Abstract
The role of tumor necrosis factor alpha (TNF-α) in host defense against chlamydial infection remains unclear. In order to further evaluate the relevance of TNF-α to host resistance in chlamydial genital tract infection, we examined the effect of local inhibition of the TNF-α response in normal C57 mice and in interferon gamma gene-deficient C57 mice infected intravaginally with the mouse pneumonitis agent of Chlamydia trachomatis. Since the guinea pig model of female genital tract infection more closely approximates the human in terms of ascending infection and development of pathology, we also examined the effect of local inhibition of the TNF-α response in guinea pigs infected intravaginally with the guinea pig strain of Chlamydia psittaci. We successfully blocked the early TNF-α response in the respective animal models. This blockade had no effect on the numbers of organisms isolated from the genital tract during the time of TNF-α inhibition in mice or guinea pigs. Analysis of interleukin-1β, macrophage inflammatory protein-2, and granulocyte macrophage-colony stimulating factor in the mouse model revealed that blockade of the TNF-α response did not alter the release of these proinflammatory proteins. Yet, in TNF-α-depleted mice, increased numbers of neutrophils were detected in the genital tract, and, in TNF-α-depleted guinea pigs, increased numbers of neutrophils as well as infiltrating lymphocytes were seen in the endocervix. Blockade of TNF-α does not affect the level of infection in mice or guinea pigs, but it may decrease TNF-α-induced apoptosis of infiltrating inflammatory cells.
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine released primarily from monocytes and macrophages upon invasion of the host by a wide variety of pathogens. It contributes in various ways to defense against pathogenic agents. In the late 1980s, Manor and Sarov reported that human monocyte-derived macrophages inhibit growth of Chlamydia trachomatis (L2/4434/Bu) in human laryngeal carcinoma cells (HEp-2 cells), and this inhibition is reduced by the addition of anti-TNF-α antibodies (17). Further, Shomer-Avni et al. reported a direct inhibitory effect of human recombinant TNF-α on the in vitro growth of C. trachomatis in HEp-2 cells (34). However, recent work published by Perry and colleagues reported that TNF-α has no effect on the in vitro growth of the human strains of C. trachomatis serovar D or L2 or the mouse pneumonitis agent of C. trachomatis (MoPn) when murine intestinal epithelial cell lines are used for culture (25). These different conclusions may simply indicate that TNF-α has different effects on chlamydiae in human versus rodent cells in vitro, but they point out that further investigations of the role of TNF-α in host defense against chlamydiae are needed.
There is evidence that TNF-α plays a role in vivo in host defense against chlamydiae. In a murine model of chlamydial pneumonia, Williams et al. demonstrated that exogenous administration of anti-TNF-α antibody significantly accelerated mortality and caused an increase in MoPn counts in the lung (39). As regards genital tract infection, mice genetically deficient in the p55 type I receptor for TNF-α have a statistically significant delay in clearance of MoPn and of human serovar D from the genital tract (25). As no direct inhibitory effect of TNF-α was determined in vitro in this study, the investigators proposed that the delay in clearance was due to indirect effects of TNF-α on local effectors of host defense.
We reported the detection of high levels of TNF-α in C57BL/6 (C57) mice infected with MoPn or human serovar E during the first week of primary infection (10). Significantly lower levels of TNF-α were found in C3H/HeN mice, and the C3H strain was found to have a prolonged course of infection and increased oviduct pathology compared to the C57 strain, suggesting that increased TNF-α levels enhance host defense in the C57 strain. Further, C57 mice deficient in gamma interferon (IFN-γ) continue to exhibit near eradication of MoPn from the genital tract mucosa (9, 23). The effector mechanism responsible for this IFN-γ-independent clearance of MoPn is unknown. Although studies have not shown a compensatory increase in mRNA levels of TNF-α in IFN-γ gene-deficient mice, local protein levels of TNF-α have not been reported. Since TNF-α biosynthesis is largely controlled at a translational level, a significant increase in secretion of the protein may occur without detection of a significant increase in transcription of the TNF-α gene (15).
Compared to the mouse model, the guinea pig model of female genital tract infection more closely approximates the human infection in terms of ascending infection, hormonal influences, and degree of oviduct pathology after primary infection (27, 28, 30–32). Data suggest that guinea pigs and mice may use different inflammatory effector molecules in host defense against intracellular pathogens (26) and different isolates of Chlamydia may exhibit differential sensitivity to inflammatory mediators (25). We have examined TNF production in female guinea pigs infected with the Chlamydia psittaci agent of guinea pig inclusion conjunctivitis (GPIC) and reported high levels of TNF-α in genital tract secretions during the first week of primary infection (11). Further, we have shown that treatment of guinea pigs with physiologic levels of estradiol enhances the level of infection (20) and leads to a significant increase in the level of TNF-α-detected in secretions (R. G. Rank, A. K. Bowlin, L. K. Reddy, and T. Darville, submitted for publication). Dose response experiments in ovariectomized guinea pigs demonstrated that the level of TNF-α was directly related to the infecting dose of chlamydiae (Rank, et al., submitted).
In order to further evaluate the relevance of TNF-α to host resistance and to the development of pathology in chlamydial genital tract infection, we have examined the effect of local inhibition of the TNF-α response in normal and IFN-γ gene-deficient C57 mice infected with MoPn and in guinea pigs infected with GPIC.
MATERIALS AND METHODS
Animals.
Female C57BL/6 (C57; H-2b) mice (6 weeks old) and IFN-γ-deficient C57Bl/6-Ifgtm1 mice (IFN-γ−/−) with disrupted IFN-γ genes were purchased from Jackson Laboratories (Bar Harbor, Maine). These mice were free of pathogenic bacteria and viruses as determined by culture and serology. They were used at between 6 and 12 weeks of age in these experiments.
Female 20-week-old outbred Hartley strain (Sasco Labs, Omaha, Neb.) guinea pigs were also used for experiments. Guinea pigs were given food and water ad libitum in an environmentally controlled room with a cycle of 12 h of light and 12 h of darkness.
Infection.
Mice received 2.5 mg of medroxyprogesterone acetate (Depo-Provera) (Upjohn, Kalamazoo, Mich.) subcutaneously at 7 days before vaginal infection. The mice were infected by placing 30 μl of 250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (pH 7.2) containing 107 inclusion-forming units (IFU) (2,000 50% infective doses) of McCoy cell-grown MoPn into the vaginal vault. Infectious organisms were administered with the mice under sodium pentobarbital anesthesia. Infection was monitored by swabbing the vaginal vault and exocervix with a Calgiswab (Spectrum Medical Industries, Los Angeles, Calif.) at various times following infection and by enumerating IFU by isolation on McCoy cell monolayers (16). The number of inclusion bodies within 20 fields (×40 magnification) was counted under a fluorescence microscope, and IFU were calculated.
Guinea pigs were infected with approximately 107 IFU of McCoy cell-grown GPIC elementary bodies in 0.05 ml via intravaginal inoculation. All inoculations were performed at random times during the animals' estrous cycles. Animals were sacrificed on day 5 or 35 of infection. In animals sacrificed on day 5, the kinetics of lower genital tract infection were monitored by isolation of GPIC from cervical swabs (29). Immunohistochemical staining for chlamydiae was also performed on guinea pig genital tract tissues from animals sacrificed on day 5. In animals sacrificed on day 35, the percentages of inclusion-bearing cells were determined on Giemsa-stained smears of vaginal wall scrapings obtained at intervals throughout infection (3).
Immunohistochemistry of GPIC inclusions.
Immunohistochemistry analysis for the detection of chlamydiae was performed on paraffin-embedded tissue sections, using the indirect conjugate method with pooled guinea pig GPIC-immune serum as the primary antibody. Four-micrometer sections were deparaffinized and hydrated through xylene and graded alcohol series. Endogenous peroxidase activity was quenched by incubation in methanol containing 0.3% H2O2 for 30 min and then washing in phosphate-buffered saline (PBS) (0.9% at pH 7.5). Nonspecific staining was blocked by incubation in 10% nonimmune rabbit serum. The primary antibody was applied for 1 h in a humidified chamber, diluted 1:40 in PBS. Following washes in buffer, tissues were incubated for 1 h with horseradish-peroxidase-labeled rabbit anti-guinea pig antibody diluted 1:20 in PBS. Visualization was accomplished with Sigma Fast 3,3′-diaminobenzidine tablets (Sigma Chemical Co., St. Louis, Mo.) as the chromogen. Sections were then counterstained with hematoxylin, dehydrated, cleared, and mounted in Cytoseal (CMS, Houston, Tex.). A semiquantitative scoring system was used to enumerate positive cells: 0, no staining; 1+, any reactivity up to 25% of cells; 2+, 25% to 50% reactivity; 3+, >50% to 75% reactivity; and 4+, >75% to 100% reactivity.
Histopathology.
Mice were sacrificed at day 7, and guinea pigs at day 5 or 35, and the genital tract was removed, fixed in 10% buffered formalin, and embedded in paraffin. Longitudinal 4-μm sections were cut, stained with hematoxylin and eosin, and evaluated by a pathologist blinded to the experimental design. Each anatomic site (exocervix, endocervix, uterine horn, oviduct, and mesosalpinx) was independently assessed for the presence of acute inflammation (neutrophils), chronic inflammation (lymphocytes), plasma cells, and erosion of the mucosa. Right and left uterine horns and right and left oviducts were evaluated individually. A four-tiered semiquantitative scoring system was used to quantitate the inflammation: 0, normal; 1+, rare foci (minimal presence) of parameter; 2+, scattered (one to four) aggregates or mild diffuse increase in parameter; 3+, numerous aggregates (more than four) or moderate diffuse or confluent areas of parameter; and 4+, severe diffuse infiltration or confluence of parameter.
TNF-α inhibition.
Groups of six mice were injected intravaginally with 150 μg of rabbit anti-murine TNF-α antibodies (Endogen, Woburn, Mass.) twice a day on days 0, 2, 4, and 6 of infection. These antibodies have been shown to block or neutralize TNF-α activity in vivo (6). Control infected mice were injected with 150 μg of normal rabbit immunoglobulin G (IgG) (Endogen). Each mouse was anesthetized by methoxyflurane inhalation, and 75 μl of antibody was injected into each side of the vagina, using a 28-gauge needle attached to an insulin syringe. The needle was advanced approximately 2 to 3 mm into the vaginal wall. The optimum dose and interval were determined in preliminary experiments. Intravaginal injection resulted in more consistent inhibition than intravenous injection.
For the guinea pig experiments, we used a recombinant human p80 TNF-α receptor linked to the Fc region of human IgG1 kindly provided by Immunex Corp., Seattle, Wash. This dimeric receptor TNF-α inhibitor protein binds TNF-α with high affinity and acts as an antagonist of TNF-α biologic activity in both in vitro and in vivo assays (21). A dose of 1 mg of inhibitor protein was administered via intracardiac injection every other day starting on the day prior to infection. Control infected guinea pigs received the same volume of drug vehicle. Groups of six control and six inhibitor-treated guinea pigs were sacrificed on day 5. In a second protocol, guinea pigs were treated with inhibitor protein every other day through day 15 and were sacrificed on day 35.
Cytokine analysis of murine genital tract secretions.
Murine genital tract secretions were collected on the day prior to infection and on days 2 through 7 postinoculation, after which the mice were sacrificed. An aseptic surgical sponge (Weck Ophthalmologicals, Atlanta, Ga.) was inserted into the vagina of an anesthetized mouse and retrieved 30 min later. The sponges were held at −70°C until they were eluted individually in 0.3 ml of PBS containing 0.05% Tween and 0.05% sodium azide for use in a murine cytokine enzyme-linked immunosorbent assay (ELISA). The murine ELISAs for TNF-α and IFN-γ (Endogen) and for interleukin-1β (IL-1β) macrophage inflammatory protein-2 (MIP-2), and granulocyte-macrophage colony stimulating factor (GM-CSF) (Research and Development, Minneapolis, Minn.) were performed as per manufacturers' instructions. Precision was >95% between duplicate samples.
Assay of guinea pig genital tract secretions for TNF-α.
Guinea pig genital tract secretions were collected on multiple days throughout the course of infection and also via vaginal sponges as described previously (11). Guinea pig sponges were held at −70°C until they were eluted individually in 0.5 ml of Eagle minimal essential medium, and the TNF-α activity of cell-free eluates was measured by L929 cytotoxicity assay as previously described (11). Optical density of L929 cells incubated with medium alone represented 0% lysis, and cells treated with 3 M guanidine hydrochloride represented 100% lysis. One unit of TNF-α was defined as that amount of TNF-α required to produce 50% lysis of L929 cells. To determine the specificity of the assay, appropriate dilutions of selected samples or standard recombinant murine TNF-α (Genentech, San Francisco, Calif.) were preincubated with five neutralizing units of polyclonal rabbit anti-mouse TNF-α (Genentech) or nonspecific rabbit IgG for 4 h at 4°C before addition to the plates. The lower limit of detectability of the assay is 2.5 U/ml.
Statistics.
Statistical comparisons between groups over time for levels of infection and for TNF-α were made by a two-way analysis of variance (ANOVA). The Kruskal-Wallis one-way ANOVA on ranks was used to determine significant differences in the pathological data between groups. Comparisons of pathological data within groups were made by use of the Student-Newman-Keuls method of all-pairwise multiple comparisons. The z test for determination of significant differences in sample proportions was used to compare frequencies of pathological findings between specific groups.
RESULTS
Effect of TNF-α inhibition on level of infection and cellular inflammation in normal C57 mice.
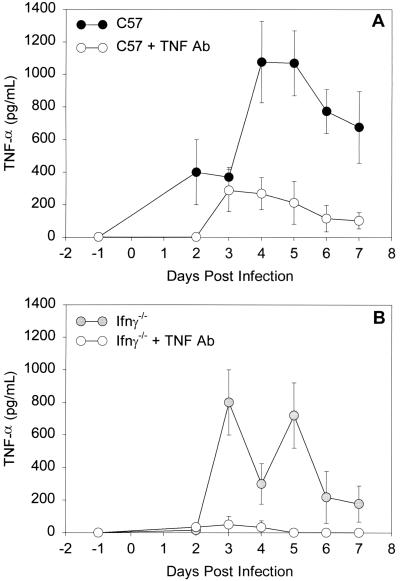
We have detected high levels of TNF-α in lower genital tract secretions of C57 mice during the first week of infection, after which levels fall to baseline (10). Thus, we chose to inhibit the local TNF-α response for 7 days and examine what effect this might have on numbers of organisms and on the cellular inflammatory response. Levels of TNF-α in endocervical secretions were determined by ELISA. Although we were unable to completely block the local TNF-α response, it was reduced by 75 to 90% on days 4 through 7. Figure 1A shows the results from a representative experiment. A two-way ANOVA showed significantly lower levels of TNF-α in the antibody-treated mice over the 7 days examined (P < 0.001).
FIG. 1.
TNF-α levels (means ± standard errors of the mean) in genital tract secretions of C57 mice (A) and C57 IFN-γ−/− mice (B) over the first 7 days of primary infection with MoPn. ○, mice that received anti-TNF-α antibody; ●, mice that received control rabbit Ig. Levels of TNF-α were determined with an ELISA kit specific for murine TNF-α. Data represent one representative experiment (n = 6 per group). Each sample was run in duplicate.
Despite our success in inhibiting the local TNF-α response, analyses from vaginal swabs revealed no difference in the numbers of organisms isolated from control and antibody-treated mice (Table 1). Numbers of organisms decreased from day 4 to day 7 in the TNF-α antibody-treated mice as they did in each of the control infected mice, suggesting that host defense mechanisms were not compromised despite significant inhibition of the TNF-α response.
TABLE 1.
Isolations of C. trachomatis MoPn from control and anti-TNF-α-antibody-treated mice
| Mouse groupa | IFU (log10 [mean ± SD]) on day of infectionb
|
|
|---|---|---|
| 4 | 7 | |
| C57 control | 6.2 ± 0.6 | 5.5 ± 0.3 |
| C57 + TNF-α antibody | 6.4 ± 0.4 | 5.9 ± 0.5 |
| C57 IFN-γ−/− control | 6.4 ± 0.3 | 5.6 ± 0.6 |
| C57 IFN-γ−/− + TNF-α antibody | 6.3 ± 0.5 | 5.6 ± 0.5 |
Six female mice per group were infected with 107 IFU of murine MoPn, and isolations were performed from vaginal swabs as described in Materials and Methods.
Means from vaginal swabs of six mice. Results are representative of two separate experiments.
Interestingly, when the levels of acute and chronic inflammatory cells were graded histologically, a significant increase in numbers of neutrophils was seen in the endocervix and uterine horns of the TNF-α antibody-treated mice compared to controls (median pathology scores, 3 and 4, respectively, for endocervix and uterine horns of antibody-treated mice versus 2 and 3 for control mice [P < 0.05 by ANOVA on ranks and Student-Newman-Keuls multiple comparison procedure]). Although higher numbers of neutrophils were also seen in the oviducts of the antibody-treated mice, this difference was not significant. Numbers of lymphocytes were slightly higher in the antibody-treated mice in the endocervix and uterine horns, but again the differences were not statistically significant; plasma cells were equal in the two groups (data not shown).
Effect of TNF-α inhibition on level of infection and cellular inflammation in C57 IFN-γ−/− mice.
Increased levels of TNF-α were found in infected IFN-γ−/− mice on days 3 through 7, and intravaginal injections of anti-TNF-α antibody completely depleted the response (Fig. 1B). As seen in the immunologically intact mice, inhibition of the TNF-α response had no effect on the numbers of organisms isolated from the lower genital tract on days 4 and 7 of infection (Table 1), and numbers of organisms decreased in both groups from day 4 to day 7. The TNF-α levels detected in control IFN-γ−/− mice (Fig. 1B) were not significantly different than those seen in control C57 mice in two separate experiments (Fig. 1A). Thus, a compensatory increase in TNF-α is not the mechanism for early control of genital tract MoPn infection in mice lacking IFN-γ.
Histological examination of the genital tract tissues of IFN-γ−/− mice revealed increased numbers of neutrophils in the antibody-treated mice compared to controls, although a significant increase was determined only for the uterine horns. The median pathology score was 3 in the endocervix of antibody-treated IFN-γ−/− mice and 2 in control IFN-γ−/− mice (P < 0.05 by ANOVA on ranks). Lymphocyte and plasma cell infiltrations were the same in both groups (data not shown).
Effect of TNF-α inhibition on other proinflammatory cytokines in the mouse.
Neutrophils are known to be effective mediators of host defense against chlamydiae; thus, it is possible that, despite a relative lack of TNF-α-mediated inhibition of chlamydial growth, a compensatory increase in the neutrophil response resulted in similar numbers of organisms being detected in the control and antibody-treated mice. This prompted us to examine the effect of TNF-α inhibition on other proinflammatory cytokines and chemokines that are known to induce neutrophil influx into a tissue. Since IL-1β, MIP-2, and GM-CSF are proinflammatory mediators that are potent inducers of neutrophil chemotaxis and activation (1, 5, 12), we examined levels of these proteins in genital tract secretions from the control and TNF-α antibody-treated groups of mice by ELISA.
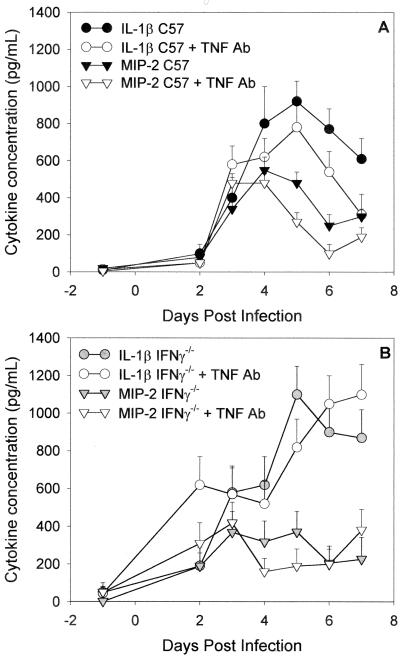
In Fig. 2A and B it can be seen that in normal and IFN-γ−/− mice, respectively, inhibition of the TNF-α response did not lead to increases in IL-1β or in the neutrophil chemokine MIP-2. In fact, on most days, the levels were slightly lower in the antibody-treated mice. Both cytokines were elevated above baseline on days 3 through 7 of infection, and the kinetics of the responses were similar in control and antibody-treated mice. Levels of GM-CSF were also increased in all four groups in response to infection on days 3 through 7, but no differences were seen between control and antibody-treated mice (data not shown). Secretions were also examined for levels of IFN-γ in the normal C57 mice, and no difference was seen in the kinetics of the response in control and antibody-treated C57 mice (data not shown). Thus, the relative increase in acute inflammatory cells seen in the TNF-α-inhibited mice was not explained by a compensatory increase in levels of other proinflammatory cytokines or the chemokine MIP-2.
FIG. 2.
IL-1β (circles) and MIP-2 (inverted triangles) levels (means ± standard errors of the mean) in genital tract secretions of C57 mice (A) and C57 IFN-γ−/− mice (B) over the first 7 days of primary infection with MoPn. Levels of the respective cytokines were determined with ELISA kits specific for murine IL-1β and MIP-2. Open symbols represent mice that received anti-TNF-α antibody, and filled symbols represent mice that received control rabbit immunoglobulin. Data are pooled from two experiments (n = 12 per group). Each sample was run in duplicate.
Effect of TNF-α inhibition on level of infection, cellular inflammation, and tissue pathology in guinea pigs infected with GPIC.
Previous studies in our lab with the GPIC model revealed that a marked acute inflammatory response characterized by infiltration of the cervical epithelium with neutrophils is present on day 5 of primary infection (3). We have also detected high levels of TNF-α in genital tract secretions from vaginally infected guinea pigs during the first 5 days of infection (11). Thus, in an initial experimental protocol, groups of infected drug vehicle-treated (control group) and TNF-α inhibitor-treated guinea pigs were sacrificed on day 5, and their tissues were examined for levels of inflammatory cells and for chlamydial organisms. The kinetics of lower genital tract infection were determined by culture of vaginal swabs. The experiment was repeated, and pathological data and isolation data were pooled for analysis.
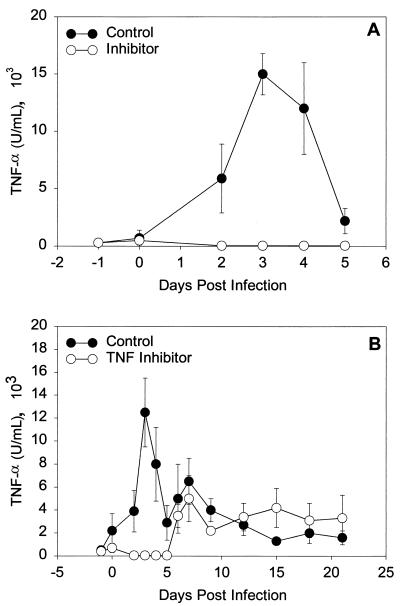
As depicted in Fig. 3A, we were able to completely deplete the local TNF-α response in infected guinea pigs with the TNF-α inhibitor protein for the first 5 days of infection. Despite blockade of the TNF-α response, immunohistochemical staining of genital tract tissues for chlamydiae revealed no difference in the degree of staining (median score for infected control endocervix, 2.0, and for inhibitor-treated endocervix, 1.5 [P = 0.62 by the rank sum test]; median score for infected control exocervix, 1.5, and for inhibitor-treated exocervix, 2.0 [P = 0.49]). No chlamydial inclusions were seen in the fundus, uterine horns, or oviducts. This was expected on day 5 of infection, as it takes approximately 7 days post-vaginal inoculation for guinea pigs to become isolation positive above the endocervix. Isolations from vaginal swabs also revealed no difference in the level of infection (day 5 median log10 IFU in control, 6.6, and median log10 IFU in inhibitor treated, 6.7 [P = 0.4 by the rank sum test]). Thus, as seen in the mouse after vaginal infection with the murine strain of C. trachomatis, depletion of the early TNF-α response had no effect on the degree or level of guinea pig C. psittaci infection after vaginal inoculation.
FIG. 3.
TNF-α levels (means ± standard errors of the mean) in genital tract secretions of guinea pigs over the first 7 days of primary infection (A) or over 3 weeks of primary infection with GPIC (B). Open circles (○) represent guinea pigs that received the TNF-α inhibitor protein, and filled circles (●) represent guinea pigs that received injections of drug vehicle. Levels of TNF-α were determined by L929 cytotoxicity assay. Data represent one representative experiment (n = 6 per group). Each sample was run in duplicate.
Examination of genital tract tissues for relative degrees of inflammatory cells and mucosal erosion revealed a significant increase in the level of acute and chronic inflammatory cells in the endocervix of inhibitor-treated animals versus controls. The median pathology score for acute inflammatory cells in endocervix of inhibitor-treated animals was 3, compared to 2 in controls; the median pathology score for chronic inflammatory cells was 2 in inhibitor-treated animals versus 1 in controls (P < 0.05 by ANOVA on ranks and the Student-Newman-Keuls multiple comparison procedure). Plasma cell infiltration and mucosal erosion were similar in the two groups (data not shown). Pathological parameters for the exocervix were not different in the two groups, and low and equal degrees of pathology were seen in tissues above the endocervix (data not shown). Thus, just as in the mouse, early TNF-α depletion had no effect on the level of chlamydial infection but led to increased numbers of neutrophils and lymphocytes in the endocervix, the primary site of infection during this early time period.
A second experimental protocol sought to inhibit the TNF-α response for a prolonged period so as to evaluate what effects these early changes in the inflammatory response might have on the overall course of infection and on chronic tissue pathology. Groups of guinea pigs were injected with drug vehicle or with TNF-α inhibitor every other day through day 15 of infection, and then the animals were sacrificed on day 35. As depicted in Fig. 3B, we were successful once again in depleting the early TNF-α response through day 5 of infection, but after day 5, TNF-α levels increased above baseline in both groups. The guinea pigs likely developed an antibody response to the TNF-α inhibitor protein. When inclusion scores over time and chronic tissue pathology were compared in these two groups, no differences were found (data not shown). A repeat experiment yielded the same results. Inhibition of the early TNF-α response did not lengthen the duration of infection nor influence the degree of chronic tissue pathology that developed as a result of infection. The increased levels of inflammation detected in the lower genital tract on day 5 of infection had undetectable effects on lasting tissue pathology in the upper genital tract.
DISCUSSION
In previously published articles, we have described the detection of high levels of TNF-α in genital tract secretions of mice and guinea pigs during the first week of chlamydial infection (10, 11). After this early surge of TNF-α, levels fall to baseline by days 7 to 10 in both animal species. The intensity of the TNF-α response is proportional to the intensity of infection (11; Rank et al., submitted), with significantly higher levels of TNF-α being found during primary infection than during challenge infection in both mice and guinea pigs (10, 11). In both animal species, high TNF-α levels are present at a time of marked neutrophil influx, suggesting a role for TNF-α in the early local acute inflammatory response in the genital tract. Using anti-TNF-α antibody treatment of mice and TNF-α inhibitor protein administration in guinea pigs, we successfully blocked this early TNF-α response in the respective animal models. Interestingly, this blockade had no effect on the numbers of organisms isolated from the genital tract during the time of TNF-α inhibition in mice or guinea pigs. Since the guinea pig more closely resembles the human in terms of ascending infection, we attempted a prolonged inhibition of the TNF-α response in this model. Although prolonged inhibition was not achieved, blockade of the early burst of TNF-α in the guinea pig model had no effect on the course of ascending infection or on resultant chronic tissue pathology.
TNF-α induces neutrophil influx and has a strong activating effect on these cells. Despite these known effects of TNF-α, it is apparent from this study that blockade of TNF-α released in response to chlamydial invasion of the genital tract does not compromise the early acute inflammatory response in mice and guinea pigs. Surprisingly, an augmented neutrophil response was detected in the mouse genital tract, and increased numbers of neutrophils as well as infiltrating lymphocytes were seen in the endocervix of infected guinea pigs. In addition, it is obvious from our analysis of IL-1β, MIP-2, and GM-CSF in the mouse model that blockade of the TNF-α response did not alter the release of other proinflammatory proteins. It is likely that release of these mediators, together with other chemokines released from Chlamydia-infected epithelial cells (33), led to an effective and robust acute inflammatory response despite significant inhibition of TNF-α-mediated effects.
Despite significant inhibition of the local TNF-α response in our study, no effect on the infection was seen. In mice genetically deficient in the TNF-α p55 receptor molecule (TNFRI), Perry et al. found a marginal but statistically significant delay in the rate of clearance of either MoPn or human serovar D of C. trachomatis from the mouse genital tract, and this difference was evident by day 6 of infection (25). Perry et al. did not examine other parameters of the response in the TNFRI−/− mice, and so we cannot determine whether the delay was due to direct or indirect effects. The discrepancy in our results and those of Perry et al. might be explained by a more effective removal of TNF-α effects with the use of mice genetically deficient in the TNFRI gene. It is possible that our detection methods are insensitive to low levels of TNF-α and, as seen in Fig. 1A, we were unable to completely deplete the TNF-α response in normal C57 mice.
We chose to examine IFN-γ−/− mice to maximize our chance of detecting a role for TNF-α in the host response to chlamydial infection. Despite an absence of IFN-γ expression, these mice have been shown to eliminate MoPn organisms from the local genital tract at rates not significantly different than those of wild-type mice, suggesting that alternative mechanisms of early host defense are in effect (9, 23). When these mice were depleted of TNF, there was no effect on numbers of organisms isolated from the genital tract or on levels of the proinflammatory mediators MIP-2, IL-1β (Fig. 2), and GM-CSF. Thus, from this double-depletion experiment, it can be concluded that the in vivo response to MoPn infection in the mouse genital tract is intact without IFN-γ and TNF-α and that alternate mechanisms are important in early host defense.
Williams et al. showed, via in vivo antibody inhibition of TNF-α, that TNF-α has a significant role in host defense against MoPn in the murine pneumonia model (38, 39). There is an obvious discrepancy between these data and ours. This discrepancy can most likely be explained by differences in the sites of infection. Since a predominant response to chlamydial infection of the lung would likely include alveolar macrophages, one would expect to find high levels of TNF-α in this model, as was indeed shown by Williams et al. (39), and to find that inhibition of TNF-α would have an impact on the infection. Previously published data have also shown a role for IL-6 in host defense in the MoPn pneumonia model (37), but not in genital tract infection (24).
In vivo inhibition of TNF-α in murine models of infection with other intracellular organisms, including Leishmania donovani (36), Listeria monocytogenes (8), Mycobacterium avium (2), and Trypanosoma cruzi (35), has shown a marked increase in pathogen burden and increased morbidity and mortality in the antibody-treated mice. These pathogens are primarily killed by activated monocytes and macrophages of the reticuloendothelial system. The development of intracellular killing activity by activated monocytes and macrophages requires the autocrine effects of TNF-α (19), and so one would expect inhibition of the TNF-α response to have a profound effect on these infections. Although chlamydiae may infect resident genital tract macrophages, they primarily infect genital tract epithelial cells. Thus, early control of chlamydial infection must rely on inhibitory activities of the epithelial cells themselves and the rapid recruitment of effective inflammatory cells to the genital tract mucosa.
In our comparisons of chlamydial genital tract infection in different strains of mice, the C57 strain has significantly shorter and less intense infection than the C3H strain (10). We not only found increased levels of TNF-α in the C57 strain compared to strain C3H but also found significantly increased numbers of neutrophils in the lower genital tract of the C57 strain on days 3 and 7 of infection with either MoPn or human serovar E when compared to strain C3H. Barteneva et al. revealed that BALB/c mice that received an antineutrophil antibody had a more intense genital tract infection with MoPn than those that received nonspecific rat IgG (4). The number of IFU shed per mouse was 2- to 100-fold higher in the experimental group than in the control group on day 7, but the experimental animals eventually eradicated the infection. These data, taken together, indicate that neutrophils play a critical role in the early control of chlamydial genital tract infection.
The reason for the detection of increased numbers of neutrophils in the groups of mice treated with anti-TNF-α antibody and of increased numbers of neutrophils and lymphocytes in the TNF-α inhibitor-treated guinea pigs is possibly the effects of TNF-α on apoptosis of inflammatory cells. Apoptosis of inflammatory cells is an important mechanism underlying the resolution of an inflammatory focus. TNF-α induces apoptosis of multiple target cells (13), including neutrophils (14, 40) and lymphocytes (7). In a recent study we examined the effect of MoPn infection and subsequent TNF-α secretion on apoptosis in the murine genital tract (22). We found that mice infected with MoPn had higher numbers of apoptotic cells in the genital tract and that apoptosis occurred in both infected and neighboring uninfected cells, in both the epithelium and submucosa. Inhibition of TNF-α led to a significant decrease in apoptosis, suggesting that chlamydial infection induced apoptosis directly and indirectly through the release of cytokines. Consideration of data from the present study indicates that blockade of TNF-α may decrease TNF-α induced apoptosis not only of neighboring epithelial cells but also of infiltrating inflammatory cells.
This study does not indicate that TNF-α has no effect on growth of chlamydia in vivo. It simply indicates that, as regards the genital tract, alternate mediators of host defense are in place to effect early control of chlamydial infection. Proinflammatory mediators released directly from the infected epithelial cells themselves likely stimulate rapid neutrophil influx, and these cells are effective mediators of host defense until antigen-specific mechanisms are induced to eliminate the pathogen. In addition, this study does not indicate that TNF-α has no contribution in the development of tissue pathology. However, it indicates that the early burst of TNF-α seen during primary chlamydial genital tract infection is not necessary for pathology to be induced.
ACKNOWLEDGMENTS
We acknowledge the superb technical assistance of Anne Bowlin and Jim D. Sikes.
This work was supported by Public Health Service grants AI43337 and AI23044 from the National Institutes of Health and by the Horace C. Cabe Foundation and the Bates-Wheeler Foundation, Arkansas Children's Hospital Research Institute.
REFERENCES
- 1.Appelberg R. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J Leukoc Biol. 1992;52:303–306. doi: 10.1002/jlb.52.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Bali S, Hastings K L, Kazempour K, Inglis S, Dempsey W L. Inhibition of tumor necrosis factor-alpha alters resistance to Mycobacterium avium complex infection in mice. Antimicrob Agents Chemother. 1998;42:2336–2341. doi: 10.1128/aac.42.9.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron A L, White H J, Rank R G, Soloff B L. Target tissues associated with genital infection of female guinea pigs by the chlamydial agent of guinea pig inclusion conjunctivitis. J Infect Dis. 1979;139:60–68. doi: 10.1093/infdis/139.1.60. [DOI] [PubMed] [Google Scholar]
- 4.Barteneva N, Theodor I, Peterson E M, de la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell M D, Taub D D, Kunkel S J, Strieter R M, Foley R, Gauldie J, Perry V H. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur J Neurosci. 1996;8:1803–1811. doi: 10.1111/j.1460-9568.1996.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Thompson C B. Hierarchical control of lymphocyte survival. Science. 1996;274:67–68. doi: 10.1126/science.274.5284.67. [DOI] [PubMed] [Google Scholar]
- 8.Conlan J W. Neutrophils and tumor necrosis factor-alpha are important for controlling early gastrointestinal stages of experimental murine listeriosis. J Med Microbiol. 1997;46:239–250. doi: 10.1099/00222615-46-3-239. [DOI] [PubMed] [Google Scholar]
- 9.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darville T, Andrews C W, Lafoon K K, Shymasani W, Kishen L R, Rank R G, Andrews C W, Jr, Laffoon K K. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darville T, Laffoon K K, Kishen L R, Rank R G. Tumor necrosis factor-alpha activity in genital tract secretions of guinea pigs infected with chlamydiae. Infect Immun. 1995;63:4675–4681. doi: 10.1128/iai.63.12.4675-4681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello C A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 13.Golstein P, Ojcius D M, Young J D. Cell death mechanisms and the immune system. Immunol Rev. 1991;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 14.Gon S, Gatanaga T, Sendo F. Involvement of two types of TNF receptor in TNF-alpha induced neutrophil apoptosis. Microbiol Immunol. 1996;40:463–465. doi: 10.1111/j.1348-0421.1996.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Brown T, Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990;171:465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manor E, Sarov I. Inhibition of Chlamydia trachomatis replication in HEp-2 cells by human monocyte-derived macrophages. Infect Immun. 1988;56:3280–3284. doi: 10.1128/iai.56.12.3280-3284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Megran D W, Stiver H G, Bowie W R. Complement activation and stimulation of chemotaxis by Chlamydia trachomatis. Infect Immun. 1985;49:670–673. doi: 10.1128/iai.49.3.670-673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nacy C A, Meierovics A I, Belosevic M, Green S J. Tumor necrosis factor-alpha: central regulatory cytokine in the induction of macrophage antimicrobial activities. Pathobiology. 1991;59:182–184. doi: 10.1159/000163640. [DOI] [PubMed] [Google Scholar]
- 20.Pasley J N, Rank R G, Hough A J, Jr, Cohen C, Barron A L. Effects of various doses of estradiol on chlamydial genital infection in ovariectomized guinea pigs. Sex Transm Dis. 1985;12:8–13. doi: 10.1097/00007435-198501000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfettini J-L, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius D M. Effect of Chlamydia trachomatis infection and subsequent TNFα secretion on apoptosis in the murine genital tract. Infect Immun. 2000;68:2237–2244. doi: 10.1128/iai.68.4.2237-2244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 24.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-γ-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 26.Rajagopalan-Levasseur P, Lecointe D, Bertrand G, Fay M, Gougerot-Pocidalo M A. Differential nitric oxide production by macrophages from mice and guinea pigs infected with virulent and avirulent Legionella pneumophila serogroup 1. Clin Exp Immunol. 1996;104:48–53. doi: 10.1046/j.1365-2249.1996.d01-644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rank R G. Animal models for urogenital infections. In: Clark V L, Bavoil P M, editors. Bacterial pathogenesis. Part A. Identification and regulation of virulence factors. San Diego, Calif: Academic Press, Inc.; 1994. pp. 83–92. [Google Scholar]
- 28.Rank R G, Barron A L. Specific effect of estradiol on the genital mucosal antibody response in chlamydial ocular and genital infections. Infect Immun. 1987;55:2317–2319. doi: 10.1128/iai.55.9.2317-2319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rank R G, Batteiger B E, Soderberg L S F. Susceptibility to reinfection after a primary chlamydial genital infection. Infect Immun. 1988;56:2243–2249. doi: 10.1128/iai.56.9.2243-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rank R G, Sanders M M. Ascending genital tract infection as a common consequence of vaginal inoculation with the guinea pig inclusion conjunctivitis agent in normal guinea pigs. In: Bowie W R, Caldwell H D, Jones R B, Mardh P-A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections. New York, N.Y: Cambridge University Press; 1990. pp. 249–252. [Google Scholar]
- 31.Rank R G, Sanders M M. Pathogenesis of endometritis and salpingitis in a guinea pig model of chlamydial genital infection. Am J Pathol. 1992;140:927–936. [PMC free article] [PubMed] [Google Scholar]
- 32.Rank R G, Sanders M M, Kidd A T. Influence of the estrous cycle on the development of upper genital tract pathology as a result of chlamydial infection in the guinea pig model of pelvic inflammatory disease. Am J Pathol. 1993;142:1291–1296. [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shemer-Avni Y, Wallach D, Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988;56:2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva J S, Vespa G N, Cardoso M A, Aliverti J C, Cunha F Q. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumang M C, Keogh C, Moldawer L L, Helfgott D C, Teitelbaum R, Hariprashad J, Murray H W. Role and effect of TNF-alpha in experimental visceral leishmaniasis. J Immunol. 1994;153:768–775. [PubMed] [Google Scholar]
- 37.Williams D M, Grubbs B, Darville T, Kelly K, Rank R. A role for interleukin 6 in host defense against Chlamydia trachomatis. Infect Immun. 1998;66:4564–4567. doi: 10.1128/iai.66.9.4564-4567.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams D M, Grubbs B, Kelly K A, Rank R G. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams D M, Magee D M, Bonewald L F, Smith J G, Bleicker C A, Byrne G I, Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990;58:1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita K, Takahashi A, Kobayashi S, Hirata H, Mesner P W, Jr, Kaufmann S H, Yonehara S, Yamamoto K, Uchiyama T, Sasada M. Caspases mediate tumor necrosis factor-alpha-induced neutrophil apoptosis and downregulation of reactive oxygen production. Blood. 1999;93:674–685. [PubMed] [Google Scholar]