Abstract
Purpose
Diabetic retinopathy (DR) is a complication of type 2 diabetes mellitus (T2DM). Lipoprotein(a) (Lp(a)) contributes to the progression of DR, but how is unclear. In homeostasis of the retinal microvasculature, myeloid-derived pro-angiogenic cells (PACs) also play a pivotal role, and fail to function properly in diabetic conditions. Here, we explored the putative contribution of Lp(a) from patients with T2DM with/without DR and healthy controls on inflammation and angiogenesis of retinal endothelial cells (RECs), and on PAC differentiation. Subsequently, we compared the lipid composition of Lp(a) from patients to that from healthy controls.
Methods
Lp(a)/LDL obtained from patients and healthy controls were added to TNF-alpha-activated RECs. Expression of VCAM-1/ICAM-1 was measured using flowcytometry. Angiogenesis was determined in REC-pericyte co-cultures stimulated by pro-angiogenic growth factors. PAC differentiation from peripheral blood mononuclear cells was determined by measuring expression of PAC markers. The lipoprotein lipid composition was quantified using detailed lipidomics analysis.
Results
Lp(a) from patients with DR (DR-Lp(a)) failed to block TNF-alpha-induced expression of VCAM-1/ICAM-1 in REC whereas Lp(a) from healthy controls (healthy control [HC]-Lp(a)) did. DR-Lp(a) increased REC angiogenesis more than HC-Lp(a) did. Lp(a) from patients without DR showed intermediate profiles. HC-Lp(a) reduced the expression of CD16 and CD105 in PAC, but T2DM-Lp(a) did not. Phosphatidylethanolamine content was lower in T2DM-Lp(a) than in HC-Lp(a).
Conclusions
DR-Lp(a) does not show the anti-inflammatory capacity seen with HC-Lp(a), but increases REC angiogenesis, and affects PAC differentiation less than HC-Lp(a). These functional differences in Lp(a) in T2DM-related retinopathy are associated with alterations in the lipid composition as compared to healthy conditions.
Keywords: angiogenesis, diabetic retinopathy (DR), lipoprotein(a) (Lp(a)), lipidomics, microvascular dysfunction, myeloid-derived pro-angiogenic cells, retinal endothelial cells (RECs)
Diabetic retinopathy (DR), with a worldwide prevalence of around 35% among people with diabetes, is a major cause of decreased eyesight and blindness.1 Based on the severity of retinal neovascularization, DR is classified into sequential stages of disease progression, that is, nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR).2 NPDR is characterized by retinal microaneurysms, hemorrhages, intraretinal microvascular abnormalities (IRMAs), and lipid exudates, whereas the prominent characteristic of PDR is aberrant new blood vessel formation in the retina.2
Retinal blood vessels are complex multicellular units consisting of endothelial cells, pericytes, vascular smooth muscle cells, and immune cells.3 DR onset is characterized by morphologic and functional changes in some of these cells, which eventually lead to retinal hypoxia.3 In addition, perivascular macrophages play a critical supportive role in homeostasis of the neurovascular network to which the retinal vasculature belongs.4 In diabetic conditions, myeloid-derived pro-angiogenic cells (PACs; formerly referred to as endothelial progenitor cells [EPCs]) fail in their protective functions toward damaged and ischemic vasculature. In these conditions, PACs display an altered phenotype due to the hypoxic and inflammatory microenvironment.5 In addition, diabetes-associated chronic low-grade inflammation leads to dysfunctional neurovascular crosstalk and dysregulated retinal angiogenesis, especially in the later stages of DR.6
Several mechanisms are associated with vascular wall disintegrity in type-2 diabetes mellitus (T2DM) and DR, including increased formation of advanced glycation end products (AGEs), oxidative stress, increased expression of growth factors, and leukostasis.7 Especially in people with T2DM, lipoprotein(a) [Lp(a)] is an independent risk factor for cardiovascular diseases.8 Lp(a) is a low density, LDL-like lipoprotein with an apolipoprotein(a) [apo(a)] that is covalently bound to apo B100.9 Atherogenic Lp(a) is associated with increased macrovascular inflammation, trans-endothelial migration of monocytes, and vascular remodeling.10 Although the association between Lp(a) and macrovascular diseases has been well established,11–13 the relationship between Lp(a) and microvascular complications, including DR, is a topic of debate. Some studies have identified high circulating Lp(a) concentrations as an independent risk factor for DR in patients with T2DM.14,15 However, data on this are inconclusive.16,17
Studies on the association between Lp(a) and DR mostly focus on epidemiological perspectives,14,15,17–20 but the mechanisms by which Lp(a) might contribute to DR remain elusive so far. Therefore, the overall aim of this study was to explore cellular mechanisms by which Lp(a) may contribute to retinal vascular dysfunction in T2DM-associated retinopathy. We first explored the relationship between circulating Lp(a) concentrations and DR in a cohort of patients with T2DM with or without DR. Second, to unravel its potential pathogenic role functionally, Lp(a) was obtained from healthy controls and patients and its direct impact on in vitro inflammatory responses and angiogenic capacity by retinal endothelial cells (RECs) was explored. In addition, the interaction of Lp(a) with myeloid-derived PACs in the context of DR was studied. Detailed lipidomics was conducted to determine putative compositional differences in Lp(a) in patients with T2DM compared to controls.
Methods
Study Design and Patients
Patients with T2DM referred to the Rotterdam Eye Hospital or the Department of Ophthalmology, Erasmus MC, were included and assigned to one of the following three groups: T2DM without DR (NoDR), with NPDR or with PDR. This diagnosis was established according to the International Disease Severity Scale based on the Early Treatment Diabetic Retinopathy Study (ETDRS) classification.21 The patients with PDR were in active status. Patients with PDR and some NPDR cases were treated with intravitreal anti-VEGF therapies. In addition, healthy controls aged 50 years or older were recruited from hospital staff. Exclusion criteria were presence of any kind of diabetes or Cushing's disease. The healthy controls were not under any specific diet or medication. In accordance with the American Diabetes Association (ADA) and World Health Organization (WHO) guidelines, diabetes was defined as a fasting plasma glucose ≥7.0 mmol/L and/or a non-fasting plasma glucose level ≥11.1 mmol/L and/or treatment with oral glucose-lowering medication or insulin or the diagnosis of T2DM registered by a medical specialist. Patients with type-1 diabetes or other types of diabetes mellitus (DM) were excluded from the study.
Patients with NoDR did not have retinal alterations seen with ophthalmoscopy. Patients with NPDR had more than one of the following retinal alterations: microaneurysms, blood, hard exudates, cotton wool spots, venous looping or beading, and IRMAs. Patients with PDR exhibited vitreous or preretinal hemorrhage with neovascularization of the disc or elsewhere. Written informed consent was obtained from all participants. The study was approved by the local medical ethics committee of Erasmus MC (MEC-2018-148 and MEC-2016-202) and is conducted in accordance with the ethical principles of the Declaration of Helsinki.
Serum, Plasma, and PBMC Isolation
Peripheral blood was collected from patients for serum, plasma, and peripheral blood mononuclear cells (PBMCs) isolation. Serum was isolated through centrifugation (1850 × g for 10 minutes) and stored at -80°C until further use. PBMCs and plasma were isolated through standard Ficoll-Paque gradient centrifugation.22 Plasma was stored at -80°C until further use. PBMCs were transferred into new tubes, washed (with phosphate-buffered saline [PBS] and centrifuged for 10 minutes at 760 × g) and subsequently resuspended in RPMI freezing medium (containing 40% fetal bovine serum [FBS] + 10% dimethyl sulfoxide; Sigma-Aldrich, St Louis, MO, USA) and stored in liquid nitrogen until further use.
Lp(a) and LDL Isolation
Lipoproteins were isolated from 10 mL of plasma and serum (5 mL from each). The plasma or serum was brought to a density (d) = 1.210 g/mL with crystalized potassium bromide (KBr) and a discontinuous gradient was formed by overlayering with 2.8 mL of d = 1.063 g/mL KBr, followed by 2.8 mL of d = 1.019 g/mL KBr, and finally 2.6 mL of d = 1.006 g/mL KBr. Samples were centrifuged at 273,620.3 × g (18 hours at 4°C) in a Beckman centrifuge (SW41-rotor; Beckman Coulter Inc., Brea, CA). Subsequently, the gradient was fractionated in 0.25 mL fractions and Lp(a)- and LDL-containing fractions were selected based on density. Cholesterol was measured in all fractions using CHOD-PAP (an enzymatic photometric test; Diasys Diagnostic System, GmbH, Holzheim, Germany). Lp(a) was measured in fractions 14 to 23, applying a Kringle IV-independent immunoturbidimetric assay (DiaSys Diagnostic system; GmbH, Holzheim, Germany) with an apo(a) concentration range of 3 to 300 mg/dL. The 3 fractions with the highest Lp(a) concentration were pooled as were the 3 fractions with the highest LDL concentration level, and dialyzed overnight with at least one refreshment of 1 × adapted PBS (175 g NaCl, 28 g Na2HPO4, 4.3 g KH2PO4, in 2 liter MilliQ, pH 7.1). Next, samples were filter-sterilized using 0.2 µm pore filters (Sigma Aldrich) and reconcentrated at 4500 rpm (20 minutes at 20°C) using a Vivaspin 6 concentrator (10 kDa MWCO polyethersulfone; GE Healthcare).
Immunoblotting
The apo(a) KIV-2 repeat number was determined using Western blot, as previously described.9 In subjects with two distinct apo(a) isoforms, the band representing the smaller isoform was used as a continuous variable.
3-D In Vitro Tubule Formation Assay
To explore the effect of lipoproteins on retinal vessel formation, we made use of the 3-D retinal tubule formation assay we described previously.23 Briefly, human microvascular RECs (cat # ACBRI 181; Cell Systems, Troisdorf, Germany) were transduced with GFP-tagged lentiviral vector and co-cultured with human brain vascular pericytes (cat # SCC1200-KIT; Sciencell research, San Diego, CA, USA) in a collagen matrix (bovine collagen type-1, cat # A1064401; Gibco) in the presence of three pro-angiogenic growth factors (interleukin [IL]-3, stem cell factor [SCF], and stromal cell-derived factor 1-alpha [SDF1-alpha]; R&D systems, Abingdon, UK; each factor 25 ng/mL [low concentration {Lo GF}] or 200 ng/mL (high concentration {Hi GF}] as indicated). After 24 hours, successful sprouting of endothelial cells was typically observed, and the lipoproteins were added to the medium above the collagen matrix. After another 3 days of culture, tubule formation of the RECs was imaged at 20 times magnification with an inverted fluorescence microscope (Olympus SC30, Shinjuku, Japan). Total surface area and total tubule length were quantified and expressed as percentages of total image surface by FIJI software (version 1.51n).
TNF-Alpha-Induced Adhesion Molecule Expression by REC
RECs (passages 4-7) were seeded on gelatin-coated 48-well plates (40,000 cells per well; Thermo Fisher) in 250 µL endothelial cell growth medium (EGM) containing 5% FBS (Gibco) and cultured for 48 hours (37°C at 5% CO2). Subsequently, after 3 hours of incubation with 0.3 g/L Lp(a) or cholesterol-matched LDL, TNF-alpha (100 ng/mL, cat # 210-TA-005; R&D systems) was added to the cells, and incubation was continued for another 17 to 18 hours. Next, the culture medium was collected, centrifuged, and the supernatant was stored at -80°C until analysis for cytokines. The adherent cells were harvested in PBS (calcium- and magnesium-free) containing 1 mM EDTA, and analyzed for adhesion molecule expression by flow cytometry using antihuman fluorescently labeled monoclonal antibodies against CD106 (VCAM-1)-PE (STA, eBioscience) and CD54 (ICAM-1)-PerCP-eFluor710 (HA58, eBioscience). Flow cytometric measurement was conducted with a FACSCanto II cell analyzer (BD Biosciences, Piscataway, NJ, USA). Viable cells were gated and data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). At least 10,000 events were acquired per sample.
Myeloid-Derived PAC Formation Assay
PBMCs isolated from a healthy blood bank donor were thawed and cultured in M199, Earle's Salts medium (cat # 11150059; Gibco) supplemented with 2% FBS and plated on fibronectin-coated tissue culture 48-well plates (0.4 × 106 cells/200 µL). From day zero, 0.3 g/L of Lp(a) or a cholesterol-matched LDL, or PBS was present. After 4 days incubation at 37°C (5% CO2), the medium was discarded, the adherent cells were harvested, washed with staining buffer (MACSima running buffer; Miltenyi Biotec, Bergisch Gladbach, Germany), and exposed to antihuman monoclonal fluorescent antibodies in a dark, cold environment according to the manufacturer's instructions. The following antibodies were used: CD14-PE-Cy7 (61D3, eBioscience), CD16 (FcγRIII)-APC-Cy7 (3G8 [RUO]; BD Pharmingen), CD105 (endoglin)-APC (166707; R&D Systems), CD133-PE (170411; R&D Systems), CD163-PerCP (GHI/61; eBioscience), CD31 (PECAM-1)-PerCP (WM59; BioLegend), anti-HLA-DR (MHC class II)-FITC (G46-6; BD Pharmingen), and CD206-FITC (15-2, MRC1; BioLegend). After 20 minutes of incubation, the cells were washed and resuspended in 200 µL staining buffer, and measured with FACSCanto II cell analyzer. Viable cells were gated and data were analyzed using FlowJo software. At least 10,000 events were acquired per sample.
Cytokine Measurement
REC culture media were analyzed for CCL2 (MCP-1), CCL5 (RANTES), G-CSF, GM-CSF, HGF, IL-1β, IL-6, PDGF-BB, and VEGF-A using a Luminex multiplex bead immunoassay (R&D Systems, Abingdon, UK) according to the manufacturer's instruction. Data acquisition was done on a Luminex MAGPIX machine and data were analyzed applying Bio-Plex Manager MP software (Bio-Rad, Hercules, CA, USA). IL-8 levels were measured by standard enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience, San Diego, CA).
Lipidomic Analysis
Of the isolated Lp(a) and LDL, 20 µg of protein was diluted in 700 µL water and mixed with 800 µL 1 N HCl:CH3OH 1:8 (v/v), 900 µL CHCl3, 200 µg/mL of the antioxidant 2,6-di-tert-butyl-4-methylphenol (BHT; Sigma Aldrich), and 3 µL of SPLASH LIPIDOMIX Mass Spec Standard (# 330707; Avanti Polar Lipids). After vortexing and centrifugation, the lower organic fraction was collected and evaporated using a Savant Speedvac SPD111V (Thermo Fisher Scientific) at room temperature. The remaining lipid residue was stored at -20°C under argon. Lipid species were measured with a hydrophilic interaction chromatography (HILIC) liquid-chromatography tandem mass spectrometry (LC-MS/MS) method on a Nexera X2 UHPLC system (Shimadzu) coupled with hybrid triple quadrupole/linear ion trap mass spectrometer (6500+ QTRAP system; AB SCIEX), as described previously.24 Lipids were quantified based on internal standard signals in accordance with the guidelines of the Lipidomics Standards Initiative (LSI; level 2 type quantification as defined by the LSI).25
Statistics
All statistical analyses were performed using GraphPad Prism (version 5.04). Differences were analyzed by Student's t-test, Mann Whitney U t-test, Kruskal-Wallis nonparametric test, or 1-way ANOVA followed by a post hoc Tukey or Dunn's multiple comparison test. A P value < 0.05 was considered to indicate a statistically significant difference. All data are presented in means ± standard error of the mean (SEM).
Results
Demographic data and patient categories are summarized in the Table.
Table.
Demographic Data From the Healthy Controls and Patients Included in the Study
| Total Number of Patients Included in Each Experimental Setting | Healthy Control (n = 10) | T2DM + NoDR (n = 13) | T2DM + NPDR (n = 41) | T2DM + PDR (n = 7) |
|---|---|---|---|---|
| Male (%) | 5 (50) | 10 (77) | 23 (56) | 5 (71) |
| BMI (mean ± SD) | 25 ± 1 | 29.4 ± 4.3 | 30 ± 6 | 24 ± 1.5 |
| Disease duration in years, mean ± SD | NA | 8–22, 16 ± 6 | 9–40, 19 ± 8 | 9–14, 10 ± 4 |
| Insulin injection | NA | 2 (15%) | 20 (48%) | 1 (14%) |
BMI, body mass index; NA, not applicable.
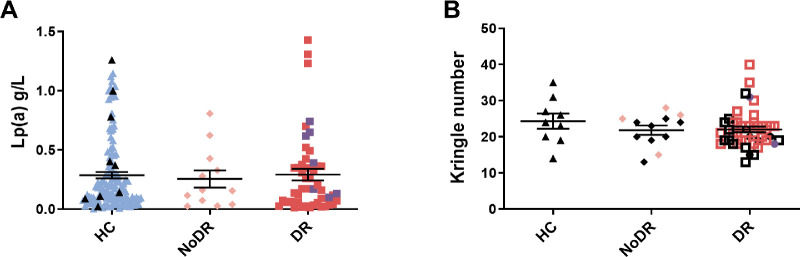
Circulating Lp(a) Concentration and Apo(a) Size do not Correlate With DR Status and Disease Progression
In our patient cohort, we observed no differences in serum Lp(a) concentrations between patients with T2DM patients and healthy controls, or between patients without DR, with NPDR, or with PDR (Fig. 1A). It is well documented that concentrations of Lp(a) correlate inversely with the size of the apo(a) determined by the number of Kringle IV-type 2 (KIV-2) repeats, and therefore are highly genetically determined.26 To validate our findings further we investigated the sizes of apo(a) of patients and healthy controls using Western blot. No differences in the KIV-2 repeat numbers were observed between patients and healthy controls, nor between patients with T2DM without DR, with NPDR, or with PDR (Fig. 1B, Supplementary Fig. S1).
Figure 1.
Lipoprotein(a) (Lp(a)) concentration and apolipoprotein(a) (apo(a)) size do not differ between healthy controls and patients with T2DM with diabetic retinopathy (DR) or without DR (NoDR). (A) Lp(a) measured in plasma of a large healthy control cohort (blue symbol, n = 118) and the healthy controls that were included in the functional experiments (black symbol, n = 9, patients with T2DM without DR (NoDR, n = 12), and patients with T2DM with DR (including nonproliferative DR [red; n = 41] and proliferative DR [purple; n = 7]). (B) The apo(a) KIV-2 repeat numbers for healthy controls (n = 9), patients with NoDR (n = 12), and patients with DR (NPDR [squares; n = 39] and PDR [circles; n = 7]). Black symbols indicate the participants who were included in the functional experiments.
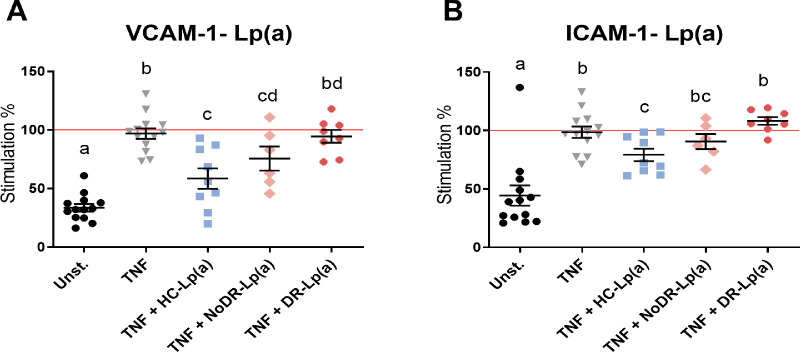
DR-Lp(a) Elicits Decreased Capacity to Inhibit Retinal Endothelial Inflammation and Secretion of Angiogenic Mediators Compared to HC-Lp(a)
We next hypothesized that when Lp(a) concentrations do not differ, putative functional differences between patients and HC-Lp(a) might exist and contribute to microvascular dysfunction in DR. Therefore, we subsequently tested the effect of Lp(a) from patients and healthy controls on the inflammatory response of human primary REC. Flow cytometry showed that the expression of adhesion molecules VCAM-1 (CD106) and ICAM-1 (CD54) was enhanced in REC after 24 hours of stimulation with TNF-alpha (Figs. 2A, 2B). Lp(a)/LDL from healthy controls and patients were added to the cells 3 hours before TNF-alpha stimulation. Lp(a) from healthy controls significantly inhibited TNF-alpha-induced upregulation of the expression of VCAM-1 (P = 0.0006) and ICAM-1 (P = 0.01; see Figs. 2A, 2B). Lp(a) from patients with T2DM without DR (NoDR) also inhibited TNF-alpha-induced upregulation of VCAM-1, but to a lesser extent than Lp(a) from healthy controls (P = 0.037). Lp(a) from patients with DR did not affect TNF-alpha-induced upregulation of VCAM-1 and ICAM-1. Under basal (non-TNF-stimulated) conditions, Lp(a) from healthy controls and the patient groups did not affect VCAM-1 and ICAM-1 expression (data not shown). In comparison, similar but less pronounced effects on VCAM-1 were observed with LDL (Supplementary Figs. S2A, S2B), whereas LDL had no effect on TNF-alpha-induced ICAM-1 expression. Collectively, these data suggest that Lp(a) from patients with T2DM, and particularly Lp(a) from patients with DR, has a decreased capacity to inhibit inflammatory retinal endothelial activation.
Figure 2.
Lipoprotein(a) (Lp(a)) from patients with diabetic retinopathy (DR) are incapable of inhibiting TNF-alpha-induced expression of adhesion molecules in RECs. Lp(a) was isolated from healthy controls (HCs, n = 9), patients with T2DM without DR (NoDR, n = 6), and patients with T2DM with DR (DR, n = 8). In a 24-hour culture condition, RECs were activated with TNF-alpha and further stimulated with Lp(a). The expression of (A) VCAM-1 (CD106) and (B) ICAM-1 (CD54) in Lp(a)-stimulated RECs was determined using flow cytometry and expressed as median fluorescence intensity (MFI), and normalized to TNF-alpha control stimulated cells. Different letters indicate statistically significant differences. Significance was calculated compared to the TNF-alpha + PBS condition (TNF) using 1-way ANOVA followed by post hoc Tukey multiple comparison test (P < 0.05).
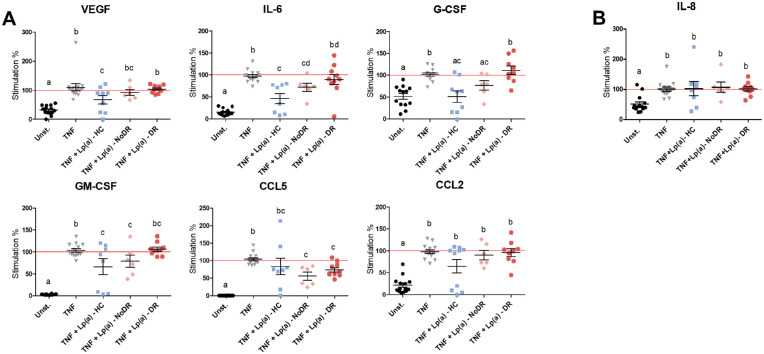
To investigate whether REC activation and the Lp(a) effect on this was also reflected in inflammatory cytokine production, we analyzed the cell culture media of the experiments described above for different cytokines known to be involved in angiogenesis using a cytometric bead array. Incubation of REC for 24 hours with TNF-alpha increased the release of VEGF, IL-6, G-CSF, GM-CSF, CCL5, CCL2, and IL-8 into the extracellular medium (Figs. 3A, 3B). Lp(a) from healthy controls, and to some extent Lp(a) from patients with NoDR, inhibited TNF-alpha-induced release of VEGF, IL-6, G-CSF, and GM-CSF (see Fig. 3). In contrast, Lp(a) from patients with DR displayed a significantly reduced capacity to inhibit the TNF-alpha-induced release of these cytokines in REC. In addition, Lp(a) from patients with T2DM, both without or with DR, reduced the TNF-alpha-induced release of CCL5 in RECs. Lp(a) from healthy controls and patients did not affect the basal production (in the absence of TNF-alpha stimulation) of these factors by RECs (data not shown). At cholesterol-matched concentrations, LDL from healthy controls and patients did not affect the release of the cytokines measured (Supplementary Fig. S3).
Figure 3.
Lipoprotein(a) (Lp(a)) from patients with diabetic retinopathy (DR) shows decreased ability to inhibit inflammatory and angiogenic cytokine production in RECs. The RECs were incubated simultaneously for 24 hours with TNF-alpha and Lp(a). Cytokine levels were determined in the extracellular medium using (A) multiplex assay or (B) ELISA. Levels are normalized to the TNF-alpha control. Different letters indicate significant differences. Significance was calculated compared to the TNF-alpha + PBS condition (TNF) using 1-way ANOVA followed by post hoc Tukey or Dunn's multiple comparison test (P < 0.05).
DR- and HC-Lp(a) Differently Affect Retinal Microvascular In Vitro Neovascularization
As apo(a) was shown to inhibit in vitro tubule/vessel formation of macrovascular endothelial cells (EC),27 we investigated the effect of Lp(a) on microvascular retinal neovascularization in an in vitro 3D tubule formation assay. Lp(a) obtained from a non-T2DM donor with a high-plasma Lp(a) level was added in two concentrations (0.3 g/L and 0.9 g/L) to the assay with high growth factor (Hi GF; 200 ng/mL) and low growth factor (Lo GF; 25 ng/mL) culture conditions. Non-T2DM donor Lp(a) significantly reduced tubule formation by RECs in a dose-dependent manner, both when co-incubated with Hi GF and Lo GF (data not shown, and Supplementary Fig. S4, respectively). This was observed both for total tubule surface area (Supplementary Fig. S4B) and for total tubule length (Supplementary Fig. S4C). In marked contrast, cholesterol-matched LDL from the same donor slightly increased in vitro tubule formation by RECs in the presence of Hi GF and Lo GF (data not shown).
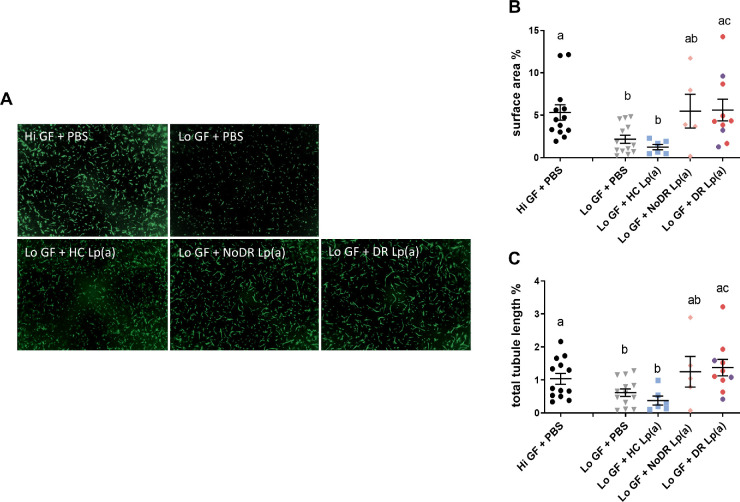
Because Lp(a) from the non-T2DM donor significantly inhibited tubule formation at a concentration of 0.3 g/L, and blood volumes from patients were restricted, we decided to use 0.3 g/L in the in vitro tubule formation assay as standard concentration, which appeared to be achievable for all patient samples. In a subsequent pilot assay, we tested the effect of Lp(a) from two patients with DR and observed that this did not inhibit, but even increased retinal vessel formation (data not shown). Accordingly, we decided to use the Lo GF culture conditions to study the modulatory effects of Lp(a) isolated from DR patients in the endothelial tubule formation assay as this allowed detection of both inhibitory and stimulatory effects.
Next, we assessed Lp(a) and LDL from patients with T2DM without DR (NoDR, n = 5), patients with T2DM with DR (n = 13, 9 with NPDR and 4 with PDR) as well as healthy controls in the tubule formation assay. In line with our previous observation, Lp(a) from healthy controls slightly, but not significantly, reduced in vitro tubule formation (Figs. 4A-C). In contrast, DR-Lp(a) significantly enhanced in vitro tubule formation compared to Lo GF HC-Lp(a) and Lo GF PBS control (see Figs. 4A-C). NoDR-Lp(a) stimulated tubule formation to a similar level, but this did not reach statistical significance, due to the high degree of variability and small sample size. Cholesterol-matched LDL from patients with DR stimulated in vitro tubule formation when determined by total tubule surface area, but not LDL from healthy controls or from NoDR patients (Supplementary Figs. S5A, S5B). Collectively, these results show that Lp(a) in patients with T2DM with DR displays altered functionality and stimulates retinal vessel formation in vitro, in contrast to Lp(a) from healthy controls.
Figure 4.
Lipoprotein(a) (Lp(a)) from patients with diabetic retinopathy (DR) increase RECs in vitro angiogenesis. Lp(a) was isolated from healthy controls (HCs, n = 6), patients with T2DM without DR (NoDR, n = 5), and patients with T2DM with DR (nonproliferative DR [red, n = 10] and proliferative DR [purple, n = 3]). The 3D co-cultures of RECs and pericytes were stimulated to form tubules using 200 ng/mL (Hi GF) or 25 ng/mL (Lo GF) of angiogenic growth factors in the presence or absence of Lp(a). (A) Representative images from different culture conditions imaged after 4 days. The tubules were quantified as (B) total surface area percentage and (C) total tubule length of the vessels, both expressed as percentage of total pixels. Values show means ± SEM from 13 independent experiments. Different letters indicate significant difference. Significance was calculated using 1-way ANOVA followed by post hoc Tukey or Dunn's multiple comparison test (P < 0.05).
HC- and DR-Lp(a) Affect Myeloid-Derived Pro-Angiogenic Cell Formation Differently
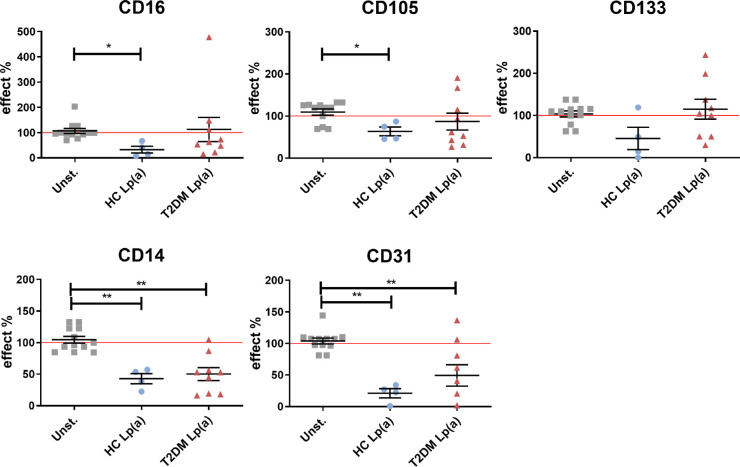
There are several indications that hampered myeloid-derived PAC formation and function contributes to vascular complications in diabetic conditions.28,29 To investigate whether Lp(a) could play a role in PAC dysfunction in diabetes, we cultured PBMC isolated from a healthy control donor under PAC-differentiating conditions for 4 days and stimulated the cultures with HC- and T2DM-Lp(a) or -LDL from the initiation of the culture. As indicators of PAC differentiation, phenotypic markers were used, in particular CD105 (endoglin) and CD133 (prominin-1), as indicated by literature,30 as well as the endothelial marker CD31 (PECAM-1), and monocyte-/ macrophage markers CD14 and CD16. Due to the small number of patients with NoDR and patients with DR, and because no difference was found between Lp(a) effects from the two groups in PAC marker expression, both were grouped together (referred to as T2DM). Flow cytometric analysis revealed that HC-Lp(a) reduced expression of CD16 and CD105 in PAC differentiation (Fig. 5). In contrast, Lp(a) from patients with T2DM did not affect the expression of these markers. Expression of the monocyte and endothelial cell markers CD14 and CD31 was reduced by Lp(a) from healthy controls as well as patients with T2DM (see Fig. 5). In comparison, LDL from healthy controls only reduced the expression of CD133, whereas LDL from patients with T2DM did not affect expression of any of the markers examined (Supplementary Fig. S6). Based on these data, we propose that Lp(a) from healthy controls and patients with T2DM have different impacts on the differentiation of monocyte-derived PACs.
Figure 5.
Lipoprotein(a) (Lp(a)) from healthy controls (HCs) and patients with T2DM affects myeloid-derived pro-angiogenic cell (PAC) differentiation. Lp(a) was present in the PAC formation culture from day zero. On day 4, expression of cell differentiation markers was determined by flow cytometry. Marker expression was expressed as median fluorescence intensity (MFI) after correction for the auto-fluorescence, and normalized to unstimulated controls. Error bars represent the standard error of the mean (SEM). Differences were analyzed by Mann Whitney U t-test. * P < 0.05, ** P < 0.01.
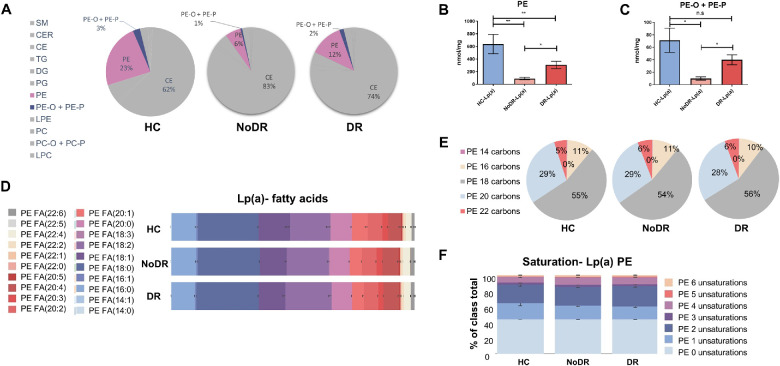
The Lipid Composition of Both Lp(a) and LDL From Patients With T2DM With and Without DR Differs from that of Healthy Controls
To investigate whether observed functional differences between Lp(a) and LDL from patients with T2DM and healthy controls are reflected in particle lipid composition, we performed detailed lipidomics analysis. Data found that Lp(a) and LDL from patients with T2DM with and without DR differ markedly in lipid composition compared to Lp(a) and LDL from healthy controls (Fig. 6 for Lp(a), see Supplementary Fig. S7 for LDL). The most remarkable difference was found in phosphatidylethanolamine (PE) content (see Fig. 6A). PE content was much lower in Lp(a) from patients without DR than from patients with DR, and both contained significantly less PE than Lp(a) from healthy controls (see Fig. 6B). This relative paucity in PE content was compensated in particular by increased cholesterol ester content. The levels of the plasmalogens 1-alkyl,2-acylphosphatidylcholine and 1-alkenyl,2-acylphosphatidylcholine (PE-O + PE-P) differed in parallel with PE among the three groups (see Figs. 6A, 6C). Similar pronounced differences in PE and plasmalogen content were found for LDL particles isolated from the two patient groups (Supplementary Figs. S7A, S7B, S7C). The distribution of PE-associated fatty acid species was similar in Lp(a) and LDL from healthy controls, patients with NoDR, and patients with DR (see Figs. 6D, 6E, Supplementary Figs. S7D, S7E). We also did not find differences in the degree of unsaturation of acyl chains associated with PE between healthy controls and patient groups, neither for Lp(a) nor for LDL particles (see Fig. 6F, Supplementary Fig. S7F). No correlation was found between the Lp(a) composition and the age of the patients and healthy individuals (data not shown).
Figure 6.
Lipoprotein(a) (Lp(a)) from healthy controls (HCs) and patients with T2DM without diabetic retinopathy (NoDR) and T2DM with diabetic retinopathy (DR) show compositional differences. Lp(a) was isolated from healthy controls (n = 8), patients with NoDR (n = 7), and patients with DR (n = 16), cholesterol content was measured and particles were subjected to lipidomic analysis. All lipid concentrations were normalized to cholesterol content of the Lp(a) particles. (A) An overview of the lipid classes analyzed in Lp(a) of healthy controls, NoDR, and DR groups. SM, sphingomyelins; CER, ceramides; CE, cholesterol esters; TG, triacylglycerides; DG, diacylglycerides; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; PE-O + PE-P, 1-alkyl,2-acylphosphatidylethanolamines + 1-alkenyl,2-acylphosphatidylethanolamines; LPE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PC-O + PC-P, 1-alkyl,2-acylphosphatidylcholine + 1-alkenyl,2-acylphosphatidylcholine; LPC, lysophosphatidylcholine. Quantification of (B) PE and (C) PE-O + PE-P contents in Lp(a) from different patient groups. (D) Relative fatty acid composition of PE in Lp(a). (E) PE acyl chain length of Lp(a). (F) Number of unsaturations in the acyl chains in PE of Lp(a). The values show means ± SEM in Lp(a) isolated from different healthy controls or patients. Differences were analyzed by Student t-test or Mann Whitney U t-test. * P < 0.05, ** P < 0.01.
Discussion
Our data demonstrate that Lp(a) from patients with T2DM with DR shows major functional differences compared to Lp(a) from healthy controls, and minor differences with that of patients with T2DM but not diagnosed with DR. These alterations are reflected in inflammatory and angiogenic responses of microvascular REC and differentiation of myeloid-derived PAC. These findings might be related to the different lipid composition of Lp(a) in patients with DR and healthy people.
In our relatively small cohort of patients, we could not find any association between the circulating Lp(a) concentration and different stages of DR. Similarly, Singh et al.17 found no association between Lp(a) level and development of DR in a large cohort of controls and patients of European descent. However, most studies performed in Asian populations found a positive correlation between serum Lp(a) concentration and development of DR.15,18,31 It is likely, therefore, that ethnicity plays a role in the link between Lp(a) and DR, but additional investigations in larger populations are required to clarify this.
Increased levels of pro-inflammatory cytokines and activated adhesion molecules, along with vascular dysfunction in patients with diabetes play key roles in the initiation and progression of DR.32 Here, we demonstrate that HC-Lp(a) inhibits the induction of the expression of pro-inflammatory VCAM-1 and ICAM-1, and also the release of some pro-inflammatory and pro-angiogenic cytokines by TNF-alpha-activated RECs. These data add to earlier works that showed contributions of Lp(a) from healthy controls to wound healing and tissue repair.33 In contrast, such anti-inflammatory and anti-angiogenic properties of Lp(a) were remarkably impaired in patients with DR. Apo(a) has previously been shown to induce the expression of adhesion molecules by endothelial cells through triggering of Rho/Rho kinase signaling.34,35 It is conceivable that the absence of anti-inflammatory capacity of DR-Lp(a) contributes to an exacerbated pro-inflammatory status in the presence of other pro-inflammatory factors in hyperglycemic conditions, such as T2DM.
Lp(a) has been identified as an inhibitor of angiogenesis and tumor growth (reviewed in ref. 36). In our 3-D in vitro REC angiogenesis model, Lp(a) from healthy individuals indeed showed a slight anti-angiogenic effect although this was not statistically significant, likely due to the small sample size. Apo(a) has some shared features with angiostatin, including the inhibition of angiogenesis, which may be related to the high sequence homology between these molecules.27 In accordance, a recombinant form of apo(a) (r-apo(a)) was found to suppress tubule formation induced by basic fibroblast growth factor (b-FGF)/TNF-alpha in 3-D human microvascular endothelial cells (hMVEC).37 The anti-angiogenic function of HC-Lp(a) observed in our REC-based model might be attributed to the apo(a) suppression of b-FGF, which was a growth factor in our culture system. In marked contrast, Lp(a) isolated from patients with T2DM with DR stimulated REC tubule formation in our in vitro angiogenesis model. Interestingly, we also found that DR-LDL slightly stimulates REC in vitro angiogenesis. Lp(a) and LDL from patients with T2DM with DR may share similar compositional properties, such as shown here for PE content, or may have similar molecules attached to the particles, which may be responsible for the comparable pro-angiogenic characteristics. However, this appears not to be the case for Lp(a) and LDL derived from healthy controls or from patients with T2DM without DR.
Diabetes-related vascular complications are associated with hampered myeloid-derived PAC formation and function.38 PAC represent an in vitro derived subtype of CD14+CD16+ macrophages, characterized by CD105, CD133, and CD31 expression.39 These cells are known to contribute to angiogenesis and vascular integrity. However, due to their high plasticity, PAC phenotype may vary depending on the local microenvironments to which they are exposed in different disorders.40 We observed that PACs generated from PBMCs isolated from patients with T2DM showed a different phenotype with different angiogenic capacities compared to PACs generated from healthy controls-derived PBMC, implying intrinsic changes in these cells in T2DM (manuscript in preparation). In the current study, we focused on the role of Lp(a) from healthy controls versus patients with T2DM on PAC development from healthy control PBMC. These results showed that HC-Lp(a) causes a reduction in the expression of prototypical PAC markers compared to control cultures without added lipoprotein. These findings add to a previous study showing that the number of M2-like circulating monocytes with hematopoietic and PAC phenotype is decreased in T2DM.30 There is evidence indicating that Lp(a) promotes macrophage differentiation towards an M2 phenotype with pro-inflammatory capacity, and the ability to secrete several pro-inflammatory cytokines, such as IL-1 and IL-6, likely through apo(a)-associated upregulation of cyclooxygenase-2 (COX-2) mediated by Akt and glycogen synthase kinase 3-β.41,42 However, the T2DM-Lp(a) did not downmodulate the expression of CD16 and CD105, in PAC-generating cultures. This is in line with the observed anti-angiogenic function of HC-Lp(a) and pro-angiogenic function of DR-Lp(a) in REC tubule formation. The presence of myeloid-derived PACs in damaged endothelium and their contribution in neovascularization has been well established.43 It is, therefore, tempting to conclude that DR-Lp(a) indirectly stimulates retinal pathological angiogenesis via maintaining PAC pro-angiogenic properties.
Considering the functional differences of Lp(a) and LDL between patients with T2DM and healthy controls, we sought to identify the lipid profiles of the Lp(a) and LDL samples included in our functional assays. Our targeted lipidomics analyses showed that Lp(a) from patients with T2DM, contains less PE and plasmalogens as compared to HC-Lp(a), and Lp(a) from patients with NoDR contains less PE and plasmalogens than Lp(a) from patients with DR. Phospholipids have been previously reported to be markers for T2DM and associated complications.44 PE, in particular, has been associated with activation of pro-inflammatory responses in ECs in several studies.45–47 It has also been shown that PE, when expressed on the outer surface of the apoptotic cells, modulates the phagocytic function of macrophages through binding CD300a.48 It therefore remains to be explained how the low-PE content of Lp(a) from patients with T2DM is associated with the observed loss of anti-inflammatory capacity, as observed in our study. Fatty acids can also influence insulin function through stimulating inflammation and oxidative stress.44 In oxidative stress conditions, such as diabetes, reactive oxygen species (ROS) may lead to the formation of OxPLs in cell membranes, whereas Lp(a) is shown to bind OxPLs.49 Lp(a), when present at low plasma concentration, might serve as a protector against oxidative stress and inflammation-related damage.50,51 On the other hand, when present at high concentrations, Lp(a) is atherogenic possibly due to its high affinity to bind to arterial intimal proteoglycans and subsequent increase in intimal concentration of LDL with associated pro-inflammatory OxPLs.50,51 OxPLs bound to Lp(a) can also induce pro-inflammatory responses in ECs and increase trans-endothelial migration of monocytes.10,52 This pro-inflammatory activity has been linked to the interaction between Lp(a) and hyperglycemia-induced ROS, and the subsequent increased production of oxidation-specific epitopes on Lp(a) particles.53 It is tempting to speculate that the inability of DR-Lp(a) to inhibit activation of pro-inflammatory phenotype of RECs, as indicated by adhesion molecules and cytokine profile, might be ascribed to increased OxPLs content compared to HC-Lp(a).
In conclusion, the pathogenesis of DR is very complex, and involves several aspects related to microvascular regulation. In this respect, the role of lipids on the development of DR is not well known. We have shown here that Lp(a) functionality is altered in patients with T2DM, and patients with DR in particular. As a result, DR-Lp(a) loses its anti-inflammatory and anti-angiogenic activity on RECs, and directly stimulates REC angiogenesis. It also does not control PAC differentiation, which may indirectly contribute to the pathological angiogenesis of RECs in patients with DR. These functional differences are associated with changes in lipid composition, including the decrease of PE content in patients with T2DM. Therefore, we propose that metabolic alterations in patients with T2DM affect Lp(a) and LDL lipid composition. These lipoprotein particles undergo compositional changes that might play a role in the microvascular dysfunctions in T2DM and the pathogenesis of DR, as evidenced in our functional assays.
A limitation of our study is that only a small number of samples per group could be included in the functional assays. This was caused on the one hand by the limited amount of Lp(a) that could be isolated from each individual, particularly in those with low serum Lp(a) concentration, and on the other hand the high number of experimental settings. This issue restricts the ability to draw firm conclusions on putative distinctions between patients with PDR and patients with NPDR. Another limitation is that other potential factors like age, sex, and ethnicity were not considered in this study.
The mechanisms and the responsible factors by which Lp(a) exerts its anti-angiogenic and anti-inflammatory capacities, and which are affected in patients with T2DM with DR, still need to be identified. More in depth lipid profiling of Lp(a) by non-targeted lipidomics may reveal specific lipid species. Moreover, proteomic analysis may help to identify Lp(a) associated proteins. In parallel, studying the functionality of Lp(a)-stimulated PAC using the in vitro angiogenesis model may further extend our knowledge on the role of Lp(a) in the development of DR.
Supplementary Material
Acknowledgments
The authors thank their students for their invaluable contributions in collection of data, in particular Fleur de Bie, Renske den Dekker, Trishika Binda, Liona van Deursen, and Ihsane el Allali.
Supported by grants from Uitzicht (# 2015-10) and Rotterdamse Stichting Blindenbelangen. Lipidomics experiments were performed at the KU Leuven lipidomics core facility Lipometrix (http://www.lipometrix.be/).
Disclosure: M. Shariatzadeh, None; N.M.A. Nagtzaam, None; L. van Vark-van der Zee, None; C. van Holten-Neelen, None; A.J.M. Verhoeven, None; J. Dehairs, None; J.V. Swinnen, None; F. Leijten, None; J.C. ten Berge, None; J.P.M. Ciriano, None; K.T. Wong, None; M. Mulder, None; P.J.M. Leenen, None; W.A. Dik, None
References
- 1. Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018; 61(1): 29–38. [DOI] [PubMed] [Google Scholar]
- 2. Duh EJ, Sun JK, Stitt AW.. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight. 2017; 2(14): e93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lechner J, O'Leary OE, Stitt AW.. The pathology associated with diabetic retinopathy. Vision Res . 2017; 139: 7–14. [DOI] [PubMed] [Google Scholar]
- 4. Lapenna A, De Palma M, Lewis CE.. Perivascular macrophages in health and disease. Nat Rev Immunol . 2018; 18(11): 689–702. [DOI] [PubMed] [Google Scholar]
- 5. Chambers SE, O'Neill CL, O'Doherty TM, Medina RJ, Stitt AW. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013; 218(11): 1370–1375. [DOI] [PubMed] [Google Scholar]
- 6. Rübsam A, Parikh S, Fort PE.. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018; 19(4): 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Semeraro F, Cancarini A, dell'Omo R, et al.. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res . 2015; 2015: 582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berk KA, Yahya R, Verhoeven AJM, et al.. Effect of diet-induced weight loss on lipoprotein(a) levels in obese individuals with and without type 2 diabetes. Diabetologia. 2017; 60(6): 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vongpromek R, Bos S, Ten Kate GJ, et al.. Lipoprotein(a) levels are associated with aortic valve calcification in asymptomatic patients with familial hypercholesterolaemia. J Intern Med. 2015; 278(2): 166–173. [DOI] [PubMed] [Google Scholar]
- 10. Schnitzler JG, Hoogeveen RM, Ali L, et al.. Atherogenic lipoprotein(a) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Circ Res. 2020; 126(10): 1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maranhão RC, Carvalho PO, Strunz CC, Pileggi F.. Lipoprotein (a): structure, pathophysiology and clinical implications. Arq Bras Cardiol. 2014; 103(1): 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang J, Hu B, Kong L, Cai H, Zhang C.. Native, oxidized lipoprotein(a) and lipoprotein(a) immune complex in patients with active and inactive rheumatoid arthritis: plasma concentrations and relationship to inflammation. Clin Chim Acta. 2008; 390(1-2): 67–71. [DOI] [PubMed] [Google Scholar]
- 13. Enkhmaa B, Anuurad E, Zhang W, et al.. HIV disease activity as a modulator of lipoprotein(a) and allele-specific apolipoprotein(a) levels. Arterioscler Thromb Vasc Biol. 2013; 33(2): 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malaguarnera G, Gagliano C, Bucolo C, et al.. Lipoprotein(a) serum levels in diabetic patients with retinopathy. Biomed Res Int. 2013; 2013: 943505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim CH, Park HJ, Park JY, et al.. High serum lipoprotein(a) levels in Korean type 2 diabetic patients with proliferative diabetic retinopathy. Diabetes Care. 1998; 21(12): 2149–2151. [DOI] [PubMed] [Google Scholar]
- 16. Haffner SM, Klein BE, Moss SE, Klein R.. Lp(a) is not related to retinopathy in diabetic subjects. Eur J Ophthalmol. 1995; 5(2): 119–123. [DOI] [PubMed] [Google Scholar]
- 17. Singh SS, Rashid M, Lieverse AG, et al.. Lipoprotein(a) plasma levels are not associated with incident microvascular complications in type 2 diabetes mellitus. Diabetologia. 2020; 63(6): 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yun JS, Lim TS, Cha SA, et al.. Lipoprotein(a) predicts the development of diabetic retinopathy in people with type 2 diabetes mellitus. J Clin Lipidol. 2016; 10(2): 426–433. [DOI] [PubMed] [Google Scholar]
- 19. Lin J, Hu FB, Mantzoros C, Curhan GC.. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia. 2010; 53(2): 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ergün UG, Oztüzün S, Seydaoglu G. Lipoprotein (A) levels in type 2 diabetic patients with diabetic retinopathy. Med J Malaysia. 2004; 59(3): 406–410. [PubMed] [Google Scholar]
- 21. Ghanchi F, Diabetic Retinopathy Guidelines Working Group. The Royal College of Ophthalmologists' clinical guidelines for diabetic retinopathy: a summary. Eye (Lond.). 2013; 27(2): 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nilsson C, Aboud S, Karlén K, et al.. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin Vaccine Immunol . 2008; 15(4): 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shariatzadeh M, Brandt MM, Cheng C, et al.. Three-dimensional tubule formation assay as therapeutic screening model for ocular microvascular disorders. Eye (Lond.). 2018; 32(8): 1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nassar ZD, Mah CY, Dehairs J, et al.. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife. 2020; 9: e54166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liebisch G, Ahrends R, Arita M, et al.. Lipidomics needs more standardization. Nature Metabolism. 2019; 1(8): 745–747. [DOI] [PubMed] [Google Scholar]
- 26. Banach M. Lipoprotein (a) - We know so much yet still have much to learn …. J Am Heart Assoc. 2016; 5(4): e003597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu L, Boffa MB, Koschinsky ML.. Apolipoprotein(a) inhibits in vitro tube formation in endothelial cells: identification of roles for Kringle V and the plasminogen activation system. PLoS One. 2013; 8(1): e52287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menegazzo L, Albiero M, Avogaro A, Fadini GP. Endothelial progenitor cells in diabetes mellitus. BioFactors. 2012; 38(3): 194–202. [DOI] [PubMed] [Google Scholar]
- 29. Chambers SEJ, O'Neill CL, Guduric-Fuchs J, et al.. The vasoreparative function of myeloid angiogenic cells is impaired in diabetes through the induction of IL1β. Stem Cells. 2018; 36(6): 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terenzi DC, Al-Omran M, Quan A, et al.. Circulating pro-vascular progenitor cell depletion during type 2 diabetes: translational insights into the prevention of ischemic complications in diabetes. JACC Basic Transl Sci. 2019; 4(1): 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tu W-J, Liu H, Liu Q, Cao J-L, Guo M.. Association between serum lipoprotein(a) and diabetic retinopathy in Han Chinese patients with type 2 diabetes. J Clin Endocrinol Metab . 2017; 102(7): 2525–2532. [DOI] [PubMed] [Google Scholar]
- 32. Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R.. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013; 2013: 343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho T, Jung Y, Koschinsky ML. Apolipoprotein(a), through its strong lysine-binding site in KIV(10'), mediates increased endothelial cell contraction and permeability via a Rho/Rho kinase/MYPT1-dependent pathway. J Biol Chem . 2008; 283(45): 30503–30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lippi G, Guidi G.. Lipoprotein(a): from ancestral benefit to modern pathogen? QJM. 2000; 93(2): 75–84. [DOI] [PubMed] [Google Scholar]
- 35. Orsó E, Schmitz G.. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017; 12(Suppl 1): 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jawi MM, Frohlich J, Chan SY. Lipoprotein(a) the insurgent: a new insight into the structure, function, metabolism, pathogenicity, and medications affecting lipoprotein(a) molecule. J Lipids. 2020; 2020: 3491764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulter V, Koolwijk P, Peters E, et al.. Impact of apolipoprotein(a) on in vitro angiogenesis. Arterioscler Thromb Vasc Biol. 2001; 21(3): 433–438. [DOI] [PubMed] [Google Scholar]
- 38. Duong HT, Erzurum SC, Asosingh K.. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis. 2011; 14(4): 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoder MC, Mead LE, Prater D, et al.. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007; 109(5): 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chambers SEJ, O'Neill CL, O'Doherty TM, Medina RJ,, Stitt AW. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013; 218(11): 1370–1375. [DOI] [PubMed] [Google Scholar]
- 41. Buechler C, Ullrich H, Ritter M, et al.. Lipoprotein (a) up-regulates the expression of the plasminogen activator inhibitor 2 in human blood monocytes. Blood. 2001; 97(4): 981–986. [DOI] [PubMed] [Google Scholar]
- 42. Buechler C, Ullrich H, Aslanidis C, et al.. Lipoprotein (a) downregulates lysosomal acid lipase and induces interleukin-6 in human blood monocytes. Biochim Biophys Acta. 2003; 1642(1-2): 25–31. [DOI] [PubMed] [Google Scholar]
- 43. Kim S-J, Kim J-S, Papadopoulos J, et al.. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol . 2009; 174(5): 1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bagheri M, Tiwari HK, Murillo AL, et al.. A lipidome-wide association study of the lipoprotein insulin resistance index. Lipids Health Dis . 2020; 19(1): 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stafford JH, Thorpe PE.. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia (New York, NY). 2011; 13(4): 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donovan EL, Pettine SM, Hickey MS, Hamilton KL, Miller BF.. Lipidomic analysis of human plasma reveals ether-linked lipids that are elevated in morbidly obese humans compared to lean. Diabetol Metab Syndr . 2013; 5(1): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo L, Chen Z, Cox BE, et al.. Phosphatidylethanolamines modified by γ-ketoaldehyde (γKA) induce endoplasmic reticulum stress and endothelial activation. J Biol Chem. 2011; 286(20): 18170–18180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simhadri VR, Andersen JF, Calvo E, et al.. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012; 119(12): 2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tselepis AD. Oxidized phospholipids and lipoprotein-associated phospholipase A(2) as important determinants of Lp(a) functionality and pathophysiological role. J Biomed Res . 2018; 31(1): 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsimikas S, Witztum JL.. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. 2008; 19(4): 369–377. [DOI] [PubMed] [Google Scholar]
- 51. Tsimikas S, Lau HK, Han KR, et al.. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004; 109(25): 3164–3170. [DOI] [PubMed] [Google Scholar]
- 52. van der Valk FM, Bekkering S, Kroon J, et al.. Oxidized phospholipids on lipoprotein (a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016; 134(8): 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leibundgut G, Scipione C, Yin H, et al.. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J Lipid Res. 2013; 54(10): 2815–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.