Abstract
Using polarized epithelial cells, primarily MDCK-1, we assessed the mode of binding and effects on epithelial cell structure and permeability of Yersinia pseudotuberculosis yadA-deficient mutants. Initially, all bacteria except the invasin-deficient (inv) mutant adhered apically to the tight junction areas. These contact points of adjacent cells displayed β1-integrins together with tight junction-associated ZO-1 and occludin proteins. Indeed, β1-integrin expression was maximal in the tight junction area and then gradually decreased along the basolateral membranes. Wild-type bacteria also opened gradually the tight junction to paracellular permeation of different-sized markers, viz., 20-, 40-, and 70-kDa dextrans and 45-kDa ovalbumin, as well as to their own translocation between adjacent cells in intimate contact with β1-integrins. The effects on the epithelial cells and their barrier properties could primarily be attributed to expression of the Yersinia outer membrane protein YopE, as the yopE mutant bound but caused no cytotoxicity. Moreover, the apical structure of filamentous actin (F-actin) was disturbed and tight junction-associated proteins (ZO-1 and occludin) were dispersed along the basolateral membranes. It is concluded that the Yersinia bacteria attach to β1-integrins at tight junctions. Via this localized injection of YopE, they perturb the F-actin structure and distribution of proteins forming and regulating tight junctions. Thereby they promote paracellular translocation of bacteria and soluble compounds.
Enteropathogenic Yersinia enterocolitica and Y. pseudotuberculosis are fecal-oral pathogens that cause a range of gastrointestinal diseases including perturbation of barrier functions in reactive arthritis (8, 38, 57). They have distinct cytotoxic effects on eukaryotic cells (32, 53–56). A common 70-kb plasmid encodes a set of virulence proteins, among them the Yops (Yersinia outer membrane proteins) (10, 15, 16, 61) and Ysc (Yersinia se\cretion) proteins involved in type III secretion (45). Most of these Yop proteins, YopH, YopE, YopM, YopJ, and YpkA (Yersinia protein kinase A), are translocated into the eukaryotic cells upon cell-cell contact and have specific effects (5, 24, 51, 55, 56, 59). YopB and YopD are required for translocation of Yop effectors into the host cell (5, 24, 27, 51, 55). A wide range of cytotoxic effects can thus be anticipated. YopM is homologous to GPIbα, the α chain of the platelet receptor for the von Willebrand factor (40), thereby preventing platelet aggregation by interacting with thrombin (41, 52). YpkA is a Ser/Thr protein kinase that is homologous to eukaryotic protein kinases (19, 23). YopJ triggers apoptosis of macrophages (46, 47) and counteracts the release of tumor necrosis factor alpha (6, 50). YopH is a highly potent protein tyrosine phosphatase (4, 20, 65), and it has been suggested that YopE mediates contact-dependent cytotoxicity by disruption of cellular filamentous actin (F-actin) in the target cell (54, 55). Together, the latter two proteins are assumed to inhibit bacterial engulfment by eukaryotic cells (2, 12, 54). In mice, both yopE and yopH mutants of Y. pseudotuberculosis are avirulent and rapidly cleared from the lymphoid organs (32). In contrast, a yopE mutant of Y. enterocolitica was as cytotoxic as the wild-type strain (26). This could be explained by the presence of a new effector protein, YopT (34), which also induces disruption of F-actin, like YopE in Y. pseudotuberculosis. The plasmid-encoded YopK is not cytotoxic, but it restricts translocation of the ensemble of virulence proteins across the target cell membrane. Accordingly, a yopK mutant exhibits an enhanced cytotoxic effect (32, 33). Finally, the chromosomally encoded invasin binds with high affinity to β1-integrins when present on the surface of mammalian cells (35, 36, 39).
The tight junctions (3, 17, 18, 37, 44, 63) of the epithelial cells limit the permeation of substances and translocation of bacteria via the paracellular space besides distinguishing between the apical and basolateral plasma membrane domains of the cells. Several cytoplasmic, peripheral membrane proteins, e.g., ZO-1 (22, 60), ZO-2 (21), and ZO-3 (28), and two integral membrane protein families, occludin and claudins (17, 18), are all localized in the vicinity of tight junctions. F-actin is assumed to interact with tight junctions via ZO-1 and ZO-2 (28, 30, 42).
We demonstrate here that the barrier properties of two epithelial cell lines, Madin-Darby canine kidney cell line 1 (MDCK-1) and Caco-2, are markedly impaired by apical infection with wild-type bacteria but are little affected by yopE and other mutant strains.
All bacteria except the inv mutant adhered to the tight junction area, where β1-integrins were seemingly exposed apically close to the tight-junction-associated proteins ZO-1 and occludin. Incidentally, wild-type bacteria, but not the YopE mutant, perturbed the permeability barrier in a graduated way. They decreased the transepithelial resistance (TER) and opened the paracellular path to larger molecules (45-kDa ovalbumin and 20- to 70-kDa dextrans) and to translocation of the bacteria themselves along the basolateral membrane. The results suggest that bacteria recognize the tight junction area where they inject, among other cytotoxins, YopE. This is proposed to perturb F-actin locally and thereby also the structure and functions of tight junctions.
(This report was presented in part as a poster at Yersinia session D/B-244, 99th General Meeting of the American Society for Microbiology, 30 May to 3 June 1999, Chicago, Ill.)
MATERIALS AND METHODS
Bacterial strains, growth conditions, and Yop expression.
The Y. pseudotuberculosis strains used (Table 1) were grown overnight on a rotary shaker at 26°C in brain heart infusion (Oxoid) medium supplemented with 5 mM EGTA and 20 mM MgCl2. The cultures were subsequently diluted 1:20 with fresh medium and incubated for 1 h at 26°C and then at 37°C for an additional 3 h. Construction of the mutant strains is described in detail elsewhere (31).
TABLE 1.
Yersinia strains used
| Strain | Relevant genotypea | Reference |
|---|---|---|
| YpIII(pIB102) | Wild type | 7 |
| YpIII(pIB155) | ΔyopK | 32 |
| YpIII(pIB522) | ΔyopE | 54 |
| YpIII(pIB522, ΔK) | ΔyopE ΔyopK | 32 |
| YpIII(pIB605) | ΔyopD | 24 |
| YpIII(pIB604) | ΔyopB | 24 |
| Yp100(pIB103) | Δyad ΔinvA | 26 |
All bacterial strains are yadA deficient.
Cell culture.
MDCK-1 cells (passages 62 to 86) were grown for 3 to 4 days in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 10 mM HEPES. For comparison, the human intestinal epithelial cell line Caco-2 was also used in some experiments. Caco-2 cells (passages 80 to 85) were grown essentially according to standard procedures (29) in DMEM containing 10% heat-inactivated fetal calf serum (GIBCO, BRL Life Technologies, Paisley, Scotland), 2 mM l-glutamine, 100 U of penicillin per ml, and 1% nonessential amino acids (NordCell, Stockholm, Sweden). The cells were cultured to confluency on Transwell filters (3-μm pore size; no. 3415; Costar, Badhoevedorp, The Netherlands) or in 24-well microtiter plates with 13-mm-diameter circular coverslips at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. In the first case, they had to reach characteristic TER values, i.e., around 1 to 3 and 0.5 to 1 kohm · cm2 for MDCK-1 and Caco-2 cells, respectively.
Bacterial infection.
The cell monolayers grown on filter inserts or glass coverslips were washed free of penicillin, placed in 24-well tissue culture plates covered with DMEM without antibiotics, and incubated for 1 h at 37°C in a 5% CO2 atmosphere to stabilize the cells. The cells were then infected for 1 to 5 h from the apical side only with the selected strains of Y. pseudotuberculosis (2 × 107 bacteria/filter) (Table 1). Before staining and mounting, the cells were washed with DMEM without antibiotics to remove nonadherent bacteria.
TER.
The TER of the cell monolayers was determined using an epithelial voltohmmeter (EVOM, World Precision Instruments, Sarasota, Fla.) before and during 1 to 5 h of infection with the different mutant strains of Y. pseudotuberculosis.
Epithelial barrier characteristics.
To assess paracellular permeability, fluorescein isothiocyanate-labeled dextrans (20 to 70 kDa) were dissolved in Krebs-Ringer glucose phosphate buffer, pH 7.3 (KRG), to 20 mg/ml. Subsequently, 200 μl of this marker was added to the apical surface of the MDCK-1 monolayers. Aliquots of 100 μl were removed from the basolateral compartment at 1-h intervals for a period of 5 h and placed in 2 ml of KRG. Fluorescence measurements were performed with a fluorescence spectrophotometer (Perkin-Elmer Ltd., Beaconsfield, Buckinghamshire, England).
To follow the route of permeation, the cell monolayers were also incubated with fixable 40-kDa dextran (D-1845 dextran, fluorescein labeled; Molecular Probes, Eugene, Oreg.) and 45-kDa ovalbumin (O-837 ovalbumin, Texas red conjugated; Molecular Probes). The probes were added simultaneously for 15 min (at the same concentrations as the nonfixable dextran markers) after 3 h of infection with the selected bacterial strains (wild type and yopE mutant) and then fixed with paraformaldehyde. Due to the large difference in emission wavelength between fluorescein and Texas red (520 and 615 nm, respectively), the two markers could be visualized with the confocal microscope simultaneously (58).
Cell viability test.
The viability of polarized MDCK-1 monolayers infected with selected Y. pseudotuberculosis strains was assessed by incubation with trypan blue (0.1 mg/ml; Merck, Darmstadt, Germany) for 8 min and then examined in a light microscope (Carl Zeiss, Oberkochen, Germany) at intervals during the 1- to 5-h infection period. By this rough criterion, the cells remained viable during the experimental period.
Fluorescence microscopy of Yersinia, β1-integrins, F-actin, ZO-1, and occludin.
Monolayers of MDCK-1 cells were grown to confluence on either glass coverslips or Transwell filters. After the incubation with bacteria, the specimens were washed twice in phosphate-buffered saline (PBS) and fixed with 2.5% paraformaldehyde for 45 min on ice, washed in PBS, and incubated in NaBH4 (0.5 mg/ml) for 10 min to reduce autofluorescence. The cells were subsequently permeabilized with 0.3% Triton X-100 for 7 min at room temperature. Single staining of F-actin was done with rhodamine-labeled phalloidin (diluted 1/20 in PBS; Molecular Probes) for 45 min at 37°C in the dark. For dual staining of Yersinia and β1-integrins, a rabbit antiserum against Yersinia (diluted 1/500; 45) and mouse anti-human monoclonal antibodies (MAbs) against β1-integrin subunit (MAb 2253) and β1-integrin receptor (MAb 1959; Chemicon, Temecula, Calif.) were used. These antibodies were also used for blocking bacterial binding. As secondary antibodies were used goat anti-rabbit Alexa 594 and goat anti-mouse Alexa 488, respectively. The parallel localization of ZO-1 and β1-integrins was assessed using a MAb directed against rat anti-ZO-1 (diluted 1/200; Chemicon) and the previous antibodies against β1-integrins. Secondary antibodies were goat anti-rat Alexa 488 and goat anti-mouse Alexa 594. For colocalization of occludin and ZO-1, we first used a rabbit anti-occludin polyclonal antibody (diluted 1/150; Zymed, San Francisco, Calif.) and the above antibody against ZO-1 and then the appropriate secondary antibodies, i.e., Alexa 594-tagged goat anti-rabbit and Alexa 488-labeled goat anti-rat antibodies (Molecular Probes). After staining and a final wash in PBS, the coverslips were mounted on glass microscope slides in the Ultimate mounting medium containing antifade agent (CITIFLUOR Ltd., London, United Kingdom). The specimens were examined in a confocal microscope (Sarastro 2000; Molecular Dynamics, Sunnyvale, Calif.) using a 60× immersion objective (numerical aperture = 1.4). For fluorescence activation, either all lines or the 514-nm line of the argon laser was used in combination with a laser power of maximum 10 mW and a 535-nm primary beam splitter. In dual-stained samples, a secondary beam splitter (595 DRLP) was matched with barrier filter 600 EFLP (red channel) and interference filter 540 DF30 (green channel).
Statistics.
Data are presented as standard error of the mean (SEM). Comparisons between groups were made with Student's t test.
RESULTS
Yersinia YopE decreases TER of MDCK-1 monolayers.
During apical infection with the different Y. pseudotuberculosis strains (Table 1), alterations of tight junction integrity were followed by TER measurements (Table 2). First, it is noticeable that only a minor effect was achieved after 5 h with the yopE mutant (−3%), whereas both wild-type and yopK mutant bacteria caused a significant decrease in TER, by 44 and 70%, respectively. The latter data suggest enhanced translocation and intracellular effects of YopE. With the yopK yopE double mutant there was a small impairment of TER (−10%), which may reflect the combined effect of Yops other than YopE. Second, even for the wild-type bacteria the reduction of TER was gradual over the time of incubation.
TABLE 2.
Changes in TER of polarized MDCK-1 cells during 1 to 5 h of apical infection with Y. pseudotuberculosis strains
| Straina | Change in TER (% ± SEM; n = 6)
|
||
|---|---|---|---|
| 1 h | 3 h | 5 h | |
| None | 2 ± 5 | 7 ± 6 | 6 ± 4 |
| YpIII(pIB522) | 5 ± 3 | 6 ± 8 | −3 ± 1 |
| YpIII(pIB522, ΔK) | 5 ± 4 | 0 ± 5 | −10 ± 1 |
| YpIII(pIB102) | −6 ± 3 | −26 ± 6 | −44 ± 8 |
| YpIII(pIB155) | −10 ± 8 | −45 ± 10 | −70 ± 8 |
See Table 1 for genotypes.
Yersinia YopE increases the paracellular flux to fluorescent dextrans and ovalbumin across MDCK-1 monolayers.
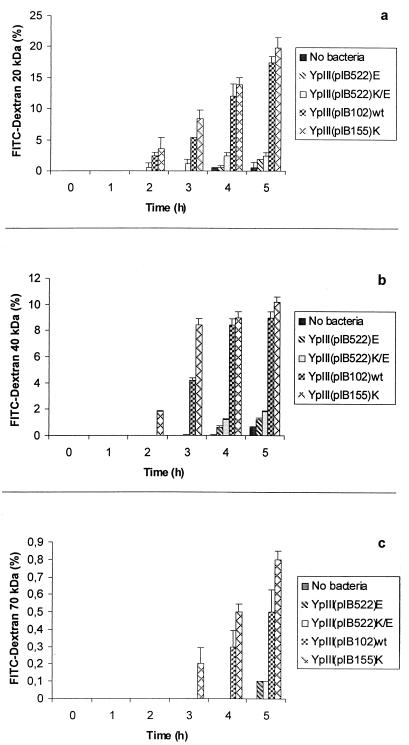
To further analyze the effects of the Y. pseudotuberculosis strains on epithelial integrity, we studied the passage of different-sized fluorescent dextrans (20, 40, and 70 kDa). These probes are assumed to be paracellular markers. Clear time- and size-dependent effects were seen for the different strains (Fig. 1). For the yopE mutant, the passage of probes across the cell layer was almost equivalent to that without bacteria. The strong dependence on probe size suggests that the barrier was impaired in a graded way.
FIG. 1.
Size-dependent flux of fluorescent dextrans (20 [a], 40 [b], and 70 [c] kDa) across MDCK-1 monolayers infected apically with different strains of Y. pseudotuberculosis. Incubation of confluent monolayers with wild-type and yopK mutant strains increased in a graduated way the paracellular permeation to the probe molecules. Values are expressed as means ± standard error of the mean for triplicate samples from six independent experiments.
Using fixable 40-kDa dextran and 45-kDa ovalbumin, confocal microscopy revealed that the paracellular route was indeed opened up by wild-type but not yopE mutant bacteria (Fig. 2). Ovalbumin was also endocytosed to some extent, as indicated by some intracellular fluorescence. This was, however, seen to a similar extent in the noninfected controls (not shown). A similar pattern was observed for Caco-2 cells (not shown).
FIG. 2.
Route of transepithelial permeation of fluorescein-labeled 40-kDa dextran (fixable; green fluorescence) and rhodamine-labeled 45-kDa ovalbumin (red fluorescence) across MDCK-1 cell monolayers infected with yopE mutant (a, merged dextran and ovalbumin images) or wild-type (b, merged dextran and ovalbumin images; c, dextran image; d, ovalbumin image) Y. pseudotuberculosis, as assessed by horizontal confocal sectioning. Bars, 5 μm.
The trypan blue test indicated retained viability of the polarized MDCK-1 cells during the 5-h infection with Y. pseudotuberculosis strains. We think, however, that the gradual change in TER (Table 2) and in permeability of the dextran probes of different sizes (Fig. 1) are better signs of a functioning epithelium, albeit strongly affected by some of the bacteria.
Apicolateral expression and bacterial binding to β1-integrins on polarized MDCK-1 cells.
As expected, both of the tight junction markers ZO-1 and occludin formed a string-like pattern in control cells (see Fig. 7a), indicating well-established tight junctions. Incidentally, ZO-1 and β1-integrins also seemed to colocalize at these sites (see Fig. 4b). Moreover, both horizontal and vertical sectioning showed that β1-integrins are found on the lateral membranes and in increasing concentrations toward the tight junction area (not shown). Evidently, some β1-integrins are exposed on the apical side of the junction, allowing for invasin-mediated bacterial attachment (Fig. 3b). Even more interesting, after 3 to 4 h of infection, wild-type bacteria had penetrated the paracellular space while still in contact with β1-integrins on the neighboring cells.
FIG. 7.
Distribution of tight junction proteins (ZO-1 and occludin) of polarized MDCK-1 monolayers, as assessed with horizontal (top) and vertical (bottom) confocal sectioning. (a) Control cells; (b) cells infected with the yopE mutant; (c) cells infected with wild-type bacteria for 3 h. Both ZO-1 and occludin appear similarly in panels a and b but disintegrated in panel c. In comparison to control cells (a) and cells treated with the yopE mutant (b), wild-type bacteria exhibited punctate ZO-1 and occludin distributions (horizontal sections) and dispersion along the basolateral membrane of the proteins (vertical sections). Bars, 2 μm.
FIG. 4.
Polarized MDCK cell monolayers incubated with wild-type Y. pseudotuberculosis and then dually stained for bacteria (red) and ZO-1 (green) (a) or ZO-1 (green) and β1-integrins (red) (b). The horizontal confocal sections in panel b show that β1-integrins and ZO-1 proteins colocalize at the level of tight junctions and that the string-like distribution of ZO-1 is partially interrupted by the bacterial interaction. Bar, 2 μm.
FIG. 3.
Apical bacterial binding and β1-integrin distribution on polarized MDCK-1 cell monolayers. Dual staining of β1-integrins with green Alexa 488 and of bacteria with red Alexa 594 on cells incubated with the Y. pseudotuberculosis inv mutant (yadA inv−) (a) and Y. pseudotuberculosis wild-type (yadA inv+) (b) strains, as assessed by horizontal confocal sectioning. Bar, 2 μm.
It is generally assumed that the Yersinia strains used in this study, which are yadA deficient, adhere to β1-integrins via their invasin (Inv) (36, 43). If there are no β1-integrins on the apical side, the bacteria should not be able to infect the cells unless there is yet another mode of binding and invading the epithelial cell layer. We therefore first tested for the role of the invasin on bacterial adhesion. Only randomly scattered inv-negative bacteria were seen, however, when the cell monolayer was screened for fluorescent bacteria. This suggests that β1-integrins mediate the initial apical attachment of bacteria that occurred only at the cell peripheries (Fig. 3b and 4b), where adjacent cells touch. The same pattern was also observed for Caco-2 cells (not shown).
The relative binding was quantified by viable count of 2-h attaching wild-type and inv bacteria. In relation to wild-type bacteria, the inv strain displayed only around 7 and 5% apical affinity for MDCK-1 and Caco-2 cells, respectively.
It has recently been shown that the invasin expresses lectin activity that can be blocked with 20 mM sialic acid (N-acetylneuraminic acid (25). We thus tested sialic acid at 20 mM but found no effect on the extent of bacterial binding. However, the distribution of both wild-type bacteria and β1-integrins became more patchy but still localized to the tight junction regions (not shown).
To further assess the specificity of invasin–β1-integrin interaction, we added anti-β1-integrin antibodies 1 h before and during 2 h of infection of MDCK-1 cells with wild-type bacteria. Out of the two MAbs tested (1959 and 2253; Chemicon), MAb 1959 reduced the binding to around 18 and 10%, as assessed by viable count and fluorescence intensity measurements on confocal images, respectively. Incidentally, both antibodies stain β1-integrins equally well in MDCK-1 cells. These findings, together with those in Fig. 3 and 4, suggest that apically exposed β1-integrins are indeed essential for the attachment.
To further investigate this issue, we stained MDCK-1 monolayers without prior 0.3% Triton X-100 permeabilization (Fig. 5a and b). The horizontal and vertical confocal sectioning clearly confirm that β1-integrins are apically exposed for antibody binding, but also that the wild-type bacteria (Fig. 5c and d) open the tight junctions and thereby make basolaterally located β1-integrins more accessible to the antibodies.
FIG. 5.
β1-Integrin distribution in MDCK-1 cells incubated for 2 h with cell culture medium (DMEM) (a and b), wild-type bacteria (c and d), and inv bacteria (e and f), as revealed by horizontal and vertical confocal sections of nonpermeabilized cells, labeled from the apical side. Bars, 5 μm.
Yersinia YopE is the key effector of disruption of F-actin, and it redistributes tight junctions proteins ZO-1 and occludin in MDCK-1 cells.
First, we confirm that F-actin forms a cage-like structure in epithelial cells (Fig. 6), characterized by high concentrations in the microvillus-brush border area, in the actomyosin ring, along the basolateral membranes, and at the substratum (31, 58), where stress fibers may be seen (not shown). When MDCK-1 monolayers were incubated with a yopE mutant of Y. pseudotuberculosis for 3 h, F-actin was normally distributed, displaying, for instance, an intact peripheral ring just beneath the intracellular tight junction (Fig. 6b). It was also the case with the yopE yopK double mutant (not shown). By contrast, the F-actin structure was clearly rearranged and diminished in cells infected with the wild-type strain (Fig. 6c). Quantitatively, the wild-type bacteria diminished the F-actin content by around 15%, as assessed with the NIH Image software (Fig. 6; Table 3). This was even more evident with the yopK mutant, i.e., when unrestricted translocation of Yops can take place. Consequently, no disruption was seen in monolayers infected with yopD- or yopB-deficient strains, i.e., bacteria without a functioning type III secretion machinery (not shown).
FIG. 6.
Structure of filamentous actin in polarized MDCK-1 cell monolayers as visualized with tetramethyl rhodamine isothiocyanate-labeled phalloidin and vertical confocal sectioning in uninfected cells (a), cells incubated with the yopE mutant (b), and cells incubated with wild-type Y. pseudotuberculosis for 3 h (c). In panels a and b there are equivalent amounts of F-actin basolaterally, and apically in the brush border region, but in panel c apical F-actin staining is weaker; the relative amount of F-actin in the different cell compartments is shown in Table 3. The strip on the left indicates the relative F-actin concentration on an 8-bit pseudocolor scale (0 [black] to 255 [white] levels).
TABLE 3.
Relative amounts of F-actin in MDCK-1 cells, as labeled with fluorescent phalloidin in control cells and in cells incubated with yopE mutant or wild-type bacteria (Fig. 6a to c)
| Specimen | Relative amta (mean ± SEM of F-actin)
|
||
|---|---|---|---|
| Apical | Lateral | Basal | |
| Control | 79.4 ± 0.8 | 34.1 ± 0.2 | 48.6 ± 0.2 |
| yopE mutant | 84.7 ± 0.7 | 33.6 ± 0.2 | 49.1 ± 0.2 |
| Wild-type bacteria | 67.8 ± 0.6 | 29.1 ± 0.2 | 42.5 ± 0.2 |
On a scale of 0 to 255.
We further assessed the effect of infection on the distribution of the two tight junction-associated proteins ZO-1 and occludin (Fig. 7). Interestingly, a relocalization of these proteins occurred in parallel to F-actin reorganization, but only in monolayers infected with the wild-type bacteria (Fig. 7c), not with the yopE mutant (Fig. 7b). Indeed, horizontal, confocal sectioning revealed an alteration in ZO-1 from a string-like to a more punctate pattern (Fig. 7c). In addition, vertical sectioning displayed a reduction in the amount of ZO-1, since immunofluorescence intensity was reduced in the infected area (Fig. 7c). Occludin was reorganized too, as if the protein had become free to move along the lateral membranes. Below the tight junction area there was also increasing amounts of intracellular, vesicle-like fluorescence, as a sign of protein up-regulation and/or turnover (not shown).
DISCUSSION
Bacterial adhesion is a crucial step in the infectious process. This allows effective translocation of cytotoxic proteins like YopH and YopE into the host cells and is essential for the virulence of Y. pseudotuberculosis (7, 51, 53–56). Clark and coworkers (9) have suggested that the targeting of Y. pseudotuberculosis to mouse membranous (M) cells is mediated by specific interaction between the bacterial invasin and β1-integrins on the M cells. McCormick et al. (44) have shown that β1-integrins are expressed along the lateral membranes in T84 epithelial cell monolayers. Accordingly, we have observed that basolateral infection of polarized MDCK cells by wild-type and especially by yopK-deficient Y. pseudotuberculosis strains decreases TER (33). Such findings are in line with the general concept that the bacteria invade the host via M cells and then also perturb the epithelium from the basolateral side in vivo.
Our present observations show that β1-integrins are exposed also apically in the tight junction area, which appears sufficient for inv-mediated binding of Yersinia (Fig. 3 to 5). Such apical binding of Y. pseudotuberculosis to multiple β1-integrins allows type III secretion and YopE translocation, which consequently modifies host F-actin organization and affects tight junction structure and function (Fig. 6 and 7).
Tight junctions hold cells to each other and play an integral role in maintaining cellular architecture (63). The dynamic link between these occluding junctions and the F-actin cytoskeleton has been demonstrated in several studies (48). Treatment of polarized T84 cells with cytochalasin D also influenced the paracellular pathway permeability (42). Incidentally, many bacterial toxins including factors modify the F-actin arrangement in the host cells (11), including several ADP-ribosylating toxins of Clostridium (1), the hemagglutinin/protease of Vibrio cholerae (64), and the Escherichia coli cytotoxic necrotizing factor type 1 (13). Recently, it was also found that cytochalasin D counteracted Helicobacter pylori infection of AGS gastric epithelial cells (62). The Bacteroides fragilis toxin fragilysin has been shown to decrease TER of cultured monolayers of epithelial cell lines from the colon, lung, and kidney in a dose- and time-dependent manner (49), and Fasano et al. (14) have shown that intestinal secretion is induced by the zonula occludens toxin (ZOT) upon opening of tight junctions.
When MDCK-1 cells were apically infected with wild-type Y. pseudotuberculosis YpIII(pIB102), there was both a decrease in TER (Table 2) and a graduated increase in the paracellular flux of fluorescent markers (Fig. 1). We interpret this as the result of dynamic changes in the organization of the F-actin cytoskeleton and tight junctions proteins ZO-1 and occludin. Since the yopE mutant did not change the paracellular permeability or the arrangement of the tight junction proteins ZO-1 and occludin (Fig. 7), we propose that YopE is a major epithelial cytotoxin of Y. pseudotuberculosis (55). Notably, it was recently reported that YopT also perturbs the F-actin structure (34). However, the yopT gene, which is present in Y. enterocolitica, does not exist in Y. pseudotuberculosis (Å. Forsberg et al., unpublished data). Iriarte and coworkers (34) report that less YopT than YopE is secreted. It is not known whether YopT also disrupts the barrier properties and the tight junction structure of polarized epithelial cells. If so, it might explain why Y. enterocolitica with two collaborating cytotoxins (YopE and YopT) causes a more severe diarrheal disease (57).
In summary, our findings propose that the apical surfaces of polarized epithelial cells, like MDCK-1, expose β1-integrins in areas of cell-to-cell contact. This allows Y. pseudotuberculosis bacteria to interact via their invasin close to the tight junctions, where Yops and other effector proteins are injected and focus their signals. Interestingly, YopE evidently affects the barrier functions through rearrangement of F-actin cytoskeleton in contact with tight junction-associated proteins (ZO-1 and occludin). What happens to proteins of the newly discovered tight junction-associated claudin protein family (63) remains to be elucidated.
ACKNOWLEDGMENTS
We thank Kai Simons (EMBL, Heidelberg, Germany) for providing the MDCK-1 cells, Ann-Britt Jeppson (ASTRA-Zeneca, Lund, Sweden) for Caco-2 cells, Virginia L. Miller (UCLA, Los Angeles, Calif.) for the inv yadA mutant, and Patricia Ödman and Maurice Devenney for revising the English text.
This work was supported by grants from the Swedish Medical Research Council (project no. 6251 and 11221), Swedish Research Council for Engineering Sciences, King Gustaf V 80 Year Foundation, Professor Nanna Svartz Foundation, Lions Research Foundation for Young Scientists, and Swedish Society for Medical Research.
REFERENCES
- 1.Aktories K. Clostridial ADP-ribosylating toxins: effects on ATP and GTP-binding proteins. Mol Cell Biochem. 1994;138:167–176. doi: 10.1007/BF00928459. [DOI] [PubMed] [Google Scholar]
- 2.Andersson K, Carballeira N, Magnusson K-E, Persson C, Stendahl O, Wolf-Watz H, Fällman M. YopH of Y. pseudotuberculosis interrupts early phosphotyrosine signalling associated with phagocytosis. Mol Microbiol. 1996;20:1057–1069. doi: 10.1111/j.1365-2958.1996.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 3.Balda M S, Matter K. Tight junctions. J Cell Sci. 1998;111:541–547. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- 4.Bliska J B, Guan K, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland A, Sory M-P, Iriate M, Kerbourch C, Wattiau P, Cornelis G R. Status of Yop M and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Boland A, Cornelis G R. Role of YoP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bölin I, Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984;43:72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrow T, Bacon G A. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956;37:481–493. [PMC free article] [PubMed] [Google Scholar]
- 9.Clark M A, Hirst B H, Jepson M A. M-cells surface β1-integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis G R. Yersinia, finely tuned pathogens. In: Hormache C E, Penn C W, Smythe C J, editors. Molecular biology of bacterial infections. Cambridge, England: Cambridge University Press; 1992. pp. 231–265. [Google Scholar]
- 11.Donelli G, Fiorentini C. Bacterial protein toxins acting on the cell cytoskeleton. Microbiologica. 1994;14:345–362. [PubMed] [Google Scholar]
- 12.Fällman M, Andersson K, Håkansson S, Magnusson K-E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falzano L, Fiorentini C, Boquet P, Doneli G. Interaction of Escherichia coli cytotoxic necrotizing factor type 1 (CNF1) with cultured cells. Cytotechnology. 1993;11:S56–S58. [PubMed] [Google Scholar]
- 14.Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology. 1997;112:839–846. doi: 10.1053/gast.1997.v112.pm9041245. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg Å, Wolf-Watz H. The virulence protein Yop5 of Y. pseudotuberculosis is regulated at transcriptional level by plasmid-pIB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988;2:121–133. doi: 10.1111/j.1365-2958.1988.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 16.Forsberg Å, Rosqvist R, Wolf-Watz H. Regulation and polarized transfer of the Yersinia outer proteins (Yops) involved in antiphagocytosis. Trends Microbiol. 1994;2:14–19. doi: 10.1016/0966-842x(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin, a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse M, Fujita K, Hiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galyov E E, Håkansson S, Forsberg Å, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 20.Guan K, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 21.Gumbiner B. Structure, biochemistry and assembly of epithelial tight junctions. Am J Physiol. 1987;253:749–758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 22.Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 24.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 25.Hamburger Z A, Brown M S, Isberg R R, Bjorkman P J. Crystal structure of invasin: a bacterial integrin-binding protein. Science. 1999;286:291–295. doi: 10.1126/science.286.5438.291. [DOI] [PubMed] [Google Scholar]
- 26.Han Y W, Miller V L. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect Immun. 1997;65:327–330. doi: 10.1128/iai.65.1.327-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartland E L, Green S P, Phillips Q A, Robins-Browne R M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haskins J, Gu L, Wittchen E S, Hibbard J, Stevensson B R. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidalgo I J, Raub T J, Borchardt R T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 30.Hirokawa N, Tilney L G. Interactions between actin filaments and membranes in quick-frozen and deeply etched hair cells of the chick ear. J Cell Biol. 1982;95:249–261. doi: 10.1083/jcb.95.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmgren K, Magnusson K-E, Franki N, Hays R. ADH-induced depolymerization of F-actin in the toad bladder granular cell: a confocal microscopy study. Am J Physiol. 1992;262(3 Pt. 1):C672–C677. doi: 10.1152/ajpcell.1992.262.3.C672. [DOI] [PubMed] [Google Scholar]
- 32.Holmström A, Rosqvist R, Wolf-Watz H. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infect Immun. 1995;63:2269–2276. doi: 10.1128/iai.63.6.2269-2276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fällman M, Magnusson K-E, Wolf-Watz H, Forsberg Å. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 34.Iriarte M, Cornelis G R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 35.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 36.Isberg R R, Leong M J. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 37.Keon B H, Schfer S, Kuhn C, Franke W W. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahesmaa-Rantala R, Magnusson K-E, Granfors K, Leino R, Sundqvist T, Toivanen A. Increased permeability following infection with Yersinia enterocolitica. Ann Rheum Dis. 1991;50:91–94. doi: 10.1136/ard.50.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leong M J, Fournier R S, Isberg R R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung K Y, Straley S C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIbα. J Bacteriol. 1989;171:4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung K Y, Reisner B S, Straley S C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990;58:3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madara J L, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability. J Cell Biol. 1986;102:2125–2135. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marra A, Isberg R R. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCormick A B, Nusrat A, Parkos C A, Andrea L D, Hofman P M, Carnes D, Liang T W, Madara J L. Unmasking of intestinal epithelial lateral membrane β1 integrin consequent to transepithelial neutrophil migration in vitro facilitates Inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills S D, Boland A, Sory M P, Van Der Smissen P, Kerbourch C, Finlay B B, et al. YopP, a novel Yersinia effector protein, is delivered into macrophages to induce apoptosis. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nybom P, Magnusson K-E. Modulation of the junctional integrity by low or high concentrations of cytochalasin B and dihydrocytochalasin B is associated with distinct changes in F-actin and ZO-1. Biosci Rep. 1996;16:265–272. doi: 10.1007/BF01855015. [DOI] [PubMed] [Google Scholar]
- 49.Obiso R J, Jr, Azghani A O, Wilkins T D. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect Immun. 1997;65:1431–1439. doi: 10.1128/iai.65.4.1431-1439.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophages TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 51.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 52.Reisner B S, Straley S C. Yersinia pestis YopM: thrombin binding and overexpression. Infect Immun. 1992;60:3355–3363. doi: 10.1128/iai.60.12.5242-5252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosqvist R, Forsberg Å, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;56:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serrander R, Magnusson K-E, Kihlström E, Sundqvist T. Acute Yersinia infections in man increase intestinal permeability towards low-molecular weight polyethyleneglycols (PEG 400) Scand J Infect Dis. 1986;18:409–413. doi: 10.3109/00365548609032356. [DOI] [PubMed] [Google Scholar]
- 58.Söderholm J D, Holmgren Peterson K, Olaison G, Franzen L E, Weström B, Magnusson K-E, Sjödahl R. Increased epithelial permeability to antigenic proteins in the non-inflamed ileum—a pathogenic factor in Crohn's disease? Gastroenterology. 1999;117:65–72. doi: 10.1016/s0016-5085(99)70551-2. [DOI] [PubMed] [Google Scholar]
- 59.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 60.Stevensson B R, Siliciano J D, Mooseker M S, Goodenough D A. Identification of ZO-1: a high molecular weight polypeptide associated with tight junctions (zonula occludens) in a variety of cells. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 62.Su B, Johansson S, Fällman M, Patarroyo M, Granström M, Normark S. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology. 1999;117:595–604. doi: 10.1016/s0016-5085(99)70452-x. [DOI] [PubMed] [Google Scholar]
- 63.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 64.Wu Z, Nybom P, Sundqvist T, Magnusson K-E. Endogeneous nitric oxide in MDCK-I cells modulates the Vibrio cholerae hemagglutinin/protease (HA/P)-mediated cytotoxicity. Microb Pathog. 1998;24:321–326. doi: 10.1006/mpat.1998.0201. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z Y, Clemens J C, Schubert H L, Stuckey J A, Fischer M W F, Hume D M, Saper M A, Dixon J E. Expression, purification, and physiochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J Biol Chem. 1992;267:23759–23766. [PubMed] [Google Scholar]