Abstract
DNA topoisomerases are important enzymes that stabilize DNA supercoiling and resolve entanglements. There are two main types of topoisomerases in all cells: type I, which causes single-stranded DNA breaks, and type II, which cuts double-stranded DNA. Topoisomerase activity is particularly increased in rapidly dividing cells, such as cancer cells. Topoisomerase inhibitors have been an effective chemotherapeutic option for the treatment of several cancers. In addition, combination cancer therapy with topoisomerase inhibitors may increase therapeutic efficacy and decrease resistance or side effects. Topoisomerase inhibitors are currently being used worldwide, including in the United States, and clinical trials on the combination of topoisomerase inhibitors with other drugs are currently underway. The primary objective of this review was to comprehensively analyze the current clinical landscape concerning the combined application of irinotecan, an extensively investigated type I topoisomerase inhibitor for colorectal cancer, and doxorubicin, an extensively researched type II topoisomerase inhibitor for breast cancer, while presenting a novel approach for cancer therapy.
Keywords: topoisomerases, irinotecan, doxorubicin, clinical trial, combination chemotherapy regimens
1. Introduction
Cancer is expected to become the leading cause of death and the most significant barrier to increasing life expectancy worldwide in the 21st century [1]. Cancer is an important public health issue worldwide and ranks second among the causes of death in the United States. In 2023, 1,958,310 new cancer cases and 609,820 cancer-related deaths are expected in the United States [2]. Therefore, the development of novel and more specific chemotherapeutic agents against the most aggressive tumors and the identification of new biological targets are vital goals in cancer research [1,3]. One of the main drug targets used in chemotherapy to inhibit the abnormal proliferation of cancer cells is topoisomerase (TOPO) [4].
DNA TOPOs are a group of enzymes that regulate DNA topology. TOPO activity increases especially in rapidly dividing cancer cells [5]. They are involved in many important cellular biological processes, including DNA replication, transcription, recombination, and chromosome condensation [6]. These enzymes covalently attach to groups of DNA phosphorus, causing the DNA strands to split and finally recombine [5]. Depending on the number of DNA strands cut, TOPO can be classified into types I and II. Type I enzymes cleave only one DNA strand, whereas type II enzymes cleave both strands to prevent supercoiling or entanglement [7]. Many anticancer drugs act as TOPO poison inhibitors that trap covalent complexes of human TOPOs, causing DNA damage and cancer cell death [8,9]. Clinically approved TOPO-targeting drugs include the camptothecin analog irinotecan (a prodrug of SN-38), topotecan, and belotecan as TOPO I inhibitors and pixantrone, etoposide, etopophos (etopoposide phosphate), teniposide, doxorubicin, epirubicin, valrubucin, daunorubucin, idarubicin, amrubicin, aclarubicin, amsacrine, and mitoxantrone as TOPO II inhibitors [5,10,11,12]. Despite their clinical efficacy, current anticancer therapies that use TOPO-directed agents have several important limitations and adverse effects. Traditional TOPO I inhibitors (e.g., such as camptothecin) exhibit significant dose-limiting toxicity and cancer cells develop resistance to these drugs. In addition, treatment with drugs targeting TOPO II can induce secondary malignancies such as acute myeloid leukemia due to its inhibition [13,14,15]. Therefore, new approaches are required to improve the efficacy of cancer treatment with TOPO inhibitors and counteract their side effects. One approach is combination therapy using a topoisomerase-targeting drug and another drug.
Combination therapy—the combination of two or more treatments—is the cornerstone of cancer treatment. Combinations of anticancer agents show improved efficacy compared to monotherapy because they characteristically target key pathways in a synergistic or additive manner. This approach potentially diminishes drug resistance while providing therapeutic anticancer benefits, such as reduced tumor growth, mitosis arrest, reduced metastatic potential, reduced cancer stem cell populations, and induction of apoptosis [16]. This review presents the clinical outcomes of combination therapies for colorectal cancer (CRC) using irinotecan, the most widely studied TOPO I inhibitor, and doxorubicin, the most widely studied TOPO II inhibitor, for breast cancer.
2. Combination Therapy
Combination therapies increase the efficacy of cancer treatments and cope with multiple genetic changes in different cancer cells. This involves administering one or more types of treatment simultaneously, such as two or more chemotherapies or a combination of chemotherapy and radiation/adjuvant therapy. Occasionally, one or more natural products with antitumor activities, such as low-molecular-weight components of herbs or fungi, may be used in combination therapies [17]. In addition, combination therapies can be applied in cancer cell cultures, animal xenograft models, and clinical trials of patients with cancer. Although the monotherapy approach remains an extremely common treatment for many types of cancer, this conventional method is usually considered less effective than the combined therapy approach [16]. Conventional single-treatment approaches target proliferating cells non-selectively, eventually destroying both healthy and cancer cells. The combination of two drugs may exhibit synergistic, antagonistic, or additive effects compared with their attributes in monotherapy. This approach is intended to minimize the side effects of monotherapy, such as chemical drug resistance, low efficacy, final dose reduction with biological effects, and other side effects leading to patient death [18].
3. TOPO I Inhibitors
3.1. TOPO I Mechanism and Inhibitors
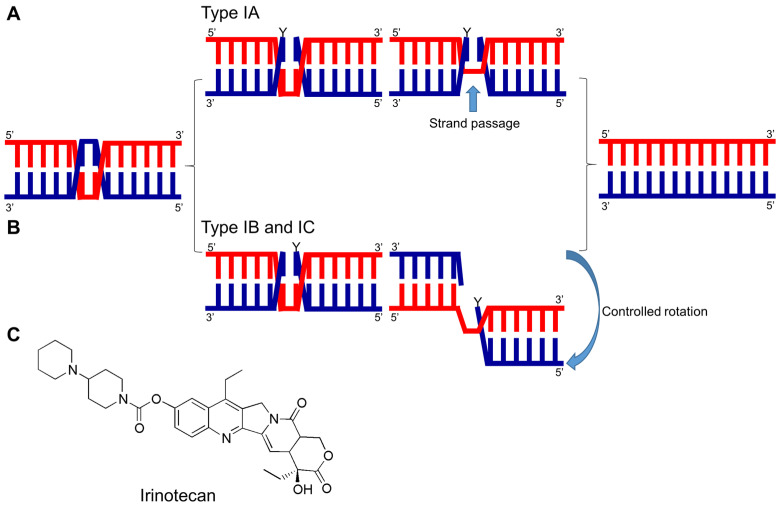
TOPO I induces temporary breaks in a single strand of DNA, leading to changes in its topology. TOPO I can be divided into three subfamilies: type IA, IB, and IC [8,11,19]. Altering the DNA topology by breaking the phosphodiester bonds between nucleotides in DNA strands is based on the same general mechanism in all subtypes of TOPO I. The phosphoryl group of the DNA is attacked by the tyrosyl group of TOPO I, resulting in the formation of a covalent bond between the tyrosyl group and one side of the broken DNA. Simultaneously, the free hydroxylated strands unwind and rotate. The hydroxyl ends of the free DNA strand attack the produced phosphotyrosine bonds. The phosphodiester bonds between the two strands are reconstructed, and topoisomerase is released to participate in the next catalytic cycle [20]. In most cases, TOPO changes the phase of DNA by type 1, which does not require external energy (e.g., ATP hydrolysis) [21]. Type IA TOPOs require a nick- or a single-stranded region to bind to the DNA. They change the DNA topology by cleaving one strand of double-stranded DNA, covalently attaching an active site tyrosine to a 5′-phosphoryl group and utilizing a ‘strand passage’ mechanism. In contrast, type IB and IC TOPOs cleave one strand of double-stranded DNA, attach with the active site tyrosine to the 3′-phosphoryl group to form covalent bonds, and utilize a ‘controlled rotation’ mechanism to relax the DNA supercoil [8,22,23] (Figure 1A,B).
Figure 1.
Catalytic mechanism of type I DNA TOPOs and chemical structure of irinotecan. (A) Each of the three types of DNA TOPOs has a distinct mechanism for catalyzing changes in DNA topology. Type IA functions as a monomer, cleaving one DNA strand and creating a 5′-phospho-tyrosyl bond within the protein-DNA complex. This creates an opening in the cleaved strand, allowing the uncut strand to pass through for relaxation or decatenation of the DNA. The ends of the cut strand are then reconnected, restoring the DNA backbone, and the enzyme can dissociate from the 5′-end of the DNA. (B) Type IB and IC also act as monomers but cleave one strand of duplex DNA and form a temporary 3-phospho-tyrosyl bond. DNA relaxation is achieved by the controlled rotation of the free 5′-end of the DNA around the uncut strand. (C) Chemical structural formula of irinotecan.
For a long time, camptothecins were the only class of compounds demonstrated to target TOPO I. Camptothecin (CPT) was isolated from the stem and bark of Camptotheca acuminate in 1966 by M. E. Wall and M. C. Wani in a natural product screening for anticancer drugs [24]. CPT interacts with DNA and TOPO I enzymes via a hydrogen bond to form the TOPO I–DNA–camptothecin ternary complex. This ternary complex collides with the DNA replication fork causing DNA damage and eventually leading to cell death [19]. Clinically approved TOPO I inhibitors include the camptothecin analog irinotecan (a prodrug of SN-38), topotecan, and belotecan [5,10,11,12].
3.2. Irinotecan Combination Therapy
Irinotecan is an extensively studied TOPO I inhibitor. The approval for the use of irinotecan (Camptosar®) as a treatment for cervical, lung, and ovarian cancer was granted in Japan in 1994, and in 1995 and 1996, it was approved for use in Europe and the United States, respectively [25] (Figure 1C). Irinotecan is a prodrug that is metabolically active in the body, similar to 7-ethyl-10-hydroxycamptothecin (SN-38) [26]. Irinotecan is used in the treatment of advanced CRC and other solid tumors, including pancreatic and non-small cell lung cancer, biliary tract cancer, and advanced gastric and cervical cancer. To date, various clinical trials have revealed the survival advantages of irinotecan-based therapy in patients with metastatic CRC, making it one of the main drugs used for the treatment of metastatic CRC [26]. It is used in pediatric and adult oncology. Although irinotecan can be used as monotherapy, it is used in combination with other cytotoxic agents, such as oxaliplatin and 5-fluorouracil, and monoclonal antibodies, such as bevacizumab and cetuximab. Experimental and clinical studies have shown that irinotecan can be combined with kinase inhibitors, such as apatinib, fruquintinib, dasatinib, regorafenib, and sunitinib, as well as cell cycle checkpoint inhibitors [27]. Irinotecan-based combinations vary widely. These drugs can be appropriately combined with DNA repair inhibitors, agents affecting epigenetic modifications, signal modulators, and immunotherapies [10].
3.3. Clinical Status of Irinotecan Combination Therapy in CRC
According to clinical trial reports, clinical studies on combination therapy with irinotecan are the most common in CRC. Therefore, we summarized the studies that reported the results of clinical trials of combination therapy with irinotecan in CRC (Table 1).
Table 1.
Clinical status of irinotecan combination therapy in CRC.
| Drugs | Target Cancer | Purpose of Study | Clinical Trials Status | Clinical Trials Identifier | Refs. |
|---|---|---|---|---|---|
| Capecitabine, Irinotecan |
CRC | A study on the combined effect of capecitabine and irinotecan | Phase 2 | NCT00022698 | [28,29,30] |
| Ascorbic acid, Irinotecan |
Stage IV CRC | Phase I/II study of ascorbic acid infusion versus irinotecan monotherapy combined with irinotecan treatment in patients with relapsed or advanced CRC who have failed at least one treatment regimen with a fluorouracil-based regimen | Phase 1 Phase 2 |
NCT01550510 | [31] |
| Pemetrexed, Irinotecan |
Metastatic CRC | To determine the efficacy and safety of the combination of pemetrexed and irinotecan | Phase 2 | NCT00191984 | [32,33] |
| ISIS 183750, Irinotecan |
Colorectal neoplasms Colorectal carcinoma Colorectal tumors |
Testing the safety and efficacy of irinotecan against ISIS 183750 and advanced solid or CRC | Phase 1 Phase 2 | NCT01675128 | [34,35,36] |
| MM-121, Irinotecan, Cetuximab |
CRC | To evaluate the safety and tolerability of escalating doses of MM-121 + cetuximab and MM-121 + cetuximab + irinotecan combinations | Phase 1 | NCT01451632 | [37,38] |
| S-1, Irinotecan, Bevacizumab |
CRC | To determine if it is safe to treat unresectable or recurrent colorectal cancer | Phase 2 | NCT00569790 | [39,40] |
| Tivantinib, Cetuximab, Irinotecan |
Metastatic CRC | ARQ 197 or placebo plus irinotecan and cetuximab, defines the recommended dose for phase 2 | Phase 1 Phase 2 | NCT01075048 | [41,42] |
| Guadecitabine (SGI-110), Regorafenib, Lonsurf (TAS-102), Irinotecan |
Previously treated metastatic CRC | Enrollment in phase 1 study of SGI-110 combined with irinotecan and after the MTD was determined, patients were enrolled in a 2:1 randomized phase 2 study of SGI-110 and irinotecan versus standard-of-care regorafenib or TAS-102 |
Phase 1 Phase 2 |
NCT01896856 | [43,44] |
| Cetuximab, FOLFIRI | EGFR expressing metastatic CRC | To investigate the effect of cetuximab in combination with chemotherapy (FOLFIRI) compared to the same chemotherapy for patient EGF receptors | Phase 3 | NCT00154102 | [45,46,47,48,49] |
| Regorafenib, FOLFIRI | CRC metastatic | Comparison of PFS between regorafenib + FOLFIRI chemotherapy and placebo + FOLFIR in mCRC patients previously treated with FOLFOX therapy | Phase 2 | NCT01298570 | [50,51,52] |
| ABT-165, Bevacizumab, FOLFIRI | Previously treated metastatic adenocarcinoma of the colon or rectum | Study evaluating efficacy and tolerability of ABT-165 + FOLFIRI compared to bevacizumab + FOLFIRI | Phase 2 | NCT03368859 | [53,54] |
| Encorafenib, Binimetinib, Cetuximab, FOLFIRI |
BRAF V600E-mutant metastatic CRC | To evaluate encorafenib plus cetuximab plus or minus binimetinib versus choosing either irinotecan/cetuximab or FOLFIRI/cetuximab as control in patients with BRAFV600E mCRC | Phase 3 | NCT02928224 | [55,56,57,58,59,60] |
| Napabucasin, Bevacizumab, FOLFIRI |
CRC | Trial of cancer stem cell pathway inhibitor napabucacin plus standard biweekly FOLFIRI versus standard biweekly FOLFIRI | Phase 3 | NCT02753127 | [61] |
| FOLFIRI, Panitumumab |
Recurrent colorectal carcinoma Stage IVA CRC Stage IVB CRC |
A study on how well FOLFIRI works in combination with panitumumab in the treatment of CRC patients | Phase 2 | NCT02508077 | [62] |
| Ramucirumab, FOLFIRI |
CRC | Comparing overall survival of participants with metastatic CRC treated with ramucirumab plus FOLFIRI or placebo plus FOLFIRI | Phase 3 | NCT01183780 | [63,64,65,66,67,68,69,70] |
| Bevacizumab, FOLFOXIRI |
Colorectal neoplasms | Efficacy and safety evaluation of FOLFOXIRI/bevacizumab regimen (concurrent and sequential) versus FOLFOX/bevacizumab | Phase 2 | NCT01765582 | [71,72,73] |
| Panitumumab, FOLFOXIRI | CRC | A plan to determine the ORR of the combination of FOLFOXIRI and panitumumab | Phase 2 | NCT01226719 | [74,75] |
EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; FOLFIRI, folinic acid, fluorouracil, and irinotecan; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; FOLFOXIRI, folinic acid, fluorouracil, oxaliplatin, and irinotecan; ORR, objective response rate; PFS, progression-free survival.
According to the 2023 United States cancer statistics, CRC is the third most commonly diagnosed cancer in both males and females and the third leading cause of estimated deaths in both sexes [2]. The treatment of CRC typically involves a combination of surgery, chemotherapy, and radiation therapy, depending on the stage and location of the cancer and the patient’s overall health and other individual factors [76]. Surgery is the primary treatment for CRC and involves the removal of the tumor and surrounding tissue. In some cases, the entire colon may require removal (colectomy). After surgery, chemotherapy can be administered to kill any remaining cancer cells and reduce the risk of cancer recurrence. The National Comprehensive Cancer Network (NCCN) guidelines recommend the use of chemotherapy regimens, including CAPOX (capecitabine and oxaliplatin), FOLFIRI (folinic acid, fluorouracil, and irinotecan), FOLFOX (folinic acid, fluorouracil, and oxaliplatin), or FOLFOXIRI (folinic acid, fluorouracil, oxaliplatin, and irinotecan), for unresectable metastatic CRC [77]. The most commonly used chemotherapeutic regimens for CRC are FOLFOX and FOLFIRI [78].
Many clinical studies on FOLFIRI and FOLFOXIRI combined with irinotecan for CRC treatment have been reported. The aim of the NCT01183780 trial, which has been referenced the most among clinical trials on FOLFIRI, was to evaluate the overall survival of metastatic CRC patients who received either ramucirumab plus FOLFIRI or placebo plus FOLFIRI [63]. Ramucirumab is a human IgG-1 monoclonal antibody that interacts with the extracellular part of the vascular endothelial growth factor (VEGF) receptor 2, which is important for blood vessel growth. Targeting angiogenesis is crucial for CRC treatment. Ramucirumab has been proven effective in treating several types of cancer, including gastric, lung, urothelial, colorectal, and advanced liver cancers [79]. The NCT01183780 study, which involved 1072 patients, showed that ramucirumab, in combination with FOLFIRI, as a second-line treatment for metastatic CRC, significantly enhanced the overall survival rate compared to placebo with FOLFIRI. Moreover, no unexpected negative events were observed, and the adverse effects were controllable [64]. In CRC, the identification of activating RAS/RAF mutations early in the disease is a crucial molecular discovery, and these mutations have been suggested as biomarkers for predicting treatment outcomes and disease prognosis [80]. In the NCT01183780 study, adding ramucirumab to FOLFIRI resulted in improved patient outcomes, regardless of RAS/RAF mutation status or tumor location [65,68].
4. TOPO II Inhibitors
4.1. TOPO II Mechanism and Inhibitors
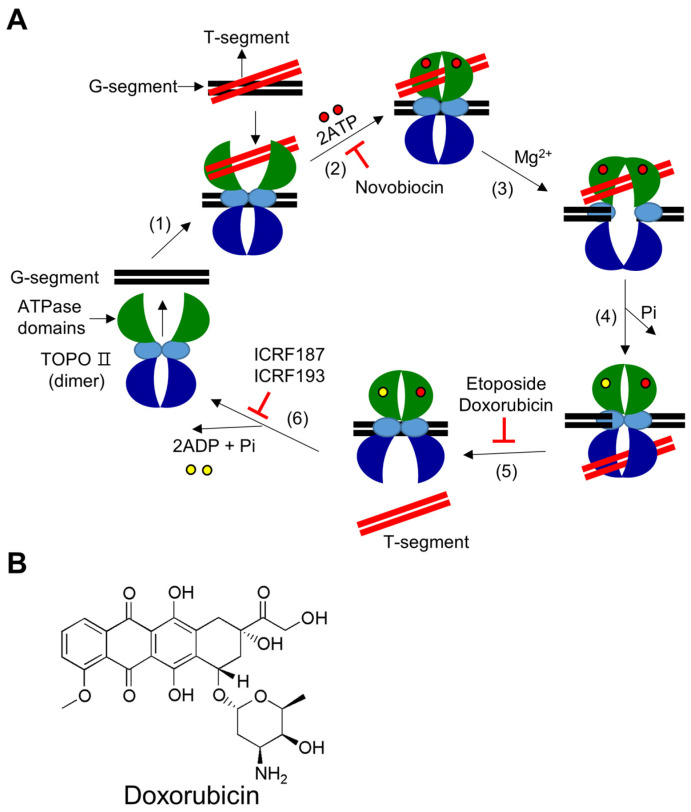
TOPO II are enzymes that cleave both strands of the DNA double helix at the same time and are used to untangle and relieve supercoils in DNA [81]. There are two subtypes of TOPO II, TOPO IIA and TOPO IIB, which are found in different organisms. TOPO IIA exists in bacteria, eukaryotes, and a small number of archaea species, whereas TOPO IIB is mainly found in archaea, plants, and some algae [5]. TOPO IIA is primarily involved in DNA replication and mitosis, whereas TOPO IIB regulates gene expression during transcription. The activity of TOPO II (or TOPO IIA) during mitosis is crucial for the survival of cells [82]. The main mechanism through which TOPO II alters DNA topology involves cutting both DNA strands using Mg2+ and ATP hydrolysis. These enzymes can relax both positive and negative supercoils in DNA and pass a second DNA duplex through a gap after covalently attaching tyrosine to the 5′-end of broken DNA and releasing a free 3′-end (Figure 2A). TOPO II plays a vital role in various nuclear processes, including transcription, replication, and recombination, because of its exceptional ability to untangle double strands of DNA. Loss of TOPO II activity results in double-stranded DNA breaks and cell death, whereas increased DNA cleavage can lead to DNA translocation [5].
Figure 2.
Catalytic mechanism of type II DNA TOPOs and chemical structure of doxorubicin. (A) (1) DNA binding: The enzyme’s homo-dimer preferentially binds to catenated, knotted, and supercoiled DNA segments. The segment of double-stranded DNA that is cleaved during the enzymatic reaction cycle is referred to as the “G segment” (with “G” for gate), and the segment of double-stranded DNA that passes through the cleaved G segment is referred to as the “T segment” (with “T” for transported). The enzyme binds to the G segment and then to the T segment. (2) ATP binding: The binding of two ATP molecules in the ATPase domains alters the conformation of the ATPase domains from an open to a closed state. Novobiocin prevents ATP binding. (3) DNA cleavage: In the presence of Mg2+ ions, the enzyme temporarily cleaves the G segment of DNA by initiating a nucleophilic attack and forming two 5′-phosphotyrosyl bonds with the DNA backbone. (4) Strand passage: After the G segment is cleaved, the T segment is threaded through it. (5) T segment release and re-ligation: Once the T segment has passed through, it is released from the enzyme, and the cleaved G segment is rejoined. Etoposide and doxorubicin prevent the rejoining process. (6) G segment releases when the ATPase domain is opened: After the T segment is released, the enzyme stays in a closed clamp shape. Hydrolysis of ATP causes the closed clamp to open, allowing the G segment to be released and preparing the enzyme for the next reaction cycle. Bisdioxopiperazines, such as ICRF187 and ICRF193, inhibit the ATPase activity of the enzyme. (B) Chemical structural formula of doxorubicin.
TOPO II inhibitors are categorized into two types based on their mode of action: catalytic inhibitors and TOPO II poisons. Catalytic inhibitors of TOPO II hinder its enzymatic functions. They obstruct the enzyme either before the cleavage of DNA or after the re-ligation of DNA is completed. As a result, these inhibitors do not cause the accumulation of TOPO II-DNA cleavage complexes. The lack of TOPO II activity in relaxing DNA supercoils or disentangling sister chromatids during mitosis can lead to unsuccessful cell division and, ultimately, cell death [83]. TOPO II poisons prevent TOPO II from completing the catalytic cycle after DNA cleavage. As a result, they increase the accumulation of TOPO II-DNA cleavage complexes, which can cause DNA damage that the cell’s DNA repair system cannot handle. This leads to the accumulation of DNA breaks, ultimately triggering programmed cell death. TOPO II poisons include etoposide, doxorubicin, and amsacrine [84].
4.2. Doxorubicin Combination Therapy
Doxorubicin is one of the most widely studied TOPO II inhibitors (Figure 2B). Doxorubicin was isolated from Streptomyces peucetius actinobacteria in the 1960s and was subsequently developed as a cancer drug [85]. Doxorubicin is a chemotherapeutic drug belonging to the anthracycline antibiotic family, with the trade name adriamycin. It is widely recognized as one of the most effective treatments for solid tumors and is used to treat several types of cancers, including breast cancer, bladder cancer, Kaposi’s sarcoma, lymphoma, and acute lymphocytic leukemia [86]. Doxorubicin is widely used for the treatment of breast cancer [87,88]. Doxorubicin was approved for medical use in the United States in 1974 [12]. It is considered an essential medicine by the World Health Organization [89]. Although doxorubicin is an effective chemotherapy for various types of malignant tumors, its application is limited owing to the risk of cardiotoxicity [90]. Therefore, doxorubicin is often used in combination with other drugs or therapies to increase its efficacy and reduce its side effects or the risk of drug resistance. One of the most commonly used doxorubicin-based combination therapies is the AC-T regimen, which involves a combination of doxorubicin and cyclophosphamide (AC), followed by taxane drugs, such as paclitaxel or docetaxel (T). This combination effectively reduces the risk of recurrence in early-stage breast cancer [91]. Doxorubicin can also be used in combination with targeted therapies, such as trastuzumab (Herceptin®) or pertuzumab (Perjeta®), in breast cancers that overexpress the human epidermal growth factor receptor 2 (HER2) protein. These targeted therapies function by blocking HER2 protein, which promotes the growth of cancer cells. When used in combination with doxorubicin, these targeted therapies can improve treatment effectiveness [92,93].
4.3. Clinical Status of Doxorubicin Combination Therapy in Breast Cancer
According to clinical trial reports, combination therapy with doxorubicin is the most widely studied treatment for breast cancer. Therefore, we have summarized the studies reporting the results of clinical trials on doxorubicin combination therapy for breast cancer (Table 2).
Table 2.
Clinical status of doxorubicin combination therapy in breast cancer.
| Drugs | Target Cancer | Purpose of Study | Clinical Trials Status | Clinical Trials Identifier | Refs. |
|---|---|---|---|---|---|
| Cisplatin, AC | Breast cancer | To evaluate cisplatin, a chemotherapy drug that has been shown to be active in the treatment of breast cancer and women with BRCA mutations | Phase 2 | NCT01670500 | [94,95,96,97] |
| Eribulin, AC | Inflammatory breast cancer HER2-negative carcinoma of breast |
Studying a drug called eribulin combined with standard therapy as a possible preoperative treatment for HER2-negative inflammatory breast cancer | Phase 2 | NCT02623972 | [98] |
| Bevacizumab, Paclitaxel, Gemcitabine hydrochloride, Pegfilgrastim, AC |
HER2-negative breast cancer Stage II breast cancer Stage IIIA breast cancer Stage IIIB breast cancer Stage IIIC breast cancer |
To investigate the efficacy and side effects of adding bevacizumab to the chemotherapy regimen in the treatment of stage 2 or 3 HER2-neu negative breast cancer in women | Phase 2 | NCT00679029 | [99] |
| AC, Paclitaxel, Tipifarnib |
Breast cancer Male breast cancer |
To study the side effects and optimal dose of tipifarnib when given with combination chemotherapy and how effective it is in treating patients with stage 2 or 3 breast cancer | Phase 1 Phase 2 |
NCT00470301 | [100,101] |
| AC-T (Docetaxel) |
Breast cancer | Comparison of disease-free survival after TAC versus AC-T in HER2-neu negative breast cancer patients who are eligible for surgery | Phase 3 | NCT00312208 | [102,103,104] |
| AC-T (Paclitaxel), Ixabepilone |
Breast cancer | Comparison of patients receiving AC and ixabepilone and patients receiving AC and weekly paclitaxel | Phase 3 | NCT00789581 | [105,106] |
| AC-T (Paclitaxel), Epoetin alfa, Filgrastim, Epirubicin hydrochloride, Fluorouracil |
Breast cancer | Comparing the effectiveness of chemotherapy with or without epoetin alfa for the treatment of women who have undergone surgery for stage I, II, or III breast cancer | Phase 3 | NCT00014222 | [107] |
| AC-T (Docetaxel), Trastuzumab, Carboplatin |
Breast neoplasms | Comparing the treatment outcomes of women with HER2-positive breast cancer who had positive lymph nodes or high-risk negative lymph nodes and were treated with adjuvant therapy including doxorubicin, cyclophosphamide, and docetaxel, with or without trastuzumab, versus those who received trastuzumab, docetaxel, and carboplatin | Phase 3 | NCT00021255 | [104,108,109,110,111,112,113] |
| FAC, Docetaxel |
Breast cancer | Comparison of disease-free survival rates after TAC combination therapy and FAC combination therapy | Phase 3 | NCT00688740 | [114,115,116] |
| FAC, Paclitaxel, Eribulin, Epirubicin |
Breast cancer | To find out if and how well eribulin given in combination with standard chemotherapy can treat early-stage breast cancer compared to paclitaxel given in combination with standard chemotherapy | Phase 2 | NCT01593020 | [117,118] |
| Doxorubicin, FEC | Breast cancer | Two combination chemotherapy regimens were studied to compare how effective they were in treating women who had surgery for breast cancer that had not spread to the lymph nodes | Phase 3 | NCT00087178 | [119] |
| TAC | Breast cancer | To see if we can find out if taxotere and/or adriamycin/cytoxan can make tumors smaller | Phase 2 | NCT00206518 | [120] |
| TAC | Breast cancer | To find out what effect (good or bad) TC or TAC has on early-stage HER2- breast cancer | Phase 3 | NCT00493870 | [121,122] |
| TAC, Paclitaxel, Gemcitabine |
Breast cancer | Studying three different combination chemotherapy regimens and comparing how effective they are in treating women who have had surgery for node-positive breast cancer | Phase 3 | NCT00093795 | [123,124] |
| AC-TH, Carboplatin |
Breast cancer | To evaluate the safety of trastuzumab for the treatment of HER2-positive nodule-positive or high-risk nodule-negative | Phase 4 | NCT02419742 | [125] |
| AC-THP, Atezolizumab, Trastuzumab emtansine |
Breast cancer | To evaluate the efficacy and safety of atezolizumab compared with placebo when given in combination with neoadjuvant dose-dense doxorubicin + cyclophosphamide followed by paclitaxel + trastuzumab + pertuzumab in patients with early HER2-positive breast cancer | Phase 3 | NCT03726879 | [126,127] |
AC, doxorubicin and cyclophosphamide; AC-T, sequential doxorubicin–cyclophosphamide and paclitaxel or docetaxel; AC-TH, doxorubicin plus cyclophosphamide, followed by paclitaxel plus trastuzumab; AC-THP, doxorubicin and cyclophosphamide, followed by paclitaxel, trastuzumab, and pertuzumab; FAC, fluorouracil, doxorubicin and cyclophosphamide; FEC, fluorouracil, epirubicin and cyclophosphamide; HER2, human epidermal growth factor receptor 2; TAC, docetaxel, doxorubicin, and cyclophosphamide; TC, docetaxel and cyclophosphamide.
According to 2023 cancer statistics in the United States, breast cancer accounts for 31% of new diagnoses in women, ranking first, and is also the second leading cause of estimated deaths in women [2]. The primary objectives of treatment for breast cancer that has not spread to other parts of the body (non-metastatic) are to eliminate the tumor from the breast and nearby lymph nodes and prevent cancer from returning and spreading to other areas. Local treatment for nonmetastatic breast cancer typically involves surgery to remove the tumor and nearby lymph nodes; radiation therapy may also be considered after surgery [128]. The use of adjuvant chemotherapy is crucial in lowering the likelihood of breast cancer recurrence and enhancing the survival rate of patients. The NCCN’s guidelines for breast cancer treatment suggest several adjuvant chemotherapy plans, such as AC-T (sequential doxorubicin–cyclophosphamide and paclitaxel or docetaxel), ACT (concurrent doxorubicin–cyclophosphamide and paclitaxel or docetaxel), AC (doxorubicin–cyclophosphamide), CMF (cyclophosphamide, methotrexate, and fluorouracil), and TC (docetaxel and cyclophosphamide). Sequential AC-T therapy is the most widely used regimen [91].
Numerous clinical studies have reported the use of doxorubicin-based AC-T regimens for breast cancer treatment. The aim of the NCT00312208 study was to compare the disease-free survival of patients with operable breast cancer with positive axillary lymph nodes who were HER2-neu negative and treated either with docetaxel combined with doxorubicin and cyclophosphamide (TAC) or with doxorubicin and cyclophosphamide, followed by docetaxel (AC-T) [102]. The NCT00312208 study, which included 3299 patients, analyzed the data after 10 years and found that TAC was not more effective than AC-T in women with early-stage breast cancer and positive lymph nodes. The toxicity profiles of the two treatment groups were different, which is consistent with previous reports [103].
The aim of NCT00021255, which has the highest number of references among the studies of doxorubicin-based AC-T therapy, was to evaluate the disease-free survival of women diagnosed with operable breast cancer and showing HER2-neu expression with positive or high-risk node-negative lymph nodes. In this study, the researchers compared the effectiveness of two adjuvant treatment regimens during the treatment period: doxorubicin, cyclophosphamide, and docetaxel with or without trastuzumab, docetaxel, and carboplatin [108]. HER2 (ERBB2) is a member of the human type 1 receptor tyrosine kinases [109]. In a certain percentage of breast cancers (approximately 15–20%), this gene is amplified, leading to the overexpression of the HER2 protein, resulting in the transformation of normal cells to cancerous cells [129,130]. Normally, HER2 is activated only when a ligand binds to one of the other three members of the HER family—epidermal growth factor receptor (EGFR)/HER1, HER3, or HER4)—leading to the formation of heterodimers with HER2 and the activation of its kinase activity [131]. However, when HER2 is overexpressed, it associates with itself and other HER family members in a ligand-independent manner [109]. Trastuzumab is a monoclonal antibody used to treat breast cancer overexpressing HER2 [132]. The NCT00021255 study, which involved 3222 patients, showed that the addition of adjuvant trastuzumab for one year resulted in significant improvements in disease-free and overall survival rates among women diagnosed with HER2-positive breast cancer [113]. Additionally, the loss of the tumor suppressor gene phosphatase and tensin homolog (PTEN) is associated with a worse prognosis in patients with HER2-amplified breast cancer; however, this is not related to trastuzumab resistance. This study demonstrated that PTEN deficiency is not a predictive factor for trastuzumab resistance in HER2-positive breast cancer [109].
5. Conclusions
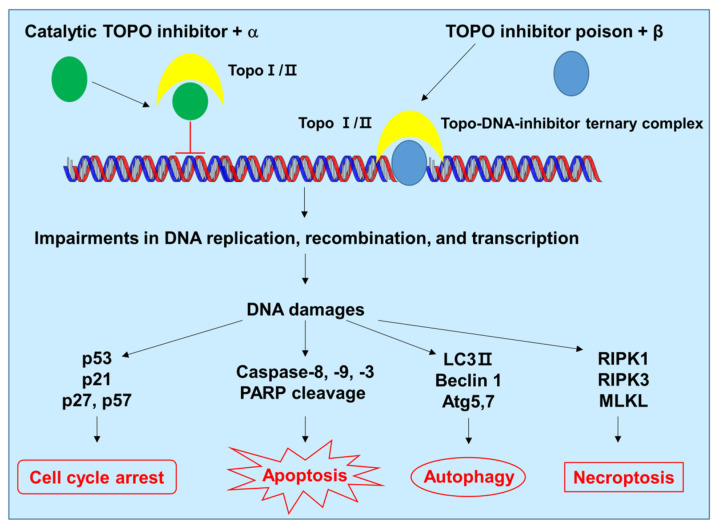
In this review, we described the clinical status of combination chemotherapy for CRC, primarily using irinotecan, the most extensively studied TOPO I inhibitor, and for breast cancer, primarily using doxorubicin, the most extensively studied TOPO II inhibitor. DNA replication, transcription, and repair are essential for every cell, and TOPOs play crucial roles in these processes. Owing to their significant biological functions, enzyme structures, and mechanisms of action, TOPOs have been a major focus in the development of novel anticancer agents. Combination chemotherapy with TOPO inhibitors induces cellular stress and cell death by causing cell cycle arrest, apoptosis, autophagy, and necroptosis pathways (Figure 3). However, TOPO inhibitors are subject to drug resistance, have significant dose-limiting toxicity, and can induce secondary cancers. Therefore, clinical studies on combination chemotherapy using TOPO inhibitors, together with other cancer therapeutic agents, are continually evolving to decrease these phenomena. Examples of current studies include ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) for Hodgkin’s lymphoma, CBV (cyclophosphamide, carmustine, and etoposide) for lymphoma, and CAV (cyclophosphamide, doxorubicin, and vincristine) for small cell lung cancer. These studies demonstrate new potential for cancer treatment using TOPO inhibitors.
Figure 3.
Proposed model of cell death induced by combination of chemotherapeutic drugs and TOPO inhibitors. The combination chemotherapy causes DNA damage, generates cellular stress, and induces cell death by activating cell cycle arrest, apoptosis, autophagy, and necroptosis pathways. α and β refer to other chemotherapeutic drugs used in combination therapy. Atg, autophagy related; LC3, microtubule-associated protein 1A/1B-light chain 3; MLKL, mixed lineage kinase domain-like protein; RIPK, receptor-interacting protein kinase; PARP, poly(ADP-ribose) polymerase.
Author Contributions
Conceptualization, J.Y.J., D.K. and N.D.K.; Investigation, J.Y.J. and D.K.; Writing—Original Draft Preparation, J.Y.J.; Writing—Review and Editing, D.K. and N.D.K.; Supervision, N.D.K.; Project Administration, J.Y.J. and N.D.K.; Funding Acquisition, N.D.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1A6A3A01085858) (J.Y.J.). This study was supported by the National Research Foundation of Korea (NRF) and funded by the Korean government (MSIT) (2021R1F1A1051265) (N.D.K.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Liang X., Wu Q., Luan S., Yin Z., He C., Yin L., Zou Y., Yuan Z., Li L., Song X., et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur. J. Med. Chem. 2019;171:129–168. doi: 10.1016/j.ejmech.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Singh V., Afshan T., Tyagi P., Varadwaj P.K., Sahoo A.K. Recent development of multi-targeted inhibitors of human topoisomerase II enzyme as potent cancer therapeutics. Int. J. Biol. Macromol. 2023;226:473–484. doi: 10.1016/j.ijbiomac.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Greco G., Pellicioni V., Cruz-Chamorro I., Attisani G., Stefanelli C., Fimognari C. Marine-derived compounds targeting topoisomerase II in cancer cells: A review. Mar. Drugs. 2022;20:674. doi: 10.3390/md20110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzun K., Bielawska A., Bielawski K., Gornowicz A. DNA topoisomerases as molecular targets for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2020;35:1781–1799. doi: 10.1080/14756366.2020.1821676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal S., Bajaj P., Pandey S., Tandon V. Topoisomerases: Resistance versus sensitivity, how far we can go? Med. Res. Rev. 2017;37:404–438. doi: 10.1002/med.21417. [DOI] [PubMed] [Google Scholar]
- 7.Matias-Barrios V.M., Dong X. The implication of topoisomerase II inhibitors in synthetic lethality for cancer therapy. Pharmaceuticals. 2023;16:94. doi: 10.3390/ph16010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado J.L., Hsieh C.M., Chan N.L., Hiasa H. Topoisomerases as anticancer targets. Biochem. J. 2018;475:373–398. doi: 10.1042/BCJ20160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuya S.M., Bjornsti M.A., van Waardenburg R. DNA topoisomerase-targeting chemotherapeutics: What’s new? Cancer Chemother. Pharmacol. 2017;80:1–14. doi: 10.1007/s00280-017-3334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Tse-Dinh Y.C. Recent advances in use of topoisomerase inhibitors in combination cancer therapy. Curr. Top. Med. Chem. 2019;19:730–740. doi: 10.2174/1568026619666190401113350. [DOI] [PubMed] [Google Scholar]
- 11.Bjornsti M.A., Kaufmann S.H. Topoisomerases and cancer chemotherapy: Recent advances and unanswered questions. F1000Research. 2019;8:F10000. doi: 10.12688/f1000research.20201.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hevener K., Verstak T.A., Lutat K.E., Riggsbee D.L., Mooney J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B. 2018;8:844–861. doi: 10.1016/j.apsb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felix C.A. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim. Biophys. Acta. 1998;1400:233–255. doi: 10.1016/S0167-4781(98)00139-0. [DOI] [PubMed] [Google Scholar]
- 14.Mistry A.R., Felix C.A., Whitmarsh R.J., Mason A., Reiter A., Cassinat B., Parry A., Walz C., Wiemels J.L., Segal M.R., et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N. Engl. J. Med. 2005;352:1529–1538. doi: 10.1056/NEJMoa042715. [DOI] [PubMed] [Google Scholar]
- 15.Azarova A.M., Lyu Y.L., Lin C.P., Tsai Y.C., Lau J.Y., Wang J.C., Liu L.F. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc. Natl. Acad. Sci. USA. 2007;104:11014–11019. doi: 10.1073/pnas.0704002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayat Mokhtari R., Homayouni T.S., Baluch N., Morgatskaya E., Kumar S., Das B., Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozhat Z., Heydarzadeh S., Memariani Z., Ahmadi A. Chemoprotective and chemosensitizing effects of apigenin on cancer therapy. Cancer Cell Int. 2021;21:574. doi: 10.1186/s12935-021-02282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redondo-Blanco S., Fernández J., Gutiérrez-Del-Río I., Villar C.J., Lombó F. New insights toward colorectal cancer chemotherapy using natural bioactive compounds. Front. Pharmacol. 2017;8:109. doi: 10.3389/fphar.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You F., Gao C. Topoisomerase inhibitors and targeted delivery in cancer therapy. Curr. Top. Med. Chem. 2019;19:713–729. doi: 10.2174/1568026619666190401112948. [DOI] [PubMed] [Google Scholar]
- 20.Jain C.K., Majumder H.K., Roychoudhury S. Natural compounds as anticancer agents targeting DNA topoisomerases. Curr. Genom. 2017;18:75–92. doi: 10.2174/1389202917666160808125213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker N.M., Rajan R., Mondragón A. Structural studies of type I topoisomerases. Nucleic Acids Res. 2009;37:693–701. doi: 10.1093/nar/gkn1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talukdar A., Kundu B., Sarkar D., Goon S., Mondal M.A. Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur. J. Med. Chem. 2022;236:114304. doi: 10.1016/j.ejmech.2022.114304. [DOI] [PubMed] [Google Scholar]
- 23.Capranico G., Marinello J., Chillemi G. Type I DNA topoisomerases. J. Med. Chem. 2017;60:2169–2192. doi: 10.1021/acs.jmedchem.6b00966. [DOI] [PubMed] [Google Scholar]
- 24.Wall M.E., Wani M.C., Cook C.A., Palmer K.H., McPhail A.T., Sim G.A. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata. J. Am. Chem. Soc. 1966;88:3888–3890. doi: 10.1021/ja00968a057. [DOI] [Google Scholar]
- 25.Kciuk M., Marciniak B., Kontek R. Irinotecan-still an important player in cancer chemotherapy: A comprehensive overview. Int. J. Mol. Sci. 2020;21:4919. doi: 10.3390/ijms21144919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita K., Kubota Y., Ishida H., Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015;21:12234–12248. doi: 10.3748/wjg.v21.i43.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailly C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019;148:104398. doi: 10.1016/j.phrs.2019.104398. [DOI] [PubMed] [Google Scholar]
- 28.Capecitabine and Irinotecan in Treating Patients with Locally Advanced, Recurrent, or Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2004 Available online: https://ClinicalTrials.gov/show/NCT00022698.
- 29.Meropol N.J., Gold P.J., Diasio R.B., Andria M., Dhami M., Godfrey T., Kovatich A.J., Lund K.A., Mitchell E., Schwarting R. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 2006;24:4069–4077. doi: 10.1200/JCO.2005.05.2084. [DOI] [PubMed] [Google Scholar]
- 30.Carlini L.E., Meropol N.J., Bever J., Andria M.L., Hill T., Gold P., Rogatko A., Wang H., Blanchard R.L. UGT1A7 and UGT1A9 polymorphisms predict response and toxicity in colorectal cancer patients treated with capecitabine/irinotecan. Clin. Cancer Res. 2005;11:1226–1236. doi: 10.1158/1078-0432.1226.11.3. [DOI] [PubMed] [Google Scholar]
- 31.Research Study of IV Vitamin C in Combination with Irinotecan vs. Irinotecan Alone for Advanced Colorectal Cancer. [(accessed on 17 March 2023)];2015 Available online: https://ClinicalTrials.gov/show/NCT01550510.
- 32.A Study of the Combination of Pemetrexed and Irinotecan Every Two Weeks in Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2007 Available online: https://ClinicalTrials.gov/show/NCT00191984.
- 33.Louvet C., André T., Gamelin E., Hebbar M., Mabro M., Bennamoun M., Rassam H., de Gramont A. Phase II study of biweekly pemetrexed plus irinotecan as second-line therapy for metastatic colorectal cancer. J. Oncol. 2010;2010:785934. doi: 10.1155/2010/785934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ISIS 183750 with Irinotecan for Advanced Solid Tumors or Colorectal Cancer. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT01675128.
- 35.Duffy A.G., Makarova-Rusher O.V., Ulahannan S.V., Rahma O.E., Fioravanti S., Walker M., Abdullah S., Raffeld M., Anderson V., Abi-Jaoudeh N., et al. Modulation of tumor eIF4E by antisense inhibition: A phase I/II translational clinical trial of ISIS 183750-an antisense oligonucleotide against eIF4E-in combination with irinotecan in solid tumors and irinotecan-refractory colorectal cancer. Int. J. Cancer. 2016;139:1648–1657. doi: 10.1002/ijc.30199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makarova-Rusher O.V., Duffy A.G., Ulahannan S.V., Fioravanti S., Walker M., Raffeld M., Compton K., Lee S., Tomita Y., Trepel J.B. Phase I/II study of ISIS 183750 in combination with irinotecan for advanced solid tumors or colorectal cancer: Final results. J. Clin. Oncol. 2015;33:639. doi: 10.1200/jco.2015.33.3_suppl.639. [DOI] [Google Scholar]
- 37.A Safety Study of MM-121 with Cetuximab and Irinotecan in Patients with Advanced Cancers. [(accessed on 17 March 2023)];2013 Available online: https://ClinicalTrials.gov/show/NCT01451632.
- 38.Cleary J.M., McRee A.J., O’Neil B.H., Sharma S., Pearlberg J., Manoli S., Kubasek W.L., Korn W.M. A phase 1 study of MM-121 (a fully human monoclonal antibody targeting the epidermal growth factor receptor family member ErbB3) in combination with cetuximab and irinotecan in patients with advanced cancers. J. Clin. Oncol. 2014;32:3076. doi: 10.1200/jco.2014.32.15_suppl.3076. [DOI] [Google Scholar]
- 39.Phase II Trial of Combination Therapy with Irinotecan, S-1, and Bevacizumab (IRIS/Bev) in Patients with Unresectable or Recurrent Colorectal Cancer. [(accessed on 17 March 2023)];2010 Available online: https://ClinicalTrials.gov/show/NCT00569790.
- 40.Komatsu Y., Yuki S., Sogabe S., Fukushima H., Nakatsumi H., Kobayashi Y., Iwanaga I., Nakamura M., Hatanaka K., Miyagishima T., et al. Phase II study of combined chemotherapy with irinotecan and S-1 (IRIS) plus bevacizumab in patients with inoperable recurrent or advanced colorectal cancer. Acta Oncol. 2012;51:867–872. doi: 10.3109/0284186X.2012.682629. [DOI] [PubMed] [Google Scholar]
- 41.Arq 197 in Combination With Chemotherapy in Patients with Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2012 Available online: https://ClinicalTrials.gov/show/NCT01075048.
- 42.Eng C., Hart L.L., Severtsev A., Gladkov O., Mueller L., Kopp M.V., Vladimirov V.I., Langdon R.M., Kotiv B., Barni S., et al. A randomized, placebo-controlled, phase I/II study of tivantinib (ARQ 197) in combination with cetuximab and irinotecan in patients (pts) with KRAS wild-type (WT) metastatic colorectal cancer (CRC) who had received previous front-line systemic therapy. J. Clin. Oncol. 2013;31:3508. doi: 10.1200/jco.2013.31.15_suppl.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phase I/II Study of SGI-110 with Irinotecan Versus Regorafenib or TAS-102 in Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2019 Available online: https://ClinicalTrials.gov/show/NCT01896856.
- 44.Sharma A., Vatapalli R., Abdelfatah E., Wyatt McMahon K., Kerner Z., Guzzetta A., Singh J., Zahnow C., Baylin S.B., Yerram S., et al. Hypomethylating agents synergize with irinotecan to improve response to chemotherapy in colorectal cancer cells. PLoS ONE. 2017;12:e0176139. doi: 10.1371/journal.pone.0176139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYSTAL) [(accessed on 17 March 2023)];2006 Available online: https://ClinicalTrials.gov/show/NCT00154102.
- 46.Van Cutsem E., Köhne C.H., Hitre E., Zaluski J., Chang Chien C.R., Makhson A., D’Haens G., Pintér T., Lim R., Bodoky G., et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 47.Cutsem E.V., Köhne C.-H., Láng I., Folprecht G., Nowacki M.P., Cascinu S., Shchepotin I., Maurel J., Cunningham D., Tejpar S., et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 48.Tejpar S., Stintzing S., Ciardiello F., Tabernero J., Van Cutsem E., Beier F., Esser R., Lenz H.J., Heinemann V. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3:194–201. doi: 10.1001/jamaoncol.2016.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Licitra L., Störkel S., Kerr K.M., Van Cutsem E., Pirker R., Hirsch F.R., Vermorken J.B., von Heydebreck A., Esser R., Celik I., et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: Analysis of data from the EXTREME and CRYSTAL studies. Eur. J. Cancer. 2013;49:1161–1168. doi: 10.1016/j.ejca.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Regorafenib+FOLFIRI Versus Placebo+FOLFIRI as 2nd Line Tx in Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2016 Available online: https://ClinicalTrials.gov/show/NCT01298570.
- 51.Quintanilha J.C.F., Geyer S., Etheridge A.S., Racioppi A., Hammond K., Crona D.J., Peña C.E., Jacobson S.B., Marmorino F., Rossini D., et al. KDR genetic predictor of toxicities induced by sorafenib and regorafenib. Pharm. J. 2022;22:251–257. doi: 10.1038/s41397-022-00279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultheis B., Folprecht G., Kuhlmann J., Ehrenberg R., Hacker U.T., Köhne C.H., Kornacker M., Boix O., Lettieri J., Krauss J., et al. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: Results of a multicenter, phase Ib study. Ann. Oncol. 2013;24:1560–1567. doi: 10.1093/annonc/mdt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A Study of ABT-165 plus FOLFIRI vs. Bevacizumab plus FOLFIRI in Subjects with Metastatic Colorectal Cancer Previously Treated with Fluoropyrimidine, Oxaliplatin and Bevacizumab. [(accessed on 17 March 2023)];2019 Available online: https://ClinicalTrials.gov/show/NCT03368859.
- 54.Strickler J.H., Cubillo A., Liang J.T., Matrana M., Kozloff M., Lowe T., Blaney M., Sahtout M., Naumovski L., Wainberg Z.A. Efficacy and safety of dilpacimab (ABT-165) versus bevacizumab plus FOLFIRI in metastatic colorectal cancer: A phase II study. Future Oncol. 2022;18:3011–3020. doi: 10.2217/fon-2021-1603. [DOI] [PubMed] [Google Scholar]
- 55.Study of Encorafenib + Cetuximab plus or Minus Binimetinib vs. Irinotecan/Cetuximab or Infusional 5-Fluorouracil (5-FU)/Folinic Acid (FA)/Irinotecan (FOLFIRI)/Cetuximab with a Safety Lead-in of Encorafenib + Binimetinib + Cetuximab in Patients with BRAF V600E-Mutant Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2019 Available online: https://ClinicalTrials.gov/show/NCT02928224.
- 56.Stintzing S., Seufferlein T., Rosé C., Reichenbach F., Lüftner D. Encorafenib in combination with cetuximab after systemic therapy in patients with BRAF(V600E) mutant metastatic colorectal cancer: German health technology assessment-driven analyses from the BEACON CRC study. Clin. Color. Cancer. 2022;21:244–251. doi: 10.1016/j.clcc.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Kopetz S., Grothey A., Van Cutsem E., Yaeger R., Wasan H., Yoshino T., Desai J., Ciardiello F., Loupakis F., Hong Y.S., et al. Quality of life with encorafenib plus cetuximab with or without binimetinib treatment in patients with BRAF V600E-mutant metastatic colorectal cancer: Patient-reported outcomes from BEACON CRC. ESMO Open. 2022;7:100477. doi: 10.1016/j.esmoop.2022.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabernero J., Grothey A., Van Cutsem E., Yaeger R., Wasan H., Yoshino T., Desai J., Ciardiello F., Loupakis F., Hong Y.S., et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: Updated survival results and subgroup analyses from the BEACON study. J. Clin. Oncol. 2021;39:273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kopetz S., Grothey A., Yaeger R., Van Cutsem E., Desai J., Yoshino T., Wasan H., Ciardiello F., Loupakis F., Hong Y.S., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N. Engl. J. Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 60.Van Cutsem E., Huijberts S., Grothey A., Yaeger R., Cuyle P.J., Elez E., Fakih M., Montagut C., Peeters M., Yoshino T., et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: Safety lead-in results from the phase III BEACON colorectal cancer study. J. Clin. Oncol. 2019;37:1460–1469. doi: 10.1200/JCO.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.A Study of Napabucasin (BBI-608) in Combination with FOLFIRI in Adult Patients with Previously Treated Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2020 Available online: https://ClinicalTrials.gov/show/NCT02753127.
- 62.FOLFIRI and Panitumumab in Treating Patients with RAS and BRAF Wild-Type Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2017 Available online: https://ClinicalTrials.gov/show/NCT02508077.
- 63.A Study in Second Line Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT01183780.
- 64.Tabernero J., Yoshino T., Cohn A.L., Obermannova R., Bodoky G., Garcia-Carbonero R., Ciuleanu T.E., Portnoy D.C., Van Cutsem E., Grothey A., et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/s1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 65.Obermannová R., Van Cutsem E., Yoshino T., Bodoky G., Prausová J., Garcia-Carbonero R., Ciuleanu T., Garcia Alfonso P., Portnoy D., Cohn A., et al. Subgroup analysis in RAISE: A randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann. Oncol. 2016;27:2082–2090. doi: 10.1093/annonc/mdw402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tabernero J., Hozak R.R., Yoshino T., Cohn A.L., Obermannova R., Bodoky G., Garcia-Carbonero R., Ciuleanu T.E., Portnoy D.C., Prausová J., et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann. Oncol. 2018;29:602–609. doi: 10.1093/annonc/mdx767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grothey A., Yoshino T., Bodoky G., Ciuleanu T., Garcia-Carbonero R., García-Alfonso P., Van Cutsem E., Muro K., Mytelka D.S., Li L., et al. Association of baseline absolute neutrophil counts and survival in patients with metastatic colorectal cancer treated with second-line antiangiogenic therapies: Exploratory analyses of the RAISE trial and validation in an electronic medical record data set. ESMO Open. 2018;3:e000347. doi: 10.1136/esmoopen-2018-000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshino T., Portnoy D.C., Obermannová R., Bodoky G., Prausová J., Garcia-Carbonero R., Ciuleanu T., García-Alfonso P., Cohn A.L., Van Cutsem E., et al. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann. Oncol. 2019;30:124–131. doi: 10.1093/annonc/mdy461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim H.H., Hopkins A.M., Rowland A., Yuen H.Y., Karapetis C.S., Sorich M.J. Effect of early adverse events on survival outcomes of patients with metastatic colorectal cancer treated with ramucirumab. Target. Oncol. 2019;14:743–748. doi: 10.1007/s11523-019-00683-z. [DOI] [PubMed] [Google Scholar]
- 70.Taniguchi H., Yoshino T., Yamaguchi K., Yamazaki K., Nixon A.B., Tabernero J., Van Cutsem E., Robling K.R., Abada P.B., Hozak R.R., et al. Clinical development and evaluation of a VEGF-D assay in plasma from patients with metastatic colorectal cancer in the RAISE study. Curr. Med. Res. Opin. 2021;37:1769–1778. doi: 10.1080/03007995.2021.1940908. [DOI] [PubMed] [Google Scholar]
- 71.Sequential and Concurrent FOLFOXIRI/Bevacizumab Regimens versus FOLFOX/Bevacizumab in First-Line Metastatic Colorectal Cancer. [(accessed on 17 March 2023)];2016 Available online: https://ClinicalTrials.gov/show/NCT01765582.
- 72.Cremolini C., Antoniotti C., Stein A., Bendell J., Gruenberger T., Rossini D., Masi G., Ongaro E., Hurwitz H., Falcone A., et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J. Clin. Oncol. 2020;38:3314–3324. doi: 10.1200/JCO.20.01225. [DOI] [PubMed] [Google Scholar]
- 73.Hurwitz H.I., Tan B.R., Reeves J.A., Xiong H., Somer B., Lenz H.J., Hochster H.S., Scappaticci F., Palma J.F., Price R., et al. Phase II randomized trial of sequential or concurrent FOLFOXIRI-bevacizumab versus FOLFOX-bevacizumab for metastatic colorectal cancer (STEAM) Oncologist. 2019;24:921–932. doi: 10.1634/theoncologist.2018-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.FOLFOXIRI plus Panitumumab Patients with Metastatic KRAS Wild-Type Colorectal Cancer with Liver Metastases Only. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT01226719.
- 75.Bendell J.C., Zakari A., Peyton J.D., Boccia R., Moskowitz M., Gian V., Lipman A., Waterhouse D., LoCicero R., Earwood C., et al. A phase II study of FOLFOXIRI plus panitumumab followed by evaluation for resection in patients with metastatic KRAS wild-type colorectal cancer with liver metastases only. Oncologist. 2016;21:279–280. doi: 10.1634/theoncologist.2015-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang Y.J., Jang J.Y., Kwon Y.H., Lee J.H., Lee S., Park Y., Jung Y.S., Im E., Moon H.R., Chung H.Y., et al. MHY2245, a sirtuin inhibitor, induces cell cycle arrest and apoptosis in HCT116 human colorectal cancer cells. Int. J. Mol. Sci. 2022;23:1590. doi: 10.3390/ijms23031590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi Y.J., Choi C.Y., Rhie S.J., Shin S. Safety assessment on serious adverse events of targeted therapeutic agents prescribed for RAS wild-type metastatic colorectal cancer: Systematic review and network meta-analysis. Int. J. Environ. Res. Public Health. 2022;19:9196. doi: 10.3390/ijerph19159196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ridouane Y., Lopes G., Ku G., Masud H., Haaland B. Targeted first-line therapies for advanced colorectal cancer: A Bayesian meta-analysis. Oncotarget. 2017;8:66458–66466. doi: 10.18632/oncotarget.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turkes F., Chau I. Ramucirumab and its use in the treatment of hepatocellular carcinoma. Future Oncol. 2019;15:979–988. doi: 10.2217/fon-2018-0822. [DOI] [PubMed] [Google Scholar]
- 80.Dolatkhah R., Dastgiri S., Eftekhar Sadat A.T., Farassati F., Nezamdoust M., Somi M.H. Impact of RAS/RAF mutations on clinical and prognostic outcomes in metastatic colorectal cancer. Bioimpacts. 2021;11:5–14. doi: 10.34172/bi.2021.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okoro C.O., Fatoki T.H. A mini review of novel topoisomerase II inhibitors as future anticancer agents. Int. J. Mol. Sci. 2023;24:2532. doi: 10.3390/ijms24032532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J.H., Berger J.M. Cell Cycle-Dependent Control and Roles of DNA Topoisomerase II. Genes. 2019;10:859. doi: 10.3390/genes10110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matias-Barrios V.M., Radaeva M., Song Y., Alperstein Z., Lee A.R., Schmitt V., Lee J., Ban F., Xie N., Qi J., et al. Discovery of new catalytic topoisomerase II inhibitors for anticancer therapeutics. Front. Oncol. 2021;10:633142. doi: 10.3389/fonc.2020.633142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vann K.R., Oviatt A.A., Osheroff N. Topoisomerase II poisons: Converting essential enzymes into molecular scissors. Biochemistry. 2021;60:1630–1641. doi: 10.1021/acs.biochem.1c00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Can M.M., Shawuti S., Kalindemirtas F.D., Erdemir G., Kuruca D.S., Kaneko S., Aktas Z., Oncul O. Anticancer drug doxorubicin (DOX) loading performance of functionalized polyaniline (PANI) surface with active carbon. J. Mater. Sci. 2023;58:4726–4738. doi: 10.1007/s10853-023-08291-z. [DOI] [Google Scholar]
- 86.Roychoudhury S., Kumar A., Bhatkar D., Sharma N.K. Molecular avenues in targeted doxorubicin cancer therapy. Future Oncol. 2020;16:687–700. doi: 10.2217/fon-2019-0458. [DOI] [PubMed] [Google Scholar]
- 87.Argenziano M., Gigliotti C.L., Clemente N., Boggio E., Ferrara B., Trotta F., Pizzimenti S., Barrera G., Boldorini R., Bessone F., et al. Improvement in the anti-tumor efficacy of doxorubicin nanosponges in in vitro and in mice bearing breast tumor models. Cancers. 2020;12:162. doi: 10.3390/cancers12010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran Q.H., Hoang D.H., Song M., Choe W., Kang I., Kim S.S., Ha J. Melatonin and doxorubicin synergistically enhance apoptosis via autophagy-dependent reduction of AMPKα1 transcription in human breast cancer cells. Exp. Mol. Med. 2021;53:1413–1422. doi: 10.1038/s12276-021-00675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baxi S.M., Beall R., Yang J., Mackey T.K. A multidisciplinary review of the policy, intellectual property rights, and international trade environment for access and affordability to essential cancer medications. Global. Health. 2019;15:57. doi: 10.1186/s12992-019-0497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rawat P.S., Jaiswal A., Khurana A., Bhatti J.S., Navik U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021;139:111708. doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 91.Fujii T., Le Du F., Xiao L., Kogawa T., Barcenas C.H., Alvarez R.H., Valero V., Shen Y., Ueno N.T. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: A systematic review and network meta-analysis. JAMA Oncol. 2015;1:1311–1318. doi: 10.1001/jamaoncol.2015.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.López-Miranda E., Pérez-García J.M., Di Cosimo S., Brain E., Ravnik M., Escrivá-de-Romaní S., Vidal M., Gligorov J., Borštnar S., Calabuig L., et al. Trastuzumab emtansine plus non-pegylated liposomal doxorubicin in HER2-positive metastatic breast cancer (thelma): A single-arm, multicenter, phase Ib trial. Cancers. 2020;12:3509. doi: 10.3390/cancers12123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Medina E.A.G., Caballero B.B., Miguel K.L., Gutiérrez Z.A., Fernández B.M., Tul L.E.A., Rodríguez L.E.M., Guerrero O.V., Varela I.G.S., Bernardo M.C.C., et al. Neoadjuvant trastuzumab and pertuzumab in combination with standard chemotherapy for HER2-positive early breast cancer: Real-world practice in Cuba. Cancer Treat. Res. Commun. 2023;34:100670. doi: 10.1016/j.ctarc.2022.100670. [DOI] [PubMed] [Google Scholar]
- 94.Cisplatin vs. Doxorubicin/Cyclophosphamide in BrCa. [(accessed on 17 March 2023)];2019 Available online: https://ClinicalTrials.gov/show/NCT01670500.
- 95.Tung N., Arun B., Hacker M.R., Hofstatter E., Toppmeyer D.L., Isakoff S.J., Borges V., Legare R.D., Isaacs C., Wolff A.C., et al. TBCRC 031: Randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial) J. Clin. Oncol. 2020;38:1539–1548. doi: 10.1200/JCO.19.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tung N., Hacker M.R., Garber J.E. Reply to S. Takamizawa et al. J. Clin. Oncol. 2020;38:2700–2701. doi: 10.1200/JCO.20.01190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takamizawa S., Ishiki H., Shimoi T., Shimizu M., Satomi E. Neoadjuvant cisplatin in BRCA carriers with HER2-negative breast cancer. J. Clin. Oncol. 2020;38:2699–2700. doi: 10.1200/JCO.20.00789. [DOI] [PubMed] [Google Scholar]
- 98.A Phase 2 Study of Eribulin Followed by AC as Preoperative Therapy for HER2-Negative Inflammatory Breast Cancer. [(accessed on 17 March 2023)];2021 Available online: https://ClinicalTrials.gov/show/NCT02623972.
- 99.Combination Chemotherapy and Bevacizumab in Treating Women with HER2/Neu-Negative Stage II or Stage III Breast Cancer. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT00679029.
- 100.Tipifarnib and Combination Chemotherapy in Treating Patients with Stage II or Stage III Breast Cancer. [(accessed on 17 March 2023)];2013 Available online: https://ClinicalTrials.gov/show/NCT00470301.
- 101.Andreopoulou E., Vigoda I.S., Valero V., Hershman D.L., Raptis G., Vahdat L.T., Han H.S., Wright J.J., Pellegrino C.M., Cristofanilli M., et al. Phase I-II study of the farnesyl transferase inhibitor tipifarnib plus sequential weekly paclitaxel and doxorubicin-cyclophosphamide in HER2/neu-negative inflammatory carcinoma and non-inflammatory estrogen receptor-positive breast carcinoma. Breast Cancer Res. Treat. 2013;141:429–435. doi: 10.1007/s10549-013-2704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Docetaxel in Breast Cancer. [(accessed on 17 March 2023)];2008 Available online: https://ClinicalTrials.gov/show/NCT00312208.
- 103.Mackey J.R., Pieńkowski T., Crown J., Sadeghi S., Martin M., Chan A., Saleh M., Sehdev S., Provencher L., Semiglazov V., et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann. Oncol. 2016;27:1041–1047. doi: 10.1093/annonc/mdw098. [DOI] [PubMed] [Google Scholar]
- 104.Press M.F., Sauter G., Buyse M., Fourmanoir H., Quinaux E., Tsao-Wei D.D., Eiermann W., Robert N., Pienkowski T., Crown J., et al. HER2 gene amplification testing by fluorescent in situ hybridization (FISH): Comparison of the ASCO-college of American pathologists guidelines with FISH scores used for enrollment in breast cancer international research group clinical trials. J. Clin. Oncol. 2016;34:3518–3528. doi: 10.1200/JCO.2016.66.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.A Randomized Trial of Ixempra versus Taxol in Adjuvant Therapy of Triple Negative Breast Cancer. [(accessed on 17 March 2023)];2015 Available online: https://ClinicalTrials.gov/show/NCT00789581.
- 106.Yardley D.A., Arrowsmith E.R., Daniel B.R., Eakle J., Brufsky A., Drosick D.R., Kudrik F., Bosserman L.D., Keaton M.R., Goble S.A., et al. TITAN: Phase III study of doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early-stage triple-negative breast cancer. Breast Cancer Res. Treat. 2017;164:649–658. doi: 10.1007/s10549-017-4285-6. [DOI] [PubMed] [Google Scholar]
- 107.Combination Chemotherapy with or Without Colony-Stimulating Factors in Treating Women with Breast Cancer. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT00014222.
- 108.Combination Chemotherapy with or without Trastuzumab in Treating Women with Breast Cancer. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT00021255.
- 109.Stern H.M., Gardner H., Burzykowski T., Elatre W., O’Brien C., Lackner M.R., Pestano G.A., Santiago A., Villalobos I., Eiermann W., et al. PTEN loss is associated with worse outcome in HER2-amplified breast cancer patients but is not associated with trastuzumab resistance. Clin. Cancer. Res. 2015;21:2065–2074. doi: 10.1158/1078-0432.CCR-14-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Press M.F., Sauter G., Buyse M., Bernstein L., Guzman R., Santiago A., Villalobos I.E., Eiermann W., Pienkowski T., Martin M., et al. Alteration of topoisomerase II-alpha gene in human breast cancer: Association with responsiveness to anthracycline-based chemotherapy. J. Clin. Oncol. 2011;29:859–867. doi: 10.1200/JCO.2009.27.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez E.A., Press M.F., Dueck A.C., Jenkins R.B., Kim C., Chen B., Villalobos I., Paik S., Buyse M., Wiktor A.E., et al. Immunohistochemistry and fluorescence in situ hybridization assessment of HER2 in clinical trials of adjuvant therapy for breast cancer (NCCTG N9831, BCIRG 006, and BCIRG 005) Breast Cancer Res. Treat. 2013;138:99–108. doi: 10.1007/s10549-013-2444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Au H.J., Eiermann W., Robert N.J., Pienkowski T., Crown J., Martin M., Pawlicki M., Chan A., Mackey J., Glaspy J., et al. Health-related quality of life with adjuvant docetaxel- and trastuzumab-based regimens in patients with node-positive and high-risk node-negative, HER2-positive early breast cancer: Results from the BCIRG 006 Study. Oncologist. 2013;18:812–818. doi: 10.1634/theoncologist.2013-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Slamon D., Eiermann W., Robert N., Pienkowski T., Martin M., Press M., Mackey J., Glaspy J., Chan A., Pawlicki M., et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Docetaxel in Node Positive Adjuvant Breast Cancer. [(accessed on 17 March 2023)];2010 Available online: https://ClinicalTrials.gov/show/NCT00688740.
- 115.Mackey J.R., Martin M., Pienkowski T., Rolski J., Guastalla J.P., Sami A., Glaspy J., Juhos E., Wardley A., Fornander T., et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14:72–80. doi: 10.1016/S1470-2045(12)70525-9. [DOI] [PubMed] [Google Scholar]
- 116.Martin M., Pienkowski T., Mackey J., Pawlicki M., Guastalla J.P., Weaver C., Tomiak E., Al-Tweigeri T., Chap L., Juhos E., et al. Adjuvant docetaxel for node-positive breast cancer. N. Engl. J. Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 117.Neoadjuvant Study of Sequential Eribulin followed by FAC Compared to Sequential Paclitaxel followed by FEC in Early Stage Breast Cancer not Overexpressing HER-2. [(accessed on 17 March 2023)];2020 Available online: https://ClinicalTrials.gov/show/NCT01593020.
- 118.Lim B., Song J., Ibrahim N.K., Koenig K.B., Chavez-MacGregor M., Ensor J.E., Jr., Gomez J.S., Krishnamurthy S., Caudle A.S., Shaitelman S.F., et al. A randomized phase II study of sequential eribulin versus paclitaxel followed by FAC/FEC as neoadjuvant therapy in patients with operable HER2-negative breast cancer. Oncologist. 2021;26:e230–e240. doi: 10.1002/onco.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Comparison of Two Combination Chemotherapy Regimens in Treating Women with Breast Cancer. [(accessed on 17 March 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT00087178.
- 120.Taxotere and Adriamycin/Cytoxan (AC) Validation in Breast Cancer Patients. [(accessed on 17 March 2023)];2016 Available online: https://ClinicalTrials.gov/show/NCT00206518.
- 121.TAC Versus TC for Adjuvant Breast Cancer. [(accessed on 17 March 2023)];2015 Available online: https://ClinicalTrials.gov/show/NCT00493870.
- 122.Blum J.L., Flynn P.J., Yothers G., Asmar L., Geyer C.E., Jr., Jacobs S.A., Robert N.J., Hopkins J.O., O’Shaughnessy J.A., Dang C.T., et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology) J. Clin. Oncol. 2017;35:2647–2655. doi: 10.1200/JCO.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Positive Breast Cancer. [(accessed on 17 March 2023)];2012 Available online: https://ClinicalTrials.gov/show/NCT00093795.
- 124.Swain S.M., Tang G., Geyer C.E., Jr., Rastogi P., Atkins J.N., Donnellan P.P., Fehrenbacher L., Azar C.A., Robidoux A., Polikoff J.A., et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 trial. J. Clin. Oncol. 2013;31:3197–3204. doi: 10.1200/JCO.2012.48.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Safety and Efficacy of Trastuzumab as Part of Breast Cancer Treatment Regimen. [(accessed on 17 March 2023)];2021 Available online: https://ClinicalTrials.gov/show/NCT02419742.
- 126.A Study to Evaluate the Efficacy and Safety of Atezolizumab or Placebo in Combination with Neoadjuvant Doxorubicin + Cyclophosphamide followed by Paclitaxel + Trastuzumab + Pertuzumab in Early Her2-Positive Breast Cancer. [(accessed on 17 March 2023)];2021 Available online: https://ClinicalTrials.gov/show/NCT03726879.
- 127.Huober J., Barrios C.H., Niikura N., Jarząb M., Chang Y.C., Huggins-Puhalla S.L., Pedrini J., Zhukova L., Graupner V., Eiger D., et al. Atezolizumab with neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2-positive early breast cancer: Primary results of the randomized phase III IMpassion050 trial. J. Clin. Oncol. 2022;40:2946–2956. doi: 10.1200/JCO.21.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Waks A.G., Winer E.P. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 129.Portnow L.H., Kochkodan-Self J.M., Maduram A., Barrios M., Onken A.M., Hong X., Mittendorf E.A., Giess C.S., Chikarmane S.A. Multimodality imaging review of HER2-positive breast cancer and response to neoadjuvant chemotherapy. Radiographics. 2023;43:e220103. doi: 10.1148/rg.220103. [DOI] [PubMed] [Google Scholar]
- 130.Suppan C., Balic M. Current standards and future outlooks in metastatic Her2-positive breast cancer. Breast Care. 2023;18:69–75. doi: 10.1159/000528756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vega Cano K.S., Marmolejo Castañeda D.H., Escrivá-de-Romaní S., Saura C. Systemic therapy for HER2-positive metastatic breast cancer: Current and future trends. Cancers. 2022;15:51. doi: 10.3390/cancers15010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peliçário Vargas B., Sari M.H.M., Ferreira L.M. Trastuzumab in breast cancer treatment: The era of biosimilars. Anticancer Agents Med. Chem. 2022;22:2507–2516. doi: 10.2174/1871520622666220302114313. [DOI] [PubMed] [Google Scholar]