Background:
The COVID-19 pandemic has evolved through multiple phases characterized by new viral variants, vaccine development, and changes in therapies. It is unknown whether rates of cardiovascular disease (CVD) risk factor profiles and complications have changed over time.
Methods:
We analyzed the American Heart Association COVID-19 CVD registry, a national multicenter registry of hospitalized adults with active COVID-19 infection. The time period from April 2020 to December 2021 was divided into 3-month epochs, with March 2020 analyzed separately as a potential outlier. Participating centers varied over the study period. Trends in all-cause in-hospital mortality, CVD risk factors, and in-hospital CVD outcomes, including a composite primary outcome of cardiovascular death, cardiogenic shock, new heart failure, stroke, and myocardial infarction, were evaluated across time epochs. Risk-adjusted analyses were performed using generalized linear mixed-effects models.
Results:
A total of 46 007 patient admissions from 134 hospitals were included (mean patient age 61.8 years, 53% male, 22% Black race). Patients admitted later in the pandemic were younger, more likely obese, and less likely to have existing CVD (Ptrend ≤0.001 for each). The incidence of the primary outcome increased from 7.0% in March 2020 to 9.8% in October to December 2021 (risk-adjusted Ptrend=0.006). This was driven by an increase in the diagnosis of myocardial infarction and stroke (Ptrend<0.0001 for each). The overall rate of in-hospital mortality was 14.2%, which declined over time (20.8% in March 2020 versus 10.8% in the last epoch; adjusted Ptrend<0.0001). When the analysis was restricted to July 2020 to December 2021, no temporal change in all-cause mortality was seen (adjusted Ptrend=0.63).
Conclusions:
Despite a shifting risk factor profile toward a younger population with lower rates of established CVD, the incidence of diagnosed cardiovascular complications of COVID increased from the onset of the pandemic through December 2021. All-cause mortality decreased during the initial months of the pandemic and thereafter remained consistently high through December 2021.
Keywords: COVID-19, heart failure, mortality, myocardial infarction, stroke
What is Known
Preexisting cardiovascular disease (CVD) and CVD risk factors are associated with poor outcomes in COVID-19 infection, while CVD complications represent an important part of the morbidity and mortality of COVID-19.
What the Study Adds
As the pandemic evolved from March 2020 to December 2021, the profile of hospitalized patients with COVID-19 changed toward a younger population with a lower prevalence of prior established CVD.
The incidence of diagnosed cardiovascular complications increased over time, driven by an increase in the rates of myocardial infarction, stroke, and pulmonary embolism.
All-cause in-hospital mortality and use of mechanical ventilation decreased during the initial months of the pandemic but remained stable from July 2020 onward through December 2021.
Increased incidence of CVD complications may reflect greater awareness of CVD risk from COVID-19, changing patient demographics, and the influence of newer viral variants and treatments.
Since the first cases of infection with the SARS CoV-2 virus were reported in December 2019, the COVID-19 pandemic has evolved through multiple phases and claimed the lives of millions of individuals worldwide.1,2 The early phases were marked by limited data and scant therapeutic options. New treatment strategies emerged as the pandemic progressed, including dexamethasone, remdesivir, and monoclonal and polyclonal antibody treatments.3–6 Later, research led to broader use of more intensive anticoagulant prophylaxis for thromboembolic disease, the recognition of a selective role for targeted anti-inflammatory agents such as tocilizumab, and the introduction of new antiviral agents targeted toward key features of the SARS CoV-2 virus.7–10 In addition, recommendations for the management of hypoxemia associated with COVID-19 evolved.11,12 The development of effective vaccines shifted the course of the pandemic, while evolutionary pressures contributed to the emergence of viral variants with unique properties.13–17
The intersection of COVID-19 with cardiovascular disease (CVD) was recognized early in the pandemic. Patients with CVD risk factors were identified as more likely to develop severe illness leading to hospitalization and death,18–21 while CVD complications have been recognized as important contributors to the morbidity and mortality associated with COVID-19 infection.22–25 Myocardial injury commonly occurs and is associated with more severe illness.26,27 Multiple studies have described myocardial inflammatory changes with cardiac magnetic resonance imaging among some survivors of COVID-19 infection.28–30
Despite these rapid changes in the treatment strategies, vaccine availability, and the virus itself (through multiple variants), it is unknown whether the CVD risk factor profile or CVD complications associated with COVID-19 infection have changed over the course of the pandemic. To address this question, we examined the longitudinal prevalence of CVD risk factors and the incidence of diagnosed CVD complications in hospitalized patients with COVID-19 using the American Heart Association’s COVID-19 Cardiovascular Disease Registry. We also examined multiple additional secondary outcomes, including all-cause in-hospital mortality, thromboembolic events, and resource utilization. Given advances in the prevention and treatment of severe COVID-19 infection, we hypothesized that the rate of cardiovascular complications from COVID-19 has decreased over the course of the pandemic.
Methods
Study Population and Data Source
We studied patients included in the American Heart Association COVID-19 CVD registry, part of the Get With The Guidelines® program. Details of this registry have been previously described.31–33 Briefly, the COVID-19 CVD registry is available to all hospitals in the United States. Trained data abstractors at each participating institution collect medical record data from consecutive adult patients (age ≥18 years old) who are hospitalized with active COVID-19 as the primary diagnosis. The registry collects over 400 data elements, including baseline demographics, prior medical history, clinical characteristics, and in-hospital outcomes. IQVIA (Parsippany, New Jersey) hosts data collection for the American Heart Association COVID-19 registry. For all institutions, participation in the COVID-19 CVD registry was approved or review was waived by a local institutional review board. Informed consent was not required as there is no participant interaction in this quality improvement registry. Dr de Lemos had full access to all data in the study and takes responsibility for the data analyses and integrity. Requests to access the registry dataset may be sent from qualified researchers to the American Heart Association at qualityresearch@heart.org.
Time Epochs
This was an observational study using a repeated cross-sectional design to analyze separate groups of patient admissions over distinct time periods. To identify trends over time, the study period from March 2020 to December 2021 was divided into 8 time epochs. March 2020 was considered as a separate time epoch given the very large numbers of COVID-19 admissions from centers in limited geographical distribution during this initial period of the pandemic in the United States. The remaining study period was divided into 3-month epochs. Hospital admissions were classified into a specific time epoch based on the date of hospital admission. Data analysis was performed using a de-identified database within a secure workspace on the American Heart Association Precision Medicine Platform (https://precision.heart.org/).34
Exposures
Demographics, CV risk factors, and other major comorbidities were summarized, both for the overall study duration and for each time epoch. Race and ethnicity were obtained from the medical record by local data abstractors. Prior CVD was defined as a history of myocardial infarction (MI), coronary artery bypass surgery, prior percutaneous coronary intervention, stroke, peripheral arterial disease, heart failure, congenital heart disease, atrial fibrillation, or atrial flutter. Atherosclerotic cardiovascular disease was defined as history of MI, stroke, coronary artery bypass surgery, percutaneous coronary intervention, or peripheral vascular disease. A composite of CVD risk factors was also defined, which included diabetes, dyslipidemia, hypertension, smoking, and obesity (body mass index ≥30). Severity of illness at time of presentation was assessed using the quick sequential organ failure assessment score.35,36 Medications, including use of glucocorticoids, remdesivir, hydroxychloroquine, convalescent plasma, and tocilizumab, were abstracted from the medical record. Vaccination status was not captured systematically in the registry.
Outcomes
The primary CVD composite outcome included MI, stroke, new heart failure, cardiogenic shock, and in-hospital death due to cardiovascular causes. Secondary outcomes included the composite of major adverse cardiovascular events (defined as cardiovascular death, MI, stroke, or coronary revascularization), and the individual outcomes of MI, stroke, new heart failure, cardiogenic shock, atrial fibrillation, deep vein thrombosis, pulmonary embolism, in-hospital all-cause death, and cardiovascular death. Outcomes were captured by abstractors at each of the hospitals participating in the registry and were not centrally adjudicated.
We also evaluated time trends in therapeutic interventions for hospitalized patients, including COVID-19 medications, use and duration of mechanical ventilation, hospital and intensive care unit (ICU) length of stay, use of kidney replacement therapy, and use of venovenous extracorporeal membrane oxygenation. The use of cardiovascular procedures, including echocardiography and coronary angiography, was also evaluated across time epochs.
Statistical Analysis
Patient characteristics were described for the study period as a whole as well as for each time epoch. The primary and secondary outcomes were described using incidence estimates for the overall study period and for each time epoch. Categorical variables were analyzed across time epochs using the Cochran-Armitage test, while continuous variables were analyzed using trend tests for continuous variables.
We also performed risk-adjusted analyses of the composite outcome, use of mechanical ventilation, and all-cause in-hospital mortality. This adjusted analysis used prespecified variables that were identified in a prior risk-adjusted analysis of the registry examining the period from March to November 2020.37 Variables included in the model were age, sex, history of coronary revascularization, cancer, cerebrovascular disease, chronic kidney disease, diabetes, heart failure, hypertension, pulmonary disease, smoking, body mass index, admission vital signs, need for supplemental O2 on admission, interstitial infiltrate present on admission, and admission creatinine. Risk-adjusted time trends were performed using generalized linear mixed-effects models, with random effects for each hospital to account for the clustering of patients within hospitals. A logit link function with a binary distribution was used for the models. Exposure and covariates in the main analysis had <5% missing data and thus no imputation was performed; only patients with complete data were included in the regression analysis. However, as a sensitivity analysis, we used the missing-indicator method so that each participant could still be included in the analysis to maintain statistical power. We found no appreciable differences in results, and for clarity and ease of interpretation, we show the complete-case analysis. Secondary adjusted analyses were repeated after censoring data from March to June 2020 to allow assessment of time trends after the initial months of the pandemic. All analyses were performed on the American Heart Association Precision Medicine Platform using SAS software (version 9.4), with statistical significance defined as a 2-sided P value <0.05.
Results
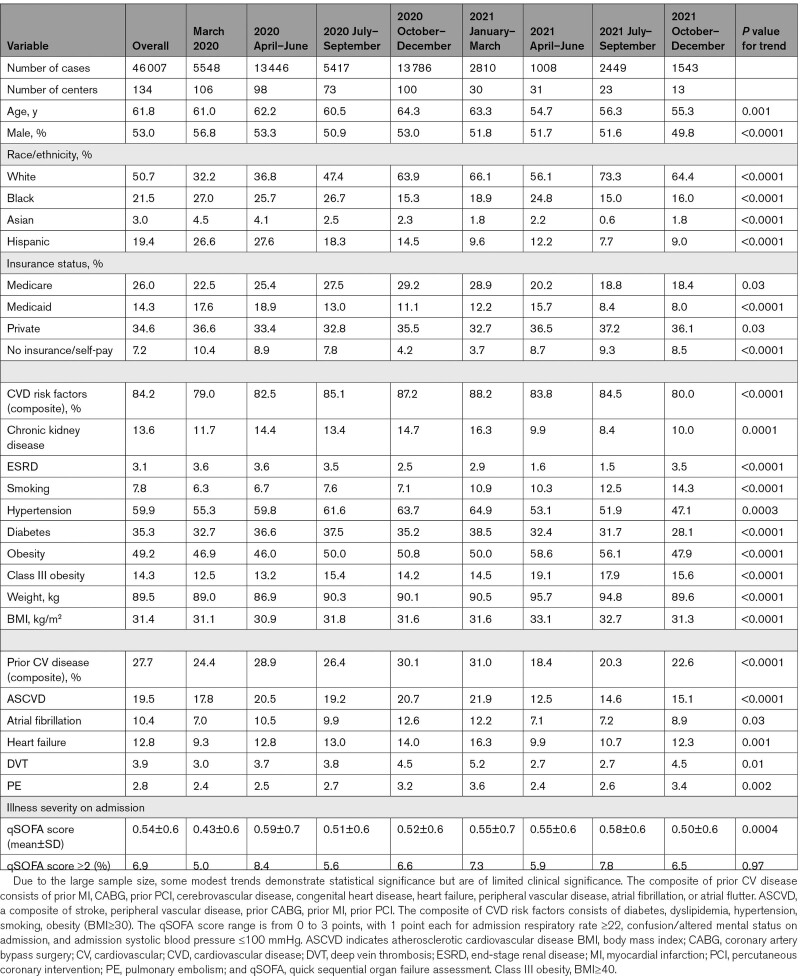
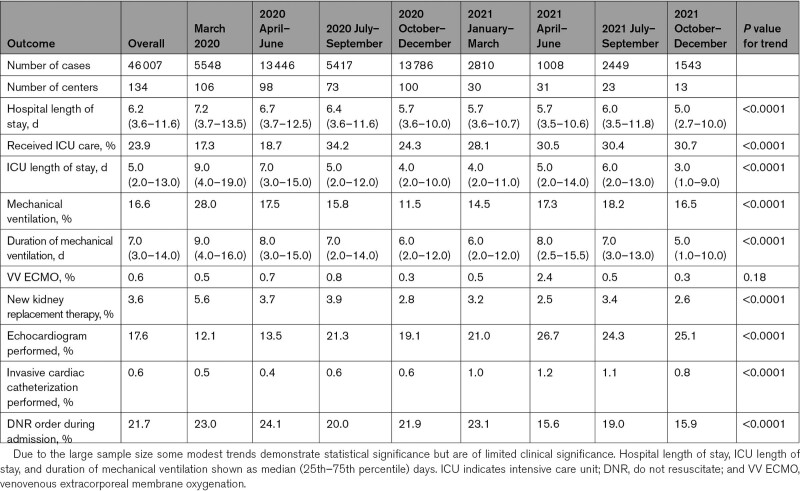
A total of 46 007 patient admissions from 134 hospitals were included in the present study (Table 1). Mean±SD age was 62±18 years, and males comprised 53% of the population. White patients represented 51% of the overall cohort, Black patients 22%, and patients of Hispanic ethnicity 19%. CVD risk factors were present in 84% of patients. A large proportion of patients also had preexisting CVD (28%), of which the most common preexisting cardiovascular condition was atherosclerotic cardiovascular disease in 20% of patients.
Table 1.
Baseline Characteristics of Admitted Patients With COVID-19
Changes in Demographics and Clinical Characteristics Over the Course of the Pandemic
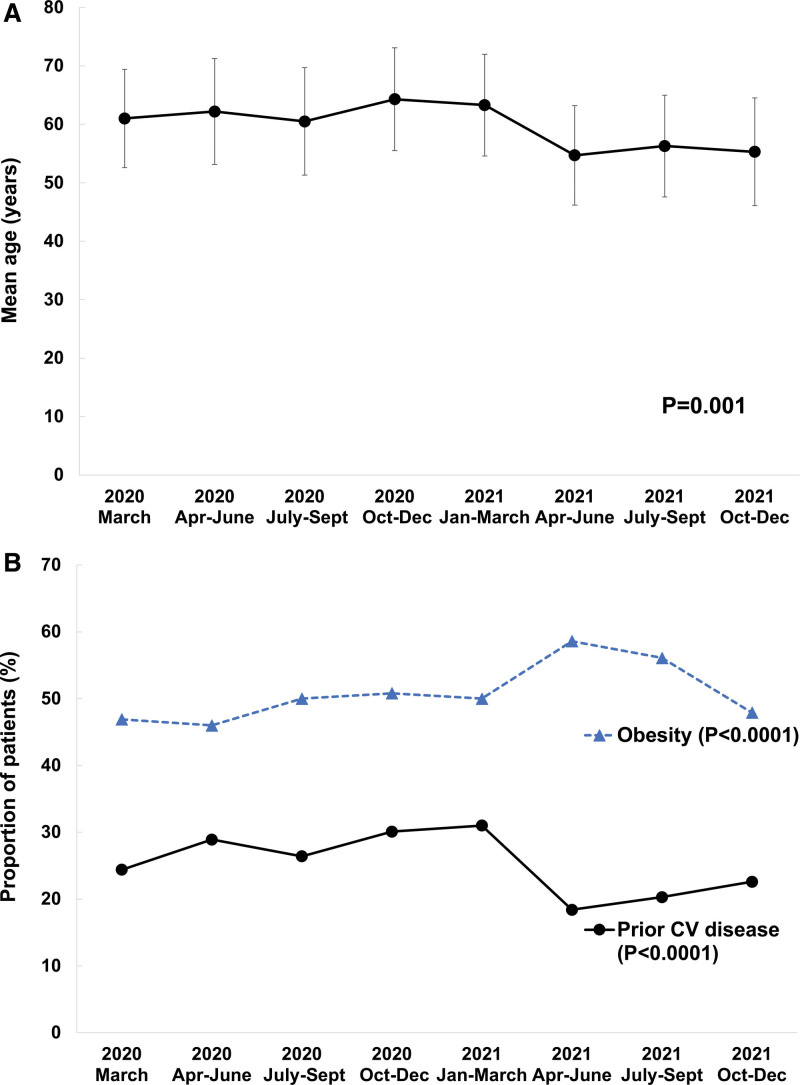
Patients admitted during the earlier time epochs portions tended to be older than those admitted from March 2021 onward and were less likely to have preexisting CVD (Ptrend<0.0001, Figure 1A and 1B; Table 1). The proportion of obese patients was 49% overall, with a modest trend towards increased rates of obesity later in the study period (Ptrend<0.0001). Severity of illness on admission, as assessed by quick sequential organ failure assessment score, did not change significantly over the study period.
Figure 1.
Temporal trends in the age and past medical history of patients admitted with COVID-19. A, Age. Error bars represent the SD. B, Medical history. The composite of prior cardiovascular (CV) disease consists of prior myocardial infarction, coronary artery bypass surgery, prior percutaneous coronary intervention, cerebrovascular disease, congenital heart disease, heart failure, peripheral vascular disease, atrial fibrillation, or atrial flutter.
COVID-19 Therapies
Medical therapy for COVID-19 evolved throughout the pandemic (Figure S1). The earliest stages of the pandemic saw a large proportion of patients receive hydroxychloroquine, which decreased rapidly to very low levels during subsequent periods of the pandemic (≤1% of patients from January 2021 onward). The administration of convalescent plasma increased from the early portion of the pandemic with peak usage in October to December 2020 followed subsequently by decreased use. Treatment with glucocorticoids and remdesivir increased significantly over time; ultimately 54% and 33% of the entire cohort received glucocorticoids or remdesivir, respectively. Tocilizumab was used in 7% of the overall cohort.
Cardiovascular Outcomes and Diagnostics
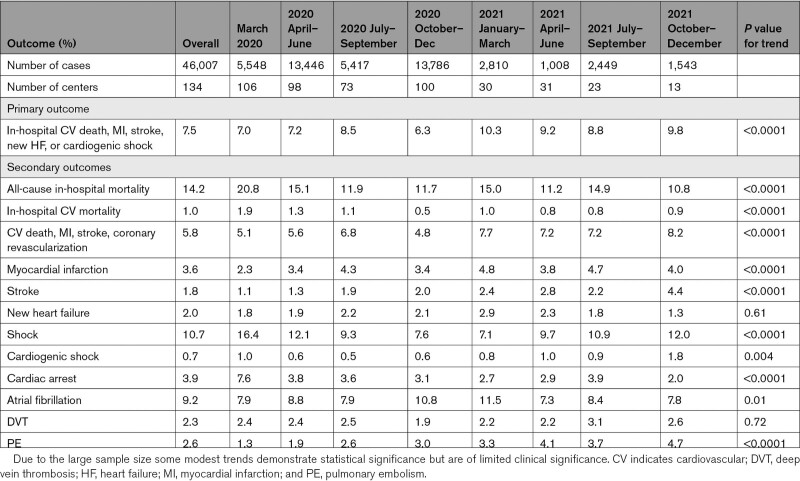
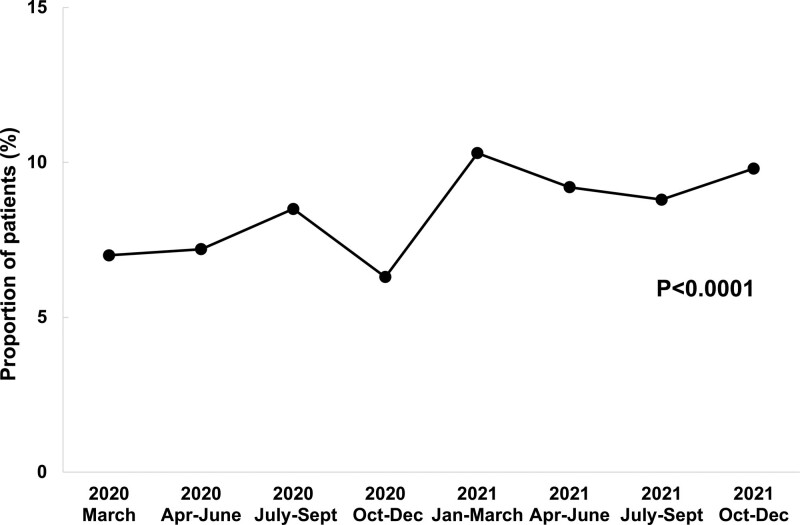
Over the entire study period, the crude incidence of the primary composite outcome was 7.5% (Table 2; Figure 2); among those with a history of prior CVD, the incidence was 12.2%, compared with 5.9% in those with no history of prior CVD. The incidence of the primary outcome increased from 7.0% in March 2020 to 9.8% in the October to December 2021 epoch (Ptrend<0.0001). After adjustment for baseline demographics, comorbidities, and illness severity on admission, the rate of the primary cardiovascular outcome was higher in later time epochs (adjusted P=0.006), as well as in the secondary analysis restricted to the period from July 2020 to December 2021 (adjusted P=0.006).
Table 2.
Cardiovascular Outcomes of Hospitalized Patients
Figure 2.
Incidence of the primary composite cardiovascular outcome in hospitalized patients with COVID-19. The primary composite outcome consists of cardiovascular death, cardiogenic shock, new heart failure, stroke, and myocardial infarction.
Among components of the primary outcome, MI and stroke were relatively uncommon, occurring in 3.6% and 1.8% of the cohort over the entire study period, but the incidence of both diagnoses rose modestly with time (Table 2; Figure S2A and S2B). ST-elevation MI (STEMI) was rare, reported in only 0.3% of patients in the registry. New heart failure occurred in 2% of patients with no change over time (Figure S2C). A total of 10.7% of patients experienced shock of any type, of which cardiogenic shock was rare throughout the study period (0.7% of all patients). The overall incidence of venous thromboembolism increased over time, driven by an increase in diagnosed pulmonary embolism (Ptrend<0.0001; Table 2; Figure S2D). Rates of diagnosed deep vein thrombosis were stable during the study period (Table 2).
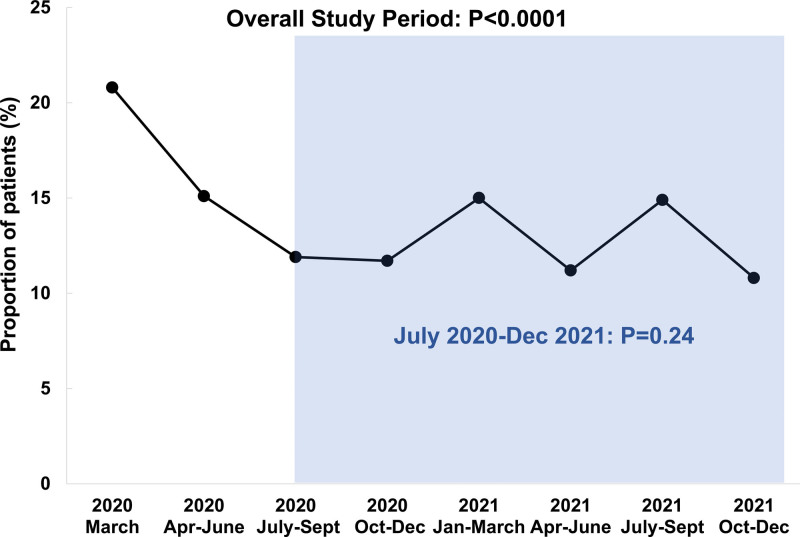
All-cause in-hospital mortality for the entire study period was 14.2%. Mortality was highest in March 2020 (20.8%), with significantly decreased rates of crude and risk-adjusted hospital mortality over subsequent time epochs (Figure 3; unadjusted Ptrend<0.0001 and adjusted Ptrend<0.0001). When the analysis was restricted to the period between July 2020 and December 2021, no significant change in mortality was observed (unadjusted Ptrend=0.24, adjusted Ptrend=0.63). Cardiovascular mortality was rare throughout the study period with 1.0% of the overall cohort experiencing death due to MI, heart failure, cardiogenic shock, or stroke. Overall, 9% of deaths were attributed to a cardiovascular cause, with respiratory causes representing the 72% of deaths. A “do-not-resuscitate” order was placed on 21.7% of all patients in the study with declining rates over the course of the pandemic (Table 3).
Figure 3.
All-cause in-hospital mortality among patients hospitalized with COVID-19. Incidence of in-hospital mortality. P values shown are from the unadjusted trend analysis.
Table 3.
Resource Utilization and Noncardiovascular Outcomes Among Hospitalized Patients
Noncardiac Outcomes and Resource Utilization
A total of 17% of the cohort required mechanical ventilation. Similar to all-cause mortality, rates of mechanical ventilation fell markedly in the initial months of the pandemic, with no consistent change thereafter (Figure S3). When the analysis was restricted to the period from July 2020 to December 2021, no change in the use of mechanical ventilation was observed (unadjusted Ptrend=0.34, adjusted Ptrend=0.12). The proportion of patients who received ICU care increased from 17.3% during March 2020 to 30.7% by October to December 2021(Ptrend<0.001; Table 3). Hospital and ICU length of stay and duration of mechanical ventilation all showed modest decreases over the study period. Echocardiography was used more frequently in later time epochs (Figure S4). Invasive coronary angiography was rarely used, with 0.6% of all patients undergoing invasive angiography and about half of those undergoing percutaneous coronary intervention.
Discussion
In this analysis of a large multicenter registry of hospitalized patients with COVID-19, we found that despite a changing risk factor profile towards a younger patient population with lower rates of preexisting CVD, the incidence of diagnosed cardiovascular complications of COVID-19 increased from the beginning of the pandemic through the end of 2021. This was driven predominantly by increased rates of MI and stroke. All-cause mortality and the use of mechanical ventilation declined during the initial months of the pandemic but remained stable from July 2020 through December 2021.
Shifting Risk Factor Profiles and Therapies
Within the registry cohort, the early stages of the pandemic were marked by an older population, a large number of whom had existing CVD. Black and Hispanic patients also made up a large proportion of COVID-19 patients during this period, consistent with findings seen in other cohorts.38,39 In contrast, the patient population during the later stages of the pandemic tended to be younger with a lower proportion of Black and Hispanic patients, fewer comorbidities, and a lower prevalence of prior CVD. This shift toward a younger population after March 2021 may be a reflection of widespread vaccination of older adults during early 2021, as close to three-quarters of Americans age ≥65 years old had received at least one dose of a vaccine by April 1, 2021.40 Unvaccinated individuals comprised the majority of COVID-19 cases and deaths from April to December 2021 in data gathered from 25 US jurisdictions by the Centers for Disease Control.41 While vaccination status was not assessed in our study and thus no conclusions can be drawn from our data regarding the effect of vaccination on CV outcomes, it is likely that an increasing proportion of patients in our registry who were admitted with COVID-19 from April 2021 onward were unvaccinated.
We observed significant changes in therapies for COVID-19 in response to rapidly evolving evidence. Hydroxychloroquine was used in more than one-third of patients during the first portion of the pandemic, with use declining significantly to ~1% after it was found to lack benefit in a randomized controlled trial.42 Likewise, the use of remdesivir and glucocorticoids both increased significantly after preliminary reports of the Adaptive Covid-19 Treatment Trial (ACTT-1) and Recovery trials in May 2020 and July 2020, respectively.3,4
Increased Diagnosis of CVD Complications
Reported cardiovascular complications of COVID-19 increased over time, driven primarily by modest increases in MI and stroke, as well as increased diagnosis of pulmonary embolism. These trends are likely mediated at least in part by greater recognition and intensified screening for CVD and venous thromboembolism complications. Diagnosis of venous thromboembolism, in particular, may have been influenced by early reports that described a high incidence of deep vein thrombosis/pulmonary embolism.24,43 It remains noteworthy, however, that these increases in diagnosed MI and stroke occurred despite the shift toward a younger population with lower rates of established cardiovascular disease. There remained a statistically significant increase in cardiovascular complications on risk-adjusted analysis as well. As expected, the rate of the primary outcome was higher among patients with a prior history of CVD but occurred not infrequently even among those without a prior CVD history.
Consistent with other cohorts, new heart failure and cardiogenic shock were uncommon within our study population.22,23 Likewise, cardiovascular mortality represented a small fraction of the overall mortality seen among the study cohort, with the bulk of mortality driven by respiratory causes.
Mortality, Mechanical Ventilation, and Resource Utilization
We observed a decrease in both crude and adjusted all-cause mortality over the course of the study period, data which are consistent with reports from the initial phases of the pandemic.37,44,45 We also observed a decrease in the use and duration of mechanical ventilation over the entire study period. However, large reductions in these outcomes appeared early in the pandemic with no significant further improvement over the period from July 2020 to December 2021. These early improvements likely in part reflect rapid introduction of new therapies such as remdesivir and corticosteroids as well as increasing expertise in managing COVID-19 respiratory complications by clinicians, including increased use of noninvasive ventilation, delayed intubation, proning, and more rapid extubation.11 It is noteworthy that all-cause mortality and use of mechanical ventilation remained high during the later stages of the study period despite the changing risk profile of admitted patients as well as advances in therapy, with 12% mortality and 15% requiring mechanical ventilation from July 2020 onwards.
ICU utilization increased significantly over time, suggesting that significant rationing of ICU level care may have occurred during the early phases of the pandemic. The increased availability of critical care resources may have also played a role in the improvement in overall mortality seen after the initial months of the pandemic. Both hospital and ICU length of stay declined with time, suggesting a potential benefit of medical therapies as well as increasing comfort with discharging patients for continued outpatient management. The use of echocardiography increased substantially during the pandemic, likely due to heightened awareness of potential cardiovascular complications of COVID-19 as well as greater comfort performing echocardiography among COVID-19 infected patients.
Limitations
Our observational study of hospitalized patients has several limitations, which should be considered. Sample size and the number of centers contributing to the registry varied over the course of the pandemic, due to both fluctuating numbers of patients admitted with COVID-19 as well as the voluntary nature of the registry. Given this variability, we did not use joint point regression to assess changes over shorter time intervals than the epochs described above. The number of patients enrolled and sites contributing data in the registry declined over the study period, a potential source of bias which should be considered when drawing inferences from our repeated cross-sectional analyses. As a retrospective registry without central adjudication or a standardized protocol to screen for CV complications, additional unmeasured factors may also contribute to biases, most notably ascertainment bias. It is not possible to determine from our data to what degree the observed increase in the incidence of the primary outcome occurred due to changes in the natural history of CVD complications associated with COVID-19 infection versus increased screening and awareness of cardiovascular complications of COVID-19. We did not analyze factors such as bed capacity or resource limitation during surges which may have affected both rates of diagnosis and outcomes, and our data did not capture vaccination status. Data are not available from January 2022 onward and thus we did not assess CVD complications during the Omicron surge. Novel therapeutic strategies such as paxlovid which emerged after December 2021 may affect CVD outcomes and were not captured within our study period. Multiple variants of the SARS-CoV-2 virus with differing biological properties predominated at different points over the study period.46 Our data did not capture viral genomics, and no inference can be made from our data on the impact of any specific viral variant on the risk of cardiovascular complications. Additionally, COVID-19 surges impacted different regions of the U.S. at different times, and the approach used to categorize time epochs in our study does not account for these potential regional differences.
Conclusions
Through a longitudinal analysis of a large multicenter registry of hospitalized patients with COVID-19 through December 2021, we observed a shift over the course of the pandemic to a younger population of patients with fewer comorbidities and lower rates of established cardiovascular disease. Despite this changing risk factor profile, the incidence of reported cardiovascular complications of COVID-19 rose, driven primarily by modest increases in the diagnosis of stroke and MI. All-cause mortality and use of mechanical ventilation declined during the early months of the pandemic, consistent with advances in the management of severe COVID-19 infection, but remained persistently high throughout the rest of the study period. These data suggest that patients with COVID-19 infection severe enough to require admission may remain at substantial risk of cardiovascular morbidity and all-cause mortality.
Article Information
Sources of Funding
The American Heart Association (AHA)’s suite of Registries is funded by multiple industry sponsors. The AHA’s COVID-19 CVD Registry is supported by The Moore Foundation. Dr Kolkailah is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL125247.
Disclosures
Dr Elkind reports serving as an unpaid Officer of the American Heart Association. Dr Hall, C. Rutan, J. Walchok, J.H. Williams, Dr Stevens, and P. Mallya are employees of the American Heart Association. Dr Wang reports receiving research grants to the Duke Clinical Research Institute from Abbott, AstraZeneca, Bristol Myers Squibb, Boston Scientific, Cryolife, Chiesi, Merck, Portola, and Regeneron, as well as consulting honoraria from AstraZeneca, Bristol Myers Squibb, Cryolife, and Novartis. All other authors have no relationships to report.
Supplemental Material
Figures S1–S4
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CVD
- cardiovascular disease
- ICU
- intensive care unit
- MI
- myocardial infarction
- VV ECMO
- venovenous extracorporeal membrane oxygenation
For Sources of Funding and Disclosures, see page 349.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.122.009652.
Contributor Information
Eric J. Hall, Email: jennifer.hall@heart.org.
Colby R. Ayers, Email: colby.ayers@utsouthwestern.edu.
Ahmed A. Kolkailah, Email: a.kolkailah@gmail.com.
Christine Rutan, Email: Christine.rutan@heart.org.
Jason Walchok, Email: jason.walchok@heart.org.
Joseph H. Williams, IV, Email: Joseph.williams@heart.org.
Tracy Y. Wang, Email: tracy.wang@duke.edu.
Fatima Rodriguez, Email: frodrigu@stanford.edu.
Steven M. Bradley, Email: steven.bradley@allina.com.
Laura Stevens, Email: laura.stevens@cuanschutz.edu.
Jennifer L. Hall, Email: jennifer.hall@heart.org.
Pratheek Mallya, Email: pratheek.mallya@heart.org.
Gregory A. Roth, Email: rothg@uw.edu.
David A. Morrow, Email: dmorrow@partners.org.
Mitchell S.V. Elkind, Email: mse13@columbia.edu.
Sandeep R. Das, Email: sandeep.das@utsouthwestern.edu.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Center for Disease Control and Prevention. Daily Updates of Totals by Week and State Print: Provisional Death Counts for Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/nchs/nvss/vsrr/covid19/index.htm.
- 3.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vazquez C, Savoy N, Giunta DH, Perez LG, Sanchez MDL, et al. ; PlasmAr Study Group. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Investigators A, Investigators AC-a, Investigators R-C, Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martin-Quiros A, Caraco Y, Williams-Diaz A, Brown ML, et al. ; MOVe-OUT Study Group. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasa P, Azoulay E, Khanna AK, Jain R, Gupta S, Javeri Y, Juneja D, Rangappa P, Sundararajan K, Alhazzani W, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021;25:106. doi: 10.1186/s13054-021-03491-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, Bradley JM, Dark P, Dave C, De Soyza A, et al. ; RECOVERY-RS Collaborators. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327:546–558. doi: 10.1001/jama.2022.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, Zhang X, Muruato AE, Zou J, Fontes-Garfias CR, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592:116–121. doi: 10.1038/s41586-020-2895-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, Seaman SR, Harris RJ, Hope R, Lopez-Bernal J, et al. ; COVID-19 Genomics UK (COG-UK) consortium. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22:35–42. doi: 10.1016/S1473-3099(21)00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, Rosenblatt A, Walchok J, Omar W, Khera R, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936 [DOI] [PubMed] [Google Scholar]
- 20.Ponsford MJ, Gkatzionis A, Walker VM, Grant AJ, Wootton RE, Moore LSP, Fatumo S, Mason AM, Zuber V, Willer C, et al. Cardiometabolic traits, sepsis, and severe COVID-19: a mendelian randomization investigation. Circulation. 2020;142:1791–1793. doi: 10.1161/CIRCULATIONAHA.120.050753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolkailah AA, Riggs K, Navar AM, Khera A. COVID-19 and cardiometabolic health: lessons gleaned from the pandemic and insights for the next wave. Curr Atheroscler Rep. 2022;24:607–617. doi: 10.1007/s11883-022-01033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez-Garcia J, Jaladanki S, Rivas-Lasarte M, Cagliostro M, Gupta A, Joshi A, Ting P, Mitter SS, Bagiella E, Mancini D, et al. New heart failure diagnoses among patients hospitalized for COVID-19. J Am Coll Cardiol. 2021;77:2260–2262. doi: 10.1016/j.jacc.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg DD, Alviar CL, Bhatt AS, Baird-Zars VM, Barnett CF, Daniels LB, DeFilippis AP, Fagundes A, Jr, Katrapati P, Kenigsberg BB, et al. Epidemiology of acute heart failure in critically ill patients with COVID-19: an analysis from the critical care cardiology trials network. J Card Fail. 2022;28:675–681. doi: 10.1016/j.cardfail.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG, Jensen JUS, Fralick M, Schou M, Lamberts M, Gerds T, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smilowitz NR, Jethani N, Chen J, Aphinyanaphongs Y, Zhang R, Dogra S, Alviar CL, Keller N, Razzouk L, Quinones-Camacho A, et al. Myocardial injury in adults hospitalized with COVID-19. Circulation. 2020;142:2393–2395. doi: 10.1161/CIRCULATIONAHA.120.050434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, Slaughter JC, Fitch W, Hughes SG, Soslow JH. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alger HM, Rutan C, Williams JH, Walchok JG, Bolles M, Hall JL, Bradley SM, Elkind MSV, Rodriguez F, Wang TY, et al. American Heart Association COVID-19 CVD registry powered by get with the guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006967. doi: 10.1161/CIRCOUTCOMES.120.006967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao A, Ranka S, Ayers C, Hendren N, Rosenblatt A, Alger HM, Rutan C, Omar W, Khera R, Gupta K, et al. Association of kidney disease with outcomes in COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. J Am Heart Assoc. 2021;10:e020910. doi: 10.1161/JAHA.121.020910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez F, Solomon N, de Lemos JA, Das SR, Morrow DA, Bradley SM, Elkind MSV, Williams JH, Holmes D, Matsouaka RA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens LM, de Lemos JA, Das SR, Rutan C, Alger HM, Elkind MSV, Zhao J, Iyer K, Figueroa CA, Hall JL. American Heart Association precision medicine platform addresses challenges in data sharing. Circ Cardiovasc Qual Outcomes. 2021;14:e007949. doi: 10.1161/CIRCOUTCOMES.121.007949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, Dunning J, Fairfield CJ, Gamble C, Green CA, et al. ; ISARIC4C investigators. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE)Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:290–300. doi: 10.1001/jama.2016.20328 [DOI] [PubMed] [Google Scholar]
- 37.Roth GA, Emmons-Bell S, Alger HM, Bradley SM, Das SR, de Lemos JA, Gakidou E, Elkind MSV, Hay S, Hall JL, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open. 2021;4:e218828. doi: 10.1001/jamanetworkopen.2021.8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of patients with coronavirus disease 2019 (COVID-19) presenting to the emergency department. Clin Infect Dis. 2022;74:387–394. doi: 10.1093/cid/ciab290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Center for Disease Control and Prevention. The Race to Vaccinate. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-reports/04022021.html.
- 41.Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, Avoundjian T, Awofeso AO, Barnes J, Bayoumi NS, et al. ; MSHI. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76:2060–2072. doi: 10.1016/j.jacc.2020.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray WK, Navaratnam AV, Day J, Wendon J, Briggs TWR. Changes in COVID-19 in-hospital mortality in hospitalised adults in England over the first seven months of the pandemic: an observational study using administrative data. Lancet Reg Health Eur. 2021;5:100104. doi: 10.1016/j.lanepe.2021.100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Vidal C, Cozar-Llisto A, Meira F, Duenas G, Puerta-Alcalde P, Cilloniz C, Garcia-Pouton N, Chumbita M, Cardozo C, Hernandez M, et al. ; COVID-19-researcher group. Trends in mortality of hospitalised COVID-19 patients: a single centre observational cohort study from Spain. Lancet Reg Health Eur. 2021;3:100041. doi: 10.1016/j.lanepe.2021.100041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Telenti A, Hodcroft EB, Robertson DL. The evolution and biology of SARS-CoV-2 variants. Cold Spring Harb Perspect Med. 2022;12:a041390. doi: 10.1101/cshperspect.a041390 [DOI] [PMC free article] [PubMed] [Google Scholar]