Abstract
CNI-1493, a potent macrophage deactivator, was used to treat infant rats systemically infected with Haemophilus influenzae type b (Hib). CNI-1493 was injected 1 h prior to bacterial inoculation and 24 h later and resulted in a 75 percent increased rate of survival compared to that for untreated controls. The effect of CNI-1493 on the inflammatory response was studied by immunohistochemical detection of individual tumor necrosis factor alpha (TNF-α)-, interleukin 1 beta (IL-1β)-, and gamma interferon (IFN-γ)-producing cells in the spleen. A significant reduction of the incidence of TNF-α- and IL-1β-expressing cells was found for CNI-1493-treated animals. IFN-γ expression was not suppressed by CNI-1493, indicating that cytokine inhibition was specific in macrophages. CNI-1493 significantly reduced the number of infiltrating granulocytes in the brain from that for controls. This study provides evidence that CNI-1493 protects against lethal Hib infection by deactivating the inflammatory cascade in infant rats.
During the onset of acute invasive bacterial infections such as sepsis and meningitis, activation of cytokine cascades plays a major role in pathogenesis (8, 26). An early event in the host's inflammatory response is the production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) (8, 26). These cytokines can be produced by different cells, including monocytes and macrophages and cells within the central nervous system (CNS), such as astrocytes and microglia (13, 20, 27). In sepsis, high levels of these cytokines in serum are associated with mortality (30, 31). During the onset of bacterial meningitis, TNF-α and IL-1β are also present in the cerebrospinal fluid (CSF) (13, 20, 27) and contribute to accumulation of leukocytes in CSF, development of brain edema, and damage of cells within the CNS (13, 20, 27).
Specific therapy targeted against TNF-α has been tried in the search for an effective anti-inflammatory adjuvant treatment. Anti-TNF-α antibodies or soluble TNF-α receptors have been used in experimental sepsis models showing protection against mortality (18). However, clinical studies have not been able to demonstrate such a beneficial effect (18, 26). In recent studies of experimental bacterial meningitis, inhibition of the release of TNF-α with pentoxifylline, thalidomide, glucocorticoids, or specific antibodies to TNF-α significantly reduced meningeal inflammation (20, 27). However, treatment of patients with corticosteroids has had a limited efficacy (21). Recent work with a new class of TNF-α synthesis inhibitors, the tetravalent guanylhydrazone CNI-1493, indicates that it acts by inhibition of the phosphorylation of the p38 mitogen-activated protein (MAP) kinase (2, 3, 6). A major effect of CNI-1493 is suppression of TNF-α synthesis, mediated by a dose-dependent inhibition of the translation of TNF mRNA (9, 10). Secondarily, the synthesis of other cytokines and chemokines is also inhibited, including that of IL-1β, macrophage inflammatory protein 1 alpha (MIP-1α) and MIP-1β (3). CNI-1493 suppresses the production of TNF-α and IL-1 even in the presence of gamma interferon (IFN-γ) (3), which is in contrast to the macrophage-inhibitory action mediated by corticosteroids, the latter appearing to have anti-inflammatory effects only in the absence of IFN-γ (17). Administration of CNI-1493 in a murine model of polymicrobial sepsis significantly reduced levels of TNF-α in serum and increased survival rates (28).
In the present study, we evaluated the protective effects of CNI-1493 on the pathogenic sequelae of cytokine release in an experimental model of Haemophilus influenzae type b (Hib) infection in infant rats. Bacteria were inoculated intraperitoneally (i.p.), resulting in a systemic infection with a hematogenous spread to the CNS (22). The results indicate that CNI-1493 significantly reduces mortality and infiltration of granulocytes in brain tissue and attenuates the systemic proinflammatory cytokine response.
MATERIALS AND METHODS
Hib strain LCR 528 was originally isolated from the CSF of a child with bacterial meningitis. The strain was grown in brain heart infusion broth supplemented with 5% Fildes enrichment medium to late log phase and then frozen at −70°C in Trypticase soy broth with 10% glycerol (pH 7.3) until use. For each experiment, an overnight growth was subcultured on chocolate agar plates and allowed to grow for 8 h in late log phase to facilitate maximal capsule expression and then was centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) to an approximate concentration of 5 × 106 CFU per ml, determined by quantitative subcultures. The final inoculum was 5 × 105 CFU/rat. The weight of each rat was approximately 10 to 15 g.
CNI-1493.
The tetravalent guanylhydrazone CNI-1493, CAS registration no. 164301-51-3, was synthesized and purified as previously described (2). CNI-1493 is a powerful inhibitor of synthesis of TNF and IL-1. It inhibits macrophage activation and subsequent proinflammatory cytokine production while having no inhibitory activity on T cell proliferation or activation (2, 3). The mechanism by which CNI-1493 inhibits macrophage TNF synthesis is suppression of TNF translation efficiency (9). The purity was >98% as estimated by the melting point, nuclear magnetic resonance, elution from high-performance liquid chromatography, and elemental analyses. A stock solution was prepared in sterile, deionized, lipopolysaccharide-free water. Rats were injected with 5 mg/kg of body weight, given as a 0.1-ml injection i.p.
Infant rat model.
Five- to seven-day-old outbred Sprague-Dawley rats (Charles River, Uppsala, Sweden) in different litters with their mothers were used as previously described (22). The animals were fed and housed under standard conditions. Animals were inoculated by an i.p. injection of 0.1 ml of the Hib suspension (5 × 105 CFU). The animals were divided into five different groups with 40 to 44 animals per group (three litters per group) and treated as follows. Group 1 received two injections of CNI-1493 (5 mg/kg, i.p.), the first 1 h before bacterial inoculation and the second 24 h after. Group 2 received CNI-1493 (5 mg/kg, i.p.) under the same conditions as group 1. This group received additional treatment with a suboptimal dose of the antibiotic cefotaxime (Claforan; Hoechst Marion Roussel, Stockholm, Sweden) of 50 mg/kg, i.p., started 12 h after bacterial inoculation and given twice daily for 4 days. Group 3 received only cefotaxime, in the same way as group 2. Group 4 was treated with dexamethasone (Decadron; Merck Sharp & Dohme, Sollentuna, Sweden) in two doses of 0.15 mg/kg, given i.p. 1 h before bacterial injection and 24 h after. The animals received cefotaxime in the same suboptimal dosage as that for groups 2 and 3. Group 5 served as the control group; i.e., the animals were inoculated and received two injections daily with PBS for 2 days. In each group, five animals chosen at random were sacrificed at 3, 24, and 48 h, respectively, after Hib infection. Following decapitation, the spleen and brain were removed and immediately snap-frozen in isopentane and dry ice and stored at −70°C until sectioned. For the remaining rats, mortality was assessed twice daily for 7 days. The survival data were based on three different experiments. In one set of experiments, animals were monitored for 14 days. The experiment was approved by an animal ethics committee at Huddinge Court House.
Immunohistochemical detection of granulocyte markers in brain sections.
Cryostat sections (12 μm) of the brain at 24 h after Hib infection were cut and mounted on glass slides (SuperFrost Plus; Menzel-Gläser). Sections were fixed for 10 min in 2% formaldehyde (Sigma Chemical Co., St. Louis, Mo.) in PBS at room temperature. All slides were subsequently stored at −20°C until stained.
The brain section was stained with a monoclonal antibody directed against a granulocyte antigen (Mouse anti-granulocyte human/rat; Serotec, Oxford, United Kingdom) (diluted 1:10). The staining method was the same as that described below for TNF-α, but without using saponin. The secondary antibody used was a biotin-labeled Fab2-fragmented donkey anti-mouse antibody (Jackson Immunoresearch Labs) (diluted 1:1,000). The infiltrating granulocytes in the whole section of the brain were then quantified in a computerized image analyzer, as described below.
The specificity of the granulocyte antibody was tested by staining of a blood smear from rat, using the same staining method as for the brain sections.
Immunohistochemical detection of intracellular TNF-α, IL-1β, and IFN-γ in spleen sections.
Cryostat sections (10 μm each) of the spleen were cut and mounted on glass slides (SuperFrost Plus; Menzel-Gläser). Sections were fixed for 10 min in 2% formaldehyde (Sigma Chemical Co.) in PBS at room temperature. All slides were subsequently stored at −20°C until stained.
TNF-α.
The cryopreserved sections were stained for intracellular production of TNF-α as previously described (16, 25). Briefly, permeabilization of the cell membrane and the Golgi organelle was performed by use of a balanced salt solution (BSS) (GIBCO Ltd., Paisley, United Kingdom) supplemented with 0.1% saponin (Riedel de Haen AG, Seelze, Germany) in all subsequent washes and incubation steps. Endogenous peroxidase activity was blocked with 1% hydrogen peroxidase and 2% sodium azide dissolved in BSS-saponin for 1 h at room temperature in the dark. Sections were then washed three times in BSS-saponin and were thereafter blocked with either 2% normal goat sera or 2% normal human AB sera in BSS-saponin for 30 min at room temperature to reduce background staining due to nonspecific binding sites. Slides were thoroughly washed, and endogenous biotin was blocked with avidin-saponin for 30 min and biotin-saponin for an additional 15 min (avidin/biotin blocking kit; Vector, Burlingame, Calif.). After additional thorough washes in BSS-saponin, sections were incubated overnight at room temperature in a humidified chamber with 50 μl of cytokine-specific antigen, affinity-purified antibody (polyclonal rabbit anti-rat TNF-α, lot no. 8-14; P. Van der Meide, Biomedical Primate Research Centre, Rijswijk, The Netherlands) (1 μg/ml). Control staining was done by omitting the primary antibody and by a negative control, consisting of an irrelevant mouse immunoglobulin G1 (IgG1) antibody and rabbit IgG (rabbit IgG, mouse IgG1 negative control; Dako, Glostrup, Denmark). The slides were then washed and incubated with appropriate biotin-labeled antibody (Fab2-fragmented donkey anti-rabbit; Jackson Immunoresearch Labs) (diluted 1:1,000) for 30 min at room temperature. Sections were again rinsed in BSS-saponin, and 50 μl of a solution of Vectastain avidin-biotin-horseradish peroxidase (Vector) prepared in BSS-saponin according to the manufacturer's directions was applied for 30 min at room temperature. After a final wash, the substrate diaminobenzidine (Peroxidase Substrate Kit; Vector) was added. The reaction was stopped after 5 min by washes in BSS, after which sections were counterstained with Mayer's hematoxylin. Finally, the slides were left to air dry and mounted with buffered glycerol.
IL-1β.
The same method as that described above for TNF-α was used. However, the cytokine-specific antibody used was a polyclonal goat anti-rat IL-1β (lot no. YRO 1/AF-501-NA; R&D Systems, Minneapolis, Minn.) (2 μg/ml) (diluted 1:500). The biotin-labeled antibody used was a Fab2-fragmented donkey anti-goat antibody (Jackson Immunoresearch Labs) (diluted 1:1,000).
IFN-γ.
The same method as that described above was used. The cytokine-specific antigen affinity-purified antibody used was a monoclonal mouse anti-rat IFN-γ (DB-1; P. Van der Meide, Biomedical Primate Research Centre, Rijswijk, The Netherlands) (2 μg/ml). The biotin-labeled antibody used was a Fab2-fragmented donkey anti-mouse antibody (Jackson Immunoresearch Labs) (diluted 1:1,000).
Semiquantification of cytokine-producing cells by computerized in-situ imaging.
The immunocytochemically stained cells were examined with a Leica DMX microscope (Leica, Wetzlar, Germany) equipped with a 3-chip charge-coupled device color camera (CXC-750P; Sony, Tokyo, Japan). The images were analyzed in an image analyzer (Quantimet QW 550; Leica, Cambridge, United Kingdom). The image was directed by a PC computer. Special software for cell detection and measurement of intensity (Detect A Cell) was written in QUIPS. The methodology has recently been described (1, 4, 5). For data analysis, cell size was expressed in cell area (square microns), mean total cell intensity was expressed in gray levels (0 to 255), and the frequencies of positive and negative cells were calculated for at least 1.5 × 104 cells per section. The data acquired were imported to a Microsoft Excel dedicated macro setup (IMAGE2XL, developed by T. E. Fehniger, Department of Infectious Diseases, Huddinge University Hospital, Stockholm, Sweden), which provided statistical analysis using the Astute program (University of Leeds, Leeds, United Kingdom) by calculating the positive stained area versus the total stained area.
Culture of Hib in presence of CNI-1493.
In order to evaluate if CNI-1493 had any direct antimicrobial effects, the Hib strain used in the animal experiments was cultured in broth with and without addition of CNI-1493 in a high concentration. A Hib inoculum of 5.5 × 107 CFU/ml was cultured in Müller-Hinton broth with CNI-1493 at a concentration of 30 mg/liter. Control cultures were grown without CNI-1493. Quantitative subcultures were done at 5 and 24 h, respectively.
Statistical analysis.
Analysis of variance was used for comparison of multiple groups, while the Mann-Whitney U test was used to compare two groups. Differences were considered significant at a P value of <0.05. Data are expressed as means ± standard errors.
RESULTS
Effect of CNI-1493 on survival in an infant rat model.
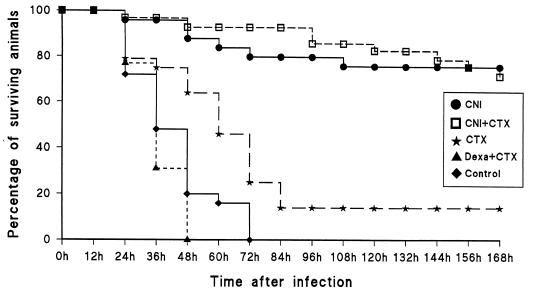
Mortality rates were assessed for all groups twice daily for 7 days (Fig. 1). For group 1, among animals treated with CNI-1493 only (n = 25), 76% survived 1 week after bacterial inoculation (P = 0.0001 compared to results for the control; P = 0.0001 compared to results for animals treated with cefotaxime only). In group 2, treatment with CNI-1493 plus (n = 29), 72% survived after 1 week (P = 0.0001 compared to results for the control). For group 3, treatment with cefotaxime alone (n = 28), a 14% survival rate was seen after 1 week. Group 4, treatment with dexamethasone plus cefotaxime (n = 26), showed a mortality rate of 100% within 2 days. Group 5, the control animals (infected without treatment; n = 25), showed a mortality rate of 100% within 3 days. Animals surviving to day 7 appeared to be in an unaffected and normal condition and were considered cured of Hib infection. Observation of a subgroup for a total of 14 days indicated that all animals were healthy (as judged by observation of movements, fur, suckling, and growth). These data indicate that the treatment with CNI-1493 conferred protection against lethality.
FIG. 1.
Life table analysis illustrating the percentages of surviving animals at the indicated time points after i.p. injection of Hib at time point 0 h (all drugs were also given i.p.). Treatment groups are indicated by the following abbreviations. CNI, CNI-1493 (5 mg/kg) injected 1 h before bacterial inoculation and 24 h after (n = 25). CNI + CTX, CNI-1493 (as previous dosage) with cefotaxime (50 mg/kg), twice daily for 4 days, first dose 12 h after bacterial inoculation (n = 29). CTX, cefotaxime alone (as previous dosage) (n = 28). Dexa + CTX, dexamethasone (0.15 mg/kg), first dose 1 h before bacterial inoculation and 24 h later with cefotaxime (as previous dosage) (n = 26). Control, infected animals without any treatment (n = 25). A significant increase in the survival rate over that for controls was noticed for all CNI-1493-treated animals both with and without antibiotic treatment.
Detection of granulocyte markers in brain sections.
Brain sections at 24 h after Hib inoculation from the group of animals treated with CNI-1493 only and from the control group were analyzed to determine the number of infiltrating granulocytes. In both groups, single granulocytes were found scattered in the brain parenchyma and the meninges, and clusters of infiltrating cells were detected, especially in perivascular areas (see Fig. 3). The granulocyte antibody was also incubated with a normal rat blood smear in order to test its specificity, showing that granulocytes stained in the same way as the granulocytes in the brain sections.
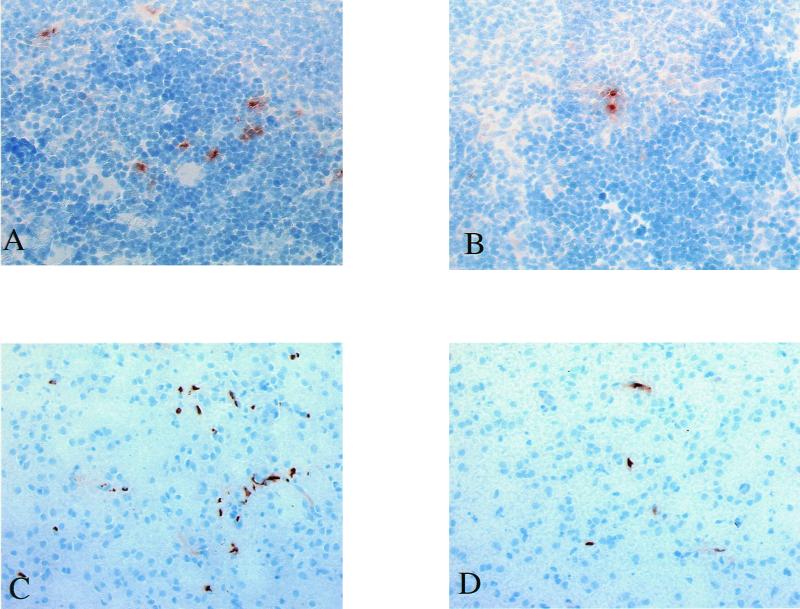
FIG. 3.
(A and B) Microphotographs of cryopreserved spleen sections immunohistochemically stained for TNF-α 3 h after Hib inoculation. Sections from an untreated control animal (A) and a CNI-1493 treated animal, where the number of positively stained cells was reduced (B), are shown. TNF-α-expressing cells stain brown with diaminobenzidine. Please note the extracellular deposition of TNF surrounding producer cells. The nuclei of all cells were counterstained with hematoxylin (blue). (C and D) Microphotographs of cryopreserved brain sections immunohistochemically stained for infiltrating granulocytes 24 h after Hib inoculation. Sections from an untreated control animal (C) and a CNI-1493-treated animal, showing a reduced number of granulocytes (D), are shown. Granulocytes stained brown with diaminobenzidine. The nuclei of all cells were counterstained with hematoxylin (blue).
Semiquantification by computerized in-situ imaging of positively stained cells was done for both groups of animals (n = 5 per group) and showed that control animals (infected but not treated) had a mean presence of infiltrating granulocytes of 3.52% ± 0.61% of the total cell area. In the group of animals pretreated with CNI-1493, the mean number of infiltrating granulocytes was significantly reduced at 1.76% ± 0.37% (P = 0.019) positively stained cells per total cell area (see Fig. 3C and D).
Effect of CNI-1493 on TNF-α-producing cells in spleen.
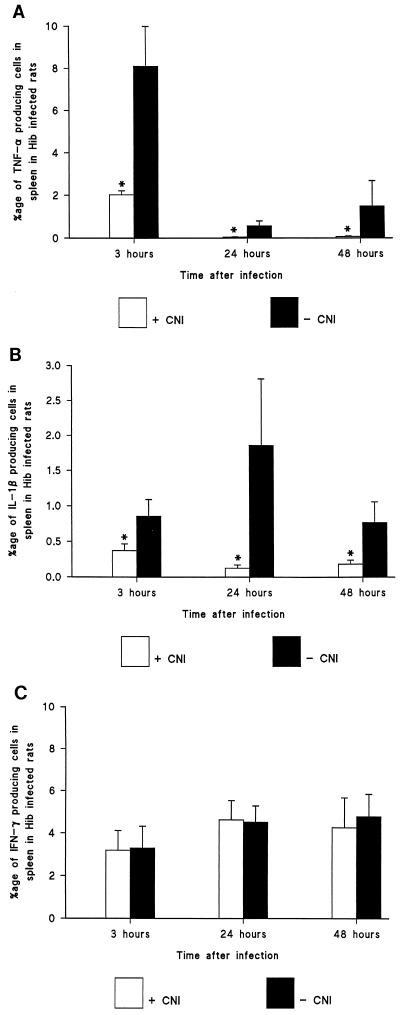
To determine the effect of treatment on cellular cytokine synthesis, splenic cells were analyzed at time points of 3, 24, and 48 h (n = 5 at each time point) after bacterial inoculation. We did perform two color staining procedures using MAC 387 and CD68 monoclonal antibodies in order to show that all IL-1β- and IL-1α-expressing cells were CD68- and MAC 387-positive cells, while the TNF-α-expressing cells costained with CD68 and MAC 387 at a rate of 80 to 95%. The rest of the TNF-α-positive cells were CD3-positive T cells in the spleen. Semiquantification by computerized in-situ imaging of TNF-α-producing cells showed that the control animals had a mean prevalence of TNF-α-producing cells per total cell area of 8.13% ± 1.88%, 0.57% ± 0.23%, and 1.52% ± 1.19% positive cells at 3, 24, and 48 h, respectively. Pretreatment with CNI-1493 significantly reduced the mean number of TNF-α-producing cells at every time point compared with results for the control group: 2.03% ± 0.19% (P = 0.007), 0.04% ± 0.02% (P = 0.01), and 0.07% ± 0.02% (P = 0.02) positive cells, respectively (Fig. 2A and 3A and B).
FIG. 2.
Immunohistochemical staining and calculation by computerized imaging of cytokine-producing cells in spleen. Cells producing TNF-α (A), IL-1β (B), and IFN-γ (C) in the spleen were analyzed. The bars illustrate mean percentages ± standard errors of positively stained cells per total cell area at 3, 24, and 48 h after i.p. Hib injection, with and without CNI-1493 treatment (5 mg/kg, given i.p.) (n = 5 per group). P was <0.05 between the groups at each time point for TNF-α and IL-1β. No significant difference was found at any point of time for IFN-γ.
Effect of CNI-1493 on IL-1β-producing cells in the spleen.
Semiquantification by computerized in-situ imaging of IL-1β producing cells was done at the same time points as those used for TNF-α, with and without CNI-1493 treatment. This showed that the control animals had a mean prevalence of IL-1β-producing cells per total cell area of 0.86% ± 0.23%, 1.87% ± 0.95%, and 0.78% ± 0.29% positive cells at 3, 24, and 48 h, respectively. In animals pretreated with CNI-1493, the mean number of IL-1β-producing cells was significantly reduced at all time points compared with results for the control group: 0.37% ± 0.10% (P = 0.04), 0.12% ± 0.05% (P = 0.007), and 0.18% ± 0.06% (P = 0.04) positive cells at 3, 24, and 48 h, respectively (Fig. 2B).
Effect of CNI-1493 on IFN-γ-producing cells in the spleen.
To obtain evidence that the cytokine-suppressing effects of CNI-1493 were specific, semiquantification by computerized in-situ imaging of IFN-γ-producing cells was done for both groups. The control animals had a mean prevalence of IFN-γ-producing cells per total cell area of 3.33% ± 1.02%, 4.55% ± 0.77%, and 4.86% ± 1.06% positive cells at 3, 24, and 48 h, respectively. In animals pretreated with CNI-1493, the mean number of IFN-γ-producing cells was not significantly different at any time point compared with results for the control group: 3.2% ± 0.93%, 4.63% ± 0.92%, and 4.35% ± 1.42% positive cells, respectively (Fig. 2C).
Antibacterial effect of CNI-1493 on Hib.
No antibacterial effect of CNI-1493 could be demonstrated. Quantitative cultures at 5 h in media without CNI-1493 resulted in 4.0 × 107 CFU/ml, and in media with CNI-1493, results were 3.9 × 107 CFU/ml. At 24 h, the corresponding values were 4.5 × 107 and 4.3 × 107 CFU/ml, respectively.
DISCUSSION
These results indicate that treatment with CNI-1493 reduced mortality by 75% with experimental Hib infection of infant rats. CNI-1493 therapy resulted in a significant decrease in the number of TNF-α- and IL-1β-producing cells in the spleen and the number of infiltrating granulocytes in the brain, compared with results for untreated animals. The meningeal inflammation seen with Hib infection in the infant rat model has previously been well described (19, 22) and has recently been used for studies of the CNS inflammatory response in Hib infection (11, 12). However, this model has some drawbacks, including the difficulties of obtaining CSF or blood samples due to the small size of the animal, and if CSF is taken, the amount of blood contamination has to be determined to avoid false pleocytosis or a positive CSF culture (29). In the present study, CNS inflammation was measured immunohistochemically by determining the number of infiltrating granulocytes in the whole brain section, including the meninges. Despite the 50% reduction in the number of infiltrating granulocytes in CNI-1493-treated animals, our belief is that the significant reduction in mortality mainly results from suppression of the systemic inflammation rather than of the local CNS inflammation. The model used here therefore more closely resembles neonatal sepsis with CNS engagement than meningitis alone.
Previous in vitro studies revealed that CNI-1493 effectively down-regulates proinflammatory cytokine synthesis, in particular, TNF-α synthesis in cultured endotoxin-stimulated murine and human macrophages (3, 6). However, suppression of TNF-α is not complete, since CNI-1493-treated macrophages still can produce about 10% of the amount of TNF-α produced by nontreated macrophages (3, 6). It has recently been deduced that the mechanism by which CNI-1493 exerts its inhibitory effects on macrophages is predominantly suppression of TNF-α mRNA translation, while the expression of TNF-α mRNA is not significantly affected by CNI-1493 (9). The p38 MAP kinase signaling cascade has been demonstrated to be crucial in the posttranscriptional regulation in the synthesis of some proinflammatory cytokines (15). Recent findings indicate that CNI-1493 inhibits the phosphorylation of p38 MAP kinase, thereby providing the molecular background for its action as an inhibitor of cytokine translation in the macrophage (10). It has previously been shown in vitro that CNI-1493 fails to have any suppressive effect on cytokine expression in lymphocytes and that IFN-γ production is not blocked (6). In that study, proinflammatory cytokine synthesis was studied at the single-cell level (using computerized image analysis) following different routes of cell activation, and it was demonstrated that the production of IL-2, IFN, and TNF by activated T cells was not affected by CNI-1493 treatment (6). On the other hand, similar treatment resulted in a profound inhibition of lipopolysaccharide-induced production of TNF, IL-1, IL-6, and IL-8 by macrophages, independently of IFN priming (6). This is in line with the present study, because the number of IFN-γ-producing cells in the spleen was unaffected by the administration of CNI-1493. Additionally, the capacity of CNI-1493 to override IFN-γ-induced steroid-resistant inflammation was also shown in this study, in accordance with previous reports (3, 6, 17).
Our results indicate that inhibition of TNF-α production in the spleen in CNI-1493-treated animals was associated with reduced mortality rates compared with results for nontreated animals. Previously, it has been demonstrated in vitro that secondarily to inhibition of TNF-α by CNI-1493, the production of other proinflammatory cytokines and chemokines, including IL-1, IL-6, IL-8, MIP-1α, and MIP-1β, was also suppressed (3, 6). In the present study, numbers of IL-1β-producing cells in the spleen were significantly reduced in CNI-1493-treated animals, possibly also contributing to the low mortality rate in this group.
The accumulation of leukocytes in CSF has been demonstrated to be one main contributing factor in the CNS injury associated with bacterial meningitis (24). It has previously been shown that blocking of receptors for leukocyte-endothelial adhesion prevents transmigration of leukocytes into the CSF, reduces neuronal cell apoptosis (7), and increases survival in experimental bacterial meningitis (24). Our findings that the numbers of infiltrating granulocytes in brain sections were significantly reduced in CNI-1493-treated animals compared to results for controls indicated a protective effect on the CNS inflammatory response by CNI-1493. It is likely that this contributed to increased survival rates in this model, although the mechanism by which CNI-1493 inhibited granulocyte traversal of the blood-brain barrier was not clear. One explanation could be that several mediators in the granulocyte extravasation process were suppressed by CNI-1493. TNF-α and IL-1β are known to activate leukocyte adhesion receptors (selectins and integrins), which is a prerequisite for extravasation of leukocytes to the site of inflammation (14). Chemokines, such as MIP-1α, MIP-1β, and MIP-2, activate and attract leukocytes, leading to extravasation and accumulation of these cells in the inflamed area (23). Moreover, it has recently been shown with the infant rat model with Hib-induced CNS inflammation that neutralization of MIP-1α and MIP-2 with monoclonal antibodies significantly reduced the number of neutrophils in brain tissue (11). Other possible protective effects exerted by CNI-1493 in vivo could be the reduction of nitric oxide and cellular apoptosis, as indicated by Villa et al. (28). There is evidence that CNI-1493 has the capacity to interfere in the inflammatory cascade on several different levels, conferring protection against lethal Hib infection in infant rats. However, the compound does not seem to have any direct antimicrobial properties in itself, since bacterial growth was unaffected in the presence of even a high concentration of CNI-1493.
In the present study, three groups of animals received a suboptimal dose of cefotaxime (50 mg/kg twice daily for 4 days; first dose, 12 h after infection). The main reason for this treatment was not to sterilize the CSF compartment but rather to observe possible differences in survival rates of CNI-1493-treated animals with different kinetics of bacterial growth. The survival rate, however, was the same for the group treated with CNI-1493 only and the group treated with CNI-1493 plus cefotaxime. Treatment with cefotaxime alone protected 14% of the animals from death in this model. When animals receiving cefotaxime were pretreated with glucocorticosteroids (dexamethasone, 1 h before infection and 24 h later), mortality was increased to 100% within 48 h. One explanation for this fast death could be that it is due to the immunosuppressive effects exerted by glucocorticosteroids. With this dosage (pretreatment) of corticosteroids the negative effects obviously outweighed the positive, immunomodulatory effects documented for the treatment of bacterial meningitis when an adequate dose of antibiotics is given at the same time. Additionally, IFN-γ expression was already noticed in the spleen 3 h after Hib exposure, leaving a very short time frame for steroid-mediated antiinflammatory action.
In conclusion, our data indicate that by down-modulating the very initial inflammatory response with prophylactic treatment with a tetravalent guanylhydrazone (CNI-1493) (5 mg/kg, given i.p.), survival rates were increased by 75% for infant rats with systemic and CNS inflammation induced with Hib. Treatment with CNI-1493 was demonstrated to strikingly reduce the number of TNF-α- and IL-1β-producing cells in the spleen and the number of infiltrating granulocytes in the brain. Our findings illustrate a potential treatment strategy with a macrophage suppressive compound as a novel therapeutic approach to reduce CNS inflammatory damage in bacterial meningitis.
ACKNOWLEDGMENTS
We thank Lena Radler for excellent technical assistance and Ragaa Eltayeb and Ahmed Sharafeldin for skilled experimental support. We are grateful for the donations of cytokine-specific antibodies from Peter van der Meide (TNF-α and IFN-γ) and Monica Sang (IL-1β).
This work was supported by The Swedish Society for Medical Research, the Swedish Medical Research Council (10850), the Clas Groschinski Memorial Foundation, the Bert von Kantzow Foundation, and “Förenade Liv” Mutual Group Life Insurance Company, Stockholm, Sweden.
REFERENCES
- 1.Andersson J, Abrams J, Björk L, et al. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994;83:16–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi M, Ulrich P, Bloom O, et al. An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality. Mol Med. 1995;1:254–266. [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi M, Bloom O, Raabe T, et al. Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone. J Exp Med. 1996;183:927–936. doi: 10.1084/jem.183.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björk L, Andersson U, Chauvet J M, Skansén-Saphir U, Andersson J. Quantification of superantigen-induced IFN-gamma production by computerised image analysis—inhibition of cytokine production and blast transformation by pooled human IgG. J Immunol Methods. 1994;175:201–213. doi: 10.1016/0022-1759(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 5.Björk L, Fehniger T, Andersson U, Andersson J. Computerized assessment of production of multiple human cytokines at the single-cell level using image analysis. J Leukoc Biol. 1996;59:287–295. doi: 10.1002/jlb.59.2.287. [DOI] [PubMed] [Google Scholar]
- 6.Björk L, Tracey K J, Ulrich P, Bianchi M, Fehniger T, Åkerlund K, Andersson U, Andersson J. Targeted inhibition of cytokine production by a tetravalent guanylhydrazone (CNI-1493) in monocytes but not in T-lymphocytes. J Infect Dis. 1997;176:1303–1312. doi: 10.1086/514126. [DOI] [PubMed] [Google Scholar]
- 7.Braun J S, Novak R, Herzog K H, Bodner S M, Cleveland J L, Tuomanen E I. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 8.Braun J S, Tuomanen E I. Molecular mechanisms of brain damage in bacterial meningitis. Adv Pediatr Infect Dis. 1999;14:49–71. [PubMed] [Google Scholar]
- 9.Cohen P, Nakshatri H, Dennis J, Caragine T, Bianchi M, Cerami A, Tracey K J. CNI-1493 inhibits monocyte/macrophage tumor necrosis factor by suppression of translation efficiency. Proc Natl Acad Sci USA. 1996;176:1303–1312. doi: 10.1073/pnas.93.9.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen P S, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey K J. The critical role of p38 MAP kinase in T-cell HIV-1 replication. Mol Med. 1997;3:339–346. [PMC free article] [PubMed] [Google Scholar]
- 11.Diab A, Abdalla H, Li H L, Shi F D, Zhu J, Höjeberg B, Lindquist L, Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diab A, Zhu J, Lindquist L, Wretlind B, Link H, Bakhiet M. Cytokine mRNA profiles during the course of experimental Haemophilus influenzae bacterial meningitis. Clin Immunol Immunopathol. 1997;85:236–245. doi: 10.1006/clin.1997.4430. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 14.Lawrence M B, Springer T A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 15.Lee J C, Laydon J T, McDonnell P C, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1995;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 16.Litton M, Sander B, Murphy E, O'Garra A, Abrams J. Early expression of cytokines in lymph nodes after treatment in vivo with SEB. J Immunol Methods. 1994;175:47–58. doi: 10.1016/0022-1759(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 17.Luedke C E, Cerami A. Interferon-gamma overcomes glucocorticoid suppression of cachectin/tumor necrosis factor biosynthesis by murine macrophages. J Clin Investig. 1990;86:1234–1240. doi: 10.1172/JCI114829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn W A, Cohen J. Adjunctive therapy for septic shock: a review of experimental approaches. Clin Infect Dis. 1995;20:143–158. doi: 10.1093/clinids/20.1.143. [DOI] [PubMed] [Google Scholar]
- 19.Moxon E R, Smith A L, Averill D R, Smith D H. Haemophilus influenzae in infant rats after intranasal inoculation. J Infect Dis. 1974;129:154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- 20.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental Gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaad U B, Kaplan S L, McCracken G H., Jr Steroid therapy for bacterial meningitis. Clin Infect Dis. 1995;20:685–690. doi: 10.1093/clinids/20.3.685. [DOI] [PubMed] [Google Scholar]
- 22.Smith A L, Smith D H, Averill D R, Jr, Marino J, Moxon E R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973;8:278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–322. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 24.Tuomanen E I, Saukkonen K, Sande S, Cioffe C, Wright S D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989;170:959–968. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulfgren A K, Lindblad S, Klareskog L, Andersson J, Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Poll T, van Deventer S J H. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin N Am. 1999;13:413–426. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 27.van Furth A M, Roord J J, van Furth R. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun. 1996;64:4883–4890. doi: 10.1128/iai.64.12.4883-4890.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villa P, Meazza C, Sironi M, Bianchi M, Ulrich P, Botchkina G, Tracey K J, Ghezzi P. Protection against lethal poly-microbial sepsis by CNI-1493, an inhibitor of pro-inflammatory cytokine synthesis. J Endotoxin Res. 1997;4:197–204. [Google Scholar]
- 29.Vogel U, Steinmetz I, Frosch M. Avoiding artifacts in the infant rat model for bacterial meningitis: use of Sangur test strips for the rapid quantification of blood contamination in cerebrospinal fluid. Med Microbiol Immunol. 1996;185:27–30. doi: 10.1007/s004300050011. [DOI] [PubMed] [Google Scholar]
- 30.Waage A, Brandtzæg P, Halstensen A, Espevik T. Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;i:355. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 31.Waage A, Brandtzæg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 1, interleukin 6, and fatal outcome. J Exp Med. 1989;169:333. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]