Abstract
In the treatment and prevention of osteoporosis and more generally of neoplastic and metabolic pathologies affecting bone tissues, antiresorption drugs such as bisphosphonates and monoclonal antibody are used. Bisphosphonates have been linked to cases of osteonecrosis of the jaws since 2003 by Marx, with more and more evidence over the next two decades; together with bisphosphonate drugs, cases relating to the use of monoclonal drugs have been subsequently added. Among the main independent risk factors, we have extraction procedures in oral surgery that can affect both the mandible and the maxilla and the anterior or posterior sectors. The incidence of MRONJ treated with oral bisphosphonates ranges from 0.5% to 3% according to studies; this incidence would appear to be higher in patients treated with antiresorptive agents with neoplastic diseases. Many pathologies including those in which antiresorptive drugs are used show differences in prevalence in relation to sex; similarly, there could be differences in the incidence of cases of osteonecrosis based on gender in patients undergoing dentoalveolar surgery. Therefore, the objective of this systematic review and trial sequential analysis was to identify and quantify whether there is a proportionally greater risk of MRONJ in male or female subjects and whether there is evidence of greater involvement of osteonecrosis at several extraction sites, differentiating them into mandibular or maxilla and in the anterior or posterior sector. The revision protocol followed the indications of the Cochrane Handbook, and were recorded in Prospero, while the drafting of the manuscript was based on PRISMA. The results of the systematic review, after the study identification and selection process, included a total of 24 studies. The results of the meta-analysis reports: odds ratio (random effects model): 1.476 (0.684, 3.184) between male and female; odds ratio (random effects model): 1.390 (0.801, 2.412) between mandible and maxillary, and an odds ratio value of 0.730 (0.250, 2.137) between the anterior and posterior extraction sites. In conclusion, we can see that there was a trend in the onset of MRONJ as a complication of dentoalveolar surgical procedures, which proportionally mostly involved the male sex and the posterior mandibular sectors, however, this trend must be further confirmed by additional studies.
Keywords: osteonecrosis, MRONJ, BRONJ, ONJ, antiresorptive drugs
1. Introduction
The bone tissue constantly undergoes a remodeling characterized by resorption and formation, in which a key role is played by two cell types such as osteoclasts and osteoblasts; these two moments are generally in balance in the adult, but with the advance in age, the number and activity of osteoblasts decrease while the action of osteoclasts increases.
Differentiation such as the maturation and activation of osteoclasts is programmed and influenced by osteoblasts through the expression of OPG (osteoprotegerin) and RANKL (receptor activator of nuclear factor kappa-B ligand) [1].
RANKL is expressed on the cytoplasmic surface by binding to its receptor in osteoclastic precursors; it stimulates hematopoietic cells, differentiating them into osteoclasts [2]. On the other hand, OPG is excreted by osteoblasts and leads to the inactivation of RANKL by preventing the differentiation and activation of osteoclasts [3].
This reabsorption mechanism, determined by an upregulation of RANKL, is widely demonstrated in menopausal female subjects suffering from osteoporosis [4]. Furthermore, bone loss in menopausal women is accelerated by the production of pro-inflammatory cytokines TNF-α, IL-6, and IL-1 [5]. The inflammation act plays a clear and evident role in bone resorption as occurs in periodontitis [6,7], peri-implantitis, and in healing tissue following tooth extractions.
In the treatment and prevention of osteoporosis, and more generally in neoplastic and metabolic pathologies involving bone tissues, antiresorptive drugs are used such as bisphosphonates (zoledronate, zoledronate, pamidronate, risedronate, ibandronate) [8] and the human monoclonal antibody: denosumab [9]. In addition, other antineoplastic monoclonal antibodies such as bevacizumab, sunitinib, and temsirolimus have also been associated with the development of osteonecrosis of the jaw (ONJ) [9].
The use of bisphosphonates in oncology is widely consolidated, and in some classes of carcinomas such as non TNBC or non HER2+ breast cancer [10], treatment with this class of drugs leads to a modification of the tumor microenvironment as well as a significant reduction in the mortality and recurrence rates [11].
Bisphophonates have been linked to cases of osteonecrosis of the jaw since 2003 by Marx with more and more evidence in the following two decades [12]; together with bisphosphonate drugs, cases were subsequently added to cases related to the use of monoclonal drugs, thus moving from a definition of BRONJ (bisphosphonate-related osteonecrosis of the jaw) [13] to that of MRONJ (medication-related osteonecrosis of the jaw) [14] and ARONJ (antiresorptive agent-related osteonecrosis of the jaw) [15].
Among the main independent risk factors, we have the extraction procedures in oral surgery [16]; in fact, extractions of the dental elements should be avoided if possible in patients with high dose therapies of antiresorptive agents [17].
The extractions of the dental elements are among the main surgical dental procedures and can affect both the mandible and the maxilla and the anterior sectors (incisors and canines) or the posterior sectors (molars and premolars) [18]. The different extraction sites have anatomical and tissue characteristics with different qualities and densities of the bone structures, which diversify the different extraction techniques [19,20].
The incidence of MRONJ treated with oral bisphosphonates ranges from 0.5% [21] to 3% [22] according to studies: the incidence would seem higher in patients treated with antiresorptive agents with neoplastic pathologies [23] while the incidence of MRONJ is higher in patients treated with antiresorptive agents for malignancy due to the fact that these agents are generally given IV in higher doses and with higher frequency [24].
Many pathologies including those in which antiresorptive drugs are used present differences in prevalence in relation to sex; similarly, there could be differences in the incidence of cases of osteonecrosis according to sex in patients undergoing dentoalveolar surgery [25].
The characteristics of the bone can vary in relation to the different anatomical positions. The bone remodeling processes following extractions undergo significant differences between the posterior and anterior regions, with greater evidence in the posterior sectors where the post-extraction atrophy of the mandible begins and progresses faster [26]. Moreover, at the level of the posterior maxilla, the remodeling and trabecular organization is more random, therefore, post-extraction bone resorption (which is inhibited by bisphosphonate drugs) could occur in a non-equal way in the maxillary bones, consequently, the characteristics of the bone in formation are influenced by the location in which they form [27].
Other evidence on a different bone characteristic comes from studies conducted by Mish [28], where in the posterior and maxillary sectors, there was the presence of bone with a thin porous layer and with fine trabecular bone (D3–D4), while there was denser bone in the anterior and posterior mandibles (D1–D2) [28].
These differences in bone histo-morphological composition are the reflection of a different bone remodeling process that occurs in these areas [29], differences that could be the cause of an altered incidence in the localization of MRONJ cases.
The knowledge of these differences by the oral surgeon who performs the tooth extractions can be clinically relevant in the choice of surgical technique. In fact, in these extraction sites, the execution of extraction techniques that are more respectful of the crestal bone as well as the use of suitable techniques in the preparation of a flap and sutures in the most difficult cases appear to be fundamental in the prevention of ONJ in patients taking drugs related to osteonecrosis.
Previous systematic reviews of the literature did not focus on the localization of MRONJ in relation to the extraction sites and gender. In fact, among the most recent, Schwech in 2020 [30] highlighted the incidence of cases of osteonecrosis in cancer patients; Aboalela et al. analyzed the aspects of the drug holiday in tooth extraction in patients treated with antiresorptive drugs in 2022 [31], and Cabras et al. in 2021 analyzed the possible efficacy of antibiotic therapy in the prevention of MRONJ during dentoalveolar surgery [32,33].
Therefore, the objective of this systematic review was to identify and quantify whether there is a proportionally greater risk of ONJ in male or female subjects, and whether there is evidence of a greater involvement of osteonecrosis in the different extraction sites, separating them into mandibular or maxillary and in the anterior or posterior sector.
2. Materials and Methods
2.1. Protocol and Registration
The systematic review was written following the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines [34]. All of the research, selection, and data extraction procedures followed the indications of the Cochrane Handbook, and the revision protocol was submitted and registered on the PROSPERO Platform with a registration number of CRD42023400788.
2.2. Eligibility Criteria
All prospective and retrospective studies and RCTs reporting data on the number of BRONJ, MRONJ, or ARONJ in patients who underwent dentoalveolar surgery and who made use of bisphosphonates and more generally of antiresorptive agents were considered potentially eligible.
In particular, studies were selected that reported the data on the osteonecrosis that occurred in the maxillary and mandibular surgical sites and in the anterior and posterior sectors, and further attention was paid to the differences in prevalence between the female and male sexes.
The PICO question formulated was therefore the following: whether there are differences in proportion in the onset of osteonecrosis in patients receiving the antiresorptive agent: in the surgical sites (between the mandibular and maxillary sectors, between the anterior and posterior sectors); between the female and male; (P)articipants (patients taking antiresorptive agents and undergoing dentoalveolar surgery); (I)ntervention (presence of osteonecrosis of the jaws in the different extraction sites), (C)ontrol (patients without osteonecrosis); and (O)utcome (odds ratio between the frequency of cases of osteonecrosis in the different surgical sites and between the sexes).
The inclusion criteria were as follows: studies reporting the data on the osteonecrosis experienced in patients using antiresorptive agents undergoing dentoalveolar surgery and reporting the location of the osteonecrosis (mandibular, maxillary, or anterior or posterior) or reporting the number of osteonecrosis between the two sexes.
The exclusion criteria were as follows: studies that did not report data on osteonecrosis cases or that only reported osteonecrosis cases, as a study population, studies published in a language other than English, and those at high risk of bias.
2.3. Sources of Information, Research and Selection
Studies were identified through literature searches of electronic databases by two authors (M.D. and A.B.). Publication language restrictions were enforced and non-English language articles were excluded. The literature search was conducted on the PubMed, Scopus, and Cochrane library databases. The last literature search was conducted on 16 February 2023. In addition, a gray literature search was also conducted by consulting Google Scholar, Science Direct, and Open Gray and the bibliographic sources of previous systematic reviews on the topic were also investigated.
We used the following terms to search the databases: BRONJ, MRONJ, Osteonecrosis, ARONJ, Bisphosphonates.
The following search terms were used on PubMed:
Search: BRONJ OR MRONJ OR Osteonecrosis jaw OR ARONJ OR Bisphosphonates osteonecrosis Sort by: Most Recent
“BRONJ” [All Fields] OR “MRONJ” [All Fields] OR ((“osteonecrosis” [MeSH Terms] OR “osteonecrosis” [All Fields] OR “osteonecroses” [All Fields]) AND (“jaw” [MeSH Terms] OR “jaw” [All Fields])) OR “ARONJ” [All Fields] OR ((“bisphosphonated” [All Fields] OR “bisphosphonic” [All Fields] OR “diphosphonates” [MeSH Terms] OR “diphosphonates” [All Fields] OR “bisphosphonate” [All Fields] OR “bisphosphonates” [All Fields]) AND (“osteonecrosis” [MeSH Terms] OR “osteonecrosis” [All Fields] OR “osteonecroses” [All Fields])).
Translations:
Osteonecrosis: [MeSH Terms] OR “osteonecrosis” [All Fields] OR “osteonecroses” [All Fields];
Jaw: “jaw” [MeSH Terms] OR “jaw” [All Fields];
Bisphosphonates: “bisphosphonated” [All Fields] OR “bisphosphonic” [All Fields] OR “diphosphonates” [MeSH Terms] OR “diphosphonates” [All Fields] OR “bisphosphonate” [All Fields] OR “bisphosphonates” [All Fields];
On the Scopus platform, instead, the following search terms and criteria were used:
TITLE-ABS-KEY (bronj OR mronj OR osteonecrosis AND jaw OR aronj OR bisphosphonates AND osteonecrosis).
Duplicates were removed using EndNote and manually. The identified articles were independently evaluated and reviewed by two reviewers (M.D. and A.B.), the evaluation of potentially eligible articles was carried out considering the title and abstract, while the full text was evaluated for inclusion in the systematic review. In addition, the k agreement between the two reviewers was assessed and a third reviewer resolved any disagreements.
2.4. Data Collection Process and Data Characteristics
The type of data and information to be extracted were previously determined by the two authors responsible for screening the articles and were independently transcribed into tables to be subsequently compared to minimize and reduce the risk of bias.
The data that were extracted from the articles concerned the first author, the year of publication, type of study, the country that conducted the study, the number of patients, the average age, the gender, the primary pathology for which antiresorptive agent was administered, the type of antiresorptive agent taken, the route of administration, the average duration of administration of the drug, the number of extraction sites or extracted teeth, the location of the surgical sites, the number of osteonecrosis and their location, and the distribution of cases between the sexes.
2.5. Risk of Bias within Individual Studies, Summary Measures, Summary of Results, Risk of Bias across Studies, Publication Bias and Additional Measures
The ROBINS-I tool was used to measure the risk of bias, and it was evaluated by the two authors (A.B. and M.D.) appointed to select the studies. The studies with a high risk of bias were excluded from the systematic review and meta-analysis.
The results were extracted and reported in tables while the aggregated data were represented in figures such as the forest plot with the respective numerical values of odds ratio (OdRa) and heterogeneity indices such as the Higgins index (I2).
The risk of bias between studies was assessed visually (funnel plot) by analyzing of the overlaps of the confidence intervals (C.I.), through the index of inconsistency I2 (a value of I2 greater than 30% was considered medium and a random analysis was applied-effects in specific cases), and through a funnel chart. If the meta-analysis presented high indices of heterogeneity, a sensitivity analysis was performed excluding only the studies that presented a low overlap of the C.I. or that emerged graphically from the funnel plot.
For the meta-analysis, and in particular for the calculation of the pooled odds radio, the Reviewer Manager 5.4 software (Cochrane Collaboration, Copenhagen, Denmark), and Open Meta-Analyst version 10 were used. The GRADE pro-Guideline Development Tool online software (GRADE pro-GDT, Evidence Prime) and TSA (trial sequential analyses) using a Java-based software, the TSA software (Copenhagen Trial Unit, Center for Clinical Intervention Research, Copenhagen, Denmark) were also performed.
3. Results
3.1. Selection of Studies
The research question that guided the selection of the studies was as follows: whether there are proportional differences in the onset of osteonecrosis in patients treated with antiresorptive and anti-neoplastic agents at the surgical sites (between the mandibular and maxillary sectors, and/or between the anterior and posterior) and between the females and males.
The research phase was performed by consulting and extracting the bibliographic references on two databases, SCOPUS (7234 records) and PubMed (5036 records), and on a Cochrane Central Trials register (348 trials), providing a number of 12,618 records. The references were uploaded to EndNote X8 and the duplicates removed using software while the duplicates not identified by the software were identified manually and removed, obtaining a number of records equal to 7408.
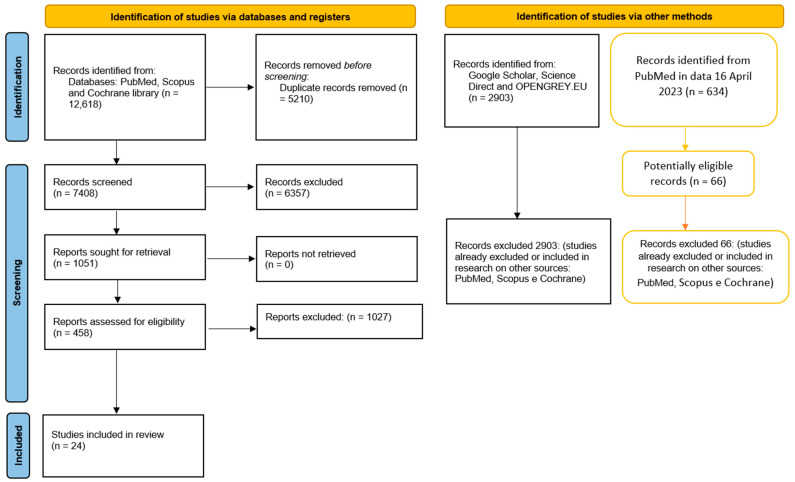
After reading the record’s title and abstract, there reached an equal number of 458 items potentially eligible, and at the end of the selection, the articles included for the qualitative evaluation totaled 24. A further search of the gray literature (Google Scholar, Open Gray, and Science Direct) and previous systematic reviews was conducted that did not allow us to identify further studies to be included in the revision (Figure 1). The records were screened by two authors (M.D. and A.B.), independently, doubtful situations were addressed at the end of the selection involving a third author (FS) to resolve potential conflicts.
Figure 1.
The entire selection and screening procedures are described in the PRISMA flowchart; tables with the orange lines are the searches performed subsequently (on 16 April 2022), with the addition of new keywords on PubMed.
An update of the PubMed keywords was performed on 16 April 2023 with the addition of the following key words:
Search: denosumab AND osteonecrosis: (“denosumab” [MeSH Terms] OR “denosumab” [All Fields] OR “denosumab s” [All Fields]) AND (“osteonecrosis” [MeSH Terms] OR “osteonecrosis” [All Fields] OR “osteonecroses” [All Fields]); Translations: denosumab: “denosumab” [MeSH Terms] OR “denosumab” [All Fields] OR “denosumab’s” [All Fields];
Osteonecrosis: “osteonecrosis” [MeSH Terms] OR “osteonecrosis” [All Fields] OR “osteonecroses” [All Fields].
We obtained a number of records equal to 634, which were screened by the two authors in search of any clinical studies to be included; the results of this selection are highlighted in Figure 1.
3.2. Data Characteristics
The articles included in the review are as follows: Shudo et al. 2018 [35], Jeong et al., 2017 [36], Lain and Ajwani 2016 [37], Hasegawa et al., 2017 [22], Ferlito et al., 2011 [38], Hutcheson et al., 2014 [39], Lazarovici et al., 2010 [40], Lodi et al., 2010 [41], Migliorati et al., 2013 [42], Mozzati et al., 2013 [43], Mozzati et al., 2012 [44], Mozzati et al., 2011 [45], O’Connell et al., 2012 [46], Saia et al., 2010 [47], Scoletta et al., 2013 [48], Scoletta et al., 2011 [49], Vescovi et al., 2013 [50], Ottesen et al., 2021 [51], Kang et al., 2020 [52], Kawakita et al., 2017 [53], Fujieda et al., 2020 [54], Bodem et al., 2015 [55], O’Ryan and Lo 2012 [56], and Kunchur et al., 2009 [21].
The data extracted are reported in three tables. Table 1 represents the data concerning the first author, the country of the study, the type of study, the total number of patients, the average age or the range, the primary disease for which the drug is being administered, the type of drug administered, the route and duration of administration, and the number of extraction sites or extracted teeth.
Table 1.
Characteristics of the studies included in the systematic review; the main characteristics of the groups of patients included in the studies are also reported, F (female), M (male), DS (deviation standard), Y (years), m (month), OR (oral administration), IV (intravenous administration), BF (bisphosphonates), RTC (randomized controlled trial), retrospective study (RS), retrospective multicenter study (RMS), prospective study (PS), case-control study (CCS), case series (CS), observational longitudinal noncontrolled study (OLNS), \ data not present or not reportable, 1 A total of 184 teeth in 102 extraction sites, 2 The number of extraction sites is not specified, ? data reported but not clearly specified in the study.
| First Autor, Data | Country | Study Design | Population (F, M) | Mean Age (y), DS, Range Age (y) | Primary Disease | Type of Administration (OR, IV) | Duration of Administration, Mean DS (m), Range (m) | Extraction\Procedure Site |
|---|---|---|---|---|---|---|---|---|
| Shudo et al., 2018 [35] | Japan | PS | 132 (112, 20) | 71.9 ± 11.4, (40–94) | Primary osteoporosis, prevention osteoporosis | OR: Alendronate (59), Risedronate (37), Minodronate (31), Ibandronato (5). | 40.4 ± 38.0, 1–162 | 274 |
| Jeong et al., 2017 [36] | Korea | PS | 320 (298, 22) | 111 patients < 65 y | Osteoporosis | OR: Alendronate (161) Risedronate (73), Ibandronato (20). | 140 patients < 3 y | 651 |
| Lain and Ajwani, 2016 [37] | Australia | RS | 266 (OR) (208, 58) | 73.3 ± 6.9 | Osteoporosis and cancer | OR: 266 Alendronate (203), Risedronate (55), Etidronate (1) Conbination (3), Unknown (4) IV:9 |
\ | 266 |
| Hasegawa et al., 2017 [22] | Japan | RMS | 1175 (1014, 161) | 70.7 ± 11.7 (23–102) |
Osteoporosis and cancer | OR: Alendronate (695), Risedronate (304), Minodronate (106), Others (8), Alendronate/Risedronate (27), Alendronate/Minodronate (19), Alendronate/Others (1), Risedronate/Minodronate (3), Minodronate/Others (1), Unknown (11). | 38.5 ± 37.7, 1–246 | 2458 |
| Ferlito et al., 2011 [38] | Italy | OLNS | 43 | 56.4 ± 5.8 | Multiple myeloma, breast cancer, prostate cancer, lung cancer | IV: Zolendronato | 16.2 ± 3.2 | 102 |
| Hutcheson et al., 2014 [39] | Australia | PS | 950 (727, 403) | 71 | Osteoporosis | OR: Alendronate (560) Risedronate (373), Other combinations (17). | 199 patients >5 y | 2461 |
| Lazarovici et al., 2010 [40] | Israel | PS | 78 (63, 15) | F 64.2, (20–89); M 62.63, (9–81). |
Osteoporosis, breast carcinoma, multiple myeloma, prostate carcinoma, neurogenic carcinoma | OR: Alendronate (44), Risedronate (3), Zoledronic acid (10), Pamidronate (7). IV: Alendronate and Risendronate (4), Zoledronic acid and Clodronate (2), Pamidronate and Clodronate (1). |
Or: 42–144, IV: 24–61 | 78 |
| Lodi et al., 2010 [41] | Italy | PS | 23 | 68.2, (44–83) | Multiple myeloma, bone metastasis of breast cancer or other solid tumors and severe osteoporosis | IV: Zoledronate (20), Pamidronate (2), Clodronate (1). | 17.5, 3–36 | 38 |
| Migliorati et al., 2013 [42] | Canada, Norway, USA | PS | 53 (43, 10) | 70, (40–92) | Osteoporosis metastatic bone cancer | IV 13\45, OR 32\45 |
60 | 53 |
| Mozzati et al., 2013 [43] | Italy | PS | 700 (677, 23) | (52–79) | Osteoporosis, rheumatoid arthritis, and Paget’s disease. | OR: Alendronate | \ | \ |
| Mozzati et al., 2012 [44] | Italy | CCS | 176 (101, 75) | (44–83) | Prostatic carcinoma, breast carcinoma, multiple myeloma, lung carcinoma, ovarian carcinoma | IV: Zoledronic acid | \ | \ |
| Mozzati et al., 2011 [45] | Italy | CCS | 100 (75, 25) | (44–83) | Prostatic carcinoma, breast carcinoma, multiple myeloma, lung carcinoma, ovarian carcinoma | IV: Zoledronic acid (53), Pamidronate (47) | \ | \ |
| O’Connell et al., 2012 [46] | Ireland | PS | 23 (22, 1) | 59, (44–78) | Osteoporosis | OR: Acid Alendronic (19), Risendronate (2); IV: Zoledronic Acid (2) |
30 (8–72) |
23 ? |
| Saia et al., 2010 [47] | Italy | PS | 60 (42, 18) | 65 ± 13, (17–84) | Cancer | IV: Zoledronate (38), Pamidronate (24), Neridronate (4), OR: Risedronate (2); |
\ | 185 |
| Scoletta et al., 2013 [48] | Italy | PS | 63 (45, 18) | 65.82 ± 8.82 | Cancer and osteoporosis | IV: Zoledronic acid (54), Pamidronate (4), Ibandronate (5) | 19.03 months | 202 |
| Scoletta et al., 2011 [49] | Italy | PS | 64 (44, 20) | 64.81 ± 10.98 | Cancer and osteoporosis | IV: Zoledronic acid (57), Pamidronate (2), Zoledronic acid + Pamidronate (5) | 16.20 | 220 |
| Vescovi et al., 2013 [50] | Italy | CS | 217 (179, 38) | 68.72, (30–83) | Cancer and osteoporosis | Zoledronate (87), Zoledronate + Pamidronate (1), Alendronate 54, Risedronate 18, Alendronate + Zoledronate (3), Clodronate (24), in 30 cases different association of BPs. | 17 (cancer) 53 (osteoporosis) | 589 |
| Ottesen et al., 2021 [51] | Denmark | RTC | 23 (12, 11) | 69 (59–77), 67 (56–78) | Cancer | IV: Denosumab (13), Bisphosphonate (10). | 9 (range 2–30) 17.5 (range 4–96) | 31 |
| Kang et al., 2020 [52] | Korea | RS | 465 (420, 45) | M 3.7 ± 10.5; F 69.3 ± 8.8 | Osteoporosis and cancer | OR: 410 Alendronate IV and OR: 30 Ibandronate and Alendronate. IV: Ibandronate 26 |
OR: 39.0 ± 35.5; IV:(40.0 ± 35.6) |
1323 |
| Kawakita et al., 2017 [53] | Japan | RS | 341 (352, 43) | 72.4 ± 10.6, 74.1 ± 9.62 | Osteoporosis and cancer | OR | 43.4 ± 36.3; 31.3 ± 31.0 | 850 |
| Fujieda et al., 2020 [54] | Japan | RS | 232 (202, 30) | 71 (24–94) | Autoimmune disease | Alendronate (111), Risedronate (80), Minodronate (23), Ibantronate (4), Denousumab (14) | 37 (17–51) | \ |
| Bodem et al., 2015 [55] | Germany | PS | 61 (42, 19) | 65.65 ± 12.69 (34–87) | Cancer | IV: Zoledronic acid (38), Ibandronate (17), and Pamidronate (6). | 40.25 ± 32.91; (4–245) | 102 (184) 1 |
| O’Ryan and Lo, 2012 [56] | USA | RS | 30 (26, 4) | (54–89) | Osteoporosis | \ | \ | \ |
| Kunchur et al., 2009 [21] | Australia | PS | 222 (165, 57) | OR 71 ± 11.6, IV 61 ± 11 | Osteoporosis and cancer | OR: Alendronate (139) Risedronate (76); IV: Pamidronate (6), Zoledronic acid (1) |
OR: 50.5 ± 32 (2–180) IV: 26.9 ± 25 (21–72) | 194 procedure and 21 endodontic therapy 2 |
The type of studies were heterogeneous: there were six retrospective studies and 13 prospective, to which must be added two case controls, one case series, one randomized study, and one non-randomized observational study. The total number of patients included taking antiresorptive drugs was 5817, of which 1106 were male (excluding studies of Ferlito et al. (2011) [38] and Lodi et al. (2010) [41], which did not provide indications on gender).
Table 2 shows the data relating to the number of extraction sites for the dentoalveolar surgery procedures and the number of patients as well as the number of MRONJs for the different extraction sites.
Table 2.
Number of MRONJ events that occurred in the different extractive sites. \ Data not present or not reportable, F (female), M (male), 1 One patient underwent surgery in both jaws. 2 Two patients involved both jaws, 3 Ten sites in eight patients, ? data reported but not clearly specified in the study.
| First Autor, Data | Population (F, M) | Extraction Site Total | Extraction Site Maxillary\MRONJ Site | Extraction Site Mandibular\MRONJ | Extraction Site Anterior\MRONJ | Extraction Site Posterior\MRONJ | MRONJ Total |
|---|---|---|---|---|---|---|---|
| Shudo et al., 2018 [35] | 132 (112, 20) | 274 | 165\0 | 109\0 | 97 | 177 | 0 |
| Jeong et al., 2017 [36] | 320 (298, 22) | 651 | 365\3 | 286\15 | 168\5 | 483\13 | 11 patients, 18 sites |
| Lain and Ajwani 2016 [37] | 266 (208, 58) | 266 | 136\12 | 130\14 | 93\5 | 173\21 | 26 sites |
| Hasegawa et al., 2017 [22] | 1175 (1014, 161) | 2458 | 1240\14 | 1218\27 | 1231\5 | 1591\36 | 41 sites |
| Ferlito et al., 2011 [38] | 43 | 102 | 59\0 | 43\0 | \ | \ | 102 sites |
| Hutcheson et al., 2014 [39] | 950 (727, 403) | 2461 | \ | \ | \ | \ | 4 |
| Lazarovici et al., 2010 [40] | 78 (63, 15) | 78 | 33 1\10 | 44\8 | 20\7 | 57\11 | 18 patients |
| Lodi et al., 2010 [41] | 23 (15, 8) | 38 | 7 2\0 | 25\0 | 4\0 | 33\0 | 0 |
| Migliorati et al., 2013 [42] | 53 (43, 10) | 53 | \ | \ | \ | \ | 1 |
| Mozzati et al., 2013 [43] | 700 (677, 23) | 1480 | 616 | 864 | \ | \ | 0 |
| Mozzati et al., 2012 [44] | 176 (101, 75) | 542 | 255\0 | 287\5 | \ | \ | 5 |
| Mozzati et al., 2011 [45] | 100 | 222 | 108 | 114 | \ | \ | 2 |
| O’Connell et al., 2012 [46] | 23 (22, 1) | 23 ? | \ | \ | \ | \ | 0 |
| Saia et al., 2010 [47] | 60 (42,18) | 185 | 82 | 103 | \ | \ | 5 patients |
| Scoletta et al., 2013 [48] | 63 (45, 18) | 202 | 91\2 | 111\0 | \ | \ | 2 |
| Scoletta et al., 2011 [49] | 64 (44, 20) | 220 | 107\0 | 113\5 | \ | \ | 5 |
| Vescovi et al., 2013 [50] | 217 (179, 38) | 589 | 304\4 | 285\1 | \ | \ | 5 |
| Ottesen et al., 2021 [51] | 23 (12, 11) | 31 | 18 | 13 | \ | \ | 4 |
| Kang et al., 2020 [52] | 465 (420, 45) | 1323 | 740\0 | 583\1 | \ | \ | 1 |
| Kawakita et al., 2017 [53] | 341 (352, 43) | 850 | 203\3 | 199\4 | \ | \ | 7 |
| Fujieda et al., 2020 [54] | 232 (202, 30) | \ | \ | \ | \ | \ | 10 |
| Bodem et al., 2015 [55] | 61 (42, 19) | 102 | 45\5 | 55\5 | \ | \ | 10 (8 3) |
The total number of MRONJ in the included studies involving the male gender was 20 cases, while for the female gender, it was a total of 88 (Table 3).
Table 3.
Number of MRONJ in the male and female sex; \ Data not present or not reportable.
| First Autor, Data | Male (MRONJ) | Male Total | Female (MRONJ) | Female Total |
|---|---|---|---|---|
| Shudo et al., 2018 [35] | 0 | 20 | 0 | 212 |
| Jeong et al., 2017 [36] | 0 | 22 | 11 | 298 |
| Lain and Ajwani, 2016 [37] | 5 | 58 | 21 | 208 |
| Hasegawa et al., 2017 [22] | 5 | 161 | 36 | 1014 |
| Ferlito et al., 2011 [38] | \ | \ | \ | \ |
| Hutcheson et al., 2014 [39] | 1 | 403 | 3 | 727 |
| Lazarovici et al., 2010 [40] | \ | 15 | \ | 63 |
| Lodi et al., 2010 [41] | 0 | \ | 0 | \ |
| Migliorati et al., 2013 [42] | 1 | 10 | 0 | 43 |
| Mozzati et al., 2013 [43] | 0 | 23 | 0 | 677 |
| Mozzati et al., 2012 [44] | \ | 75 | \ | 101 |
| Mozzati et al., 2011 [45] | \ | \ | \ | \ |
| O’Connell et al., 2012 [46] | 0 | 1 | 0 | 22 |
| Saia et al., 2010 [47] | 2 | 18 | 3 | 42 |
| Scoletta et al., 2013 [48] | 0 | 18 | 1 | 45 |
| Scoletta et al., 2011 [49] | 1 | 44 | 4 | 20 |
| Vescovi et al., 2013 [50] | 4 | 38 | 1 | 179 |
| Ottesen et al., 2021 [51] | \ | 11 | \ | 12 |
| Kang et al., 2020 [52] | 0 | 45 | 1 | 420 |
| Kawakita et al., 2017 [53] | 0 | 43 | 7 | 352 |
| Fujieda et al., 2020 [54] | \ | 30 | \ | 202 |
| Bodem et al., 2015 [55] | \ | 19 | \ | 42 |
| Kunchur et al., 2009 [21] | 1 | 57 | 0 | 165 |
| O’Ryan and Lo, 2012 [56] | \ | 4 | \ | 26 |
3.3. Risk of Bias
The risk of bias was rated as acceptable for all studies included in the systematic review, but a more rigorous approach was taken in choosing to include the studies in the meta-analysis as well excluding some studies that reported non-homogeneous data or that, for example, concerned a panel of patients whose population of origin was not clearly defined and stratified, or not comparable with others (O’Ryan and Lo, 2012 [56]).
The studies were evaluated using the following parameters (ROBINS-I): due to confounding factors, bias in selection of participants into the study, bias in the classification of interventions, bias due to deviations from the intended interventions, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of reported outcome; for each parameter, the assessment could be critical risk, severe risk, moderate risk, low risk or unmeasured risk (Table 4).
Table 4.
Risk of bias: low risk +, moderate risk -, serious risk x, critical risk !, unmeasured risk ?.
| First Autor, Data | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | |
|---|---|---|---|---|---|---|---|---|
| Shudo et al., 2018 [35] | + | + | + | + | + | + | + | + |
| Jeong et al., 2017 [36] | - | + | + | + | + | + | + | + |
| Lain and Ajwani, 2016 [37] | + | + | + | + | + | + | + | + |
| Hasegawa et al., 2017 [22] | + | + | + | + | + | + | + | + |
| Ferlito et al., 2011 [38] | + | + | + | + | ? | + | ? | + |
| Hutcheson et al., 2014 [39] | + | + | + | + | ? | + | + | + |
| Lazarovici et al., 2010 [40] | + | + | + | + | + | + | + | + |
| Lodi et al., 2010 [41] | + | + | + | + | + | + | + | + |
| Migliorati et al., 2013 [42] | + | + | ? | + | ? | + | ? | + |
| Mozzati et al., 2013 [43] | + | + | ? | + | ? | + | ? | + |
| Mozzati et al., 2012 [44] | + | + | + | + | ? | + | + | + |
| Mozzati et al., 2011 [45] | + | + | + | + | ? | + | ? | + |
| O’Connell et al., 2012 [46] | + | + | + | + | ? | + | ? | + |
| Saia et al., 2010 [47] | + | + | + | + | ? | + | ? | + |
| Scoletta et al., 2013 [48] | + | + | + | + | ? | + | ? | + |
| Scoletta et al., 2011 [49] | + | + | + | + | ? | + | ? | + |
| Vescovi et al., 2013 [50] | + | + | + | + | ? | + | ? | + |
| Ottesen et al., 2021 [51] | + | + | + | + | + | + | + | + |
| Kang et al., 2020 [52] | + | + | + | + | + | + | + | + |
| Kawakita et al., 2017 [53] | + | + | + | + | ? | + | ? | + |
| Fujieda et al., 2020 [54] | + | + | + | + | ? | + | ? | + |
| Bodem et al., 2015 [55] | + | + | + | + | ? | + | ? | + |
| Kunchur et al., 2009 [21] | + | + | + | + | ? | + | + | + |
| O’Ryan and Lo, 2012 [56] | + | - | + | + | ? | + | + | + |
3.4. Meta-Analysis
The meta-analysis of the data was conducted using the Open Meta-Analyst version 10 (forest plot), and the Reviewer Manager 5.4 software (Cochrane Collaboration, Copenhagen, Denmark) for the construction of the funnel plot.
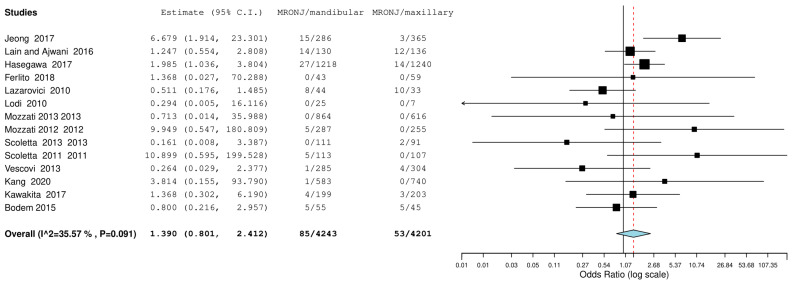
The first meta-analysis conducted concerned the mandibular or maxillary localization of the MRONJ, and random effects were applied according to DerSimonian and Laird by calculating the OdRa (the probability that the MRONJ occurs in the mandibular site compared to the probability that the MRONJ occurs in the maxillary site). The value of the odds ratio turned out to be slightly in favor of a smaller number of probabilistic events in the maxillary area—OdRa: 1.390 C.I. (0.801, 2.412), p value 0.241, however, the central rhombus that gives the size of the effect intercepted the central line of no effect (Figure 2).
Figure 2.
Binary random effects model metric; odds ratio: 1.390; C.I. (Confidence Interval): (lower bound) 0.801 (upper bound) 2.412; p-value 0.241; Q = Q statistic (measure of weighted squared deviations); df = degrees of freedom; I2 (I^2) = Higgins heterogeneity index, I2 < 50%, heterogeneity low; P = p value; heterogeneity (Het.): tau^2: 0.315; Q (df = 13) 20.178, Het. p-value: 0.091, I^2: 35.574; Results (log scale): 0.329 (−0.221, 0.880), Standard error (SE): 0.281; Weights: Jeong: 10.945%, Lain and Ajwani: 16.231%, Hasegawa: 18.572%, Ferlito: 1.814%, Lazarovici: 12.918%, Lodi: 1.760%, Mozzati 2013: 1.829%, Mozzati 2012: 3.154%, Scoletta 2013: 2.887%, Scoletta 2011: 3.140%, Vescovi: 5.024%, Kang: 2.646%, Kawakita: 8.692%, Bodem: 10.390%. Correction factor = 0.5 (applied only to values of 0). The graph of each study shows the first author and the date of publication as well as the measurement of the number of MRONJs on the total and the relative OdRa with the confidence intervals reported. The final value with the relative confidence intervals is expressed in bold. Jeong et al., 2017 [36], Lain and Ajwani, 2016 [37], Hasegawa et al., 2017 [22], Ferlito et al., 2011 [38], Lazarovici et al., 2010 [40], Lodi et al., 2010 [41], Mozzati et al., 2013 [43], Mozzati et al., 2012 [44], Scoletta et al., 2013 [48], Scoletta et al., 2011 [49], Vescovi et al., 2013 [50], Kang et al., 2020 [52], Kawakita et al., 2017 [53], Bodem et al., 2015 [55].
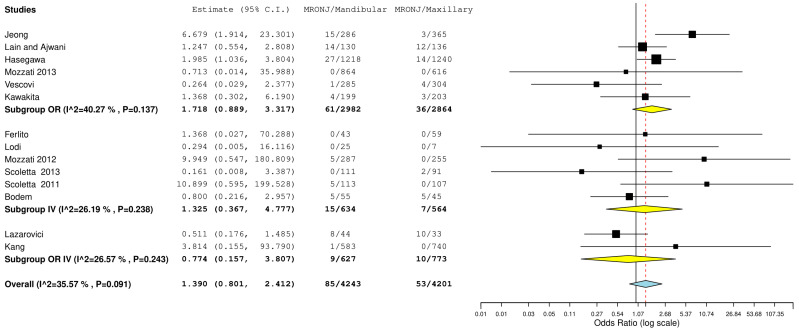
An analysis of subgroups was also conducted according to the route of administration of the antiresorptive drug adopted in the studies, whether intravenous (IV) or oral (OR), or whether they included patients whose therapy could be either IV or OR. The value of the odds ratio aggregated for the subgroups depicted in Figure 3 did not deviate in individual values from the overall odds ratio given by the inclusion of patients from all studies.
Figure 3.
Forest plot analysis subgroup; subgroup OR: 6 studies, OdRa: 1.718 (0.889, 3.317), SE: 0.336, p-Val: 0.107, z-Val: 1.611, Q (df): 8.371 (5), Het. p-Val: 0.137, I^2: 40.27%; Subgroup IV: 6 studies, OdRa: 1.325 (0.367, 4.77), SE: 0.654, p-Val: 0.667, z-Val: 0.430, Q (df): 6.774 (5), Het. p-Val: 0.238, I^2: 26.19%; Subgroup OR IV: 2 studies, OdRa: 0.774 (0.157, 3.807), SE: 0.813, p-Val: 0.752, z-Val: −0.316, Q (df): 1.362 (1), Het. p-Val: 0.243, I^2: 26.57%. Jeong et al., 2017 [36], Lain and Ajwani, 2016 [37], Hasegawa et al., 2017 [22], Ferlito et al., 2011 [38], Lazarovici et al., 2010 [40], Lodi et al., 2010 [41], Mozzati et al., 2013 [43], Mozzati et al., 2012 [44], Scoletta et al., 2013 [48], Scoletta et al., 2011 [49], Vescovi et al., 2013 [50], Kang et al., 2020 [52], Kawakita et al., 2017 [53], Bodem et al., 2015 [55].
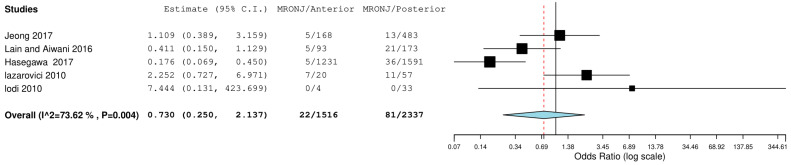
The second meta-analysis was performed including data from studies that reported information on the location of the MRONJ (anterior or posterior); the data in the meta-analysis reported an OdRa value of 0.730 (0.250 2.137), slightly in favor of a minor involvement of the anterior sectors by osteonecrosis. Additionally in this case, as graphically deduced from the forest plot (Figure 4), the central rhombus intercepted the central line of the non-effect.
Figure 4.
Binary random effects model: OdRa: 0.730 (0.250, 2.137), p-value: 0.566, tau^2: 0.996, Q (df = 4): 15.165, Het. p-value: 0.004, I^2: 73.624. Results (log scale) −0.314 (−1.388, 0.760) SE: 0.548. weights: Jeong: 23.420%, Lain and Aiwani: 23.784%, Hasegawa: 24.489%, Lazarovici: 22.588%, Lodi: 5.718%. Jeong et al., 2017 [36], Lain and Ajwani, 2016 [37], Hasegawa et al., 2017 [22], Lazarovici et al., 2010 [40], Lodi et al., 2010 [41].
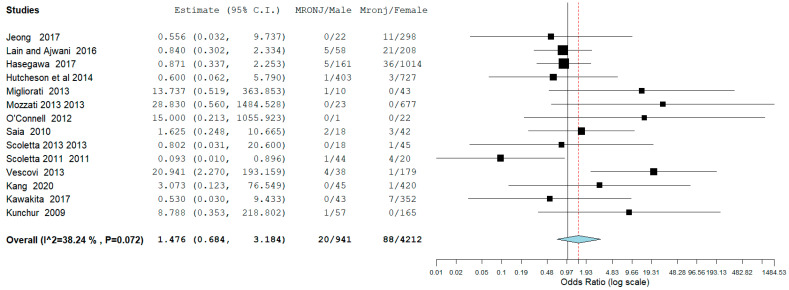
The result of the third meta-analysis concerned the probability (odds ratio) that MRONJ occurs in the male gender compared to the probability that MRONJ occurs in the female gender. The meta-analysis was slightly in favor for females—OdRa: 1.476 (0.684, 3.184), with fewer events in proportion to the male gender (Figure 5).
Figure 5.
Binary random effects model: OdRa: 1.476 (0.684, 3.184), p-value: 0.321, tau^2: 0.692, Q (df = 13): 21.049, Het. p-value: 0.072, I^2: 38.239. Results (log scale) 0.389 (−0.380, 1.158) SE: 0.392; Weights: Jeong: 5.444%, Lain and Ajwani: 15.968%, Hasegawa: 16.599%, Hutcheson et al.: 7.585%, Migliorati: 4.414%, Mozzati 2013: 3.250%, O’Connell: 2.849%, Saia: 9.539%, Scoletta 2013: 4.480%, Scoletta 2011: 7.590%, Vescovi: 7.785%, Kang: 4.549%, Kawakita: 5.398%, Kunchur: 4.550%. Jeong et al., 2017 [36], Lain and Ajwani, 2016 [37], Hasegawa et al., 2017 [22], Hutcheson et al., 2014 [39], Migliorati et al., 2013 [42], Mozzati et al., 2013 [43], O’Connell et al., 2012 [46], Saia et al., 2010 [47], Scoletta et al., 2013 [48], Scoletta et al., 2011 [49], Vescovi et al., 2013 [50] Kang et al., 2020 [52], Kawakita et al., 2017 [53], Kunchur et al., 2009 [21].
3.5. Risk of Bias across Studies: Publication Bias
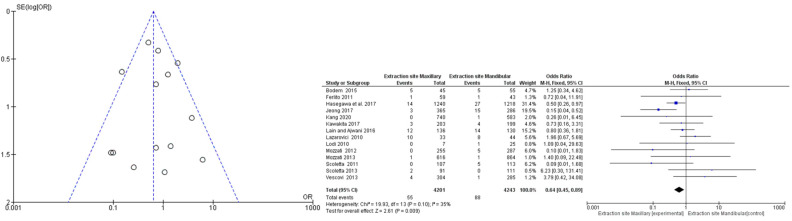
For the first meta-analysis, the publication bias was evaluated through the graphical construction of a funnel plot using the Reviewer Manager 5.4 software program (Cochrane Collaboration, Copenhagen, Denmark), resulting in the distribution of the 14 studies seeming to be homogeneous (Figure 6). Furthermore, the absence of sources of heterogeneity was highlighted, which confirmed a low heterogeneity of the studies as graphically evidenced by the overlapping of the confidence intervals, the heterogeneity values, and by the value of the Higgins index I2: 35 (Figure 6).
Figure 6.
Funnel plot and forest plot (RevManger 5.4): OR, odds ratio; SE, standard error. Graphically, there are no sources of heterogeneity. The odds ratio value mirrors Figure 2, a correction factor d of 1 was applied to studies with mandibular and maxillary MRONJ events equal to 0. Jeong et al., 2017 [36], Lain and Ajwani, 2016 [37], Hasegawa et al., 2017 [22], Ferlito et al., 2011 [38], Lazarovici et al., 2010 [40], Lodi et al., 2010 [41], Mozzati et al., 2013 [43], Mozzati et al., 2012 [44], Scoletta et al., 2013 [48], Scoletta et al., 2011 [49], Vescovi et al., 2013 [50], Kang et al., 2020 [52], Kawakita et al., 2017 [53], Bodem et al., 2015 [55].
3.6. Trial Sequential Analysis: Grade
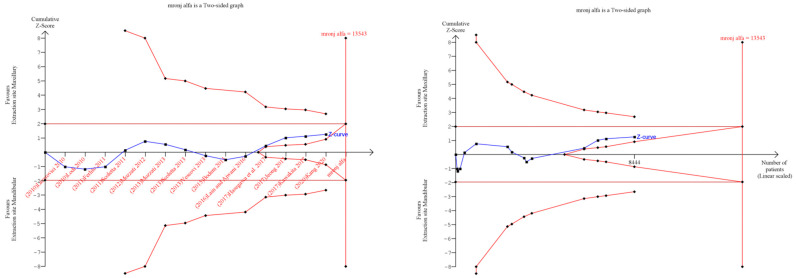
Trial sequential analysis (TSA) was executedto estimate the potency of the result of the first outcome, and by adjusting the results to avoid type II and I errors. The program used was TSA free software. The O’Brien–Fleming spending function was utilized by applying random effects; for the purpose of determining the optimal sample size and for the power of the results, a RRR (relative risk reduction) of 20%, an alpha value of 5% (type 1 error), and a beta value of 80% (type 2 error) were used (Figure 7).
Figure 7.
TSA: Red lines represent the sequential trial monitoring limits and futility limits. The solid blue line is the cumulative Z-curve that requires the information dimension to demonstrate or reject a 20% relative increase in benefit at the maxillary versus mandibular extraction site (5% alpha and 80% beta), whose results included 13,543 patients (vertical red line). The cumulative Z-curve not crossing the Z line (horizontal red line), Z = 1.96, indicates an absence of evidence because the meta-analysis included fewer patients than the required information size, which is a false negative result. Jeong et al., 2017 [36], Lain and Ajwani, 2016 [37], Hasegawa et al., 2017 [22], Ferlito et al., 2011 [38], Lazarovici et al., 2010 [40], Lodi et al., 2010 [41], Mozzati et al., 2013 [43], Mozzati et al., 2012 [44], Scoletta et al., 2013 [48], Scoletta et al., 2011 [49], Vescovi et al., 2013 [50], Kang et al., 2020 [52], Kawakita et al., 2017 [53], Bodem et al., 2015 [55].
The authors also used GRADE pro-GDT to assess the quality of the evidence on the outcome. The results suggest that the quality of evidence is low (Table 5).
Table 5.
Evaluation of GRADE pro GDT: ⊕◯◯◯ Very low, ⊕⊕◯◯ Low, ⊕⊕⊕◯ Moderate, ⊕⊕⊕⊕ High.
| Certainty Assessment | No. of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | MRONJ Maxillary | MRONJ Mandibular | Relative (95% CI) | Absolute (95% CI) | |
| 14 | Observational studies | Not serious | not serious | not serious | not serious | none | 55/4201 (1.4%) | 88/4243 (2.1%) | OR 0.68 (0.48 to 0.95) |
7 fewer per 1.000 (from 11 fewer to 1 fewer) | ⨁⨁◯◯ Low |
4. Discussion
The authors performed a systematic review of the literature, in order to ascertain whether there were differences in the gender and localization of osteonecrosis of the jaws from bone antiresorptive drugs following dentoalveolar surgical procedures. The present systematic review is currently the first review with meta-analysis and trial sequential analyses to assess the power of the findings of the meta-analysis conducted on these specific outcomes. The review work involved 14 articles and the total number of patients included in this study was 5817, of which 1106 were male.
The first report concerning osteonecrosis of the jaw as a complication of tooth extractions in patients taking antiresorptive drugs came from Marx et al. and it was clear that tooth extraction became the main trigger for osteonecrosis [12]. From subsequent studies, it became evident that osteoporotic patients, who were mainly female, were largely involved by MRONJ. Besides being involved with osteonecrosis of the jaw, there are also cancer patients (prostatic carcinoma, breast carcinoma, multiple myeloma, lung carcinoma, ovarian carcinoma, oral cancer) including those of the male sex, and it was strongly advised that the prevention of osteonecrosis should be performed by extracting compromised and no longer recoverable teeth before starting drug therapies [57].
With the emergence of new antiresorptive drugs, it is important to give clear answers on which kinds of patients are at risk of osteonecrosis complications as well as the related surgical extraction sites together with the procedures that may be mainly involved in this complication [58].
The aspects related to the age of MRONJ onset were extensively investigated by a very recent systematic literature review conducted by Rosales et al. (2023) [59] where it was concluded that there was a low presence of MRONJ in the infantile and juvenile population treated with antiresorptive drugs [59,60], while the incidence in patients with cancer receiving high doses of antiresorptive drugs was investigated by Schwech et al. [30].
The aspect of a drug holiday in the onset of osteonecrosis following dentoalveolar surgery was not covered in this meta-analysis as it was performed and extensively discussed by Aboalela et al. (2022) [31] and Ottesen et al. (2020) [61], who stated the absence of high-level evidence for the use of the drug holiday, mainly due to the high heterogeneity of the patients and therapies in the included studies.
From a previous systematic review in 2015 with the meta-analysis of data (comparison between cancer patients and osteoporotic patients) conducted by Gaudin [62], data on the differences between the two sexes and osteonecrosis localization was reported, with eight cases of MRONJ in male patients, and 13 for the females, presenting a number of patients included equal to 2566 (females 2098, males 468), with 13 presenting mandibular, and 23 maxillary localizations. No data were reported on the localization of osteonecrosis at the anterior or posterior extraction sites [62].
The data emerging from this meta-analysis reported a number of MRONJ in patients undergoing dentoalveolar surgery equal to 20 out of 941 for males and 88 out of 4212 for females with an aggregate OdRa (random effects model): 1476 (0.684, 3.184) (Figure 5), slightly in favor for the female sex (smaller number of MRONJ in proportion). For the localization of osteonecrosis, the reported data were 85 MRONJ following 4243 mandibular extraction sites, and 53 MRONJ following of 4202 maxillary extraction sites with the aggregate OdRa (random effects model) of 1.390 (0.801, 2.412) (Figure 2), reporting 22 MRONJ after 1516 anterior sector extractions, and 81 MRONJ after 2337 posterior sector extractions, the aggregate OdRa (random effects model) was 0.730 (0.250, 2.137) (Figure 4), with values slightly in favor for a location in the maxillary and anterior sites (favor: with proportionally fewer cases of MRONJ).
The subgroup analysis differentiating between the routes of administration did not reveal substantial differences between the locations of the MRONJ (Figure 3). Furthermore, the TSA (Figure 7) indicated that assuming an RRR equal to 20% between the maxillary and mandibular sites, the optimal number of patients was not reached.
The biological rationale for which the BRONJ, and more generally the MRONJ, is more probable (in proportion) in male subjects who undergo tooth extractions must be sought in the nature of the primary pathologies [63].
In the males, patients taking drugs related to osteonecrosis, bisphosphonates, denosumab, and antineoplastic drugs (bevacizumab, sunitinib, temsirolimus [64]) were generally subjects with malignancies who took intravenous drugs at high doses, while for the female sex, in the majority of cases, oral antiresorptive drugs were administered for the treatment and prevention of osteoporosis [65].
These two conditions, the tumor and osteoporotic scenarios, are likely to determine the differences in the proportion of the tendency in the onset of osteonecrosis in the two genders in patients undergoing dentoalveolar procedures [66].
The results of our meta-analysis partially agreed with the data of Suryani et al. [66] in cases of osteonecrosis of the jaw in patients taking non-resorptive drugs.
These authors identified a total of 867 patients with MRONJ (33% female, 55% male, 12% unspecified) in the literature, in which the mandibular region developed the greatest number of osteonecrosis (35%), followed by the maxilla (14%) [66].
The more compact nature of the bone (D1, D2), could lead to greater cases of osteonecrosis in the posterior mandibular sectors, which could lead to greater bone trauma during tooth extraction, if techniques that protect the alveolar bone are not adopted. Furthermore, bone remodeling (inhibited by antiresorptive agents) as well as edentulous ridge atrophy occur more quickly in the posterior mandibular sector following extraction.
Among the limitations of the review is the heterogeneity of the included studies, which were non-randomized retrospective; additionally, we confirm that most of the studies presented a small population sample size. Finally, the GRADE evaluation reported a low result, therefore the results of the meta-analysis outlining a trend should be considered with the proper limitation.
5. Conclusions
In conclusion, we can evaluate that within the limits of the systematic review and meta-analysis, there is a trend in the onset of MRONJ as a complication of dentoalveolar surgical procedures, which proportionally mostly involves the male sex and the posterior mandibular sector. However, this trend must be further confirmed by further studies as emerges from the TSA.
Author Contributions
Conceptualization, M.D. and A.B.; Methodology, M.D., G.A.C. and M.A.; Software, M.D.; Data analysis, M.D. and F.S.; Visualization, M.D., R.A. and D.G.; Bibliographic research, F.S. and A.B.; Supervision and project administration, L.L.M., V.C. and A.B.; Writing review and editing, M.D. and A.B.; Critical revision of the manuscript for important intellectual content, M.D. and A.B.; Accuracy or integrity of any part of the work appropriately investigated and resolved: All authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Udagawa N., Koide M., Nakamura M., Nakamichi Y., Yamashita T., Uehara S., Kobayashi Y., Furuya Y., Yasuda H., Fukuda C., et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021;39:19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 2.Troiano G., Laino L., Dioguardi M., Giannatempo G., Lo Muzio L., Lo Russo L. Mandibular Class II Furcation Defect Treatment: Effects of the Addition of Platelet Concentrates to Open Flap: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Periodontol. 2016;87:1030–1038. doi: 10.1902/jop.2016.160058. [DOI] [PubMed] [Google Scholar]
- 3.Tao H., Li W., Zhang W., Yang C., Zhang C., Liang X., Yin J., Bai J., Ge G., Zhang H., et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol. Res. 2021;174:105967. doi: 10.1016/j.phrs.2021.105967. [DOI] [PubMed] [Google Scholar]
- 4.Gambacciani M., Levancini M. Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Med. 2014;56:115–131. [PubMed] [Google Scholar]
- 5.Zhurakivska K., Troiano G., Caponio V.C.A., Dioguardi M., Laino L., Maffione A.B., Lo Muzio L. Do Changes in Oral Microbiota Correlate With Plasma Nitrite Response? A Systematic Review. Front Physiol. 2019;10:1029. doi: 10.3389/fphys.2019.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kany S., Vollrath J.T., Relja B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dioguardi M., Alovisi M., Crincoli V., Aiuto R., Malagnino G., Quarta C., Laneve E., Sovereto D., Lo Russo L., Troiano G., et al. Prevalence of the Genus Propionibacterium in Primary and Persistent Endodontic Lesions: A Systematic Review. J. Clin. Med. 2020;9:739. doi: 10.3390/jcm9030739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papapetrou P.D. Bisphosphonate-associated adverse events. Hormones. 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q.Z., Liu J.Y., Pan J. Progress on medication-related osteonecrosis of the jaw. Hua Xi Kou Qiang Yi Xue Za Zhi. 2018;36:568–572. doi: 10.7518/hxkq.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grassini D., Cascardi E., Sarotto I., Annaratone L., Sapino A., Berrino E., Marchiò C. Unusual Patterns of HER2 Expression in Breast Cancer: Insights and Perspectives. Pathobiology. 2022;89:278–296. doi: 10.1159/000524227. [DOI] [PubMed] [Google Scholar]
- 11.Annaratone L., Cascardi E., Vissio E., Sarotto I., Chmielik E., Sapino A., Berrino E., Marchiò C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology. 2020;87:125–142. doi: 10.1159/000507055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marx R.E., Sawatari Y., Fortin M., Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Colella G., Campisi G., Fusco V. American Association of Oral and Maxillofacial Surgeons position paper: Bisphosphonate-Related Osteonecrosis of the Jaws-2009 update: The need to refine the BRONJ definition. J. Oral Maxillofac. Surg. 2009;67:2698–2699. doi: 10.1016/j.joms.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 14.Di Fede O., Canepa F., Panzarella V., Mauceri R., Del Gaizo C., Bedogni A., Fusco V., Tozzo P., Pizzo G., Campisi G., et al. The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols. Int. J. Environ. Res. Public Health. 2021;18:8432. doi: 10.3390/ijerph18168432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi A., Shiraki M., Morrison A., Khan A.A. Antiresorptive agent-related osteonecrosis of the jaw in osteoporosis patients from Asian countries. Osteoporos Sarcopenia. 2017;3:64–74. doi: 10.1016/j.afos.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laino L., Troiano G., Giannatempo G., Graziani U., Ciavarella D., Dioguardi M., Lo Muzio L., Lauritano F., Cicciù M. Sinus Lift Augmentation by Using Calcium Sulphate. A Retrospective 12 Months Radiographic Evaluation over 25 Treated Italian Patients. Open Dent. J. 2015;9:414–419. doi: 10.2174/1874210601509010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith S.J., AlQranei M., Alagl A.S., Almas K. Tooth Extraction Protocols for Patients on Bisphosphonate Therapy: An Update. J. Int. Acad. Periodontol. 2017;20:38–47. [PubMed] [Google Scholar]
- 18.Troiano G., Dioguardi M., Cocco A., Giuliani M., Fabiani C., D’Alessandro A., Ciavarella D., Lo Muzio L. Centering Ability of ProTaper Next and WaveOne Classic in J-Shape Simulated Root Canals. ScientificWorldJournal. 2016;2016:1606013. doi: 10.1155/2016/1606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Weijden F., Dell’Acqua F., Slot D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009;36:1048–1058. doi: 10.1111/j.1600-051X.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 20.Dioguardi M., Quarta C., Sovereto D., Troiano G., Melillo M., Di Cosola M., Cazzolla A.P., Laino L., Lo Muzio L. Autotransplantation of the Third Molar: A Therapeutic Alternative to the Rehabilitation of a Missing Tooth: A Scoping Review. Bioengineering. 2021;8:120. doi: 10.3390/bioengineering8090120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunchur R., Need A., Hughes T., Goss A. Clinical Investigation of C-Terminal Cross-Linking Telopeptide Test in Prevention and Management of Bisphosphonate-Associated Osteonecrosis of the Jaws. J. Oral Maxillofac. Surg. 2009;67:1167–1173. doi: 10.1016/j.joms.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa T., Kawakita A., Ueda N., Funahara R., Tachibana A., Kobayashi M., Kondou E., Takeda D., Kojima Y., Sato S., et al. A multicenter retrospective study of the risk factors associated with medication-related osteonecrosis of the jaw after tooth extraction in patients receiving oral bisphosphonate therapy: Can primary wound closure and a drug holiday really prevent MRONJ? Osteoporos Int. 2017;28:2465–2473. doi: 10.1007/s00198-017-4063-7. [DOI] [PubMed] [Google Scholar]
- 23.Anastasilakis A.D., Pepe J., Napoli N., Palermo A., Magopoulos C., Khan A.A., Zillikens M.C., Body J.J. Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS. J. Clin. Endocrinol. Metab. 2022;107:1441–1460. doi: 10.1210/clinem/dgab888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel V., Kelleher M., Sproat C., Kwok J., McGurk M. New cancer therapies and jaw necrosis. Br. Dent. J. 2015;219:203–207. doi: 10.1038/sj.bdj.2015.680. [DOI] [PubMed] [Google Scholar]
- 25.Aspray T.J., Hill T.R. Osteoporosis and the Ageing Skeleton. Subcell Biochem. 2019;91:453–476. doi: 10.1007/978-981-13-3681-2_16. [DOI] [PubMed] [Google Scholar]
- 26.Ulm C., Tepper G., Blahout R., Rausch-Fan X., Hienz S., Matejka M. Characteristic features of trabecular bone in edentulous mandibles. Clin. Oral Implants Res. 2009;20:594–600. doi: 10.1111/j.1600-0501.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindhe J., Cecchinato D., Bressan E.A., Toia M., Araújo M.G., Liljenberg B. The alveolar process of the edentulous maxilla in periodontitis and non-periodontitis subjects. Clin. Oral Implants Res. 2012;23:5–11. doi: 10.1111/j.1600-0501.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 28.Misch C.E. Bone classification, training keys to implant success. Dent Today. 1989;8:39–44. [PubMed] [Google Scholar]
- 29.Monje A., Chan H.L., Galindo-Moreno P., Elnayef B., Suarez-Lopez del Amo F., Wang F., Wang H.L. Alveolar Bone Architecture: A Systematic Review and Meta-Analysis. J. Periodontol. 2015;86:1231–1248. doi: 10.1902/jop.2015.150263. [DOI] [PubMed] [Google Scholar]
- 30.Schwech N., Nilsson J., Gabre P. Incidence and risk factors for medication-related osteonecrosis after tooth extraction in cancer patients—A systematic review. Clin. Exp. Dent. Res. 2023;9:55–65. doi: 10.1002/cre2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aboalela A.A., Farook F.F., Alqahtani A.S., Almousa M.A., Alanazi R.T., Almohammadi D.S. The Effect of Antiresorptive Drug Holidays on Medication-Related Osteonecrosis of the Jaw: A Systematic Review and Meta-Analysis. Cureus. 2022;14:e30485. doi: 10.7759/cureus.30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabras M., Gambino A., Broccoletti R., Sciascia S., Arduino P.G. Lack of evidence in reducing risk of MRONJ after teeth extractions with systemic antibiotics. J. Oral Sci. 2021;63:217–226. doi: 10.2334/josnusd.21-0016. [DOI] [PubMed] [Google Scholar]
- 33.Troiano G., Dioguardi M., Cocco A., Laino L., Cervino G., Cicciu M., Ciavarella D., Lo Muzio L. Conservative vs. Radical Approach for the Treatment of Solid/Multicystic Ameloblastoma: A Systematic Review and Meta-analysis of the Last Decade. Oral Health Prev. Dent. 2017;15:421–426. doi: 10.3290/j.ohpd.a38732. [DOI] [PubMed] [Google Scholar]
- 34.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shudo A., Kishimoto H., Takaoka K., Noguchi K. Long-term oral bisphosphonates delay healing after tooth extraction: A single institutional prospective study. Osteoporos. Int. 2018;29:2315–2321. doi: 10.1007/s00198-018-4621-7. [DOI] [PubMed] [Google Scholar]
- 36.Jeong H.G., Hwang J.J., Lee J.H., Kim Y.H., Na J.Y., Han S.S. Risk factors of osteonecrosis of the jaw after tooth extraction in osteoporotic patients on oral bisphosphonates. Imaging Sci. Dent. 2017;47:45–50. doi: 10.5624/isd.2017.47.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lain R., Ajwani S. Minor post-extraction complications other than BRONJ in older patients on oral bisphosphonates—A retrospective study. Gerodontology. 2017;34:171–179. doi: 10.1111/ger.12239. [DOI] [PubMed] [Google Scholar]
- 38.Ferlito S., Puzzo S., Liardo C. Preventive protocol for tooth extractions in patients treated with zoledronate: A case series. J. Oral Maxillofac. Surg. 2011;69:e1–e4. doi: 10.1016/j.joms.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 39.Hutcheson A., Cheng A., Kunchar R., Stein B., Sambrook P., Goss A. A C-terminal crosslinking telopeptide test-based protocol for patients on oral bisphosphonates requiring extraction: A prospective single-center controlled study. J. Oral Maxillofac. Surg. 2014;72:1456–1462. doi: 10.1016/j.joms.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 40.Lazarovici T.S., Mesilaty-Gross S., Vered I., Pariente C., Kanety H., Givol N., Yahalom R., Taicher S., Yarom N. Serologic bone markers for predicting development of osteonecrosis of the jaw in patients receiving bisphosphonates. J. Oral Maxillofac. Surg. 2010;68:2241–2247. doi: 10.1016/j.joms.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 41.Lodi G., Sardella A., Salis A., Demarosi F., Tarozzi M., Carrassi A. Tooth extraction in patients taking intravenous bisphosphonates: A preventive protocol and case series. J. Oral Maxillofac. Surg. 2010;68:107–110. doi: 10.1016/j.joms.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 42.Migliorati C.A., Saunders D., Conlon M.S., Ingstad H.K., Vaagen P., Palazzolo M.J., Herlofson B.B. Assessing the association between bisphosphonate exposure and delayed mucosal healing after tooth extraction. J. Am. Dent. Assoc. 2013;144:406–414. doi: 10.14219/jada.archive.2013.0134. [DOI] [PubMed] [Google Scholar]
- 43.Mozzati M., Arata V., Gallesio G. Tooth extraction in osteoporotic patients taking oral bisphosphonates. Osteoporos. Int. 2013;24:1707–1712. doi: 10.1007/s00198-012-2239-8. [DOI] [PubMed] [Google Scholar]
- 44.Mozzati M., Arata V., Gallesio G. Tooth extraction in patients on zoledronic acid therapy. Oral Oncol. 2012;48:817–821. doi: 10.1016/j.oraloncology.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Mozzati M., Arata V., Gallesio G., Carossa S. A dental extraction protocol with plasma rich in growth factors (PRGF) in patients on intravenous bisphosphonate therapy: A case-control study. Joint Bone Spine. 2011;78:648–649. doi: 10.1016/j.jbspin.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 46.O’Connell J.E., Ikeagwani O., Kearns G.J. A role for C-terminal cross-linking telopeptide (CTX) level to predict the development of bisphosphonate-related osteonecrosis of the jaws (BRONJ) following oral surgery? Ir. J. Med. Sci. 2012;181:237–242. doi: 10.1007/s11845-011-0790-5. [DOI] [PubMed] [Google Scholar]
- 47.Saia G., Blandamura S., Bettini G., Tronchet A., Totola A., Bedogni G., Ferronato G., Nocini P.F., Bedogni A. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J. Oral Maxillofac. Surg. 2010;68:797–804. doi: 10.1016/j.joms.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Scoletta M., Arata V., Arduino P.G., Lerda E., Chiecchio A., Gallesio G., Scully C., Mozzati M. Tooth extractions in intravenous bisphosphonate-treated patients: A refined protocol. J. Oral Maxillofac. Surg. 2013;71:994–999. doi: 10.1016/j.joms.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Scoletta M., Arduino P.G., Pol R., Arata V., Silvestri S., Chiecchio A., Mozzati M. Initial experience on the outcome of teeth extractions in intravenous bisphosphonate-treated patients: A cautionary report. J. Oral Maxillofac. Surg. 2011;69:456–462. doi: 10.1016/j.joms.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Vescovi P., Meleti M., Merigo E., Manfredi M., Fornaini C., Guidotti R., Nammour S. Case series of 589 tooth extractions in patients under bisphosphonates therapy. Proposal of a clinical protocol supported by Nd:YAG low-level laser therapy. Med. Oral Patol. Oral Cir. Bucal. 2013;18:e680–e685. doi: 10.4317/medoral.18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ottesen C., Schiodt M., Jensen S.S., Kofod T., Gotfredsen K. Tooth extractions in patients with cancer receiving high-dose antiresorptive medication: A randomized clinical feasibility trial of drug holiday versus drug continuation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022;133:165–173. doi: 10.1016/j.oooo.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Kang S.H., Park S.J., Kim M.K. The effect of bisphosphonate discontinuation on the incidence of postoperative medication-related osteonecrosis of the jaw after tooth extraction. J. Korean Assoc. Oral Maxillofac. Surg. 2020;46:78–83. doi: 10.5125/jkaoms.2020.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakita A., Yanamoto S., Morishita K., Naruse T., Hayashida S., Soutome S., Rokutanda S., Inokuchi S., Matsuo T., Umeda M. Discontinuing oral bisphosphonate therapy during dental extraction does not prevent osteonecrosis of the jaw: A multicenter retrospective study of 341 patients with propensity score matching analysis. J. Oral Maxillofac. Surg. Med. Pathol. 2017;29:522–526. doi: 10.1016/j.ajoms.2017.07.008. [DOI] [Google Scholar]
- 54.Fujieda Y., Doi M., Asaka T., Ota M., Hisada R., Ohnishi N., Kono M., Kameda H., Nakazawa D., Kato M., et al. Incidence and risk of antiresorptive agent-related osteonecrosis of the jaw (ARONJ) after tooth extraction in patients with autoimmune disease. J. Bone Miner. Metab. 2020;38:581–588. doi: 10.1007/s00774-020-01089-y. [DOI] [PubMed] [Google Scholar]
- 55.Bodem J.P., Kargus S., Eckstein S., Saure D., Engel M., Hoffmann J., Freudlsperger C. Incidence of bisphosphonate-related osteonecrosis of the jaw in high-risk patients undergoing surgical tooth extraction. J. Cranio-Maxillofac. Surg. 2015;43:510–514. doi: 10.1016/j.jcms.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 56.O’Ryan F.S., Lo J.C. Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: Clinical course and outcomes. J. Oral Maxillofac. Surg. 2012;70:1844–1853. doi: 10.1016/j.joms.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 57.Troiano G., Perrone D., Dioguardi M., Buonavoglia A., Ardito F., Lo Muzio L. In vitro evaluation of the cytotoxic activity of three epoxy resin-based endodontic sealers. Dent. Mater. J. 2018;37:374–378. doi: 10.4012/dmj.2017-148. [DOI] [PubMed] [Google Scholar]
- 58.Laino L., Troiano G., Dioguardi M., Perillo L., Laino G., Lo Muzio L., Cicciù M. Patient Discomfort During and After Surgically Assisted Rapid Maxillary Expansion Under Local Anaesthesia. J. Craniofac. Surg. 2016;27:772–775. doi: 10.1097/SCS.0000000000002535. [DOI] [PubMed] [Google Scholar]
- 59.Rosales H.D., Garcia Guevara H., Requejo S., Jensen M.D., Acero J., Olate S. Medication-Related Osteonecrosis of the Jaws (MRONJ) in Children and Young Patients-A Systematic Review. J. Clin. Med. 2023;12:1416. doi: 10.3390/jcm12041416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dioguardi M., Gioia G.D., Caloro G.A., Capocasale G., Zhurakivska K., Troiano G., Russo L.L., Muzio L.L. The Association between Tooth Loss and Alzheimer’s Disease: A Systematic Review with Meta-Analysis of Case Control Studies. Dent. J. 2019;7:49. doi: 10.3390/dj7020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ottesen C., Schiodt M., Gotfredsen K. Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ): A systematic review. Heliyon. 2020;6:e03795. doi: 10.1016/j.heliyon.2020.e03795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaudin E., Seidel L., Bacevic M., Rompen E., Lambert F. Occurrence and risk indicators of medication-related osteonecrosis of the jaw after dental extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2015;42:922–932. doi: 10.1111/jcpe.12455. [DOI] [PubMed] [Google Scholar]
- 63.Srivastava A., Nogueras Gonzalez G.M., Geng Y., Won A.M., Myers J., Li Y., Chambers M.S. Medication-Related Osteonecrosis of the Jaw in Patients Treated Concurrently with Antiresorptive and Antiangiogenic Agents: Systematic Review and Meta-Analysis. J. Immunother. Precis. Oncol. 2021;4:196–207. doi: 10.36401/JIPO-21-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fusco V., Santini D., Armento G., Tonini G., Campisi G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert. Opin. Drug. Saf. 2016;15:925–935. doi: 10.1080/14740338.2016.1177021. [DOI] [PubMed] [Google Scholar]
- 65.Yong E.L., Logan S. Menopausal osteoporosis: Screening, prevention and treatment. Singapore Med. J. 2021;62:159–166. doi: 10.11622/smedj.2021036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suryani I.R., Ahmadzai I., Shujaat S., Ma H., Jacobs R. Non-antiresorptive drugs associated with the development of medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. Clin. Oral Investig. 2022;26:2269–2279. doi: 10.1007/s00784-021-04331-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.