Abstract
It has been widely demonstrated that the gut microbiota is responsible for essential functions in human health and that its perturbation is implicated in the development and progression of a growing list of diseases. The number of studies evaluating how the gut microbiota interacts with and influences other organs and systems in the body and vice versa is constantly increasing and several ‘gut–organ axes’ have already been defined. Recently, the view on the link between the gut microbiota (GM) and the peripheral nervous system (PNS) has become broader by exceeding the fact that the PNS can serve as a systemic carrier of GM-derived metabolites and products to other organs. The PNS as the communication network between the central nervous system and the periphery of the body and internal organs can rather be affected itself by GM perturbation. In this review, we summarize the current knowledge about the impact of gut microbiota on the PNS, with regard to its somatic and autonomic divisions, in physiological, regenerative and pathological conditions.
Keywords: gut microbiota, peripheral nerve, nerve injury and regeneration, short chain fatty acid
1. Introduction
The collection of bacteria, viruses, fungi, and protozoans that colonize the gastrointestinal tract (GIT) is termed the ‘gut microbiota’ (GM). GM shares a mutually beneficial relationship with its host, and contributes to host tissue homeostasis through a series of physiological functions. These include digestion, vitamin synthesis, maintenance of the integrity of the mucosal barrier, protection against pathogens, immune system development and maturation, and central nervous system (CNS) modulation, thus making the GM a ‘vital-organ’ itself [1,2].
It has been estimated that the GIT is inhabited by more than 1014 microorganisms, which encompasses ∼10 times more bacterial cells than the number of cells in the human body and over 100 times the amount of genomic content (microbiome) as the human genome [3]. The GM is mainly dominated by the phyla Firmicutes and Bacteroidetes (up to 75% of all gut flora), and to a lesser extent by Actinobacteria, Tenericutes, Proteobacteria and Verrumicrobia [4,5].
At birth, the GIT is rapidly colonized by microorganisms; the microbial composition in early life is strongly influenced by the mode of delivery, the feeding method, the use of antibiotics and the maternal microbial composition. By around 2.5 years of age, the composition, diversity and functional capabilities of the human infant microbiota resemble those of human adult microbiota [3]. Various factors, such as geographical location, diet, exercise, use or abuse of antibiotics, age and diseases continue to shape and modify the GM composition during adulthood, to old age [3,6].
The GM transforms dietary nutrients and endogenous molecules into microbial-derived metabolites that can signal to peripheral organs and tissues in the body, allowing their communication through various mechanisms such as humoral, endocrine, immune and others, thus establishing complex ‘gut–organ axes’ [1]. The crosstalk between the GM and distal organs has therefore emerged as a research field of utmost interest and such interactions are being delineated piece by piece. In fact, in the last two decades, the number of studies showing the impact that the composition of the GM has on host physiology and metabolism and its interaction with various organs has grown substantially. The same is true for increasing evidence of an impact the body conditions itself, e.g., stress or drug treatment, have on the GM composition [7]. Alterations or imbalances in the amount, composition and diversity of the microbiota, a condition termed dysbiosis, has been linked to dysregulation of bodily functions and diseases such as inflammatory bowel disease, cardiovascular diseases, cancer, respiratory diseases, diabetes, brain disorders, chronic kidney diseases, liver diseases and others [8]. Therefore, identifying and controlling intestinal bacterial function with probiotics and prebiotics and/or modulating the release of bioactive factors from microbes represents an innovative approach for the treatment of multiple pathologies.
It is now clear that the various components of the peripheral nervous system (PNS) serve not only as a carrier system between the GIT and other organs including the central nervous system (CNS), but are also influenced by the intestinal microbiota themselves. In particular, the importance of a healthy or well-balanced GM for the undisturbed development of the enteric nervous system has been widely demonstrated in recent years. To date, only few data exist in the literature on the possible interaction between the GM and the somatic part of the PNS, but recent findings support that there is indeed a direct interaction [9,10,11,12].
In this review, we will start describing the anatomy of the PNS and will then highlight currently available knowledge on how the different divisions of the PNS are influenced by the GM in physiological, regenerative and pathological conditions.
2. Anatomy of the Peripheral Nervous System
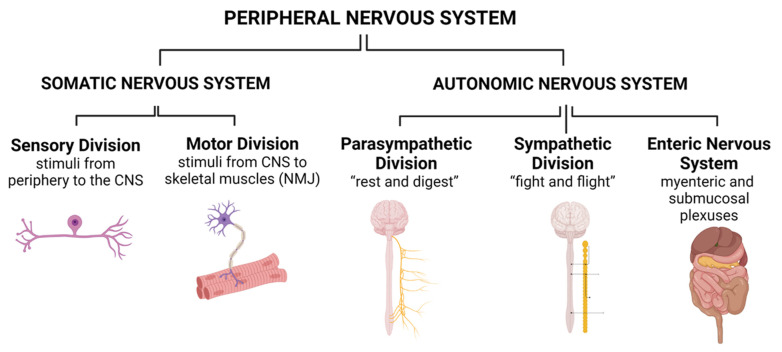
In this section, we briefly recapitulate the anatomical organization of the PNS. The PNS can be divided into the somatic nervous system (SNS) and the autonomic nervous system (ANS). Both can be further subdivided into anatomically and functionally distinct subcategories (Figure 1).
Figure 1.
Organization of the Peripheral Nervous system. CNS: Central Nervous System. NMJ: Neuromuscular Junction. Created with BioRender.com.
The GM can communicate with its host organism via immunological, hormonal, and neuronal signaling for the regulation of digestion and metabolism [13]. Therefore, the mammalian organism needs to receive and process different kinds of information for maintaining a healthy condition and for interacting with its environment. The PNS with its neurons, axons, nerves, and plexuses, represents the “wiring” connection between the organisms’ outer and inner surfaces and the CNS (spinal cord and higher centers in the brain). Signals transmitted via peripheral nerves are processed towards the perception of internal and external stimuli (interoception or exteroception) followed by a motor response [13].
The axons of the peripheral nervous system therefore belong to different functional categories and either work as afferents, e.g., transmitting signals from an end organ to a neuron, or as efferents, e.g., transmitting signals in the opposite way.
2.1. Somatic Nervous System (SNS)
The afferents in the SNS are represented by sensory axons transmitting tactile or painful stimuli towards the spinal cord or brainstem (sensory spinal or cranial nerves). The neurons responsible for this transmission have a pseudo-unipolar morphology and their cell bodies reside in the peripheral sensory dorsal root ganglia (DRG). The central sections of the sensory axons connect via the sensory, dorsal spinal roots to the spinal cord. Depending on the origin of the signal and the specific function of the DRG neuron, specific subclasses of DRG neurons form activating synapses with second-order neurons at different locations of the CNS. Second-order sensory neurons can be located in the dorsal horn of the spinal cord grey matter (e.g., for protopathic processing [14]), in the gracile or cuneate nucleus in the dorsal brainstem (e.g., for epicritic processing), or in the cerebellar cortex (e.g., for proprioceptive processing). Sensory stimuli processed via the SNS become conscious depending on the gating function of the diencephalic thalamus.
The efferents in the SNS are represented by motor axons originating from neurons with a multipolar morphology residing in the ventral horn of the spinal cord grey matter or motor brain stem nuclei. The respective motor axons connect via motor cranial or spinal nerves (e.g., via the motor, ventral spinal roots) with skeletal muscle fibers, where they form specialized synapses, the neuromuscular junctions (NMJs) [15], representing a specialized excitatory chemical synapse. At the NMJs, electrical impulses, transmitted via the efferent motor axons, are converted into muscle fiber action potentials. The conversion is achieved by the release of the neurotransmitter acetylcholine from presynaptic boutons formed at the distal end of a motor axon and high-density expression of acetylcholine receptors at postsynaptic invaginations of the sarcolemma [15]. Motor signals processed via the SNS are largely attributed to the voluntary control of skeletal muscles.
2.2. Autonomic Nervous System (ANS)
The ANS, in contrast to the SNS, processes stimuli that do mainly remain unconscious and control smooth muscle fibers (e.g., in the vascular system or the GIT or the activity of the heart or lung). The ANS can be further subdivided into the parasympathetic, the sympathetic, and the enteric nervous system (ENS) [16].
The parasympathetic and sympathetic nervous systems contain afferents for processing sensory input signals and efferents for processing motor output signals that control the body homeostasis [13]. Signaling in the sympathetic system is transmitted via the sympathetic trunk and paravertebral and prevertebral ganglia to the peripheral organs [16]. The sympathetic signaling can be simplified as the one being responsible for the “fight and flight” conditions of the body [13]. The respective motor neurons are located in the intermedial nuclei of the lower cervical to the upper lumbar spinal cord segments. Signaling in the parasympathetic system is transmitted mainly via the vagus nerve with motor nuclei in the brainstem (medulla oblongata) and ganglia close to the target organs [16]. The system can be simplified as the one being responsible for the body “rest and digest” conditions [13].
The ENS is expanded as an intrinsic ganglia-rich neuronal network in the wall of the GIT and can be subdivided into the myenteric and the submucosal plexuses [17]. The myenteric plexus is located between the circular and longitudinal smooth muscle layers along the whole GIT from the esophagus to the anus. The presence of the submucosal plexus is restricted to the small and large intestines, where it is located beneath the mucosal layer of the gut [17]. The physical state of the intestine is detected by intrinsic primary afferent neurons in the submucosal and myenteric plexuses that can sense the interaction of chemicals with the mucosa as well as the distension caused by a food bolus [13,17]. The ENS forms a sensorimotor reflex circuit, independent of the CNS [18]. Enteric motor neurons can be classified as excitatory, inhibitory, secretomotor, vasomotor, and neurons for the enteroendocrine control [17]. Those located in the submucosal plexus regulate secretion and blood flow and those located in the myenteric plexus control gut motility [13]. Mainly located in the myenteric plexus, interneurons can also be found. They can be classified into orally or anally directed and form longitudinally expanded chains along the GIT [17]. The activity of the interneurons links the ascending and descending motor networks towards the autonomous functioning of the GIT [13].
We have now briefly reviewed the anatomy of the PNS. How GM can use the PNS in influencing developmental processes, in maintaining healthy conditions, or in the manifestation of diverse pathologies, is an active field of investigation [19]. The vagus nerve, however, represents an obvious interconnecting structure. Signaling molecules, produced in the GIT (e.g., microbiota-derived metabolites), could use the vagus nerve or also spinal nerves for reaching other parts of the body or the spinal cord, brainstem and brain. The vagus nerve is the main afferent pathway from the GIT and inner organs; it therefore provides an anatomical link between the internal organs and GIT to the brainstem with 80–90% afferent fibers and 10–20% efferent fibers. Sensory vagal inputs arrive in the nucleus of the solitary tract via the nodose ganglion. From there, fibers ramify to widespread areas of the CNS, including the cerebral cortex and medulla oblongata [20,21]. The vagus nerve is therefore able to sense the GM metabolites through its afferents, to transfer this gut information to the CNS where it is integrated into the central autonomic network for generating a well-adapted or an inappropriate response [22]. The integrity of vagal afferents is therefore critical for the communication of the GM via the PNS to the brain.
Molecular mechanisms that could mediate the communication between GM and the different PNS components have been recently reviewed elsewhere [13]. On the other hand, there is increasing evidence of a more direct impact of the GM on the proper development and function of the subdivisions of the PNS and their target tissues, which will be reviewed in the upcoming sections.
3. Effect of Gut Microbiota on the Somatic Peripheral Nervous System
The increasing knowledge that the composition of the GM can influence the regeneration of diverse tissues [23], including the liver [24], skin [25], and bones [26], triggered the evaluation of such impact also in the central and peripheral nervous systems. In the CNS, models of both traumatic brain and spinal cord injuries demonstrated that such insults are responsible for leaks in the gut barrier integrity, rapidly damaging intestinal mucosa. From there, toxins are systemically released in the plasma and further increase the intestinal permeability and malabsorption of nutrients. Moreover, the balance in gut bacteria populations is altered and even bacteria can be translocated to other organs [27,28,29]. As well, dysbiosis itself was recognized to influence the inflammatory response to injury and impairing functional recovery, therefore clarifying a bidirectional impact between the GM and the CNS in acute injury [28,30].
Evidence of a role for GM in contributing to peripheral nerve repair plasticity has emerged in the last years in the context of both the SNS and ENS. The following paragraphs will summarize updated knowledge from this field.
3.1. The Process of Somatic Nerve Injury and Regeneration
Long lasting effort in biomedical research was dedicated to the design of therapeutic approaches able to improve nerve regeneration after injury. Indeed, despite the peripheral neuron ability to regenerate, traumatic nerve injuries are conditions frequently leading to persistent motor and sensory impairment due to inefficient or delayed regeneration [31]. The regeneration process can be affected by many variables including the injury type, its extension, the requirement for surgery, the patient’s age, comorbidities and the lifestyle, including the diet [32,33,34]. Therefore, the research focusing on strategies promoting regeneration has still extensive room for application. In this context, a peerless contribution has come from the use of animal models of induced injury, that by unraveling cellular and systemically orchestrated mechanisms underlying the ability of peripheral nerves to regenerate, highlighted a number of relevant therapeutic targets [35]. A paradigm of timely defined events occurs upon nerve injury, starting from the interruption of the injured axonal fibers, leading to a disconnection between neuronal bodies and their distal terminals; these in turn undergo Wallerian degeneration, while a retrograde signaling from the injury site sets a communication with the neural nuclear core [36,37]. Schwann cells respond to the loss of axonal contact by triggering a re-programming that is essential in inducing cell proliferation, myelin debris removal, guidance of axon regrowth and remyelination [38,39]. This, together with immune cell recruitment, supports the establishment of a microenvironment that promotes the required regenerative process [40,41].
3.2. The Role of Gut Microbiota on Somatic Nerve Injury and Regeneration
The interest in the impact of GM on nerve injury and recovery has been growing very recently. In the last few years, studies emerged showing that GM modulation by antibiotics, prebiotics, or in pathological alterations such as dysbiosis can affect the recovery from peripheral nerve injury [42]. Liu et al. studied regeneration after corneal nerve injury induced by epithelial abrasion and demonstrated, for the first time, that the oral application of antibiotics results in a brake to the regeneration of the corneal branch of the trigeminal nerve (V cranial nerve) after injury. The application route for antibiotics had an impact on the GM composition, which was not affected after local application of antibiotics at the injury site via eye drop administration. Interestingly, the latter did not delay corneal nerve regeneration [9]. Indeed, mice orally treated with antibiotics displayed altered corneal gene expression pointing to a reduced neurogenesis, compared to control mice, as well as histological defects in corneal nerve regrowth. Of note, fecal microbiota transplantation (FMT) was able to rescue corneal gene expression and the density of regrown nerves; this was found to be mediated by CC chemokine receptor 2 (CCR2)-negative macrophages, a population of corneal macrophages, able to express neurotrophic cues and likely to be contributing to a regeneration promoting milieu [9]. When these cells were depleted by injection with anti-colony stimulating factor 1 receptor (CSF1R) antibody, regeneration was impaired, while CCR2-negative macrophages transfer from normal mice to antibiotics-treated ones normalized nerve healing. The expression of macrophage-related genes found to be downregulated upon oral antibiotic treatment was rescued not only with FMT, but also via probiotic administration, stressing the fact that GM composition has a direct impact on the immune cell state and distribution in peripheral districts [9]. Similarly, Rodenhouse et al. demonstrated that probiotics were able to counteract the delaying impact of oral antibiotic administration on motor functional recovery after traumatic sciatic nerve injury [10]. Therefore, in a scenario in which oral antibiotic treatment preceding nerve injury induced a reduction in the diversity of GM composition and consequently impaired nerve recovery, the administration of probiotics appeared to prevent regeneration deficits both in corneal and sciatic nerves. This contributes to the more general idea that dietary approaches targeting the balance in GM composition could promote peripheral nerve regeneration [9,10]. The probiotics applied so far in nerve regeneration, consisted in a commercial mixture, medical grade, of eight bacterial strains including Lactobacillaceae (L. acidophilus, L. plantarum, L. casei, and L. bulgaricus), Bifidobacteriaceae (B. breve, B. longum, and B. infantis) and Streptococcus thermophilus, referred to as VSL#3. This mixture was previously shown to exert beneficial effects on experimental models of irritable bowel syndrome, colitis and hepatic inflammation [43,44,45].
In line with the idea that a dietary approach can affect nerve regeneration by modulating systemic availability of the GM-derived products and metabolites, intermittent fasting was recently shown to enhance nerve regeneration and to sustain DRG neurite outgrowth upon sciatic nerve crush [11]. Despite the acknowledged key roles executed by Schwann cells and macrophages in the regeneration process, intermittent fasting, acting at a systemic level on cell metabolism, did not show any impact on the Schwann cell proliferation/dedifferentiation state, or on the recruitment of macrophages at the injury site [11]. Instead, this approach led to increased levels of detectable indole-3-propionate (IPA) in animal serum, paralleled by shifts in GM composition towards an enrichment in gram-positive bacteria and Clostridiaceae [11]. Indeed, oral antibiosis able to deplete gram-positive bacteria prevented the positive impact of intermittent fasting on nerve regeneration [11]. Fecal transplantation from intermittently fasting animals to control mice definitely demonstrated that the promoted nerve regeneration in intermittent fasted animals was due to the modulated GM. On the other hand, a direct impact of IPA on nerve recovery was confirmed via the oral administration of such metabolites. This, not only induced an improvement in nerve regeneration, but also a DRG-specific response in terms of the overexpression of genes involved in the recruitment of neutrophils, known to be among the first responders to injury and involved in Wallerian degeneration and axon regeneration also in the CNS [46,47]. The direct link between a higher presence of neutrophils within DRGs upon IPA treatment and axonal regeneration was evidenced by significantly impaired axonal regeneration upon neutrophil depletion [11]. If IPA treatment (or intermittent fasting) will become clinically relevant in the context of peripheral nerve injury has to be demonstrated. Some authors challenge the translational impact from rodent pre-clinical studies, because it has a long history of use as a neuroprotective reactive oxygen species scavenger in the brain in preclinical models, but was never reported to be used in patients, maybe due to differences between human and rodent PNS proteomes [48,49].
Short chain fatty acids (SCFAs) are monocarboxylic acids produced via bacterial fermentation of dietary fibers and resistant starch, and exert multiple beneficial effects on the host via multiple signaling mechanisms. Acetate, propionate and butyrate are the most abundant SCFAs (≥95%) and are present in an approximate molar ratio of 60:20:20 in the colon and stools [50]. The short-chain free fatty acid receptor FFAR is a G-protein coupled receptor (GPCR) activated by free fatty acids. In particular, FFA2 (GPR43) and FFA3 (GPR41) are activated by SCFAs. Free fatty acid receptor (FFAR) 3, but not FFAR2, has been recently shown to be expressed by Schwann cells and dorsal root ganglia [12]. Schwann cells and dorsal root ganglia exposed to oxidative stress and treated with propionate were reported to have better protection against oxidative damage by the downregulation of histone deacetylases (HDAC) 2 and upregulation of HDAC 8 expression with hyperacetylation of histone 3, resulting in the upregulation of the major antioxidative enzyme catalase; moreover, treatment with propionate also resulted in better DRGs axon outgrowth with increased expression of the growth associated protein 43 (gap-43) [12]. Taken together, these results demonstrated a neuroprotective and neuroregenerative effect of propionate in vitro.
3.3. The Influence of the GM on Motor Target Tissues of the Somatic Nervous System: The Gut–(Skeletal) Muscle Axis
The SNS is responsible for controlling voluntary skeletal muscle movements through neurotransmission at the level of the NMJ. Due to its ability to exploit insulin-mediated glucose uptake, fatty acid oxidation and breakdown of stored proteins for energy production, skeletal muscle is considered the major metabolic organ in the body. Moreover, it is highly plastic, being capable of responding to environmental stimuli, such as the training or nutritional states, by remodeling itself [51]. Interestingly, although no direct impact of the GM on somatic nerves has been reported to date, emerging scientific evidence supports the existence of a bidirectional communication between the intestinal microbiota and skeletal muscle, defined as the “gut–muscle axis”. In particular, within the past few years, an increasing number of animal studies reported a positive impact of a balanced GM on the maintenance of skeletal muscle mass and function. Compared to specific pathogen-free (SPF) mice, with usually undefined complex GM, germ-free (GF) mice and antibiotic-treated mice were found to display a reduction in muscle mass with signs of skeletal muscle atrophy [52,53,54,55]. Impaired grip strength and locomotor activity were also described in GF mice together with reduced serum levels of choline, the precursor of the neurotransmitter acetylcholine, and altered expression of NMJ-related genes, such as the ones encoding the different AChR subunits and the ones encoding rapsyn, low-density lipoprotein receptor-related protein 4 (Lrp4), and muscle-specific kinase (MuSK), key proteins involved in the formation and maintenance of NMJs [52]. Based on these findings, authors speculated that a defective nerve–muscle communication could occur under GM depletion [52]. In other studies, the ablation of the intestinal microbiota in mice via antibiotic treatment was similarly associated with reduced muscle endurance capacity and increased ex vivo muscle fatigue, but its impact on the NMJ structure and function was not investigated [53,54,56]. Interestingly, in both models, recolonization of the gut with a complex GM via either transplantation of fecal microbiota samples from specific pathogen-free animals or bacteria natural reseeding was effective in ameliorating overall muscle health, by increasing muscle mass and strength and, in some cases, also by improving endurance and fatigability [52,54]. A similar recovery was observed when GM-depleted mice were administered with a mixture of short chain fatty acids (SCFAs) or subjected to continuous acetate infusion, suggesting that GM-derived metabolites, and in particular SCFAs, could be important for promoting muscle anabolism and for regulating muscle energy expenditure [52,53].
Besides its role in the modulation of skeletal muscle mass and functioning, the GM has also been demonstrated to influence skeletal muscle adaptation to exercise training. Indeed, in a recent work, gut dysbiosis was reported to prevent exercise-induced fiber-type shift and fiber-type-specific hypertrophy in mice [57]. On the other hand, several animal and human studies supported the idea that regular and moderate physical exercise positively modulates GM composition and activity by promoting microbial diversity and richness of beneficial bacterial strains involved in protein and carbohydrate metabolism as well as in SCFA production. Vice versa, training overload and sedentary behavior have been linked to dysbiosis with a decrease in anti-inflammatory bacteria and outgrowth of harmful opportunistic pathogens [58,59,60,61]. It is now clear that different mechanisms, both direct and indirect, concur for the regulation of skeletal muscle metabolism by the GM. An intact GM needs to be balanced with a healthy diet and an active lifestyle to increase glycogen storage capacity and mitochondrial function and to reduce oxidative and inflammatory stress, to promote muscle anabolism. Conversely, a dysbiotic microbiome in conjunction with an unbalanced diet and an inadequate exercise regimen are associated with insulin resistance, loss of mitochondria function, autophagy dysregulation, metabolic endotoxemia and consequent oxidative stress and systemic inflammation. Overall, these conditions lead to muscle proteolysis and therefore to a decline in muscle mass and function (Figure 2) [62,63]. Most of these observations, however, come from studies performed on young animals or humans, whereas less is known about this bidirectional interaction during aging. Physiological age-dependent changes in the GM composition occur in elderly people, where a reduction in bacterial diversity with an overgrowth of pathogens and a concomitant drop in anti-inflammatory species and butyrate-producers have been described [64]. Of note, a high inter-individual variability in the GM composition has been reported among older adults, also depending on their healthy status. Frail low-functioning older individuals, indeed, display a more altered GM and lower muscle strength compared to high-functioning volunteers of the same age, suggesting that a dysregulated gut–muscle axis may play a key role in the development and progression of age-related physical frailty and sarcopenia [65]. The mechanisms that have been proposed to mediate such an association comprise inflammation and anabolic resistance consequent to GM alterations, which could ultimately impact muscle size and function, as well as the modulation of appetite by microbial metabolites, which could on the other hand promote malnutrition [66,67]. Strategies aiming at modulating the intestinal microbiota have begun to be explored to counteract the loss of muscle mass and function in pathological conditions, including not only sarcopenia, but also cachexia and neuromuscular diseases, as well as to improve physical performance. Healthy diets (characterized by a high content of fibers and a low content of fat) and prebiotics/probiotics have been proven beneficial for skeletal muscle maintenance by several studies. A variety of bacterial species belonging mainly to the Bifidobacterium and Lactobacillus genera were found able to increase muscle mass, strength and endurance when they have been administered, sometimes in combination with training, to either adult or aged mice. The proposed mechanisms are restoration of mitochondrial dysfunction, attenuation of inflammation and increased glucose utilization [68,69,70,71,72,73]. Interestingly, similar results were reproduced in human studies [74,75,76]. Future studies aimed at deepening the understanding of the interplay between the GM and skeletal muscle in a more systemic view, e.g., considering also the PNS, could be of interest to clarify the mechanisms underlying GM regulation of host physiology. This type of studies could pave the way for the development of novel therapeutic approaches targeting the intestinal microbiota to fight muscle wasting and PNS abnormalities in the context of many physiological and pathological conditions.
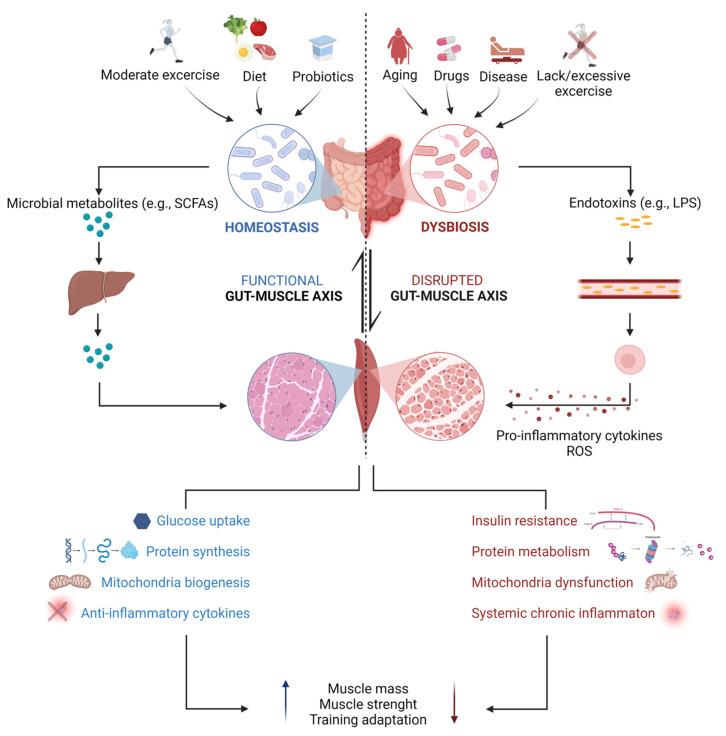
Figure 2.
Schematic diagram of gut microbiota-mediated regulation of skeletal muscle under physiological and pathological conditions. The shaping of the gut microbiota composition is driven by both genetic and environmental factors. Regular moderate physical activity, consumption of dietary fibers and supplementation with prebiotics/probiotics promote an increase in the relative abundance of potentially beneficial bacteria, involved in the production of metabolites, among which short chain fatty acids (SCFAs). Following absorption by intestinal cells, SCFAs (mostly propionate and acetate, while butyrate is mainly used as a source of energy for colonocytes) reach the liver, where most of the propionate serves as a substrate for hepatic gluconeogenesis, while part of the acetate is destined to undergo hepatic lipid synthesis. Eventually, just small amounts of SCFAs (primarily acetate) reach the systemic circulation and directly interact with skeletal muscles [77]. Thus, both direct and indirect mechanisms concur with SCFA-mediated regulation of skeletal muscle metabolism, including modulation of glucose and energy metabolism, protein balance, mitochondrial biogenesis and regulation of inflammation, overall improving skeletal muscle mass and function. On the contrary, dysbiosis, a state that can be caused by aging, insufficient/excessive physical activity, drug consumption or disease state, is associated with increased intestinal permeability and gut leaking, which facilitates the passage of endotoxins (e.g., lipopolysaccharide, LPS) and other microbial products (e.g., indoxyl sulfate) into the peripheral circulation, from where these molecules trigger the production of pro-inflammatory cytokines and reactive oxygen species (ROS) by macrophages and promote insulin resistance, muscle proteolysis, and mitochondrial dysfunction in skeletal muscles. This results in skeletal muscle changes that may manifest as decreases in muscle mass and function, ultimately affecting muscle physical performance. Created with Biorender.com.
4. Gut Microbiota and the Autonomic Nervous System
4.1. Link between the Gut Microbiota and the Sympathetic and Parasympathetic Nervous System
Is the regenerative process of the ANS also influenced by gut microbiota? Do injuries of the ANS affect the GM? Both these questions are still open since, to date, few publications are available regarding the correlation between the GM and ANS in its damaged/regeneration condition.
Some researchers use an injury model of autonomic nerves for demonstrating how their lesions influence GM composition. For example, recently, Zhang et al. [78] demonstrated that a bilateral superior cervical ganglionectomy significantly caused abnormal GM composition, implicating a role for the sympathetic pathway in regulating the GM. Moreover, they demonstrated that this GM may participate in several biological pathways suggesting the important role of the cervical sympathetic ganglion in regulating the GM–brain axis, and further confirming that the sympathetic nervous system regulates the same.
Most of the articles use the denervation of the ANS for demonstrating the correlation between the GM and the brain. Liu et al. [21] emphasize the role of the vagus nerve in the GM to brain signaling as an integral component of a bi-directional neuroimmunoendocrine pathway. This way the vagus nerve does not act simply as a direct-line between the gut and brain; instead, the vagal signaling is required for an immune response that is a necessary and sufficient requirement for the effects of the bacteria on behavior.
Surgical vagotomy has been used to investigate the physiological role of the vagus nerve in the gut–brain signaling and in certain diseases, including neurodegenerative diseases [79,80], depression-like behaviors [81] and others.
For example, two different register-based matched-cohort studies of vagotomized patients showed a reduced risk for subsequent Parkinson Disease among patients treated with truncal vagotomy [82,83], suggesting a critical role of the vagus nerve in the pathogenesis of the disease. Intriguingly, when the rat gut was injected with human brain lysate from patients with Parkinson Disease or with recombinant and pathological α-synuclein, α-synuclein has been demonstrated to be retrogradely transported into the brain via the vagus nerve, and this process was avoided via vagotomy [84,85].
Subdiaphragmatic vagotomy has also been shown to ameliorate demyelination and microglial activation in cuprizone-treated mice (an animal model for multiple sclerosis disease) compared to non-vagotomized animals [86]. It was also shown to block depression-like behaviors in Chrna7 knock-out mice (the gene coding for the α7 subtype of the nicotinic acetylcholine receptor), an animal model that demonstrates depression-like behaviors through abnormal composition of the GM and systemic inflammation [81], demonstrating the central role of the vagus nerve in the gut–microbiota–brain axis.
The indirect effect of ANS injury is also demonstrated by Guo et al. [87]. In fact, they demonstrated the beneficial effect of renal denervation on cardiac function and that this effect may be mediated by influencing intestinal bacteria, since it improves intestinal barrier function and ameliorates intestinal dysbiosis. On the other side, gut–microbiome aberrations and intestinal dysfunction are potential contributors to the development of heart failure [87].
Moreover, the renal denervation exerts beneficial effects on blood pressure control and perivascular fibrosis in rats exposed to chronic intermittent hypoxia, as demonstrated by Lu et al. [88]. Finally, Ma et al. [89], following a lesion in the splenic nerve, demonstrated that the GM might regulate microglial function in the brain via the gut–microbiota–brain axis.
4.2. Interactions between the Gut Microbiota and the Enteric Nervous System
In recent years, the interaction between the GM and the ENS has been extensively studied in both physiological and pathological conditions, as demonstrated by several recently published reviews that exhaustively describe this communication [13,18,90,91,92,93,94,95,96,97,98]. In this review, we will therefore provide only an overview of the impact of GM on the ENS structure and function, rather than describing in detail the complexity of the system.
Abnormalities in the adult ENS morphology in GF animals have been demonstrated several years ago by the observation that the myenteric neurons of GF rats were hypertrophic compared to the controls [99]. Moreover, electrophysiological recordings showed decreased neuronal excitability in myenteric neurons of GF animals [100] with associated deficits in gut motility [101]. Alteration in terms of the loss of enteric neurons were also observed in systemically antibiotic (Abx)-treated mice [19]. GF mice display structural abnormalities of the ENS already during postnatal development (postnatal day 3) characterized by a decrease in nerve density and in the number of neurons per ganglion, and an increase in the proportion of myenteric nitrergic neurons with consequent functional alterations in terms of the frequency of amplitude of muscle contractions [102]. Going further back in the developmental stages of the ENS in utero, it has been demonstrated that exposing a pregnant sheep to Ureaplasma parvum infection results in the loss of enteric neurons in the ovine fetal ENS [103]. Interestingly, microbiota has also been demonstrated to influence enteric glial cell development [19,98,103]. GF mice showed lower numbers and density of enteric glial cells in the mucosa compared to control mice, but after recolonization with a normal GM, the number of enteric glial cells was restored [98]. Taken together, all these studies demonstrate that a healthy, or well balanced, GM is essential for the proper structural and functional development of both neurons and glial cells of the ENS.
While the ENS is formed during fetal life (from embryonic day 8 to postnatal day 14 in mice and from week 4 in human embryos to the postnatal period) [104], plasticity persists in the postnatal period during which the GIT is colonized by bacteria. GF mice retain a higher degree of plasticity in the ENS with a higher proportion of the cells expressing nestin, a neuronal stem cell marker. Furthermore, the colonization of GF mice with conventional, complex GM induced the proliferation of enteric neurons with increased intestinal transit rates, through the release of serotonin (5-HT) and activation of 5-HT4 receptors [105]. Enteric neurogenesis has also been observed after microbiota recolonization in Abx-treated animals, with an increase in the number of enteric glia and neurons [19].
Several different mechanisms have been proposed to explain how the GM influences ENS functions. Different metabolites, by-products and molecules are secreted, modified, or stimulated by GM; among these, there are lipopolysaccharide (LPS) and Lipoteichoic Acid (LTA), tryptophan metabolites, serotonin, gamma-aminobutyric acid (GABA), bile acids, short chain fatty acids (SCFAs), cocaine-amphetamine regulated transcript (CART), substance P and calcitonin gene-regulated peptide (CGRP) [13].
Recent expression analysis indicated that enteric neurons and glia express receptors that can sense these metabolites, suggesting that they have the potential to respond directly to stimuli derived from the GM. For example, Toll-like receptors (TLRs) -3 and -7, which recognize viral RNA, and TLR4, which recognizes LPS (a membrane component of Gram-negative bacteria), are expressed in the myenteric and submucous plexuses (by both neurons and glial cells) as well as in the DRG neurons of the somatic PNS [106]. Mice lacking TLR4 displayed significant delay in gastrointestinal motility and reduced numbers of nitrergic ENS neurons, like GF mice. Furthermore, LPS activation of TLR4 in enteric neurons in vitro led to the activation of the transcription factor nuclear factor (NF)-κB and increased cell survival, suggesting that interactions between enteric neurons and microbes increases neuron survival and gastrointestinal motility in mice [107]. Moreover, TLR2, the receptor of LTA, is expressed by enteric neurons and it has been shown that the inhibition of TLR2 signaling suppresses neurogenesis whereas TLR2 agonists promote neurogenesis and restore myenteric neurons upon microbiota depletion [108].
Indole, an aromatic heterocyclic product of microbiota tryptophan metabolism, binds the aryl hydrocarbon receptor (AHR), a nuclear receptor controlling gene expression. Recently, AHR signaling in enteric neurons has been shown to be a key player in the maintenance of gut homeostasis. Mice lacking AHR, specifically in enteric neurons, showed reduced intestinal transit time, like GM-depleted mice. Intestinal motility is restored once AHR is expressed in enteric neurons of Abx-treated mice [109].
The most extensively studied gut microbe-derived metabolites in the ENS are SCFAs. FFA3 has been shown to be expressed by enteric neurons in both submucosal and myenteric ganglia [110,111]. A wide range of studies demonstrated the involvement of SCFAs as modulators of multiple colonic function, as well as of inflammatory and metabolic processes. SCFAs might be also involved in maintaining ENS homeostasis.
Butyrate, but not propionate or acetate, has been shown to significantly increase the proportion of cholinergic, but not nitrergic myenteric neurons both in vivo and in primary culture of ENS, in association with the ex vivo increase of colonic motility and contractile response; this neurochemical plasticity was reported to be mediated by Type 2 monocarboxylate transporter (MCT) and involves the Src kinase signaling pathway and the acetylation of histone H3 lysine 9 (H3K9) in enteric neurons [112]. Butyrate has also been shown to enhance both the cholinergic and nitrergic phenotypes of myenteric neurons and neuromuscular transmission postnatally [113]. Butyrate treatment also affects the postnatal development of enteric glial cells, since it has been reported to inhibit the proliferation of EGC in vitro and in vivo, without induction of cell death [114]. Propionate, on the other hand, has been shown to reduce the motility of the guinea pig colon [115].
A recent study showed that SCFA supplementation in Abx-treated mice rescues neuronal loss induced by Abx-treatment through enhancing neuronal survival and promoting enteric neurogenesis, but no effect on glial cells and only a minor effect on gastrointestinal function is reported [19].
The GM has been reported to also influence the extrinsic neurons of the gut [116,117]. Microbiota depletion resulted in elevated levels of gut-extrinsic sympathetic activity, as shown by the increased expression of the neuronal transcription factor c-Fos in the sympathetic coeliac-superior mesenteric ganglia. FMT from specific pathogen-free mice to GF mice restored the expression of c-Fos in the intestinal sympathetic ganglia, suggesting that microbiota can suppress the gut-extrinsic sympathetic neurons [116].
5. Microbiota in Pathological States of the PNS
Based on numerous observational findings collected in the last past decades in both pre-clinical and clinical settings, it is nowadays clear that an altered and less diverse GM is associated with a variety of pathological conditions. Interestingly, some recent studies highlighted how GM-related modulation of host physiology, especially through immunological and hormonal regulation, requires an interaction with the PNS. Moreover, both sympathetic and parasympathetic neurons are key regulators of gut functions and, consequently, of GM diversity and activity [118]. Given this close communication, it is easy to speculate that harmful alterations of this fascinating microbial community could lead to functional changes of the PNS, and vice versa, even in the context of non-peripheral pathologies.
5.1. Microbiota and Neuropathic Pain
Neuropathic pain is a sequela arising from micro- and macroscopic nerve lesions and diseases affecting the SNS and limiting patients’ quality of life. A large number of in vivo models has been established to investigate the molecular mechanisms underlying the development and chronification of neuropathic pain [14]. Studies performed in these models led to the hypothesis that alterations in GM may affect the development of neuropathic pain [119,120,121].
5.1.1. Chemotherapy-Induced Neuropathic Pain
The GM was previously found to be a key player in the tumor growth-inhibiting effect promoted by different chemotherapeutic drugs in mice [122]. However, Shen et al. recently showed that it also mediates chemotherapy-induced neuropathic pain, an important dose-limiting side effect developed by more than 30% of patients undergoing continuous usage of chemotherapeutics, such as paclitaxel and oxaliplatin. Indeed, they found that the absence of a complete GM in both ABX-treated and GF-mice prevented oxaliplatin-induced mechanical hyperalgesia, a major symptomatic manifestation of neuropathic pain, and that this effect was abrogated when GM was restored with FMT [120]. The relevance of these pre-clinical findings is supported by a clinical study that revealed a change in the composition of the GM of patients who received oxaliplatin, compared to the one registered prior to chemotherapy [123]. Interestingly, several recent publications have demonstrated that microbiome composition is affected by host genetics in humans and that consequent variations in GM composition may impact the development of pathologies other than neuropathic pain [124,125]. In accordance with this finding, in their animal study, Ramakrishna et al. additionally observed different susceptibility to chemotherapy-induced peripheral neuropathies of different mouse strains, emphasizing the impact of the genome on the GM and thus on its associated pathologies [126].
Further data obtained from animal models suggest that the modulation of inflammation is involved in the protective effect of GM ablation on the development of neuropathic pain after chemotherapy. Shen et al. demonstrated that the DRG inflammatory response to the chemotherapeutic agent was dampened in the absence of a complete GM via the LPS-TLR4 pathway. In fact, following oxaliplatin treatment, the DRG from ABX-treated mice were found to display a decreased presence of infiltrated macrophages and inflammatory cytokines (IL-6 and TNF-α), compared to those of water-fed mice. The restoration of the inflammatory response upon exogenous LPS administration and the evidence that the specific ablation of TLR4 on hematopoietic cells was sufficient to phenocopy GM eradication support the role of GM-released LPS in promoting and intensifying the pro-inflammatory response of macrophages against oxaliplatin, while simultaneously leading to a hypersensitization of peripheral nociceptors [120]. The involvement of the LPS–TLR4 pathway in the insurgence of chemotherapy-induced pain is further confirmed in a study by Wardill et al. In their work, the authors show that irinotecan treatment in mice induced an increase in the permeability of the intestinal barrier, allowing LPS to be released from the GM and triggering neuropathic pain associated with spinal cord astrogliosis, both attenuated in treated TLR4−/− mice [127]. Finally, another work proved that the administration of the probiotic formulation DSF (DeSimone formulation) to an in vitro model of DRG neurons was able to attenuate paclitaxel-induced neurotoxicity through the modulation of the inflammatory response [128], providing in this way, a rationale for the adoption of microbiota-targeting therapeutic strategies in the treatment of chemotherapy-induced peripheral neuropathy-associated neuropathic pain.
5.1.2. Diabetic Neuropathic Pain
Diseases of metabolic origin, such as obesity and type II diabetes, one of the most frequent comorbidities associated with obesity, are often accompanied by both autonomic and somatic alterations of the PNS as a result of dysregulated metabolic pathways, leading to the development of a painful peripheral neuropathy [129]. In this context, dysbiosis seems to play a pivotal role as a risk factor. Microbiome signatures from patients with diabetic neuropathic pain were recently found to differ from the ones of both healthy controls and patients with diabetes without diabetic neuropathic pain [130]. Interestingly, the observed enrichment in specific phyla and genera of bacteria, such as Megasphaera and Parabacteroidetes, positively correlated with insulin resistance, inflammation and dyslipidemia [130]. Of note, microbiota-modulating therapies, such as supplementation with probiotics or FMT, are effective in ameliorating insulin resistance [131,132]. A recent case report described remission from a painful neuropathy in an obese type II diabetic woman after FMT from a healthy donor [133]. In addition, FMT from lean to western diet-fed obese, insulin-resistant neuropathic mice was shown to prevent mechanical allodynia and thermal hyperalgesia and to decrease nerve fibers loss [134]. Pain relief was mainly achieved through FMT-mediated reduction of DRG neuronal hyperexcitability consequent to reduced Ryanodine receptor 2-dependent Ca2+ release from the endoplasmic reticulum. Furthermore, FMT was found to modulate PNS immune cells by promoting a shift in the polarization of DRG macrophages towards an anti-inflammatory phenotype and to increase circulating butyrate levels [134]. Even though such evidence points to microbiota-modulating interventions as promising tools to prevent and to alleviate peripheral neuropathies associated with obesity and type II diabetes, further studies will be necessary to elucidate the molecular mechanisms linking GM to pain and to discriminate between beneficial and detrimental bacterial strains.
5.1.3. Trauma-Induced Neuropathic Pain
Injuries of the PNS are another major cause of neuropathic pain. Two independent groups of researchers draw a link between chronic constriction injury (CCI)-induced neuropathic pain and GM in rodents. Chen et al. reported an altered composition of intestinal microbiota in animals with CCI-induced neuropathic pain compared to sham controls [121]. This indicates that the condition of CCI-induced neuropathic pain somehow influences GM structure. An impact in the opposite direction, however, can also be postulated. Ding et al. demonstrated that changes in GM composition induced by ABX pre-treatment influenced the parameter values of CCI-induced neuropathic pain [135]. Indeed, following antibiotic treatment, animals presented with damped mechanical allodynia and thermal hyperalgesia. The translatability of these findings into human clinics, however, might prove difficult, because a large patient study, in contradiction to the pre-clinical observation of Ding et al. [135], demonstrated an increased incidence in neuropathic pain after antibiosis [136].
The mouse studies reported above [121,135] unfortunately do not provide information about pain management potentially applied to the operated animals and do not investigate whether the pain medication or the antibiotics might have directly influenced the observed development of neuropathic pain. This specific question was addressed in a study by Ma et al. demonstrating that FMT from healthy to neuropathic mice can reverse the amelioration of neuropathic pain arising from nerve injury, chemotherapy, and diabetes caused by antibiotic depletion of GM [137]. These observations are in accordance with the fact that in vivo treatment in mice with the phytosteroid diosgenin reversed oxaliplatin-induced peripheral neuropathy parameter values (e.g., mechanical withdrawal threshold and cold hyperalgesia). Again, treatment of the animals with FMT indicated that the pain ameliorating effect of diosgenin is GM-dependent [138].
To our knowledge, most research approaches investigating the influence of the microbiome on the development of neuropathic pain are based on the comparison of different mouse strains or of ABX-induced microbiome depletion models. Consequently, it has first to be considered that different genetic backgrounds might influence pain responsiveness per se and that therefore sensitivity to pain does not depend only on GM composition. Secondly, ABX may not alter the intestinal microbiota of different individual animals in the same way. Pane et al. questioned, in their systematic review, whether there is a correlation or a cause–effect relationship between the intestinal microbiome and neuropathic pain states [139].
For excluding confounders, future research should rather compare neuropathic pain development and related parameter values in long-term stable GF or gnotobiotic animals with that in animals from the same genetic background, but carrying a complex GM. The advantage could be obtained from the use of well-established and highly standardized models, such the axenic GF mice used by Shen et al. [120] or the oligo-mouse-microbiota (OMM12) model [140], containing only 12 defined bacterial strains. In our opinion, the comparison of standardized models of GM depletion would allow to increase the reproducibility of data and possibly to better elucidate whether there is a verifiable causality between GM composition and the prevalence in the development of neuropathic pain caused by different inducing conditions.
5.2. Microbiota and Peripheral Implications in Autism Spectrum Disorder
Autism Spectrum Disorder is a heterogeneous and multifactorial neurodevelopmental disability that, in addition to core clinical manifestations (difficulties in social communication and interaction and repetitive behaviors), is often defined by the presence of GIT-related symptoms, whose severity positively correlates with the one of the pathology itself [141]. Many studies have documented that, compared to neurotypical people, autistic patients display gut dysbiosis with an enrichment of the Clostridium genus and an imbalance in the Firmicutes/Bacteroidetes ratio [142]. Another common pathogenic event in Autism Spectrum Disorder is ANS dysfunction, characterized by a hypoactivation of the parasympathetic branch with a concomitant hyperactivation also of the sympathetic branch [143]. Interestingly, in recent years, such autonomic imbalance has been hypothesized to be responsible for Autism Spectrum Disorder-associated dysbiosis through deregulation of the gut–brain axis at multiple levels [144]. First, parasympathetic stimulation is a known modulator of intestinal innate immunity, and deficiencies in its inputs may weaken the production of anti-microbial peptides from cells of the colonic mucosa, facilitating bacterial translocation and promoting both metabolic and oxidative stress. Second, ANS dysfunction could at the same time attenuate the enteric inflammatory response, thereby sustaining the already established dysbiosis state. Finally, functional alterations of ANS, which is responsible, together with ENS, for regulating water and electrolyte balance, could cause a perturbation of the intestinal osmotic equilibrium, stabilizing the GM in its altered form [144]. The instauration of a vicious cycle in which ANS dysfunction and gut dysbiosis mutually sustain each other could explain why therapeutic approaches targeting the microbiota have been applied so far to Autism Spectrum Disorder with only conflicting and transient results.
5.3. Gut Microbiota and the Sympathetic Nervous System in Hypertension
Gut dysbiosis has also been linked to hypertension [4,145,146,147]. Chronically enhanced sympathetic nervous system activity accompanied by a high release of noradrenaline and dampened parasympathetic activity are hallmarks of hypertension, thus suggesting a neurogenic component that contributes to the development of this disease [148]. Spontaneously hypertensive rats, as well as hypertensive patients, showed a significant decrease in microbial richness, diversity, and evenness, in addition to an increased Firmicutes/Bacteroidetes ratio (F/B ratio), which is a signature of gut dysbiosis [146]. On the other hand, under normotensive conditions, the reduction or the absence of GM (obtained with antibiotic treatment or with GF mice, respectively) does not significantly affect blood pressure [149,150].
A study in which reciprocal FMT from normotensive rats to spontaneously hypertensive rats showed that blood pressure was reduced in the hypertensive rats transplanted with feces from the normotensive rats and vice versa. Moreover, plasma noradrenaline levels were reduced in the first case and increased in the latter one, showing an impact of GM on the sympathetic nervous system activity. FMT from normotensive to hypertensive rats also induced a reduction of the inflammation in the hypothalamic paraventricular nucleus, a brain area for cardiovascular control, which also showed altered expression of receptors for SCFAs, suggesting a possible connection between GM metabolites and blood pressure control [151]. Indeed, spontaneously hypertensive rats were characterized by reduced acetate and butyrate-producing bacteria [146]. SCFAs have been reported to have anti-hypertensive properties, since their administration lowers blood pressure, and this effect is supposed to be mediated, at least in part, by G protein–coupled receptors, such as olfactory receptor 78 and G protein–coupled receptor 41 (GPR41) [152,153,154].
Moreover, intraperitoneal injection of acetate (the most abundant SCFAs in the plasma) results in a decrease of blood pressure and heart rate, and this last effect was blocked after treatment with a selective β-1 adrenergic receptor antagonist, demonstrating a role of sympathetic tone in the acetate-mediated response [155]. Authors hypothesized that SCFAs act upstream of the sympathetic nerve terminal to block the release of norepinephrine to β-1 receptors in the heart, inhibiting the canonical sympathetic signaling pathway that acts through G proteins. This effect is attenuated by the treatment with sympathomimetics. They also hypothesize that acetate acts directly on the heart in a different cell type by binding to a currently unknown G protein–coupled receptor [155].
Interestingly, the elevated splanchnic sympathetic nerve activity and mild gut pathology in juvenile prehypertensive rodents precede hypertension-related gut dysbiosis, suggesting that elevated gut sympathetic nerve activity modulates the gastrointestinal environment before the development of hypertension [156].
Dysfunctional autonomic nervous system in gut dysbiosis seems therefore to contribute to the development and maintenance of hypertension [4]. However, the cellular and molecular mechanisms involved in the gut–brain interconnection to control blood pressure are not yet fully addressed. Two main hypotheses have been proposed.
According to the first hypothesis, increased activity of the sympathetic nervous system in the gut in pre-hypertensive set-ups contributes to epithelial dysfunction and gut leakage, leading to intestinal dysbiosis. This results in an imbalance in the production of bacterial metabolites, including SCFAs, that is accompanied by an increased production and secretion of gastrointestinal hormones, such as 5HT, by the enterochromaffin cells as a consequence of an overstimulation of the adrenergic signaling. Local 5HT thus decreases the parasympathetic activity of intestinal vagal afferents via 5HT3 receptors (5HT3R), while 5HT released into circulation may affect vasculature and cause vasoconstriction. Furthermore, circulating 5HT can negatively modulate the transmission of feedback from the gut to the cardioregulatory regions of the brain. In the end, the resulting concomitant dysfunction of both the two branches of the ANS would, if not cause a hypertensive phenotype, at least promote its persistence in time [147].
Recently, a second mechanism has been proposed that involves miR-204, a microRNA that plays an important role in CNS function, and that is known to be affected by gut dysbiosis. According to the hypothesized mechanism, gut dysbiosis decreases miR-204 levels in the hypothalamus, which, in turns, increases brain derived neurotrophic factor (BDNF) and glutamate ionotropic receptor NMDA type subunit 2B (Grin2B) and consequently, sympathetic activity and noradrenaline release. Noradrenaline, by acting on ß1 receptors on the sinoatrial node and on α1 receptors on the vasculature, produces cardiac and vessel contraction, respectively. In parallel, parasympathetic nerve activity is decreased, leading to lower levels of acetylcholine and a reduction in cardiac contraction. Deregulation of both sympathetic and parasympathetic nerve activity, and the consequent deregulation of the levels of noradrenaline and acetylcholine, will induce abnormalities in the heart and vessels, ultimately resulting in hypertension [157].
5.4. Gut Microbiota and Autoimmune Disorders: A Focus on Myasthenia Gravis
Over the years, expanding scientific evidence pointed at disturbances of GM as a major risk factor for the development of both central and peripheral autoimmune diseases, among which multiple sclerosis [158,159] and rheumatoid arthritis [160]. In the wake of these findings, attention has recently been focused on the role of microbiota aberrancies in the pathogenesis of myasthenia gravis (MG), an acquired autoimmune disease of the NMJ characterized by impaired neuromuscular transmission that clinically manifests as focal or generalized muscle weakness and fatigability [161]. Gut dysbiosis and altered metabolite profiles have been described in patients with MG in several studies. Qiu et al. were the first to provide evidence that MG is accompanied by a dysbiotic GM, as expressed by a reduced overall phylogenetic diversity and altered structural composition. Specifically, at the phylum level, a depletion in Firmicutes and Actinobacteria with a concomitant enrichment in Bacteroidetes and Proteobacteria was described in MG subjects compared to healthy controls [162,163,164]. The consequent drop in the Firmicutes/Bacteroidetes ratio is consistent with what has been reported for other autoimmune diseases, such as irritable bowel syndrome and Crohn’s disease [165,166]. It is also reflective of a pro-inflammatory environment in which the altered GM weakens intestinal epithelial integrity, initiating an immune response that exacerbates the immunological imbalance already underlying the autoimmune disorder [162]. Consistently with these findings, an altered fecal metabolome with a decrease in the overall content of SCFAs, and in particular of propionate and butyrate, was observed in individuals with MG [162]. Since most of the differential metabolites detected in MG patients have a role in amino acid, microbial and nucleotide metabolism, it was suggested that the GM may contribute to the development of MG through the modulation of metabolic pathways and oxidative stress [164]. Interestingly, Zheng et al. managed to identify several altered microbial features that correlated with disease severity and were able to discriminate MG subjects from healthy controls with a 100% accuracy based solely on a combination of microbial and metabolic markers. Using a similar approach, Tan et al. were capable of distinguishing between two clinical subtypes of MG, ocular and generalized myasthenia gravis. Overall, these studies demonstrated the existence of a dysregulated GM composition and activity in patients with MG and proved the feasibility and the potential of fecal GM and metabolomic analysis as a novel non-invasive diagnostic tool for such pathology.
The association between GM disturbances and the development of MG has been confirmed also in preclinical settings. In fact, GF-mice, transplanted with the microbiota of MG patients and subsequently immunized to induce MG, exhibited impaired motor activity, upregulation of inflammatory cytokines and dysregulated fecal metabolic pathways compared to mice transplanted with either a healthy GM or a combination of the two [164]. However, how these two events are correlated is still under investigation. Recently, it was hypothesized that alterations in the composition of GM could impinge on one of the main pathogenic events in MG, the presence of an immune disequilibrium in the Th17/Treg (T helper 17/T regulatory cells) axis. Indeed, contrary to Th17 cells, whose levels are dramatically increased in MG patients, where they stimulate B cells to activate and to produce pathogenic autoantibodies [167,168], Treg cells are significantly depleted in the blood of MG patients, contributing to the exacerbation of autoreactive immune responses [168]. Curiously, CD4 + CD25 + Foxp3 + Treg are highly abundant in the colonic lamina propria, where they affect and can be affected by GM [169]. It has been proposed that a reduction in the proportion of Clostridium strains and, consequently, in the production of SCFAs, could profoundly impact the T cell development, favoring the conversion of naïve CD4+ T cells towards Th17 cells rather than Treg cells [162,170]. Increased proportions of Streptococcus are likely to similarly restrain CD4 + CD25 + Foxp3 + Treg differentiation through the inhibition of the activity of peroxisome proliferator-activated receptor γ (PPARγ) [162,170]. Moreover, increased levels of Desulfovibrio were suggested to contribute to MG pathogenesis by reducing butyrate levels and therefore by promoting an inflammation state [171]. Improving the current understanding of the types of microbial species and metabolites and of the mechanisms that underlie MG pathogenesis could help in the identification of novel potential therapeutic targets. Meanwhile, the validity of the rationale of using microbiota-targeting interventions to restore immune homeostasis through correction of the Th17/Treg imbalance has been proven in a number of autoimmune diseases, including not only MG [172,173,174], but also irritable bowel syndrome [175] and autoimmune encephalomyelitis [176]. Nonetheless, to better apply these findings in clinical practice, further studies are needed to determine the types and the dosages of probiotics that could provide benefits to MG patients as well as the appropriate frequency and duration of the FMT approach.
5.5. Gut Microbiota and Myopathies
Apart from their primary effect on skeletal muscle structure and function, genetic myopathies are generally linked to alterations in muscle and lipid metabolism as well as to endocrine disturbances and chronic systemic low-grade inflammation. Even though myopathic patients often suffer from gastrointestinal dysfunction and altered gastrointestinal motility, a causal link between a dysregulated gut–muscle axis and the pathophysiology of musculoskeletal disorders has not been proven yet. Interestingly, two recent studies demonstrated for the first time a role for GM in shaping progression and severity of Duchenne muscular dystrophy (DMD), the most frequent and severe form of human myopathy. Indeed, both studies independently showed that mdx mice, the main DMD mouse model, are characterized by an altered GM composition with increased Prevotellaceae and significantly different metabolic profiles compared to age-matched wild type animals [177,178]. The enrichment of Prevotella in mdx mice was strongly correlated with aberrant immunity activation, gut inflammation and damaged muscular integrity [177]. Of note, microbiota depletion in mdx mice reduced chronic muscle inflammation and fibrosis deposition, but simultaneously aggravated the deregulation of glucose and lipid metabolism, enhancing fatty acid oxidation and promoting a shift in fiber-type toward a more oxidative phenotype. On the other hand, FMT with an eubiotic microbiota reduced muscle immunity and ameliorated muscle pathological features of mdx mice, including reduced myofiber size, increased fibrotic infiltrate and impaired muscle force [177]. Similarly, oral supplementation of sodium butyrate in late-stage dystrophic mdx mice restored muscle locomotion and strength to the same extent as deflazacort, a synthetic glucocorticoid widely employed in the standard care of DMD [178]. Kalkan et al. further demonstrated that a dysregulated GM participates in DMD pathology by exacerbating inflammation and autophagy impairment through the promotion of endocannabinoid system overactivity. Consistent with this finding, sodium butyrate exerted anti-inflammatory and pro-autophagic effects and reduced endocannabinoid system overactivity in mdx mice as well as in LPS-stimulated C2C12 myotubes and even in primary human myoblasts from DMD patients [178]. Even though still confined to preclinical settings, the results presented in these two studies open up the possibility to use dietary and microbiota modulating interventions to ameliorate or reverse symptoms in dystrophic patients and to slow down the progression of neuromuscular diseases.
6. Conclusions
In this review, we aimed at gathering and presenting a concise overview of the currently available knowledge of the impact of GM composition on the different components of the PNS in physiological, regenerative, and pathological conditions.
From this, it is obvious that the way to intensify the research in this field is open, given that promising potential for the modulation of the GM in the treatment of PNS pathologies still needs to be fully addressed. This opens the field to further experimental and clinical research, eventually leading to translational applications.
A main limitation from a translational point of view is, however, represented by the low similarity between the different experimental models and the human being. There is a need for first securely proving causality and second defining the exact identity and optimal balance of microbe phyla or species that will be decisive for limiting specific pathologies. This is also envisioned by a new approach for specific depletion of well selected bacterial species in the human GM [7]. The strict use of highly standardized GF-models or gnotobiotic models appears more promising in this than the “simple” depletion and modification of the GM by systemic antibiosis [179].
Author Contributions
Conceptualization, M.C., K.H.-T. and G.R.; writing—original draft preparation, S.C., S.K., S.R. and G.R.; writing—review and editing, M.C., K.H.-T., G.G. and G.R. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ahlawat S., Asha, Sharma K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021;72:636–668. doi: 10.1111/lam.13333. [DOI] [PubMed] [Google Scholar]
- 2.Amon P., Sanderson I. What is the microbiome? Arch. Dis. Child. Educ. Pract. Ed. 2017;102:257–260. doi: 10.1136/archdischild-2016-311643. [DOI] [PubMed] [Google Scholar]
- 3.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robles-Vera I., Toral M., Duarte J. Microbiota and Hypertension: Role of the Sympathetic Nervous System and the Immune System. Am. J. Hypertens. 2020;33:890–901. doi: 10.1093/ajh/hpaa103. [DOI] [PubMed] [Google Scholar]
- 5.Foster J.A., McVey Neufeld K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcos-Fernandez R., Ruiz L., Blanco-Miguez A., Margolles A., Sanchez B. Precision modification of the human gut microbiota targeting surface-associated proteins. Sci. Rep. 2021;11:1270. doi: 10.1038/s41598-020-80187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou K., Wu Z.X., Chen X.Y., Wang J.Q., Zhang D., Xiao C., Zhu D., Koya J.B., Wei L., Li J., et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022;7:135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Wu M., He J., Xiao C., Xue Y., Fu T., Lin C., Dong D., Li Z. Antibiotic-Induced Dysbiosis of Gut Microbiota Impairs Corneal Nerve Regeneration by Affecting CCR2-Negative Macrophage Distribution. Am. J. Pathol. 2018;188:2786–2799. doi: 10.1016/j.ajpath.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodenhouse A., Talukder M.A.H., Lee J.I., Govindappa P.K., O’Brien M., Manto K.M., Lloyd K., Wandling G.D., Wright J.R., Chen See J.R., et al. Altered gut microbiota composition with antibiotic treatment impairs functional recovery after traumatic peripheral nerve crush injury in mice: Effects of probiotics with butyrate producing bacteria. BMC Res. Notes. 2022;15:80. doi: 10.1186/s13104-022-05967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serger E., Luengo-Gutierrez L., Chadwick J.S., Kong G., Zhou L., Crawford G., Danzi M.C., Myridakis A., Brandis A., Bello A.T., et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature. 2022;607:585–592. doi: 10.1038/s41586-022-04884-x. [DOI] [PubMed] [Google Scholar]
- 12.Gruter T., Mohamad N., Rilke N., Blusch A., Sgodzai M., Demir S., Pedreiturria X., Lemhoefer K., Gisevius B., Haghikia A., et al. Propionate exerts neuroprotective and neuroregenerative effects in the peripheral nervous system. Proc. Natl. Acad. Sci. USA. 2023;120:e2216941120. doi: 10.1073/pnas.2216941120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook T.M., Mansuy-Aubert V. Communication between the gut microbiota and peripheral nervous system in health and chronic disease. Gut Microbes. 2022;14:2068365. doi: 10.1080/19490976.2022.2068365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kankowski S., Grothe C., Haastert-Talini K. Neuropathic pain: Spotlighting anatomy, experimental models, mechanisms, and therapeutic aspects. Eur. J. Neurosci. 2021;54:4475–4496. doi: 10.1111/ejn.15266. [DOI] [PubMed] [Google Scholar]
- 15.Iyer S.R., Shah S.B., Lovering R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021;22:8058. doi: 10.3390/ijms22158058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karemaker J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017;38:R89–R118. doi: 10.1088/1361-6579/aa6782. [DOI] [PubMed] [Google Scholar]
- 17.Fleming M.A., 2nd, Ehsan L., Moore S.R., Levin D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020;2020:8024171. doi: 10.1155/2020/8024171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng Z.H., Zhu Y., Li Q.L., Zhao C., Zhou P.H. Enteric Nervous System: The Bridge between the Gut Microbiota and Neurological Disorders. Front. Aging Neurosci. 2022;14:810483. doi: 10.3389/fnagi.2022.810483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicentini F.A., Keenan C.M., Wallace L.E., Woods C., Cavin J.B., Flockton A.R., Macklin W.B., Belkind-Gerson J., Hirota S.A., Sharkey K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9:210. doi: 10.1186/s40168-021-01165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Forsythe P. Vagotomy and insights into the microbiota-gut-brain axis. Neurosci. Res. 2021;168:20–27. doi: 10.1016/j.neures.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Bonaz B., Bazin T., Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shavandi A., Saeedi P., Gerard P., Jalalvandi E., Cannella D., Bekhit A.E. The role of microbiota in tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2020;14:539–555. doi: 10.1002/term.3009. [DOI] [PubMed] [Google Scholar]
- 24.Liu H.X., Keane R., Sheng L., Wan Y.J. Implications of microbiota and bile acid in liver injury and regeneration. J. Hepatol. 2015;63:1502–1510. doi: 10.1016/j.jhep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi J., Xie C., Zeng L., Zhu Z., Chen N., He Q., Xu X., Xie H., Zhou J., Li L., et al. Bacillus subtilis WB800N alleviates diabetic wounds in mice by regulating gut microbiota homeostasis and TLR2. J. Appl. Microbiol. 2022;133:436–447. doi: 10.1111/jam.15547. [DOI] [PubMed] [Google Scholar]
- 26.Wallimann A., Magrath W., Pugliese B., Stocker N., Westermann P., Heider A., Gehweiler D., Zeiter S., Claesson M.J., Richards R.G., et al. Butyrate Inhibits Osteoclast Activity In Vitro and Regulates Systemic Inflammation and Bone Healing in a Murine Osteotomy Model Compared to Antibiotic-Treated Mice. Mediat. Inflamm. 2021;2021:8817421. doi: 10.1155/2021/8817421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hang C.H., Shi J.X., Li J.S., Wu W., Yin H.X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J. Gastroenterol. 2003;9:2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kigerl K.A., Hall J.C., Wang L., Mo X., Yu Z., Popovich P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 2016;213:2603–2620. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W., Yuan Q., Li Z., Du Z., Wu G., Yu J., Hu J. Translocation and Dissemination of Gut Bacteria after Severe Traumatic Brain Injury. Microorganisms. 2022;10:2082. doi: 10.3390/microorganisms10102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celorrio M., Abellanas M.A., Rhodes J., Goodwin V., Moritz J., Vadivelu S., Wang L., Rodgers R., Xiao S., Anabayan I., et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 2021;9:40. doi: 10.1186/s40478-021-01137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin J.W., Hogan M.V., Chhabra A.B., Deal D.N. Peripheral nerve repair and reconstruction. J. Bone Jt. Surg. Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 32.Kuffler D.P., Foy C. Restoration of Neurological Function Following Peripheral Nerve Trauma. Int. J. Mol. Sci. 2020;21:1808. doi: 10.3390/ijms21051808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Soury M., Fornasari B.E., Carta G., Zen F., Haastert-Talini K., Ronchi G. The Role of Dietary Nutrients in Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2021;22:7417. doi: 10.3390/ijms22147417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muratori L., Fregnan F., Maurina M., Haastert-Talini K., Ronchi G. The Potential Benefits of Dietary Polyphenols for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2022;23:5177. doi: 10.3390/ijms23095177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheib J., Hoke A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 36.Hanz S., Perlson E., Willis D., Zheng J.Q., Massarwa R., Huerta J.J., Koltzenburg M., Kohler M., van-Minnen J., Twiss J.L., et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/S0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 37.Coleman M.P., Hoke A. Programmed axon degeneration: From mouse to mechanism to medicine. Nat. Rev. Neurosci. 2020;21:183–196. doi: 10.1038/s41583-020-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthur-Farraj P.J., Latouche M., Wilton D.K., Quintes S., Chabrol E., Banerjee A., Woodhoo A., Jenkins B., Rahman M., Turmaine M., et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Sanchez J.A., Carty L., Iruarrizaga-Lejarreta M., Palomo-Irigoyen M., Varela-Rey M., Griffith M., Hantke J., Macias-Camara N., Azkargorta M., Aurrekoetxea I., et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen P., Cescon M., Zuccolotto G., Nobbio L., Colombelli C., Filaferro M., Vitale G., Feltri M.L., Bonaldo P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015;129:97–113. doi: 10.1007/s00401-014-1369-9. [DOI] [PubMed] [Google Scholar]
- 41.Cattin A.L., Burden J.J., Van Emmenis L., Mackenzie F.E., Hoving J.J., Garcia Calavia N., Guo Y., McLaughlin M., Rosenberg L.H., Quereda V., et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtovic Z., Svensson C.I., Krock E. Bugs improve nerve regeneration: Fasting-induced, microbiome-derived metabolite enhances peripheral nerve regeneration. Signal Transduct. Target. Ther. 2022;7:351. doi: 10.1038/s41392-022-01186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Distrutti E., Cipriani S., Mencarelli A., Renga B., Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS ONE. 2013;8:e63893. doi: 10.1371/journal.pone.0063893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jena P.K., Sheng L., Li Y., Wan Y.Y. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model. Hepatobiliary Surg. Nutr. 2020;9:170–182. doi: 10.21037/hbsn.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C.S., Li W.B., Wang H.Y., Ma Y.M., Zhao X.H., Yang H., Qian J.M., Li J.N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018;24:4254–4262. doi: 10.3748/wjg.v24.i37.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindborg J.A., Mack M., Zigmond R.E. Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration. J. Neurosci. 2017;37:10258–10277. doi: 10.1523/JNEUROSCI.2085-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sas A.R., Carbajal K.S., Jerome A.D., Menon R., Yoon C., Kalinski A.L., Giger R.J., Segal B.M. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020;21:1496–1505. doi: 10.1038/s41590-020-00813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwaid A.G., Krasowka-Zoladek A., Chi A., Cornella-Taracido I. Comparison of the Rat and Human Dorsal Root Ganglion Proteome. Sci. Rep. 2018;8:13469. doi: 10.1038/s41598-018-31189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leigh S.J., Clarke G., Cryan J.F. Rewiring bugs: Diet, the gut microbiome, and nerve regeneration. Dev. Cell. 2022;57:1917–1919. doi: 10.1016/j.devcel.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 50.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sartori R., Romanello V., Sandri M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021;12:330. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lahiri S., Kim H., Garcia-Perez I., Reza M.M., Martin K.A., Kundu P., Cox L.M., Selkrig J., Posma J.M., Zhang H., et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019;11:eaan5662. doi: 10.1126/scitranslmed.aan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto T., Morino K., Ugi S., Nakagawa F., Lemecha M., Ida S., Ohashi N., Sato D., Fujita Y., Maegawa H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019;316:E956–E966. doi: 10.1152/ajpendo.00510.2018. [DOI] [PubMed] [Google Scholar]
- 54.Nay K., Jollet M., Goustard B., Baati N., Vernus B., Pontones M., Lefeuvre-Orfila L., Bendavid C., Rue O., Mariadassou M., et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019;317:E158–E171. doi: 10.1152/ajpendo.00521.2018. [DOI] [PubMed] [Google Scholar]
- 55.Manickam R., Oh H.Y.P., Tan C.K., Paramalingam E., Wahli W. Metronidazole Causes Skeletal Muscle Atrophy and Modulates Muscle Chronometabolism. Int. J. Mol. Sci. 2018;19:2418. doi: 10.3390/ijms19082418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang W.C., Chen Y.H., Chuang H.L., Chiu C.C., Huang C.C. Investigation of the Effects of Microbiota on Exercise Physiological Adaption, Performance, and Energy Utilization Using a Gnotobiotic Animal Model. Front. Microbiol. 2019;10:1906. doi: 10.3389/fmicb.2019.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valentino T.R., Vechetti I.J., Jr., Mobley C.B., Dungan C.M., Golden L., Goh J., McCarthy J.J. Dysbiosis of the gut microbiome impairs mouse skeletal muscle adaptation to exercise. J. Physiol. 2021;599:4845–4863. doi: 10.1113/JP281788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert J.E., Myslicki J.P., Bomhof M.R., Belke D.D., Shearer J., Reimer R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl. Physiol. Nutr. Metab. 2015;40:749–752. doi: 10.1139/apnm-2014-0452. [DOI] [PubMed] [Google Scholar]
- 59.Petersen L.M., Bautista E.J., Nguyen H., Hanson B.M., Chen L., Lek S.H., Sodergren E., Weinstock G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. doi: 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barton W., Penney N.C., Cronin O., Garcia-Perez I., Molloy M.G., Holmes E., Shanahan F., Cotter P.D., O’Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 61.Allen J.M., Mailing L.J., Niemiro G.M., Moore R., Cook M.D., White B.A., Holscher H.D., Woods J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sport. Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 62.Przewlocka K., Folwarski M., Kazmierczak-Siedlecka K., Skonieczna-Zydecka K., Kaczor J.J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients. 2020;12:1451. doi: 10.3390/nu12051451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G., Jin B., Fan Z. Mechanisms Involved in Gut Microbiota Regulation of Skeletal Muscle. Oxid. Med. Cell. Longev. 2022;2022:2151191. doi: 10.1155/2022/2151191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bischoff S.C. Microbiota and aging. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:26–30. doi: 10.1097/MCO.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 65.Fielding R.A., Reeves A.R., Jasuja R., Liu C., Barrett B.B., Lustgarten M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019;127:110722. doi: 10.1016/j.exger.2019.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grosicki G.J., Fielding R.A., Lustgarten M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018;102:433–442. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ticinesi A., Nouvenne A., Cerundolo N., Catania P., Prati B., Tana C., Meschi T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients. 2019;11:1633. doi: 10.3390/nu11071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y.M., Wei L., Chiu Y.S., Hsu Y.J., Tsai T.Y., Wang M.F., Huang C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients. 2016;8:205. doi: 10.3390/nu8040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheiman J., Luber J.M., Chavkin T.A., MacDonald T., Tung A., Pham L.D., Wibowo M.C., Wurth R.C., Punthambaker S., Tierney B.T., et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ni Y., Yang X., Zheng L., Wang Z., Wu L., Jiang J., Yang T., Ma L., Fu Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019;63:e1900603. doi: 10.1002/mnfr.201900603. [DOI] [PubMed] [Google Scholar]
- 71.Chen L.H., Huang S.Y., Huang K.C., Hsu C.C., Yang K.C., Li L.A., Chan C.H., Huang H.Y. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging. 2019;11:756–770. doi: 10.18632/aging.101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee M.C., Hsu Y.J., Ho H.H., Hsieh S.H., Kuo Y.W., Sung H.C., Huang C.C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms. 2020;8:545. doi: 10.3390/microorganisms8040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang W.C., Hsu Y.J., Huang C.C., Liu H.C., Lee M.C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients. 2020;12:1145. doi: 10.3390/nu12041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang W.C., Hsu Y.J., Li H., Kan N.W., Chen Y.M., Lin J.S., Hsu T.K., Tsai T.Y., Chiu Y.S., Huang C.C. Effect of Lactobacillus plantarum TWK10 on Improving Endurance Performance in Humans. Chin. J. Physiol. 2018;61:163–170. doi: 10.4077/CJP.2018.BAH587. [DOI] [PubMed] [Google Scholar]
- 75.Huang W.C., Pan C.H., Wei C.C., Huang H.Y. Lactobacillus plantarum PS128 Improves Physiological Adaptation and Performance in Triathletes through Gut Microbiota Modulation. Nutrients. 2020;12:2315. doi: 10.3390/nu12082315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin C.L., Hsu Y.J., Ho H.H., Chang Y.C., Kuo Y.W., Yeh Y.T., Tsai S.Y., Chen C.W., Chen J.F., Huang C.C., et al. Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle- and Long-Distance Runners: A Double-Blind Controlled Trial. Nutrients. 2020;12:1972. doi: 10.3390/nu12071972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giron M., Thomas M., Dardevet D., Chassard C., Savary-Auzeloux I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle. 2022;13:1460–1476. doi: 10.1002/jcsm.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W., Li Y., Sun T., Li Z., Manyande A., Xu W., Feng M., Xiang H. Superior cervical ganglionectomy alters gut microbiota in rats. Am. J. Transl. Res. 2022;14:2037–2050. [PMC free article] [PubMed] [Google Scholar]
- 79.Han Y., Wang B., Gao H., He C., Hua R., Liang C., Zhang S., Wang Y., Xin S., Xu J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022;15:6213–6230. doi: 10.2147/JIR.S384949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh A., Dawson T.M., Kulkarni S. Neurodegenerative disorders and gut-brain interactions. J. Clin. Investig. 2021;131:e143775. doi: 10.1172/JCI143775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y., Eguchi A., Wan X., Chang L., Wang X., Qu Y., Mori C., Hashimoto K. A role of gut-microbiota-brain axis via subdiaphragmatic vagus nerve in depression-like phenotypes in Chrna7 knock-out mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2023;120:110652. doi: 10.1016/j.pnpbp.2022.110652. [DOI] [PubMed] [Google Scholar]
- 82.Svensson E., Horvath-Puho E., Thomsen R.W., Djurhuus J.C., Pedersen L., Borghammer P., Sorensen H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 83.Liu B., Fang F., Pedersen N.L., Tillander A., Ludvigsson J.F., Ekbom A., Svenningsson P., Chen H., Wirdefeldt K. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holmqvist S., Chutna O., Bousset L., Aldrin-Kirk P., Li W., Bjorklund T., Wang Z.Y., Roybon L., Melki R., Li J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 85.Kim S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X., Eguchi A., Yang Y., Chang L., Wan X., Shan J., Qu Y., Ma L., Mori C., Yang J., et al. Key role of the gut-microbiota-brain axis via the subdiaphragmatic vagus nerve in demyelination of the cuprizone-treated mouse brain. Neurobiol. Dis. 2023;176:105951. doi: 10.1016/j.nbd.2022.105951. [DOI] [PubMed] [Google Scholar]
- 87.Guo Z., Chen Y., Chen S., Liu C., Li S., Chen P. Renal Denervation Mitigated Fecal Microbiota Aberrations in Rats with Chronic Heart Failure. Evid.-Based Complement. Altern. Med. 2021;2021:1697004. doi: 10.1155/2021/1697004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu D., Wang J., Zhang H., Shan Q., Zhou B. Renal denervation improves chronic intermittent hypoxia induced hypertension and cardiac fibrosis and balances gut microbiota. Life Sci. 2020;262:118500. doi: 10.1016/j.lfs.2020.118500. [DOI] [PubMed] [Google Scholar]
- 89.Ma L., Zhang J., Fujita Y., Shinno-Hashimoto H., Shan J., Wan X., Qu Y., Chang L., Wang X., Hashimoto K. Effects of spleen nerve denervation on depression-like phenotype, systemic inflammation, and abnormal composition of gut microbiota in mice after administration of lipopolysaccharide: A role of brain-spleen axis. J. Affect. Disord. 2022;317:156–165. doi: 10.1016/j.jad.2022.08.087. [DOI] [PubMed] [Google Scholar]
- 90.Jacobson A., Yang D., Vella M., Chiu I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joly A., Leulier F., De Vadder F. Microbial Modulation of the Development and Physiology of the Enteric Nervous System. Trends Microbiol. 2021;29:686–699. doi: 10.1016/j.tim.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Kuwahara A., Matsuda K., Kuwahara Y., Asano S., Inui T., Marunaka Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020;41:199–216. doi: 10.2220/biomedres.41.199. [DOI] [PubMed] [Google Scholar]
- 93.Foong J.P.P., Hung L.Y., Poon S., Savidge T.C., Bornstein J.C. Early life interaction between the microbiota and the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;319:G541–G548. doi: 10.1152/ajpgi.00288.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fried S., Wemelle E., Cani P.D., Knauf C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology. 2021;197:108721. doi: 10.1016/j.neuropharm.2021.108721. [DOI] [PubMed] [Google Scholar]
- 95.Heiss C.N., Olofsson L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019;31:e12684. doi: 10.1111/jne.12684. [DOI] [PubMed] [Google Scholar]
- 96.Obata Y., Pachnis V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology. 2016;151:836–844. doi: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 98.Kabouridis P.S., Pachnis V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Investig. 2015;125:956–964. doi: 10.1172/JCI76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dupont J.R., Jervis H.R., Sprinz H. Auerbach’s plexus of the rat cecum in relation to the germfree state. J. Comp. Neurol. 1965;125:11–18. doi: 10.1002/cne.901250103. [DOI] [PubMed] [Google Scholar]
- 100.McVey Neufeld K.A., Mao Y.K., Bienenstock J., Foster J.A., Kunze W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013;25:183-e88. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 101.Abrams G.D., Bishop J.E. Effect of the normal microbial flora on gastrointestinal motility. Proc. Soc. Exp. Biol. Med. 1967;126:301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- 102.Collins J., Borojevic R., Verdu E.F., Huizinga J.D., Ratcliffe E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 103.Heymans C., de Lange I.H., Hutten M.C., Lenaerts K., de Ruijter N.J.E., Kessels L., Rademakers G., Melotte V., Boesmans W., Saito M., et al. Corrigendum: Chronic Intra-Uterine Ureaplasma parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses. Front. Immunol. 2020;11:672. doi: 10.3389/fimmu.2020.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rao M., Gershon M.D. Enteric nervous system development: What could possibly go wrong? Nat. Rev. Neurosci. 2018;19:552–565. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Vadder F., Grasset E., Manneras Holm L., Karsenty G., Macpherson A.J., Olofsson L.E., Backhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barajon I., Serrao G., Arnaboldi F., Opizzi E., Ripamonti G., Balsari A., Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J. Histochem. Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anitha M., Vijay-Kumar M., Sitaraman S.V., Gewirtz A.T., Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016.e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yarandi S.S., Kulkarni S., Saha M., Sylvia K.E., Sears C.L., Pasricha P.J. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology. 2020;159:200–213.e8. doi: 10.1053/j.gastro.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Obata Y., Castano A., Boeing S., Bon-Frauches A.C., Fung C., Fallesen T., de Aguero M.G., Yilmaz B., Lopes R., Huseynova A., et al. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578:284–289. doi: 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 110.Nohr M.K., Egerod K.L., Christiansen S.H., Gille A., Offermanns S., Schwartz T.W., Moller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 111.Kaji I., Akiba Y., Konno K., Watanabe M., Kimura S., Iwanaga T., Kuri A., Iwamoto K., Kuwahara A., Kaunitz J.D. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J. Physiol. 2016;594:3339–3352. doi: 10.1113/JP271441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soret R., Chevalier J., De Coppet P., Poupeau G., Derkinderen P., Segain J.P., Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 113.Suply E., de Vries P., Soret R., Cossais F., Neunlist M. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G1373–G1380. doi: 10.1152/ajpgi.00338.2011. [DOI] [PubMed] [Google Scholar]
- 114.Cossais F., Durand T., Chevalier J., Boudaud M., Kermarrec L., Aubert P., Neveu I., Naveilhan P., Neunlist M. Postnatal development of the myenteric glial network and its modulation by butyrate. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G941–G951. doi: 10.1152/ajpgi.00232.2015. [DOI] [PubMed] [Google Scholar]
- 115.Hurst N.R., Kendig D.M., Murthy K.S., Grider J.R. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol. Motil. 2014;26:1586–1596. doi: 10.1111/nmo.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muller P.A., Schneeberger M., Matheis F., Wang P., Kerner Z., Ilanges A., Pellegrino K., Del Marmol J., Castro T.B.R., Furuichi M., et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McVey Neufeld K.A., Perez-Burgos A., Mao Y.K., Bienenstock J., Kunze W.A. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol. Motil. 2015;27:627–636. doi: 10.1111/nmo.12534. [DOI] [PubMed] [Google Scholar]
- 118.Mayer E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brandon-Mong G.J., Shaw G.T., Chen W.H., Chen C.C., Wang D. A network approach to investigating the key microbes and stability of gut microbial communities in a mouse neuropathic pain model. BMC Microbiol. 2020;20:295. doi: 10.1186/s12866-020-01981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shen S., Lim G., You Z., Ding W., Huang P., Ran C., Doheny J., Caravan P., Tate S., Hu K., et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017;20:1213–1216. doi: 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen P., Wang C., Ren Y.N., Ye Z.J., Jiang C., Wu Z.B. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol. Brain. 2021;14:50. doi: 10.1186/s13041-021-00765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iida N., Dzutsev A., Stewart C.A., Smith L., Bouladoux N., Weingarten R.A., Molina D.A., Salcedo R., Back T., Cramer S., et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wan L., Li H., Sun G., Zhang L., Xu H., Su F., He S., Xiao F. Mutational Pattern Induced by 5-Fluorouracil and Oxaliplatin in the Gut Microbiome. Front. Microbiol. 2022;13:841458. doi: 10.3389/fmicb.2022.841458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lopera-Maya E.A., Kurilshikov A., van der Graaf A., Hu S., Andreu-Sanchez S., Chen L., Vila A.V., Gacesa R., Sinha T., Collij V., et al. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat. Genet. 2022;54:143–151. doi: 10.1038/s41588-021-00992-y. [DOI] [PubMed] [Google Scholar]
- 125.Qin Y., Havulinna A.S., Liu Y., Jousilahti P., Ritchie S.C., Tokolyi A., Sanders J.G., Valsta L., Brozynska M., Zhu Q., et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022;54:134–142. doi: 10.1038/s41588-021-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramakrishna C., Corleto J., Ruegger P.M., Logan G.D., Peacock B.B., Mendonca S., Yamaki S., Adamson T., Ermel R., McKemy D., et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci. Rep. 2019;9:20324. doi: 10.1038/s41598-019-56832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wardill H.R., Gibson R.J., Van Sebille Y.Z., Secombe K.R., Coller J.K., White I.A., Manavis J., Hutchinson M.R., Staikopoulos V., Logan R.M., et al. Irinotecan-Induced Gastrointestinal Dysfunction and Pain Are Mediated by Common TLR4-Dependent Mechanisms. Mol. Cancer Ther. 2016;15:1376–1386. doi: 10.1158/1535-7163.MCT-15-0990. [DOI] [PubMed] [Google Scholar]
- 128.Castelli V., Palumbo P., d’Angelo M., Moorthy N.K., Antonosante A., Catanesi M., Lombardi F., Iannotta D., Cinque B., Benedetti E., et al. Probiotic DSF counteracts chemotherapy induced neuropathic pain. Oncotarget. 2018;9:27998–28008. doi: 10.18632/oncotarget.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Brien P.D., Hinder L.M., Rumora A.E., Hayes J.M., Dauch J.R., Backus C., Mendelson F.E., Feldman E.L. Juvenile murine models of prediabetes and type 2 diabetes develop neuropathy. Dis. Model. Mech. 2018;11:dmm037374. doi: 10.1242/dmm.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Ye X., Ding D., Lu Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 2020;48:300060520936806. doi: 10.1177/0300060520936806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vrieze A., Van Nood E., Holleman F., Salojarvi J., Kootte R.S., Bartelsman J.F., Dallinga-Thie G.M., Ackermans M.T., Serlie M.J., Oozeer R., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 132.Sabico S., Al-Mashharawi A., Al-Daghri N.M., Wani K., Amer O.E., Hussain D.S., Ahmed Ansari M.G., Masoud M.S., Alokail M.S., McTernan P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38:1561–1569. doi: 10.1016/j.clnu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 133.Cai T.T., Ye X.L., Yong H.J., Song B., Zheng X.L., Cui B.T., Zhang F.M., Lu Y.B., Miao H., Ding D.F. Fecal microbiota transplantation relieve painful diabetic neuropathy: A case report. Medicine. 2018;97:e13543. doi: 10.1097/MD.0000000000013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bonomo R.R., Cook T.M., Gavini C.K., White C.R., Jones J.R., Bovo E., Zima A.V., Brown I.A., Dugas L.R., Zakharian E., et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA. 2020;117:26482–26493. doi: 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ding W., You Z., Chen Q., Yang L., Doheny J., Zhou X., Li N., Wang S., Hu K., Chen L., et al. Gut Microbiota Influences Neuropathic Pain through Modulating Proinflammatory and Anti-inflammatory T Cells. Anesth. Analg. 2021;132:1146–1155. doi: 10.1213/ANE.0000000000005155. [DOI] [PubMed] [Google Scholar]
- 136.Morales D., Pacurariu A., Slattery J., Pinheiro L., McGettigan P., Kurz X. Association between Peripheral Neuropathy and Exposure to Oral Fluoroquinolone or Amoxicillin-Clavulanate Therapy. JAMA Neurol. 2019;76:827–833. doi: 10.1001/jamaneurol.2019.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma P., Mo R., Liao H., Qiu C., Wu G., Yang C., Zhang Y., Zhao Y., Song X.J. Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice. J. Neuroinflamm. 2022;19:169. doi: 10.1186/s12974-022-02523-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Man S., Xie L., Liu X., Wang G., Liu C., Gao W. Diosgenin relieves oxaliplatin-induced pain by affecting TLR4/NF-κB inflammatory signaling and the gut microbiota. Food Funct. 2023;14:516–524. doi: 10.1039/D2FO02877H. [DOI] [PubMed] [Google Scholar]
- 139.Pane K., Boccella S., Guida F., Franzese M., Maione S., Salvatore M. Role of gut microbiota in neuropathy and neuropathic pain states: A systematic preclinical review. Neurobiol. Dis. 2022;170:105773. doi: 10.1016/j.nbd.2022.105773. [DOI] [PubMed] [Google Scholar]
- 140.Eberl C., Ring D., Munch P.C., Beutler M., Basic M., Slack E.C., Schwarzer M., Srutkova D., Lange A., Frick J.S., et al. Reproducible Colonization of Germ-Free Mice with the Oligo-Mouse-Microbiota in Different Animal Facilities. Front. Microbiol. 2019;10:2999. doi: 10.3389/fmicb.2019.02999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adams J.B., Audhya T., McDonough-Means S., Rubin R.A., Quig D., Geis E., Gehn E., Loresto M., Mitchell J., Atwood S., et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011;8:34. doi: 10.1186/1743-7075-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mangiola F., Ianiro G., Franceschi F., Fagiuoli S., Gasbarrini G., Gasbarrini A. Gut microbiota in autism and mood disorders. World J. Gastroenterol. 2016;22:361–368. doi: 10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kong X., Liu J., Liu K., Koh M., Tian R., Hobbie C., Fong M., Chen Q., Zhao M., Budjan C., et al. Altered Autonomic Functions and Gut Microbiome in Individuals with Autism Spectrum Disorder (ASD): Implications for Assisting ASD Screening and Diagnosis. J. Autism Dev. Disord. 2021;51:144–157. doi: 10.1007/s10803-020-04524-1. [DOI] [PubMed] [Google Scholar]
- 144.Beopoulos A., Gea M., Fasano A., Iris F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome-Host Interaction Mechanistic Hypothesis. Nutrients. 2021;14:65. doi: 10.3390/nu14010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mell B., Jala V.R., Mathew A.V., Byun J., Waghulde H., Zhang Y., Haribabu B., Vijay-Kumar M., Pennathur S., Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zubcevic J., Richards E.M., Yang T., Kim S., Sumners C., Pepine C.J., Raizada M.K. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ. Res. 2019;125:104–116. doi: 10.1161/CIRCRESAHA.119.313965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schlaich M.P., Lambert E., Kaye D.M., Krozowski Z., Campbell D.J., Lambert G., Hastings J., Aggarwal A., Esler M.D. Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 149.Karbach S.H., Schonfelder T., Brandao I., Wilms E., Hormann N., Jackel S., Schuler R., Finger S., Knorr M., Lagrange J., et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J. Am. Heart Assoc. 2016;5:e003698. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santisteban M.M., Kim S., Pepine C.J., Raizada M.K. Brain-Gut-Bone Marrow Axis: Implications for Hypertension and Related Therapeutics. Circ. Res. 2016;118:1327–1336. doi: 10.1161/CIRCRESAHA.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Toral M., Robles-Vera I., de la Visitacion N., Romero M., Yang T., Sanchez M., Gomez-Guzman M., Jimenez R., Raizada M.K., Duarte J. Critical Role of the Interaction Gut Microbiota—Sympathetic Nervous System in the Regulation of Blood Pressure. Front. Physiol. 2019;10:231. doi: 10.3389/fphys.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L.X., Rey F., Wang T., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N.A., Berkowitz D.E., Pluznick J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bruning J., Chapp A., Kaurala G.A., Wang R., Techtmann S., Chen Q.H. Gut Microbiota and Short Chain Fatty Acids: Influence on the Autonomic Nervous System. Neurosci. Bull. 2020;36:91–95. doi: 10.1007/s12264-019-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Poll B.G., Xu J., Jun S., Sanchez J., Zaidman N.A., He X., Lester L., Berkowitz D.E., Paolocci N., Gao W.D., et al. Acetate, a Short-Chain Fatty Acid, Acutely Lowers Heart Rate and Cardiac Contractility along with Blood Pressure. J. Pharmacol. Exp. Ther. 2021;377:39–50. doi: 10.1124/jpet.120.000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santisteban M.M., Qi Y., Zubcevic J., Kim S., Yang T., Shenoy V., Cole-Jeffrey C.T., Lobaton G.O., Stewart D.C., Rubiano A., et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kassan A., Ait-Aissa K., Kassan M. Hypothalamic miR-204 Induces Alteration of Heart Electrophysiology and Neurogenic Hypertension by Regulating the Sympathetic Nerve Activity: Potential Role of Microbiota. Cureus. 2021;13:e18783. doi: 10.7759/cureus.18783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Esmaeil Amini M., Shomali N., Bakhshi A., Rezaei S., Hemmatzadeh M., Hosseinzadeh R., Eslami S., Babaie F., Aslani S., Torkamandi S., et al. Gut microbiome and multiple sclerosis: New insights and perspective. Int. Immunopharmacol. 2020;88:107024. doi: 10.1016/j.intimp.2020.107024. [DOI] [PubMed] [Google Scholar]
- 159.Takewaki D., Suda W., Sato W., Takayasu L., Kumar N., Kimura K., Kaga N., Mizuno T., Miyake S., Hattori M., et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2020;117:22402–22412. doi: 10.1073/pnas.2011703117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang D.W., Pang X.T., Zhang H., Gao H.X., Leng Y.F., Chen F.Q., Zhang R., Feng Y., Sun Z.L. Gut microbial dysbiosis in rheumatoid arthritis: A systematic review protocol of case-control studies. BMJ Open. 2022;12:e052021. doi: 10.1136/bmjopen-2021-052021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gilhus N.E., Tzartos S., Evoli A., Palace J., Burns T.M., Verschuuren J. Myasthenia gravis. Nat. Rev. Dis. Prim. 2019;5:30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 162.Qiu D., Xia Z., Jiao X., Deng J., Zhang L., Li J. Altered Gut Microbiota in Myasthenia Gravis. Front. Microbiol. 2018;9:2627. doi: 10.3389/fmicb.2018.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moris G., Arboleya S., Mancabelli L., Milani C., Ventura M., de Los Reyes-Gavilan C.G., Gueimonde M. Fecal microbiota profile in a group of myasthenia gravis patients. Sci. Rep. 2018;8:14384. doi: 10.1038/s41598-018-32700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng P., Li Y., Wu J., Zhang H., Huang Y., Tan X., Pan J., Duan J., Liang W., Yin B., et al. Perturbed Microbial Ecology in Myasthenia Gravis: Evidence from the Gut Microbiome and Fecal Metabolome. Adv. Sci. 2019;6:1901441. doi: 10.1002/advs.201901441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Walker A.W., Sanderson J.D., Churcher C., Parkes G.C., Hudspith B.N., Rayment N., Brostoff J., Parkhill J., Dougan G., Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stojanov S., Berlec A., Strukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Masuda M., Matsumoto M., Tanaka S., Nakajima K., Yamada N., Ido N., Ohtsuka T., Nishida M., Hirano T., Utsumi H. Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J. Neuroimmunol. 2010;225:123–131. doi: 10.1016/j.jneuroim.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 168.Cao Y., Amezquita R.A., Kleinstein S.H., Stathopoulos P., Nowak R.J., O’Connor K.C. Autoreactive T Cells from Patients with Myasthenia Gravis Are Characterized by Elevated IL-17, IFN-gamma, and GM-CSF and Diminished IL-10 Production. J. Immunol. 2016;196:2075–2084. doi: 10.4049/jimmunol.1501339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nagano Y., Itoh K., Honda K. The induction of Treg cells by gut-indigenous Clostridium. Curr. Opin. Immunol. 2012;24:392–397. doi: 10.1016/j.coi.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 170.Thye A.Y., Law J.W., Tan L.T., Thurairajasingam S., Chan K.G., Letchumanan V., Lee L.H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients. 2022;14:1647. doi: 10.3390/nu14081647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen P., Tang X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients with Myasthenia Gravis. Front. Immunol. 2021;12:803101. doi: 10.3389/fimmu.2021.803101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chae C.S., Kwon H.K., Hwang J.S., Kim J.E., Im S.H. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PLoS ONE. 2012;7:e52119. doi: 10.1371/journal.pone.0052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ruiz L., Delgado S., Ruas-Madiedo P., Sanchez B., Margolles A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Microbiol. 2017;8:2345. doi: 10.3389/fmicb.2017.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rinaldi E., Consonni A., Cordiglieri C., Sacco G., Crasa C., Fontana A., Morelli L., Elli M., Mantegazza R., Baggi F. Therapeutic Effect of Bifidobacterium Administration on Experimental Autoimmune Myasthenia Gravis in Lewis Rats. Front. Immunol. 2019;10:2949. doi: 10.3389/fimmu.2019.02949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park J.S., Choi J.W., Jhun J., Kwon J.Y., Lee B.I., Yang C.W., Park S.H., Cho M.L. Lactobacillus acidophilus Improves Intestinal Inflammation in an Acute Colitis Mouse Model by Regulation of Th17 and Treg Cell Balance and Fibrosis Development. J. Med. Food. 2018;21:215–224. doi: 10.1089/jmf.2017.3990. [DOI] [PubMed] [Google Scholar]
- 176.Kwon H.K., Kim G.C., Kim Y., Hwang W., Jash A., Sahoo A., Kim J.E., Nam J.H., Im S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013;146:217–227. doi: 10.1016/j.clim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 177.Farini A., Tripodi L., Villa C., Strati F., Facoetti A., Baselli G., Troisi J., Landolfi A., Lonati C., Molinaro D., et al. Microbiota dysbiosis influences immune system and muscle pathophysiology of dystrophin-deficient mice. EMBO Mol. Med. 2023;15:e16244. doi: 10.15252/emmm.202216244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kalkan H., Pagano E., Paris D., Panza E., Cuozzo M., Moriello C., Piscitelli F., Abolghasemi A., Gazzerro E., Silvestri C., et al. Targeting gut dysbiosis against inflammation and impaired autophagy in Duchenne muscular dystrophy. EMBO Mol. Med. 2023;15:e16225. doi: 10.15252/emmm.202216225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Basic M., Dardevet D., Abuja P.M., Bolsega S., Bornes S., Caesar R., Calabrese F.M., Collino M., De Angelis M., Gerard P., et al. Approaches to discern if microbiome associations reflect causation in metabolic and immune disorders. Gut Microbes. 2022;14:2107386. doi: 10.1080/19490976.2022.2107386. [DOI] [PMC free article] [PubMed] [Google Scholar]