Abstract
Purpose:
Angiotensin-(1–12) [Ang-(1–12)] serves as a primary substrate to generate angiotensin II (Ang II) by angiotensin-converting enzyme and/or chymase suggests it may be an unrecognized source of Ang II-mediated microvascular complication in hypertension-mediated retinopathy. We investigated Ang-(1–12) expression and internalization in adult retinal pigment epithelial-19 (ARPE-19) cultured cells. We performed the internalization of Ang-(1–12) in ARPE-19 cells in the presence of a highly specific monoclonal antibody (mAb) developed against the C-terminal end of the Ang-(1–12) sequence.
Methods:
All experiments were performed in confluent ARPE-19 cells (passage 28–35). We employed high-performance liquid chromatography to purify radiolabeled, 125I-Ang-(1–12) and immuno-neutralization with Ang-(1–12) mAb to demonstrate Ang-(1–12)'s internalization in ARPE-19 cells. Internalization was also demonstrated by immunofluorescence (IF) method.
Results:
These procedures revealed internalization of an intact 125I-Ang-(1–12) in ARPE-19 cells. A significant reduction (∼53%, P < 0.0001) in 125I-Ang-(1–12) internalization was detected in APRE-19 cells in the presence of the mAb. IF staining experiments further confirms internalization of Ang-(1–12) into the cells from the extracellular culture medium. No endogenous expression was detected in the ARPE-19 cells. An increased intensity of IF staining was detected in cells exposed to 1.0 μM Ang-(1–12) compared with 0.1 μM. Furthermore, we found hydrolysis of Ang-(1–12) into Ang II by ARPE-19 cells' plasma membranes.
Conclusions:
Intact Ang-(1–12) peptide is internalized from the extracellular spaces in ARPE-19 cells and metabolized into Ang II. The finding that a selective mAb blocks cellular internalization of Ang-(1–12) suggests alternate therapeutic approaches to prevent/reduce the RPE cells Ang II burden.
Keywords: retinal pigment epithelial cells, angiotensin-(1–12), angiotensin II, internalization, peptide metabolism, monoclonal antibody therapy
Introduction
The discovery of angiotensin-(1–12) [Ang-(1–12)] as a new member of the renin–angiotensin system (RAS) led us to explore the pathological role of this upstream angiotensin (Ang) II precursor in retinal vascular disorder associated with primary hypertension. Ang-(1–12) was first detected in a strain of Japanese Wistar rats' blood and various tissues.1 Since then, our laboratory is actively engaged in characterizing the role of this novel member of the RAS family in hypertension and vascular disease.
In circulation, primarily angiotensin-converting enzyme (ACE) sequentially cleaves the 2 amino acids from C-terminal end of the Ang-(1–12) peptide to release first Ang I and then Ang II; in cardiac tissue chymase mainly cleaves Ang-(1–12) directly into Ang II.2–8 Recently, we showed Ang-(1–12) as a circulating RAS component in normal and hypertensive subjects.9 In another study we found a significant increase of Ang-(1–12) levels in plasma of hypertensive patients with systolic blood pressure (SBP) ≥140 mm Hg compared with the group with SBP <140 mm Hg. Furthermore, our analysis shows that compared with Ang I levels reported in normal subjects, Ang-(1–12) levels in plasma and urine were severalfold higher in hypertensive patients.10
A robust literature shows that RAS is a key player in the regulation of various ocular disorders, including retinal vascular damage. However, the influence of systemic RAS at cellular and molecular levels in retinal pathology is not yet fully understood. The classical RAS including the biologically active Ang peptides, Ang II-forming enzymes (chymase and ACE), and receptors are expressed locally in the human eyes (retina, vitreous body, and aqueous humor).11–15 Ang II is considered a main instigator of an inflammatory cascade causing retinal neurodegeneration through AT1R.16 Significantly high levels of Ang II have been detected in the vitreous fluids of patients with proliferative diabetic retinopathy (DR) and diabetic macular edema.17,18 Circulating and cellular Ang II plays an important role in the development of vascular diseases in the eye.11,19–21
The human retinal pigment epithelium (RPE) is responsible for the development and maintenance of the photoreceptors in retinas. Adult retinal pigment epithelial-19 (ARPE-19) is a spontaneously immortalized cell line of the human RPE derived from the normal eyes of a 19-year-old male donor who died in a vehicle accident.22 Because of structural and functional similarities with in vivo RPE cells, the ARPE-19 cell line is widely used for in vitro eye research. This cell line holds epithelial characteristics after in vitro culturing for an extended period, making it beneficial for RPE physiological studies.22–24 For the first time, we have utilized the ARPE-19 cells to investigate the Ang-(1–12) endogenous expression, cellular internalization, and hydrolysis. Furthermore, we investigated Ang-(1–12) uptake in the presence of a highly specific monoclonal antibody (mAb) developed by us toward the C-terminal end of the Ang-(1–12).
Methods
ARPE-19 cells (passage 27) were kindly provided by Dr. Goldis Malek lab (Duke University Medical Center, Durham, NC) and all experiments reported in this article were performed from passages 28 to 35. Ang peptides (purity ≥98.5%) were custom synthesized by GenScript (Piscataway, NJ). Radiolabeled iodine-125 (125I) was obtained from PerkinElmer (Waltham, MA). The remaining analytical grade chemicals were purchased from Sigma (St. Louis, MO) and Fisher Scientific (Atlanta, GA). The hybridoma clones producing the mAb directed toward the C-terminus of the human Ang-(1–12) were developed by us in collaboration with GenScript USA, Inc. The hybridoma clones were maintained, purified, and characterized by us, as described previously.25 Protein L column purified Ang-(1–12) mAb IgG from hybridoma clone# 14B3 is highly specific to detect the C-terminus of the human Ang-(1–12) amino acids and does not cross-react with the angiotensinogen protein and any other closely related Ang peptides.25
Radiolabeling of Ang peptides and high-performance liquid chromatography purification
Ang peptides [human Ang-(1–12), Ang I, and Ang II] radiolabeling with 125I and high-performance liquid chromatography (HPLC) purifications were done by us as described elsewhere.10 The purified 125I-Ang-(1–12) trace (purity ≥99%) was free of unlabeled Ang-(1–12) and 125I.10 The purity of 125I-Ang-(1–12) peptide was routinely monitored on the HPLC before each experiment at the time it was used for internalization, neutralization, and metabolism studies.
Radiolabeled Ang-(1–12) internalization and hydrolysis by ARPE-19 cells
In this study, we used ARPE-19 cells (a primary cell line, passage 28) grown in 10% fetal bovine serum (FBS) supplemented Dulbecco's modified Eagle medium/nutrient mixture F-12 (DMEM/F12) medium at 37°C in a humidified incubator with an atmosphere of 5% CO2. We replaced the culture media with fresh 10% FBS supplemented DMEM/F12 medium every 2–3 days. All experiments were performed between passages 28–35.
For internalization experiments, we grew the ARPE-19 cells (seeding density 1 × 105 cells/3.5 cm2) in 12-well plates in 10% FBS supplemented DMEM/F12. Internalization of the radiolabeled 125I-Ang-(1–12) peptide was performed when cells reached confluence as described by us.26 On the day of internalization study, the 12-well plates were washed 3 times with serum-free media and 1.0 mL of FBS-free media containing RAS and peptidase inhibitors cocktail was added as described in Table 1. After 10 min preincubation in the inhibitors cocktail media, the radiolabeled 125I-Ang-(1–12) peptide (1 nM) alone or with human Ang-(1–12) mAb (3.0 mg/mL) were added in each well and placed at 37°C for 4 h in a humidified incubator with an atmosphere of 5% CO2.
Table 1.
The Renin–Angiotensin System and Peptidase Inhibitor Cocktail Used to Stop the Angiotensin-(1–12) Hydrolysis
| Enzyme inhibitor cocktail (final concentration) | Inhibits, enzyme |
|---|---|
| RAS inhibitors | |
| Lisinopril (50 μM) | ACE |
| Chymostatin (50 μM) | Chymase |
| SCH39370 (50 μM) | Neprilysin |
| MLN-4760 (50 μM) | ACE2 |
| Peptidase inhibitors | |
| Amastatin hydrochloride (10 μM) | Leucine aminopeptidase |
| Bestatin hydrochloride (50 μM) | Aminopeptidase enzyme |
| Benzylsuccinic acid (50 μM) | Carboxypeptidase A |
| 4-Chloromercuribenzoic acid (250 μM) | Cysteine proteases |
The inhibitor cocktail contains all these RAS and peptidase inhibitors. This inhibitor cocktail completely stops the Ang-(1–12) peptide hydrolysis and is reported by us previously.3,26 To demonstrate the ACE and chymase-mediated hydrolytic products formation from the substrate Ang-(1–12), the ACE and chymase inhibitors (lisinopril and chymostatin) were removed from the reaction mixture.
ACE, angiotensin-converting enzyme; Ang, angiotensin; RAS, renin–angiotensin system.
The cultured media were collected after 4 h from each well and stored at −80°C for HPLC analysis. Finally, each well was washed with 3 mL of ice-cold phosphate-buffered saline (PBS) 3 times and 1 time with 3 mL of ice-cold glycine-HCl (0.05 M, pH 4.0) to remove the free and outer membrane-bound radiolabeled tracers. The thoroughly washed cells were lysed with 1 N NaOH (1.0 mL) overnight on a plate shaker and counted (gamma counter) to detect the internalized radiolabeled 125I-Ang-(1–12) contents. Internalization of 125I-Ang-(1–12) by ARPE-19 cells were expressed as counts per min per mg protein.
HPLC analysis was also performed to demonstrate that the intact radiolabeled 125I-Ang-(1–12) peptide was internalized in ARPE-19 cells. For this, the cells (seeding density 1 × 106 cells/60 cm2) were grown in 100 mm culture dishes. The ARPE-19 cells were incubated with radiolabeled 125I-Ang-(1–12) with or without mAb for 4 h at 37°C in a humidified incubator with an atmosphere of 5% CO2. After aspirating the medium, dishes were washed with 5 mL of ice-cold PBS 3 times and 1 time with 5 mL of 0.05 M ice-cold glycine-HCl (pH 4.0) to remove the free and membrane-bound radiolabeled tracers.
The thoroughly washed cells were harvested in hypotonic buffer (10 mM NaCl, 25 mM HEPES, and 5 mM EDTA, pH 7.4). The harvested cells were lysed using a tight-fitting glass mortar and pestle Dounce homogenizer with 7–10 strokes. The lysed cells were centrifuged (28,000 g) for 20 min to remove the cell membranes and debris. The pellet [native plasma membranes (nPMs)] and cytosolic cell fractions were counted on a gamma counter. The clear supernatants (pooled cytosolic fractions) were analyzed by HPLC described as follows.
HPLC of ARPE-19 cells culture media and cytosolic fraction
As described earlier, the collected cytosolic fractions and cultured medium were processed on C18 HPLC column to detect the 125I-Ang-(1–12) cellular internalization and hydrolytic products generation in culture medium by ARPE-19 cells. In brief, we added an equal volume of 1% phosphoric acid (PA) to the pooled cytosolic fractions and cultured medium, filtered the mixture through a 0.2-μm syringeless filter device and injected (∼50,000 cpm) on C18 HPLC column to separate the 125I-Ang-(1–12) substrate and its metabolic products as previously described by us.3,5,6,26
Immunofluorescence imaging of Ang-(1–12) internalization
Immunofluorescence (IF) technique was also utilized to demonstrate the internalization of Ang-(1–12) by ARPE-19 cells using Ang-(1–12) mAb and anti-mouse Alexa Fluor 488 secondary antibody.27 For IF study, cells (2.5 × 104 cells/1.7 cm2 in 1.0 mL DMEM/F12 medium) were seeded in 4-well glass chamber slides (Lab-Tek, Rochester, NY). The cells were washed as described earlier and 1.0 mL of serum-free medium containing a chemical preparation of RAS and peptidase inhibitors (Table 1) was added to stop the hydrolysis of Ang-(1–12). After 10 min of preincubation, the Ang-(1–12) was added only in third well (0.1 μM) and fourth well (1 μM) and incubated for 4 h at 37°C with an atmosphere of 5% CO2. Ang-(1–12) added was not added in first and second wells and served as untreated control.
After 4 h of exposure, the culture media were aspirated and each well was washed 4 times with 1 mL of Dulbecco's phosphate-buffered saline (DPBS) and 1 time with 1.0 mL of 0.05 M glycin-HCl (pH 4.0) to remove free and outer membrane-bound Ang-(1–12) peptide. The thoroughly washed cells were fixed with 1.0 mL of 4% paraformaldehyde for 10 min, permeabilized with 0.3% Triton X-100 in DPBS for 15 min, and blocked with 1.0 mL of 5% fat-free bovine serum albumin with 0.1% Tween-20 in DPBS for 1 h at room temperature (RT). After 1 h, the blocking buffer was removed from the wells (second, third, and fourth) and 1.0 mL of Ang-(1–12) mAb (primary antibody 1:5,000 dilution in blocking buffer) was added in the wells (second, panel B; third, panel C and fourth, panel D) and incubated overnight at 4°C.
No primary antibody was added in well-1 (panel A) serves as the negative control. After overnight incubation at 4°C, the wells were washed 4 times with 1.0 mL of PBS and anti-mouse Alexa flour 488 fluorescent secondary antibody (1:1,000 dilution in blocking buffer) was added in each well and incubated for 1 h (RT) in the dark. At the end of treatment, wells were washed with 1 mL of PBS 4 times. Finally, the chamber wall and gasket assembly were removed and 10–15 μL of Mounting Medium containing DAPI (Vector Laboratories, Burlington, CA) was added to each well and sealed by a glass coverslip. The immunoreactive fluorescent staining of the internalized Ang-(1–12) in ARPE-19 cells was visualized and imaged using a confocal microscope (Nikon Instruments, Melville, NY).
Metabolism of 125I-Ang-(1–12) by ARPE-19 cells membrane
The metabolism of radiolabeled Ang-(1–12) substrate by ACE and chymase using ARPE-19 cells membrane was performed in the presence or absence of RAS inhibitors cocktail as previously described by us.3,5,6,26 In this study, the ARPE-19 cells were cultured and the postconfluence cells were collected in PBS buffer. The cells were lysed by sonication and centrifuged (28,000 g) for 20 min at 4°C to collect the nPMs. The nPMs were solubilized in triton X-100 (0.5%) containing PBS for overnight (4°C) and insoluble nPMs were removed by centrifuging the tubes at 16,000 g for 10 min at 4°C.
The solubilized proteins were used for 125I-Ang-(1–12) hydrolysis. In brief, the soluble proteins (∼50 μg/200 μL) were preincubated (10 min at 37°C) with all RAS inhibitor cocktail or inhibitors cocktail without lisinopril and chymostatin (Table 1). After preincubation for 10 min, the reactions were started by adding 125I-Ang-(1–12) substrate [1 nM] and stopped after 60 min by adding 1% PA (200 μL). Finally, the reaction mixtures were centrifuged at 16,000 g for 10 min, filtered, and injected (∼100,000 cpm) to separate the 125I-Ang-(1–12) substrate and its metabolic products on C18 column by HPLC, and the radiolabeled peaks were analyzed as described by us.3,5,6,26
Statistical analysis
All experiments were performed at least 3 or more times. Values are presented as mean ± SD (standard deviation). The statistical analysis between 2 groups were performed by Student's t-test where P < 0.05 was set as statistical significance (GraphPad Prism 9 Software, San Diego, CA).
Results
HPLC purification of 125I-Ang-(1–12) and mAb cross-reactivity
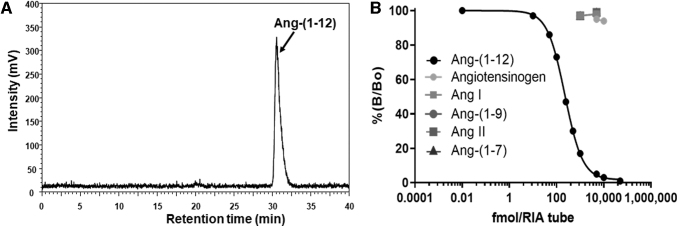
As shown in Fig. 1A, our radiolabeled human 125I-Ang-(1–12) substrate was highly purified (purity ≥99%) and utilized in cellular internalization and metabolism studies. Also the purified Ang-(1–12) mAb used in these experiments were highly specific in recognizing the C-terminal end of the Ang-(1–12) sequence, possessed no cross-reactivity with angiotensinogen and other Ang peptides, including Ang I and Ang II (Fig. 1B).
FIG. 1.
HPLC chromatogram of purified 125I-Ang-(1–12) and mAb specificity and cross-reactivity. The human Ang-(1–12) was radiolabeled with Iodine-125 and was purified on C18 column by HPLC. As shown in HPLC chromatogram, (A) the purified radiolabeled 125I-Ang-(1–12) peptide fraction was highly pure (≥99% purity) and was used in internalization and metabolism studies. (B) The Protein L column purified mAb shows high specificity for Ang-(1–12) (filled black circle) and does not cross-react with closely related Ang peptides (1,000 and 5,000 fmol) and human angiotensinogen (5,000 and 10,000 fmol) as demonstrated by RIA. Ang, angiotensin; HPLC, high-performance liquid chromatography; mAb, monoclonal antibody.
Cellular internalization of Ang-(1–12)
Figure 2 shows that radiolabeled Ang-(1–12) was internalized in ARPE-19 cells from extracellular culture medium. Importantly, our finding further shows that the internalization of radiolabeled Ang-(1–12) in ARPE-19 cells significantly decreased (P < 0.0001) when co-incubated with Ang-(1–12) mAb. The cell associated radiolabeled counts (mean ± SD) after 4 h exposure was 15,307 ± 3,711 cpm/mg protein for Ang-(1–12) alone and 8,058 ± 1,926 cpm/mg protein in the presence of the Ang-(1–12) mAb. We found a 53% decrease in ARPE-19 cellular internalization of 125I-Ang-(1–12) during co-incubation with the mAb.
FIG. 2.
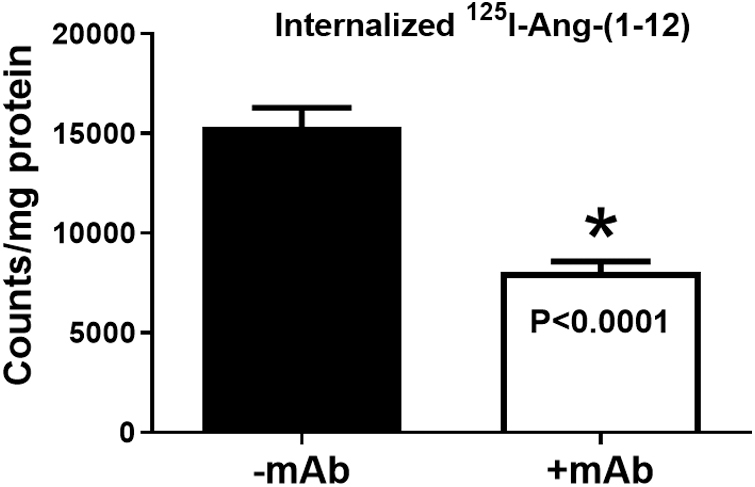
Radiolabeled 125I-Ang-(1–12) Internalization −/+mAb. Internalization of radiolabeled 125I-Ang-(1–12) in confluent ARPE-19 cells at 37°C for 4 h in the presence and absence of mAb (1 mg/mL). All experiments were performed at least 3 times or more on different passages. Values are expressed in terms of counts per milligram protein (*P < 0.0001 vs. −mAb). Details are in Methods section. ARPE-19, adult retinal pigment epithelial-19.
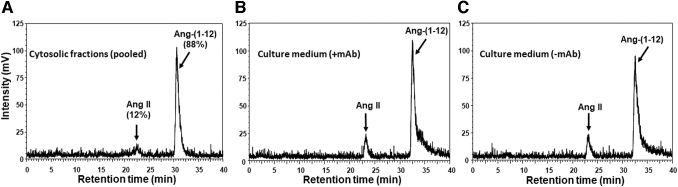
The internalization of 125I-Ang-(1–12) in ARPE-19 was calculated based on a total radiolabeled trace (1.8 × 106 cpm) added into the culture medium. We found that after 4 h of exposure, a total 5.5 × 104 cpm (3.1%) and 3.5 × 103 cpm (0.2%) were detected in the cells' cytosolic and membrane fractions, respectively. Furthermore, the cytosolic cell fraction was injected on C18 column and performed HPLC analysis to confirm that an intact 125I-Ang-(1–12) is internalized in ARPE-19. As shown in Fig. 3A, the internalized radiolabeled content (88%) eluted at a retention time identical to standard intact 125I-Ang-(1–12) peptide. A small peak (∼12%) corresponds to the retention time of Ang II, which was also detected in the cytosolic fraction.
FIG. 3.
HPLC chromatograms of cytosolic fractions (from 100 mm dishes) and culture media (from 12-well plates). ARPE-19 cells were treated with 125I-Ang-(1–12) for 4 h at 37°C. The cells and culture media were collected after 4 h of exposure. The radiolabeled substrate and its metabolic products were analyzed by HPLC. Chromatograms; (A) pooled cytosolic fractions, (B) culture medium in the presence of mAb, and (C) culture medium in the absence of mAb. Details are in Methods section.
The cultured supernatants collected after 4 h exposure from ARPE-19 cells treated with radiolabeled Ang-(1–12) with or without mAb added to culture medium were injected on HPLC C18 column to identify the metabolic products. We found that the majority of radiolabeled Ang-(1–12) peptide remained intact and not hydrolyzed in the presence of inhibitors cocktail (Fig. 3B, C). As shown in the HPLC chromatograms, the Ang-(1–12) peptide remained intact (84% and 86% for −mAb and +mAb, respectively) and only a small peak (<16% for −mAb and 14% for +mAb, respectively) corresponding to the retention time of Ang II was detected in the culture supernatants.
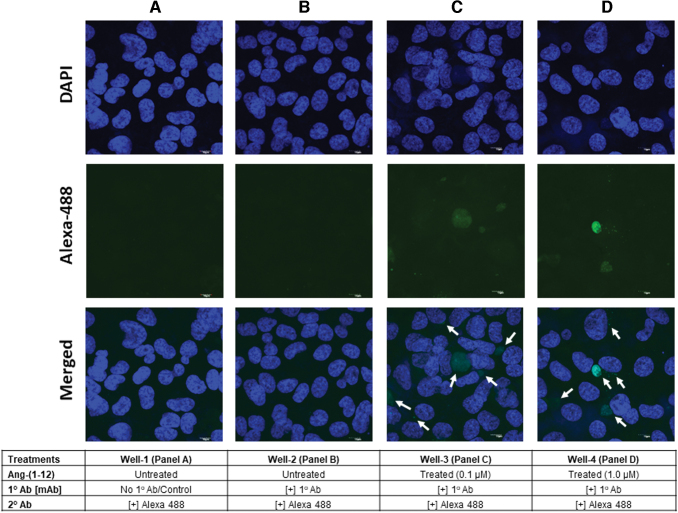
The IF staining results (Fig. 4A–D) demonstrate that Ang-(1–12) is not expressed endogenously in ARPE-19 cells and is internalized from extracellular space. As shown in Fig. 4B, in the absence of exogenous Ang-(1–12) in culture media, no immunoreactive fluorescent green staining of Alexa flour 488 was detected in ARPE-19 cells. Whereas, in the presence of exogenous Ang-(1–12) in the culture media, the immunoreactive fluorescent green staining was detected in the ARPE-19 cells (Fig. 4C, D). An increased intensity in immunoreactive fluorescent green staining was detected with increasing concentration of Ang-(1–12) in the culture medium (panel C, 0.1 μM vs. panel D, 1.0 μM). Figure 4A serves as the negative control (secondary antibody only).
FIG. 4.
ARPE-19 cells immunofluorescence staining. The ARPE-19 cells grown on 4-well chambered slides and treated with or without Ang-(1–12) for 4 h at 37°C. Well-1 [A, untreated Ang-(1–12) and mAb] and well-2 [B, untreated Ang-(1–12)] serve as negative control and endogenous Ang-(1–12) expression, respectively. Well-3 (C) and well-4 were treated with Ang-(1–12) (0.1 and 1.0 μM, respectively) serve as internalization of Ang-(1–12) from extracellular culture medium. Endogenous (B) and arrows indicate the Ang-(1–12) internalization (C, D) were detected by immunofluorescence staining using Ang-(1–12) mAb as primary and anti-mouse Alexa Fluor 488 for secondary antibody as described in Methods section. Color images are available online.
Metabolism of 125I-Ang-(1–12)
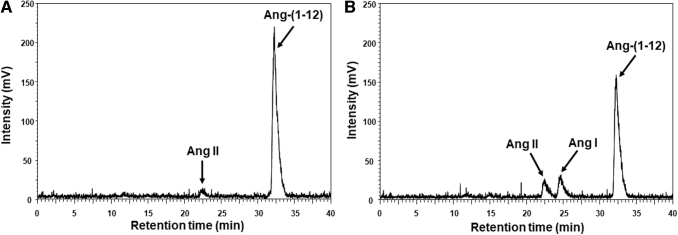
The HPLC chromatogram (Fig. 5A, B) shows the metabolism of radiolabeled Ang-(1–12) peptide by soluble membrane fractions of ARPE-19 cells. We found that in the presence of all inhibitors, including lisinopril and chymostatin (Table 1), >94% of the radiolabeled Ang-(1–12) substrate had remained intact and only a minute Ang II peak (<6%) was detected on the HPLC chromatogram (Fig. 5A). However, in the absence of both ACE and chymase enzyme inhibitors (lisinopril and chymostatin), only 70% of the 125I-Ang-(1–12) substrate remained intact (Fig. 5B) and the remaining 30% hydrolyzed into Ang I (17%) and Ang II (13%) products.
FIG. 5.
HPLC chromatograms of radiolabeled 125I-Ang-(1–12) substrate and its hydrolytic products generation by ARPE-19 cells. HPLC analysis of the hydrolytic products generated from 125I-Ang-(1–12) substrate by solubilized plasma membranes in the absence of both ACE and chymase inhibitors. The HPLC chromatograms, represent the hydrolytic products are generated in presence of ACE and chymase inhibitors (panel A) and in the absence of both inhibitors (panel B). Details are in Methods section. ACE, angiotensin-converting enzyme.
Discussion
The characterization, by our laboratory, that an Ang I upstream precursor “Ang-(1–12)” serves as a primary substrate for Ang II generation in humans is expanded in this study, which revealed a significant intake of 125I-Ang-(1–12) in a cell line that is a critical tool in exploring the role of endocrine and cytokine mechanisms of retinopathy. Our current HPLC data show that an intact radiolabeled Ang-(1–12) peptide is uptaken by ARPE-19 cells. This internalization was blocked with co-incubation of Ang-(1–12) mAb present in the culture medium. The cellular internalization depends on the amount of Ang-(1–12) present in the extracellular culture medium. We found that the IF intensity was increased with increasing the Ang-(1–12) concentrations in the culture medium.
Recently, we reported that Ang-(1–12) levels were severalfold elevated in the plasma of human hypertensive patients compared with the Ang I in healthy humans.10 The high levels of circulating Ang-(1–12) may contribute as a source for extracellular and intracellular production of Ang II. Earlier, we demonstrated an uptake of intact Ang-(1–12) peptide in myocytes isolated from 1- to 3-day-old pups of spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto rats (WKY).26 We showed that radiolabeled Ang-(1–12) was internalized in neonatal myocytes (SHR and WKY) from the culture medium. Furthermore, we found that at all time points Ang-(1–12) internalization rate was significantly higher in SHR myocytes when compared with WKY myocytes. In another study, a significantly higher level of endogenous Ang-(1–12) content was detected in adult SHR rats (heart and kidney) compared with WKY.28 A recent study shows that radiolabeled angiotensinogen protein is internalized by ARPE-19 cells.29
The blood-retinal barrier (BRB) is made of prominent types of cells, the endothelial cells present on the inner side and a monolayer RPE cells on the outer side of BRB.30 This RPE is heavily involved in the membrane trafficking burden (transports the nutrients into the retinal neurons and removes the waste products from the retina into the blood through the transporters, receptors, and channels present on both apical-side and basolateral-side plasma membranes of the cells).31–34 Receptors for proteins and peptides (such as AT1R, MasR, natriuretic peptides receptors, and glucagon-like peptide-1 receptor) are present in human RPE and cultured ARPE-19 cells at the apical and basal plasma membrane.35–38 The multiligand megalin and cubilin receptors, heavily involved in the endocytic uptake of many ligands (peptides, hormones, proteins, enzymes, and drugs), are contained by ARPE-19 cells.39–43
Megalin has been recognized to regulate homeostasis of many RAS molecules and significantly involved in pathophysiological functions and neurodegenerative disorders.44 Several studies demonstrated that angiotensinogen, renin, Ang II, and Ang-(1–7) are endocytosed through megalin present on the cell surface, including the retinal cells.29,41,42,44–48 Our current study on human ARPE-19 cells and previous data from the cultured neonatal hypertensive rat myocytes26 suggest that circulating Ang-(1–12) may be a source of tissue and cellular accumulation through receptor-mediated internalization process. Plasma-derived angiotensinogen and renin in the interstitial fluid is a potential source of cardiac angiotensins.49 Further studies are in progress to demonstrate the receptor-ligand internalization process of Ang-(1–12) from the basal-to-apical and apical-to-basal compartments using transwell inserts.
In this study, we have demonstrated that in the absence of both lisinopril and chymostatin, the solubilized plasma membranes from ARPE-19 cells metabolize Ang-(1–12) substrate into Ang I and Ang II. Our current findings are consistent with our previous studies that ACE sequentially cleaves the 2 residues at a time from the C-terminal end by cleaving the bond (first Leu10-Val11 and then Phe8-His9) of the Ang-(1–12) substrate to release the products Ang I and Ang II, whereas chymase directly generates Ang II product by cleaving the Phe8-His9 bond.2,5,8 The RAS components (including ACE and chymase enzymes) necessary for the generation of biologically active Ang II peptide have been demonstrated in experimental animals and human retinal tissue, subretinal fluid or vitreous.13,18,50–53
Chymase is an enzyme highly expressed in various tissues, including blood vessels and ocular tissue. Study shows that in idiopathic macular hole disease an increased levels of chymase was detected in experimental animals.53 Extracellularly and intracellularly produced Ang II are exerting trophic, pro-fibrotic, pro-inflammatory, and proliferative actions in target organs of diabetes (including eye).54–56 Earlier, we have demonstrated that the generation of Ang II primarily depends on ACE in circulation and on chymase in tissues/cells.2,3,5–7 Regardless of the generation Ang II by ACE and/or chymase, this biologically active peptide responsible for reactive oxygen species production, extracellular matrix remodeling, apoptosis, and gene expression can damage the tissues in various organs by activating the intracellular signaling pathways (including retinal microvasculature).11,16,57
Both laboratory and clinical studies indicate that Ang II plays a critical role in retinal tissue pathogenesis (retinopathy). However, involvement of the circulatory RAS [particularly Ang-(1–12)] at the cellular and molecular level in progression and development of retinopathy remains to be elucidated. Hypertension is linked in the development and progression of retinopathy and significantly contributes in several other retinal diseases in nondiabetic subjects.
However, the retinal disease risk factors are increased in hypertensive patients with diabetes. Studies indicate that the DR relative risk factor is increased by 70% with coexistence of both hypertension and diabetes.58–60 Our recent data suggest that Ang-(1–12) may represent a source of intracellular Ang II production escaping the alternative hydrolysis by ACE or uptake by Ang II receptor blockers (ARBs).7 Administration of anti-hypertension drugs [ACE inhibitor (ACEi) and ARBs or both] have the expected effects on plasma Ang II, but we showed that cellular (myocardial) Ang II content remains unchanged.61
To date, a precise medical treatment to prevent RAS-mediated retinopathy remains elusive. Although there is no cure for RAS-mediated retinopathy, the progression of the disease can be prevented, delayed, or reduced by strict control of blood sugar, antihypertensive medications, laser surgery, vitrectomy, and anti-inflammatory medicines.62–66 Lack of therapies targeting specific pathogenic mechanisms remain a serious limitation to prevent blindness in diabetic patients with hypertension. Experimental evidence suggests the involvement of circulating and ocular RAS components in neurodegenerative progression of DR.11,16,19,67–69 ACEi and/or ARBs are currently favored to treat patients with hypertension and diabetes. These medications are only partially effective to control the retinopathy progression.70,71
Intracellular Ang II generation and effects are not improved by ACEi and/or receptor blockers as these molecules cannot penetrate the cell plasma membrane layers.72–76 This discrepancy may result from renin-independent noncanonical pathways where Ang-(1–12) serves as a substrate to generate in circulation and in cellular/tissue levels. In retinal tissues, the Ang-(1–12)/Ang II/chymase/ACE axis may be a primary pathogenic factor for retinopathy progression complicated by hypertension and diabetes. In this study, we have found that the mAb against Ang-(1–12) neutralizes the Ang II-forming substrate and blocks the internalization of Ang-(1–12) into the ARPE-19 cells from the extracellular space. The data suggest that the mAb could be used as a therapeutic agent to prevent/reduce the hypertension-mediated retinopathy by neutralizing the effects of the circulatory Ang-(1–12).
For the past few decades, the FDA has approved a number of mAb to treat cancers, atherosclerosis, and immunologic diseases.77–80 To date, the therapeutic use of mAb has not been investigated in RAS-associated neurovascular disease in hypertension and diabetes. We demonstrated in a humanized transgenic hypertensive rat model that circulating Ang-(1–12) serves as an Ang II-forming substrate endogenously and showed that this mAb directed toward Ang-(1–12) by itself decreases arterial pressure.25 Neutralization of Ang-(1–12) by mAb will block the nonrenin pathway of Ang II generation and also prevent the circulating Ang-(1–12) internalization into retinal cells. Our mAb has high specificity for Ang-(1–12) and does not cross-react with the angiotensinogen protein and angiotensinogen-derived angiotensin peptides. Also our mAb completely blocks the Ang-(1–12) hydrolytic sites and prevents Ang II formation through ACE and chymase.25
Conclusions
This is the first line of evidence that ARPE-19 cells do not express Ang-(1–12) endogenously and as intact Ang-(1–12) peptide sequence is internalized from extracellular space and is metabolized into Ang II. Furthermore, the selective mAb blocks the cellular internalization of Ang-(1–12) suggests alternate therapeutic approaches to prevent/reduce the RPE cells Ang II burden.
Acknowledgments
We thank Dr. Goldis Malek for providing the ARPE-19 cells and Mr. Jonathan H Diaz (Graduate Student, Wake Forest PhD Graduate Program) for technical assistance to acquire and analyze the confocal images (Fig. 4). The author (MSO) would like to thank the funding of Researchers Supporting Project Number (RSPD2023R710), King Saud University, Riyadh, Saudi Arabia.
Authors' Contributions
S.A., C.M.F., and R.M.S. designed the experiments and wrote original draft of the article. S.A., K.N.W., and J.R.G. conducted the experiments. S.A., K.N.W., and C.M.F. performed the data analysis. J.L.V., K.N.W., M.S.O., M.C., G.M., J.R.G., and R.M.S. were involved in proof reading and editing the article. All contributing authors approved the final version of this article and agreed for its submission.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Support for this study was provided by NIH grants P01 HL-051952 and R21AG070371 to Carlos M. Ferrario.
References
- 1. Nagata S, Kato J, Sasaki, K, et al. . Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 2006;350(4):1026–1031. [DOI] [PubMed] [Google Scholar]
- 2. Moniwa N, Varagic J, Simington SW, et al. . Primacy of angiotensin converting enzyme in angiotensin-(1–12) metabolism. Am J Physiol Heart Circ Physiol 2013;305(5):H644–H650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmad S, Simmons T, Varagic J, et al. . Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One 2011;6(12):e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmad S, Varagic J, Groban L, et al. . Angiotensin-(1–12): A chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep 2014;16(5):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmad S, Varagic J, VonCannon JL, et al. . Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme. Biochem Biophys Res Commun 2016;478(2):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmad S, Wei CC, Tallaj J, et al. . Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens 2013;7(2):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrario CM, Ahmad S, Nagata S, et al. . An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 2014;126(7):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrario CM, Groban L, Wang H, et al. . The Angiotensin-(1–12)/Chymase axis as an alternate component of the tissue renin angiotensin system. Mol Cell Endocrinol 2021;529:111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrario CM, Iyer SR, Burnett JCJr., et al. . Angiotensin (1–12) in humans with normal blood pressure and primary hypertension. Hypertension 2021;77(3):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad S, Punzi HA, Wright KN, et al. . Newly developed radioimmunoassay for Human Angiotensin-(1–12) measurements in plasma and urine. Mol Cell Endocrinol 2021;529:111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marin Garcia PJ, Marin-Castano ME. Angiotensin II-related hypertension and eye diseases. World J Cardiol 2014;6(9):968–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaajanen A, Kalesnykas G, Vapaatalo H, et al. . The expression of Mas-receptor of the renin-angiotensin system in the human eye. Graefes Arch Clin Exp Ophthalmol 2015;253(7):1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Senanayake P, Drazba J, Shadrach K, et al. . Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci 2007;48(7):3301–3311. [DOI] [PubMed] [Google Scholar]
- 14. Vita JB, Anderson JA, Hulem CD, et al. . Angiotensin-converting enzyme activity in ocular fluids. Invest Ophthalmol Vis Sci 1981;20(2):255–257. [PubMed] [Google Scholar]
- 15. Holappa M, Vapaatalo H, Vaajanen A.. Many faces of renin-angiotensin system—Focus on eye. Open Ophthalmol J 2017;11:122–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danser AH, Derkx FH, Admiraal PJ, et al. . Angiotensin levels in the eye. Invest Ophthalmol Vis Sci 1994;35(3):1008–1018. [PubMed] [Google Scholar]
- 17. Funatsu H, Yamashita H. Pathogenesis of diabetic retinopathy and the renin-angiotensin system. Ophthalmic Physiol Opt 2003;23(6):495–501. [DOI] [PubMed] [Google Scholar]
- 18. Senanayake PD, Bonilha VL, Peterson JW, et al. . Retinal angiotensin II and angiotensin-(1–7) response to hyperglycemia and an intervention with captopril. J Renin Angiotensin Aldosterone Syst 2018;19(3):1470320318789323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ola MS, Alhomida AS, Ferrario CM, et al. . Role of tissue renin-angiotensin system and the chymase/angiotensin-(1–12) axis in the pathogenesis of diabetic retinopathy. Curr Med Chem 2017;24(28):3104–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rojas M, Zhang W, Lee DL, et al. . Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Invest Ophthalmol Vis Sci 2010;51(3):1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Otani A, Takagi H, Suzuma K, et al. . Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res 1998;82(5):619–628. [DOI] [PubMed] [Google Scholar]
- 22. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 1996;62(2):155–169. [DOI] [PubMed] [Google Scholar]
- 23. Davis AA, Bernstein PS, Bok D, et al. . A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci 1995;36(5):955–964. [PubMed] [Google Scholar]
- 24. Rettinger CL, Wang HC. Current advancements in the development and characterization of full-thickness adult neuroretina organotypic culture systems. Cells Tissues Organs 2018;206(3):119–132. [DOI] [PubMed] [Google Scholar]
- 25. Ferrario CM, VonCannon JL, Zhang J, et al. . Immunoneutralization of human angiotensin-(1–12) with a monoclonal antibody in a humanized model of hypertension. Peptides 2022;149:170714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmad S, Varagic J, Westwood BM, et al. . Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One 2011;6(1):e15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrario CM, VonCannon J, Ahmad S, et al. . Activation of the human angiotensin-(1–12)-chymase pathway in rats with human angiotensinogen gene transcripts. Front Cardiovasc Med 2019;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jessup JA, Trask AJ, Chappell MC, et al. . Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 2008;294(6):H2614–H2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulgar VM, Cruz-Diaz N, Westwood BM, et al. . Angiotensinogen uptake and stimulation of oxidative stress in human pigment retinal epithelial cells. Peptides 2022;152:170770. [DOI] [PubMed] [Google Scholar]
- 30. Diaz-Coranguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: Cellular basis and development. Vis Res 2017;139:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehmann GL, Benedicto I, Philp NJ, et al. . Plasma membrane protein polarity and trafficking in RPE cells: Past, present and future. Exp Eye Res 2014;126:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storm T, Burgoyne T, Futter CE. Membrane trafficking in the retinal pigment epithelium at a glance. J Cell Sci 2020;133(16):jcs238279. [DOI] [PubMed] [Google Scholar]
- 33. Sugasawa K, Deguchi J, Okami T, et al. . Immunocytochemical analyses of distributions of Na, K-ATPase and GLUT1, insulin and transferrin receptors in the developing retinal pigment epithelial cells. Cell Struct Funct 1994;19(1):21–28. [DOI] [PubMed] [Google Scholar]
- 34. Philp NJ, Wang D, Yoon H, et al. . Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 2003;44(4):1716–1721. [DOI] [PubMed] [Google Scholar]
- 35. Striker GE, Praddaude F, Alcazar O, et al. . Regulation of angiotensin II receptors and extracellular matrix turnover in human retinal pigment epithelium: Role of angiotensin II. Am J Physiol Cell Physiol 2008;295(6):C1633–C1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dahrouj M, Alsarraf O, Liu Y, et al. . C-type natriuretic peptide protects the retinal pigment epithelium against advanced glycation end product-induced barrier dysfunction. J Pharmacol Exp Ther 2013;344(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prasad T, Verma A, Li Q.. Expression and cellular localization of the Mas receptor in the adult and developing mouse retina. Mol Vis 2014;20:1443–1455. [PMC free article] [PubMed] [Google Scholar]
- 38. Puddu A, Sanguineti R, Montecucco F, et al. . Retinal pigment epithelial cells express a functional receptor for glucagon-like peptide-1 (GLP-1). Mediators Inflamm 2013;2013:975032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christensen EI, Birn H. Megalin and cubilin: Multifunctional endocytic receptors. Nat Rev Mol Cell Biol 2002;3(4):256–266. [DOI] [PubMed] [Google Scholar]
- 40. Langston Suen WL, Chau Y. Size-dependent internalisation of folate-decorated nanoparticles via the pathways of clathrin and caveolae-mediated endocytosis in ARPE-19 cells. J Pharm Pharmacol 2014;66(4):564–573. [DOI] [PubMed] [Google Scholar]
- 41. Storm T, Burgoyne T, Dunaief JL, et al. . Selective ablation of megalin in the retinal pigment epithelium results in megaophthalmos, macromelanosome formation and severe retina degeneration. Invest Ophthalmol Vis Sci 2019;60(1):322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Storm T, Heegaard S, Christensen EI, et al. . Megalin-deficiency causes high myopia, retinal pigment epithelium-macromelanosomes and abnormal development of the ciliary body in mice. Cell Tissue Res 2014;358(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hollborn M, Birkenmeier G, Saalbach A, et al. . Expression of LRP1 in retinal pigment epithelial cells and its regulation by growth factors. Invest Ophthalmol Vis Sci 2004;45(6):2033–2038. [DOI] [PubMed] [Google Scholar]
- 44. Kukida M, Sawada H, Daugherty A, et al. . Megalin: A bridge connecting kidney, the renin-angiotensin system, and atherosclerosis. Pharmacol Res 2020;151:104537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pohl M, Kaminski H, Castrop H, et al. . Intrarenal renin angiotensin system revisited: Role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 2010;285(53):41935–41946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ye F, Wang Y, Wu C, et al. . Angiotensinogen and megalin interactions contribute to atherosclerosis-brief report. Arterioscler Thromb Vasc Biol 2019;39(2):150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonzalez-Villalobos R, Klassen RB, Allen PL, et al. . Megalin binds and internalizes angiotensin-(1–7). Am J Physiol Renal Physiol 2006;290(5):F1270–F1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gonzalez-Villalobos R, Klassen RB, Allen PL, et al. . Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol 2005;288(2):F420–F427. [DOI] [PubMed] [Google Scholar]
- 49. Danser AH, van Kats JP, Admiraal PJ, et al. . Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension 1994;24(1):37–48. [DOI] [PubMed] [Google Scholar]
- 50. Wagner J, Jan Danser AH, Derkx FH, et al. . Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: Evidence for an intraocular renin-angiotensin system. Br J Ophthalmol 1996;80(2):159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sakaguchi H, Takai S, Sakaguchi M, et al. . Chymase and angiotensin converting enzyme activities in a hamster model of glaucoma filtering surgery. Curr Eye Res 2002;24(5):325–331. [DOI] [PubMed] [Google Scholar]
- 52. Shiota N, Saegusa Y, Nishimura K, et al. . Angiotensin II-generating system in dog and monkey ocular tissues. Clin Exp Pharmacol Physiol 1997;24(3–4):243–248. [DOI] [PubMed] [Google Scholar]
- 53. Sugiyama T, Katsumura K, Nakamura K, et al. . Effects of chymase on the macular region in monkeys and porcine muller cells: Probable involvement of chymase in the onset of idiopathic macular holes. Ophthalmic Res 2006;38(4):201–208. [DOI] [PubMed] [Google Scholar]
- 54. Behl T, Kotwani A.. Potential of angiotensin II receptor blockers in the treatment of diabetic retinopathy. Life Sci 2017;176:1–9. [DOI] [PubMed] [Google Scholar]
- 55. Chawla T, Sharma D, Singh A. Role of the renin angiotensin system in diabetic nephropathy. World J Diabetes 2010;1(5):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ribeiro-Oliveira A Jr., Nogueira AI, Pereira RM, Boas WW, Dos Santos RA, Simoes e Silva AC.. The renin-angiotensin system and diabetes: An update. Vasc Health Risk Manag 2008;4(4):787–803. [PMC free article] [PubMed] [Google Scholar]
- 57. Silva KC, Rosales MA, Biswas SK, et al. . Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes 2009;58(6):1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walraven I, Mast MR, Hoekstra T, et al. . Real-world evidence of suboptimal blood pressure control in patients with type 2 diabetes. J Hypertens 2015;33(10):2091–2098. [DOI] [PubMed] [Google Scholar]
- 59. Raum P, Lamparter J, Ponto KA, et al. . Prevalence and cardiovascular associations of diabetic retinopathy and maculopathy: Results from the Gutenberg Health Study. PLoS One 2015;10(6):e0127188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wong TY, Cheung N, Tay WT, et al. . Prevalence and risk factors for diabetic retinopathy: The Singapore Malay Eye Study. Ophthalmology 2008;115(11):1869–1875. [DOI] [PubMed] [Google Scholar]
- 61. Ferrario CM, Jessup J, Chappell MC, et al. . Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 62. Do DV, Wang X, Vedula SS, et al. . Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev 2015;1:CD006127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marozas LM, Fort PE. Diabetic retinopathy-update on prevention techniques, present therapies, and new leads. US Ophthalmic Rev 2014;7(1):54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bain SC, Klufas MA, Ho A, et al. . Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes Metab 2019;21(3):454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hooymans JM, Ballegooie EV, Schweitzer NM, et al. . Worsening of diabetic retinopathy with strict control of blood sugar. Lancet 1982;2(8295):438. [DOI] [PubMed] [Google Scholar]
- 66. Zhang W, Liu H, Rojas M, et al. . Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 2011;3(5):609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ola MS, Alhomida AS. Neurodegeneration in diabetic retina and its potential drug targets. Curr Neuropharmacol 2014;12(4):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ola MS, Nawaz MI, Khan HA, et al. . Neurodegeneration and neuroprotection in diabetic retinopathy. Int J Mol Sci 2013;14(2):2559–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ola MS, Nawaz MI, Siddiquei MM, et al. . Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications 2012;26(1):56–64. [DOI] [PubMed] [Google Scholar]
- 70. Sjolie AK, Klein R, Porta M, et al. . Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet 2008;372(9647):1385–1393. [DOI] [PubMed] [Google Scholar]
- 71. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: A systematic review. JAMA 2007;298(8):902–916. [DOI] [PubMed] [Google Scholar]
- 72. Moniwa N, Varagic J, Ahmad S, et al. . Hemodynamic and hormonal changes to dual renin-angiotensin system inhibition in experimental hypertension. Hypertension 2013;61(2):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dell'italia LJ, Balcells E, Meng QC, et al. . Volume-overload cardiac hypertrophy is unaffected by ACE inhibitor treatment in dogs. Am J Physiol 1997;273(2 Pt 2):H961–H970. [DOI] [PubMed] [Google Scholar]
- 74. Ferrario CM. Addressing the theoretical and clinical advantages of combination therapy with inhibitors of the renin-angiotensin-aldosterone system: Antihypertensive effects and benefits beyond BP control. Life Sci 2010;86(9–10):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Toto R, Palmer BF. Rationale for combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor treatment and end-organ protection in patients with chronic kidney disease. Am J Nephrol 2008;28(3):372–380. [DOI] [PubMed] [Google Scholar]
- 76. Burnier M, Maillard M. Angiotensin II receptor antagonists: Where do we stand? IDrugs 2000;3(3):304–309. [PubMed] [Google Scholar]
- 77. Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies (Basel) 2020;9(3):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Elgundi Z, Reslan M, Cruz E, et al. . The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev 2017;122:2–19. [DOI] [PubMed] [Google Scholar]
- 79. Lu RM, Hwang YC, Liu IJ, et al. . Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020;27(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ridker PM, Everett BM, Thuren T, et al. . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]