S, Bakken J S. Protection against Ehrlichia equi is conferred by prior infection with the human granulocytotropic ehrlichia (HGE agent) J Clin Microbiol. 1995;33:3333–3334. doi: 10.1128/jcm.33.12.3333-3334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck K A, Buchanan J A, Nelson W J. Golgi membrane skeleton: identification, localization and oligomerization of a 195-kDa ankyrin isoform associated with Golgi complex. J Cell Sci. 1997;110:1239–1249. doi: 10.1242/jcs.110.10.1239. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumler J S, Asanovich K M, Bakken J S, Richter P, Kimsey R, Madigan J E. Serologic cross-reaction among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic ehrlichia. J Clin Microbiol. 1995;33:1098–1103. doi: 10.1128/jcm.33.5.1098-1103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmore R D. Comparison of the rompA gene repeat regions of Rickettsiae reveals species-specific arrangements of individual repeating units. Gene. 1993;125:97–102. doi: 10.1016/0378-1119(93)90752-o. [DOI] [PubMed] [Google Scholar]
- 12.Hoock T C, Peters L L, Lux S E. Isoforms of ankyrin-3 that lack the NH2-terminal repeats associated with mouse macrophage lysosomes. J Cell Biol. 1997;136:1059–1070. doi: 10.1083/jcb.136.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh T-C, Aguero-Rosenfeld M, Wu J M, Ng C, Papanikolou N K, Varde S A, Schwartz I, Pizzolo J G, Melamed M, Horowitz H W, Nadelman R B, Wormser G P. Cellular changes and induction of apoptosis in human promyelocytic HL60 cells infected with the agent of human granulocytic ehrlichiosis (HGE) Biochem Biophys Res Commun. 1997;232:298–303. doi: 10.1006/bbrc.1997.6276. [DOI] [PubMed] [Google Scholar]
- 14.Ijdo J W, Sun W, Magnarelli L A, Fikrig E. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect Immun. 1998;66:3264–3269. doi: 10.1128/iai.66.7.3264-3269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, Rikihisa Y. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB and activation of NF-κB. Infect Immun. 1997;65:2890–2897. doi: 10.1128/iai.65.7.2890-2897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madigan J E, Richter P J, Kimsey R B, Barlough J E, Bakken J S, Dumler J S. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1141–1144. doi: 10.1093/infdis/172.4.1141. [DOI] [PubMed] [Google Scholar]
- 17.Miskin J E, Abrams C C, Goatley L C, Dixon L K. A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science. 1998;281:562–565. doi: 10.1126/science.281.5376.562. [DOI] [PubMed] [Google Scholar]
- 18.Moffat F L, Han T, Li Z-M, Peck M D, Falk R E, Spalding P B, Jy W, Ahn Y S, Chu A J, Bourguignon L Y W. Involvement of CD44 and the cytoskeletal linker protein ankyrin in human neutrophil bacterial phagocytosis. J Cell Physiol. 1996;168:638–647. doi: 10.1002/(SICI)1097-4652(199609)168:3<638::AID-JCP16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Murphy C I, Storey J R, Recchia J, Doros-Richert L A, Gingrich-Baker C, Munroe K, Bakken J S, Coughlin R T, Beltz G A. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect Immun. 1998;66:3711–3718. doi: 10.1128/iai.66.8.3711-3718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 21.Peters L L, Lux S E. Ankyrins: structure and function in normal cells and hereditary spherocytes. Semin Hematol. 1993;30:85–118. [PubMed] [Google Scholar]
- 22.Popov V L, Han V C, Chen S-M, Dumler J S, Feng H-M, Andreadis T G, Tesh R B, Walker D H. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol. 1998;47:235–251. doi: 10.1099/00222615-47-3-235. [DOI] [PubMed] [Google Scholar]
- 23.Popov V L, Chen S-M, Feng H-M, Walker D H. Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol. 1995;43:411–421. doi: 10.1099/00222615-43-6-411. [DOI] [PubMed] [Google Scholar]
- 24.Storey J R, Doros-Richert L A, Gingrich-Baker C, Munroe K, Mather T N, Coughlin R T, Beltz G A, Murphy C I. Molecular cloning and sequencing of three granulocytic Ehrlichia genes encoding high-molecular-weight immunoreactive proteins. Infect Immun. 1998;66:1356–1363. doi: 10.1128/iai.66.4.1356-1363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker D H, Dumler J S. Emergence of ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X J, Crocquet-Valdes P, Walker D H. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene. 1997;184:149–54. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhi N, Rikihisa Y, Horowitz H W, Wormser G P. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]