Abstract
Loss of function mutations in the P5-ATPase ATP13A2 are associated with Kufor-Rakeb Syndrome and Neuronal Ceroid Lipofuscinosis. While the function of ATP13A2 is unclear, in vitro studies suggest it is a lysosomal protein that interacts with the metals manganese (Mn) and zinc and the presynaptic protein alpha-synuclein. Loss of ATP13A2 function in mice causes sensorimotor deficits, enhanced autofluorescent storage material, and accumulation of alpha-synuclein. The present study sought to determine the effect of Mn administration on these same outcomes in ATP13A2-deficient mice. Wildtype and ATP13A2-deficient mice received saline or Mn at 5–9 or 12–19 months for 45 days. Sensorimotor function was assessed starting at day 30. Autofluorescence was quantified in multiple brain regions and alpha-synuclein protein levels were determined in the ventral midbrain. Brain Mn, iron, zinc, and copper concentrations were measured in 5–9 month old mice. The results show Mn enhanced sensorimotor function, increased autofluorescence in the substantia nigra, and increased insoluble alpha-synuclein in the ventral midbrain in older ATP13A2-deficient mice. In addition, the Mn regimen used increased Mn concentration in the brain and levels were higher in Mn-treated mutants than controls. These results indicate loss of ATP13A2 function leads to increased sensitivity to Mn in vivo.
Keywords: alpha-synuclein, Parkinson’s disease, manganese, sensorimotor function, mice, lipofuscin
Introduction
ATP13A2 is a P5-ATPase of the P-type ion transport ATPase superfamily (Schultheis et al., 2004). Its substrate specificity and physiological function are unknown, however studies suggest it is involved in lysosomal degradation of proteins, manganese (Mn) homeostasis, and most recently zinc (Zn) transport (Gitler et al., 2009; Chesi et al., 2012; Matsui et al., 2013; Ramonet et al., 2012; Tan et al., 2011; Park et al., 2014; Kong et al., 2014; Tsunemi and Krainc, 2014). Loss of function mutations in ATP13A2 cause Neuronal Ceroid Lipofuscinosis and Kufor-Rakeb Syndrome, the latter an autosomal recessive form of Parkinsonism (PARK9; Ramirez et al., 2006; Farias et al., 2011; Whölke et al., 2011; Bras et al., 2012). Studies in yeast and cell culture show an interaction between ATP13A2 and Mn with loss of function mutations resulting in enhanced Mn toxicity and overexpression of ATP13A2 protecting against Mn toxicity (Gitler et al., 2009, Chesi et al., 2012; Schmidt et al., 2009; Covy et al., 2012; Daniel et al., 2015). Similarly, ATP13A2 protects mammalian cell lines and rat primary neuronal cultures from Mn-induced cell death and is upregulated in response to Mn treatment (Gitler et al., 2009, Tan et al., 2011, Covy et., 2012). These data indicate ATP13A2 may be involved in regulating intracellular Mn homeostasis (Tan et al., 2011). Indeed, polymorphisms of ATP13A2 are shown to modify the effects of Mn on motor function in an elderly population (Rentschler et al., 2012).
In addition to its relationship with Mn, in vitro studies show ATP13A2 also interacts with alpha-synuclein (aSyn), a presynaptic protein that abnormally accumulates in lewy bodies in the neurodegenerative disorders Parkinson’s disease, multiple system atrophy, and lewy body dementia (Polymeropooulos et al., 1997; Spillantini et al., 1997). ATP13A2 expression can suppress aSyn toxicity in yeast and rescue aSyn-induced dopaminergic degeneration in primary neuronal culture (Gitler et al., 2009). However, a recent study did not find neuroprotection when ATP13A2 and aSyn were co-expressed using viral vector technology in the substantia nigra in rats (Daniel et al., 2015). Fibroblasts from PARK9 patients and dopaminergic cell lines made deficient in ATP13A2 exhibit a wide variety of lysosomal dysfunction including reduced lysosomal degradation capacity, accumulation of aSyn and neurotoxicity, impaired lysosomal acidification, decreased proteolytic processing of lysosomal enzymes, accumulation and enlargement of lysosomes, and decreased clearance of autophagosomes (Usenovic et al., 2012, Dehay et al., 2012). Recently, we showed that mice with loss of function of ATP13A2 develop age-dependent sensorimotor deficits, enhanced lipofuscin accumulation, and increased insoluble aSyn in the brain (Schultheis et al., 2013). While another study in a different ATP13A2 knockout mouse found age-dependent motor impairments, gliosis, accumulated ubiquitin protein aggregates, and endolysosomal abnormalities but no aberrant aSyn up to 18 months of age indicating the relationship between ATP13A2 and aSyn remains uncertain in vivo (Kett et al., 2015). This uncertainty is further supported by a lack of post mortem studies for Kufor-Rakeb Syndrome.
There are now several studies that also link Mn with aSyn oligomerization and accumulation in neurons in vitro and in the brain in vivo (Xu et al., 2013, 2014a, 2014b; Verina et al., 2013). In humans and rodents chronic Mn exposure can result in manganism, a toxic condition that presents with “psychomotor excitement” followed by hypoactivity that resembles aspects of sporadic PD (Shukla et al., 1984). The goal of the present study was to determine if loss of function of ATP13A2 leads to an enhanced sensitivity to Mn exposure in vivo.
Materials and Methods
Animals
Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were pre-approved by the Institutional Animal Care and Use Committee at Northern Kentucky University. Mice lacking ATP13A2 (13a2; Schultheis et al., 2013) were backcrossed to C57BL/6 for 8 generations. Heterozygous male and female mice were bred together to produce wildtype (WT) and homozygous 13a2 mice included in all experiments. Animals were maintained on the C57BL/6-background and littermates were never bred together. The genotype of all WT and 13a2 mice was confirmed by polymerase chain reaction (PCR) amplification analysis of DNA from tail tips. Male and female mice from 27 litters were included in the study. All mice were group housed and provided free access to water and standard rodent chow throughout the experiment except during behavioral testing procedures. Behavioral procedures were conducted during the light cycle.
Manganese Treatment
Manganese chloride tetrahydrate (MnCl2•4H2O, adjusted for the molar stoichiometry in the tetrahydrate, Sigma M8054) dissolved in saline (5mg/kg/day,ip, Sigma) or saline alone were administered daily for 45 days to male and female WT and 13a2 mice. The 5mg/kg dose and intraperitoneal administration route have been shown to cause modest effects in mice following 30 days of daily administration and reflects more of an environmentally relevant dose rather than a higher occupationally relevant regimen (Stanwood et al., 2009; Moreno et al., 2009; Kim et al., 2017). Behavioral testing was conducted over a two-week period following 30 days of manganese and saline treatment. Mice remained on their respective treatments during behavioral testing. One cohort of mice was tested at 5–9 months of age (WT-Saline= 7, WT-MnCl2= 8, 13a2-Saline= 8, 13a2-MnCl2= 8) and a separate cohort was tested at 12–19 months of age (WT-Saline= 8, WT-MnCl2= 8, 13a2-Saline= 7, 13a2-MnCl2= 8). All animals were tested at the same time and the ages chosen were based on the initial characterization of these mice to include an older group that is at the cusp of manifesting behavioral impairments and a younger group that is not expected to develop sensorimotor impairments until much older (Schultheis et al., 2013).
Sensorimotor Tests
All mice were tested for spontaneous activity, motor performance and coordination and gait in that order. Spontaneous Activity. Spontaneous movements of the mice were measured in a small, transparent cylinder 15.5 cm high and 12.7 cm in diameter (Fleming et al., 2004, 2013; Schultheis et al., 2013). The cylinder was placed on a piece of glass with a mirror positioned at an angle beneath the cylinder to allow a clear view of movements along the ground and walls of the cylinder. Videotapes were viewed and rated in slow motion by an experimenter blind to mouse genotype and treatment. The number of rears, forelimb and hindlimb steps, and time spent grooming over a three-minute period were measured for each mouse.
Motor performance and coordination was measured with the challenging beam traversal test (Fleming et al., 2004, 2013; Schultheis et al., 2013). Briefly, the beam consists of four sections (25 cm each, 1 meter total length), each section having a different width. The beam starts at a width of 3.5 cm and gradually narrows by 1 cm increments to a final width of 0.5 cm. Animals were trained to traverse the length of the beam starting at the widest section and ending at the narrowest section. Animals received two days of training prior to testing; on the day of the test a mesh grid (one cm squares) of corresponding width was placed over the beam surface leaving approximately a one cm space between the grid and the beam surface. Animals were then videotaped while traversing the grid-surfaced beam and videotapes were rated on slow motion by an experimenter blind to genotype and treatment. Errors, steps, and time to traverse the beam were measured across five trials and the means of those trials were included in the analysis.
To measure gait, animals were trained to walk through a narrow alley leading into their home-cage. Once trained, paper was placed along the alley floor and each animal’s hindlimbs were brushed with non-toxic paint. Animals were then placed at the beginning of the alley. As they walked into their home-cage they left their paw prints on the paper underneath (Schallert et al., 1978; Fleming et al., 2004, 2013; Schultheis et al., 2013). Stride length was determined by measuring the distance between hindlimb prints. Only strides made while continuously walking (no stopping) were included in the analysis. Stride lengths at the beginning and end of the alley were not counted since animals tend to make irregular steps at the beginning and typically stop and make smaller steps just before entering the cage.
Following sensorimotor testing, half of the mice were anesthetized with pentobarbital (100 mg/kg, ip) and intracardially perfused with 0.1M phosphate buffered saline (PBS) followed by ice cold 4% paraformaldehyde. Brains were removed, post-fixed for 6 hours in 4% paraformaldehyde, cryoprotected with 0.1M phosphate buffer (pH 7.4) containing 20% sucrose for 72 hours, and rapidly frozen in isopentane pre-cooled to −70ºC with dry ice. The other half of the mice were euthanized and brains were rapidly removed and fresh frozen in powdered dry ice.
Metal Analysis
Mouse brain samples were freeze dried at −80°C for 24 hours and then weighed to the nearest hundredth of a milligram. Samples were digested with trace metal-grade nitric acid and hydrogen peroxide using a method previously developed (Wegst-Uhrich et al., 2015), which was adapted from USEPA 3050B (Acid Digestion of Sediments, Sludges, and Soils 1996). Method reagent blanks were prepared using the same procedure and contained only the chemical reagents. Ten ng/mL of 103Rh was added to each sample as an internal standard for instrument drift and volume correction. Samples were diluted to 10 mL with Nanopure® water and filtered with 0.45-μm polypropylene membrane syringe filters prior to analysis. Samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) on a Thermo Scientific X-Series 2 instrument. A 1 mL sample loop was used to minimize sample volume, and collision cell technology was used to reduce polyatomic inferences. All samples were normalized to the 103Rh signal; quantification was performed with an external calibration curve in 2% nitric acid over the concentration range of 0.5 to 500 ng/mL. Standard metal mix (82026-108, BDH Aristar® Plus) was used to quantitate all tested elements, and isotopes 55Mn, 57Fe, 65Cu, and 64Zn were chosen for optimal sensitivity in quantification. Detection limits were calculated using a multipoint standard addition technique with spiking at 4 levels (0, 2.5 10 and 50 ng/mL). The detection limits were determined to be 1.82, 18.3, 6.25, and 2.98 for Mn, Fe, Cu, and Zn respectively. Acid digestion extraction recoveries were evaluated at a 50 ng/mL spiking level. Endogenous concentration levels were background subtracted from the recovery samples since analyte free matrix could not be obtained. Values are reported as average and standard errors (n=6). Recovery values were 90 ± 1, 100 ± 7, 97 ± 4, and 91 ± 2 % for Mn, Fe, Zn and Cu respectively.
Autofluorescence Quantification
Perfused brains were cut using a cryostat. Ten-micron coronal sections were taken from the prefrontal cortex (bregma 2.40 mm to 2.00 mm), hippocampus (bregma −2.10 mm to −2.40 mm), substantia nigra (bregma −3.00 mm to −3.60 mm), and cerebellum (bregma −6.40 mm to −6.60 mm) based on a mouse brain atlas and then placed on slides (Hof, 2000; Superfrost Plus, Fisher). The sections were dehydrated using serial ethanol dilutions (70%, 95%, and 100%), air-dried, and rehydrated in dH2O. Following rehydration the sections were cover-slipped with Aqua/Polymount (Polyscienes, Inc. Warrington, PA). Using a fluorescent microscope with a TRITC HYQ filter at 296x magnification, pictures for each mouse and each brain region were processed. The exposure time varied per area of the brain in order to minimize overexposure and maximize autofluorescence. For quantification, all images were processed using a custom program, coded in the MATLAB programming language. First, each image was imported and converted to a matrix of gray scale values. Gray scale values ranged from 0, which depicts black, to 65536, which depicts white. The gray scale values were then divided by the maximum gray scale value to normalize them to a 0 (black) to 1 (white) unit scale. A background noise threshold 20% of the normalized gray scale range was used in all analyses. The base dependent measure for each image was the square root of the number of pixels exceeding the noise threshold. Since the pixel counts were taken over a two-dimensional surface, the square root transform controlled the counts for area effects. The exact value of the noise threshold was not crucial to the outcome of analysis. The same quantification, completed with a range of both larger and smaller noise thresholds, yielded qualitatively identical outcomes.
Immunohistochemistry and Autofluorescence
A separate set of coronal sections (10um) from the substantia nigra (bregma −3.00 mm to −3.60 mm) were incubated in 4% PFA for 30 minutes, washed in 0.1M PBS (pH=7.4) 3x 10 minutes, and coverslips applied with an aqueous mounting medium (VECTASHIELD Antifade Mounting Medium, Catalog Number: H-1000). The sections were then scanned for autofluorescence (GFP and Texas Red) using the Leica Biosystems Aperio FL scanner at 20x magnification.
Nigral sections also were stained for tyrosine hydroxylase immunoreactivity (TH-IR), to identify dopaminergic cells, or for aSyn-IR. Sections were incubated in 0.3% hydrogen peroxide in 0.1M PBS for 15 minutes to block endogenous peroxidase activity and were washed again in 0.1M PBS 3x for 10 minutes each. Sections were incubated in 10% normal goat serum/0.3% triton X-100 in 0.1M PBS for one hour at RT and incubated overnight in primary antibody at 4 degrees Celsius. For TH staining, rabbit anti-tyrosine hydroxylase primary antibody (1:250, EMD Millipore, Catalog Number: AB152) was mixed with 5% normal goat serum in 0.1M PBS. For aSyn staining, mouse anti-alpha synuclein primary antibody (1:150, BioLegend Syn202, Previously Covance, Catalog Number: MMS-529R) was used. On day two of staining, sections were washed in 0.1M PBS 3x 10 minutes each. For TH staining all slides were incubated in biotinylated secondary goat anti-rabbit (VECTASTAIN ABC HRP Kit; Peroxidase, Rabbit IgG, PK-4001) following the manufacturer’s instructions. For aSyn staining all slides were incubated in biotinylated secondary goat anti-mouse at a concentration of 1:200 (Vector Labs, Biotinylated Goat Anti-Mouse IgG, Catalog Number BA-9200). The sections were then washed in 0.1M PBS 3x 10 minutes each. Sections were incubated in the Avidin-biotin complex kit for one hour (ABC mixture: 2 drops of A, 2 Drops of reagent B in 5ml of .1M PBS; VECTASTAIN ABC HRP KIT, Catalog Number PK 4001) to detect the secondary antibodies. Sections were washed in 0.1M PBS 2x 10 minutes each and then in 0.1M TB (pH 7.6) for 10 minutes. The sections were then incubated in DAB solution following manufacturer’s instructions (Catalog Number: SK-4100, Vector Labs, CA) using nickel (TH-IR for 10 minutes; aSyn-IR for 5 minutes). Slides were washed once more in 0.1M PBS for five minutes, briefly washed in DD water, air dried, and coverslipped (VECTASHIELD Antifade Mounting Medium, Catalog Number: H-1000). The slides were scanned on the Leica Biosystems Aperio FL scanner for brightfield images.
Autofluorescence, TH-IR, and aSyn-IR pictures of the substantia nigra pars compacta (Bregma −3.80mm to −4.30mm) were acquired using the scanned images from the Leica Biosystems scanner on the Aperio ImageScope v12.2.2.5015 software at 10x magnification. By utilizing landmarks within each section the previously scanned autofluorescence images were then merged with their TH-IR or aSyn-IR stained respective images (Kanaan et al., 2007). The merged images were then processed (contrast, brightness and gamma settings) to optimize visualization. The adjustments were kept constant between all groups. Additional pictures of the merged images were taken at 20x magnification to better visualize immunoreactivity and autofluorescence together.
Triton Soluble and Insoluble aSyn Immunoblot
Ventral midbrain was dissected using a scalpel blade and making coronal cuts adjacent to the inferior colliculi at ~bregma –6.36 mm and at ~bregma –2.54 mm. The ventral midbrain was dissected out and excluded hippocampus, cortex and cerebellum. To determine the levels of Triton-soluble (T-Sol, soluble) and Triton-insoluble (T-Insol, insoluble) alpha-synuclein, ventral midbrain tissue from young (5–9 months) and old (12–19 months) 13a2 and WT mice was subjected to a sequential extraction procedure as previously described (Schultheis et al., 2013; Mazzulli et al., 2006; Tsika et al., 2010). Briefly, fresh frozen tissues were homogenized in a Triton X-100 based lysis buffer and subjected to four successive freeze-thaw cycles. The resulting lysates were centrifuged at 16,000 RPM for 30 min at 4°C. The supernatants were collected as the T-Sol fractions. The remaining pellets were resuspended in a SDS-based lysis buffer, boiled for 20 minutes and sonicated. The lysates were centrifuged at 16,000 RPM for 30 min at 25°C and the supernatants were collected as the T-Insol fractions. Sixty micrograms of protein from each sample was fractionated on 4–12% Tris-glycine gels and then transferred to PVDF membranes. Ventral midbrain tissue from mice overexpressing human WT aSyn was included as a positive control to confirm the aSyn signal (Rockenstein et al., 2002). The positive control was not included in any of the quantification. The membranes were blocked in 5% milk for 30 min at 37°C and then incubated with primary antibodies for alpha-synuclein (Biolegend (formerly Covance), Dedham, MA:Syn202, 1:1000) and β-actin (loading control, Sigma:A5441, 1:1000) in 1% milk overnight at 4°C. The membranes were washed 5X in PBS-T and incubated in anti-mouse IgG, HRP-linked secondary antibody (Cell Signaling Technology, Danvers, MA:7076, 1:5000) for 2 hours at room temperature. The membranes were again washed 5X in PBS-T and developed using ECL Plus Western Blot Detection Kit (Amersham Biosciences, Picataway, NJ USA: RPN2132). Protein blots were normalized to β-actin and then shown as relative to WT-Saline.
Statistical Analysis
Separate analyses were conducted on the younger (5–9m) cohort and the older (12–19m) cohort of mice. ANOVA was used in most analyses except when any of the basic assumptions of ANOVA were violated and then nonparmetric analyses were used. An F-Test was performed to determine homogeneity of variance. The two key comparisons for the behavior, autofluorescence, and aSyn accumulation, were determining the effect of genotype in vehicle-treated mice (saline-treated WT vs. saline-treated 13a2) and the effect of manganese on 13a2 mice (saline-treated 13a2 vs. MnCl2-treated 13a2), therefore these two comparisons were made in each test using the Bonferroni test in addition to a 2X2 randomized ANOVA. To determine the effect of sex, a 2x2x2 ANOVA comparing sex, genotype, and treatment was performed for each age followed by Bonferroni corrected post hoc contrasts. Pearson correlation coefficients were determined for age and behavioral parameters and for autofluorescence, aSyn levels, and behavioral parameters. All analyses were conducted with StatPlus® software (5.8.3.8) for Macintosh or MATLAB. The level of significance was set at p ≤ 0.05.
Results
Overall, the Mn treatment was well tolerated by both genotypes in each age group. Body weights did not significantly differ at the start of the experiment or after treatment (Table 1).
Table 1.
Body weights (g) in wildtype and Atp13a2 knockout mice.
| Age | 5–9m | 12–19m | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Wildtype | Saline | 29.60 ± 2.01 | 29.13 ± 1.83 | 38.51 ± 1.59 | 38.46 ± 1.79 |
| MnCl2 | 27.69 ± 1.55 | 27.64 ± 1.46 | 35.64 ± 1.36 | 34.95 ± 1.24 | |
| Atp13a2 KO | Saline | 30.10 ± 1.66 | 29.76 ± 1.51 | 35.70 ± 1.07 | 34.67 ± 1.04 |
| MnCl2 | 28.35 ± 1.65 | 28.19 ± 1.45 | 35.49 ± 2.65 | 33.40 ± 2.09 | |
Mean ± SEM shown; WT=wildtype, 13a2=Atp13a2 knockout; Pre=start of experiment, Post=end of experiment
Sensorimotor Testing
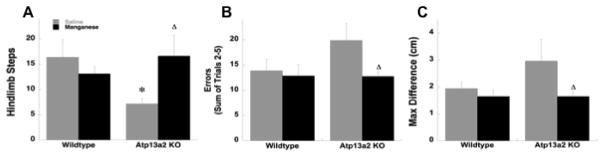
In the younger 5–9m cohort, there were no significant effects of genotype or Mn treatment on sensorimotor function (Table 2). However, in older mice there was an effect of genotype and Mn treatment on sensorimotor function in 13a2 mice. In the test of spontaneous activity, ANOVA of forelimb stepping revealed a main effect of genotype F(1,27)= 8.28, p≤0.01 with forelimb stepping significantly reduced in 13a2-Saline mice (25.00 ± 4.17) compared to WT-Saline (43.50 ± 5.58) mice (Bonferroni test, p≤0.01). For hindlimb stepping, ANOVA showed a significant genotype X treatment interaction F(1,27)= 4.82, p≤0.05. Saline-treated 13a2 mice made fewer hindlimb steps compared to WT-Saline mice (Bonferroni test, p≤0.05; Figure 1A). Further, 13a2-MnCl2 mice made more hindlimb steps compared to 13a2-Saline mice (Bonferroni test, p≤0.05; Figure 1) indicating an enhancement in stepping, specifically in 13a2 mice. Although this result may seem surprising, it is consistent with previous reports of an initial enhancement of sensorimotor function in response to Mn (Bonilla, 1984; Natchman et al., 1986; St.Pierre et al., 2001; Salehi et al., 2003; Kim et al., 2013; Krishna et al., 2014). There was no effect of genotype or Mn on rears (WT-Saline=8.5 ± 2.16, WT-MnCl2=6.13 ± 0.81, 13a2-Saline= 5.43 ± 1.29, 13a2-MnCl2=7.25 ± 1.81) or groom time (WT-Saline=20.84 ± 2.46, WT-MnCl2=17.33 ± 3.56, 13a2-Saline= 28.04 ± 4.68, 13a2-MnCl2=21.62 ± 4.00) in the cylinder. On the challenging beam a trial-by-trial analysis of errors showed that both 13a2-Saline and 13a2-MnCl2 mice made a similar number of errors on the beam on trial one but after trial one 13a2-MnCl2 mice made fewer errors. Indeed, when errors were added for trials 2–5 13a2-MnCl2 mice exhibited fewer total errors compared to 13a2-Saline mice, again suggesting an enhancement of sensorimotor function in 13a2-MnCl2 mice (Bonferroni test, p≤0.05; Figure 1B). There was no effect of genotype or Mn on time to traverse (WT-Saline=10.32 ± 1.22, WT-MnCl2=12.16 ± 0.96, 13a2-Saline= 11.24 ± 2.97, 13a2-MnCl2=8.14 ± 0.96) or steps (WT-Saline=17.95 ± 0.42, WT-MnCl2=19.33 ± 0.41, 13a2-Saline= 18.40 ± 0.52, 13a2-MnCl2=18.15 ± 0.61) on the beam. For gait analysis 13a2-Saline mice made more variable strides compared to 13a2-MnCl2 (Bonferroni test, p≤0.05; Figure 1C). There was, however, no effect of genotype or Mn treatment on stride length (WT-Saline=6.15 ± 0.18, WT-MnCl2=6.16 ± 0.20, 13a2-Saline= 6.29 ± 0.14, 13a2-MnCl2=6.27 ± 0.27).
Table 2.
Sensorimotor analysis in wildtype and Atp13a2 knockout mice at 5–9 months of age
| WT | 13a2 | ||||
|---|---|---|---|---|---|
| Saline | MnCl2 | Saline | MnCl2 | ||
| Challenging Beam | |||||
| Time | 8.94±1.19 | 8.47±1.25 | 11.13±1.04 | 9.32±1.24 | |
| Steps | 18.11±0.80 | 17.28±0.46 | 17.45±0.36 | 16.75±0.66 | |
| Errors | 3.11±0.70 | 3.83±0.42 | 3.98±0.54 | 3.43±0.35 | |
| Spontaneous Activity | |||||
| Rears | 10.86±1.71 | 8.38±1.91 | 9.00±1.66 | 13.13±3.61 | |
| Forelimb Steps | 29.71±5.14 | 29.38±4.06 | 29.50±3.49 | 37.63±7.15 | |
| Hindlimb Steps | 18.57±4.48 | 13.25±1.98 | 14.75±3.53 | 17.25±4.30 | |
| Groom Time (s) | 18.78±3.86 | 16.40±6.01 | 14.44±3.05 | 19.32±3.54 | |
| Gait | |||||
| Stride Length | 5.59±0.22 | 6.26±0.21 | 5.95±0.21 | 5.41±0.27 | |
| Max Difference | 2.03±0.20 | 2.55±0.30 | 2.00±0.23 | 2.58±0.39 | |
Mean ± SEM shown; WT=wildtype, 13a2=Atp13a2 knockout
Figure 1.
WT and 13a2 mice received daily injections of saline (WT=8, 13a2=7) or MnCl (5mg/kg, ip; WT=8, 13a2=8) for 45 days at 12–19 months of age. Starting on day 30 sensorimotor function was assessed in the cylinder (A: hindlimb steps), on the challenging beam (B: Errors), and in gait (C: maximum stride difference). * represents p≤0.05 compared to WT-Saline, Δ represents p≤0.05 compared to 13a2-Saline.
Correlations between age and behavior are shown in Table 3. There was no significant correlation between age and errors on the beam, hindlimb steps in the cylinder, or variability of stride lengths in younger WT or 13a2 mice. However, in the older cohort group, there was a positive correlation with errors on the beam in only the13a2 mice (r=0.52, p≤0.05).
Table 3.
Pearson Correlation Coefficients (r) for Age, Behavior, and Brain Pathology
| WT | 13a2 | ||||
|---|---|---|---|---|---|
| 5–9m | 12–19m | 5–9m | 12–19m | ||
| Age | |||||
| Beam | 0.25 | 0.34 | −0.37 | 0.52* | |
| Cylinder | −0.09 | −0.08 | −0.13 | 0.02 | |
| Gait | −0.39 | 0.20 | −0.41 | 0.12 | |
| WT | 13a2 | ||||
| VM aSyn (12–19m) | T-Sol | T-Insol | T-Sol | T-Insol | |
| Age | 0.66 | 0.21 | 0.37 | 0.33 | |
| Beam | −0.26 | −0.33 | 0.06 | 0.06 | |
| Cylinder | −0.42 | 0.62 | 0.75* | 0.72* | |
| Gait | −0.01 | 0.32 | −0.70 | −0.22 | |
represents p≤0.05,
VM=ventral midbrain, T-Sol=triton-soluble aSyn, T-Insol= triton-insoluble aSyn; WT=wildtype, 13a2=Atp13a2 knockout
Metal Analysis
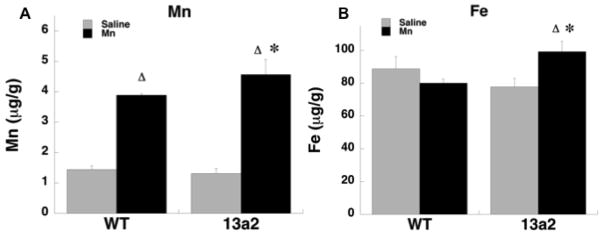
Mn, Fe, Zn, and Cu concentrations were determined in half brain (sagittal cut) in the younger cohort of mice (Figure 2; Table 4). For Mn, the F-Test showed the variance in the mutants receiving Mn was significantly higher than the other conditions, therefore Mann-Whitney U comparisons were made between the groups. Mn concentrations were significantly higher in both WT-MnCl2 and 13a2-MnCl2 mice compared to each genotype treated with saline (p≤0.05). In addition, Mn concentrations were higher in 13a2-MnCl2 compared to WT-MnCl2 mice (p≤0.05) indicating this regimen of Mn administration not only increased brain levels but also displayed an added effect in mutants. For Fe, a 2X2 ANOVA showed a significant interaction between genotype and treatment F(1,11)=6.06, p≤0.05. Fe concentrations were higher in 13a2-MnCl2 mice compared to WT-MnCl2 and 13a2-Saline mice. There were, however, no significant effects of manganese or genotype for Zn and Cu.
Figure 2.
Concentration (μg/g) of Mn (A) and Fe (B) in sagittal half brain of WT and 13a2 mice at 5–9 months of age. Δ represents p≤0.05 compared to saline treatment in the same genotype. * represents p≤0.05 compared to MnCl-treated WT. Mean ± SEM shown.
Table 4.
Metal concentration (μg/g) in brain (sagittal half) of wildtype and Atp13a2 knockout mice at 5–9 months of age
| WT | 13a2 | ||||
|---|---|---|---|---|---|
| Saline | MnCl | Saline | MnCl | ||
| Zn | 31.75 ± 5.14 | 37.82 ± 1.64 | 33.35 ± 4.97 | 34.11 ± 6.82 | |
| Cu | 15.17 ± 0.72 | 15.76 ± 0.78 | 13.14 ± 0.78 | 14.42 ± 1.33 | |
Mean ± SEM shown; WT=wildtype, 13a2=Atp13a2 knockout
Mn and Fe content in the drinking water was less than 1 and 5 parts per billion, respectively. Mn, Fe, Zn, and Cu content in the rodent chow (Lab Diet 5100, St. Louis, MO) was 75, 240, 85, and 15 parts per million, respectively.
Autofluorescence Quantification
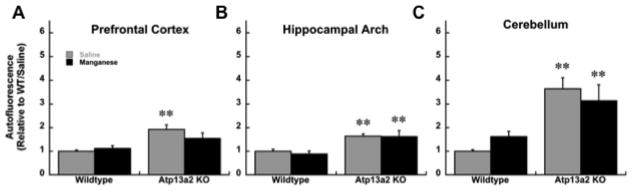
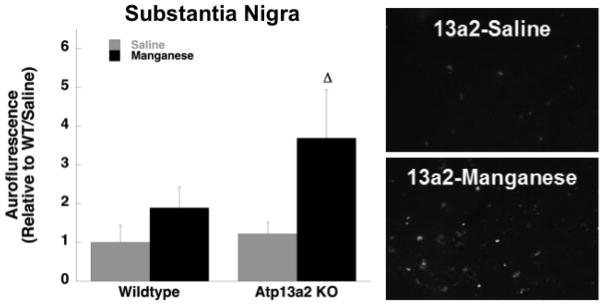
Autofluorescence was quantified in the hippocampus, cerebellum, cortex, and substantia nigra. For analysis in the cerebellum and hippocampus there was a main effect of genotype in both young and old mice (Young cerebellum- Genotype: F(1,12)= 20.86, p≤0.01; Old cerebellum- Genotype: F(1,12)=23.75, p≤0.01; Young hippocampus-Genotype: F(1,12)=18.87, p≤0.01; Old hippocampus-Genotype: F(1,12)=20.77, p≤0.01). In these regions both 13a2-Saline and 13a2-MnCl2 mice showed significantly increased autofluorescence compared to WT-Saline and WT-MnCl2, respectively (Figure 3; Table 5). In the prefrontal cortex there was a main effect of genotype in both young (F(1,12)=11.20, p≤0.01) and old (F(1,12)= 17.40, p≤0.01) mice. However, autofluorescence comparing only young WT-MnCl2 vs. 13a2-MnCl2 mice was significantly different (Table 5). In older mice, autofluorescence was significantly increased in 13a2-Saline compared to WT-Saline mice (Figure 3). In the substantia nigra there was no effect of genotype or treatment in the young mice but there was a significant main effect of Mn treatment in the older mice where 13a2-MnCl2 mice showed increased autofluorescence compared to 13a2-Saline controls (F(1,12)= 6.26, p≤0.05; Figure 4). There were no significant correlations between autofluorescence and behavior.
Figure 3.
Autofluorescent storage material was quantified in the prefrontal cortex (A), hippocampus (B), and cerebellum (C) in 12–19 month old mice treated with saline (WT=4, 13a2=4) or MnCl (WT=4, 13a2=4). Mean ± SEM relative to saline-treated WT values shown. ** represents p≤0.01 compared WT receiving the same treatment.
Table 5.
Autofluorescence and aSyn accumulation in 5–9m WT and 13a2 mice
| WT | 13a2 | ||||
|---|---|---|---|---|---|
| Saline | MnCl2 | Saline | MnCl2 | ||
| Autofluorescence (5–9m) | |||||
| PFC | 1.00 ± 0.19 | 1.58 ± 0.44 | 2.88 ± 0.63 | 4.34 ± 1.14* | |
| HPC | 1.00 ± 0.16 | 0.58 ± 0.18 | 2.10 ± 0.28* | 2.03 ± 0.45** | |
| CBL | 1.00 ± 0.31 | 1.14 ± 0.50 | 5.40 ± 1.12** | 5.64 ± 1.48** | |
| SNc | 1.00 ± 0.28 | 1.07 ± 0.61 | 1.46 ± 0.60 | 1.57 ± 0.56 | |
Mean ± SEM relative to saline-treated WT mice. PFC=prefrontal cortex, HPC= hippocampus (arch), CBL= cerebellum, SNc= substantia nigra pars compacta.
represents p<0.05, 0.01 compared to WT receiving the same treatment.
Figure 4.
Autofluorescent storage material quantified in the substantia nigra in 12–19 month old mice treated with saline (WT=4, 13a2=4) or MnCl (WT=4, 13a2=4). Mean ± SEM relative to saline-treated WT values shown. Representative photomicrographs (right) of autofluorescent storage material in substantia nigra in saline-treated 13a2 mice (top panel) and MnCl-treated 13a2 mice (bottom panel), Scale bar= 50μm, Δ represents p≤0.05 compared to saline-treated 13a2 mice.
Immunohistochemistry and Autofluorescence
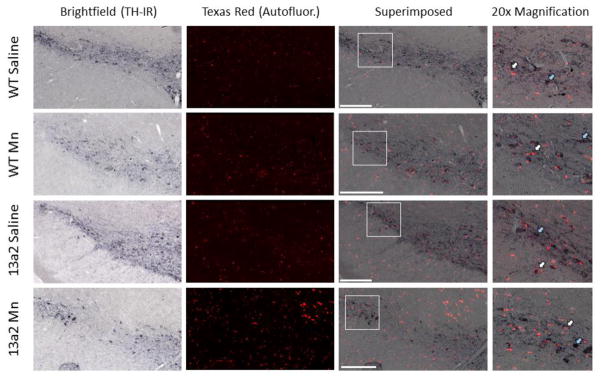
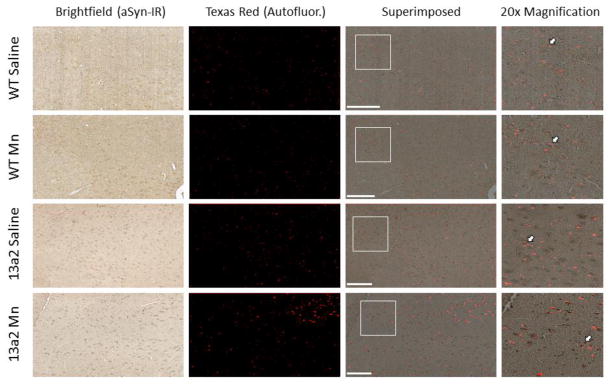
The overlap between autofluorescence and TH-IR and autofluorescence and aSyn-IR was performed on nigral sections from each condition. Overlap of superimposed pictures of TH-IR with autofluorescence was observed in some but not all cells in the substantia nigra (Figure 5). In contrast, there was a high frequency of autofluorescence overlap with aSyn-IR (Figure 6).
Figure 5.
Representative images of tyrosine hydroxylase immunoreactivity (TH-IR) overlap with autofluorescence storage material in the substantia nigra of 12–19 month WT and 13a2 mice treated with saline or MnCl. Brightfield (TH-IR) and Texas Red (Autofluorescence) images were taken at 10x magnification for each condition. Images were then superimposed. The white box on each superimposed image marks the location of the 20x magnification. The white arrows indicate an example of where overlap of TH-IR with autofluorescence occurs, the blue arrow indicates where there is no overlap. Scale bar= 200μm.
Figure 6.
Representative images of alpha-synuclein immunoreactivity (aSyn-IR) overlap with autofluorescence storage material in the substantia nigra of 12–19 month old mice treated with saline or MnCl. Brightfield (aSyn-IR) and Texas Red (Autofluorescence) images were all taken at 10x magnification for each condition. Images were then superimposed. The white box on each superimposed image marks the location of the 20x. The white arrows indicate an example of where overlap of aSyn-IR with autofluorescence occurs. Scale bar= 200μm.
Triton Soluble and Insoluble aSyn Immunoblot
Triton soluble and insoluble aSyn protein levels in the ventral midbrain were determined in young and old mice (Figure 7). In the young mice there was no effect of genotype or treatment on soluble or insoluble aSyn levels, although there was a trend for insoluble aSyn levels to be higher in 13a2-MnCl2 mice compared to 13a2-Saline (p=0.09; Figure 7C). In the older mice soluble aSyn levels in the ventral midbrain did not differ between groups (Figure 7B). However, in older mice triton-insoluble aSyn levels were significantly increased in 13a2-MnCl2 compared to 13a2-Saline and WT-MnCl2 (genotype X treatment interaction: F(1,11)=7.28, p≤0.05; Figure 7D).
Figure 7.
Triton-soluble and triton-insoluble aSyn protein levels were measured in the ventral midbrain of 5–9 (A and C) and 12–19 (B and D) month old mice treated with saline (WT=3–4, 13a2=4) or MnCl (WT=4, 13a2=4). aSyn protein levels were normalized to β-actin and calculated as relative to WT-Saline. Representative blots of aSyn (14kDa) and the loading control β-actin (42 kDa) are shown, WS=WT-saline, WM=WT-MnCl, AS=13a2-saline, and AM=13a2-MnCl treatment. * represents p≤0.05 compared to WT same treatment, Δ represents p≤0.05 compared to 13a2-saline.
Correlations were also performed on behavior and aSyn levels between WT and 13a2 mice. As indicated in Table 3, hindlimb stepping in the cylinder in 13a2 mice was significantly correlated with both soluble (r=0.75, p≤0.05) and insoluble (r=0.72, p≤0.05) aSyn levels in the ventral midbrain.
Discussion
In the present study we show for the first time in vivo that loss of function of ATP13A2 causes an increased sensitivity to Mn. Mn treatment led to an enhancement in sensorimotor function in 13a2 mice compared to saline-treated 13a2 mice. In the brain, Mn treatment in 13a2 mice significantly affected Mn concentration, autofluorescence in the substantia nigra, and insoluble aSyn in the ventral midbrain. In addition, hindlimb stepping in the cylinder was significantly correlated with aSyn levels in the ventral midbrain linking behavioral dysfunction in the Mn-treated 13a2 mice with Mn-induced pathology. The results also confirm previously reported sensorimotor deficits in older 13a2 compared to WT mice (Schultheis et al., 2013). These results provide in vivo support for earlier epidemiological and in vitro studies in yeast, C. elegan, and cultured neurons suggesting an important relationship between ATP13A2 function, Mn, and aSyn accumulation (Gitler et al., 2009; Schmidt et al., 2009; Covy et al., 2012; Daniel et al., 2015).
A neurological effect of both the loss of function of ATP13A2 and Mn treatment was observed only in the older group of mice. Behaviorally, older Mn-treated 13a2 mice showed an overall enhancement in sensorimotor function compared to older saline-treated 13a2 mice. This was demonstrated in the cylinder test, in the challenging beam test, and in gait. In the cylinder Mn-treated 13a2 mice made significantly more hindlimb steps compared to saline-treated 13a2 mice and on the challenging beam test Mn-treated mutants made significantly fewer errors than saline-treated mutant mice. In the gait test Mn treatment resulted in a decrease in stride-length variability in mutants. Mn treatment had no effect on any of the behaviors measured in WT mice indicating an increased sensitivity to a low-dose of Mn only in the 13a2 mice. This enhancement of sensorimotor function in response to Mn may seem counterintuitive but depending on the dose, administration route, and time course Mn treatment can result in the enhancement of motor function in rodents and non-human primates (Bonilla, 1984; Natchman et al., 1986; St.Pierre et al., 2001; Salehi et al., 2003; Kim et al., 2013; Krishna et al., 2014). Previous studies show that administration of Mn at doses higher than the 5mg/kg dose used in the present study in rodents also causes an enhancement in sensorimotor function (Natchman et al., 1986; Dodd et al., 2013).
It is unclear from previous studies what specific pathology contributes to the enhancement in sensorimotor function in response to Mn but, it is well established that in manganism Mn preferentially accumulates in the globus pallidus and lesions of the globus pallidus in rats are associated with increased sensorimotor function (Newland et al., 1992; Choi et al., 2005; Byler et al., 2006; Chang et al., 2009). However, Mn accumulation has also been reported in the substantia nigra in non-human primates exposed to welding fume (Park et al. 2007). This finding is particularly intriguing given that ATP13A2 is abundant in the substantia nigra (Ramirez et al., 2006). In addition, reduced dopamine release in the striatum without alterations in dopamine terminals or receptors has been reported in Mn-treated non-human primates suggesting a complex relationship between Mn, basal ganglia, nigrostriatal system, and neurotransmission (Fitsanakis et al., 2006; Guilarte et al., 2006; Racette et al., 2012). The findings in the present study also raise compelling questions regarding the role of aging as behavioral alterations were only seen in older mice. It is possible, and perhaps likely, that longer treatment and/or testing at a later time-point in the 13a2 mice may result in more severe behavioral anomalies and brain pathology in multiple brain regions.
Metal analysis in the younger cohort showed increased concentrations of Mn in both WT and 13a2 mice administered Mn. In addition, Mn concentrations were higher in Mn-treated mutants compared to Mn-treated WT mice establishing a gene X environment interaction between ATP13A2 and Mn in vivo. Fe, Zn, and Cu concentrations were also measured. While there was no effect of genotype or treatment on Zn and Cu, there was a significant interaction with Fe accumulation. Mn-treated mutants had higher Fe concentrations compared to Mn-treated WT and saline-treated 13a2 mice. While the effect on Fe in 13a2 mice is very interesting it is not quite surprising given the intimate relationship between Mn and Fe. Altering either metal can have significant consequences on the other (Fitsanakis et al., 2006). In addition, both metals share various transporters including the divalent metal transporter-1 and transferrin receptors (Gunshin et al., 1997; Roth et al., 2002; Au et al., 2008). The effect on iron is also highly relevant since mutations in ATP13A2 have been linked to neurodegeneration with brain iron accumulation (Schneider et al., 2010; Behrens et al., 2010; Bruggemann et al., 2010). More recently, in vitro research indicates ATP13A2 can protect against Fe toxicity supporting a potential role for ATP13A2 in multiple types of neurodegenerative conditions (Rinaldi et al., 2015). ATP13A2 is abundant in the brain in the mice but is also present to a lesser extent in the in the colon, kidney, liver, and stomach (Schultheis et al., 2004). The present study focused on half brain metal analysis limiting any interpretation regarding Mn exposure and behavior. It will be important for future studies in the 13a2 mouse line to determine metal accumulation in specific regions within the brain (ex. basal ganglia structures and midbrain) but also in peripheral organs as well.
For the autofluorescence quantification, the only region that showed a significant interaction between the loss of ATP13A2 function and Mn exposure was the substantia nigra in older mice. Mn administration in 13a2 mice resulted in an increase in autofluorescent storage material compared to saline-treated 13a2 mice. Further analysis of the autofluorescence in the nigra showed that it sometimes overlapped with TH-IR but almost always overlapped with aSyn. Similar to the initial characterization of the 13a2 mice, autofluorescence was increased in older 13a2 mice in the hippocampus and cerebellum (Schultheis et al., 2013). The present study also shows an increase in autofluorescence in younger 13a2 mice compared to WT indicating that enhanced autofluorescence develops early in the 13a2 mice which is consistent with the characterization of ATP13A2 knockout mice (Kett et al., 2015). Accumulation of autofluorescent storage material is a normal event in the aging brain and is reported to be associated with oxidative stress (Brunk et al., 2002). Enhanced autofluorescence is observed in the lysosomal storage disorder Neuronal Ceroid Lipofuscinosis, a condition linked to loss of ATP13A2 function, and accumulation of Mn has been shown to precede neurodegeneration in animal models of neuronal ceroid lipofuscinosis supporting an important link between ATP13A2 and Mn in neurodegenerative disease (Farias et al., 2011; Wohlke et al., 2011; Bras et al., 2012; Grubman et al., 2014).
Both ATP13A2 and Mn have also been shown to interact with the presynaptic protein aSyn (Gitler et al., 2009, Verina et al., 2013). Synucleinopathies are a group of diseases characterized by abnormal accumulation of aSyn in Lewy bodies, the most common synucleinopathy being PD (Polymeropoulos et al., 1997 Spillantini et al., 1997). A gene-dosage effect of aSyn in PD is suggested based on patients with triplication or duplication of the WT aSyn locus where the severity of the disease is worse in patients with aSyn triplication (Singleton et al., 2003; Chartier-Harlin et al., 2004). Therefore, it is hypothesized that genetic and/or environmental factors that increase aSyn may contribute to the development of synucleinopathies. In the present study triton-soluble and triton-insoluble aSyn were measured in the ventral midbrain of young and older mice. In young mice there was no difference in aSyn protein levels between the groups, but in the older mice triton-insoluble aSyn protein levels were significantly higher in Mn-treated 13a2 than in saline-treated 13a2 and Mn-treated WT mice indicating a significant gene-environment effect on aSyn accumulation. In vitro studies show aSyn is initially protective against Mn toxicity in dopaminergic neurons, but following prolonged Mn exposure aSyn will then aggregate (Harischandra et al., 2015). Although it is unclear how the biphasic effect of aSyn would would translate in vivo, it is intriguing that 13a2 mice treated with Mn show increased motor function and increased aSyn, suggesting the possibility of a protective role for aSyn in this case. Mn toxicity or “manganism” has been linked to PD because several of the motor symptoms resemble those observed in PD. However, Mn toxicity is associated with more severe damage to basal ganglia structures such as the globus pallidus rather than the substantia nigra (Guilarte, 2013). The results in the present study suggest that modest increases in exposure to Mn could potentially contribute to the development of aSyn pathology in select brain regions given a certain genetic vulnerability, in this case loss of function of ATP13A2. It will be important to determine the effect of aSyn accumulation from exposure to Mn on actual cell function and whether it eventually leads to cell death.
While the function of ATP13A2 remains unclear, in vitro studies show the protein is localized to lysosomes and interacts with heavy metals and aSyn (Gitler et al., 2009; Ramonet et al., 2012; Tan et al., 2011; Ramirez et al., 2006). Initial studies implicated primarily Mn in ATP13A2 function but more recent studies now suggest Zn homeostasis is also affected by ATP13A2 (Park et al., 2014; Kong et al., 2014; Tsunemi and Krainc, 2014). The present study now supports a relationship between ATP13A2, Mn, and aSyn accumulation in vivo and provides strong rationale for more in extensive studies to determine the impact of loss of ATP13A2 function and Mn exposure on lysosomal protein degradation, the nigrostriatal system, and basal ganglia function.
Supplementary Material
Highlights.
Manganese accumulation in the brain is significantly increased in Atp13a2-deficient mice receiving systemic manganese compared to wildtype mice receiving manganese.
Manganese administration in Atp13a2-deficient mice is associated with age-dependent enhancement in sensorimotor function.
Manganese administration in Atp13a2-deficient mice is associated with increased insoluble alpha-synuclein accumulation and enhanced autofluorescence in the ventral midbrain.
Acknowledgments
This work was supported by the National Institutes of Health NS077022 (S.M.F.) and NS070268 (P.J.S.), the UC Gardner Family Center for Parkinson’s Disease andMovement Disorders, and the University of Cincinnati Neuroscience Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29(4):569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MI, Brüggemann N, Chana P, Venegas P, Kägi M, Parrao T, Orellana P, Garrido C, Rojas CV, Hauke J, Hahnen E, González R, Seleme N, Fernández V, Schmidt A, Binkofski F, Kömpf D, Kubisch C, Hagenah J, Klein C, Ramirez A. Clinical spectrum of Kufor-Rakeb syndrome in the Chilean kindred with ATP13A2 mutations. Mov Disord. 2010;25(12):1929–1937. doi: 10.1002/mds.22996. [DOI] [PubMed] [Google Scholar]
- Bonilla E. Chronic manganese intake induces changes in the motor activity of rats. Exp Neurol. 1984;84(3):696–700. doi: 10.1016/0014-4886(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet. 2012;21(12):2646–2650. doi: 10.1093/hmg/dds089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann N, Hagenah J, Reetz K, Schmidt A, Kasten M, Buchmann I, Eckerle S, Bähre M, Münchau A, Djarmati A, van der Vegt J, Siebner H, Binkofski F, Ramirez A, Behrens MI, Klein C. Recessively inherited parkinsonism: effect of ATP13A2 mutations on the clinical and neuroimaging phenotype. Arch Neurol. 2010;67(11):1357–1363. doi: 10.1001/archneurol.2010.281. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33(5):611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Byler SL, Shaffer MC, Barth TM. Unilateral pallidotomy produces motor deficits and excesses in rats. Restor Neurol Neurosci. 2006;24(3):133–145. [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chang Y, Kim Y, Woo ST, Song HJ, Kim SH, Lee H, Kwon YJ, Ahn JH, Park SJ, Chung IS, Jeong KS. High signal intensity on magnetic resonance imaging is a better predictor of neurobehavioral performances than blood manganese in asymptomatic welders. Neurotoxicology. 2009;30(4):555–563. doi: 10.1016/j.neuro.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Chesi A, Kilaru A, Fang X, Cooper AA, Gitler AD. The role of the Parkinson's disease gene PARK9 in essential cellular pathways and the manganese homeostasis network in yeast. PLoS One. 2012;7(3):e34178. doi: 10.1371/journal.pone.0034178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Park JK, Park NH, Shin JW, Yoo CI, Lee CR, Lee H, Kim HK, Kim SR, Jung TH, Park J, Yoon CS, Kim Y. Whole Blood and Red Blood Cell Manganese Reflected Signal Intensities of T1-Weighted Magnetic Resonance Images better than Plasma Manganese in Liver Cirrhotics. J of Occup Health. 2005;47(1):68–73. doi: 10.1539/joh.47.68. [DOI] [PubMed] [Google Scholar]
- Covy JP, Waxman EA, Giasson BI. Characterization of cellular protective effects of ATP13A2/PARK9 expression and alterations resulting from pathogenic mutants. J Neurosci Res. 2012;90(12):2306–2316. doi: 10.1002/jnr.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G, Musso A, Tsika E, Fiser A, Glauser L, Pletnikova O, Schneider BL, Moore DJ. α-Synuclein-induced dopaminergic neurodegeneration in a rat model of Parkinson's disease occurs independent of ATP13A2 (PARK9) Neurobiol Dis. 2015;73C:229–243. doi: 10.1016/j.nbd.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, Vital A, Vila M, Klein C, Bezard E. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A. 2012;109(24):9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd CA, Bloomquist JR, Klein BG. Consequences of manganese administration for striatal dopamine and motor behavior in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-exposed C57BL/6 mice. Hum Exp Toxicol. 2013;32(8):865–880. doi: 10.1177/0960327112469043. [DOI] [PubMed] [Google Scholar]
- Farias FH, Zeng R, Johnson GS, Wininger FA, Taylor JF, Schnabel RD, McKay SD, Sanders DN, Lohi H, Seppälä EH, Wade CM, Lindblad-Toh K, O'Brien DP, Katz ML. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol Dis. 2011;42(3):468–474. doi: 10.1016/j.nbd.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Ekhator OR, Ghisaps B. Assessment of sensorimotor function in mouse models of Parkinson's disease. J Vis Exp. 2013;(76) doi: 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut P-O, Rockenstein E, Masliah E, Levine MS, Chesselet M-F. Early and progressive motor abnormalities in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Au C, Erikson KM, Aschner M. The effects of manganese on glutamate, dopamine and gamma- aminobutyric acid regulation. Neurochem Int. 2006;48:426–433. doi: 10.1016/j.neuint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41(3):308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman A, Pollari E, Duncan C, Caragounis A, Blom T, Volitakis I, Wong A, Cooper J, Crouch PJ, Koistinaho J, Jalanko A, White AR, Kanninen KM. Deregulation of biometal homeostasis: the missing link for neuronal ceroid lipofuscinoses? Metallomics. 2014;6(4):932–943. doi: 10.1039/c4mt00032c. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci. 2013;5:23. doi: 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. α-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson's disease. Toxicol Sci. 2015;143(2):454–468. doi: 10.1093/toxsci/kfu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ. Age-related accumulation of marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. The J of Comp Neurol. 2007;502:683–700. doi: 10.1002/cne.21333. [DOI] [PubMed] [Google Scholar]
- Kett LR, Stiller B, Bernath MM, Tasset I, Blesa J, Jackson-Lewis V, Chan RB, Zhou B, Di Paolo G, Przedborski S, Cuervo AM, Dauer WT. α-Synuclein-Independent Histopathological and Motor Deficits in Mice Lacking the Endolysosomal Parkinsonism Protein Atp13a2. J Neurosci. 2015;5(14):5724–542. doi: 10.1523/JNEUROSCI.0632-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Jin H, Anantharam V, Gordon R, Kanthasamy A, Kanthasamy AG. p73 gene in dopaminergic neurons is highly susceptible to manganese neurotoxicity. Neurotoxicology. 2017;59:231–239. doi: 10.1016/j.neuro.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CY, Sung JH, Chung YH, Park JD, Han JH, Lee JS, Heo JD, Yu IJ. Home cage locomotor changes in non-human primates after prolonged welding-fume exposure. Inhal Toxicol. 2013;25(14):794–801. doi: 10.3109/08958378.2013.849316. [DOI] [PubMed] [Google Scholar]
- Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, Cooper AA. Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23(11):2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- Krishna S, Dodd CA, Hekmatyar SK, Filipov NM. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch Toxicol. 2014;88(1):47–64. doi: 10.1007/s00204-013-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Sato F, Sato S, Koike M, Taruno Y, Saiki S, Funayama M, Ito H, Taniguchi Y, Uemura N, Toyoda A, Sakaki Y, Takeda S, Uchiyama Y, Hattori N, Takahashi R. ATP13A2 deficiency induces a decrease in cathepsin D activity, fingerprint-like inclusion body formation, and selective degeneration of dopaminergic neurons. FEBS Lett. 2013;587(9):1316–1325. doi: 10.1016/j.febslet.2013.02.046. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26(39):10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol Sci. 2009;112(2):405–415. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtman JP, Tubben RE, Commissaris RL. Behavioral effects of chronic manganese administration in rats: locomotor activity studies. Neurobehav Toxicol Teratol. 1986;8(6):711–715. [PubMed] [Google Scholar]
- Newland MC, Weiss B. Persistent effects of manganese on effortful responding and their relationship to manganese accumulation in the primate globus pallidus. Toxicol Appl Pharmacol. 1992;113(1):87–97. doi: 10.1016/0041-008x(92)90012-h. [DOI] [PubMed] [Google Scholar]
- Park JD, Chung YH, Kim CY, Ha CS, Yang SO, Khang HS, Yu IK, Cheong HK, Lee JS, Song CW, Kwon IH, Han JH, Sung JH, Heo JD, Choi BS, Im R, Jeong J, Yu IJ. Comparison of high MRI T1 signals with manganese concentration in brains of cynomolgus monkeys after 8 months of stainless steel welding-fume exposure. Inhal Toxicol. 2007;19(11):965–971. doi: 10.1080/08958370701516108. [DOI] [PubMed] [Google Scholar]
- Park JS, Koentjoro B, Veivers D, Mackay-Sim A, Sue CM. Parkinson's disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum Mol Genet. 2014;23(11):2802–2815. doi: 10.1093/hmg/ddt623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology. 2012;33(4):881–886. doi: 10.1016/j.neuro.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type 13A2ase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, Tsika E, Pletnikova O, Troncoso JC, Glauser L, Moore DJ. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet. 2012;21(8):1725–1743. doi: 10.1093/hmg/ddr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler G, Covolo L, Haddad AA, Lucchini RG, Zoni S, Broberg K. ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology. 2012;33(4):697–702. doi: 10.1016/j.neuro.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi DE, Corradi GR, Cuesta LM, Adamo HP, de Tezanos Pinto F. The Parkinson-associated human P5B-ATPase ATP13A2 protects against the iron-induced cytotoxicity. Biochim Biophys Acta. 2015;1850(8):1646–1655. doi: 10.1016/j.bbamem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68(5):568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Dolan KG, Lis A, Garrick MD. Effect of the iron chelator desferrioxamine on manganese-induced toxicity of rat pheochromocytoma (PC12) cells. J Neurosci Res. 2002;68(1):76–83. doi: 10.1002/jnr.10207. [DOI] [PubMed] [Google Scholar]
- Salehi F, Krewski D, Mergler D, Normandin L, Kennedy G, Philippe S, Zayed J. Bioaccumulation and locomotor effects of manganese phosphate/sulfate mixture in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl Pharmacol. 2003;191(3):264–271. doi: 10.1016/s0041-008x(03)00238-2. [DOI] [PubMed] [Google Scholar]
- Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. 6-hydroxydopamine and anticholinergic drugs. Science. 1978;202(4373):1216–1217. doi: 10.1126/science.202.4373.1216. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Wolfe DM, Stiller B, Pearce DA. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem Biophys Res Commun. 2009;383(2):198–202. doi: 10.1016/j.bbrc.2009.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SA, Paisan-Ruiz C, Quinn NP, Lees AJ, Houlden H, Hardy J, Bhatia KP. ATP13A2 mutations (PARK9) cause neurodegeneration with brain iron accumulation. Mov Disord. 2010;25(8):979–984. doi: 10.1002/mds.22947. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Hagen TT, O'Toole KK, Tachibana A, Burke CR, McGill DL, Okunade GW, Shull GE. Characterization of the P5 subfamily of P-type transport ATPases in mice. Biochem Biophys Res Commun. 2004;323(3):731–8. doi: 10.1016/j.bbrc.2004.08.156. [DOI] [PubMed] [Google Scholar]
- Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, Giasson B, Dickson DW, Mazzulli JR, Bardgett ME, Haik KL, Ekhator O, Chava AK, Howard J, Gannon M, Hoffman E, Chen Y, Prasad V, Linn SC, Tamargo RJ, Westbroek W, Sidransky E, Krainc D, Shull GE. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited α-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet. 2013;22(10):2067–2082. doi: 10.1093/hmg/ddt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla GS, Singhal RL. The present status of biological effects of toxic metals in the environment: lead, cadmium, and manganese. Can J Physiol Pharmacol. 1984;62(8):1015–1031. doi: 10.1139/y84-171. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J Neurochem. 2009;110(1):378–389. doi: 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre A, Normandin L, Carrier G, Kennedy G, Butterworth R, Zayed J. Bioaccumulation and locomotor effect of manganese dust in rats. Inhal Toxicol. 2001;13(7):623–632. doi: 10.1080/08958370117066. [DOI] [PubMed] [Google Scholar]
- Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, Wang D, Zhang Z. Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. J Biol Chem. 2011;286(34):29654–29662. doi: 10.1074/jbc.M111.233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsika E, Moysidou M, Guo J, Cushman M, Gannon P, Sandaltzopoulos R, Giasson BI, Krainc D, Ischiropoulos H, Mazzulli JR. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30(9):3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Krainc D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet. 2014;23(11):2791–2801. doi: 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32(12):4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verina T, Schneider JS, Guilarte TR. Manganese exposure induces α-synuclein aggregation in the frontal cortex of non-human primates. Toxicol Lett. 2013;217(3):177–183. doi: 10.1016/j.toxlet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegst-Uhrich SR, Mullin EJ, Ding D, Manohar S, Salvi R, Aga DS, Roth JA. Endogenous concentrations of biologically relevant metals in rat brain and cochlea determined by inductively coupled plasma mass spectrometry. Biometals. 2015;28(1):187–196. doi: 10.1007/s10534-014-9814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhlke A, Philipp U, Bock P, Beineke A, Lichtner P, Meitinger T, Distl O. A one base pair deletion in the canine ATP13A2 gene causes exon skipping and late-onset neuronal ceroid lipofuscinosis in the Tibetan terrier. PLoS Genet. 2011;7(10):e1002304. doi: 10.1371/journal.pgen.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Jin CH, Deng Y, Liu W, Yang TY, Feng S, Xu ZF. Alpha-synuclein oligomerization in manganese-induced nerve cell injury in brain slices: a role of NO-mediated S-nitrosylation of protein disulfide isomerase. Mol Neurobiol. 2014a;50(3):1098–110. doi: 10.1007/s12035-014-8711-z. [DOI] [PubMed] [Google Scholar]
- Xu B, Liu W, Deng Y, Yang TY, Feng S, Xu ZF. Inhibition of calpain prevents manganese-induced cell injury and alpha-synuclein oligomerization in organotypic brain slice cultures. PLoS One. 2015;10(3):e0119205. doi: 10.1371/journal.pone.0119205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Wu SW, Lu CW, Deng Y, Liu W, Wei YG, Yang TY, Xu ZF. Oxidative stress involvement in manganese-induced alpha-synuclein oligomerization in organotypic brain slice cultures. Toxicology. 2013;305:71–78. doi: 10.1016/j.tox.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Xu B, Wang F, Wu SW, Deng Y, Liu W, Feng S, Yang TY, Xu ZF. α-Synuclein is involved in manganese-induced ER stress via PERK signal pathway in organotypic brain slice cultures. Mol Neurobiol. 2014b;49(1):399–412. doi: 10.1007/s12035-013-8527-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.