Abstract
High NaCl (200 mM) increases the transcription of phospholipase Dδ (PLDδ) in roots and leaves of the salt-resistant woody species Populus euphratica. We isolated a 1138 bp promoter fragment upstream of the translation initiation codon of PePLDδ. A promoter–reporter construct, PePLDδ-pro::GUS, was introduced into Arabidopsis plants (Arabidopsis thaliana) to demonstrate the NaCl-induced PePLDδ promoter activity in root and leaf tissues. Mass spectrometry analysis of DNA pull-down-enriched proteins in P. euphratica revealed that PeGLABRA3, a basic helix–loop–helix transcription factor, was the target transcription factor for binding the promoter region of PePLDδ. The PeGLABRA3 binding to PePLDδ-pro was further verified by virus-induced gene silencing, luciferase reporter assay (LRA), yeast one-hybrid assay, and electrophoretic mobility shift assay (EMSA). In addition, the PeGLABRA3 gene was cloned and overexpressed in Arabidopsis to determine the function of PeGLABRA3 in salt tolerance. PeGLABRA3-overexpressed Arabidopsis lines (OE1 and OE2) had a greater capacity to scavenge reactive oxygen species (ROS) and to extrude Na+ under salinity stress. Furthermore, the EMSA and LRA results confirmed that PeGLABRA3 interacted with the promoter of AtPLDδ in transgenic plants. The upregulated AtPLDδ in PeGLABRA3-transgenic lines resulted in an increase in phosphatidic acid species under no-salt and saline conditions. We conclude that PeGLABRA3 activated AtPLDδ transcription under salt stress by binding to the AtPLDδ promoter region, conferring Na+ and ROS homeostasis control via signaling pathways mediated by PLDδ and phosphatidic acid.
Keywords: Populus euphratica, PeGLABRA3, phospholipase D, phosphatidic acid, ROS, Na+/H+ anti-transporter, DNA pull-down, luciferase reporter assay, virus-induced gene silencing, electrophoretic mobility shift assay, noninvasive micro-test technique, NaCl
1. Introduction
Phospholipase D (PLD) hydrolyzes membrane phospholipids to produce a secondary signaling molecule, phosphatidic acid (PA), to mediate salt stress signaling in plants [1,2,3,4,5,6,7,8,9,10,11,12,13]. Various types of PLD, particularly PLDα, PLDδ, and PLDζ, have been shown to mediate ionic and reactive oxygen species (ROS) homeostasis under salt stress. Cucumber (Cucumis sativus) CsPLDα-produced PA alleviates the salt damage in tobacco plants by accumulating osmoprotective compounds, maintaining Na+/K+ homeostasis and scavenging ROS [14,15]. AtPLDα-overexpressed poplars increase the activity of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) under NaCl treatment [16]. PLDa1 interacts with the downstream Cys-rich receptor-like kinase 2 (CRK2) to promote Arabidopsis (Arabidopsis thaliana) callose deposition and regulate plasmodesmal permeability during salt stress [17]. Phopholipase Dζ1 is considered necessary for regulating ion transport and antioxidant response in terms of the pldζ1 mutant response to salt stress [18]. Cotton (Gossypium hirsutum) GhPLDδ and PA are involved in regulating plant defense against salt stress [19]. We found that PePLDδ, isolated from the salt-resistant woody species Populus euphratica, was able to increase PA content and improve salt tolerance by regulating K+/Na+ and ROS homeostasis in Arabidopsis [20]. PLDδ genes are induced by NaCl in rice (Oryza sativa) [5] and soybean (Glycine max) [6]; however, the NaCl-induced transcriptional regulation of PLDδ in poplar trees and its relevance to salinity tolerance have been less investigated.
The basic helix–loop–helix (bHLH) transcription factors have various functions in regulating plant growth, development, and stress response [21,22,23,24,25,26,27,28,29,30]. The expression of members of the bHLH gene family in response to salt stress has been characterized in Solanum lycopersicum [31], common bean (Phaseolus vulgaris) [32], and finger millet (Eleusine coracana L.) [33]. Moreover, NaCl was found to upregulate a number of bHLH genes in Arabidopsis [34], rice [35], and sugar beet (Beta vulgaris) [36]. The overexpression of AtbHLH92 increases tolerance to salt stress in Arabidopsis [37]. AtbHLH112 confers salinity tolerance through an increased ability for proline accumulation and ROS scavenging [34]. Ectopic expression of sugar beet BvBHLH93 [36], grape (Vitis vinifera) VvbHLH1 [38], and apple (Malus xiaojinensis) MxbHLH18 [39] in Arabidopsis enhanced the activities of antioxidant enzymes and salt-responsive genes. Furthermore, tobacco (Nicotiana tabacum) transcription factor bHLH123 was found to mediate the rapid accumulation of ROS and the response to salinity during the early stages of stress [40]. bHLH transcription factors may also improve ionic homeostasis in salinized plants. For example, BvBHLH93 ectopic expression lines accumulated less Na+ but more K+ than the wild type (WT) [36]. bHLH122 regulates AtKUP2 expression to confer K+/Na+ homeostasis in Arabidopsis plants [41]. The bHLH transcription factors AtMYC2 and AtbHLH122 regulate AtNHX1 and AtNHX6 to mediate the salinity response in Arabidopsis plants overexpressing mangrove (Avicennia officinalis) AoNHX1 [42]. In rice seedlings, OsbHLH035 confers recovery from salt stress through the ABA-independent activation of OsHKT1s, thus preventing Na+ over-accumulation and toxicity in aerial tissues [35]. bHLH transcription factors regulate the expression of genes involved in salt stress tolerance by binding to their E-box and GCG-box motifs [34]. bHLH106 locates a branching point in the abiotic stress response network by interacting directly with the G-box in genes conferring salt tolerance on plants [43]. We also found that the P. euphratica PePLDδ promoter regions harbored a cis-acting G-box; however, it is unknown whether the bHLH transcription factor can mediate PePLDδ expression under salt stress.
Here, we report that PeGLABRA3, a bHLH transcription factor, regulates PePLDδ expression and plays an important role in salt stress tolerance. The binding of PeGLABRA3 to the PePLDδ promoter (PePLDδ-pro) was identified by DNA pull-down and mass spectrometry assay, virus-induced gene silencing (VIGS), luciferase reporter assay (LRA), yeast one-hybrid assay (Y1H), and electrophoretic mobility shift assay (EMSA). We investigated the salt-induced expression of PePLDδ-pro in root and leaf tissues in transgenic A. thaliana. PeGLABRA3 was isolated from P. euphratica and transferred into A. thaliana under the control of the CaMV35S promoter. We examined the AtPLDδ expression and major PA species in WT and PeGLABRA3-transgenic Arabidopsis. Our data showed that the PeGLABRA3 domain can bind to the G-box in the AtPLDδ promoter regions, thus exerting regulation of the gene transcription. AtPLDδ produced a higher abundance of specific PA species, such as 34:2 (16:0–18:2), 34:3 (16:0–18:3), 36:4 (18:2–18:2), and 36:5 (18:2–18:3), contributing to the maintenance of ionic and ROS homeostasis in PeGLABRA3-transgenic plants under NaCl stress.
2. Results
2.1. NaCl-Induced Transcription of PePLDδ in P. euphratica
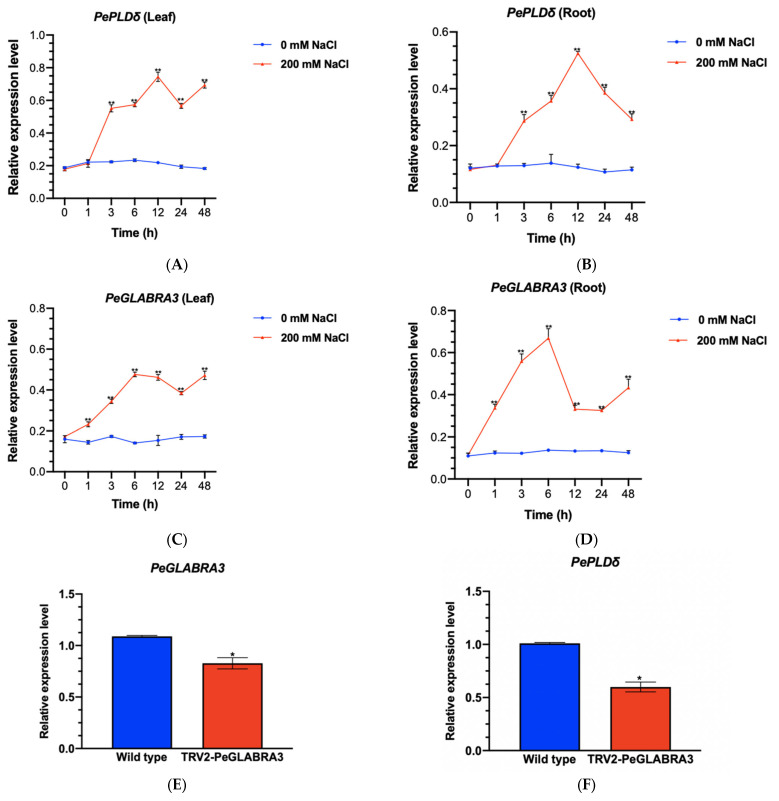
Populus euphratica PLDδ has previously been shown to increase salt tolerance in Arabidopsis plants [20]. In this study, quantitative real-time PCR (RT-qPCR) was used to determine the salt-induced transcription of the PePLDδ gene in the roots and leaves of P. euphratica seedlings. The expression of PePLDδ significantly increased after 3 h of NaCl treatment (200 mM), reaching peak levels at 12 h (Figure 1A,B). Then, PePLDδ remained at relatively high levels in the hours following salt treatment (24–48 h), although a decline was observed in the roots (Figure 1A,B).
Figure 1.
Transcription of PeGLABRA3 and PePLDδ in salt-stressed Populus euphratica. (A–D) PePLDδ and PeGLABRA3 transcription in NaCl-stressed P. euphratica. Populus euphratica seedlings were exposed to 0 or 200 mM of NaCl for 48 h. Leaves and roots were sampled at 0, 1, 3, 6, 12, 24, and 48 h for RT-qPCR analysis. (E,F) PeGLABRA3 and PePLDδ transcription in PeGLABRA3-silenced P. euphratica. Agrobacterium carrying the TRV1 empty vector and TRV2-PeGLABRA3 vector were injected into P. euphratica leaves. After 45 days of virus-induced gene silencing (VIGS), the P. euphratica seedlings were subjected to 200 mM of NaCl for 24 h. Leaves were sampled from TRV-infected P. euphratica for RT-qPCR analysis. Populus euphratica PeACT7 was used as an internal reference. Primers for PeGLABRA3, PePLDδ, and PeACT7 are shown in Supplementary Table S1. Each point (A–D) or column (E,F) represents the average of three independent replicates, and the error bar represents the standard error of the mean. * p < 0.05, ** p < 0.01.
2.2. PePLDδ Promoter Cloning and Analysis
The upstream regulatory region was characterized to determine its importance for the salt-induced expression of PePLDδ. The 1138 bp promoter fragment of PePLDδ was sequenced and analyzed using the PLACE and PlantCARE databases (Figure 2). Its promoter sequence was found to contain multiple cis-acting elements, including an ethylene-responsive element, a cis-acting element involved in the abscisic acid responsiveness (ABRE motif), a cis-acting regulatory element essential for the anaerobic induction (ARE), a cis-acting regulatory element involved in MeJA responsiveness (CGTCA motif), a gene element involved in drought and ABA signaling response (MYB), AT1 motif (part of a light-responsive module), and a G-box (a cis-regulatory element involved in light response) (Figure 2).
Figure 2.
Promoter sequence analysis of Populus euphratica PePLDδ. The P. euphratica PePLDδ promoter was isolated via PCR amplifications, and the primer sequences used for PePLDδ promoter isolation are shown in Supplementary Table S1. The sequences of the two probes (probe 1 and probe 2) that were amplified and subsequently used for PePLDδ promoter isolation are shown. Different colors represent the predicted cis-acting elements in the promoter region.
2.3. NaCl Activates the PePLDδ Promoter in Root and Leaf Tissues
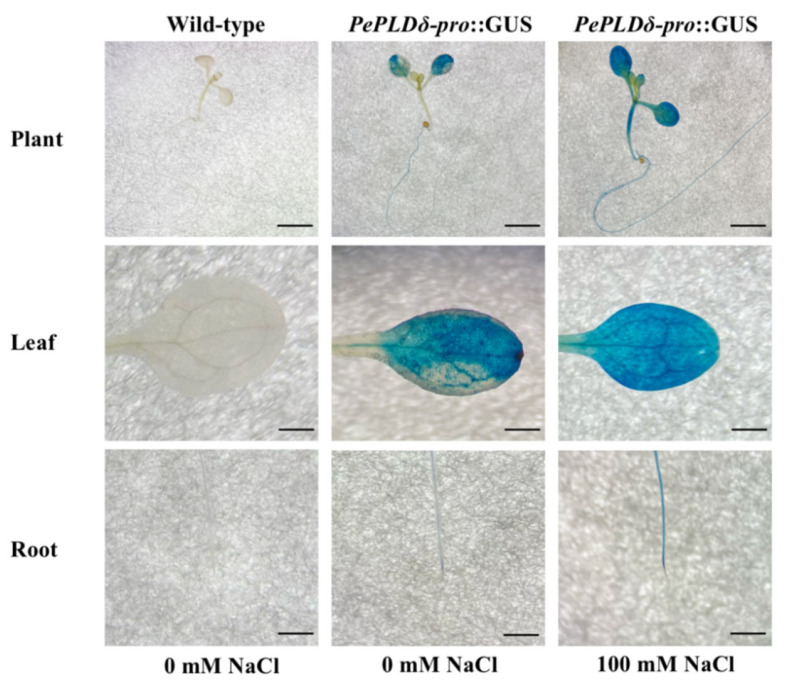
The PePLDδ-pro::GUS fusion was constructed and transferred to A. thaliana. GUS activity was observed in the root tip, mesophyll, and vein tissues (Figure 3). Furthermore, an increase in GUS activity in root and leaf tissues was observed following NaCl treatment (100 mM, 24 h). Therefore, the salt-enhanced expression of PePLDδ in P. euphratica was due to the promoter activity.
Figure 3.
GUS histochemical assays. The promoter of PePLDδ was combined with the β-glucuronidase gene (GUS) and transformed into Arabidopsis. Ten-day-old wild type seedlings and PePLDδ-pro::GUS transgenic plants (T3 generation) were treated with 0 or 100 mM of NaCl for 24 h. Roots and leaves were sampled for GUS histochemical analysis. Scale bar = 500 μm.
2.4. Transcription Factor Identification by DNA Pull-Down
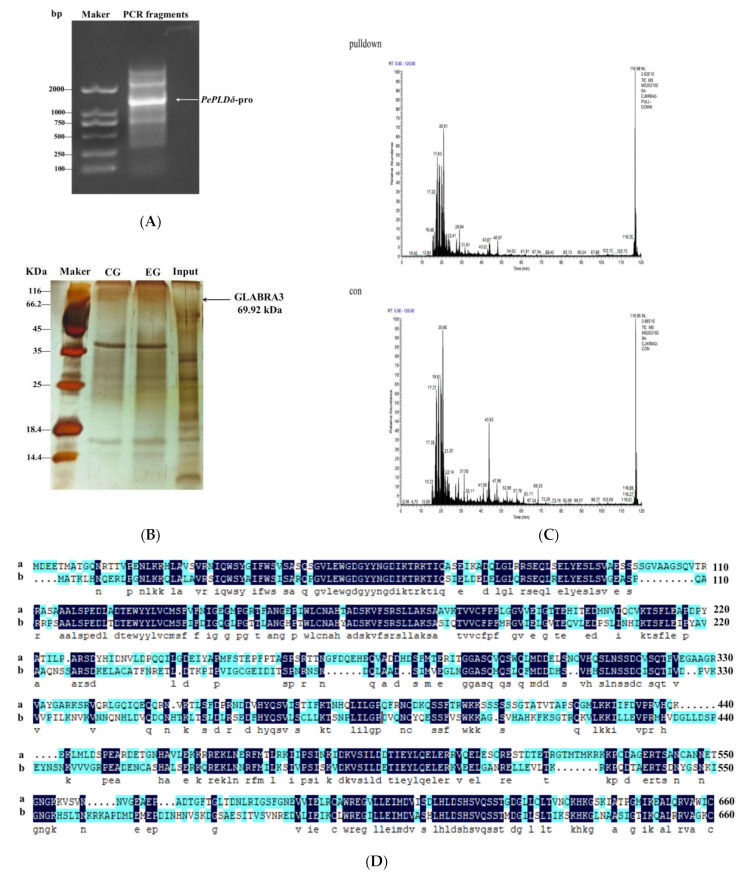
The identification of regulatory transcription factors for salt-induced PePLDδ was performed by DNA pull-down and mass spectrometry. Specific DNA probes were designed based on the sequence of the PePLDδ promoter region and labeled with desulfurizing biotin, which can bind to streptavidin coupled to magnetic beads (Figure 4A). Then, the protein extract was incubated with the magnetic bead DNA probe to obtain the PePLDδ-interacted transcription factor (Figure 4B). The target DNA probe–protein complex was obtained and identified by mass spectrometry (Figure 4C). Based on a comparison of protein type and amino acid sequence available in the NCBI database, the amino acid sequence identified by DNA pull-down covered and matched with that of PeGLABRA3, and the percentage identity and coverage reached 50.15% (Figure 4D). Accordingly, the bHLH transcription factor GLABRA3 was identified as a target transcription factor for binding the promoter region of PePLDδ (Figure 4D).
Figure 4.
DNA pull-down and mass spectrometry analysis. (A) PCR amplification of the PePLDδ probe. The PePLDδ promoter fragment was amplified by PCR, cloned, and sequenced. The probes were biotin-labeled and purified with a gel extraction kit and used for DNA pull-down. (B) DNA pull-down-enriched proteins. The biotin-labeled DNA and nucleic acid were incubated with beaver magnetic beads to form a DNA magnetic bead complex. Then, the DNA magnetic bead complex was incubated with protein extracts to form the protein-DNA-magnetic bead complex. The DNA pull-down-enriched proteins were separated using SDS-PAGE denaturing polyacrylamide gel electrophoresis and stained with a silver staining kit (Sigma-Aldrich). CG: control group; EG: experimental group. (C) Mass spectrometry analysis of DNA pull-down-enriched proteins. The green marks represent the matching isotope peaks. (D) The amino acid sequence identified by DNA pull-down covered and matched with that of PeGLABRA3; a, the protein sequence identified by DNA pull-down; b, the PeGLABRA3 protein sequence. Black shading indicates identical amino acid residues and blue shadings indicate conserved amino acids, respectively. The obtained protein sample was analyzed with mass spectrometry (Ultraflex III mass spectrometer, Bruker, Germany). The database used for protein determination was Uniprot_Arabidopsis thaliana (https://www.uniprot.org/uniprotkb?query=Arabidopsis%20thaliana, accessed on 13 August 2020), Uniprot_Populus euphratica (https://www.uniprot.org/uniprotkb?query=Populus%20euphratica, accessed on 16 December 2020), and Uniprot_Populus trichocarpa (https://www.uniprot.org/uniprotkb?query=Populus%20trichocarpa, accessed on 16 December 2020). By comparison of protein type and amino acid sequence available in the NCBI database, Arabidopsis GLABRA3 was most similar to the obtained protein, and the basic helix–loop–helix (bHLH) transcription factor GLABRA3 (GL3) was identified as a target transcription factor for binding the promoter region of PePLDδ. Con: control group.
2.5. PeGLABRA3 Cloning and Sequence Analysis
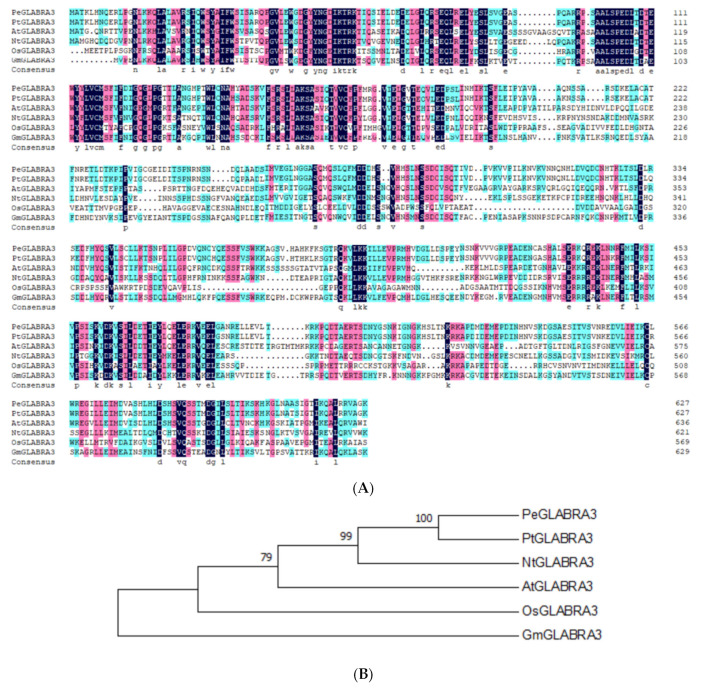
The PeGLABRA3 gene was isolated from the leaves of P. euphratica seedlings and used for sequence analysis. A comparison of the amino acid sequences of GLABRA3 from different species revealed that PeGLABRA3 is highly similar to the GLABRA3 of P. tricocarpa (Figure 5A). Furthermore, comparative phylogenetic analysis showed that PeGLABRA3 is homologous to tobacco NtGLABRA3 and P. tricocarpa PtGLABRA3 (Figure 5B).
Figure 5.
Multiple sequence alignment and phylogenetic analysis of Populus euphratica PeGLABRA3. (A) Multiple sequence alignment of PeGLABRA3 with GLABRA3 from different species. Black shading indicates identical amino acid residues and blue and pink shadings indicate conserved amino acids, respectively. (B) A phylogenetic relationship between PeGLABRA3 and GLABRA3 proteins from other different species. The phylogenetic tree was constructed using the nearest neighbor joining method with MEGA 7.0 software. At, Arabidopsis thaliana; Nt, Nicotiana tabacum; Pe, Populus euphratica; Pt, Populus trichocarpa; Os, Oryza sativa; Gm, Glycine max. GLABRA3 accession numbers for different species are shown in Supplementary Table S2.
2.6. NaCl-Induced PeGLABRA3 Expression
Populus euphratica increased PeGLABRA3 transcription in roots and leaves after 1 h of NaCl treatment, and the peak level was observed at 6 h (Figure 1C,D). The pattern of salt-induced PeGLABRA3 was similar to that of PePLDδ during the period of salt stress (Figure 1A–D). Notably, PeGLABRA3 increased earlier than PePLDδ in both roots and leaves (Figure 1A–D).
2.7. PeGLABRA3 Binds to the PePLDδ Promoter
To determine whether PeGLABRA3 interacts with PePLDδ, the binding of PeGLABRA3 to the PePLDδ promoter region was verified by EMSA, Y1H, LRA, and VIGS.
2.7.1. Electrophoretic Mobility Shift Assay
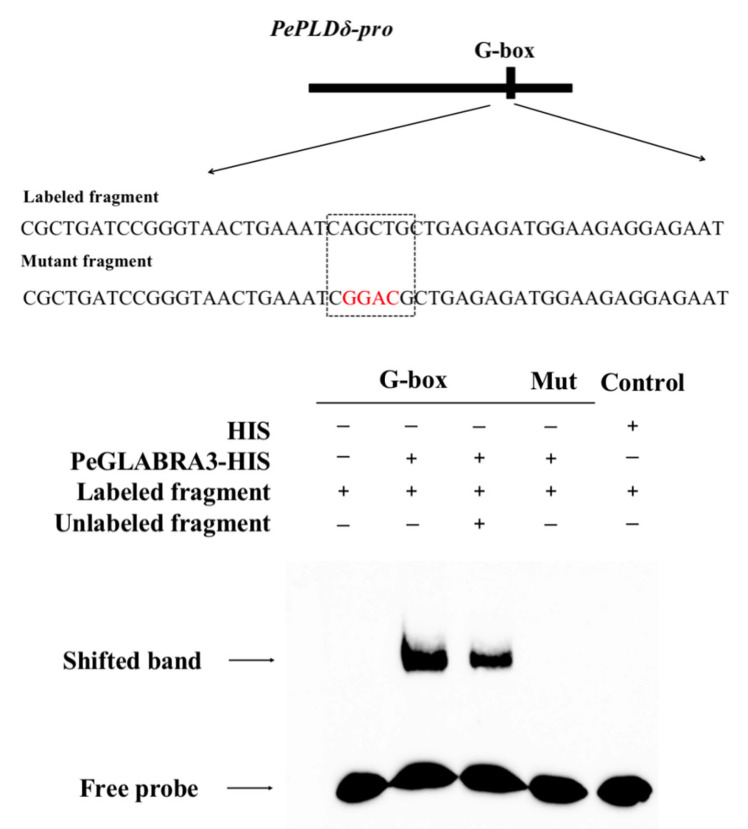
Previous research showed that the bHLH protein mediates the transactivation of target genes through binding to E- and G-box elements in the promoter region [34,43,44]. The PePLDδ promoter region contains a cis-acting element G-box (Figure 2). An EMSA was performed to evaluate the interaction of PeGLABRA3 with the G-box in vitro. Biotin labeling indicated that the PeGLABRA3 protein bound to the G-box motif in the PePLDδ promoter, while the negative control, HIS-protein, was unable to bind to the probe (Figure 6, lanes 1, 2, and 5). Furthermore, an increasing ratio of competing probes interfered with the binding of PeGLABRA3 to the biotin-labeled G-box (Figure 6, lane 3). The binding specificity of the G-box to PeGLABRA3 was also confirmed by the mutant G-box. The G-box CAGCTG was mutated to CGGACG and labeled with biotin, and the presence of a G-box mutation probe was found to inhibit the binding of PeGLABRA3 to G-box (Figure 6, lane 4).
Figure 6.
Electrophoretic mobility shift assay (EMSA) verified the interaction of PeGLABRA3 with the PePLDδ promoter region. PeGLABRA3-HIS protein purified from prokaryotic expression was used for in vitro EMSA, while HIS-tagged protein was used as a negative control. The mutant probes (Mut, CAGCTG to CGGACG) were used to confirm the binding specificity of G-box to PeGLABRA3. The bases marked in red indicate the mutated bases in the mutant probe. In each loading panel, “+” and “−” indicate the presence or absence of protein and probe, respectively. The cold probe concentration was 10× and the concentration of the polyacrylamide gel was 6%. The EMSA experiment was repeated three times and the representative images are shown.
2.7.2. Y1H Assay
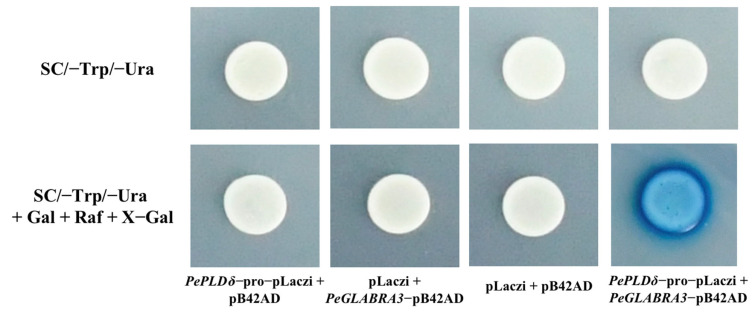
The 1138 bp promoter sequence of PePLDδ was fused to the yeast vector pLaczi, and the full-length coding sequence region of PeGLABRA3 was fused to the yeast vector pB42AD. Single colonies could be propagated on the two deficient media (SD/-Ura/-Trp), and the blue color of X-Gal was displayed when the vectors carrying PePLDδ-pro and PeGLABRA3 were co-transformed into EGY48 competent cells (Figure 7). In the negative control, color change was not observed (Figure 7). Y1H showed that PeGLABRA3 could interact with the PePLDδ promoter.
Figure 7.
Yeast one-hybrid (Y1H) analysis of PeGLABRA3 binding to the PePLDδ promoter. The co-transformation of plasmids for Y1H analysis includes following combinations: (1) PePLDδ-pro-pLaczi + pB42AD, (2) pLaczi + PeGLABRA3-pB42AD, (3) pB42AD + pLaczi, and (4) PePLDδ-pro-pLaczi + PeGLABRA3-pB42AD. The yeast cells were cultured on an SD/-Trp/-Ura solid medium supplemented without or with X-Gal. The Y1H analysis was repeated three times and representative images are shown. Gal: galactose, Raf: raffinose.
2.7.3. Luciferase Reporter Assay
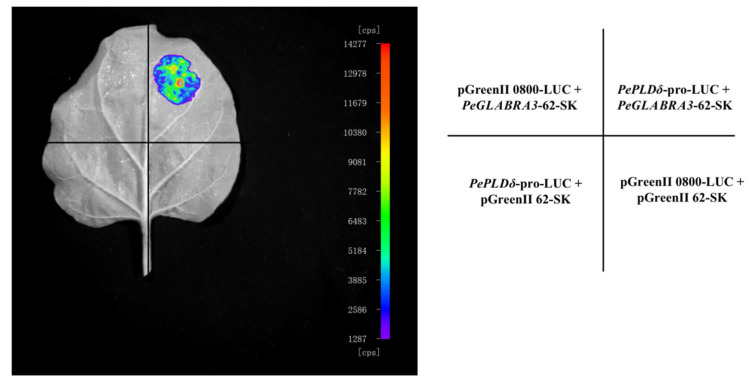
The luminescence of luciferase was extremely low and almost invisible when A. tumefaciens vector controls (VCs), pGreenII 0800-LUC, and pGreenII 62-SK were co-transformed into tobacco leaves (Figure 8). The co-transformation of PePLDδ-pro-LUC with pGreenII 62-SK and PeGLABRA3-62-SK with pGreenII 0800-LUC did not exhibit any visible luminescence (Figure 8). When PePLDδ-pro-LUC was co-transformed with PeGLABRA3-62-SK into tobacco leaves, a significant increase in luciferase luminescence was observed (Figure 8). An LRA confirmed that PeGLABRA3 activated the PePLDδ promoter.
Figure 8.
The luciferase reporter assay (LRA) validated the PeGLABRA3 interaction with the PePLDδ promoter. Nicotiana tabacum leaves were co-transformed with Agrobacterium strains containing (1) PeGLABRA3-62-SK + pGreenII 0800-LUC, (2) PeGLABRA3-62-SK + PePLDδ-pro-LUC, (3) PePLDδ-pro-LUC + pGreenII 62-SK, or (4) pGreenII 0800-LUC + pGreenII 62-SK. The LRA was repeated three times and representative luciferin luminescence images are shown.
2.7.4. Virus-Induced Gene Silencing
The VIGS was performed in P. euphratica seedlings using the tobacco rattle virus (TRV)-based factor pTRV2 [45]. Under NaCl stress, PeGLABRA3 expression declined correspondingly when Agrobacterium carrying TRV2-PeGLABRA3 was infiltrated into leaves (Figure 1E). It is worth noting that a significant reduction in PePLDδ was also observed in TRV-infected leaves (Figure 1F). Accordingly, the silencing of PeGLABRA3 led to a decrease in PePLDδ under salt conditions.
2.8. The Overexpression of PeGLABRA3 Enhances Salt Tolerance in Arabidopsis
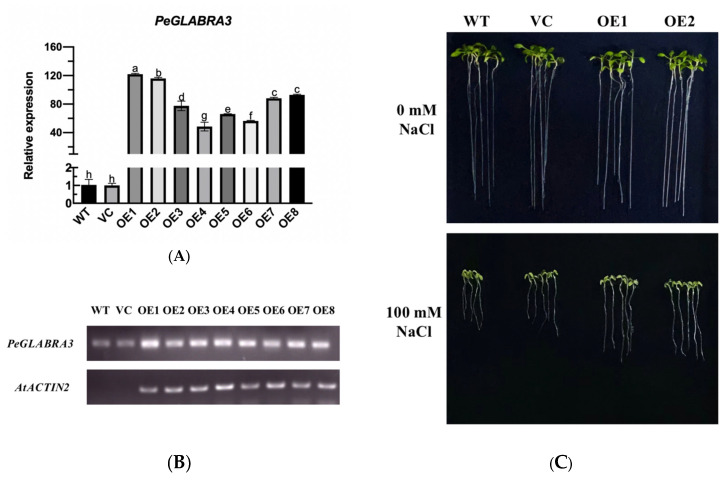
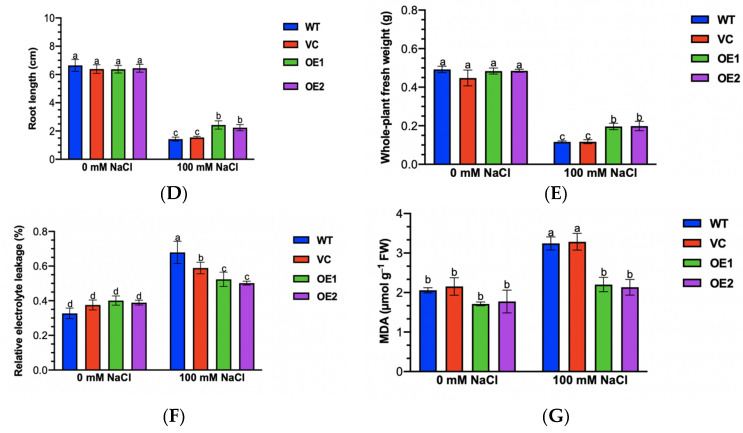
PeGLABRA3 was overexpressed in Arabidopsis to test whether it could mediate salt tolerance in higher plants. Eight PeGLABRA3-transgenic lines, OE1–OE8, were obtained and identified by RT-qPCR and semiquantitative reverse transcription PCR (Figure 9A,B). Two transgenic lines, OE1 and OE2, were used for phenotypic tests due to the higher transcript abundance of PeGLABRA3 (Figure 9A,B). Compared with WT and VC, root length and whole-plant fresh weight in transgenic lines were less inhibited after NaCl stress (100 mM, 7 d, Figure 9C–E). The salt-increased electrolyte leakage and malondialdehyde (MDA) content were also lower in OE1 and OE2 than in WT and VC (Figure 9F–G).
Figure 9.
Phenotype tests of PeGLABRA3-transgenic lines. (A,B) RT-qPCR and semiquantitative RT-PCR analysis. Total RNA was isolated from 10-day-old wild type Arabidopsis thaliana (WT) seedlings, empty vector control (VC), and transgenic lines (OE1-OE8), and used for semiquantitative PCR and real-time quantitative PCR analysis. Arabidopsis β-actin 2 (AtACTIN2) was used as an internal reference gene. Primers designed for PeGLABRA3 and the internal control gene AtACTIN2 are shown in Supplementary Table S1. Each column is the mean of three independent experiments, and the bars represent the standard error of the mean. Different letters (a–h) indicate a significant difference at p < 0.05. (C–G) Salt tolerance test. WT and VC seeds and PeGLABRA3-transgenic lines OE1 and OE2 were germinated and grown on 1/2 MS medium. Seven-day-old seedlings were exposed to 0 or 100 mM of NaCl for 7 d. Root length, whole-plant fresh weight, relative electrolyte leakage, and MDA content were examined during the period of NaCl treatment. (C) Representative pictures showing plant performance and root length under control and NaCl stress. (D) Root length. (E) Whole-plant fresh weight. (F) Relative electrolyte leakage. (G) MDA content. Each column is the mean of three independent experiments, and the bars represent the standard error of the mean. Different letters (a–d) indicate a significant difference at p < 0.05.
2.9. PeGLABRA3-Transgenic Plants Increased ROS Scavenging Capacity under Salt Stress
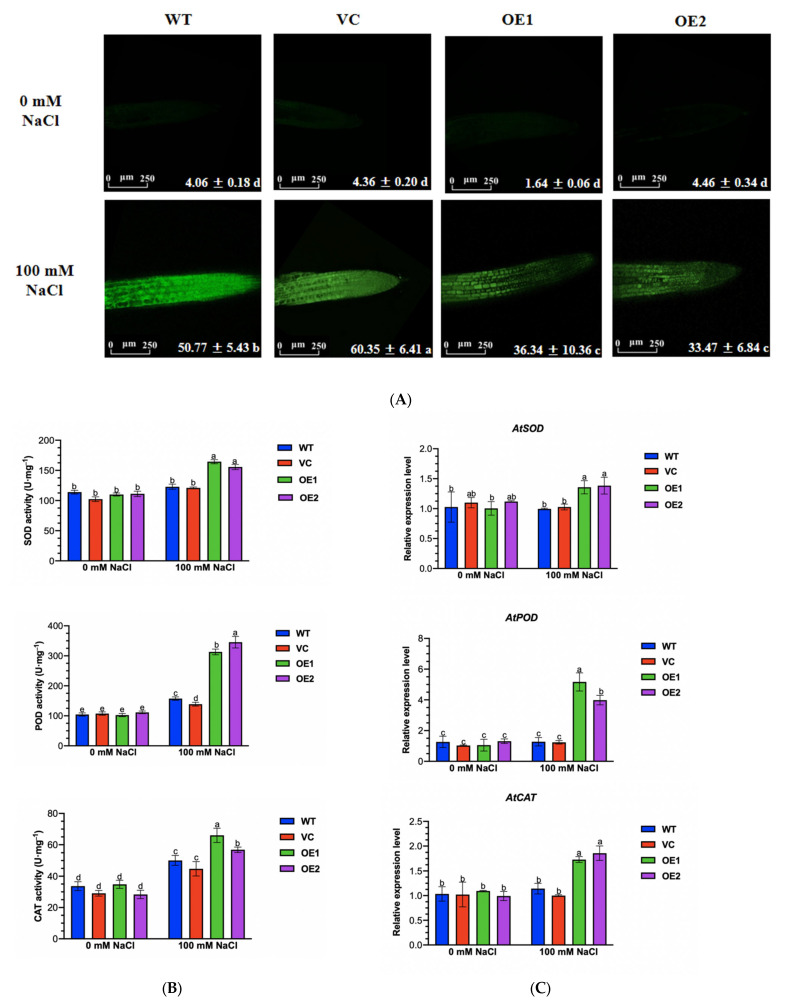
The PeGLABRA3-enhanced tolerance to NaCl was associated with increased ROS scavenging and Na+ exclusion. H2O2 concentrations were measured by a fluorescent probe, H2DCFDA, in the root cells of all tested genotypes. H2DCFDA intensity revealed that WT and VC had 1.38- to 1.81-fold higher H2O2 than the PeGLABRA3-transgenic lines OE1 and OE2 after NaCl treatment (100 mM, 12 h, Figure 10A). The increased ability to control ROS in transgenic lines was related to the enhanced activity of SOD, POD, and CAT and the increased transcription of the genes encoding these antioxidant enzymes. In PeGLABRA3-transgenic lines, the total enzyme activities of SOD, POD, and CAT increased by 10–54% under salt treatment, which was significantly higher than in WT and VC (Figure 10B). The salt-induced transcription of SOD, POD, and CAT showed a trend similar to that of antioxidant enzymes (Figure 10C).
Figure 10.
H2O2 level, activity, and transcription of antioxidant enzymes. (A) H2O2 concentration in root cells. Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were exposed to 0 or 100 mM of NaCl for 12 h. Arabidopsis roots sampled from the control and salt-stressed plants were incubated with 10 μM H2DCFDA for 15 min and washed four or five times. The intracellular green fluorescence was detected by a confocal laser microscope. Each value is the mean of three independent experiments, and different letters (a–d) indicate a significant difference at p < 0.05. Scale bar = 250 μm. (B,C) Activity and transcription of antioxidant enzymes. Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were exposed to 0 or 100 mM of NaCl for 7 days. The total activity of SOD, POD, and CAT and transcription of the encoding genes AtSOD, AtPOD, and AtCAT were examined. Arabidopsis β-actin 2 (AtACTIN2) was used as the internal reference gene. Primers for AtSOD, AtPOD, AtCAT, and AtACTIN2 are listed in Supplementary Table S1. Each column is the mean of three independent experiments, and different letters (a–e) indicate a significant difference at p < 0.05.
2.10. PeGLABRA3-Transgenic Plants Maintained Ionic Homeostasis under Salinity
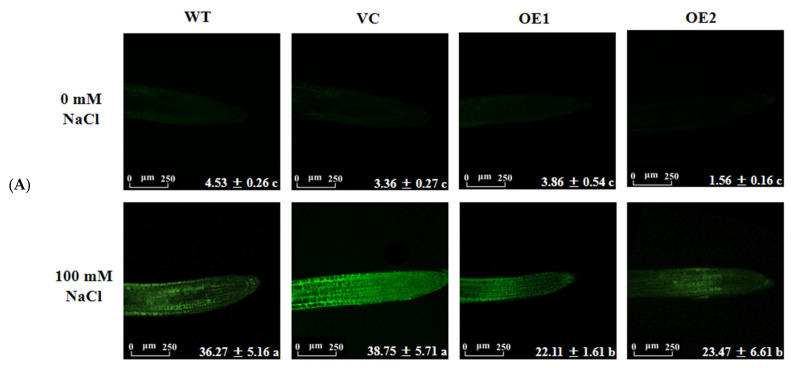
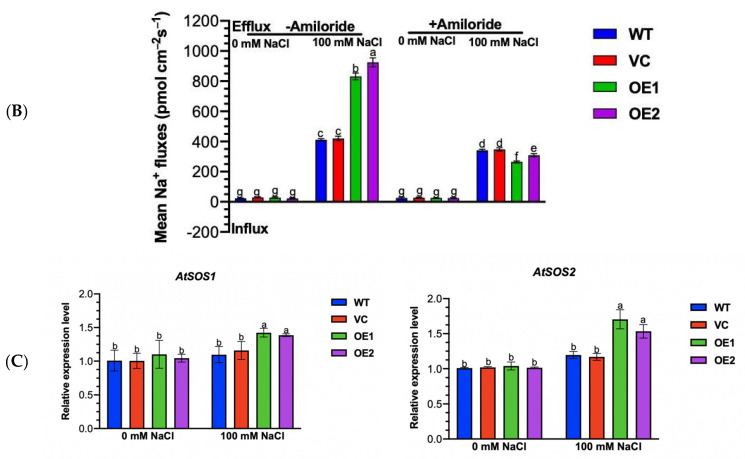
The Na+-specific probe CoroNaTMGreen was used to determine Na+ content in root cells. The fluorescence intensity significantly increased in root cells of all tested lines upon salt exposure (NaCl 100 mM, 12 h, Figure 11A). It was noteworthy that the cellular Na+ was significantly lower in transgenic lines than in WT or VC under salinity (Figure 11A). To determine whether the low Na+ accumulation in PeGLABRA3-transgenic plants was the result of an active salt extrusion, a noninvasive micro-test technique (NMT) was used to detect Na+ flow in the roots. The short-term NaCl treatment (100 mM, 12 h) resulted in a marked rise of Na+ efflux in the root tips of all tested lines (Figure 11B). We noticed that PeGLABRA3-transgenic lines exhibited a 2-fold higher flux rate than WT and VC (Figure 11B). However, the addition of amiloride, an inhibitor of Na+/H+ anti-transport, reduced the salt-elicited Na+ efflux in WT, VC, and PeGLABRA3-transgenic lines (Figure 11B), suggesting that the Na+ efflux was the result of Na+/H+ antiport across the PM. Here, we also examined the abundance of salt overly sensitive (SOS) pathway genes, SOS1 and SOS2, in Arabidopsis plants. The transcription of AtSOS1 and AtSOS2 was significantly upregulated by NaCl in PeGLABRA3-transgenic lines (Figure 11C). This finding indicates that the Na+ extrusion resulted from the Na+/H+ antiport in the roots of PeGLABRA3-transgenic plants.
Figure 11.
Na+ concentration and Na+ flux in the root. (A) Na+ concentration in root cells. Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were exposed to 0 or 100 mM of NaCl for 12 h. Arabidopsis roots sampled from control and salt-stressed plants were incubated with 20 μM CoroNa™Green for 2 h and washed four or five times. The intracellular green fluorescence was detected by a confocal laser microscope. Each value is the mean of three independent experiments and different letters (a–c) indicate a significant difference at p < 0.05. Scale bar = 250 μm. (B) Na+ flux in the root tip. Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were exposed to 0 or 100 mM of NaCl for 12 h. Then, Arabidopsis roots were incubated with amiloride (an inhibitor of Na+/H+ antiporter, 0 or 5 mM) for 30 min. Thereafter, roots were sampled and equilibrated for 30 min in the basic solution containing 0.1 mM of NaCl, 0.1 mM of CaCl2, 0.1 mM of MgCl2, 0.5 mM of KCl, and 2.5% sucrose (pH 5.8). Na+ fluxes were monitored by NMT microelectrodes at the meristematic region (200 µm from the root tip) and continuously recorded for 10 min. Each column is the mean of three independent experiments, and different letters (a–g) indicate a significant difference at p < 0.05. (C) Transcription of AtSOS1 and AtSOS2. Seven−day−old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were exposed to 0 or 100 mM of NaCl for 7 d. Transcription of AtSOS1 and AtSOS2 was examined, and Arabidopsis β-actin 2 (AtACTIN2) was used as the internal reference gene. Primers for AtSOS1, AtSOS2, and AtACTIN2 are listed in Supplementary Table S1. Each column is the mean of three independent experiments, and different letters (a and b) indicate a significant difference at p < 0.05.
2.11. PeGLABRA3 Enhances AtPLDδ Transcription by Binding Its Promoter
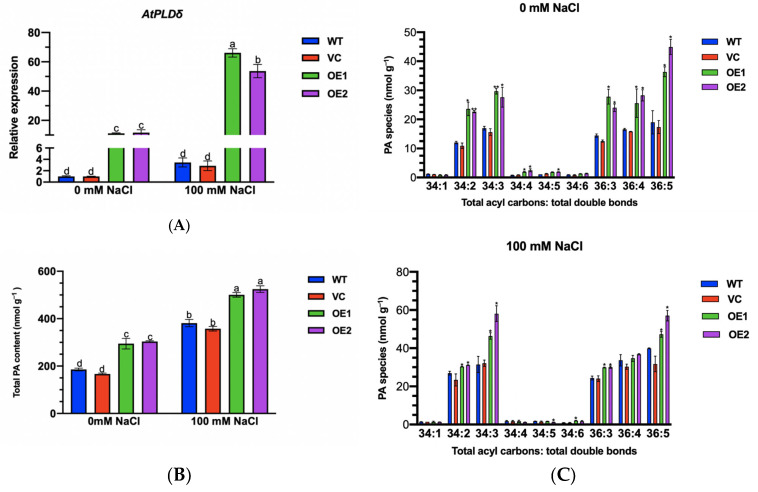
The PeGLABRA3-transgenic lines OE1 and OE2 typically had higher expression of AtPLDδ than WT and VC in the presence and absence of salt treatment (Figure 12A). To explore the transcriptional regulation of AtPLDδ, the promoter region of AtPLDδ was cloned and analyzed (Figure S1). The cis-element G-boxes were shown to be in the promoter region (Figure S1). Electrophoretic mobility transfer analysis and luciferase reporter gene assay were performed to determine whether PeGLABRA3 activated AtPLDδ transcription in transgenic plants. EMSA and LRA data revealed that PeGLABRA3 binds to the AtPLDδ promoter region (Figures S2 and S3). Therefore, PeGLABRA3 interacted with the AtPLDδ promoter to accelerate AtPLDδ transcription.
Figure 12.
AtPLDδ transcription and phosphatidic acid (PA) content. (A) AtPLDδ transcription. WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were germinated and grown on 1/2 MS medium for 7 d. The seedlings were transferred to a liquid medium containing 0 or 100 mM of NaCl for a further 7 d. Transcription of AtPLDδ was examined, and Arabidopsis β-actin 2 (AtACTIN2) was used as the internal reference gene. Primers for AtPLDδ and AtACTIN2 are listed in Supplementary Table S1. Each column is the mean of three independent experiments, and different letters (a–d) indicate a significant difference at p < 0.05. (B,C) Phosphatidic acid (PA) content. Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines were transferred to a liquid medium containing 0 or 100 mM of NaCl for 24 h. Seedlings sampled from control and stressed plants were used to measure total PA content and the concentration of specific PA species, including 34:1, 34:2, 34:3, 34:4, 34:5, 34:6, 36:3, 36:4, and 36:5. Phosphatidic acid species were identified and quantified using electrospray ionization tandem mass spectrometry. Each column is the mean of three independent plants, and different letters (a–d) or asterisks (*) represent significant differences at p < 0.05, (**) represent significant differences at p < 0.01.
2.12. Phosphatidic Acid Content of PeGLABRA3-Transgenic Lines
Membrane phospholipids can be hydrolyzed by phospholipase Dδ to produce phospholipid acid [20] and, therefore, we determined the content of PA in transgenic lines. NaCl increased the content of total PA in all tested lines, but PeGLABRA3-transgenic plants retained 1.25- to 1.50-fold higher PA than WT and VC irrespective of control and salt treatment (Figure 12B). Moreover, the major PA species were further analyzed in Arabidopsis. Compared with WT and VC, the abundance of typical PA species in PeGLABRA3-transgenic lines increased, including PA species 34:2(16:0–18:2), 34:3(16:0–18:3), 36:3(18:1–18:2), 36:4(18:1–18:3), and 36:5(18:2–18:3) (Figure 12C). Notably, the PA species 34:2(16:0–18:2), 34:3(16:0–18:3), and 36:5(18:2–18:3) significantly increased in transgenic lines after NaCl exposure (Figure 12C).
3. Discussion
3.1. PeGLABRA3 Interacts with PePLDδ to Increase Gene Expression under NaCl Stress
NaCl induced the expression of PeGLABRA3, a bHLH transcription factor, in the roots and leaves of P. euphratica (Figure 1). Similarly, NaCl was found to upregulate a number of bHLH genes in Arabidopsis [34], rice [35], and sugar beet [36]. Previously, bHLH transcription factors were shown to bind to the E-box and GCG-box motifs to regulate the expression of genes involved in salt stress tolerance [34,43,44]. PePLDδ was found to contain G-box in the promoter region (Figure 2) and became active in roots and shoots after NaCl exposure (Figure 3). By means of DNA pull-down and mass spectrometry, Y1H, LRA, and EMSA, we confirmed that PeGLABRA3 binds the PePLDδ promoter (Figure 4, Figure 6, Figure 7 and Figure 8). Furthermore, PePLDδ expression was downregulated when the PeGLABRA3 gene was silenced in salt-treated P. euphratica leaves (Figure 1). Therefore, the salt-related increase in the expression of PePLDδ was due to the binding of PeGLABRA3 to the promoter of PePLDδ. We have previously shown that P. euphratica phospholipase Dδ increases salt tolerance by regulating K+/Na+ and ROS homeostasis in Arabidopsis [20]. Here, the binding of PeGLABRA3 to the AtPLDδ promoter was able to improve salt stress in transgenic Arabidopsis.
3.2. PeGLABRA3 Interacts with AtPLDδ to Mediate Ionic and ROS Homeostasis
The PeGLABRA3-transgenic lines showed enhanced root length and whole-plant growth under salinity (Figure 9). This outcome accords with previous findings that the overexpression of various bHLHs, such as AtbHLH92 [37], AtbHLH112 [34], OrbHLH2 [46], BvBHLH93 [36], VvbHLH1 [38], and MxbHLH18 [39], enhances salt tolerance in transgenic plants. In this study, PeGLABRA3 enhanced salt tolerance through an increased ability to control ionic and ROS homeostasis in transgenic Arabidopsis. PeGLABRA3-transgenic lines increased the activities of antioxidant enzymes such as SOD, POD, and CAT by positively regulating the expression of antioxidant genes (Figure 10). The upregulated ROS scavenging enzymes enabled the salt-stressed plants to maintain a low level of H2O2 (Figure 10). As a result, salt-induced oxidative damage was reduced (Figure 9). Similarly, the overexpression of bHLHs, including BvBHLH93 [36], VvbHLH1 [38], MxbHLH18 [39], and NtbHLH123 [40], can enhance the activities of antioxidant enzymes and the expression of antioxidant genes. Tobacco transcription factor bHLH123 improves salt tolerance by activating NADPH oxidase NtRbohE expression, which mediates the rapid accumulation of ROS and the response to salt stress during the early stages [40]. Our data showed that PeGLABRA3 activated AtPLDδ transcription to maintain ROS homeostasis under salt stress. The Arabidopsis AtPLDδ contains several G-boxes in the promoter region (Figure S1). The EMSA and LRA demonstrated that PeGLABRA3 binds to the AtPLDδ promoter region (Figures S2 and S3). In accordance with those results, the expression of AtPLDδ was significantly higher in transgenic lines than in WT and VC under control conditions and upon salt exposure (Figure 12). The PeGLABRA3-activated AtPLDδ resulted in increased PA in transgenic lines, including PA species such as 34:2 (16:0–18:2), 34:3 (16:0–18:3), 36:3 (18:1–18:2), 36:4 (18:1–18:3), and 36:5 (18:2–18:3) in no-salt controls and 34:2 (16:0–18:2), 34:3 (16:0–18:3), and 36:5 (18:2–18:3) in salt-stressed plants (Figure 12). Previous studies showed that Arabidopsis overexpressing P. euphratica PePLDδ had increased PA species and upregulated antioxidant enzymes SOD, POD, and APX [20]. Therefore, PeGLABRA3 interacted with the promoter of AtPLDδ to enhance gene expression, and the AtPLDδ-derived PA contributed to enhancing antioxidant defense under salt stress. In accordance, the overexpression of Arabidopsis AtPLDα in poplars increases the activity of SOD, CAT, and POD under drought treatment [16]. The AtPLDα-mediated antioxidant enzymes are essential for Arabidopsis to adapt to osmotic stress [47]. Furthermore, Arabidopsis phospholipase Dδ is involved in the regulation of ROS-mediated microtubule dynamic organization, stomatal movement, and heat tolerance [48].
PeGLABRA3 also helped transgenic plants maintain ionic homeostasis under salt stress. PeGLABRA3-transgenic lines showed a greater capacity for Na+ extrusion under salinity (Figure 11). Similarly, the BvbHLH93 expression lines accumulated less Na+ but more K+ than the WT [36]. bHLH transcription factors have been shown to confer K+/Na+ homeostasis by mediating the expression of KUP2 [41], NHX1, and NHX6 [42] in Arabidopsis. In rice, bHLH transcription factors regulate the Na+/K+ ratio in salt stress by increasing the expression of OsHKT1s [35]. Here, PeGLABRA3 interacted with AtPLDδ to increase PA levels, contributing to Na+ homeostasis as PePLDδ overexpression increased Na+ extrusion in Arabidopsis [20]. It was previously demonstrated that NaCl-induced PA interacts with mitogen-activated protein kinase 6 (MPK6), which directly phosphorylates the downstream Na+/H+ antiporter, SOS1 [49]. Here, PeGLABRA3-transgenic lines exhibited increased levels of AtSOS1 and AtSOS2, indicating that PeGLABRA3 interacts with AtPLDδ to produce specific PA species, such as 34:2 (16:0–18:2 PA), which activated SOS1 for Na+ extrusion (Figure 11) [49].
4. Materials and Methods
4.1. Plant Material and Salt Treatment
Populus euphratica seedlings, obtained from Xinjiang Uygur Autonomous Region, were planted in 10 L pots containing a 1:1 ratio (v/v) of sand and soil. After 3 months of culture in a greenhouse at Beijing Forestry University, P. euphratica seedlings were exposed to 200 mM of NaCl for 48 h [45]. Roots and mature leaves were sampled after 0, 1, 3, 6, 12, 24, and 48 h of salt treatment and used for RT-qPCR analysis.
4.2. RNA Isolation and Full-Length Cloning of PeGLABRA3
Using the EASYspin Plus Complex Plant RNA Kit (Aidlab Biotech, Beijing, China), the total RNA was extracted from P. euphratica leaves, according to the manufacturer’s instructions. The first-strand cDNA was synthesized through a reverse transcription reaction. The forward and reverse primers were designed based on the homologous sequences of P. euphratica GLABRA3. The 5′-3′ primer sequence was as follows: forward, 5′ -ATGGCTACTAAGCTCCACAACC-3′, and reverse, 5′-TCAACATTTCCCAGCAACTCTTC-3′. The PCR products were gel-purified and ligated into a pMD18-T vector (Takara, Dalian, China) for DNA sequencing.
4.3. PeGLABRA3 Sequence and Phylogenetic Analysis
We used the ClustalW (http://www.genome.jp/tools/clustalw/, (accessed on 17 January 2021) EMBL-EBI, Hinxton, Cambridgeshire, UK) for the multiple sequence alignments of GLABRA3 proteins. A phylogenetic tree was constructed by MEGA 5.2 software (http://www.megasoftware.net/index.php (accessed on 17 January 2021), Center for Evolutionary Medicine and Informatics, Tempe, AZ, USA). The GLABRA3 homologs used for multiple sequence alignment and phylogenetic analysis are listed in Supplementary Table S2.
4.4. DNA Pull-Down
4.4.1. Probe Design and Labeling
Upper mature leaves were sampled from P. euphratica seedlings and used to isolate genomic DNA and total protein [45]. Using genomic DNA as the template, the PePLDδ promoter fragment was amplified by PCR, cloned, and sequenced. The probes were biotin-labeled and purified with a gel extraction kit (Sigma-Aldrich, St. Louis, MI, USA). The concentration of the probes was detected, and they were stored at −20 °C for DNA pull-down.
4.4.2. DNA Pull-Down and Mass Spectrometry Analysis
The biotin-labeled DNA and nucleic acid were buffered into a 500 µL reaction system, to which magnetic beads (Beaver Bio 22305-1) were added. The system was incubated at room temperature for 1 h to form a DNA-magnetic bead complex. The supernatant was removed from the magnetic rack for subsequent bead hanging efficiency testing. The DNA-magnetic bead complex was further washed with precooled nucleic acid incubation buffer (50 mM Tris-HCl, pH 7.6, 0.1 mM EDTA, 0.1% Tween 20) and with a protein incubation buffer (50 mM Tris-HCl, pH 7.6, 0.1 mM EDTA, 0.1% Tween 20, 75 mM KCl, 1 mM MgCl2, 10% glycerol). The washing was repeated twice prior to magnetic separation on a magnetic frame. Protein extracts of P. euphratica leaves were diluted to 500 µL with a protein extraction buffer and incubated with the DNA-magnetic bead complex overnight at 4 °C to form a protein-DNA-magnetic bead complex. The supernatant was removed completely after magnetic separation on a magnetic frame. Then, the magnetic beads were rinsed with a cold protein incubation buffer six or seven times to collect protein. The protein sample was diluted with 100 µL of a protein elution buffer, bathed at 95 °C for 5 min, and centrifuged at 12,000 rpm for 5 min. The enriched protein was separated via SDS-PAGE and stained with a silver staining kit (Sigma-Aldrich). Finally, the obtained proteins were analyzed with mass spectrometry (Ultraflex III mass spectrometer, Bruker, Germany). Primary mass spectra were scanned at a 250 ms ion accumulation time, and secondary mass spectra of 30 precursor ions were collected at a 50 ms ion accumulation time. The MS1 spectrum was collected in the range of 350–1200 m/z, and the MS2 spectrum was collected in the range of 100–1500 m/z. The dynamic exclusion time of precursor ions was set to 15s.
The database used for protein determination was Uniprot_Arabidopsis thaliana (https://www.uniprot.org/uniprotkb?query=Arabidopsis%20 thaliana, accessed on 13 August 2020), Uniprot_Populus euphratica (https://www.uniprot.org/uniprotkb?query=Populus%20euphratica, accessed on 16 December 2020), and Uniprot_Populus trichocarpa (https://www.uniprot.org/uniprotkb?query=Populus%20trichocarpa, accessed on 16 December 2020). The proteins identified by DNA pull-down and mass spectrometry assay are shown in Supplementary Table S3.
4.5. Promoter Cloning and Sequence Analysis
The promoter of PePLDδ, the 1138 bp promoter fragment upstream of the translation initiation codon (−1138 to 0 bp), was isolated from P. euphratica leaves via PCR amplification [45]. The isolated 1138 bp region corresponds to the intergenic region between the PePLDδ gene and its upstream gene. The site of PePLDδ is 2,467,415–2,474,267, and its upstream gene is 2,456,149–2,463,149. The PePLDδ-pro (1138 bp) sequence was analyzed using the PLACE and PlantCARE databases. The promoter of A. thaliana AtPLDδ was also cloned and analyzed, as described above. The primers used for the isolation of P. euphratica PePLDδ and A. thaliana AtPLDδ are shown in Supplementary Table S1. The 1237 bp DNA sequence of the AtPLDδ promoter contained the following cis-acting elements: ARE (a cis-acting regulatory element essential for the anaerobic induction), ABRE motif (a cis-acting element involved in the abscisic acid responsiveness), MYC (a cis-acting element in response to drought and ABA), and G-box (a cis-regulatory element involved in light response). ATG was the start codon of the AtPLDδ gene (Supplementary Figure S1).
4.6. Luciferase Reporter Assay
LRA was performed following Yao et al. (2020) [45] and Hellens et al. (2005) [50]. In brief, the coding sequence region of the PeGLABRA3 gene was cloned and constructed into the transient expression vector pGreenII 62-SK. The promoter sequence of PePLDδ was cloned into the LUC reporter gene of the pGreenII 0800-LUC vector. After vector construction, the recombinant plasmid was transformed into the competent state of Agrobacterium tumefaciens GV3101 (pSOUP-P19). According to the transformation steps, 100 μL of incubated bacterial liquid was finally absorbed and coated on an LB solid medium containing 50 μg/mL kanamycin and 25 μg/mL rifampicin, and the culture was inverted at 28 °C for 48 h. Positive clones identified by PCR were selected and incubated at 28 °C in a shaker (220 rpm) until optical density (OD600) reached 1.0. The bacteria were collected by centrifugation at 4000 rpm for 5 min at room temperature; thereafter, OD600 was diluted to 0.6 with tobacco injection buffer. The bacterial solution containing the plasmid PePLDδ-pro-LUC or pGreenII 0800-LUC was mixed with the same volume of a bacterial solution containing the plasmid PeGLABRA3-62-SK or pGreenII 62-SK, left at room temperature for 2 to 4 h, and then injected into tobacco leaves using a 1 mL syringe. After 48–60 h of dark treatment, D-luciferin (1 mM) was coated on the leaves, and the LB983 Night Owl II Living molecular Imaging system (Berthold Technologies, Bad Wildbad, Germany) was used to observe the fluorescence. The LRA was repeated at least three times.
The LRA was also used to validate the PeGLABRA3 interaction with the A. thaliana AtPLDδ promoter, as mentioned above.
4.7. Yeast One-Hybrid Assay
The promoter of PePLDδ (1138 bp) containing the G-box was cloned and ligated into the pLaczi vector. PeGLABRA3 was cloned and expressed by fusion with the pB42AD vector. The linearized PePLDδ-pro-pLaczi plasmid was co-transformed with pB42AD or PeGLABRA3-pB42AD into Y1H competent cells (EGY48), and the PeGLABRA3-pB42AD plasmid was co-transformed with pLaczi or PePLDδ-pro-pLaczi into Y1H yeast. The mixes were incubated at 30 °C for 30 min and resuspended every 15 min, followed by incubation at 42 °C for 15 min with resuspension every 7.5 min. The Y1H yeast cells collected by centrifugation were evenly cultured on an SD/-Trp/-Ura solid medium and incubated at 30 °C for 2–4 days. After growing single colonies, the cells were spread and coated on an SD/-Trp/-Ura + Gal + Raf + X-Gal color plate for observation.
4.8. Electrophoretic Mobility Shift Assay
The full-length coding sequence of PeGLABRA3 was cloned with HIS-tag at the C-terminus of the pET28a vector. An empty pET28a vector was used as a negative control. The constructed PeGLABRA3-pET28a vector was transferred to Escherichia coli competent cells (BL21) for re-transformation. The cells were incubated in LB medium at 37 °C until OD600 reached 0.4 to 0.6 and were then incubated at 16 °C for 12–16 h after induction with 0.5 mM isopropyl β-D-1-thiogalactopyranoside. The bacteria were collected by centrifugation, resuspended with an 8 mL HIS protein resuspension buffer, and then disrupted by ultrasound for 15 min before centrifugation. The supernatant was added to a packed column that had already been washed 15 times with the volume of the resuspended buffer. After 2 h of incubation on ice, the resuspended buffer was allowed to flow out from the bottom. The column was then washed four or five times with the resuspended buffer containing 50 mM imidazole (5×), followed by two or three washes with the gradient buffer containing 100 mM and 150 mM imidazole. Finally, 0.5 mL of an elution buffer containing 400 mM imidazole was added to the packed column, and samples were collected in centrifuge tubes. The protein collection was repeated three or four times, and the samples were stored for later use.
For biotin labeling, the 50 bp DNA fragment of the PePLDδ promoter,5′-CGCTGATCCGGGTAACTGAAATCAGCTGCTGAGAGATGGAAGAGGAGAAT-3′ and 5′-ATTCTCCTCTTCCATCTCTCAGCAGCTGATTTCAGTTACCCGGATCAGCG-3′ (harboring the G-box base sequence) were biotin-labeled at the 3′ ends using an EMSA Probe Biotin Labeling Kit (Beyotime, Nantong, China). A cold competition experiment was conducted with probes that were not labeled. Biotin-labeled mutational probes were used to verify the specificity of the PeGLABRA3 binding to G-boxes (CAGCTG). The mutant probes (Mut, CAGCTG to CGGACG) were used to confirm the binding specificity of the G-box to PeGLABRA3. Representative results were obtained by repeating the EMSA three times.
The EMSA verification of the PeGLABRA3 binding to the AtPLDδ promoter was performed as described above. The 50 bp DNA fragment of the AtPLDδ promoter used for biotin labeling were 5′-AACTCCCATCACGTCGTCCCTCCACCTGTCCTCTCTTCTC CTTCCTTGCT-3′ and 5′-AGCAAGGAAGGAGAAGAGAGGACAGGTGGAGGGAC GACGTGATGGGAGTT-3′ (harboring the G-box base sequence), respectively. The mutant probes (Mut, CACCTG to CTGGTG) were used to confirm the binding specificity of the G-box to PeGLABRA3.
4.9. Construction and Transformation of the PePLDδ-pro::GUS Gene
The PePLDδ promoter was cloned into the pBI121 vector. Then, PePLDδ-pro was combined with β-glucuronidase gene (GUS) and transformed into Agrobacterium tumefaciens. PBI121-PePLDδ-pro::GUS was transferred into wild type Arabidopsis. Transgenic lines were selected on 1/2 MS medium containing kanamycin resistance. WT seedlings and PePLDδ-Pro::GUS transgenic lines were germinated in 1/2 MS medium. The seedlings were treated with 0 or 100 mM of NaCl for 24 h after 10 days of culture. Roots and leaves were sampled for GUS staining [45]. The GUS staining was repeated with three to five individual plants for each treatment.
4.10. Virus-Induced Gene Silencing of PeGLABRA3 in P. euphratica
VIGS, a technique that leads to specific degradation of the mRNA of the target gene, was carried out as previously described [51,52]. Briefly, a 250 bp length of the PeGLABRA3 gene was obtained with two specific primers: F: 5′-GGTACCATGGCTACTAAGCTCCACAA-3′ and R: 5′-GAGCTCTCAACATTTCCCAGCAACTC-3′. The PeGLABRA3 fragment was reassembled to the pTRV2 expression vector. Then, the pTRV2-PeGLABRA3 and pTRV1 plasmids were transformed into Agrobacterium GV3101, respectively. Agrobacterium tumefaciens containing pTRV2-PeGLABRA3 and pTRV1 was mixed at a ratio of 1:1 and injected into the leaves of P. euphratica seedlings. After 45 days of VIGS, P. euphratica was subjected to 200 mM of NaCl for 24 h. Then, upper mature leaves were collected for RT-qPCR analysis.
4.11. Generation of PeGLABRA3-Transgenic Lines
Wild type Arabidopsis seeds were sown in a 1/2 MS solid medium, vernalized at 4 °C for 3 d, and cultured in a climate chamber. The temperature was maintained at 22 °C, and humidity was maintained at 60%. The long day photoperiod was 16 h light/8 h dark. After 10 d of culture, the germinated seedlings with four cotyledons were transferred to the nursery soil mixed with vermiculite at a ratio of 1:1 and cultured in the climate chamber.
The full-length PeGLABRA3 gene was inserted into the expression vector pCAMBIA1300-GFP containing the promoter of CaMV35S. The recombinant plasmid pCAMBIA1300-GFP-PeGLABRA3 was then transformed into A. tumefaciens. The empty vector pCAMBIA1300-GFP was introduced into WT Arabidopsis plants as a control. Homozygous seeds were screened in a solid medium containing 25 mg/L hygromycin. The expression level of PeGLABRA3 in T3 homozygous transgenic lines was quantified by semiquantitative reverse transcription PCR and RT-qPCR.
4.12. Phenotype Tests of Transgenic Lines
Wild type Arabidopsis thaliana (WT) seedlings, empty vector control (VC), and PeGLABRA3-transgenic lines (OE1 and OE2) were sterilized with 1% sodium hypochlorite for 10 min, washed five or six times, and grown in 1/2 MS medium. Uniform seedlings (seven-day-old) of all tested lines were placed vertically in a solid 1/2 MS medium supplemented with 0 or 100 mM of NaCl for another seven days of salt exposure. Thereafter, the root length and fresh weight were measured. The root length was examined using ImageJ Pro6 (https://imagej.net/ij/index.html (accessed on 27 July 2021)). The salt tolerance test of WT, VC, and transgenic lines was repeated at least three times.
Mature leaves were sampled from WT, VC, and PeGLABRA3-transgenic lines treated with or without 100 mM of NaCl. Relative electrolyte leakage and MDA content were determined as previously described [20].
4.13. Na+ Flux Measurements
Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were treated with 0 or 100 mM of NaCl for 12 h. Then, Arabidopsis roots were incubated with amiloride (an inhibitor of Na+/H+ antiporter, 0 or 5 mM) for 30 min. Roots were sampled and equilibrated for 30 min in the basic solution containing 0.1 mM of NaCl, 0.1 mM of CaCl2, 0.1 mM of MgCl2, 0.5 mM of KCl, and 2.5% sucrose (pH 5.8). Root tips were sampled for Na+ flux recordings with the noninvasive micro-test technique (NMT-YG-100, Younger USA, LLC, Amherst, MA, USA). Na+ fluxes were monitored by NMT microelectrodes at the meristematic region (200 µM from the root tip) and continuously recorded for 10 min [53]. Five or six individual plants were examined for each treatment.
4.14. Cellular Na+ and H2O2 Determination Roots
The fluorescent probe CoroNaTMGreen was used to detect Na+ content in control and short-term NaCl-stressed roots (0 or 100 mM NaCl, 12 h). Roots were immersed in 5 mM of an MES-KCl loading buffer (pH 5.7) containing CoroNaTMGreen (20 µM), incubated at room temperature for 2 h in the dark, and then rinsed four to five times with MS solution. For H2O2 determination, Arabidopsis roots were cultured in 10 μM H2DCFDA (Molecular Probe, Eugene, OR, USA) for 15 min and washed four or five times. The fluorescence of CoroNaTMGreen and H2DCFDA was measured using a Leica SP8 confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 510 to 530 nm. Relative fluorescence intensity was calculated by Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA) [45].
4.15. Determination of Activity and Transcription of Antioxidant Enzymes
Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines OE1 and OE2 were exposed to 0 or 100 mM of NaCl for 7 days. Control and salt-stressed plants were sampled to measure the activities of SOD, POD, and CAT [20]. The transcription of AtSOD, AtPOD, and AtCAT in the control and salinized plants was also examined with RT-qPCR [20].
4.16. RT-qPCR Analysis
Total RNA was extracted from P. euphratica roots, leaves, and WT Arabidopsis seedlings, VC, and PeGLABRA3-overexpressed lines using the EASYspin Plus Complex Plant RNA Kit (Aidlab Biotech, Beijing, China) [45]. The templates for RT-qPCR were prepared by reverse transcription, as previously described [20,54]. PeACT7 and AtACTIN2 were used as the internal reference genes for P. euphratica and Arabidopsis, respectively. Forward and reverse primers designed to target PeGLABRA3, PePLDδ, AtSOS1, AtSOS2, AtSOD, AtPOD, AtCAT, PeACT7, and AtACTIN2 are listed in Supplementary Table S1. Three biological samples were set for each treatment.
4.17. Quantitative Analysis of PA Species
Seven-day-old WT seedlings, VC, and PeGLABRA3-transgenic lines (OE1 and OE2) were subjected to 0 or 100 mM of NaCl for 24 h in a liquid MS medium. Electrospray ionization tandem mass spectrometry was used to analyze and quantify the lipids after treatment [20,55].
4.18. Data Analysis
The experimental data were subjected to SPSS version 19.0 (IBM Corporation, Armonk, NY, USA) for normality and homogeneity of variances tests. Significant differences between means were determined by Duncan’s multiple range test. Unless otherwise stated, p < 0.05 was considered significant.
5. Conclusions
High salinity induces the expression of a basic helix–loop–helix transcription factor, PeGLABRA3, in the salt-resistant Populus euphratica. PeGLABRA3 binds to the promoter of PePLDδ to enhance its expression under salt stress. The overexpression of PeGLABRA3 increases AtPLDδ transcription in Arabidopsis, resulting in an increased concentration of phosphatidic acid species under no-salt and saline conditions. We conclude that PeGLABRA3 activated AtPLDδ transcription by binding to the AtPLDδ promoter region, conferring Na+ and ROS homeostasis control via PLDδ- and PA-mediated signaling pathways under salt stress.
Acknowledgments
We thank the Functional Genomics Unit, Plant Systems Biology (Flanders Interuniversity Institute of Biotechnology, Ghent University), for providing the Gateway destination vectors. Yule Liu (School of Life Sciences, Tsinghua University) is sincerely acknowledged for providing TRV vectors.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms24098208/s1.
Author Contributions
Y.Z. (Ying Zhang): investigation, data curation, visualization, and writing—original draft preparation. K.Y.: investigation, data curation, visualization, and writing—original draft preparation. J.Y.: investigation, data curation, and visualization. Z.Z.: investigation, visualization, and data curation. Z.L.: investigation and visualization. C.Y.: visualization and validation. Y.Z. (Yanli Zhang): investigation and data curation. J.L. (Jian Liu): software and validation. J.L. (Jing Li): methodology and software. N.Z.: methodology and visualization. R.Z.: methodology and visualization. X.Z.: methodology and software. S.C.: conceptualization, supervision, writing—review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by the National Natural Science Foundation of China (grant numbers 32071730 and 31770643), the Program of Introducing Talents of Discipline to Universities, China (111 project, grant No. B13007), and the Beijing Advanced Innovation Center for Tree Breeding by Molecular Design, Beijing Municipal Education Commission (China).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bargmann B., Munnik T. The role of phospholipase D in plant stress responses. Curr. Opin. Plant Biol. 2006;9:515–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann B.O.R., Laxalt A.M., Riet B.T., van Schooten B., Merquiol E., Testerink C., Haring M.A., Bartels D., Munnik T. Multiple PLDs required for high salinity and water deficit tolerance in plants. Plant Cell Physiol. 2009;50:78–89. doi: 10.1093/pcp/pcn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pleskot R., Li J., Zárský V., Potocký M., Staiger C.J. Regulation of cytoskeletal dynamics by phospholipase D and phosphatidic acid. Trends Plant Sci. 2013;18:496–504. doi: 10.1016/j.tplants.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Katagiri T., Takahashi S., Shinozaki K. Involvement of a novel Arabidopsis phospholipase D, AtPLDδ, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J. 2001;26:595–605. doi: 10.1046/j.1365-313x.2001.01060.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh A., Pandey A., Baranwal V., Kapoor S., Pandey G.K. Comprehensive expression analysis of rice phospholipase D gene family during abiotic stresses and development. Plant Signal. Behav. 2012;7:847–855. doi: 10.4161/psb.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Zhou D., Zhang Q., Zhang W. Genomic analysis of phospholipase D family and characterization of GmPLDαs in soybean (Glycine max) J. Plant Res. 2012;125:569–578. doi: 10.1007/s10265-011-0468-0. [DOI] [PubMed] [Google Scholar]
- 7.Distéfano A.M., Valiñas M.A., Scuffi D., Lamattina L., Ten Have A., García-Mata C., Laxalt A.M. Phospholipase Dδ knock-out mutants are tolerant to severe drought stress. Plant Signal. Behav. 2015;10:e1089371. doi: 10.1080/15592324.2015.1089371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ufer G., Gertzmann A., Gasulla F., Röhrig H., Bartels D. Identification and characterization of the phosphatidic acid-binding A. thaliana phosphoprotein PLDrp1 that is regulated by PLDα1 in a stress-dependent manner. Plant J. 2017;92:276–290. doi: 10.1111/tpj.13651. [DOI] [PubMed] [Google Scholar]
- 9.Gasulla F., Barreno E., Parages M.L., Cámara J., Jiménez C., Dörmann P., Bartels D. The role of Phospholipase D and MAPK signaling cascades in the adaption of lichen microalgae to desiccation: Changes in membrane lipids and phosphoproteome. Plant Cell Physiol. 2016;57:1908–1920. doi: 10.1093/pcp/pcw111. [DOI] [PubMed] [Google Scholar]
- 10.Deepika D., Singh A. Plant phospholipase D: Novel structure, regulatory mechanism, and multifaceted functions with biotechnological application. Crit. Rev. Biotechnol. 2022;42:106–124. doi: 10.1080/07388551.2021.1924113. [DOI] [PubMed] [Google Scholar]
- 11.Vadovič P., Šamajová O., Takáč T., Novák D., Zapletalová V., Colcombet J., Šamaj J. Biochemical and genetic interactions of Phospholipase D alpha 1 and mitogen-activated protein kinase 3 affect Arabidopsis stress response. Front. Plant Sci. 2019;10:275. doi: 10.3389/fpls.2019.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris P.C. Integrating lipid signalling, mitogen-activated protein kinase cascades and salt tolerance. New Phytol. 2010;188:640–643. doi: 10.1111/j.1469-8137.2010.03507.x. [DOI] [PubMed] [Google Scholar]
- 13.Pappan K.L., Wang X. Assaying different types of plant phospholipase D activities in vitro. Methods Mol. Biol. 2013;1009:205–217. doi: 10.1007/978-1-62703-401-2_19. [DOI] [PubMed] [Google Scholar]
- 14.Li S., Huang M., Di Q., Ji T., Wang X., Wei M., Shi Q., Li Y., Gong B., Yang F. The functions of a cucumber phospholipase D alpha gene (CsPLDα) in growth and tolerance to hyperosmotic stress. Plant Physiol. Biochem. 2015;97:175–186. doi: 10.1016/j.plaphy.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Ji T., Li S., Huang M., Di Q., Wang X., Wei M., Shi Q., Li Y., Gong B., Yang F. Overexpression of cucumber phospholipase D alpha gene (CsPLDα) in tobacco enhanced salinity stress tolerance by regulating Na+–K+ balance and lipid peroxidation. Front. Plant Sci. 2017;8:499. doi: 10.3389/fpls.2017.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T., Song Y., Liu Y., Guo X., Zhu C., Wen F. Overexpression of phospholipase Dα gene enhances drought and salt tolerance of Populus tomentosa. Chin. Sci Bull. 2008;53:3656–3665. doi: 10.1007/s11434-008-0476-1. [DOI] [Google Scholar]
- 17.Hunter K., Kimura S., Rokka A., Tran H.C., Toyota M., Kukkonen J.P., Wrzaczek M. CRK2 enhances salt tolerance by regulating callose deposition in connection with PLDα1. Plant Physiol. 2019;180:2004–2021. doi: 10.1104/pp.19.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben Othman A., Ellouzi H., Planchais S., De Vos D., Faiyue B., Carol P., Abdelly C., Savouré A. Phospholipases Dζ1 and Dζ2 have distinct roles in growth and antioxidant systems in Arabidopsis thaliana responding to salt stress. Planta. 2017;246:721–735. doi: 10.1007/s00425-017-2728-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y., Hu X., Wang P., Wang H., Ge X., Li F., Hou Y. The phospholipase D gene GhPLDδ confers resistance to Verticillium dahliae and improves tolerance to salt stress. Plant Sci. 2022;321:111322. doi: 10.1016/j.plantsci.2022.111322. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Yao J., Yin K., Liu Z., Zhang Y., Deng C., Liu J., Zhang Y., Hou S., Zhang H., et al. Populus euphratica phospholipase Dδ increases salt tolerance by regulating K+/Na+ and ROS homeostasis in Arabidopsis. Int. J. Mol. Sci. 2022;23:4911. doi: 10.3390/ijms23094911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey P.C., Martin C., Toledo-Ortiz G., Quail P.H., Huq E., Heim M.A., Jakoby M., Werber M., Weisshaar B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell. 2003;15:2497–2502. doi: 10.1105/tpc.151140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heim M., Jakoby M., Werber M., Martin C., Weisshaar B., Bailey P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003;20:735–747. doi: 10.1093/molbev/msg088. [DOI] [PubMed] [Google Scholar]
- 23.Toda Y., Tanaka M., Ogawa D., Kurata K., Kurotani K., Habu Y., Ando T., Sugimoto K., Mitsuda N., Katoh E., et al. Rice salt sensitive3 forms a ternary complex with JAZ and class-C bHLH factors and regulates jasmonate-induced gene expression and root cell elongation. Plant Cell. 2013;25:1709–1725. doi: 10.1105/tpc.113.112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carretero-Paulet L., Galstyan A., Roig-Villanova I., Martínez-García J.F., Bilbao-Castro J.R., Robertson D.L. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol. 2010;153:1398–1412. doi: 10.1104/pp.110.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ariyarathne M.A., Wone B.W.M. Overexpression of the Selaginella lepidophylla bHLH transcription factor enhances water-use efficiency, growth, and development in Arabidopsis. Plant Sci. 2022;315:111129. doi: 10.1016/j.plantsci.2021.111129. [DOI] [PubMed] [Google Scholar]
- 26.Onohata T., Gomi K. Overexpression of jasmonate-responsive OsbHLH034 in rice results in the induction of bacterial blight resistance via an increase in lignin biosynthesis. Plant Cell Rep. 2020;39:1175–1184. doi: 10.1007/s00299-020-02555-7. [DOI] [PubMed] [Google Scholar]
- 27.Moreno J.E., Moreno-Piovano G., Chan R.L. The antagonistic basic helix-loop-helix partners BEE and IBH1 contribute to control plant tolerance to abiotic stress. Plant Sci. 2018;271:143–150. doi: 10.1016/j.plantsci.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Alam M.S., Kong J., Tao R., Ahmed T., Alamin M., Alotaibi S.S., Abdelsalam N.R., Xu J.H. CRISPR/Cas9 mediated knockout of the OsbHLH024 transcription factor improves salt stress resistance in Rice (Oryza sativa L.) Plants. 2022;11:1184. doi: 10.3390/plants11091184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alam M.S., Yang Z.K., Li C., Yan Y., Liu Z., Nazir M.M., Xu J.H. Loss-of-function mutations of OsbHLH044 transcription factor lead to salinity sensitivity and a greater chalkiness in rice (Oryza sativa L.) Plant Physiol. Biochem. 2022;93:110–123. doi: 10.1016/j.plaphy.2022.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Verma D., Jalmi S.K., Bhagat P.K., Verma N., Sinha A.K. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2020;287:2560–2576. doi: 10.1111/febs.15157. [DOI] [PubMed] [Google Scholar]
- 31.Waseem M., Rong X., Li Z. Dissecting the role of a basic helix-loop-helix transcription factor, SlbHLH22, under salt and drought stresses in transgenic Solanum lycopersicum L. Front. Plant Sci. 2019;10:734. doi: 10.3389/fpls.2019.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kavas M., Baloğlu M.C., Atabay E.S., Ziplar U.T., Daşgan H.Y., Ünver T. Genome-wide characterization and expression analysis of common bean bHLH transcription factors in response to excess salt concentration. Mol. Genet. Genom. 2016;291:129–143. doi: 10.1007/s00438-015-1095-6. [DOI] [PubMed] [Google Scholar]
- 33.Babitha K.C., Vemanna R.S., Nataraja K.N., Udayakumar M. Overexpression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE. 2015;10:e0137098. doi: 10.1371/journal.pone.0137098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Ji X., Nie X., Qu M., Zheng L., Tan Z., Zhao H., Huo L., Liu S., Zhang B., et al. Arabidopsis AtbHLH112 regulates the expression of genes involved in abiotic stress tolerance by binding to their E-box and GCG-box motifs. New Phytol. 2015;207:692–709. doi: 10.1111/nph.13387. [DOI] [PubMed] [Google Scholar]
- 35.Chen H.C., Cheng W.H., Hong C.Y., Chang Y.S., Chang M.C. The transcription factor OsbHLH035 mediates seed germination and enables seedling recovery from salt stress through ABA-dependent and ABA-independent pathways, respectively. Rice. 2018;11:50. doi: 10.1186/s12284-018-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Wang S., Tian Y., Wang Q., Chen S., Li H., Ma C., Li H. Functional characterization of a sugar beet BvbHLH93 transcription factor in salt stress tolerance. Int. J. Mol. Sci. 2021;22:3669. doi: 10.3390/ijms22073669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y., Yang B., Deyholos M.K. Functional characterization of the Arabidopsis bHLH92 transcription factor in abiotic stress. Mol. Genet. Genom. 2009;282:503–516. doi: 10.1007/s00438-009-0481-3. [DOI] [PubMed] [Google Scholar]
- 38.Wang F.B., Zhu H., Chen D.H., Li Z.J., Peng R.H., Yao Q.H. A grape bHLH transcription factor gene, VvbHLH, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2016;125:387–398. doi: 10.1007/s11240-016-0953-1. [DOI] [Google Scholar]
- 39.Liang X., Li Y., Yao A., Liu W., Yang T., Zhao M., Zhang B., Han D. Overexpression of MxbHLH18 increased iron and high salinity stress tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022;23:8007. doi: 10.3390/ijms23148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D., Li Y.Y., Zhou Z.C., Xiang X., Liu X., Wang J., Hu Z.R., Xiang S.P., Li W., Xiao Q.Z., et al. Tobacco transcription factor bHLH123 improves salt tolerance by activating NADPH oxidase NtRbohE expression. Plant Physiol. 2021;186:1706–1720. doi: 10.1093/plphys/kiab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajappa S., Krishnamurthy P., Kumar P.P. Regulation of AtKUP2 expression by bHLH and WRKY transcription factors helps to confer increased salt tolerance to Arabidopsis thaliana plants. Front. Plant Sci. 2020;11:1311. doi: 10.3389/fpls.2020.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnamurthy P., Vishal B., Khoo K., Rajappa S., Loh C.S., Kumar P.P. Expression of AoNHX1 increases salt tolerance of rice and Arabidopsis, and bHLH transcription factors regulate AtNHX1 and AtNHX6 in Arabidopsis. Plant Cell Rep. 2019;38:1299–1315. doi: 10.1007/s00299-019-02450-w. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad A., Niwa Y., Goto S., Ogawa T., Shimizu M., Suzuki A., Kobayashi K., Kobayashi H. bHLH106 integrates functions of multiple genes through their G-Box to confer salt tolerance on Arabidopsis. PLoS ONE. 2015;10:e0126872. doi: 10.1371/journal.pone.0126872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim M.J., Kim J.K., Shin J.S., Suh M.C. The SebHLH transcription factor mediates trans-activation of the SeFAD2 gene promoter through binding to E- and G-box elements. Plant Mol. Biol. 2007;64:453–466. doi: 10.1007/s11103-007-9165-8. [DOI] [PubMed] [Google Scholar]
- 45.Yao J., Shen Z., Zhang Y., Wu X., Wang J., Sang G., Zhang Y., Zhang H., Deng C., Liu J., et al. Populus euphratica WRKY1 binds the promoter of H+-ATPase gene to enhance gene expression and salt tolerance. J. Exp. Bot. 2020;71:1527–1539. doi: 10.1093/jxb/erz493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J., Li F., Wang J.L., Ma Y., Chong K., Xu Y.Y. Basic helix-loop-helix transcription factor from wild rice (OrbHLH2) improves tolerance to salt and osmotic stress in Arabidopsis. J. Plant Physiol. 2009;166:1296–1306. doi: 10.1016/j.jplph.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M., Liu Q., Zhang Y., Yang N., Wu G., Li Q., Wang W. Alleviation of osmotic stress by H2S is related to regulated PLD alpha 1 and suppressed ROS in Arabidopsis thaliana. J. Plant Res. 2020;133:393–407. doi: 10.1007/s10265-020-01182-3. [DOI] [PubMed] [Google Scholar]
- 48.Song P., Jia Q., Chen L., Jin X., Xiao X., Li L., Chen H., Qu Y., Su Y., Zhang W., et al. Involvement of Arabidopsis phospholipase D δ in regulation of ROS-mediated microtubule organization and stomatal movement upon heat shock. J. Exp. Bot. 2020;71:6555–6570. doi: 10.1093/jxb/eraa359. [DOI] [PubMed] [Google Scholar]
- 49.Yu L., Nie J., Cao C., Jin Y., Yan M., Wang F. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 2010;188:762–773. doi: 10.1111/j.1469-8137.2010.03422.x. [DOI] [PubMed] [Google Scholar]
- 50.Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramegowda V., Mysore K.S., Senthil-Kumar M. Virus-induced gene silencing is a versatile tool for unraveling the functional relevance of multiple abiotic-stress-responsive genes in crop plants. Front. Plant Sci. 2014;5:323. doi: 10.3389/fpls.2014.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voinnet O. Non-cell autonomous RNA silencing. FEBS Lett. 2005;579:5858–5871. doi: 10.1016/j.febslet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 53.Sun J., Chen S., Dai S., Wang R., Li N., Shen X., Zhou X., Lu C., Zheng X., Hu Z., et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009;149:1141–1153. doi: 10.1104/pp.108.129494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆ct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 55.Devaiah S.P., Roth M.R., Baughman E., Li M., Tamura P., Jeannotte R., Welti R., Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dα1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.