Abstract
Embryonic stem cells (ESCs) are derived from the inner cell mass (ICM) of the blastocyst. ESCs have two distinctive properties: ability to proliferate indefinitely, a feature referred as “self-renewal”, and to differentiate into different cell types, a peculiar characteristic known as “pluripotency”. Self-renewal and pluripotency of ESCs are finely orchestrated by precise external and internal networks including epigenetic modifications, transcription factors, signaling pathways, and histone modifications. In this systematic review, we examine the main molecular mechanisms that sustain self-renewal and pluripotency in both murine and human ESCs. Moreover, we discuss the latest literature on human naïve pluripotency.
Keywords: CRISPR/Cas9, embryonic stem cell (ESC), epigenetics, histone modifications, human ESC (hESC) vs. mouse ESC (mESC), Klf4, naïve vs. primed pluripotency, Oct4, pluripotent stem cells (PSC), self-renewal
1. Introduction
The term pluripotency indicates the ability to generate cells from all three embryonic germ layers. Pluripotent stem cells (PSCs) are cells with an unlimited self-renewal potential that are able to differentiate into virtually every type of cell: PSCs can maintain their pluripotent state indefinitely, thereby providing a suitable model to study the mechanisms of self-renewal [1,2,3].
PSCs are derived from different stages of early embryonic development and maintain self-renewal potential in vitro using exogenous cues. There are distinctive types of PSCs, including embryonic stem cells (ESCs) and induced PSC (iPSCs): ESCs are derived from the inner cell mass (ICM) of human blastocysts (known as human ESCs, hESCs) [4,5,6] or from the ICM of the mouse pre-implantation blastocyst (known as murine ESCs, mESCs) [7,8,9,10], whereas hiPSCs are obtained from somatic cells such as dermal fibroblasts and blood cells following the activation of certain reprograming factors, including Octamer-binding protein 4 (Oct4, also known as POU5F1: POU Class 5 Homeobox 1), Nanog, Krüppel-like factor 4 (Klf4), and c-Myc [11,12,13,14,15,16,17,18].
PSCs can produce three germ layers in vitro, including endoderm, mesoderm, and ectoderm. Self-renewal is associated with cell cycle control, and there are unique mechanisms controlling the cell cycle of PSCs; these mechanisms support unlimited proliferation and capacity to differentiate (Figure 1); discriminating between self-renewal and differentiation primarily depends on the expression of specific pluripotency markers [19,20,21,22,23,24,25,26,27,28,29].
Figure 1.
ESCs are able to differentiate into three embryonic germ layers: endoderm, mesoderm, and ectoderm.
PSCs represent a tremendous tool for cell therapy, regenerative medicine, disease modeling, and drug discovery [30,31,32,33]. Several studies have shown that the pluripotent state and differentiation suppression of ESCs are regulated by core stemness transcription factors (Oct4, Nanog, and Sox2), extracellular signaling pathways, nucleus transcriptional programs, chromatin remodeling, and epigenetic modulators [21,34]. In ESCs, gene regulator networks driven by master transcription factors play a key role in pluripotency maintenance. Stemness transcription factors promote the expression of genes related to pluripotency and self-renewal, thus supporting ESC pluripotency. Understanding the molecular mechanisms underlying self-renewal and pluripotency of ESCs is vital for clinical applications.
Pluripotency is regulated by specific factors in mESCs and hESCs. Indeed, mESCs show a “naïve” state of pluripotency that is mainly dependent on Leukemia inhibitory factor (LIF) and bone morphogenetic protein-4 (BMP4) signaling, while hESCs represent a “primed” state of pluripotency which is relatively similar to the murine epiblast stem cells derived from the post-implantation blastocyst [35,36,37,38,39,40,41,42,43]. In this review, we summarize different signaling pathways and epigenetic modifiers involved in the control of pluripotency state and self-renewal of PSCs in both mESCs and hESCs.
2. Murine ESCs: Signaling Pathways Supporting Self-Renewal and Pluripotency
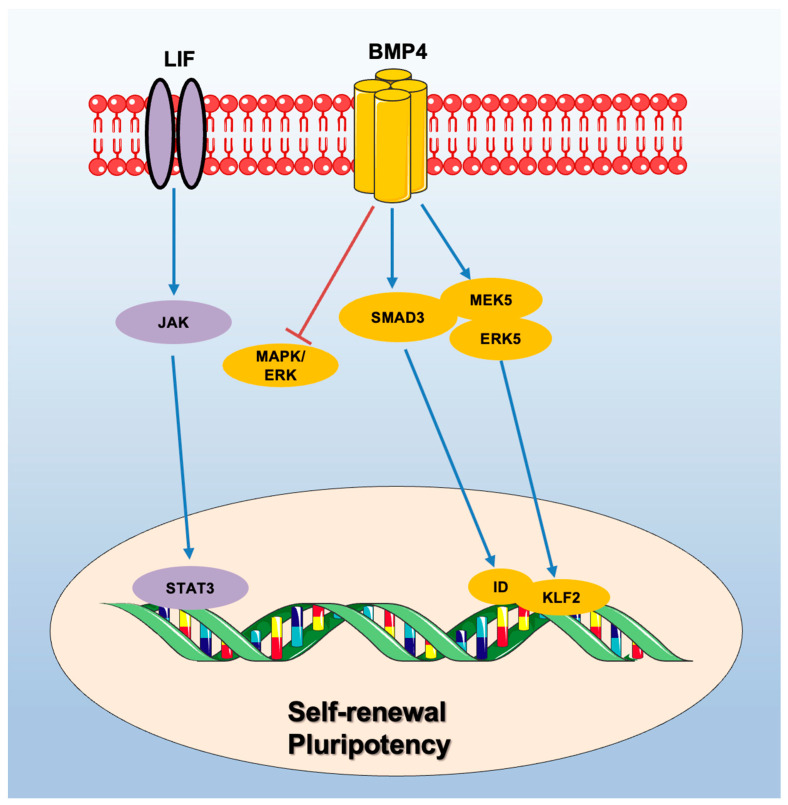
The members of the TGF-β superfamily, including TGF-β, BMP, Nodal, and Activin, are fundamental for the maintenance of pluripotency and self-renewal of stem cells during development [44,45,46]. LIF and BMP4mare two important molecular factors promoting the self-renewal capacities of mESCs. Specifically, LIF sustains the self-renewal of mESCs by activating transcription factor STAT3 (signal transducers and activators of transcription 3); however, LIF alone is insufficient to prevent neural differentiation and support self-renewal of mESCs in culture, and combination with BMP is necessary to properly induce inhibitory differentiation (Id) genes through the “Mothers Against Decapentaplegic Homolog” (Smad) pathway [47].
BMP sustains pluripotency in mESCs by maintaining an undifferentiated state through the suppression of the extracellular signal-regulated kinase ERK/MAPK pathway [48] and the activation of MEK5/ERK5 [49]. Notably, BMP signaling can induce mesodermal fate even in absence of LIF [50] (Figure 2).
Figure 2.
Major pathways involved in self-renewal and pluripotency in mouse ESCs.
The transcription factor Nanog has been shown to protect self-renewal potential of ESCs even in absence of LIF, and is generally considered a master regulator of pluripotency [51,52]. Nanog induces an undifferentiated state by repressing Gata4 and upregulating Rex1 and LIF-responsive genes, in particular Esrrb [52,53,54,55]. Oct4 is another central factor that can sustain intrinsic signaling in a LIF-independent manner to promote ES cell pluripotency and self-renewal [56,57]. Natriuretic peptide receptor A (NPR-A) upregulates gene expression of pluripotency markers (Nanog, Oct4, and Sox2) and increases levels of phosphorylated Akt, hence promoting self-renewal and pluripotency of ESCs [58]. Additionally, augmented intracellular AMP or AMP/ATP levels activate adenosine monophosphate-activated protein kinase (AMPK) signaling, which plays pivotal roles in differentiation, cell cycle, metabolism, and self-renewal of stem cells [59,60]. Indeed, Alba and co-workers demonstrated that 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), an AMPK activator, promotes stemness and self-renewal properties in mESCs via the PI3K/GSK3β regulation of Nanog expression [61].
Smad7 is a well-established negative regulator of the TGF-β/Smad signaling cascade [62]. Nevertheless, Yu and collaborators indicated that Smad7 is able to promote ESCs self-renewal in a TGF-β independent manner, by activating STAT3 signaling [63]. Epidermal growth factor receptor (EGFR) is an upstream regulator of STAT3 phosphorylation in cancer stem cells [64,65], and therefore has critical roles in proliferation, differentiation, and apoptosis [66]; specifically, EGFR inhibition in mESCs significantly reduces cell proliferation and gene expression of pluripotency markers, disrupts embryonic body (EB) formation, and upregulates differentiation genes (Gata4). These findings demonstrate the relevance of EGFR in self-renewal and pluripotency of mESCs [67]. A recent study has shown that silencing the histidine phosphatase properties of the phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) promotes self-renewal of mESCs via positively regulating the Wnt/β-Catenin pathway [68]. In addition, inhibition of PKC by CID755673 maintained pluripotency via increasing the level of AKT phosphorylation and activation of PI3K/AKT signaling [69].
Equally important, Park and colleagues reported that the knockdown of melanoma-associated antigen D1 (Maged1) causes an increase in ectodermal differentiation and reduction in ERK1/2 phosphorylation, cell cycle progression, and teratoma formation in nude mice [70]. Maged1 has been shown to be involved in many biological processes, including proliferation, differentiation, and maintenance of mESCs self-renewal [71,72].
The tumor repressor p53 gene induces cell apoptosis, senescence, and cell cycle arrest in stressed somatic cells to prevent the transmission of genetic abnormalities to their offspring [73,74]. Moreover, p53 is critical for the genomic stability of hESCs and iPSCs [75]. Chromatin immunoprecipitation (ChIP) analyses have revealed that p53 induces expression of differentiation genes and suppresses pluripotency genes [76,77]. Therefore, it has been speculated that p53 could exclude ESCs with DNA damage from a pluripotency state by repressing Nanog expression [75]. Various studies have shown that nucleolar proteins are also effective in proliferation and survival of stem cells. For instance, Nucleolin (NCL) maintains mESCs self-renewal via repression of the p53 Protein-dependent pathway; instead, NCL knock-down reduces self-renewal ability and cell proliferation, upregulating p53 protein level and inducing cell apoptosis [78].
3. Human ESCs: Signaling Pathways Involved in Self-Renewal and Pluripotency
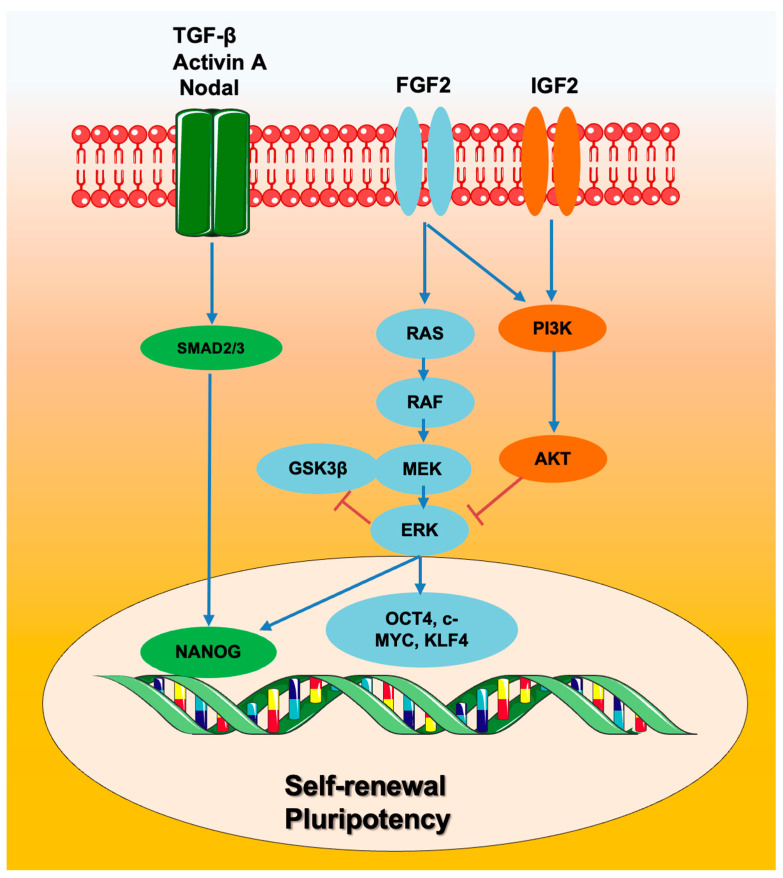
The TGF-β/Activin A/Nodal pathway has been shown to maintain the pluripotency state of hESCs by activating Smad2/3 [79,80,81]. Smad2/3 supports the self-renewal properties of hESCs through activation of Nanog [82,83]. Smad2/3 activates Nanog expression by binding the promoter of Nanog and inhibiting autocrine BMP signaling [82,84,85,86]. Inhibition of Activin A/Nodal induces differentiation by reducing gene expression of Nanog, Oct4, and Sox2. In the pluripotency state, Smad2/3 and Nanog suppress the expression of Smad-interacting protein 1 (SIP1) to inhibit neuroectodermal differentiation [87]. Furthermore, Smad2/3 can interact with the Jun N-terminal kinase (JNK)-JUN family and SnoN (SKIL) to block differentiation [88,89]. Importantly, low concentrations of Activin A (5 ng/mL) can sustain pluripotency and self-renewal of hESCs [90,91], whilst high concentrations of Activin A (50–100 ng/mL) decrease pluripotency and induce differentiation into endoderm [92]. In agreement with these finding, the chemokine (C-X-C motif) ligand 14 (CXCL14) has been shown to support the expression of self-renewal markers including Oct4 and Nanog by binding to the IGF-1R of hESCs [93]. Conditions like culturing hPSCs on simulated microgravity can enhance self-renewal properties by triggering the overexpression of pluripotent transcription factors, eventually leading to an upregulation of cell cycle regulators and reducing differentiation [94].
Generally, hESCs are cultured on Mouse Embryonic Fibroblast (MEF) or under feeder-free conditions such as on Matrigel in a medium containing basic fibroblast growth factor (bFGF) and Activin A or TGF-β. Basic Fibroblast Growth Factor (bFGF) signaling supports pluripotency, stemness, and hESCs self-renewal by activating the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, inhibiting ERK activity, WNT signaling, and dephosphorylation of glycogen synthase kinase-3 (GSK3β) [95,96,97,98,99]. The PI3K/AKT signaling pathway regulates proliferation and pluripotency of ESCs through promoting gene expression of Nanog [100,101,102]. This pathway also upregulates the gene expression of c-Myc and Tbx3 via GSK3 inhibition [103]; instead, AKT inhibition by GSK690693, AKT inhibitor VIII, and AKT inhibitor IV, induces apoptosis and reduces self-renewal of hESCs and hiPSCs [104] (Figure 3).
Figure 3.
Main signaling pathways regulating self-renewal and pluripotency in human ESCs.
Cyclin Dependent Kinase 1 (CDK1) is one of the main regulators of mitosis [105,106,107,108] and its downregulation has been shown to impair pluripotency and promote differentiation by suppressing the PDK1-PI3K/AKT pathway [109]. CDK1 is required for self-renewal of both mESCs [110] and hESCs [111], most likely because of its interaction with Oct4 [112,113,114]. Oct4 is a strategic transcription factor for the maintenance of pluripotency. During development, Oct4 is expressed in blastomers, the ICM, and epiblasts, and is then downregulated in the gastrulation stage [115]. Oct4 and Sox2 sustain hESCs self-renewal by upregulation of the Wnt-β Catenin antagonist SFRP2 and repression of the WNT trafficking protein WLS [116,117]. In agreement with these findings, Cooney and collaborators have demonstrated that germ cell nuclear factor (GCNF), an orphan nuclear receptor, suppresses the expression of Oct4 and modulates gene expression in hESCs [118].
Briefly, pluripotency and self-renewal of mESCs depend on BMP/Smad/Id and LIF/STAT3 signaling in culture, which is a hallmark of naïve pluripotency. In fact, mESCs have been shown to be able to maintain pluripotency and self-renewal without LIF, BMP4, or any external signaling by adding inhibitors of MAPK (ERK1/2 pathway) and glycogen synthase kinase 3 (so-called “2i medium”) [119]. Instead of LIF and 2i, hESCs require FGF/ERK and Activin/Nodal signaling to sustain self-renewal and pluripotency.
4. Epigenetic and Chromatin Modifications of Self-Renewal and Pluripotency in ESCs
Regulation of chromatin packaging through histone modification and DNA methylation plays an elemental role in maintaining self-renewal and pluripotency of ESCs. DNA methylation is a form of modification in which a methyl group is transferred onto cytosines in the CpG dinucleotide [120,121,122,123,124,125,126]. In this regard, there are two main histone modifications to regulate gene expression: histone H3 lysine 4 and 27 trimethylations (H3K4me3 and H3K27me3, respectively) [127,128]. H3K4me3 is related to active transcription [129], while H3K27me3 modification represents a negative regulator of gene expression and attracts chromatin inhibitors, thereby diminishing transcription [130]. During differentiation, H3K27me3 inhibits self-renewal and pluripotency. Genomic regions that present both histone modifications (H3K4me3 and H3K27me3) are known as bivalent domains [131,132,133,134]. These regions of the genome are essential for ESCs self-renewal and pluripotency. Recently, Nanog has been shown to repress differentiation genes by sustaining H3K27me3 in mESCs [135].
A member of the Ten-eleven translocation (Tet) family, Tet1, promotes gene expression of Oct4 by demethylation of CpG islands of promoter regions [136,137,138]. N6-methyladenosine (m6A) is a modification that occurs on mRNA and long noncoding RNA [139,140]. Wen at al reported that the Zinc Finger protein CCCH-Type Containing 13 (ZC3H13) is important for the localization of WTAP (WT1 Associated Protein), Virilizer (VIRMA: Vir Like m6A Methyltransferase Associated), and Hakai (CBLL1: Cbl Proto-Oncogene Like 1) in the nucleus to promote m6A methylation and to eventually modulate mESCs self-renewal [141]. DNA methyltransferase 3A and 3B (Dnmt3A, Dnmt3B)-deficient ES cells are not able to differentiate, but maintain self-renewal ability [142]. Other non-coding RNAs have also been implied in the regulation of the mechanisms underlying pluripotency and self-renewal [143,144,145,146,147,148,149,150,151,152].
Intriguingly, AICAR has been shown to sustain self-renewal of mESCs by altering the expression of epigenetic markers including Dnmt3a, Dnmt3b, Smarca2, Mbd3, and Arid1a [153]. Finally, Jia and colleagues reported that the undifferentiated embryonic cell transcription factor 1 (Utf1), the gene target of Oct4 and Sox2, promotes self-renewal and pluripotency of hESCs by inhibiting H3K27me3 modification and the polycomb repressive complex 2 (PRC2) [154].
5. Naïve Pluripotency
Up to now, two distinct states of pluripotency have been observed in the murine system: namely, naïve state and primed state. Specifically, “naïve” cells are present in an earlier developmental stage (the peri-implantation blastocyst), whereas “primed” cells exist in the later, post-implantation embryo. In mice, mESCs were shown to settle in an ICM-like state, termed “naïve pluripotency”, at E4.5. Mouse epiblast stem cells (mEpiSCs) are derived from post-implantation epiblast cells and named “primed” (from E5.5 onwards). hESCs present a primed state of pluripotency. For their self-renewal, mEpiSCs, like hESCs, depend on bFGF and Activin A/Nodal pathways that maintain the expression of OCT4. The stability of these states is controlled by endogenous and exogenous factors. For instance, culturing mESCs in medium containing primed factors including FGF-2 and TGFβ1/Activin A can convert the naïve state into the primed state and support the self-renewal of EpiSCs [155,156]. Interestingly, mESCs were converted into ground state of primed epiblast stem cells (rsEpiSC: region-selective EpiSC) by changing conditions from 2i/LIF to a medium containing FGF2 and a Wnt signaling inhibitor (IWR1); the converted cells exhibited properties of the primed state, including X chromosome inactivation [157].
Naïve and primed pluripotency show significant differences in growth, gene expression, cellular fitness, metabolism, and differentiation potential [158,159,160,161,162,163,164]. Both mESCs and hESCs express OCT3/4, SOX2, SSEA-3, GATA4; mESCs express different genes including SSEA-1, FoxD3, and LIF receptor complex (LIFR/IL6ST); they exhibit a dome-shaped colony morphology, karyotype stability, and two active X chromosomes in female cells. hESCs share a number of properties with murine naïve pluripotency while these features are absent in EpiSCs [165,166], suggesting that hESCs are less primed compared to murine EpiSCs, but they still have many features of EpiSCs; hESCs show a flat colony morphology and X chromosome inactivation in female cells, accumulation of H3K27me3, low ability to generate primordial germ cell-like cells (PGCLCs), and are identified by the expression of SSEA-4 (Stage-specific embryonic antigen 4), Podocalyxins TRA-1-81 and TRA-1-60, and Vimentin; they are also able to differentiate into trophoblast-like cells [167]. FGF/TGFβ signaling supports the inactivation of X chromosome and increases DNA methylation in the primed state of hPSCs [168,169].
During the last decade, much effort has been made to generate and evaluate human naïve pluripotency in vitro. Multiple protocols have been reported to generate human naïve pluripotency via conversion from primed PSC, reprogramming from somatic cells, and derivation from embryos [170,171,172,173,174,175,176,177,178]. Jacob Hanna and coworkers generated human naïve PSCs by ectopic expression of OCT4, Klf4, and Klf2 in a medium containing LIF and inhibitors of GSK3β and the ERK1/2 pathway [179]. Strikingly, combining 2i/LIF (2iL) and tamoxifen with forced expression of hormone-dependent STAT3-ER (ER, ligand-binding domain of the human estrogen receptor) reprogrammed hESCs from a primed state to naïve pluripotency through activation of STAT3 target genes [170]. Ohad Gafni and co-workers established a naïve human stem cell medium (NHSM) containing 2iLIF (2iL), P38i/JNKi, protein kinase C inhibitor (PKCi), Rho-kinase inhibitors (ROCKi), Activin, and FGF2, to capture and support human naïve PSCs [174]. In a similar study, Thorold Theunissen and collaborators identified five inhibitors targeting MEK, GSK3, BRAF, ROCK, and SRC together with LIF and two growth factors, i.e., FGF and Activin A (5i/L/FA), to support human naïve pluripotency and OCT4-ΔPE-GFP+ and transgene-free naïve hESCs [180]. The Oct4-ΔPE-GFP marker has been harnessed to discriminate naïve human PSCs from primed human PSCs [174,181]. Yet, Oct4-ΔPE-GFP reporter activity is not completely negative in primed hESCs and still includes a weak GFP activity; additionally, Oct4-ΔPE-GFP+ cells may still include ESCs utilizing Oct4-PE, since a mono-transgenic system is unable to discern between cells using only Oct4-DE and cells using both Oct4-DE and Oct4-PE, which may constitute an impure population of naïve PSCs. To overcome these issues, a dual reporter system for naïve and primed mouse PSCs has been generated using two fluorescent reporters, GFP and tdTomato (RFP), controlled by the cis-regulatory elements DE and PE, respectively: the expression of Oct4-ΔPE-GFP and Oct4-ΔDE-RFP was shown to accurately represent the expression of naïve and primed cells during the development of double transgenic mice [182]. Henceforth, this double transgenic system recapitulates the in vivo Oct4 regulatory system, providing an exquisite tool for assessing the regulation of naïve and primed pluripotency while enabling the separation of pure populations of naïve and primed PSCs.
Overexpressing YAP, the Hippo pathway effector, in hESCs and iPSCs was found to enhance naïve pluripotency [177]. In these conditions, hPSCs show some naïve characteristics including reduced DNA methylation, upregulation of naïve pluripotency markers, nuclear localization of TFE3, in vitro generation of PGCs, and inhibition of MEK/ERK signaling. Nonetheless, there is still a gap between mouse and human naïve pluripotency. Recently, signaling pathway modulations have been reported to enhance human naïve pluripotency induction [183].
In order to enhance efficiency of human naïve pluripotency induction, Jacob Hanna’s research team engineered WIBR3 hESC lines with inducible ablation of METTL3 expression (Tet-OFF-METTL3 hESCs): however, these cells were maintained poorly in NHSM medium [183]. Therefore, these researchers modified some previous protocols showing that adding IWR1, WNT inhibitor (WNTi), increased the expansion of their Tet-OFF-METTL3 hESCs. They also observed that culturing the DPE-OCT4-GFP naïve pluripotency reporter line described by Theunissen et al. [181] in NHSM supplemented with TNKi, JNK/P38 inhibitor, and in absence of GSK3i, significantly increased human naïve pluripotency. Screening small molecules in NHSM medium revealed a defined FGF/TGF/Activin/MEF-independent and serum-free condition named human enhanced naïve stem cell medium (HENSM), that strongly supported naïve hPSCs in the absence of DNMT1, exogenous L-glutamine, and increased oxidative phosphorylation (OXPHOS) activity, presented a dome-like morphology, formed mature teratoma, showed a normal karyotype after extended passaging, differentiation into bona fide extra-embryonic trophoblast stem cells and extra-embryonic naïve primitive endoderm cells (PrE and nEND cells), upregulation of naïve pluripotency markers including Nanog, TFCP2L1, Klf4, Klf17, DPPA3 (also known as STELLA, STELLAR, and PGC7), DPPA5, DNMT3L, KHDC1L, and ARGFX, downregulation of DNA methylation from 81% to 66%, reactivation of both X chromosomes, and lack of immediate global loss of all imprinted genes compared to NHSM, 5i/L/FA, and t2iL-Go conditions [178,184]. In this protocol, WNT/β-Catenin inhibition supported human naïve pluripotency, unlike murine naïve pluripotency, as indicated using TNK inhibitors and β-Catenin KO cells in naïve pluripotency reporter lines; similarly the inhibition of Notch/RBPj was shown to induce the expression of human naïve pluripotency markers without inhibiting ERK. Khan et al. described a protocol that included FGFR, RAF, or ERK alongside the dual inhibition of MEK and ERK with PKC, ROCK, and TNK inhibitors and Activin A to support human naïve pluripotency [185].
Besides, Klf17 was shown to enhance naïve pluripotency by suppressing the transcription of MAPK3 and ZIC2 in hESCs [186]. Remarkably, recent studies have revealed a high gene-targeting efficiency of human naïve pluripotency by CRISPR/Cas9 system compared to primed pluripotency [187]. Therefore, human naïve pluripotency provides a better model to study embryogenesis and mechanisms of pluripotency [188,189,190]. Bi and colleagues reported alkaline phosphatase placental-like 2 (ALPPL2) as an important naïve-specific surface marker in human naïve PSCs: ALPPL2 is necessary for the maintenance and establishment of naïve pluripotency [191]. ALPPL2 preserved mRNA levels of the naïve pluripotency transcription factors including TFCP2L1 (Transcription Factor CP2 Like 1, also known as CRTR1 or LBP-9) and STAT3 (Signal Transducer and Activator of Transcription 3, also known as ADMIO1 and PRF) via interacting with the RNA-binding protein IGF2BP1 (Insulin Like Growth Factor 2 MRNA Binding Protein 1) [191]. Endogenous retroviruses (ERVs) are viral elements with long terminal repeats (LTRs) that can be derived from retroviruses. ERVs are active during early embryogenesis and are silenced transcriptionally in somatic cells. Klf5 interacts with and rewires Nanog to bind the naïve-specific LTR7Y sites, and facilitate expression of naïve and trophectoderm genes in naïve hESCs; in absence of Klf5, Nanog is bound to the primed-enriched LTR7 sites in primed hESCs [192,193,194].
Sox2 is a core pluripotency factor and its ablation in mESCs results in loss of self-renewal, pluripotency, and modification of ESCs into trophoblast-like stem cells [195,196]. In fact, Sox2 modulation increased naïve pluripotency plasticity. Tremble et al. reprogrammed Sox2-low iPSCs from Sox2-/- NSCs using retroviral factors: cMyc, Klf4, Oct4, and Sox2 (rMKOS) in defined culture conditions containing inhibitors of MEK/ERK and Gsk3β signaling supplemented with LIF (2il). Sox2-low iPSCs expressed naïve pluripotency markers (Nanog, Esrrb, Klf4, Oct4) and suggested that the low expression of Sox2 is sufficient to maintain a naïve molecular signature. Likewise, Sox2-low iPSCs differentiated into both embryonic (ectoderm, mesoderm, and endoderm) and extraembryonic lineage (trophoblast), in vitro as well as in vivo [39].
6. Controversial Issues
In recent years, significant advances have been made to convert the primed state of hPSCs into naïve pluripotency. Human naïve pluripotency has been shown to differentiate better than the primed state, which is associated with the accumulation of DNA methylation and epigenetic repressive marks in primed state. The Wang laboratory highlighted the role of Klf17 in promoting naïve pluripotency in hESCs [186], while Bayerl et al. reported that Klf17 is non-necessary for mouse naïve pluripotency [183]. Further investigations into species-specific functions of Klf17 are warranted.
Chromosome X reactivation in human naïve pluripotency observed in HENSM, NHSM, 5i/L/FA, and t2iL-Go, cannot yield inactivation upon hPSC differentiation [197]. So, it will be imperative to determine which conditions strongly protect long-term genomic integrity of naïve hESCs. Another crucial finding in naïve pluripotency is the higher expression of IGF1R and phosphorylation of AKT, which should be examined in depth. Inhibition of MEK signaling blocks self-renewal in the presence of IGF1 and Activin, but whether ERK is functionally involved remains unclear. It would be also noteworthy to assess the actual role of integrins in maintenance of pluripotency.
Additionally, there are some open challenges in stem cell therapy and stem cell directed differentiation, including the attempts to improve their properties in a manner similar to the in vivo setting. Future dedicated studies on pluripotent epiblast should clarify the functional role of precise signaling pathways during early development and delineate which ones are the most effective for in vitro-derived hESC lines. Culturing hPSCs and murine ESCs in mature conditions and the combination of in vitro and in vivo assays would be useful to resolve some remaining issues regarding the functional contribution of defined signaling pathways in pluripotency, and self-renewal of hESCs and mESCs, with the ultimate goal being to promote regenerative medicine.
7. Conclusions
ESCs are derived from ICM within blastocysts. They proliferate indefinitely and are able to differentiate into three embryonic germ layers, namely endoderm, mesoderm, and ectoderm. Both self-renewal and pluripotency are dependent on a finely tuned correlation and balance of signaling pathways, transcription factor programs, and epigenetic modifications. Understanding signaling pathways to control ESCs self-renewal and pluripotency could be extremely helpful to better delineate their clinical applications [198,199,200].
The transition between different states of pluripotency represents an exquisite model to painstakingly study the development of early human pre-implantation. Culturing human naïve PSCs is possible, and screening growth factors and small molecules might be useful for optimizing conditions to close the gap between mouse and human naïve pluripotency. In this review, we summarized the main molecular mechanisms and signaling pathways involved in self-renewal and pluripotency in mESCs and hESCs, and in capturing human naïve pluripotency from primed state of pluripotency. Of course, there are still many unanswered questions regarding the exact correlation between signaling pathways, transcription factor networks, and epigenetic modifications, and further studies in this sense are warranted.
Author Contributions
Conceptualization, F.V. and G.S.; methodology and literature search, F.V., J.G., U.K., S.S.J. and G.S.; writing—original draft preparation, F.V.; writing—review and editing, G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Santulli’s Lab is currently supported in part by the National Institutes of Health (NIH): National Center for Advancing Translational Sciences (NCATS: UL1-TR002556-06, UM1-TR004400), National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST915561). J.G. is supported by a postdoctoral fellowship of the American Heart Association (AHA-20POST35211151). S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407). U.K. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Balboa D., Iworima D.G., Kieffer T.J. Human Pluripotent Stem Cells to Model Islet Defects in Diabetes. Front. Endocrinol. 2021;12:642152. doi: 10.3389/fendo.2021.642152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna J.H., Saha K., Jaenisch R. Pluripotency and Cellular Reprogramming: Facts, Hypotheses, Unresolved Issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogliotti Y.S., Wu J., Vilarino M., Okamura D., Soto D.A., Zhong C., Sakurai M., Sampaio R.V., Suzuki K., Belmonte J.C.I., et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc. Natl. Acad. Sci. USA. 2018;115:2090–2095. doi: 10.1073/pnas.1716161115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Tachibana M., Amato P., Sparman M., Gutierrez N.M., Tippner-Hedges R., Ma H., Kang E., Fulati A., Lee H.-S., Sritanaudomchai H., et al. Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekel C., Morey R., Hanna J., Laurent L.C., Ben-Yosef D., Amir H. Stabilization of hESCs in two distinct substates along the continuum of pluripotency. iScience. 2022;25:105469. doi: 10.1016/j.isci.2022.105469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 9.Plusa B., Hadjantonakis A.-K. Embryonic stem cell identity grounded in the embryo. Nature. 2014;16:502–504. doi: 10.1038/ncb2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czechanski A., Byers C., Greenstein I., Schrode N., Donahue L.R., Hadjantonakis A.-K., Reinholdt L.G. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat. Protoc. 2014;9:559–574. doi: 10.1038/nprot.2014.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varzideh F., Mone P., Santulli G. Bioengineering Strategies to Create 3D Cardiac Constructs from Human Induced Pluripotent Stem Cells. Bioengineering. 2022;9:168. doi: 10.3390/bioengineering9040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 14.Park I.-H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt R., Plath K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 2012;13:251. doi: 10.1186/gb-2012-13-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S., Li W., Zhou H., Wei W., Ambasudhan R., Lin T., Kim J., Zhang K., Ding S. Reprogramming of Human Primary Somatic Cells by OCT4 and Chemical Compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plath K., Lowry W.E. Progress in understanding reprogramming to the induced pluripotent state. Nat. Rev. Genet. 2011;12:253–265. doi: 10.1038/nrg2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton S. Signaling networks in human pluripotent stem cells. Curr. Opin. Cell Biol. 2013;25:241–246. doi: 10.1016/j.ceb.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V.B., Wong E., Orlov Y.L., Zhang W., Jiang J., et al. Integration of External Signaling Pathways with the Core Transcriptional Network in Embryonic Stem Cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Hall J., Guo G., Wray J., Eyres I., Nichols J., Grotewold L., Morfopoulou S., Humphreys P., Mansfield W., Walker R., et al. Oct4 and LIF/Stat3 Additively Induce Krüppel Factors to Sustain Embryonic Stem Cell Self-Renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Ng H.-H., Surani A. The transcriptional and signalling networks of pluripotency. Nature. 2011;13:490–496. doi: 10.1038/ncb0511-490. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W., Lei A., Tian L., Wang X., Correia C., Weiskittel T., Li H., Trounson A., Fu Q., Yao K., et al. Strategies for Genetically Engineering Hypoimmunogenic Universal Pluripotent Stem Cells. iScience. 2020;23:101162. doi: 10.1016/j.isci.2020.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidgang C.E., Seufferlein T., Kleger A., Mueller M. Pluripotency Factors on Their Lineage Move. Stem Cells Int. 2016;2016:6838253. doi: 10.1155/2016/6838253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauklin S., Vallier L. The Cell-Cycle State of Stem Cells Determines Cell Fate Propensity. Cell. 2013;155:135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avery K., Avery S., Shepherd J., Heath P.R., Moore H. Sphingosine-1-Phosphate Mediates Transcriptional Regulation of Key Targets Associated with Survival, Proliferation, and Pluripotency in Human Embryonic Stem Cells. Stem Cells Dev. 2008;17:1195–1206. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 26.Ramírez M.A., Pericuesta E., Fernandez-Gonzalez R., Moreira P., Pintado B., Gutierrez-Adan A. Transcriptional and post-transcriptional regulation of retrotransposons IAP and MuERV-L affect pluripotency of mice ES cells. Reprod. Biol. Endocrinol. 2006;4:55. doi: 10.1186/1477-7827-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Tam W.-L., Tong G.Q., Wu Q., Chan H.-Y., Soh B.-S., Lou Y., Yang J., Ma Y., Chai L., et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nature. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 28.Shi G., Jin Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. Ther. 2010;1:39. doi: 10.1186/scrt39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi R., Buchanan J.C., Paruchuri S., Morris N., Tavana H. Molecular Analysis of Stromal Cells-Induced Neural Differentiation of Mouse Embryonic Stem Cells. PLoS ONE. 2016;11:e0166316. doi: 10.1371/journal.pone.0166316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George M.N., Leavens K.F., Gadue P. Genome Editing Human Pluripotent Stem Cells to Model β-Cell Disease and Unmask Novel Genetic Modifiers. Front. Endocrinol. 2021;12:682625. doi: 10.3389/fendo.2021.682625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao X., Dani V., Dani C. Human Pluripotent Stem Cells: A Relevant Model to Identify Pathways Governing Thermogenic Adipocyte Generation. Front. Endocrinol. 2019;10:932. doi: 10.3389/fendo.2019.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sewell W., Lin R.-Y. Generation of Thyroid Follicular Cells from Pluripotent Stem Cells: Potential for Regenerative Medicine. Front. Endocrinol. 2014;5:96. doi: 10.3389/fendo.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posabella A., Alber A.B., Undeutsch H.J., Droeser R.A., Hollenberg A.N., Ikonomou L., Kotton D.N. Derivation of Thyroid Follicular Cells from Pluripotent Stem Cells: Insights from Development and Implications for Regenerative Medicine. Front. Endocrinol. 2021;12:666565. doi: 10.3389/fendo.2021.666565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hackett J.A., Surani M.A. Regulatory Principles of Pluripotency: From the Ground State Up. Cell Stem Cell. 2014;15:416–430. doi: 10.1016/j.stem.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Geng T., Zhang D., Jiang W. Epigenetic Regulation of Transition Among Different Pluripotent States: Concise Review. Stem Cells. 2019;37:1372–1380. doi: 10.1002/stem.3064. [DOI] [PubMed] [Google Scholar]
- 36.Udomlumleart T., Hu S., Garg S. Lineages of embryonic stem cells show non-Markovian state transitions. iScience. 2021;24:102879. doi: 10.1016/j.isci.2021.102879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R., Guo Y.-L. Transient inhibition of cell proliferation does not compromise self-renewal of mouse embryonic stem cells. Exp. Cell Res. 2012;318:2094–2104. doi: 10.1016/j.yexcr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devika A.S., Montebaur A., Saravanan S., Bhushan R., Koch F., Sudheer S. Human ES Cell Culture Conditions Fail to Preserve the Mouse Epiblast State. Stem Cells Int. 2021;2021:8818356. doi: 10.1155/2021/8818356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremble K.C., Stirparo G.G., Bates L.E., Maskalenka K., Stuart H.T., Jones K., Andersson-Rolf A., Radzisheuskaya A., Koo B.-K., Bertone P., et al. Sox2 modulation increases naïve pluripotency plasticity. iScience. 2021;24:102153. doi: 10.1016/j.isci.2021.102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang W., Han P., Kim E.H., Mak J., Zhang R., Torrente A.G., Goldhaber J.I., Marbán E., Cho H.C. Canonical Wnt signaling promotes pacemaker cell specification of cardiac mesodermal cells derived from mouse and human embryonic stem cells. Stem Cells. 2020;38:352–368. doi: 10.1002/stem.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecori F., Kondo N., Ogura C., Miura T., Kume M., Minamijima Y., Yamamoto K., Nishihara S. Site-specific O-GlcNAcylation of Psme3 maintains mouse stem cell pluripotency by impairing P-body homeostasis. Cell Rep. 2021;36:109361. doi: 10.1016/j.celrep.2021.109361. [DOI] [PubMed] [Google Scholar]
- 42.Yeh C.-Y., Huang W.-H., Chen H.-C., Meir Y.-J.J. Capturing Pluripotency and Beyond. Cells. 2021;10:3558. doi: 10.3390/cells10123558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollini D., Loffredo R., Maniscalco F., Cardano M., Micaelli M., Bonomo I., Licata N.V., Peroni D., Tomaszewska W., Rossi A., et al. Multilayer and MATR3-dependent regulation of mRNAs maintains pluripotency in human induced pluripotent stem cells. iScience. 2021;24:102197. doi: 10.1016/j.isci.2021.102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyer T.A., Narimatsu M., Weiss A., David L., Wrana J.L. The TGFβ superfamily in stem cell biology and early mammalian embryonic development. Biochim. Biophys. Acta. 2013;1830:2268–2279. doi: 10.1016/j.bbagen.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 45.Sakaki-Yumoto M., Katsuno Y., Derynck R. TGF-β family signaling in stem cells. Biochim. et Biophys. Acta. 2013;1830:2280–2296. doi: 10.1016/j.bbagen.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watabe T., Miyazono K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 47.Ying Q.-L., Nichols J., Chambers I., Smith A. BMP Induction of Id Proteins Suppresses Differentiation and Sustains Embryonic Stem Cell Self-Renewal in Collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/S0092-8674(03)00847-X. [DOI] [PubMed] [Google Scholar]
- 48.Li Z., Fei T., Zhang J., Zhu G., Wang L., Lu D., Chi X., Teng Y., Hou N., Yang X., et al. BMP4 Signaling Acts via Dual-Specificity Phosphatase 9 to Control ERK Activity in Mouse Embryonic Stem Cells. Cell Stem Cell. 2012;10:171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Morikawa M., Koinuma D., Mizutani A., Kawasaki N., Holmborn K., Sundqvist A., Tsutsumi S., Watabe T., Aburatani H., Heldin C.-H., et al. BMP Sustains Embryonic Stem Cell Self-Renewal through Distinct Functions of Different Krüppel-like Factors. Stem Cell Rep. 2016;6:64–73. doi: 10.1016/j.stemcr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richter A., Valdimarsdottir L., Hrafnkelsdottir H.E., Runarsson J.F., Omarsdottir A.R., Oostwaard D.W.-V., Mummery C., Valdimarsdottir G. BMP4 Promotes EMT and Mesodermal Commitment in Human Embryonic Stem Cells via SLUG and MSX2. Stem Cells. 2014;32:636–648. doi: 10.1002/stem.1592. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Yang M., Dawes J., Khillan J.S. Suppression of ES cell differentiation by retinol (vitamin A) via the overexpression of Nanog. Differentiation. 2007;75:682–693. doi: 10.1111/j.1432-0436.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 52.Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. Functional Expression Cloning of Nanog, a Pluripotency Sustaining Factor in Embryonic Stem Cells. Cell. 2003;113:643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 53.Chambers I. The Molecular Basis of Pluripotency in Mouse Embryonic Stem Cells. Cloning Stem Cells. 2004;6:386–391. doi: 10.1089/clo.2004.6.386. [DOI] [PubMed] [Google Scholar]
- 54.Shi W., Wang H., Pan G., Geng Y., Guo Y., Pei D. Regulation of the Pluripotency Marker Rex-1 by Nanog and Sox2. J. Biol. Chem. 2006;281:23319–23325. doi: 10.1074/jbc.M601811200. [DOI] [PubMed] [Google Scholar]
- 55.Festuccia N., Osorno R., Halbritter F., Karwacki-Neisius V., Navarro P., Colby D., Wong F., Yates A., Tomlinson S.R., Chambers I. Esrrb Is a Direct Nanog Target Gene that Can Substitute for Nanog Function in Pluripotent Cells. Cell Stem Cell. 2012;11:477–490. doi: 10.1016/j.stem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He R., Xhabija B., Al-Qanber B., Kidder B.L. OCT4 supports extended LIF-independent self-renewal and maintenance of transcriptional and epigenetic networks in embryonic stem cells. Sci. Rep. 2017;7:16360. doi: 10.1038/s41598-017-16611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Närvä E., Taskinen M.E., Lilla S., Isomursu A., Pietilä M., Weltner J., Isola J., Sihto H., Joensuu H., Zanivan S., et al. MASTL is enriched in cancerous and pluripotent stem cells and influences OCT1/OCT4 levels. iScience. 2022;25:104459. doi: 10.1016/j.isci.2022.104459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdelalim E.M., Tooyama I. NPR-A regulates self-renewal and pluripotency of embryonic stem cells. Cell Death Dis. 2011;2:e127. doi: 10.1038/cddis.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theret M., Gsaier L., Schaffer B., Juban G., Ben Larbi S., Weiss-Gayet M., Bultot L., Collodet C., Foretz M., Desplanches D., et al. AMPK α1- LDH pathway regulates muscle stem cell self-renewal by controlling metabolic homeostasis. EMBO J. 2017;36:1946–1962. doi: 10.15252/embj.201695273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pei S., Minhajuddin M., Adane B., Khan N., Stevens B.M., Mack S.C., Lai S., Rich J.N., Inguva A., Shannon K.M., et al. AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell. 2018;23:86–100.e6. doi: 10.1016/j.stem.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alba G., Martínez R., Postigo-Corrales F., López S., Santa-María C., Jiménez G.A., Cahuana G.M., Soria B., Bedoya F.J., Tejedo J.R. AICAR Stimulates the Pluripotency Transcriptional Complex in Embryonic Stem Cells Mediated by PI3K, GSK3β, and β-Catenin. ACS Omega. 2020;5:20270–20282. doi: 10.1021/acsomega.0c02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankauskas S.S., Mone P., Avvisato R., Varzideh F., De Gennaro S., Salemme L., Macina G., Kansakar U., Cioppa A., Frullone S., et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: Validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech. Ageing Dev. 2023. in press . [DOI] [PMC free article] [PubMed]
- 63.Yu Y., Gu S., Li W., Sun C., Chen F., Xiao M., Wang L., Xu D., Li Y., Ding C., et al. Smad7 enables STAT3 activation and promotes pluripotency independent of TGF-β signaling. Proc. Natl. Acad. Sci. USA. 2017;114:10113–10118. doi: 10.1073/pnas.1705755114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng C.-C., Liao P.-N., Ho A.-S., Lim K.-H., Chang J., Su Y.-W., Chen C.G.-S., Chiang Y.-W., Yang B.-L., Lin H.-C., et al. STAT3 exacerbates survival of cancer stem-like tumorspheres in EGFR-positive colorectal cancers: RNAseq analysis and therapeutic screening. J. Biomed. Sci. 2018;25:60. doi: 10.1186/s12929-018-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park H.-J., Min T.-R., Chi G.-Y., Choi Y.-H., Park S.-H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018;505:194–200. doi: 10.1016/j.bbrc.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 66.Prenzel N., Fischer O.M., Streit S., Hart S., Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr. Relat. Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 67.Yu M., Wei Y., Xu K., Liu S., Ma L., Pei Y., Hu Y., Liu Z., Zhang X., Wang B., et al. EGFR deficiency leads to impaired self-renewal and pluripotency of mouse embryonic stem cells. PeerJ. 2019;7:e6314. doi: 10.7717/peerj.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia R.M., Yao D.B., Cai X.M., Xu X.Q. LHPP-Mediated Histidine Dephosphorylation Suppresses the Self-Renewal of Mouse Embryonic Stem Cells. Front. Cell Dev. Biol. 2021;9:638815. doi: 10.3389/fcell.2021.638815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Z., Zhang Y., Wang X., Wang X., Ye S.-D. Inhibition of protein kinase D by CID755673 promotes maintenance of the pluripotency of embryonic stem cells. Development. 2020;147:dev185264. doi: 10.1242/dev.185264. [DOI] [PubMed] [Google Scholar]
- 70.Park S., Kwon W., Kim H.-Y., Ji Y.R., Kim D., Kim W., Han J.E., Cho G.-J., Yun S., Kim M.O., et al. Knockdown of Maged1 inhibits cell cycle progression and causes cell death in mouse embryonic stem cells. Differentiation. 2022;125:18–26. doi: 10.1016/j.diff.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Ju H., Lee S., Lee J., Ghil S. Necdin modulates osteogenic cell differentiation by regulating Dlx5 and MAGE-D1. Biochem. Biophys. Res. Commun. 2017;489:109–115. doi: 10.1016/j.bbrc.2017.05.101. [DOI] [PubMed] [Google Scholar]
- 72.Chen X., Wu R., Feng S., Gu B., Dai L., Zhang M., Zhao X. Single cell derived murine embryonic stem cell clones stably express Rex1-specific green fluorescent protein and their differentiation study. Biochem. Biophys. Res. Commun. 2007;362:467–473. doi: 10.1016/j.bbrc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Li T., Kon N., Jiang L., Tan M., Ludwig T., Zhao Y., Baer R., Gu W. Tumor Suppression in the Absence of p53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25:104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu X., Wu S., Li B., Xu Y., Liu J. Functions of p53 in pluripotent stem cells. Protein Cell. 2020;11:71–78. doi: 10.1007/s13238-019-00665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin T., Chao C., Saito S., Mazur S.J., Murphy M., Appella E., Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 77.Li M., He Y., Dubois W., Wu X., Shi J., Huang J. Distinct Regulatory Mechanisms and Functions for p53-Activated and p53-Repressed DNA Damage Response Genes in Embryonic Stem Cells. Mol. Cell. 2012;46:30–42. doi: 10.1016/j.molcel.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang A., Shi G., Zhou C., Lu R., Li H., Sun L., Jin Y. Nucleolin Maintains Embryonic Stem Cell Self-renewal by Suppression of p53 Protein-dependent Pathway. J. Biol. Chem. 2011;286:43370–43382. doi: 10.1074/jbc.M111.225185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao L., Yuan X., Sharkis S.J. Activin A Maintains Self-Renewal and Regulates Fibroblast Growth Factor, Wnt, and Bone Morphogenic Protein Pathways in Human Embryonic Stem Cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 80.Beattie G.M., Lopez A.D., Bucay N., Hinton A., Firpo M.T., King C.C., Hayek A. Activin A Maintains Pluripotency of Human Embryonic Stem Cells in the Absence of Feeder Layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 81.Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 82.Vallier L., Mendjan S., Brown S., Chng Z., Teo A., Smithers L.E., Trotter M.W.B., Cho C.H.-H., Martinez A., Rugg-Gunn P., et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bertero A., Madrigal P., Galli A., Hubner N.C., Moreno I., Burks D., Brown S., Pedersen R.A., Gaffney D., Mendjan S., et al. Activin/Nodal signaling and NANOG orchestrate human embryonic stem cell fate decisions by controlling the H3K4me3 chromatin mark. Genes Dev. 2015;29:702–717. doi: 10.1101/gad.255984.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu R.-H., Sampsell-Barron T.L., Gu F., Root S., Peck R.M., Pan G., Yu J., Antosiewicz-Bourget J., Tian S., Stewart R., et al. NANOG Is a Direct Target of TGFβ/Activin-Mediated SMAD Signaling in Human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown S., Teo A., Pauklin S., Hannan N., Cho C.H.-H., Lim B., Vardy L., Dunn N.R., Trotter M., Pedersen R., et al. Activin/Nodal Signaling Controls Divergent Transcriptional Networks in Human Embryonic Stem Cells and in Endoderm Progenitors. Stem Cells. 2011;29:1176–1185. doi: 10.1002/stem.666. [DOI] [PubMed] [Google Scholar]
- 86.Sakaki-Yumoto M., Liu J., Ramalho-Santos M., Yoshida N., Derynck R. Smad2 Is Essential for Maintenance of the Human and Mouse Primed Pluripotent Stem Cell State. J. Biol. Chem. 2013;288:18546–18560. doi: 10.1074/jbc.M112.446591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chng Z., Teo A., Pedersen R.A., Vallier L. SIP1 Mediates Cell-Fate Decisions between Neuroectoderm and Mesendoderm in Human Pluripotent Stem Cells. Cell Stem Cell. 2010;6:59–70. doi: 10.1016/j.stem.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 88.Tsuneyoshi N., Tan E.K., Sadasivam A., Poobalan Y., Sumi T., Nakatsuji N., Suemori H., Dunn N.R. The SMAD2/3 corepressor SNON maintains pluripotency through selective repression of mesendodermal genes in human ES cells. Genes Dev. 2012;26:2471–2476. doi: 10.1101/gad.201772.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Q.V., Dixon G., Verma N., Rosen B.P., Gordillo M., Luo R., Xu C., Wang Q., Soh C.-L., Yang D., et al. Genome-scale screens identify JNK–JUN signaling as a barrier for pluripotency exit and endoderm differentiation. Nat. Genet. 2019;51:999–1010. doi: 10.1038/s41588-019-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsai Z.-Y., Singh S., Yu S.-L., Kao L.-P., Chen B.-Z., Ho B.-C., Yang P.-C., Li S.S.-L. Identification of microRNAs regulated by activin A in human embryonic stem cells. J. Cell. Biochem. 2010;109:93–102. doi: 10.1002/jcb.22385. [DOI] [PubMed] [Google Scholar]
- 91.Tomizawa M., Shinozaki F., Sugiyama T., Yamamoto S., Sueishi M., Yoshida T. Activin A is essential for Feeder-free culture of human induced pluripotent stem cells. J. Cell. Biochem. 2013;114:584–588. doi: 10.1002/jcb.24395. [DOI] [PubMed] [Google Scholar]
- 92.D'Amour K.A., Agulnick A.D., Eliazer S., Kelly O.G., Kroon E., Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 93.Cheng C.-L., Yang S.-C., Lai C.-Y., Wang C.-K., Chang C.-F., Lin C.-Y., Chen W.-J., Lin P.-Y., Wu H.-C., Ma N., et al. CXCL14 Maintains hESC Self-Renewal through Binding to IGF-1R and Activation of the IGF-1R Pathway. Cells. 2020;9:1706. doi: 10.3390/cells9071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Timilsina S., Kirsch-Mangu T., Werth S., Shepard B., Ma T., Villa-Diaz L.G. Enhanced self-renewal of human pluripotent stem cells by simulated microgravity. npj Microgravity. 2022;8:22. doi: 10.1038/s41526-022-00209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levenstein M.E., Ludwig T.E., Xu R.-H., Llanas R.A., VanDenHeuvel-Kramer K., Manning D., Thomson J.A. Basic Fibroblast Growth Factor Support of Human Embryonic Stem Cell Self-Renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greber B., Lehrach H., Adjaye J. Fibroblast Growth Factor 2 Modulates Transforming Growth Factor β Signaling in Mouse Embryonic Fibroblasts and Human ESCs (hESCs) to Support hESC Self-Renewal. Stem Cells. 2007;25:455–464. doi: 10.1634/stemcells.2006-0476. [DOI] [PubMed] [Google Scholar]
- 97.Li J., Wang G., Wang C., Zhao Y., Zhang H., Tan Z., Song Z., Ding M., Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 98.Armstrong L., Hughes O., Yung S., Hyslop L., Stewart R., Wappler I., Peters H., Walter T., Stojkovic P., Evans J., et al. The role of PI3K/AKT, MAPK/ERK and NFκβ signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 99.Kang H.B., Kim J.S., Kwon H.-J., Nam K.H., Youn H.S., Sok D.-E., Lee Y., Hu F., Wang X., Liang G., et al. Basic Fibroblast Growth Factor Activates ERK and Induces c-Fos in Human Embryonic Stem Cell Line MizhES1. Stem Cells Dev. 2005;14:395–401. doi: 10.1089/scd.2005.14.395. [DOI] [PubMed] [Google Scholar]
- 100.Paling N.R.D., Wheadon H., Bone H.K., Welham M.J. Regulation of Embryonic Stem Cell Self-renewal by Phosphoinositide 3-Kinase-dependent Signaling. J. Biol. Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 101.Storm M.P., Bone H.K., Beck C.G., Bourillot P.-Y., Schreiber V., Damiano T., Nelson A., Savatier P., Welham M.J. Regulation of Nanog Expression by Phosphoinositide 3-Kinase-dependent Signaling in Murine Embryonic Stem Cells. J. Biol. Chem. 2007;282:6265–6273. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- 102.Storm M.P., Kumpfmueller B., Thompson B., Kolde R., Vilo J., Hummel O., Schulz H., Welham M.J. Characterization of the Phosphoinositide 3-Kinase-Dependent Transcriptome in Murine Embryonic Stem Cells: Identification of Novel Regulators of Pluripotency. Stem Cells. 2009;27:764–775. doi: 10.1002/stem.3. [DOI] [PubMed] [Google Scholar]
- 103.Sanchez-Ripoll Y., Bone H.K., Owen T., Guedes A.M.V., Abranches E., Kumpfmueller B., Spriggs R.V., Henrique D., Welham M.J. Glycogen Synthase Kinase-3 Inhibition Enhances Translation of Pluripotency-Associated Transcription Factors to Contribute to Maintenance of Mouse Embryonic Stem Cell Self-Renewal. PLoS ONE. 2013;8:e60148. doi: 10.1371/journal.pone.0060148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romorini L., Garate X., Neiman G., Luzzani C., Furmento V.A., Guberman A.S., Sevlever G.E., Scassa M.E., Miriuka S.G. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Sci. Rep. 2016;6:35660. doi: 10.1038/srep35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galarreta A., Valledor P., Fernandez-Capetillo O., Lecona E. Coordinating DNA Replication and Mitosis through Ubiquitin/SUMO and CDK1. Int. J. Mol. Sci. 2021;22:8796. doi: 10.3390/ijms22168796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallo D., Young J.T.F., Fourtounis J., Martino G., Álvarez-Quilón A., Bernier C., Duffy N.M., Papp R., Roulston A., Stocco R., et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature. 2022;604:749–756. doi: 10.1038/s41586-022-04638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu J., Raia P., Ghent C.M., Raisch T., Sadian Y., Cavadini S., Sabale P.M., Barford D., Raunser S., Morgan D.O., et al. Structural basis of human separase regulation by securin and CDK1–cyclin B1. Nature. 2021;596:138–142. doi: 10.1038/s41586-021-03764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ulu A., Oh W., Zuo Y., Frost J.A. Cdk1 phosphorylation negatively regulates the activity of Net1 towards RhoA during mitosis. Cell. Signal. 2021;80:109926. doi: 10.1016/j.cellsig.2021.109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X.Q., Lo C.M., Chen L., Ngan E., Xu A., Poon R. CDK1-PDK1-PI3K/Akt signaling pathway regulates embryonic and induced pluripotency. Cell Death Differ. 2017;24:38–48. doi: 10.1038/cdd.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang W.W., Zhang X.J., Liu H.X., Chen J., Ren Y.H., Huang D.G., Zou X.H., Xiao W. Cdk1 is required for the self-renewal of mouse embryonic stem cells. J. Cell. Biochem. 2011;112:942–948. doi: 10.1002/jcb.23010. [DOI] [PubMed] [Google Scholar]
- 111.Neganova I., Tilgner K., Buskin A., Paraskevopoulou I., Atkinson S.P., Peberdy D., Passos J.F., Lako M. CDK1 plays an important role in the maintenance of pluripotency and genomic stability in human pluripotent stem cells. Cell Death Dis. 2014;5:e1508. doi: 10.1038/cddis.2014.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Munoz-Descalzo S., Rué P., Faunes F., Hayward P., Jakt L.M., Balayo T., Garcia-Ojalvo J., Arias A.M. A competitive protein interaction network buffers Oct4-mediated differentiation to promote pluripotency in embryonic stem cells. Mol. Syst. Biol. 2013;9:694. doi: 10.1038/msb.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J., Rao S., Chu J., Shen X., Levasseur D.N., Theunissen T.W., Orkin S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 114.Li L., Wang J., Hou J., Wu Z., Zhuang Y., Lu M., Zhang Y., Zhou X., Li Z., Xiao W., et al. Cdk1 interplays with Oct4 to repress differentiation of embryonic stem cells into trophectoderm. FEBS Lett. 2012;586:4100–4107. doi: 10.1016/j.febslet.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 115.Palmieri S.L., Peter W., Hess H., Schöler H. Oct-4 Transcription Factor Is Differentially Expressed in the Mouse Embryo during Establishment of the First Two Extraembryonic Cell Lineages Involved in Implantation. Dev. Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 116.Zhou C., Yang X., Sun Y., Yu H., Zhang Y., Jin Y. Comprehensive profiling reveals mechanisms of SOX2-mediated cell fate specification in human ESCs and NPCs. Cell Res. 2016;26:171–189. doi: 10.1038/cr.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davidson K.C., Adams A.M., Goodson J.M., McDonald C.E., Potter J.C., Berndt J.D., Biechele T.L., Taylor R.J., Moon R.T. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang H., Wang X., Xu X., Kyba M., Cooney A.J. Germ Cell Nuclear Factor (GCNF) Represses Oct4 Expression and Globally Modulates Gene Expression in Human Embryonic Stem (hES) Cells. J. Biol. Chem. 2016;291:8644–8652. doi: 10.1074/jbc.M115.694208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ying Q.-L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martisova A., Holcakova J., Izadi N., Sebuyoya R., Hrstka R., Bartosik M. DNA Methylation in Solid Tumors: Functions and Methods of Detection. Int. J. Mol. Sci. 2021;22:4247. doi: 10.3390/ijms22084247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu W., Hill S.E., Nathan W.J., Paiano J., Callen E., Wang D., Shinoda K., van Wietmarschen N., Colón-Mercado J.M., Zong D., et al. Neuronal enhancers are hotspots for DNA single-strand break repair. Nature. 2021;593:440–444. doi: 10.1038/s41586-021-03468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gambardella J., Jankauskas S., Kansakar U., Varzideh F., Avvisato R., Prevete N., Sidoli S., Mone P., Wang X., Lombardi A., et al. Ketone bodies rescue mitochondrial dysfunction via epigenetic remodeling. JACC Basic Transl. Sci. 2023. in press . [DOI] [PMC free article] [PubMed]
- 123.Mittelstaedt N.N., Becker A.L., de Freitas D.N., Zanin R.F., Stein R.T., de Souza A.P.D. DNA Methylation and Immune Memory Response. Cells. 2021;10:2943. doi: 10.3390/cells10112943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Światowy W.J., Drzewiecka H., Kliber M., Sąsiadek M., Karpiński P., Pławski A., Jagodziński P.P. Physical Activity and DNA Methylation in Humans. Int. J. Mol. Sci. 2021;22:12989. doi: 10.3390/ijms222312989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mattei A.L., Bailly N., Meissner A. DNA methylation: A historical perspective. Trends Genet. 2022;38:676–707. doi: 10.1016/j.tig.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 126.Lappalainen T., Greally J.M. Associating cellular epigenetic models with human phenotypes. Nat. Rev. Genet. 2017;18:441–451. doi: 10.1038/nrg.2017.32. [DOI] [PubMed] [Google Scholar]
- 127.Shilatifard A. The COMPASS Family of Histone H3K4 Methylases: Mechanisms of Regulation in Development and Disease Pathogenesis. Annu. Rev. Biochem. 2012;81:65–95. doi: 10.1146/annurev-biochem-051710-134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lanzuolo C., Orlando V. Memories from the Polycomb Group Proteins. Annu. Rev. Genet. 2012;46:561–589. doi: 10.1146/annurev-genet-110711-155603. [DOI] [PubMed] [Google Scholar]
- 129.Lauberth S.M., Nakayama T., Wu X., Ferris A.L., Tang Z., Hughes S.H., Roeder R.G. H3K4me3 Interactions with TAF3 Regulate Preinitiation Complex Assembly and Selective Gene Activation. Cell. 2013;152:1021–1036. doi: 10.1016/j.cell.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simon J.A., Kingston R.E. Occupying Chromatin: Polycomb Mechanisms for Getting to Genomic Targets, Stopping Transcriptional Traffic, and Staying Put. Mol. Cell. 2013;49:808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 132.Pan G., Tian S., Nie J., Yang C., Ruotti V., Wei H., Jonsdottir G.A., Stewart R., Thomson J.A. Whole-Genome Analysis of Histone H3 Lysine 4 and Lysine 27 Methylation in Human Embryonic Stem Cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 133.Zhao X.D., Han X., Chew J.L., Liu J., Chiu K.P., Choo A., Orlov Y.L., Sung W.-K., Shahab A., Kuznetsov V.A., et al. Whole-Genome Mapping of Histone H3 Lys4 and 27 Trimethylations Reveals Distinct Genomic Compartments in Human Embryonic Stem Cells. Cell Stem Cell. 2007;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 134.Xie J., Fan C., Zhang J.L., Zhang S.Q. Expression specificity and compensation effect of Ash2l-1/Ash2l-2 in mouse embryonic stem cells. Yi Chuan. 2018;40:237–249. doi: 10.16288/j.yczz.17-284. [DOI] [PubMed] [Google Scholar]
- 135.Heurtier V., Owens N., Gonzalez I., Mueller F., Proux C., Mornico D., Clerc P., Dubois A., Navarro P. The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells. Nat. Commun. 2019;10:1109. doi: 10.1038/s41467-019-09041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Olariu V., Lövkvist C., Sneppen K. Nanog, Oct4 and Tet1 interplay in establishing pluripotency. Sci. Rep. 2016;6:25438. doi: 10.1038/srep25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sohni A., Bartoccetti M., Khoueiry R., Spans L., Velde J.V., De Troyer L., Pulakanti K., Claessens F., Rao S., Koh K.P. Dynamic Switching of Active Promoter and Enhancer Domains Regulates Tet1 and Tet2 Expression during Cell State Transitions between Pluripotency and Differentiation. Mol. Cell. Biol. 2015;35:1026–1042. doi: 10.1128/MCB.01172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao Y., Chen J., Li K., Wu T., Huang B., Liu W., Kou X., Zhang Y., Huang H., Jiang Y., et al. Replacement of Oct4 by Tet1 during iPSC Induction Reveals an Important Role of DNA Methylation and Hydroxymethylation in Reprogramming. Cell Stem Cell. 2013;12:453–469. doi: 10.1016/j.stem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Lee J., Wu Y., Harada B.T., Li Y., Zhao J., He C., Ma Y., Wu X. N6 -methyladenosine modification of lncRNA Pvt1 governs epidermal stemness. EMBO J. 2021;40:e106276. doi: 10.15252/embj.2020106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sepich-Poore C., Zheng Z., Schmitt E., Wen K., Zhang Z.S., Cui X.-L., Dai Q., Zhu A.C., Zhang L., Castillo A.S., et al. The METTL5-TRMT112 N6-methyladenosine methyltransferase complex regulates mRNA translation via 18S rRNA methylation. J. Biol. Chem. 2022;298:101590. doi: 10.1016/j.jbc.2022.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wen J., Lv R., Ma H., Shen H., He C., Wang J., Jiao F., Liu H., Yang P., Tan L., et al. Zc3h13 Regulates Nuclear RNA m6A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell. 2018;69:1028–1038.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsumura A., Hayakawa T., Kumaki Y., Takebayashi S.-I., Sakaue M., Matsuoka C., Shimotohno K., Ishikawa F., Li E., Ueda H.R., et al. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 143.Dahariya S., Raghuwanshi S., Thamodaran V., Velayudhan S.R., Gutti R.K. Role of Long non-coding RNAs in Human Induced Pluripotent Stem Cells derived Megakaryocytes: A p53, HOTAIRM1 and miR-125b interaction study. J. Pharmacol. Exp. Ther. 2022 doi: 10.1124/jpet.121.001095. [DOI] [PubMed] [Google Scholar]
- 144.Rosa A., Ballarino M. Long Noncoding RNA Regulation of Pluripotency. Stem Cells Int. 2016;2016:1797692. doi: 10.1155/2016/1797692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fico A., Fiorenzano A., Pascale E., Patriarca E.J., Minchiotti G. Long non-coding RNA in stem cell pluripotency and lineage commitment: Functions and evolutionary conservation. Cell. Mol. Life Sci. 2019;76:1459–1471. doi: 10.1007/s00018-018-3000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong L., Mu H., Wen B., Zhang W., Wei Q., Gao G., Han J., Cao S. Long non-coding RNAs involved in the regulatory network during porcine pre-implantation embryonic development and iPSC induction. Sci. Rep. 2018;8:6649. doi: 10.1038/s41598-018-24863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hunkler H.J., Groß S., Thum T., Bär C. Non-coding RNAs: Key regulators of reprogramming, pluripotency, and cardiac cell specification with therapeutic perspective for heart regeneration. Cardiovasc. Res. 2022;118:3071–3084. doi: 10.1093/cvr/cvab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen J., Wang Y., Wang C., Hu J.-F., Li W. LncRNA Functions as a New Emerging Epigenetic Factor in Determining the Fate of Stem Cells. Front. Genet. 2020;11:277. doi: 10.3389/fgene.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao C., Xie W., Zhu H., Zhao M., Liu W., Wu Z., Wang L., Bin Zhu B., Li S., Zhou Y., et al. LncRNAs and their RBPs: How to influence the fate of stem cells? Stem Cell Res. Ther. 2022;13:175. doi: 10.1186/s13287-022-02851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mondal T., Kanduri C. Noncoding RNA scaffolds in pluripotency. Circ. Res. 2012;110:1162–1165. doi: 10.1161/RES.0b013e318257c489. [DOI] [PubMed] [Google Scholar]
- 151.Guttman M., Donaghey J., Carey B.W., Garber M., Grenier J.K., Munson G., Young G., Lucas A.B., Ach R., Bruhn L., et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ng S.-Y., Johnson R., Stanton L.W. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–533. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shi X., Wu Y., Ai Z., Liu X., Yang L., Du J., Shao J., Guo Z., Zhang Y. AICAR Sustains J1 Mouse Embryonic Stem Cell Self-Renewal and Pluripotency by Regulating Transcription Factor and Epigenetic Modulator Expression. Cell. Physiol. Biochem. 2013;32:459–475. doi: 10.1159/000354451. [DOI] [PubMed] [Google Scholar]
- 154.Jia J., Zheng X., Hu G., Cui K., Zhang J., Zhang A., Jiang H., Lu B., Yates J., 3rd, Liu C., et al. Regulation of Pluripotency and Self- Renewal of ESCs through Epigenetic- Threshold Modulation and mRNA Pruning. Cell. 2012;151:576–589. doi: 10.1016/j.cell.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo G., Yang J., Nichols J., Hall J.S., Eyres I., Mansfield W., Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hanna J., Markoulaki S., Mitalipova M., Cheng A.W., Cassady J.P., Staerk J., Carey B.W., Lengner C.J., Foreman R., Love J., et al. Metastable Pluripotent States in NOD-Mouse-Derived ESCs. Cell Stem Cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Okamura D., Chikushi M., Chigi Y., Shiogai N., Jafar S., Wu J. Stepwise conversion methods between ground states pluripotency from naïve to primed. Biochem. Biophys. Res. Commun. 2021;574:70–77. doi: 10.1016/j.bbrc.2021.07.097. [DOI] [PubMed] [Google Scholar]
- 158.Tiana M., Lopez-Jimenez E., de Aja J.S., Barral A., Victorino J., Badia-Careaga C., Rollan I., Rouco R., Santos E., Sanchez-Iranzo H., et al. Pluripotency factors regulate the onset of Hox cluster activation in the early embryo. Sci. Adv. 2022;8:eabo3583. doi: 10.1126/sciadv.abo3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Minchiotti G., D’aniello C., Fico A., De Cesare D., Patriarca E.J. Capturing Transitional Pluripotency through Proline Metabolism. Cells. 2022;11:2125. doi: 10.3390/cells11142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Romayor I., Herrera L., Burón M., Martin-Inaraja M., Prieto L., Etxaniz J., Inglés-Ferrándiz M., Pineda J.R., Eguizabal C. A Comparative Study of Cell Culture Conditions during Conversion from Primed to Naive Human Pluripotent Stem Cells. Biomedicines. 2022;10:1358. doi: 10.3390/biomedicines10061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yousefi M., Marashi S.-A., Sharifi-Zarchi A., Taleahmad S. The metabolic network model of primed/naive human embryonic stem cells underlines the importance of oxidation-reduction potential and tryptophan metabolism in primed pluripotency. Cell Biosci. 2019;9:71. doi: 10.1186/s13578-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsogtbaatar E., Landin C., Minter-Dykhouse K., Folmes C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020;8:87. doi: 10.3389/fcell.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takahashi S., Kobayashi S., Hiratani I. Epigenetic differences between naïve and primed pluripotent stem cells. Cell. Mol. Life Sci. 2018;75:1191–1203. doi: 10.1007/s00018-017-2703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghimire S., Van der Jeught M., Neupane J., Roost M.S., Anckaert J., Popovic M., Van Nieuwerburgh F., Mestdagh P., Vandesompele J., Deforce D., et al. Comparative analysis of naive, primed and ground state pluripotency in mouse embryonic stem cells originating from the same genetic background. Sci. Rep. 2018;8:5884. doi: 10.1038/s41598-018-24051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamaji M., Ueda J., Hayashi K., Ohta H., Yabuta Y., Kurimoto K., Nakato R., Yamada Y., Shirahige K., Saitou M. PRDM14 Ensures Naive Pluripotency through Dual Regulation of Signaling and Epigenetic Pathways in Mouse Embryonic Stem Cells. Cell Stem Cell. 2013;12:368–382. doi: 10.1016/j.stem.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 166.Chia N.-Y., Chan Y.-S., Feng B., Lu X., Orlov Y.L., Moreau D., Kumar P., Yang L., Jiang J., Lau M.-S., et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature. 2010;468:316–320. doi: 10.1038/nature09531. [DOI] [PubMed] [Google Scholar]
- 167.Ginis I., Luo Y., Miura T., Thies S., Brandenberger R., Gerecht-Nir S., Amit M., Hoke A., Carpenter M.K., Itskovitz-Eldor J., et al. Differences between human and mouse embryonic stem cells. Dev. Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 168.Smith Z.D., Chan M.M., Humm K.C., Karnik R., Mekhoubad S., Regev A., Eggan K., Meissner A. DNA methylation dynamics of the human preimplantation embryo. Nature. 2014;511:611–615. doi: 10.1038/nature13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Leary T., Heindryckx B., Lierman S., van Bruggen D., Goeman J.J., Vandewoestyne M., Deforce D., Lopes S.M.C.D.S., De Sutter P. Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat. Biotechnol. 2012;30:278–282. doi: 10.1038/nbt.2135. [DOI] [PubMed] [Google Scholar]
- 170.Chen H., Aksoy I., Gonnot F., Osteil P., Aubry M., Hamela C., Rognard C., Hochard A., Voisin S., Fontaine E., et al. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat. Commun. 2015;6:7095. doi: 10.1038/ncomms8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chan Y.-S., Göke J., Ng J.-H., Lu X., Gonzales K.A., Tan C.-P., Tng W.-Q., Hong Z.-Z., Lim Y.-S., Ng H.-H. Induction of a Human Pluripotent State with Distinct Regulatory Circuitry that Resembles Preimplantation Epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 172.Guo G., von Meyenn F., Rostovskaya M., Clarke J., Dietmann S., Baker D., Sahakyan A., Myers S., Bertone P., Reik W., et al. Epigenetic resetting of human pluripotency. Development. 2017;144:2748–2763. doi: 10.1242/dev.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guo G., von Meyenn F., Santos F., Chen Y., Reik W., Bertone P., Smith A., Nichols J. Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass. Stem Cell Rep. 2016;6:437–446. doi: 10.1016/j.stemcr.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A., et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 175.Theunissen T., Friedli M., He Y., Planet E., O’neil R.C., Markoulaki S., Pontis J., Wang H., Iouranova A., Imbeault M., et al. Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell. 2016;19:502–515. doi: 10.1016/j.stem.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takashima Y., Guo G., Loos R., Nichols J., Ficz G., Krueger F., Oxley D., Santos F., Clarke J., Mansfield W., et al. Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell. 2014;158:1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qin H., Hejna M., Liu Y., Percharde M., Wossidlo M., Blouin L., Durruthy-Durruthy J., Wong P., Qi Z., Yu J., et al. YAP Induces Human Naive Pluripotency. Cell Rep. 2016;14:2301–2312. doi: 10.1016/j.celrep.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu X., Nefzger C., Rossello F.J., Chen J., Knaupp A., Firas J., Ford E., Pflueger J., Paynter J.M., Chy H.S., et al. Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nat. Methods. 2017;14:1055–1062. doi: 10.1038/nmeth.4436. [DOI] [PubMed] [Google Scholar]
- 179.Hanna J., Cheng A.W., Saha K., Kim J., Lengner C.J., Soldner F., Cassady J.P., Muffat J., Carey B.W., Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fischer L.A., Khan S.A., Theunissen T.W. Induction of Human Naïve Pluripotency Using 5i/L/A Medium. Methods Mol. Biol. 2022;2416:13–28. doi: 10.1007/978-1-0716-1908-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Theunissen T.W., Powell B.E., Wang H., Mitalipova M., Faddah D.A., Reddy J., Fan Z.P., Maetzel D., Ganz K., Shi L., et al. Systematic Identification of Culture Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell. 2014;15:471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Choi H.W., Joo J.Y., Hong Y.J., Kim J.S., Song H., Lee J.W., Wu G., Schöler H.R., Do J.T. Distinct Enhancer Activity of Oct4 in Naive and Primed Mouse Pluripotency. Stem Cell Rep. 2016;7:911–926. doi: 10.1016/j.stemcr.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bayerl J., Ayyash M., Shani T., Manor Y.S., Gafni O., Massarwa R., Kalma Y., Aguilera-Castrejon A., Zerbib M., Amir H., et al. Principles of signaling pathway modulation for enhancing human naive pluripotency induction. Cell Stem Cell. 2021;28:1549–1565.e12. doi: 10.1016/j.stem.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pastor W.A., Chen D., Liu W., Kim R., Sahakyan A., Lukianchikov A., Plath K., Jacobsen S.E., Clark A.T. Naive Human Pluripotent Cells Feature a Methylation Landscape Devoid of Blastocyst or Germline Memory. Cell Stem Cell. 2016;18:323–329. doi: 10.1016/j.stem.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Khan S.A., Park K.-M., Fischer L.A., Dong C., Lungjangwa T., Jimenez M., Casalena D., Chew B., Dietmann S., Auld D.S., et al. Probing the signaling requirements for naive human pluripotency by high-throughput chemical screening. Cell Rep. 2021;35:109233. doi: 10.1016/j.celrep.2021.109233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang S.-H., Hao J., Zhang C., Duan F.-F., Chiu Y.-T., Shi M., Huang X., Yang J., Cao H., Wang Y. KLF17 promotes human naive pluripotency through repressing MAPK3 and ZIC2. Sci. China Life Sci. 2022;65:1985–1997. doi: 10.1007/s11427-021-2076-x. [DOI] [PubMed] [Google Scholar]
- 187.Yang Y., Zhang X., Yi L., Hou Z., Chen J., Kou X., Zhao Y., Wang H., Sun X.-F., Jiang C., et al. Naïve Induced Pluripotent Stem Cells Generated From β-Thalassemia Fibroblasts Allow Efficient Gene Correction With CRISPR/Cas9. Stem Cells Transl. Med. 2016;5:267. doi: 10.5966/sctm.2015-0157erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rossant J., Tam P.P. New Insights into Early Human Development: Lessons for Stem Cell Derivation and Differentiation. Cell Stem Cell. 2017;20:18–28. doi: 10.1016/j.stem.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 189.Zimmerlin L., Park T.S., Zambidis E.T., Ward E., Twaroski K., Tolar J., Birbrair A., Borges I.D.T., Sena I.F.G., Almeida G.G., et al. Capturing Human Naïve Pluripotency in the Embryo and in the Dish. Stem Cells Dev. 2017;26:1141–1161. doi: 10.1089/scd.2017.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou J., Hu J., Wang Y., Gao S. Induction and application of human naive pluripotency. Cell Rep. 2023;42:112379. doi: 10.1016/j.celrep.2023.112379. [DOI] [PubMed] [Google Scholar]
- 191.Bi Y., Tu Z., Zhang Y., Yang P., Guo M., Zhu X., Zhao C., Zhou J., Wang H., Wang Y., et al. Identification of ALPPL2 as a Naive Pluripotent State-Specific Surface Protein Essential for Human Naive Pluripotency Regulation. Cell Rep. 2020;30:3917–3931.e5. doi: 10.1016/j.celrep.2020.02.090. [DOI] [PubMed] [Google Scholar]
- 192.Ai Z., Xiang X., Xiang Y., Szczerbinska I., Qian Y., Xu X., Ma C., Su Y., Gao B., Shen H., et al. Krüppel-like factor 5 rewires NANOG regulatory network to activate human naive pluripotency specific LTR7Ys and promote naive pluripotency. Cell Rep. 2022;40:111240. doi: 10.1016/j.celrep.2022.111240. [DOI] [PubMed] [Google Scholar]
- 193.Göke J., Ng H.H. CTRL + INSERT: Retrotransposons and their contribution to regulation and innovation of the transcriptome. EMBO Rep. 2016;17:1131–1144. doi: 10.15252/embr.201642743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Göke J., Lu X., Chan Y.-S., Ng H.-H., Ly L.-H., Sachs F., Szczerbinska I. Dynamic Transcription of Distinct Classes of Endogenous Retroviral Elements Marks Specific Populations of Early Human Embryonic Cells. Cell Stem Cell. 2015;16:135–141. doi: 10.1016/j.stem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 195.Avilion A.A., Nicolis S.K., Pevny L.H., Perez L., Vivian N., Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Masui S., Nakatake Y., Toyooka Y., Shimosato D., Yagi R., Takahashi K., Okochi H., Okuda A., Matoba R., Sharov A.A., et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 197.Khan S.A., Audergon P.N.C.B., Payer B. X-chromosome activity in naive human pluripotent stem cells-are we there yet? Stem Cell Investig. 2017;4:54. doi: 10.21037/sci.2017.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chour T., Tian L., Lau E., Thomas D., Itzhaki I., Malak O., Zhang J.Z., Qin X., Wardak M., Liu Y., et al. Method for selective ablation of undifferentiated human pluripotent stem cell populations for cell-based therapies. J. Clin. Investig. 2021;6:e142000. doi: 10.1172/jci.insight.142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mallanna S.K., Rizzino A. Systems biology provides new insights into the molecular mechanisms that control the fate of embryonic stem cells. J. Cell. Physiol. 2012;227:27–34. doi: 10.1002/jcp.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang G., Ye S., Zhou X., Liu D., Ying Q.-L. Molecular basis of embryonic stem cell self-renewal: From signaling pathways to pluripotency network. Cell. Mol. Life Sci. 2015;72:1741–1757. doi: 10.1007/s00018-015-1833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.