Abstract
Helicobacter pylori infection is associated with the development of gastric cancer. In short-term coculture with AGS gastric cells, H. pylori inhibits cell cycle progression and induces dose-dependent apoptosis. Based on the concept that an imbalance between proliferation and apoptosis may contribute to the emergence of gastric cancer, we chronically exposed AGS cells to H. pylori as a model of chronic exposure in humans. The AGS derivatives selected by this process were stably resistant not only to H. pylori-induced apoptosis but also to apoptosis induced by other enteric bacteria and by several toxic agents including radiation and cancer chemotherapy. Like the parental AGS cells, the derivatives underwent G1/S-phase cell cycle inhibition in response to H. pylori. The AGS derivatives displayed a marked decrease in cellular levels of the cell cycle control protein p27kip1. We found a similar decrease in epithelial cell p27kip1 expression in gastric biopsy specimens from H. pylori-infected patients. These findings are consistent with observations that link decreases in the p27kip1 level to increased susceptibility to cancer in mice with p27kip1 deleted and to a poor prognosis of gastric cancer in humans. This is the first demonstration that bacterial infection can lead to apoptosis resistance and to cross-resistance to other inducers of apoptosis such as bacteria, chemotherapeutic agents, and radiation. The development of apoptosis resistance and downmodulation of p27kip1 may contribute to the increased risk for gastric cancer observed in humans chronically exposed to H. pylori.
Chronic infection of the human gastric mucosa with the bacterium Helicobacter pylori is associated with the development of gastric adenocarcinoma (14, 19). Recent experimental studies of gerbils provide direct evidence that H. pylori infection can induce gastric carcinoma (12, 46). Although H. pylori is classified as a group 1 carcinogen by the World Health Organization (15), there is little evidence that this bacterium is directly mutagenic. Instead, the development of carcinoma in a subset of infected individuals may be a result of subtle changes in the gastric epithelial cells induced by either the bacterium itself and/or the associated host inflammatory response. These changes may include altered cell cycle regulation (P. Correa, Editorial, J. Natl. Cancer Inst. 89:836, 1997). Indeed, the number of both proliferating and apoptotic gastric epithelial cells is increased in gastric biopsy specimens from H. pylori-infected individuals (5, 26). However, experiments by our group and others have demonstrated that coculture of gastric epithelial cells with H. pylori in the presence or absence of inflammatory mediators does not stimulate increased cell proliferation directly. Instead, there is apparent growth inhibition, with an increase in the number of cells in the G1 phase of the cell cycle (39), and the induction of apoptosis (7, 9, 34, 45). We hypothesize that the in vitro induction of apoptosis by H. pylori reflects an important component of the response of the gastric mucosa in vivo (26), since a resulting compensatory increase in cellular turnover in the intact human stomach could stimulate the carcinogenic process (4).
Neoplastic transformation may be further enhanced by dysregulated apoptosis or a deficiency of apoptosis relative to proliferation, as suggested by recent models of multistage carcinogenesis (42). Based on the concept that a relative defect in apoptosis may play a role in the development of gastric cancer, we chronically cocultured gastric epithelial cells with live H. pylori in an attempt to select derivatives resistant to the induction of apoptosis by H. pylori. We report that these derivatives not only are resistant to H. pylori-induced apoptosis but also exhibit increased resistance to apoptosis induced by several other agents. Furthermore, they display changes in cell cycle distribution and alterations in the expression of several apoptosis and cell cycle-regulatory genes, including a marked reduction of expression of the cyclin-dependent kinase inhibitor p27kip1. We postulate that a similar selection process driven by H. pylori in the human stomach may play a role in the development of H. pylori-associated gastric cancer.
MATERIALS AND METHODS
Cell culture and isolation of AGS cell derivatives.
The AGS human gastric epithelial cell line was obtained from the American Type Culture Collection and maintained in an atmosphere of 5% CO2 at 37°C in Ham's F12 medium supplemented with 10% fetal bovine serum (Gibco-BRL) in 180-ml tissue culture flasks (Nunclon, Rochester, N.Y.). The cells were fed with fresh medium every 2 to 3 days and split at approximately 1:4 when subconfluent. At the third passage after being thawed from frozen stock cultures, the AGS cells were cocultured with either wild-type H. pylori strain 88-23 (ATCC 49503) or its isogenic cagA-negative mutant (kindly provided by M. Blaser, Vanderbilt University [44]). To control for the possible effects of repeated passage, cells were cocultured in parallel with complete medium only (parental control cells).
After each addition of bacteria, the medium was changed every 3 days. The cells were trypsinized and reseeded into new flasks when the cultures were confluent (approximately every 7 to 10 days). The cells were reinoculated with H. pylori on the resumption of log phase growth by the resistant cells. The initial bacterium-to-epithelial cell ratio was 20:1. This ratio was increased progressively over a 3-month period to 10,000:1 at week 12. The addition of H. pylori at a 10,000:1 ratio induces apoptosis in >95% of the parental cells (7). The apoptosis-resistant derivatives were designated HS3 (chronic exposure to wild-type H. pylori) or HS4 (chronic exposure to the isogenic cagA-negative mutant H. pylori).
Bacterial cultures.
Frozen stock cultures of wild-type H. pylori 88-23 and its isogenic cagA-negative mutant were maintained in defibrinated sheep blood (PML Microbiologicals, Tualatin, Oreg.) at −70°C. H. pylori strains were subcultured from frozen stocks to Trypticase soy agar containing 5% sheep blood (BBL, Becton Dickinson, Cockeysville, Md.) and incubated at 37°C in 5% CO2 for a minimum of two and a maximum of eight passages. The isogenic cagA-negative mutants were cultured in the presence of kanamycin, which was omitted for the passage prior to coculture. Inocula for coculture with AGS cells were diluted from suspensions that had been prepared from 48- to 72-h subcultures and adjusted by comparison of absorbance to McFarland standards, as described previously (7).
Inocula of Campylobacter jejuni (ATCC 33291), Shigella boydii (ATCC 9207), Salmonella enterica serovar Typhimurium (ATCC 14028), and enteropathogenic Escherichia coli (a gift of J. Notaro, University of Maryland), were prepared from 18- to 24-h subcultures to Trypticase soy agar containing 5% sheep blood incubated at 37°C.
Assessment of apoptosis and cell cycle distribution.
Experiments to assess apoptosis and cell cycle distribution were performed with AGS cultures that were in the logarithmic phase of growth (about 50% confluent). The cells were collected and analyzed for cell cycle distribution and apoptosis by flow cytometry, using standard protocols as described previously (39).
Measurement of adherence of H. pylori to epithelial cells.
Adherence of H. pylori to AGS cells was assessed using the flow cytometric assay described by Clyne and Drumm (8). In brief, 105 cells were pelleted into microcentrifuge tubes, washed with phosphate-buffered saline, and incubated with 2.5 × 107 cells of H. pylori strain 88-23. Rabbit antiserum 84-181 to H. pylori (kindly provided by M. Blaser and G. Perez-Perez, Vanderbilt University) was then added at a concentration of 1:100 at 37°C for 30 min, and nonadherent bacteria were removed by 15% sucrose gradient centrifugation. Fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (no. F-0511; Sigma) was added at a concentration of 1:80 at 37°C for 30 min, and after further washing, the cells were resuspended in 2% formaldehyde. Fluorescence intensity and distribution were analyzed by flow cytometry of 10,000 events (FACSort; Becton Dickinson, San Jose, Calif.).
Bacterial adherence was also examined qualitatively by fluorescence microscopy. An aliquot of epithelial cells incubated with bacteria, at the concentrations indicated above, was air dried, methanol fixed, and stained with acridine orange (Difco). Bacterial adherence was visualized under oil immersion at a magnification of ×1,000 using an Olympus BX-40 fluorescence microscope.
Assay of colony-forming efficiency.
One thousand cells per 15-cm dish were seeded in triplicate and refed with fresh medium every other day. After 10 days of growth, the cells were fixed and stained with 5% Giemsa and the number of grossly visible colonies was counted.
Measurement of growth curves.
Five thousand cells per 3-cm dish were seeded, in triplicate into a series of dishes and refed with fresh medium every other day. The cells were harvested from triplicate plates at the indicated times and quantified using a Coulter counter.
Western blot analysis.
Cells in the logarithmic phase of growth were collected and proteins were extracted for immunoblotting as described previously (39). Samples of 50 to 100 μg were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nylon membranes, incubated with blocking buffer (50 mmol of Tris per liter, 200 mmol of NaCl per liter, 0.2% Tween 20, 3% bovine serum albumin) overnight at 4°C, and then incubated with the indicated antibodies for 60 min. Polyclonal antisera to the bcl-2 gene family (bcl-2, bax, bcl-X, and bak) were kindly provided by John C. Reed, Burnham Institute, La Jolla, Calif., and used at concentrations of 1:1,500 as described previously (7). Mouse monoclonal antibodies to retinoblastoma protein (pRb) (clone G3-245), proliferating-cell nuclear antigen (clone PC10; Pharmingen, San Diego, Calif.), and p53 (Ab 1801; Santa Cruz Biotechnology, Santa Cruz, Calif.) and rabbit polyclonal antibodies to cyclins A, D1, and E (Upstate Biotechnology, Lake Placid, N.Y.), p21cip1 (sc-397-G; Santa Cruz), p27kip1 (sc-528-G; Santa Cruz), and actin (Sigma) were used at concentrations of 1:5,000. Actin immunoblotting was performed to verify that equal amounts of protein had been loaded in each lane.
Northern blotting.
Cells were collected by being scraped into 4 M acid-guanidinium isothiocyanate containing 100 mM mercaptoethanol, 0.5% sodium sarcosyl, and 25 mM sodium citrate (pH 7.0), and total RNA was extracted by centrifugation through cesium chloride (16). Samples of 20 μg of total RNA were then electrophoresed on a 1% agarose denaturing gel, transferred to a nylon membrane by capillary electrophoresis, and fixed with UV light. The membrane was hybridized with a 32P-labeled p27 cDNA probe (33) to obtain autoradiographs and was then stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase to normalize for loading differences. Band intensities were quantitated by laser densitometry (Molecular Dynamics, Sunnyvale, Calif.).
p53 sequencing.
DNA was prepared from the parental control, HS3, and HS4 cells by standard techniques (35). Exons 5 to 9 of the p53 gene were amplified by PCR, and the reaction products were sequenced using the dideoxynucleotide method, as described in detail previously (13). The primers used for exons 5, 7, and 8 were as described previously (13), and the primer pairs used for exon 6 and 9 were as follows: for exon 6, 5′-GGC CTC TGA TTC CTC ACT GA-3′ (upstream) and 5′-CTC CTC CCA GAG ACC CCA GT-3′ (downstream), and for exon 9, 5′-TGC AGT TAT GCC TCA GAT TC-3′ (upstream) and 5′-CGG CAT TTT GAG TGT TAG AC-3′ (downstream).
Patients and gastric biopsy specimens.
Sixty patients with non-ulcer-related dyspepsia who were not taking any medications were studied. Informed consent was obtained to take gastric antral biopsy specimens during diagnostic upper gastrointestinal endoscopy. H. pylori infection was diagnosed by biopsy urease test, histology, and serology, and the H. pylori CagA status was determined by serology (1). A total of 21 patients were not infected, 29 were infected by CagA+ strains, and 10 were infected by CagA− mutant strains. The study was approved by the Institutional Review Board of St. Luke's-Roosevelt Hospital Center, New York, N.Y.
Immunohistochemistry.
To assess p27kip1 expression in vivo, two formalin-fixed antral biopsy specimens per patient were sectioned at 5-μm thickness, mounted on poly-l-lysine-coated slides, and stained using an avidin-biotin complex immunoperoxidase technique with microwave pretreatment. A monoclonal antibody to p27kip1 (K25020; Transduction Laboratories, Lexington, Ky.), diluted 1:500, was applied overnight at 4°C. A breast carcinoma specimen with known positive immunostaining for p27kip1 served as a positive control, and positive nuclear staining of inflammatory cells in the lamina propria served as a further internal control. The intensity of gastric epithelial cell nuclear staining and the percentage of positive nuclei were assessed by a semiquantitative scoring system (A, no staining; B, mild staining; C, moderate staining; D, strong staining). The percentage of positive cells for nuclear staining was assessed as follows: 0, no or rare positive cells; 1, <10%; 2, 10 to 25%; 3, >25 to 50%; 4, >50%. Based on the range of values found in the normal (uninfected) gastric biopsy specimens, sections were designated positive if at least 25% of epithelial cells stained with at least moderate immunointensity. Adjacent sections were immunostained to identify proliferating cells by using an antibody to Ki-67 and for apoptotic cells by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay, and proliferation and apoptotic indices were calculated as previously described (31). All immunohistochemical evaluations were performed without knowledge of the H. pylori status of any of the patients.
Statistical methods.
Differences between the characteristics of parental control cells and the AGS derivatives were compared by two-way analysis of variance with Bonferroni-adjusted t tests. Differences in the frequency of positivity for p27kip1 between H. pylori-infected and uninfected biopsy specimens were compared by Yates-corrected chi-square analysis, and correlations were analyzed by using Spearman's rank correlation coefficient.
RESULTS
Spontaneous and H. pylori-induced apoptosis.
We first compared the apoptotic response of the HS3 and HS4 AGS derivatives and the parental control cells of the same passage to wild-type H. pylori (Fig. 1). Before addition of H. pylori, 3.95% ± 1.5% (mean ± standard error of the mean) of the parental control cells displayed evidence of apoptosis. This value was significantly lower for the HS3 (0.57% ± 0.2%) and the HS4 (0.69% ± 0.2%) derivatives (P = 0.01 for each comparison). When H. pylori was added to the control cultures at a ratio of 100:1 (bacteria to epithelial cells), there was an approximately twofold increase in apoptosis 24 h later. Separate studies indicated that the 24-h time point revealed the maximum apoptotic effect (data not shown). This increase was significant (P = 0.045). In contrast, the addition of H. pylori to either the HS3 or HS4 cells at the same ratio did not cause a significant increase in apoptosis (Fig. 1). At a 10-fold-higher bacterium-to-epithelial-cell ratio (i.e., 1,000:1), H. pylori did induce a statistically significantly increase in apoptosis in both HS3 and HS4 cells compared to that in parallel untreated cultures (P < 0.01 and P < 0.02, respectively). However, under these conditions, only about 2% of the HS3 and HS4 cells were apoptotic, in contrast to 8.3% of the parental control cells (Fig. 1).
FIG. 1.
Comparison of the extent of apoptosis in AGS parental control cells and in the HS3 and HS4 derivatives. The three cell types were exposed to medium alone (0) or H. pylori at the bacterium-to-epithelial-cell ratios shown for 24 h and then analyzed by DNA flow cytometry for the extent of apoptosis. The data are the mean and standard deviation of triplicate determinations.
There was no significant difference between the HS3 and HS4 derivatives in the extent of spontaneous apoptosis or in their responses to apoptosis induced by H. pylori. Responses to the wild-type and isogenic cagA-negative mutant H. pylori strains were identical. Our results indicate that the cagA gene product did not play a significant role in the selection of these apoptosis-resistant derivatives or in their subsequent apoptotic response to H. pylori.
Specificity of apoptosis resistance.
The HS3 and HS4 cells also displayed marked resistance to the apoptosis-inducing effects of a variety of other stimuli. These included agents commonly used in the chemotherapy of gastric cancer, such as 5-fluorouracil and etoposide, irradiation, and azide-induced hypoxia. HS3 and HS4 cells were also resistant to apoptosis induced by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor lovastatin (Table 1).
TABLE 1.
Effect of various toxic agents on the induction of apoptosis in parental control AGS cells and on their apoptosis-resistant derivatives (HS3 cells)
| Agent | Dose | % of apoptotic cellsa
|
|
|---|---|---|---|
| Parental control cells | HS3 cells | ||
| 5-FUb | 100 μM | 16.7 ± 5.4 | 6.4 ± 0.57 |
| 1,000 μM | 27.1 ± 4.7 | 11.4 ± 1.13 | |
| 10,000 μM | 33.4 ± 4.5 | 13.1 ± 6.43 | |
| VP16 | 0.1 μM | 18.1 ± 7.4 | 4.8 ± 3.54 |
| 1 μM | 30.4 ± 6.7 | 7.8 ± 2.26 | |
| 10 μM | 25.4 ± 3.1 | 6.3 ± 2.40 | |
| Lovastatin | 50 μM | 12.9 ± 7.0 | 3.2 ± 1.30 |
| Irradiation | 1 Gy | 17.0 ± 1.70 | 3.0 ± 4.38 |
| 3 Gy | 22.8 ± 4.67 | 2.9 ± 3.39 | |
| 5 Gy | 32.5 ± 5.37 | 5.4 ± 4.24 | |
| Sodium azide | 1 μM | 8.3 ± 1.70 | 4.0 ± 2.83 |
| 50 μM | 12.9 ± 9.33 | 3.2 ± 1.98 | |
| 100 μM | 21.5 ± 9.05 | 4.8 ± 3.11 | |
Values are mean ± standard deviation (three samples per group).
5-FU, 5-fluorouracil.
To determine the specificity of the apoptosis-resistant cells to apoptosis induced by other bacteria, AGS cells were cocultured with C. jejuni, enteropathogenic E. coli, S. boydii, and serovar Typhimurium using inocula diluted to yield epithelial cell-to-bacterial cell ratios after 24 h of incubation comparable to those achieved with H. pylori. The H. pylori-resistant AGS derivatives were completely resistant to the moderate apoptosis induced by C. jejuni (1.5 times the control values) and partially resistant to apoptosis induced by S. boydii (control cells, 42% apoptotic; HS3, 28.4% apoptotic; HS4, 28.3% apoptotic). In contrast, incubation of AGS parental cells with enteropathogenic E. coli or serovar Typhimurium resulted in almost total cell death at 24 h. This death was associated with the loss of trypan blue exclusion in over 90% of cells and the release of lactate dehydrogenase into the medium, consistent with nonapoptotic (necrotic) cell death. Similar results were seen using the HS3 and HS4 derivatives, indicating that these apoptosis-resistant cells remained susceptible to necrotic cell death induced by enteropathogenic E. coli or serovar Typhimurium.
Growth characteristics and cell cycle distribution of HS3 and HS4 cells.
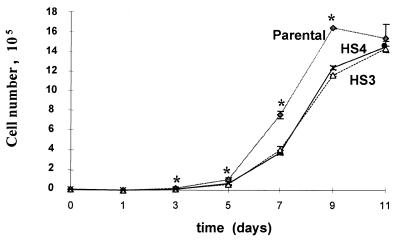
Untreated cultures of both the HS3 and HS4 cells grew more slowly than the parental control cells for the first 9 days after replating, but the saturation densities were similar to those of the parental controls (Fig. 2). These differences in cell number were reproducible and statistically significant on days 3 to 9 (P < 0.005 at each time point). In addition to exhibiting slower initial growth in mass cultures, the colony-forming efficiencies of both the HS3 and HS4 cells were less than 50% of those of the parental control cells. Thus, whereas the colony-forming efficiency of the parental control cells was 30.0 ± 4.2, the corresponding values for the HS3 and HS4 cells were 12.9 ± 2.8 and 14.7 ± 2.0, respectively.
FIG. 2.
Growth curves of AGS parental control cells and HS3 and HS4 derivatives. The data are the means and standard deviations of triplicate determinations.
Cell cycle analyses of exponentially dividing cultures demonstrated that compared with parental control cells, the HS3 and HS4 cells had a small reduction in the percentage of cells in the G0/G1 phase and a small increase in the percent of cells in the G2/M phase. No significant differences were observed in the percentage of cells in the S phase.
In contrast to their marked resistance to apoptosis, the HS3 and HS4 derivatives behaved similarly to parental control cells in their cell cycle phase response to the addition of H. pylori. Specifically, the addition of H. pylori inhibited the progression from G1 to S induced by the addition of 10% serum to cells that previously had been deprived of serum for 48 h. This response was identical in the parental control cells and in the HS3 and HS4 apoptosis-resistant derivatives (data not shown).
Levels of expression of proteins related to apoptosis and cell cycle control.
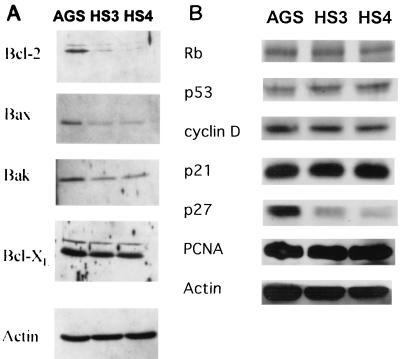
Western blot analyses of protein extracts obtained from untreated exponentially growing cultures indicated that the parental control, HS3, and HS4 cells expressed detectable levels of the apoptosis-related proteins Bcl-2 (both the ∼26- and ∼29-kDa forms), Bax, Bak, and Bcl-XL but not Bcl-Xs (Fig. 3A). Compared to the parental control cells, the HS3 and HS4 cells displayed a marked reduction in the expression of both the ∼26- and ∼29-kDa forms of Bcl-2 and reduced expression of Bax and Bak. The expression of Bcl-XL was similar in all three lines.
FIG. 3.
Immunoblot analysis of several members of the Bcl-2 protein family (A) and cell cycle-associated proteins (B). Protein extracts were isolated from untreated cultures of the parental control, HS3, and HS4 cells and analyzed by Western blotting using the indicated protein-specific antibodies. Actin served as the control for equal protein loading. Rb, retinoblastoma; PCNA, proliferating-cell nuclear antigen.
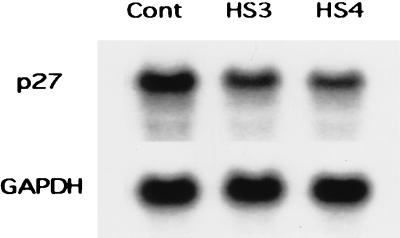
Western blot analyses also demonstrated a marked reduction in the expression of the cyclin-dependent kinase inhibitor protein p27kip1 in HS3 and HS4 cells (Fig. 3B). This decrease could be attributed, in part, to a moderate reduction in the steady-state expression of p27kip1 mRNA as determined by Northern blotting (Fig. 4). By densitometry, the normalized expression of p27kip1 mRNA was reduced by 30 and 43% and that of p27kip1 protein was reduced by 71 and 87% in HS3 and HS4 cells, respectively. The levels of expression of several other cell cycle-related proteins, pRb, p53, p21cip1, and proliferating-cell nuclear antigen, did not differ among the HS3, HS4, and parental control cells (Fig. 3B).
FIG. 4.
Northern blot analysis of p27kip1 mRNA in total RNA extracted from parental control (Cont), HS3, and HS4 cells. Densitometric analysis was normalized for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Adherence of H. pylori to AGS cells.
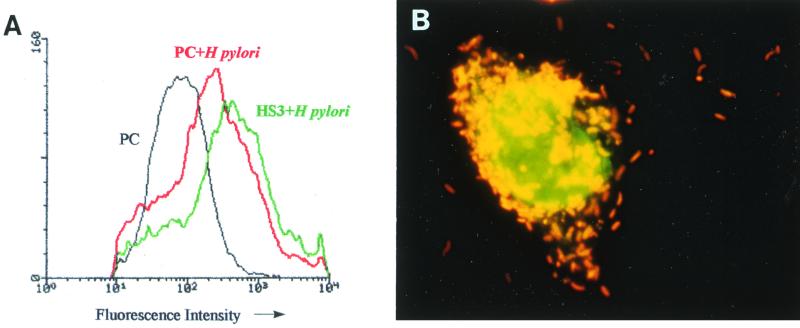
The adherence of H. pylori to the surface of the gastric epithelial cells is specific and plays a key role in mediating its effects on these cells (36). To determine whether HS3 and HS4 cells had become resistant to the induction of apoptosis by H. pylori because of changes in their cell surface that prevented the adherence of H. pylori, bacterial adherence was evaluated by an immunofluorescence flow cytometric assay and by fluorescence microscopy. The flow cytometric assay indicated that the extent of H. pylori binding was similar to (HS4) or even somewhat greater than (HS3) that obtained with the parental control cells. The results obtained with the HS4 cells were similar to those obtained with the control cells (Fig. 5A). The close adherence of H. pylori to the cell surface membrane of HS3 cells was confirmed by fluorescence light microscopy of acridine orange-stained preparations (Fig. 5B) and also by electron microscopy (data not shown). Therefore, the resistance to H. pylori-induced apoptosis in the HS3 and HS4 cells is not due to decreased bacterial adherence.
FIG. 5.
Adherence of wild-type H. pylori to parental control (PC), HS3, and HS4 cells. (A) Assessment of H. pylori binding by fluorescence using flow cytometry. The data are shown as a fluorescence histogram indicating the distribution of cells displaying binding by H. pylori, assessed using a fluorescent secondary antibody to H. pylori. The curve for HS4 plus H. pylori is superimposed upon the curve for PC plus H. pylori. (B) Numerous H. pylori organisms are adherent to the surface of HS3 cell. Acridine orange staining and oil immersion micrograph; magnification, ×100.
p53 sequencing.
Direct sequencing of exons 5 to 9 of the p53 gene did not demonstrate any mutations in the parental control AGS cells or the HS3 and HS4 derivatives (data not shown). Therefore, the parental control AGS cells contain wild-type p53 genes, confirming our previous studies (7) and demonstrating that the resistance to apoptosis acquired by the HS3 and HS4 derivatives is not due to mutations in the critical region of the p53 gene.
Stability of the resistant phenotype.
The parental control, HS3, and HS4 cells were serially passaged in parallel for 8 weeks in complete medium and without further addition of H. pylori. The HS3 and HS4 derivatives remained resistant to apoptosis induced by H. pylori. There was no reversion from the resistant phenotype (data not shown).
Expression of p27kip1 in human gastric biopsy specimens.
Decreased p27kip1 expression was seen in the HS3 and HS4 cells (Fig. 3B and 4). We therefore measured the expression of p27kip1 semiquantitatively by immunohistochemical techniques in human gastric antral biopsy specimens. In gastric biopsy specimens from patients who were not infected by H. pylori, p27kip1 was expressed predominantly in epithelial cells at the surface of gastric glands and also in the glandular base, with fewer cells stained in the middle (proliferative) zone (Fig. 6A). The mean (range) scores in the foveolar region in the uninfected cases was 3.5 (range, 1 to 4) and C (range, B to C), in the gland neck, 1.7 (range, 1 to 3) and C (range, B to C), and in the gland base, 3.0 (range, 2 to 4) and D (range, C to D). In biopsy specimens from patients infected with H. pylori (Fig. 6B), these values were 2.7 (range, 0 to 4) and B (range, A to D), 1.5 (range, 1 to 3) and C (range, B to D), and 2.6 (range, 1 to 4) and C (range, B to D), respectively. Using 3 and C as the cutoff for the normal range, positive epithelial cell staining for p27kip1 was less frequent in biopsy specimens from patients with H. pylori infection than from uninfected individuals. This reduction in the frequency of positive epithelial cell staining was observed in both surface and basal areas of gastric glands (46% of the patients infected by H. pylori had positive foveolar region staining versus 80% of the controls, and 44% of the H. pylori-infected patients had positive basal region staining versus 80% of the controls [P < 0.05 for each comparison]). No differences were observed between the reduction in p27kip1 expression in patients infected with cagA+ or cagA-negative mutant bacteria. There was no correlation between the expression of p27kip1 and markers of epithelial cell proliferation or of apoptosis.
FIG. 6.
Immunohistochemical expression of p27kip1 in the human gastric antrum. (A) Positive nuclear staining is evident in most of the epithelial cells in a biopsy specimen from a normal (uninfected) patient. (B) In a patient infected by H. pylori, strong staining for p27kip1 is seen in the mononuclear infiltrate in the lamina propria, but most epithelial cells display no or little immunostaining. Peroxidase immunohistochemistry; magnification, ×200.
DISCUSSION
In this report, we describe a model of chronic H. pylori infection. This is also a possible model for gastric carcinogenesis, based on the development of resistance to apoptosis in AGS cells after chronic exposure to H. pylori. Several gastric cancer-derived epithelial cell lines, including AGS cells, undergo apoptosis in vitro in response to H. pylori (7, 9, 34, 45) and to other stimuli, such as nonsteroidal anti-inflammatory (48) and cancer chemotherapy (27) drugs. In the present study, we derived HS3 and HS4 cells, which display marked resistance to the induction of apoptosis, by chronic coculture of AGS cells with progressively increasing doses of either wild-type H. pylori or its isogenic cagA-negative mutant. We found that the apoptosis resistance of HS3 and HS4 cells is not due to reduced adherence by H. pylori or to the selection of cells with a mutation in p53 exons 5 to 9, the most common region of inactivating mutations in p53 in human gastric and other types of carcinoma (25).
Apoptosis is a common host defense mechanism against microbial pathogens (47, 49). Conversely, some viruses have developed strategies to inhibit the host cell apoptotic response by carrying genes homologous to eukaryotic regulators of apoptosis, such as Bcl-2, or by producing proteins capable of interacting with cell cycle-regulatory proteins such as p53 (47). For example, the oncogenic Epstein-Barr virus encodes a Bcl-2 homolog which induces apoptosis resistance in human lymphoma cells (23). It is not known whether bacterial products can act in a similar fashion. The results described with H. pylori in the present study constitute the first documentation of a stable state of apoptosis resistance following chronic exposure to a bacterial pathogen. The bacterial factors responsible for this phenomenon remain to be determined. It is possible that bacteria other than H. pylori may also generate apoptosis-resistant host cells in response to chronic infection and inflammation.
It is of interest that in addition to marked resistance to H. pylori, HS3 and HS4 cells display resistance to apoptosis induced by C. jejuni and S. boydii and by 5-fluorouracil, various other chemotherapeutic agents, and lovastatin, a drug which induces apoptosis and cell cycle arrest in other cell lines (3, 17). Although enteropathogenic E. coli and Salmonella induce apoptosis in macrophages (20, 49), these organisms caused extensive nonapoptotic death in AGS cells and the apoptosis-resistant derivatives in our experiments. This may reflect differences in the target cells or the high bacterium-to-epithelial-cell ratio and late time points in our studies. Nevertheless, these results demonstrate that the HS3 and HS4 derivatives are resistant to apoptotic but not necrotic cell death induced by enteric bacteria. In general, these findings suggest that resistance to apoptosis in HS3 and HS4 cells is due to alterations in a signal transduction pathway that mediates the apoptotic response of cells to several diverse agents, not simply that induced by H. pylori. Since p53 is not mutated in the HS3 and HS4 derivatives, putative candidates for this common pathway include the Bcl-2 family (2), caspases (43), and cell cycle-regulatory proteins. The HS3 and HS4 cells display reduced expression of Bak and Bax, which in most contexts are proapoptotic, but also display reduced expression of Bcl-2, which is usually an inhibitor of apoptosis. In early models, the ratio of Bcl-2 to Bax was thought to govern the apoptotic rheostat. However, the increasing number of Bcl-2-homologous proteins and their interactions and the increasing number of pathways that both regulate and are regulated by Bcl-2 have emphasized the difficulty in predicting function based on protein expression data alone (2, 18, 38). The reduction in Bak in the HS3 and HS4 derivatives in association with an apoptosis-resistant phenotype is consistent with our previous studies demonstrating increased levels of Bak in short-term H. pylori coculture experiments with wild-type AGS cells in which apoptosis is induced (7).
Although the HS3 and HS4 cells display reduced apoptosis, they also grow more slowly than the parental AGS cells, have reduced colony-forming efficiency, and display a reduced proportion of cells in the G1 phase of the cell cycle and an increased percentage in G2/M compared with control cells. The only major change in the expression of cell cycle control proteins that we detected in these resistant cells was a marked reduction in expression of the cyclin-dependent kinase inhibitor p27kip1. This decrease was seen mainly at the level of p27kip1 protein rather than mRNA, consistent with other studies suggesting that the level of p27kip1 is regulated mainly posttranslationally via ubiquitin-proteasome-mediated proteolysis (21). Several lines of evidence suggest that this reduction in p27kip1 expression may play a role in the etiology of H. pylori-associated gastric cancer. First, reduced expression of p27kip1 is associated with a poor prognosis in several types of human cancer, including gastric cancer (11, 19, 21). For example, reduced levels of p27kip1 protein due to enhanced proteasome-mediated degradation of p27kip1 were found in 60% of a large series of colorectal carcinomas, and the extent of p27kip1 loss in these patients was found to be an independent poor prognostic marker (22). In a study of Barrett's-associated esophageal adenocarcinoma, Singh et al. reported that 83% of the tumors expressed low levels of p27kip1 and that these tumors were more aggressive histologically and were associated with decreased patient survival in comparison with tumors expressing normal levels of p27kip1 (40). Loss of p27kip1 protein has similarly been found to confer a poor prognosis in gastric cancer in two independent studies from Japan (24, 30). In both of these studies of gastric cancer, multivariate analysis found low levels of p27kip1 to be independent of routine histological prognostic factors. Taken together, these and other clinicopathological studies suggest that low p27kip1 protein expression may be a useful prognostic marker in a variety of human cancers (21). The second line of evidence suggesting that reduced p27kip1 is relevant to gastric carcinogenesis is that a tumor suppressor role for p27kip1 has been demonstrated in hemizygous and homozygus p27kip1-deleted mutant mice (10). Third, short-term exposure of AGS gastric epithelial cells to H. pylori also results in rapid downregulation of p27kip1 (39). Also, we report in this study that the immunohistochemical expression of p27kip1 is reduced in the gastric epithelial cells of patients infected with H. pylori.
The mechanisms by which reduced p27kip1 is associated with tumor progression and resistance to apoptosis are not known. p27kip1 is usually thought to act primarily as an inhibitor of the G1-to-S transition of the cell cycle, by binding to and inhibiting the cyclin E-cdk2 complex. Therefore, a decrease in the level of p27kip1 would have been predicted to increase the rate at which cells traverse the cell cycle. However, in the present study, reduced expression of p27kip1 was associated with decreased growth and with resistance to apoptosis, suggesting that p27kip1 may play additional and paradoxical roles (21). This is consistent with a recent study of gastric cancers from Japan in which reduced p27kip1 expression was associated with decreased apoptosis (30). However, whether the reduced expression of p27kip1 is responsible for mediating the resistance to apoptosis or is merely an epiphenomenon cannot be concluded from our results. It would be informative to restore p27kip1 levels to normal in the apoptosis-resistant cells (for example, with a p27kip1 expression vector) and determine whether this caused the cells to revert to an apoptosis-sensitive phenotype. If so, this would strongly suggest that the reduced expression of p27kip1 that we observed is indeed responsible for apoptosis resistance.
It is noteworthy that the expression of p27kip1 was similar in HS3 and HS4 cells and that p27kip1 expression was reduced to a similar extent in the gastric epithelium of patients infected by cagA+ or cagA-negative mutant H. pylori strains. These observations suggest that the ability of H. pylori to reduce p27kip1 expression was not related to genes within the cag pathogenicity island. This finding was unexpected, since cag+ strains are generally associated with a greater risk of gastric cancer than are cag-negative mutant strains in Western populations (6). However, in many other parts of the world, such as China, Japan, and Korea, almost all strains express cagA, and across countries the variation in the incidence of CagA seropositivity does not correlate significantly with the incidence of gastric cancer (32). Therefore, despite the recent demonstration that cagA can be transported into AGS cells in vitro (29), the importance of cag island genes in gastric carcinogenesis is still unclear.
One limitation of the present study is that we used AGS cells, which are derived from a human gastric cancer and may therefore not respond normally to apoptotic stimuli. However, there are no nontransformed gastric cell lines available with which to conduct these long-term coculture experiments. A limited comparison between another gastric cancer cell line (HMO2) and normal gastric cells in short-term primary cultures showed that the tumor cell line underwent around 50% less apoptosis in response to H. pylori (45). Of the available gastric cell lines, AGS is probably the best model for normal gastric epithelial cells because it has wild-type p53 (7) and because H. pylori adheres to AGS cells, as it does in vivo (41).
Is the ability to select apoptosis-resistant gastric epithelial cells unique to H. pylori? Chronic exposure of a variety of cancer cell lines to drugs commonly used for cancer chemotherapy has been used to select apoptosis-resistant derivatives. These have been used as a model of cancer chemotherapy resistance in vivo (28, 37). We chose H. pylori as the selection pressure on epithelial cells in culture based on the concept that gastric cancer cells are resistant to apoptosis and that gastric cancers usually arise in individuals chronically infected by H. pylori. However, it is conceivable that the ability to select gastric epithelial cells with marked apoptosis resistance is not limited to H. pylori but may also occur with other stimuli, such as chemotherapeutic agents or perhaps even other bacteria. Studies to determine whether other agents are capable of inducing stable apoptosis resistance in gastric epithelial cells are under way.
In conclusion, we have derived gastric epithelial cells that are resistant to apoptosis induced by H. pylori and several other agents. The apoptosis-resistant phenotype is stable, and the derived cells continue to be inhibited by H. pylori at the G1-to-S cell cycle phase transition. This is the first demonstration of apoptosis resistance in cells chronically infected by bacteria. Possible mechanisms for this resistance to apoptosis include altered expression of members of the Bcl-2 family of proteins and reduced levels of p27kip1. Further comparisons between these apoptosis-resistant derivatives and their parental cells may provide insights into the mechanisms by which H. pylori can induce apoptosis and the relevance of this phenomenon to gastric carcinogenesis.
ACKNOWLEDGMENTS
We thank William G. Ramey for assistance with flow cytometry and Peter R. Holt for helpful discussions.
This work was supported by an award from the Glaxo Institute for Digestive Health and the Cancer Research Foundation of America (to S.F.M.), the Charles A. Mastronardi Foundation (to E.M.S.), NCI grant R01CA63467 and an award from the National Foundation for Cancer Research (to I.B.W.), and an Award for Cancer Research from the FUSO Pharmaceutical Company, Osaka, Japan (Y.K.).
REFERENCES
- 1.Abdalla A M, Sordillo E M, Hanzely Z, Perez-Perez G I, Blaser M J, Holt P R, Moss S F. Insensitivity of the CLO test for H. pylori, especially in the elderly. Gastroenterology. 1998;115:243–244. [PubMed] [Google Scholar]
- 2.Adams J M, Cory S. The Bcl-2 family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal, B., S. Bhendwal, W. G. Ramey, B. Halmos, J. Reidy, S. F. Moss, and P. R. Holt. 1999. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin. Cancer Res. 2223–2229. [PubMed]
- 4.Ames B N, Gold L S. Chemical carcinogenesis: too many rodent carcinogens. Proc Natl Acad Sci USA. 1990;87:7772–7776. doi: 10.1073/pnas.87.19.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anti M, Armuzzi A, Gasbarrini A, Gasbarrini G. Importance of changes in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut. 1997;43(Suppl. 1):S27–S32. doi: 10.1136/gut.43.2008.s27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 7.Chen G, Sordillo E M, Ramey W G, Reidy J, Holt P R, Krajewski S, Reed J C, Blaser M J, Moss S F. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of Bak. Biochem Biophys Res Commun. 1997;39:626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- 8.Clyne M, Drumm B. Adherence of Helicobacter pylori to primary human gastrointestinal cells. Infect Immun. 1993;61:4051–4057. doi: 10.1128/iai.61.10.4051-4057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan X, Crowe S E, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley W K, Ernst P B, Reyes V E. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fero M L, Randel E, Gurley K E, Roberts J M, Kemp C J. The murine gene p27kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredersdorf S, Burns J, Milne A M, Packham G, Fallis L, Gillett C E, Royds J A, Peston D, Hall P A, Hanby A M, Barnes D M, Shousha S, O'Hare M J, Lu X. High level expression of p27kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27kip1 and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA. 1997;94:6382–6385. doi: 10.1073/pnas.94.12.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 13.Hongyo T, Buzard G S, Palli D, Weghorst C M, Amorosi A, Galli M, Caporaso N E, Fraumeni J F, Jr, Rice J M. Mutations of the K-ras and p53 genes in gastric adenocarcinomas from a high-incidence region around Florence, Italy. Cancer Res. 1995;55:2665–2672. [PubMed] [Google Scholar]
- 14.Huang J-Q, Sridhar S, Chen Y, Hunt R J. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer. Evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Kahn S M, Tomita N, Zhang Y J, Lu S H, Weinstein I B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992;52:2980–2983. [PubMed] [Google Scholar]
- 17.Keyomarsi K, Sandoval L, Band V, Pardee A B. Synchronization of tumor cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 18.Kim H-R C, Luo Y, Li G, Kessel D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999;59:3429–3432. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuipers E J. Relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;12(Suppl. 1):25–36. doi: 10.1111/j.1365-2036.1998.00009.x. [DOI] [PubMed] [Google Scholar]
- 20.Lai X H, Xu J G, Melgar S, Uhlin B E. An apoptotic response by J774 macrophage cells is common upon infection with diarrheagenic Escherichia coli. FEMS Microbiol Lett. 1999;172:29–34. doi: 10.1111/j.1574-6968.1999.tb13445.x. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd R V, Erikson L A, Jin L, Kulig E, Qian X, Cheville J C, Scheithauer B W. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancer. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Jessup J M, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 23.Marshall W L, Yim C, Gustafson E, Graf T, Sage D R, Hanify K, Williams L, Fingeroth J, Finberg R W. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T. p27 expression and gastric carcinoma. Nat Med. 1997;3:593. doi: 10.1038/nm0697-593. [DOI] [PubMed] [Google Scholar]
- 25.Moss S F. Cellular markers in the gastric precancerous process. Aliment Pharmacol Ther. 1998;12(Suppl. 1):91–109. doi: 10.1111/j.1365-2036.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- 26.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman S L, Galle P R, Stremmel W, Oren M, Krammer P H. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munker R, Zhao S, Jiang S, Snell V, Andreeff M, Andersson B S. Further characterization of cyclophosphamide resistance: expression of CD95 and of bcl-2 in a CML cell line. Leuk Res. 1998;22:1073–1077. doi: 10.1016/s0145-2126(98)00093-9. [DOI] [PubMed] [Google Scholar]
- 29.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani M, Isozaki H, Fuji K, Nomura E, Niki M, Mabuchi H, Nishiguchi K, Toyoda M, Ishibashi T, Tanigawa N. Impact of the expression of cyclin-dependent kinase inhibitor p27Kip1 and apoptosis in tumor cells on the overall survival of patients with non-early stage gastric carcinoma. Cancer. 1999;85:1711–1718. [PubMed] [Google Scholar]
- 31.Peek R M, Moss S F, Tham K T, Perez-Perez G I, Miller G G, Atherton J C, Holt P R, Blaser M J. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Perez G I, Bhat N, Gaensbauer J, Fraser A, Taylor D N, Kuipers E J, Zhang L, You W C, Blaser M J. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int J Cancer. 1997;72:453–456. doi: 10.1002/(sici)1097-0215(19970729)72:3<453::aid-ijc13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Polyak K, Lee M H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 34.Rudi J, Kuck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Segal E D, Falkow S, Tompkins L S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal-Bendirdjian E, Mannone L, Jacquemin-Sablon A. Alteration in p53 pathway and defect in apoptosis contribute independently to cisplatin-resistance. Cell Death Differ. 1998;5:390–400. doi: 10.1038/sj.cdd.4400357. [DOI] [PubMed] [Google Scholar]
- 38.Shinoura N, Yoshida Y, Nishimura M, Muramtsu Y, Asai A, Kirino T, Hamada H. Expression levels of bcl-2 determines anti- or proaptotic function. Cancer Res. 1999;59:4119–4128. [PubMed] [Google Scholar]
- 39.Shirin H, Sordillo E M, Oh S H, Yamamoto H, Delohery T, Weinstein I B, Moss S F. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 1999;59:2277–2281. [PubMed] [Google Scholar]
- 40.Singh S P, Lipman J, Goldman H, Ellis F H, Aizenman L, Cangi M G, Signoretti S, Chiaur D S, Pagano M, Loda M. Loss or subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 41.Smoot D T, Resau J H, Naab T, Desbordes B C, Gilliam T, Bull-Henry K, Curry S B, Nidiry J, Sewchand J, Mills-Robertson K. Adherence of Helicobacter pylori to cultured human gastric epithelial cells. Infect Immun. 1993;61:350–355. doi: 10.1128/iai.61.1.350-355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 43.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 44.Tummuru M K, Cover T L, Blaser M J. Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect Immun. 1994;62:2609–2613. doi: 10.1128/iai.62.6.2609-2613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner S, Beil W, Westermann J, Logan R P H, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 47.Young L S, Dawson C W, Elkiopoulos A G. Viruses and apoptosis. Br Med Bull. 1997;53:509–521. doi: 10.1093/oxfordjournals.bmb.a011627. [DOI] [PubMed] [Google Scholar]
- 48.Zhu G H, Wong B C, Eggo M C, Ching C K, Yuen S T, Chan E Y, Lai K C, Lam S K. Non-steroidal anti-inflammatory drug-induced apoptosis in gastric cancer cells is blocked by protein kinase C activation through inhibition of c-myc. Br J Cancer. 1999;79:393–400. doi: 10.1038/sj.bjc.6690062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zychlinski A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]