Abstract
It is well known that pathology caused by chlamydial infection is associated closely with the host response to the organism and that both innate and adaptive host responses contribute to tissue damage. While it is likely that the organism itself initiates the acute inflammatory response by eliciting cytokine and chemokine production from the host cell, the adaptive response is the result of activation of the cell-mediated immune response. While there are several studies describing the nature of the pathologic response in primate, guinea pig, and murine models, there is less information on the kinetics of the CD4 and CD8 response following primary and challenge infections. In this study, we have quantified by flow cytometry the mononuclear cell response to genital infection with the agent of guinea pig inclusion conjunctivitis in the cervix, endometrium, and oviducts at various times following a primary intravaginal infection and after a challenge infection. Tissues from individual animals were assessed for cells expressing CD4, CD8, or Mac-1 and for B cells. Peak responses of each subset occurred 10 to 14 days after a primary infection. The number of Mac-1-expressing cells in each tissue site was found to be dependent on the size of the inoculating dose of chlamydiae. The responses of each cell type were generally stronger in the cervix than in the upper genital tract. In contrast to the murine model but consistent with the primate models, there were equal numbers of CD4 and CD8 cells present in the infiltrates. Twenty-one days after challenge infection, which was performed 50 days after the primary infection, there was a significant increase in the number of CD4, CD8, and B cells in the oviduct compared to the number of these cells at the same time after a primary infection, providing clear cellular evidence for a cell-mediated immune pathologic response.
It has been well documented over the last two decades that Chlamydia trachomatis is a major etiologic agent in salpingitis and pelvic inflammatory disease. However, there is minimal information on the pathologic response in humans because of inherent difficulties in the acquisition of appropriate tissue for examination. Even when tissue is available, it has been virtually impossible to establish when the tissue was collected in reference to the course of the infection. Thus, our knowledge of the kinetics of the inflammatory response to chlamydial infection in the human genital tract is still elementary.
A number of animal models have been utilized in attempts to fill the holes in our knowledge base. Clearly, the model that best parallels human disease is the nonhuman primate infected with C. trachomatis. Patton and colleagues have presented excellent studies characterizing the pathologic response in the nonhuman primate infected by direct genital inoculation and in infected subcutaneous explants of genital tract tissue (8, 9, 10, 11). Nevertheless, the expense of nonhuman primates has made thorough kinetic studies of the pathologic response difficult as well. Interestingly, guinea pigs infected intravaginally with the Chlamydia psittaci agent of guinea pig inclusion conjunctivitis (GPIC) develop a disease which remarkably parallels the human and nonhuman primate diseases in infection course and pathologic response (14).
While both primate and guinea pig models have yielded much descriptive and in some cases quantitative data on the acute and chronic inflammatory responses during chlamydial infection, there is minimal information on the kinetics of the T- and B-cell response in genital tissues. This information is particularly important for understanding the mechanisms of disease, since there are pathologic data in several animal models that suggest that repeated chlamydial infection may result in the induction of an enhanced lymphocytic response with exacerbated pathology (7, 9, 17, 19). However, most data are limited to the description of mononuclear cells in the local tissue rather than a thorough assessment of the local T- and B-cell response. Van Voorhis et al. (22) presented data demonstrating both a CD4 and CD8 T-cell response in fallopian tubes of repeatedly infected monkeys, and they observed a Th1-like cytokine response (21). These data were crudely quantified and were collected only from animals repeatedly infected, so that no information is available on the response to a primary infection.
More data are available from the murine model infected with the agent of mouse pneumonitis, but the infection kinetics and pathology resulting from genital infection of the mouse are quite different from those of the primate. Because the guinea pig model so closely resembles infection in human and nonhuman primates, we undertook further characterization of the kinetics of the lymphocyte response in the cervix, endometrium, and oviducts to quantify T-cell phenotypes in relation to a primary intravaginal inoculation of GPIC and reinfection 50 days later.
MATERIALS AND METHODS
Experimental animals.
Female Hartley strain guinea pigs, each weighing 450 to 500 g, were obtained from Sasco Laboratories (Omaha, Nebr.). All animals were housed individually in an environmentally controlled room with a 12:12 light-dark cycle and were provided with food and water ad libitum.
Chlamydial infection.
Isolates of the GPIC agent were originally obtained from the late Edward Murray as a passage of his original isolate from guinea pig conjunctiva (6) and have been continuously maintained in this laboratory, initially in yolk sacs and for at least the last 10 years in McCoy cells and HeLa cells. For infection purposes in these experiments, McCoy cell-grown GPIC isolates were utilized. Chlamydiae were passaged, prepared for infection, and quantified by standard methodology.
Guinea pigs were infected intravaginally by the insertion of a blunt pipette tip to the cervix and deposition of 0.05 ml of sucrose-phosphate-glutamic acid containing either 106 or 107 inclusion-forming units (IFU) of GPIC as specified in the experiment descriptions in the Results section. All animals were confirmed to be infected by the isolation and quantification of chlamydiae from cervicovaginal swabs collected 6 days following infection. Each experiment was repeated so that four to six animals could be obtained per time point.
Flow cytometry.
Guinea pigs were euthanized at various times after infection, and their genital tracts were removed for enumeration of CD4 CD8 T cells, B cells, and cells bearing the Mac-1 cell surface marker, which include polymorphonuclear leukocytes (PMNs), monocytes, and macrophages. The genital tracts were dissected into three separate sections containing exo- and endocervix, uterine fundus and uterine horns, or oviducts and ovaries. Generally, tissues from a single animal were assessed by flow cytometry unless the cell yield was low, in which case tissues from two animals were pooled. Tissues were teased with scissors and forceps and incubated in 5 mg of type I collagenase (Sigma, St. Louis, Mo.) per ml in RPMI medium for 45 min at 37°C to produce a single cell suspension. The suspension was filtered through a cell strainer and pelleted at low speed.
For flow cytometrical analysis, the cells were suspended in Dulbecco's minimal essential medium (DMEM) containing 1% bovine serum albumin (BSA; Sigma) and 0.1% sodium azide (staining buffer) according to the microplate technique described previously (2). Cells were initially incubated with mouse anti-guinea pig cell surface markers for 25 min on ice and then were washed twice with DMEM containing 10% BSA. They were then stained with goat anti-mouse immunoglobulin G (IgG). After washing, the cells were resuspended in a solution of 1% paraformaldehyde in phosphate-buffered saline (PBS) and kept at 4°C. Flow cytometer analysis was done with a fluorescence-activated cell-sorting analyzer equipped with a 488-nm argon laser and Lysis II software (FACscan; Becton Dickinson). Calibration of the instrument was accomplished with beads (CaloBRITE; Becton Dickinson). Exclusion of dead cells was based on forward angle and 90° light scatter and 10,000 gated cells were analyzed per sample. Antibodies to guinea pig pan-B, CD4, CD8, and Mac-1 were purchased from Serotec, Oxford, United Kingdom. All antibodies were murine IgG1. The secondary antibody was fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (heavy chain specific) and was obtained from Southern Biotechnology Associates, Inc., Birmingham, Ala. A control antibody was an IgG1 antibody against influenza virus A matrix protein (M2-1C6-4R3) and was produced in our laboratory from a hybridoma obtained from the American Type Culture Collection, Manassas, Va.
Because of different masses of the tissues collected and the resulting variance in the total number of cells, the data are presented as the numbers of cells of given phenotypes per 106 total genital tract cells. This corrects for differences in the total number of cells collected.
RESULTS
Primary genital tract infection.
The initial experimentation was designed to characterize in detail the T-cell, B-cell, and Mac-1 responses in different sections of the female genital tract at various times after intravaginal infection with either 106 or 107 IFU. Both doses resulted in infection of 100% of the animals; however, we have observed that 106 IFU results in a lower tumor necrosis factor alpha (TNF-α) response in the genital tract, which could potentially affect the influx of inflammatory cells to the local tissue (R. G. Rank and T. Darville, unpublished data). Quantification of GPIC-infective chlamydia cells in the different tissues was attempted but was unsuccessful, most likely because the tissue was manipulated harshly while being prepared for flow cytometry. Previous studies with infection doses of 104 to 107 IFU have demonstrated that organisms do not reach the uterus or oviducts until after day 5 of infection (15). However, from day 7 to day 9, 80% of the guinea pigs became infected in the uterine horns and oviducts. At various days after infection, two to four animals were euthanized, and the cells from the genital tract tissues were enumerated for the presence of each of the above markers. Because of the number of tissues and the number of antibodies used for staining, it was not logistically feasible to work with more than this number of animals on a single day. The experiment was performed twice, and the data were combined for purposes of evaluation and to give a sufficient number of replicates.
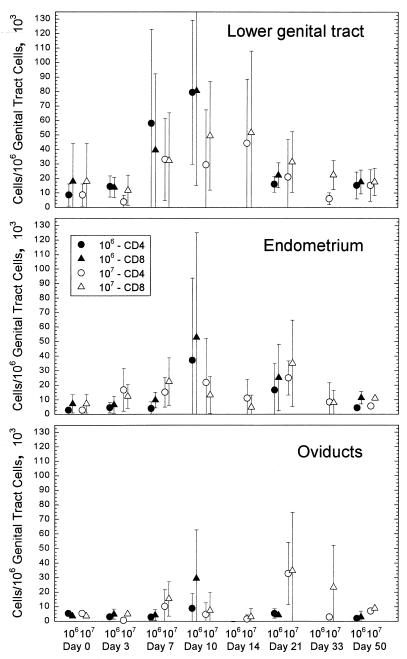
Assessment of uninfected guinea pigs resulted in the detection of low numbers (<1.7 × 105 cells/1 × 106 total cells in each tissue) of CD4 and CD8 cells in all sections of the genital tract (Fig. 1). The majority of the animals had less than 0.6 × 105 CD4 or CD8 T cells/1 × 106 total cells. In the lower genital tract, the numbers of CD4 and CD8 cells increased by day 7 after infection and were at peak levels by day 10. There was no difference in the number of cells in the lower genital tract when the groups infected with 106 versus 107 IFU (below, the “106 group” and “107 group,” respectively) were compared. The number of cells began to decrease by day 21 and was at a relatively low level by day 50. CD4 and CD8 cells did not increase in the endometrium until day 10 in the group infected with 106 IFU, and these cells decreased to control levels by day 50. In contrast, the number of T cells in the endometrium of the 107 group increased more quickly to generally higher levels on days 3 and 7, but they too dissipated by day 50. The response in the oviducts was more varied than in the other tissues, although there was a stronger response at day 21 in the 107 group. We had previously observed that only 45% of animals developed any pathology in the oviducts even though 80% were positive for organisms (16). That some animals had no increase in the number of T cells in the oviducts also suggested that not every animal had a pathologic response in the oviduct. Nevertheless, the appearance of both Mac-1-expressing cells and T cells in the current study closely paralleled the presence of PMNs and lymphocytes in our previous study with respect to time and tissue site (16).
FIG. 1.
Comparison of CD4 and CD8 cell numbers in different areas of the genital tract in response to infection with either 106 or 107 IFU of the GPIC infectious agent. The error bars represent the standard deviations of the means of results from four guinea pigs.
Of importance was the observation that CD4 and CD8 cells were present in each of the tissues in approximately a 1:1 ratio, similar to what has been reported for nonhuman primates infected with C. trachomatis (22). While B cells were present, they were not significantly increased at any time of the infection in any part of the genital tract. The size of the infecting dose of chlamydiae did not affect the number of B cells between the two groups.
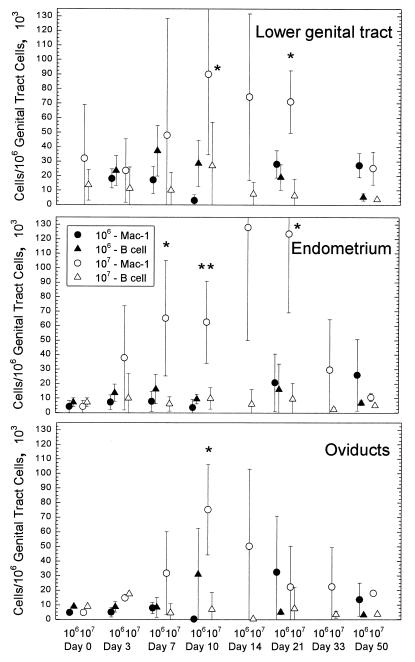
When cells expressing Mac-1 were enumerated in the various tissues of the genital tract, the number of Mac-1+ cells began to increase by day 7 after infection in the group infected with 107 IFU and reached peak levels between days 10 and 14 (Fig. 2). An increase in Mac-1+ cells in the group infected with 106 IFU was not as apparent. It was interesting that the difference between the 106 and 107 groups in each part of the genital tract was quite dramatic, suggesting that the number of organisms could influence the intensity of the inflammatory response. While at this point we cannot make any deductions about the effect of dose on the individual cell types that express Mac-1, it is clear from previous studies using this model that there is a strong acute inflammatory response consisting of PMNs in the primary infection (16). Mononuclear cells also enter the tissues during the primary response, so the Mac-1 population is likely a mixed population of PMNs and monocytes-macrophages. After a challenge infection, there is a reduced number of PMNs so that the preponderance of potential Mac-1-bearing cells is in the monocyte/macrophage group.
FIG. 2.
Comparison of B-cell and Mac-1-expressing cell numbers in different areas of the genital tract in response to infection with either 106 or 107 IFU of the GPIC infectious agent. The error bars represent the standard deviations of the means of results from four guinea pigs. The single asterisks indicate statistical significance at a P value of <0.03 according to a t test. Double asterisks indicate statistical significance at a P value of <0.01.
Reinfection.
An important premise of chlamydial genital infection is that repeated infection appears to result in enhanced disease, presumably caused by an anamnestic lymphocytic response. These observations have been made in nonhuman primate, mouse, and guinea pig models by histopathologic analyses of genital tract tissues (17, 20, 21). However, there has not been a true quantitative and comparative analysis of the T-cell response in a primary infection versus reinfection. Thus, our ability to quantify T and B cells as well as inflammatory cells bearing Mac-1 in the guinea pig model provided an ideal opportunity to evaluate the effect of repeated infection in the various tissues of the genital tract. Guinea pigs were infected intravaginally with 106 IFU of the GPIC agent and after 50 days were reinoculated intravaginally with 106 IFU. Genital tract tissues were collected from animals at 10 and 21 days after the reinoculation, and the levels of the different cell populations were quantified and compared to those in animals 10 and 21 days after a primary infection. Previously, we had documented that animals were partially immune to reinfection following recovery from a primary infection (13), as indicated by a marked reduction in IFU and the length of the challenge infection.
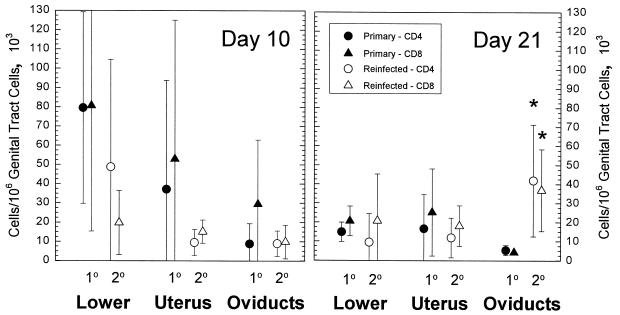
Ten days after reinfection, there was no significant difference in the number of CD4 and CD8 cells infiltrating the genital tract in comparison to those in tissues collected 10 days after a primary infection (Fig. 3). However, when guinea pigs were evaluated 21 days after reinfection, both CD4 and CD8 T-cell levels in the oviducts were significantly greater than T-cell levels in oviducts after a primary infection. These data indicated that there was indeed an enhanced cell-mediated immune response to chlamydiae or chlamydial antigen upon reinfection. This result was also particularly interesting in that a similar enhanced response was not noted in the lower genital tract and the endometrium. Previous studies showed a reduction in the PMN response but an increase in the mononuclear response in the genital tract following challenge infection (17). There was also an increased incidence of hydrosalpinx, indicating more severe upper genital tract disease.
FIG. 3.
Comparison of CD4 and CD8 cell numbers in different areas of the genital tract at 10 and 21 days after a primary GPIC intravaginal infection and after reinfection 50 days after the primary infection. The error bars represent the standard deviations of the means of results from four guinea pigs at each point in the primary group and six animals in the reinfection group.
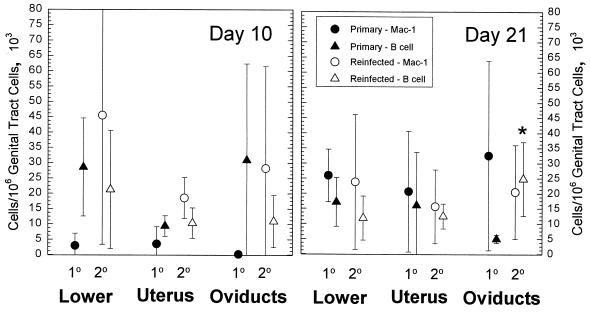
In keeping with the enhancement of the lymphocyte response, there was a significant increase in the number of B cells infiltrating the oviducts but not other tissues in the reinfected animals (Fig. 4). No differences were noted in the levels of Mac-1-bearing cells between the primary and challenge infection, suggesting that the enhanced reaction in the oviducts was the result of an anamnestic response to chlamydial antigen.
FIG. 4.
Comparison of B-cell and Mac-1-expressing-cell numbers in different areas of the genital tract 10 and 21 days after a primary GPIC intravaginal infection and after reinfection 50 days after the primary infection. The error bars represent the standard deviations of the means of results from four guinea pigs at each point in the primary group and six animals in the reinfection group. The asterisk indicates statistical significance at a P value of <0.03 according to a t test.
DISCUSSION
In this study, we have provided a quantitative assessment of the T-cell, B-cell, and Mac-1-expressing-cell responses in different sections of the guinea pig genital tract following intravaginal infection or reinfection with the agent of GPIC. Previously, we had described the pathologic response to the infection in similar groups of animals by histopathology (16). In those studies, we reported that in the primary infection, an acute and chronic inflammatory response was noted in each of the infected tissues. Initially the heaviest response was in the exo- and endocervix, but after about 7 days, pathologic changes could be seen in the endometrium and the oviducts as well. However, while organisms could be demonstrated in the endometrium and oviducts of 80% of the animals, only 45% of the animals had detectable pathology. In the current study, we also noted that the strongest reaction was in the lower genital tract with a marked influx of Mac-1-bearing cells and T cells. B cells remained relatively low in each of the tissues throughout the course of the infection. The Mac-1 response is most likely indicative of the inflammatory response in general, since this marker can be found on PMNs, monocytes, and macrophages.
Particularly in animals infected with 107 IFU of the GPIC agent, there was a much greater response of Mac-1-bearing cells in all parts of the genital tract, which closely paralleled the histopathology data previously published (16). Also, it was interesting that there was a dose response with regard to the Mac-1 response. Infection with 107 IFU yielded a higher number of cells than did infection with 106 IFU. These data are supported by observations that we have made of the TNF-α response following GPIC genital infection. In that study, the level of TNF-α detected in genital secretions was positively correlated with the number of chlamydiae in the inoculum. Thus, these data would suggest that the production of proinflammatory cytokines responsible for the acute inflammatory response might be dependent on the number of organisms present. It has been demonstrated by others that infection of macrophages with chlamydiae can elicit TNF-α production either via lipopolysaccharide (LPS) stimulation of the cells or possibly even through heat shock protein 60 (hsp60) stimulation of the cells (1, 4). Moreover, Rasmussen et al. (18) have shown that chlamydial infection of epithelial cells can also elicit interleukin 1 (IL-1) and IL-8 production, both of which are actively involved in the acute inflammatory response. In contrast to the Mac-1 response, there was no obvious dose dependency upon the T-cell or B-cell influx.
While the T-cell response is also supportive of our earlier histopathologic observations, it was surprising that the numbers of CD4 and CD8 cells were equivalent. This is in contrast to the MoPn mouse genital tract model, in which the dominant T-cell phenotype in the genital tract following infection is CD4, while CD8 T cells are generally present in lower numbers (3). Nevertheless, this ratio of CD4 to CD8 cells in the guinea pig approximates more closely what has been observed in the endocervix of humans infected with C. trachomatis (5) and nonhuman primates infected with C. trachomatis in the genital tract (21) or in the conjunctiva (24). We have also noted equivalent numbers of CD4 and CD8 cells in the guinea pig conjunctiva upon ocular infection with GPIC (R. G. Rank, unpublished data). In the murine model, CD8 cells have been shown to have a protective role, but they are not essential for resolution of the infection, nor have they been positively associated with pathology. The demonstration of a major CD8 response in the guinea pig genital tract following chlamydial infection would suggest that CD8 cells play a significant role in either protection or the production of pathology or both. Thus, as in many other parameters, the guinea pig model continues to reflect very closely what is seen in the human and nonhuman primates and can provide a convenient method for evaluating the role of this cell population in chlamydial disease.
Perhaps the most important observation in this study is the demonstration of an increased T-cell response in the oviducts upon reinfection in the genital tract. When compared to the number of T cells present in the oviduct 21 days after a primary infection, there was a significant increase in both CD4 and CD8 T cells in the oviduct 21 days after reinoculation. It was also of interest that the number of B cells was significantly increased upon reinfection. In contrast, the number of Mac-1-expressing cells in the tissues after reinfection was no different than during the primary infection. These data are all consistent with a specific pathologic cell-mediated immune reaction that has been proposed by ourselves and others to explain the more severe pathology seen upon reinfection in different animal models (17, 20, 21). Since the level of immunity to reinfection in the guinea pig is such that there is only a low level of infection upon reinoculation (13), the presence of an enhanced response in the oviducts suggests that very little antigen is required to elicit the response.
An unexpected result was the finding that the enhanced T- and B-cell responses 21 days after reinfection were limited to the oviduct and were not apparent in the lower genital tract or the endometrium. In fact, the T-cell response was lower at day 10 after reinoculation when compared to T cells at day 10 after the primary infection. This was most likely the result of a markedly reduced infection and clearance of the organisms by the serum and secretion antibody (12). It may also indicate that there are tissue differences that facilitate T-cell homing. Kelly et al. (3) recently reported that vascular cell adhesion molecule 1 (VCAM-1) and mucosal addressin cell adhesion molecule 1 (MAdCAM-1) persist longer in the upper genital tract of the mouse compared to their survival in the cervix and endometrium so that there is differential homing to the upper tract. The implications for human disease are obvious. There is evidence that there is a much greater risk of tubal obstruction in patients with repeated infection (23). The data presented here and in the mouse model (3) support the concept that fallopian tube tissue may actually facilitate enhanced disease through prolonged expression of addressins and increased homing of T cells to the site.
This study also establishes this model as an ideal tool to quantitatively examine the protective effect of a vaccine candidate on the pathologic response or the possibility that the vaccine might actually elicit a more severe pathologic response, particularly after multiple challenges. The guinea pig model has shown a remarkable resemblance to the human disease, and, coupled with the ability to demonstrate sexual transmission of chlamydial infection, it presents a unique opportunity to evaluate potential vaccine candidates.
ACKNOWLEDGMENT
This study was supported by Public Health Service grant AI23044 from the National Institutes of Health.
REFERENCES
- 1.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly K A, Walker J C, Jameel S H, Gray H L, Rank R G. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia infection. Infect Immun. 2000;68:1519–1528. doi: 10.1128/iai.68.3.1519-1528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kol A, Sukhova G K, Lichtman A H, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 5.Levine W C, Pope V, Bhoomkar A, Tambe P, Lewis J S, Zaidi A A, Farshy C E, Mitchell S, Talkington D F. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–174. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 6.Murray E S. Guinea pig inclusion conjunctivitis I. Isolation and identification as a member of the psittacosis-lymphogranuloma-trachoma group. J Infect Dis. 1964;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Patton D L, Cosgrove Sweeney Y T, Kuo C-C. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J Infect Dis. 1994;169:680–683. doi: 10.1093/infdis/169.3.680. [DOI] [PubMed] [Google Scholar]
- 8.Patton D L, Halbert S A, Kuo C C, Wang S P, Holmes K K. Host response to primary Chlamydia trachomatis infection of the fallopian tube in pig-tailed monkeys. Fertil Steril. 1983;40:829–840. [PubMed] [Google Scholar]
- 9.Patton D L, Kuo C-C. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J Reprod Fertil. 1989;85:647–656. doi: 10.1530/jrf.0.0850647. [DOI] [PubMed] [Google Scholar]
- 10.Patton D L, Kuo C-C, Wang S-P, Brenner R M, Sternfeld M D, Morse S A, Barnes R C. Chlamydial infection of subcutaneous fimbrial transplants in cynomolgus and rhesus monkeys. J Infect Dis. 1987;155:229–235. doi: 10.1093/infdis/155.2.229. [DOI] [PubMed] [Google Scholar]
- 11.Patton D L, Wolner-Hanssen P, Cosgrove S J, Holmes K K. The effects of Chlamydia trachomatis on the female reproductive tract of the Macaca nemestrina after a single tubal challenge following repeated cervical inoculations. Obstet Gynecol. 1990;76:643–650. [PubMed] [Google Scholar]
- 12.Rank R G, Barron A L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect Immun. 1983;39:463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rank R G, Batteiger B E, Soderberg L S F. Susceptibility to reinfection after a primary chlamydial genital infection. Infect Immun. 1988;56:2243–2249. doi: 10.1128/iai.56.9.2243-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rank R G, Bavoil P M. Prospects for a vaccine against Chlamydia genital disease. 2. Immunity and vaccine development. Bull Inst Pasteur. 1996;94:55–82. [Google Scholar]
- 15.Rank R G, Sanders M M. Ascending genital tract infection as a common consequence of vaginal inoculation with the guinea pig inclusion conjunctivitis agent in normal guinea pigs. In: Bowie W R, Caldwell H D, Jones R B, Mardh P-A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections. New York, N.Y: Cambridge University Press; 1990. pp. 249–252. [Google Scholar]
- 16.Rank R G, Sanders M M. Pathogenesis of endometritis and salpingitis in a guinea pig model of chlamydial genital infection. Am J Pathol. 1992;140:927–936. [PMC free article] [PubMed] [Google Scholar]
- 17.Rank R G, Sanders M M, Patton D L. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. J Sex Transm Dis. 1995;22:48–54. doi: 10.1097/00007435-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol. 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 20.Tuffrey M, Falder P, Taylor-Robinson D. Reinfection of the mouse genital tract with Chlamydia trachomatis: the relationship of antibody to immunity. Br J Exp Pathol. 1984;65:51–58. [PMC free article] [PubMed] [Google Scholar]
- 21.Van Voorhis W C, Barret L K, Sweeney Y T C, Kuo C C, Patton D L. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Voorhis W C, Barrett L K, Sweeney Y T C, Kuo C C, Patton D L. Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis. 1996;174:647–650. doi: 10.1093/infdis/174.3.647. [DOI] [PubMed] [Google Scholar]
- 23.Westrom L, Mardh P A. Chlamydial salpingitis. Br Med Bull. 1983;39:145–150. doi: 10.1093/oxfordjournals.bmb.a071806. [DOI] [PubMed] [Google Scholar]
- 24.Whittum-Hudson J A, Taylor H R, Farazdaghi M, Prendergast R A. Immunohistochemical study of the local inflammatory response to chlamydial ocular infection. Investig Ophthalmol Vis Sci. 1986;27:64–69. [PubMed] [Google Scholar]