Abstract
1,3,5 triazines, especially indole functionalized triazine derivatives, exhibit excellent activities, such as anti-tumor, antibacterial, and anti-inflammatory activities. Traditional methods for the synthesis of N-(2-triazine) indoles suffer from unstable materials and tedious operations. Transition-metal-catalyzed C-C/C-N coupling provides a powerful protocol for the synthesis of indoles by the C-H activation strategy. Here, we report the efficient ruthenium-catalyzed oxidative synthesis of N-(2-triazine) indoles by C-H activation from alkynes and various substituted triazine derivatives in a moderate to good yield, and all of the N-(2-triazine) indoles were characterized by 1H NMR, 13C NMR, and HRMS. This protocol can apply to the gram-scale synthesis of the N-(2-triazine) indole in a moderate yield. Moreover, the reaction is proposed to be performed via a six-membered ruthenacycle (II) intermediate, which suggests that the triazine ring could offer chelation assistance for the formation of N-(2-triazine) indoles.
Keywords: ruthenium, C-H activation, triazines, indoles
1. Introduction
Indoles are one of the most important nitrogen heterocycles in medicinal chemistry, natural products, and organic synthesis [1,2,3,4,5,6,7]. Additionally, indoles are termed privileged motifs because of their excellent biological activities and applications in drug discovery. For these reasons, the construction of indoles has attracted considerable attention over the past few decades [8,9,10,11]. Among these creative approaches, the transition-metal-catalyzed C-H activation strategy of indoles has taken a prominent role. Generally, the activation of the C–H bond of alkylenes requires the participation of transition-metal catalysts [12,13,14,15,16,17] and the assistance of directing groups (amide [18,19,20,21], pyridyl [22,23], pyrimidyl [24], imidazole [25], carboxylic ester [26], and methoxy [27]). In particular, Stuart reported the first example of the synthesis of indoles from N-phenyl-2-aminopyridine and alkyne or alkene via intramolecular oxidative C-N coupling and C-H activation by [Cp*RhCl2]2 [28]. This powerful protocol provided a new insight for chemists to construct indoles by C-H activation from various substrates or with cheaper catalysts. However, in contrast to rhodium, inexpensive ruthenium or nickel complexes are competent choices for these transformations. Recently, many efficient methods for indole derivatives by the Ru-catalyzed oxidative annulation of 6-anilinopurine or 2-aminopyridine and alkynes have been developed. Nevertheless, innovative approaches to diverse indole derivatives still require further exploration.
On the other hand, triazine is a privileged pharmacophore against various targets, especially indole-substituted triazines, and has been reported to be a potential antibacterial [29], anti-inflammation [30,31] agent and an acetylcholinesterase [32] and cyclic GMP-AMP synthase [33] inhibitor. Normally, unstable cyanuric chloride is used as raw material for the preparation of these compounds through a nucleophilic displacement reaction. Hence, it is significant to develop practical protocols that can meet the demand for sustainable development. More recently, Cui and their group reported the efficient copper-catalyzed sulfonamidation of arenes via C-H activation, with triazine as the directing group [34]. Inspired by our previous work [35,36,37,38,39], we reasoned that N-aryl-triazines are suitable substrates for the synthesis of indole-substituted triazines through C-H activation. We herein describe the ruthenium-catalyzed synthesis of N-triazine-substituted indoles from N-aryl-triazines and alkynes.
2. Results and Discussion
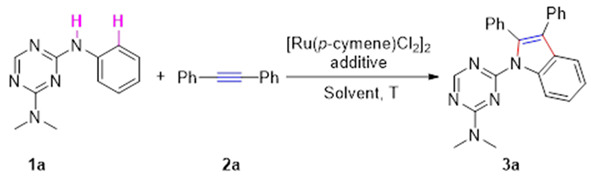
We commenced our studies by identifying the reaction of N2,N2-dimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1a) with 1,2-diphenylethyne (2a) in the presence of a ruthenium catalyst. We focused our optimization studies on a variety of additives (Cu(OAc)2, Cu (NO3)2, Cu (NO3)2, CuBr2, Cu(CF3SO3)2, NaOAc, CsOAc); the initial screening indicated that the use of Cu(OAc)2 proved to be optimal and gave a higher yield ( Table 1, entries 1–7). Notably, additional KBF6 to the reaction mixture did not improve the production of the desired product 3a (Table 1, entry 8). However, this reaction could not proceed without (Cu(OAc)2, suggesting that Cu(OAc)2 was vital for this transformation (Table 1, entry 9). Decreasing the loading of Cu(OAc)2 to 5% resulted in a 19% yield, while a slightly lower yield was observed when 20% of Cu(OAc)2 was added (Table 1, entries 10–11). The yield of 3a improved to 63% when the reaction was conducted at 100 oC (Table 1, entries 12–14). To our delight, a comparable yield (71%) was obtained by increasing the number of equivalents of 2a (Table 1, entries 15–17). Moreover, the reaction was conducted for 6 h so that it could lead to a lower yield, with many of the starting materials remaining unreacted (Table 1, entry 18). It is worth noting that the unidentified reaction occurred during the operation at 24 h (Table 1, entry 19). We also investigated a set of representative solvents, and DMF was proven to be the best choice for this synthetic methodology (Table 1, entries 20–23). Furthermore, Pd-catalysts were explored as a catalyst system, but the desired product was hardly observed (Table 1, entries 24–25). However, increasing or decreasing the amount of the ruthenium catalyst (10% or 2%) showed less efficiency for the formation of 3a (Table 1, entries 26–27).
Table 1.
Annulation reaction condition optimization a.
| Entry | Additive (%) | Solvent | Temp. (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | Cu(OAc)2 (10) | DMF | 120 | 55 |
| 2 | Cu (NO3)2(10) | DMF | 120 | - |
| 3 | CuCl2 (10) | DMF | 120 | 10 |
| 4 | CuBr2 (10) | DMF | 120 | 22 |
| 5 | Cu(CF3SO3)2 (10) | DMF | 120 | 44 |
| 6 | NaOAc (10) | DMF | 120 | 0 |
| 7 | CsOAc (10) | DMF | 120 | 0 |
| 8 | Cu(OAc)2 (10) | DMF | 120 | 50 c |
| 9 | - | DMF | 120 | 0 |
| 10 | Cu(OAc)2 (20) | DMF | 120 | 46 |
| 11 | Cu(OAc)2 (5) | DMF | 120 | 19 |
| 12 | Cu(OAc)2 (10) | DMF | 100 | 63 |
| 13 | Cu(OAc)2 (10) | DMF | 80 | 52 |
| 14 | Cu(OAc)2 (10) | DMF | 140 | 32 |
| 15 d | Cu(OAc)2 (10) | DMF | 100 | 71 |
| 16 e | Cu(OAc)2 (10) | DMF | 100 | 58 |
| 17 f | Cu(OAc)2 (10) | DMF | 100 | 42 |
| 18 g | Cu(OAc)2 (10) | DMF | 100 | 32 |
| 19 h | Cu(OAc)2 (10) | DMF | 100 | 56 |
| 20 | Cu(OAc)2 (10) | MeCN | 100 | 13 |
| 21 | Cu(OAc)2 (10) | H2O | 100 | 0 |
| 22 | Cu(OAc)2 (10) | MeOH | 100 | 32 |
| 23 | Cu(OAc)2 (10) | PEG-400 | 100 | 46 |
| 24 i | Cu(OAc)2 (10) | DMF | 100 | 0 (Pd(OAc)2) |
| 25 j | Cu(OAc)2 (10) | DMF | 100 | 0 (PdCl2) |
| 26 k | Cu(OAc)2 (10) | DMF | 100 | 64 (Ru 10%) |
| 27 l | Cu(OAc)2 (10) | DMF | 100 | 22 (Ru 2%) |
Reaction conditions: a 1a (1 mmol), 2a (1.0 mmol), [Ru(p-cymene) Cl2]2 (5 mol%), additive (10 mol%), and solvent (3 mL), 12 h. b Isolated yield. c Additional 10% of KBF4. d 1.5 mmol of 2a. e 2.0 mmol of 2a. f 0.8 mmol of 1a. f 6 h. g 24 h. h 24 h. i Pd(OAc)2 instead of [Ru(p-cymene) Cl2]2. j PdCl2 instead of [Ru(p-cymene) Cl2]2. k 10 mol% of [Ru(p-cymene) Cl2]2. l 2 mol% of [Ru(p-cymene) Cl2]2.
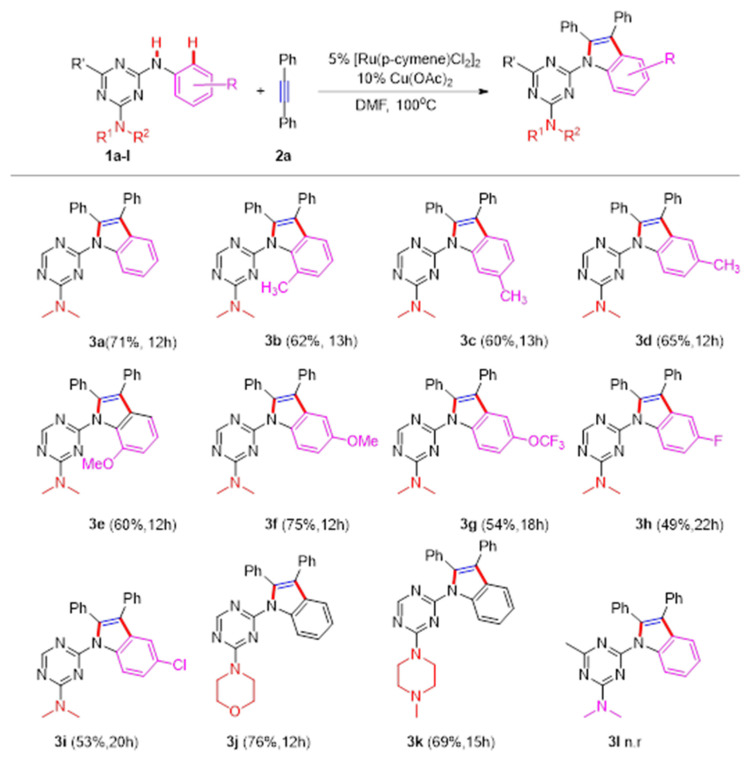
With the optimized reaction conditions in hand, a range of N-aryl triazines 1 was subjected to probe the reaction scope and generality (Scheme 1). The substrates with methyl or methoxy groups at different positions of the benzene ring exhibited good reactivity with a yield of up to 75% (3b-3f). What surprised us was that the corresponding product 3c obtained a 60% yield to the single isomer when 1c was applied. This observation indicates that the triazine ring could be an excellent directing group for improving the regioselectivity of this coupling reaction. N-aryl triazines with halogen groups (F, Cl) also underwent a reaction to give the desired products (3h, 3i). Additionally, triazines bearing morpholino or N-methyl piperazine could readily couple with 2a in 76% and 69% yields, respectively (3j, 3k). However, this reaction did not occur when the hydrogen atom at the C6 position of the triazine ring was replaced by methyl or other groups.
Scheme 1.
The synthesis of 3 from N-aryl triazines. Reaction conditions: 1a (1 mmol), 2a (1.5 mmol), [Ru(p-cymene)CL2]2 (5 mol%), Cu (OAc)2 (10 mol%), and solvent (3 mL), 12h, isolated yield.
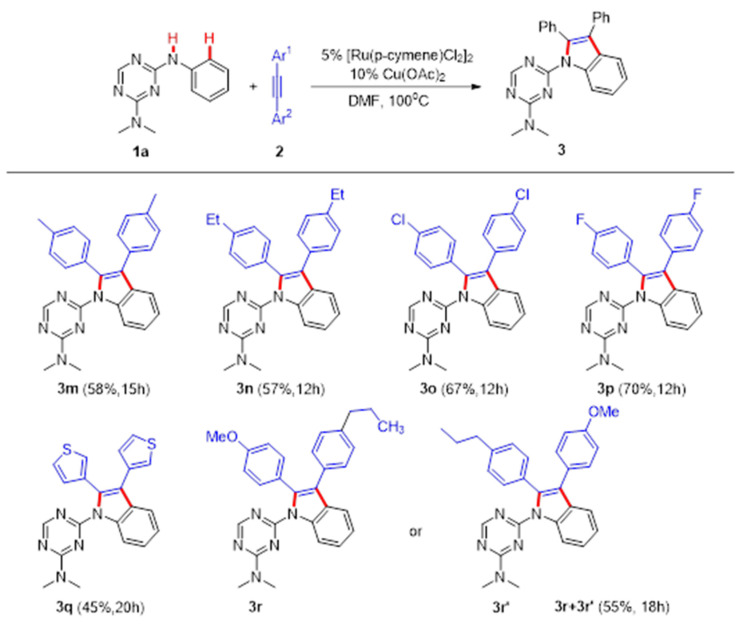
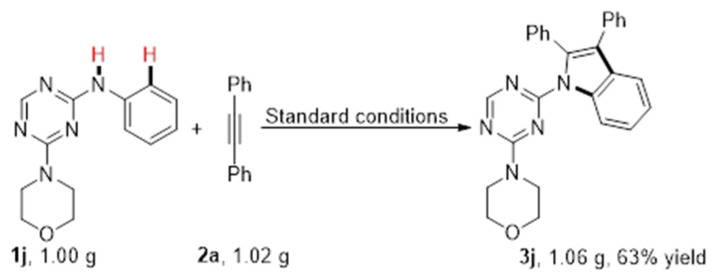
Then, various alkynes were tested to further investigate the scope of the ruthenium-catalyzed oxidative annulation strategy. Alkynes with electron-donating or withdrawing substituents reacted smoothly with 1a to generate the corresponding products (Scheme 2, 3m-3p). Furthermore, 1,2-di(thiophen-3-yl)ethyne 1q readily transformed into the desired product 3q at a 45% yield (Scheme 2, 3q). In order to explore the regioselectivity of the C−H annulation process, unsymmetrically substituted alkyne 1r was performed under optimized conditions. The results showed that 3r and 3r’ could be obtained with moderate regioselectivity and a total yield of 55% (Scheme 2, 3r and 3r’). The reaction of 1j (1.00 g) with 2a (1.02 g) under the optimal reaction conditions gave the corresponding product 3j at a 63% yield (Scheme 3).
Scheme 2.
The synthesis of 3 from various alkynes. Reaction conditions: a 1a (1 mmol), 2a (1.5 mmol), [Ru(p-cymene)CL2]2 (5 mol%), Cu (OAc)2 (10 mol%), and solvent (3 mL), isolated yield.
Scheme 3.
Gram-scale synthesis of N-(2-triazine) indole 3j.
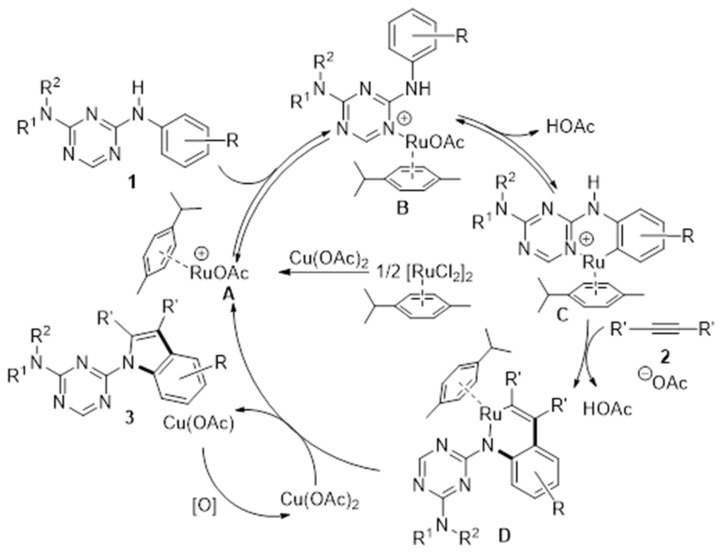
Based on the previous work [40,41,42,43], we inferred the possible mechanism for ruthenium oxidative annulation approaches. Firstly, cationic ruthenium (II) complex A, which was generated in the presence of Cu(OAc)2, reacted with 1 to produce B, and six-membered ruthenacycle (II) C was observed through the elimination of AcOH from B [41]. Subsequently, the formation of D was proposed to undergo coordination and migratory insertion with 2 [41,44]. Finally, D underwent reductive elimination to furnish the desired product 3 (Scheme 4). However, replacing the hydrogen atom at C6 of the triazine ring with other groups prevented the formation of vital intermediate B and C. It was clear that no expected corresponding product was observed when there were other groups at C6 of the triazine ring.
Scheme 4.
Proposed reaction mechanism.
3. Materials and Methods
3.1. General Information
Unless otherwise noted, materials were obtained from commercial suppliers and used without further purification. All reactions were performed in a heating mantle in a sealed tube unless otherwise noted. Thin layer chromatography (TLC) was performed using silica gel 60 F254 and was visualized using UV light. Column chromatography was performed with silica gel (mesh 300– 400). 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer in CDCl3 with Me4Si as an internal standard. Data were reported as follows: a chemical shift in ppm (δ), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, br = broad, and m = multiplet), coupling constant in Hertz (Hz) and integration. The HRMS and mass data were recorded by ESI on a TOF mass spectrometer.
3.2. General Procedure for the Synthesis of 1
The initial compound 1 was prepared as referred to in our previous work [38,39,45]. Sodium (2.5 eq) was added to anhydrous MeOH at 0 °C until the sodium was completely consumed. Then, biguanides hydrochloride (1.0 eq) was added to the reaction mixture, and 3–4 h later, methyl formate (ethyl acetate for 1l) was added. The above reaction mixture was stirred for another 24 h at 40 °C. When the reaction was complete, the mixture was concentrated under reduced pressure, and water was added, filtered, washed with water, and dried to produce a crude product. The crude product was purified by recrystallization in MeOH to obtain 1a-1l.
N2,N2-dimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1a) White solid, yield 2.65 g (74%), m.p: 186.8–187.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.30 (s, 1H, CH), 7.85 (br, 1H, NH), 7.63 (d, J = 7.8 Hz, 2H, Ar-H), 7.36 (t, J = 7.8 Hz, 2H, Ar-H), 7.09 (t, J = 7.8 Hz, 1H, Ar-H), 3.22 (s, 3H, N-CH3), 3.2 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 165.4, 164.5, 163.1, 138.7, 128.8, 123.1, 120.2, 36.4.
4-morpholino-N-phenyl-1,3,5-triazin-2-amine (1j) White solid, yield 1. 79 g (95%), m.p: 171.6–172.9 °C; 1H NMR (400 MHz, CDCl3) δ 8.35-8.30 (m, 2H, CH, NH), 7.57 (d, J = 7.7 Hz, 2H, Ar-H), 7.36 (t, J = 7.7 Hz, 2H, Ar-H), 7.11 (t, J = 7.7 Hz, 1H, Ar-H), 3.93-3.83 (m, 4H, O-CH2-), 3.82-3.73 (m, 4H, N-CH2-); 13C NMR (100 MHz, CDCl3) δ 165.7, 164.0, 163.5, 138.5, 128.8, 123.4, 120.5, 66.6, 43.7.
4-(4-methylpiperazin-1-yl)-N-phenyl-1,3,5-triazin-2-amine (1k) White solid, yield 1.73 g (90%), m.p: 187.2–188.3 °C; 1H NMR (400 MHz, CDCl3) δ 8.29 (s, 1H, CH), 7.84 (br, 1H, NH), 7.57 (d, J = 7.7 Hz, 2H, Ar-H), 7.35 (t, J = 7.7 Hz, 2H, Ar-H), 7.09 (t, J = 7.7 Hz, 1H, Ar-H), 3.97-3.81 (m, 4H, N-CH2-), 2.57-2.42 (m, 4H, N-CH2-), 2.35 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 165.8, 163.8, 163.5, 138.6, 128.8, 123.3, 120.4, 54.7, 46.1, 43.1.
N2,N2,6-trimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1l) [44] White solid, yield 1.65 g (86%), m.p: 149.2–152.1 °C; 1H NMR (400 MHz, CDCl3) δ 7.63 (dd, J = 8.6, 0.9 Hz, 2H, Ar-H), 7.58 (br, 1H, N-H), 7.35-7.29 (m, 2H, Ar-H), 7.05 (t, J = 7.4 Hz, 1H, Ar-H), 3.21 (s, 3H, N-CH3), 3.20 (s, 3H, N-CH3), 2.35 (s, 3H, -CH3); 13C NMR (100 MHz, CDCl3) δ 175.1, 165.3, 163.7, 139.1, 128.7, 122.7, 119.9, 119.8, 36.3, 25.6.
3.3. General Procedure for the Synthesis of 3
Cu(OAc)2 (10 mol%). was added to a mixture of N4-phenyl-1,3,5-triazine-2,4-diamine derivatives (0.5 mmol), [Ru(p-cymene) Cl2]2 (5 mol%) in DMF). The resultant mixture was then sealed and stirred for 12–20 h at 100 °C. After the completion of the reaction, the reaction mixture was cooled to room temperature and extracted with ethyl acetate. The organic phase was dried over anhydrous Na2SO4. The crude residue was obtained after the evaporation of the solvent in a vacuum, and the residue was purified by flash chromatography with petroleum ether and ethyl acetate (v/v 10/1) as the eluent to provide a pure product.
4-(2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3a) White solid, yield 139.6 mg (71%), m.p: 175.8–176.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.54 (s, 1H, CH), 8.53-8.51 (m, 1H, Ar-H), 7.35 (t, J = 7.6 Hz, 1H, Ar-H), 7.29-7.22 (m, 7H, Ar-H), 7.21-7.16 (m, 4H, Ar-H), 3.07 (s, 3H, N-CH3), 2.41 (s, 3H, N-CH3); 13C NMR (150 MHz, CDCl3) δ 166.3, 164.2, 162.9, 136.9, 135.9, 134.1, 133.9, 130.4, 130.2, 129.8, 128.2, 127.8, 126.8, 126.6, 124.3, 122.67, 122.1, 119.6, 115.0, 36.4, 35.6; HRMS (ESI) [M + H]+, calcd for C25H22N5: 392.1875, found: 392.1888.
4--4-(7-methyl-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3b) White solid, yield 125.8 mg (62%), m.p: 151.7–152.4 °C; 1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H, CH), 7.59 (d, J = 7.6 Hz, 1H, Ar-H), 7.36-7.29 (m, 4H, Ar-H), 7.26-7.19 (m, 6H, Ar-H), 7.18 (d, J = 7.6 Hz, 1H, Ar-H), 7.12 (d, J = 7.1 Hz, 1H, Ar-H), 3.20 (s, 3H, N-CH3), 2.88 (s, 3H, N-CH3), 2.33 (s, 3H, Ar-CH3); 13C NMR (100 MHz, CDCl3) δ 165.9, 164.5, 164.4, 137.2, 135.6, 134.3, 132.4, 131.1, 130.3, 129.4, 128.1, 127.7, 127.5, 126.5, 126.2, 122.7, 122.0, 119.4, 117.8, 36.7, 36.2, 20.6; HRMS (ESI) [M + H]+, calcd for C26H24N5: 406.2032, found: 406.2041.
N,N-dimethyl-4-(6-methyl-2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-amine (3c) White solid, yield 121.1 mg (60%), m.p: 184.3–185.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.57 (s, 1H, CH), 8.37 (s, 1H, Ar-H), 7.49 (d, J = 8.0 Hz, 1H, Ar-H), 7.31-7.27 (m, 1H, Ar-H), 7.26-7.19 (m, 4H, Ar-H), 7.20-7.16 (m, 5H, Ar-H), 7.08 (d, J = 8.0 Hz, 1H, Ar-H), 3.08 (s, 3H, N-CH3), 2.53 (s, 3H, N-CH3), 2.40 (s, 3H, Ar-CH3); 13C NMR (150 MHz, CDCl3) δ 166.2, 164.1, 162.9, 137.3, 135.3, 134.3, 134.2, 134.0, 130.3, 130.1, 128.1, 127.7, 127.6, 126.6, 126.4, 124.1, 122.0, 119.3, 115.1, 36.3, 35.6, 22.1; HRMS (ESI) [M + H]+, calcd for C26H24N5: 406.2032, found: 406.2047.
N,N-dimethyl-4-(5-methyl-2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-amine (3d) White solid, yield 131.1 mg (65%), m.p: 213.4–214.7 °C; 1H NMR (400 MHz, CDCl3) δ 8.57 (s, 1H, CH), 8.47 (d, J = 8.5 Hz, 1H, Ar-H), 7.40 (s, 1H, Ar-H), 7.37-7.30 (m, 2H, Ar-H), 7.29-7.27 (m, 2H, Ar-H), 7.27-7.24 (m, 2H, Ar-H), 7.24-7.17 (m, 5H, Ar-H), 3.12 (s, 3H, N-CH3), 2.46 (s, 3H, N-CH3), 2.43 (s, 3H, Ar-CH3); 13C NMR (100 MHz, CDCl3) δ 165.8, 163.6, 162.8, 135.9, 135.2, 134.2, 133.9, 132.3, 130.4, 130.1, 130.0, 128.1, 127.8, 126.7, 126.5, 125.7, 122.3, 119.4, 114.9, 36.5, 35.6, 21.4; HRMS (ESI) [M + H]+, calcd for C26H24N5: 406.2032, found: 406.2042.
4-(7-methoxy-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3e) White solid, yield 126.8 mg (62%), m.p: 183.7–184.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H, CH), 7.36 (d, J = 7.7 Hz, 2H, Ar-H), 7.33 (d, J = 6.9 Hz, 2H, Ar-H), 7.30-7.27 (m, 3H, Ar-H), 7.25-7.15 (m, 5H, Ar-H), 6.82 (d, J = 7.8 Hz, 1H, Ar-H), 3.84 (s, 3H, Ar-OCH3), 3.18 (s, 3H, N-CH3), 2.88 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 165.4, 164.6, 164.1, 147.3, 137.2, 134.3, 132.1, 131.2, 130.7, 130.2, 128.1, 127.7, 127.5, 126.3, 126.1, 122.5, 119.3, 112.6, 105.8, 55.9, 36.6, 36.2. HRMS (ESI) [M + H]+, calcd for C26H24N5O: 422.1981, found: 422.1989.
4-(5-methoxy-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3f) White solid, yield 158.3 mg (75%), m.p: 178.6–180.2 °C; 1H NMR (600 MHz, CDCl3) δ 8.53 (s, 1H, CH), 8.49 (d, J = 9.1 Hz, 1H, Ar-H), 7.34-7.28 (m, 2H, Ar-H), 7.25-7.23 (m, 3H, Ar-H), 7.23-7.16 (m, 5H, Ar-H), 7.06 (d, J = 2.6 Hz, 1H, Ar-H), 6.98 (dd, J = 9.1, 2.6 Hz, 1H), 3.83 (s, 3H, Ar-OCH3), 3.08 (s, 3H, N-CH3), 2.40 (s, 3H, N-CH3); 13C NMR (150 MHz, CDCl3) δ 166.2, 164.1, 162.8, 156.1, 136.6, 134.2, 133.9, 131.7, 130.5, 130.3, 130.2, 128.2, 127.7, 126.7, 126.5, 122.1, 116.2, 113.2, 101.9, 55.8, 36.3, 35.6. HRMS (ESI) [M + H]+, calcd forC26H24N5O: 422.1981, found: 422.1977.
4-(2,3-diphenyl-5-(trifluoromethoxy)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3g) White solid, yield 128.9 mg (54%), m.p: 175.8–176.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 9.2 Hz, 1H, Ar-H), 8.58 (s, 1H, CH), 7.49 (s, 1H), 7.39-7.27 (m, 7H, Ar-H), 7.26-7.22 (m, 3H, Ar-H), 3.12 (s, 3H, N-CH), 2.44 (s, 3H, N-CH); 13C NMR (100 MHz, CDCl3) δ 166.3, 164.1, 162.8, 145.1, 137.7, 135.1, 133.6, 133.1, 130.3, 130.2, 130.1, 128.4, 127.9, 127.1, 126.9, 121.7, 120.74 (q, J = 256.0 Hz, CF3).117.6, 116.2, 112.0, 36.4, 35.6. HRMS (ESI) [M + H]+, calcd for C26H21N5OF3: 476.1698, found: 476.1716.
4-(5-fluoro-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3h) White solid, yield 100.9 mg (49%), m.p: 232.9–234.3 °C; 1H NMR (400 MHz, CDCl3) δ 8.57 (s, 1H, CH), 8.55 (dd, J = 9.1, 4.7 Hz, 1H, Ar-H), 7.37-7.32 (m, 1H, Ar-H), 7.32-7.27 (m, 3H, Ar-H), 7.26-7.24 (m, 3H, Ar-H), 7.22-7.19 (m, 3H, Ar-H), 7.10 (td, J = 9.1, 2.6 Hz, 1H, Ar-H), 3.11 (s, 3H, N-CH3), 2.42 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 166.2, 164.0, 162.8, 159.5 (d, J = 238.4 Hz, C-F), 137.8, 133.9, 133.4, 133.2, 130.63 (d, J = 9.6 Hz, C-F), 130.2, 130.1, 128.3, 127.9, 127.0, 126.7, 121.9 (d, J = 4.0 Hz, C-F), 116.2 (d, J = 8.9 Hz, C-F), 111.9 (d, J = 24.9 Hz, C-F), 104.92 (d, J = 24.2 Hz, C-F), 36.4, 35.6. HRMS (ESI) [M + H]+, calcd for C25H21N5F: 410.1781, found: 410.1791.
4-(5-chloro-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3i) White solid, yield 112.5 mg (53%), m.p: 225.9–226.6 °C; 1H NMR (400 MHz, CDCl3) δ 8.58 (s, 1H, CH), 8.52 (dd, J = 8.9, 1.0 Hz, 1H, Ar-H), 7.60 (t, J = 1.0 Hz, 1H, Ar-H), 7.38-7.28 (m, 4H, Ar-H), 7.27-7.18 (m, 7H, Ar-H), 3.12 (s, 3H, N-CH3), 2.42 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 166.2, 163.9, 162.7, 137.2, 135.3, 133.7, 133.1, 130.9, 130.2, 130.1, 128.3, 127.9, 127.1, 126.8, 124.3, 121.5, 119.1, 116.3, 36.4, 35.6; HRMS (ESI) [M + H]+, calcd for C25H21N5Cl: 426.1485, found: 426.1496.
4-(4-(2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-yl)morpholine (3j) White solid, yield 164.9 mg (76%), m.p: 181.5–182.2 °C; 1H NMR (400 MHz, CDCl3) δ 8.64 (d, J = 8.4 Hz, 1H, Ar-H), 8.63 (s, 1H, CH), 7.65 (d, J = 7.8 Hz, 1H, Ar-H), 7.40 (d, J = 7.8 Hz, 1H, Ar-H), 7.35-7.15 (m, 11H, Ar-H), 3.77 (t, J = 4.1 Hz, 2H, O-CH2-), 3.65 (t, J = 4.1 Hz, 2H, O-CH2-), 3.31 (t, J = 4.1 Hz, 2H, N-CH2-), 2.85 (t, J = 4.1 Hz, 2H, N-CH2-); 13C NMR (100 MHz, CDCl3) δ 166.4, 163.1, 162.9, 136.9, 135.7, 134.4, 133.6, 130.3, 130.2, 129.9, 128.2, 127.8, 126.7, 126.7, 124.5, 122.9, 122.8, 119.7, 115.4, 66.6, 66.4, 43.7, 42.9; HRMS (ESI) [M + H]+, calcd for C27H24N5O: 434.1981, found: 434.1997.
1-(4-(4-methylpiperazin-1-yl)-1,3,5-triazin-2-yl)-2,3-diphenyl-1H-indole (3k) White solid, yield 154.1 mg (69%), m.p: 168.9–169.6 °C; 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 8.4 Hz, 1H, Ar-H), 8.60 (s, 1H, CH), 7.64 (d, J = 7.8 Hz, 1H, Ar-H), 7.39 (t, J = 7.8 Hz, 1H, Ar-H), 7.34-7.30 (m, 2H, Ar-H), 7.30-7.20 (m, 9H, Ar-H), 3.80-3.75 (m, 2H, N-CH2-), 2.96-2.82 (m, 2H, N-CH2-), 2.38 (s, 3H, N-CH3), 2.140-2.35(m, 2H, N-CH2-), 2.10-2.01(m, 2H, N-CH2-); 13C NMR (100 MHz, CDCl3) δ 166.6, 163.2, 163.1, 136.9, 135.8, 134.3, 133.7, 130.3, 130.1, 129.8, 128.2, 127.8, 126.8, 126.6, 124.4, 122.8, 122.4, 119.6, 115.3, 54.6, 54.5, 46.0, 43.0, 42.3. HRMS (ESI) [M + H]+, calcd for C28H27N6: 447.2297, found: 447.2309.
4-(2,3-di-p-tolyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3m) White solid, yield 121.5 mg (58%), m.p: 137.3–138.1 °C; 1H NMR (400 MHz, CDCl3) δ 8.57 (s, 1H, CH), 8.54 (d, J = 8.3 Hz, 1H, Ar-H), 7.63 (d, J = 7.5 Hz, 1H, Ar-H), 7.40-7.34 (m, 1H, Ar-H), 7.26 (t, J = 7.5 Hz, 1H, Ar-H), 7.19 (d, J = 8.1 Hz, 2H, Ar-H), 7.14 (d, J = 8.1 Hz, 2H, Ar-H), 7.11 (d, J = 8.0 Hz, 2H, Ar-H), 7.05 (d, J = 8.0 Hz, 2H, Ar-H), 3.14 (s, 3H, N-CH3), 2.51 (s, 2H, N-CH3), 2.38 (s, 2H, Ar-CH3), 2.34 (s, 3H Ar-CH3); 13C NMR (100 MHz, CDCl3) δ 165.9, 163.8, 162.9, 136.8, 136.4, 136.0, 135.8, 131.1, 130.9, 130.2, 130.0, 129.9, 128.9, 128.5, 124.1, 122.6, 121.8, 119.6, 114.9, 36.5, 35.6, 21.2. HRMS (ESI) [M + H]+, calcd for C27H26N5: 420.2188, found: 420.2199.
4-(2,3-bis(4-ethylphenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3n) White solid, yield 127.5 mg (57%), m.p: 192.4–193.1 °C; 1H NMR (400 MHz, CDCl3) δ 8.59 (s, 1H, CH), 8.56 (d, J = 8.3 Hz, 1H, Ar-H), 7.65 (d, J = 7.5 Hz, 1H, Ar-H), 7.36 (td, J = 7.5, 1.12 Hz, 1H, Ar-H), 7.28-7.24(m, 1H, Ar-H), 7.22 (d, J = 8.1 Hz, 2H, Ar-H), 7.16 (d, J = 7.6 Hz, 2H, Ar-H), 7.14 (d, J = 7.6 Hz, 2H, Ar-H), 7.08 (d, J = 8.1 Hz, 2H, Ar-H), 3.13 (s, 3H, N-CH3), 2.73-2.60 (m, 4H, Ar-CH2-), 2.45 (s, 3H, N-CH3), 1.28 (t, J = 7.6 Hz, 6H, -CH3),1.24 (t, J = 7.6 Hz, 6H, -CH3); 13C NMR (100 MHz, CDCl3) δ 165.9, 163.8, 162.9, 142.8, 142.3, 136.9, 135.9, 131.4, 131.1, 130.2, 130.1, 130.0, 127.6, 127.3, 124.1, 122.6, 121. 8, 119.7, 114.9, 36.4, 35.6, 28.7, 28.5, 15.8, 15.3; HRMS (ESI) [M + H]+, calcd for C29H30N5: 448.2501, found: 448.2507.
4-(2,3-bis(4-chlorophenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3o) White solid, yield 154.2 mg (67%), m.p: 173.9–174.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.58 (s, 1H, CH), 8.57 (d, J = 7.8 Hz, 1H, Ar-H), 7.59 (dd, J = 7.8, 0.7 Hz, 1H, Ar-H), 7.40 (t, J = 7.8 Hz, 1H, Ar-H), 7.34-7.29 (m, 3H, Ar-H), 7.26 (d, J = 8.5 Hz, 2H, Ar-H), 7.23-7.18 (m, 2H, Ar-H), 7.14 (d, J = 8.5 Hz, 2H, Ar-H), 3.16 (s, 3H, N-CH3), 2.55 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 165.9, 163.6, 162.7, 136.9, 134.7, 133.1, 132.7, 132.4, 131.9, 131.5, 131.4, 129.3, 128.6, 128.2, 124.7, 123.0, 121.5, 119.4, 115.3, 36.5, 35.6; HRMS (ESI) [M + H]+, calcd for C25H20N5Cl2: 460.1096, found: 460.1104.
4-(2,3-bis(4-fluorophenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3p) White solid, yield 149.6 mg (70%), m.p: 175.8–176.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.60-8.53 (m, 2H, Ar-H, CH), 7.58 (d, J = 7.7 Hz, 1H, Ar-H), 7.45-7.35 (m, 1H, Ar-H), 7.32-7.29 (m, 1H, Ar-H), 7.26-7.20 (m, 2H, Ar-H), 7.20-7.15 (m, 2H, Ar-H), 7.07-7.01 (m, 2H, Ar-H), 7.00-6.94 (m, 2H, Ar-H) 3.16 (s, 3H, N-CH3), 2.57 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 166.0, 163.8, 162.8, 161.9 (d, J = 246.9 Hz, C-F); 161.7 (d, J = 246.0 Hz, C-F); 136.7, 134.9, 131.8, 131.7, 130.0 (d, J = 3.5 Hz, C-F), 129.6, 129.5 (d, J = 3.3 Hz, C-F), 124.5, 122.9, 121.4, 119.4, 115.3 (d, J = 21.4 Hz, C-F), 115.2, 114.9 (d, J = 21.6 Hz, C-F), 36.5, 35.7; HRMS (ESI) [M + H]+, calcd for C25H20N5F2: 428.1687, found: 428.1694.
4-(2,3-di(thiophen-2-yl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3q) White solid, yield 90.6 mg (45%), m.p: 186.1–186.9 °C; 1H NMR (400 MHz, CDCl3) δ 8.59 (s, 1H, CH), 8.51 (d, J = 8.4 Hz, 1H, Ar-H), 7.85 (d, J = 7.8 Hz, 1H, Ar-H), 7.44-7.37 (m, 2H, Ar-H), 7.36-7.33 (m, 1H, thiazole-H), 7.31 (dd, J = 5.1, 1.0 Hz, 1H, thiazole-H), 7.13 (dd, J = 3.6, 1.0 Hz, 1H, thiazole-H), 7.10-7.07 (m, 1H, thiazole-H), 7.06 (dd, J = 3.6, 1.3 Hz, 1H, thiazole-H), 7.03 (dd, J = 5.1, 3.5 Hz, 1H, thiazole-H), 3.18 (s, 3H, N-CH3), 2.72 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 166.2, 164.3, 162.7, 136.7, 134.7, 134.4, 129.5, 129.0, 128.8, 127.1, 126.8, 126.7, 125.4, 124.9, 122.9, 120.0, 117.5, 115.0, 36.5, 35.9. HRMS (ESI) [M + H]+, calcd for C21H18N5S2: 404.1004, found: 404.1015.
4-(2-(4-methoxyphenyl)-3-(4-propylphenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3r, 2:1) White solid, yield 127.5 mg (55%), m.p: 177.1–178.6 °C; 1H NMR (400 MHz, CDCl3) δ 8.59 (s, 1H, CH), 8.57 (d, J = 8.4 Hz, 1H, Ar-H), 7.62 (d, J = 7.8 Hz, 1H, Ar-H), 7.37 (t, J = 7.8 Hz, 1H, Ar-H), 7.30-7.25 (m, 1H, Ar-H), 7.23-7.18 (m, 2H, Ar-H), 7.12 (d, J = 7.5 Hz, 2H, Ar-H), 7.06 (d, J = 7.5 Hz, 2H, Ar-H), 6.87 (d, J = 8.4 Hz, 2H, Ar-H), 3.84 (s, 3H, O-CH3), 3.14 (s, 3H, N-CH3), 2.58 (t, J = 7.6 Hz, 2H, Ar-CH2-), 2.45 (s, 3H, N-CH3), 1.69-1.55 (m, 2H, -CH2-), 0.96 (t, J = 7.4 Hz, 3H,-CH3); 13C NMR (100 MHz, CDCl3) δ 165.4, 162.7, 158.3, 141.3, 136.9, 135.8, 131.4, 131.4, 130.1, 129.9, 127.9, 126.1, 124.2, 122.7, 122.1, 121.9, 119.6, 115.2, 113.6, 55.2, 37.8, 36.6, 35.8, 24.6, 13.8. HRMS (ESI) [M + H]+, calcd for C29H30N5O: 464.2450, found: 464.2462.
4. Conclusions
In summary, we developed a novel ruthenium-catalyzed oxidative annulation of alkynes with N-phenyl triazines to prepare N-(2-triazine) indoles in moderate to good yields. These studies indicated that the triazine ring could act as an efficient directing group for the synthesis of bioactive indole compounds. Detailed mechanistic studies and work on substrate expansions are in progress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28093676/s1, Figure S1, 1H NMR spectrum of 4-(2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3a); Figure S2, 13C NMR spectrum of 4-(2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3a); Figure S3, 1H NMR spectrum of 4--4-(7-methyl-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3b); Figure S4, 13C NMR spectrum of 4--4-(7-methyl-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3b); Figure S5, 1H NMR spectrum of N,N-dimethyl-4-(6-methyl-2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-amine (3c); Figure S6, N,N-dimethyl-4-(6-methyl-2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-amine (3c); Figure S7, 1H NMR spectrum of N,N-dimethyl-4-(5-methyl-2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-amine (3d); Figure S8, 13C NMR spectrum of N,N-dimethyl-4-(5-methyl-2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-amine (3d); Figure S9, 1H NMR spectrum of 4-(7-methoxy-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3e); Figure S10, 13C NMR spectrum of 4-(7-methoxy-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3e); Figure S11, 1H NMR spectrum of 4-(5-methoxy-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3f); Figure S12, 13C NMR spectrum of 4-(5-methoxy-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3f); Figure S13, 1H NMR spectrum of 4-(2,3-diphenyl-5-(trifluoromethoxy)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3g); Figure S14, 13C NMR spectrum of 4-(2,3-diphenyl-5-(trifluoromethoxy)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3g); Figure S15, 1H NMR spectrum of 4-(5-fluoro-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3h); Figure S16, 13C NMR spectrum of 4-(5-fluoro-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3h); Figure S17, 1H NMR spectrum of 4-(5-chloro-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3i); Figure S18, 13C NMR spectrum of 4-(5-chloro-2,3-diphenyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3i); Figure S19, 1H NMR spectrum of 4-(4-(2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-yl)morpholine (3j); Figure S20, 13C NMR spectrum of 4-(4-(2,3-diphenyl-1H-indol-1-yl)-1,3,5-triazin-2-yl)morpholine (3j); Figure S21, 1H NMR spectrum of 1-(4-(4-methylpiperazin-1-yl)-1,3,5-triazin-2-yl)-2,3-diphenyl-1H-indole (3k); Figure S22, 13C NMR spectrum of 1-(4-(4-methylpiperazin-1-yl)-1,3,5-triazin-2-yl)-2,3-diphenyl-1H-indole (3k); Figure S23, 1H NMR spectrum of 4-(2,3-di-p-tolyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3m); Figure S24, 13C NMR spectrum of 4-(2,3-di-p-tolyl-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3m); Figure S25, 1H NMR spectrum of 4-(2,3-bis(4-ethylphenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3n); Figure S26, 13C NMR spectrum of 4-(2,3-bis(4-ethylphenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3n); Figure S27, 1H NMR spectrum of 4-(2,3-bis(4-chlorophenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3o); Figure S28, 13C NMR spectrum of 4-(2,3-bis(4-chlorophenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3o); Figure S29, 1H NMR spectrum of 4-(2,3-bis(4-fluorophenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3p); Figure S30, 13C NMR spectrum of 4-(2,3-bis(4-fluorophenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3p); Figure S31, 1H NMR spectrum of 4-(2,3-di(thiophen-2-yl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3q); Figure S32, 13C NMR spectrum of 4-(2,3-di(thiophen-2-yl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3q); Figure S33, 1H NMR spectrum of 4-(2-(4-methoxyphenyl)-3-(4-propylphenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3r and 3r’); Figure S34, 13C NMR spectrum of 4-(2-(4-methoxyphenyl)-3-(4-propylphenyl)-1H-indol-1-yl)-N,N-dimethyl-1,3,5-triazin-2-amine (3r and 3r’); Figure S35, 1H NMR spectrum of N2,N2-dimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1a); Figure S36, 13C NMR spectrum of N2,N2-dimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1a); Figure S37, 1H NMR spectrum of 4-morpholino-N-phenyl-1,3,5-triazin-2-amine (1j); Figure S38, 13C NMR spectrum of 4-morpholino-N-phenyl-1,3,5-triazin-2-amine (1j); Figure S39, 1H NMR spectrum of 4-(4-methylpiperazin-1-yl)-N-phenyl-1,3,5-triazin-2-amine (1k); Figure S40, 13C NMR spectrum of 4-(4-methylpiperazin-1-yl)-N-phenyl-1,3,5-triazin-2-amine (1k); Figure S41, 1H NMR spectrum of N2,N2,6-trimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1l); Figure S42, 13C NMR spectrum of N2,N2,6-trimethyl-N4-phenyl-1,3,5-triazine-2,4-diamine (1l); Figure S43: The analysis of relative yield of the isomers 3r and 3r’ by 1H NMR.
Author Contributions
Reaction optimization and synthesis investigation work were carried out by J.C., F.L. and H.L. Mechanism related work was conducted by D.J. and W.L. NMR and HRMS studies were carried out by J.D. and L.Z. Conceptualisation, supervision, validation, and writing of the manuscript was completed by M.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all the compounds are available from the authors.
Funding Statement
We thank the Natural Science Foundation of Jiangxi province (Nos. 20212BAB216070, 20224BAB206119), the Natural Science Foundation of China (Nos. 21967013), and the Foundation of Jiangxi Provincial Department of Education (Nos. GJJ201825, GJJ2201931) for supporting this work. We are grateful for support from the Analytical and Testing Center of Jiujiang University.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhang H.-H., Shi F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022;55:2562–2580. doi: 10.1021/acs.accounts.2c00465. [DOI] [PubMed] [Google Scholar]
- 2.Kim T., Ha M.W., Kim J. Recent Advances in Divergent Synthetic Strategies for Indole-Based Natural Product Libraries. Molecules. 2022;27:2171. doi: 10.3390/molecules27072171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George N., Jawaid Akhtar M., Al Balushi K.A., Alam Khan S. Rational drug design strategies for the development of promising multi-target directed indole hybrids as Anti-Alzheimer agents. Bioorg. Chem. 2022;127:105941. doi: 10.1016/j.bioorg.2022.105941. [DOI] [PubMed] [Google Scholar]
- 4.Asati V., Bhupal R., Bhattacharya S., Kaur K., Gupta G.D., Pathak A., Mahapatra K.D. Recent Updates on Indole Derivatives as Kinase Inhibitors in the Treatment of Cancer. Anti-Cancer Agent ME. 2023;23:404–416. doi: 10.2174/1871520622666220607143040. [DOI] [PubMed] [Google Scholar]
- 5.Sun H., Sun K., Sun J. Recent Advances of Marine Natural Indole Products in Chemical and Biological Aspects. Molecules. 2023;28:2204. doi: 10.3390/molecules28052204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Liu T., Sun J. Recent Advances in N-Heterocyclic Small Molecules for Synthesis and Application in Direct Fluorescence Cell Imaging. Molecules. 2023;28:733. doi: 10.3390/molecules28020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud E., Hayallah A.M., Kovacic S., Abdelhamid D., Abdel-Aziz M. Recent progress in biologically active indole hybrids: A mini review. Pharmaco. Rep. 2022;74:570–582. doi: 10.1007/s43440-022-00370-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., He L., Natte K., Neumann H., Beller M., Wu X.F. Palladium@Cerium(IV) Oxide-Catalyzed Oxidative Synthesis of N -(2-Pyridyl)indoles via C-H Activation Reaction. Adv. Syn. Catal. 2014;356:2955–2959. doi: 10.1002/adsc.201400466. [DOI] [Google Scholar]
- 9.Inder K., Rakesh K., Upendra S. Recent Advances in the Regioselective Synthesis of Indoles via C–H Activation/Functionalization. Synthesis. 2018;50:2655–2677. [Google Scholar]
- 10.Urbina K., Tresp D., Sipps K., Szostak M. Recent Advances in Metal-Catalyzed Functionalization of Indoles. Adv. Syn. Catal. 2021;363:2723–2739. doi: 10.1002/adsc.202100116. [DOI] [Google Scholar]
- 11.Zheng Y., Zhang W.-Y., Gu Q., Zheng C., You S.-L. Cobalt(III)-catalyzed asymmetric ring-opening of 7-oxabenzonorbornadienes via indole C–H functionalization. Nat. Commun. 2023;14:1094. doi: 10.1038/s41467-023-36723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moselage M., Li J., Ackermann L. Cobalt-Catalyzed C–H Activation. ACS Catal. 2016;6:498–525. doi: 10.1021/acscatal.5b02344. [DOI] [Google Scholar]
- 13.Liu W., Ackermann L. Manganese-Catalyzed C–H Activation. ACS Catal. 2016;6:3743–3752. doi: 10.1021/acscatal.6b00993. [DOI] [Google Scholar]
- 14.Gandeepan P., Müller T., Zell D., Cera G., Warratz S., Ackermann L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. [DOI] [PubMed] [Google Scholar]
- 15.Lu P., Boorman T.C., Slawin A.M.Z., Larrosa I. Gold(I)-Mediated C−H Activation of Arenes. J. Am. Chem. Soc. 2010;132:5580–5581. doi: 10.1021/ja101525w. [DOI] [PubMed] [Google Scholar]
- 16.Liang L.-C., Chien P.-S., Huang Y.-L. Intermolecular Arene C−H Activation by Nickel(II) J. Am. Chem. Soc. 2006;128:15562–15563. doi: 10.1021/ja065505p. [DOI] [PubMed] [Google Scholar]
- 17.Thombal R.S., Rubio P.Y.M., Lee D., Maiti D., Lee Y.R. Modern Palladium-Catalyzed Transformations Involving C–H Activation and Subsequent Annulation. ACS Catal. 2022;12:5217–5230. doi: 10.1021/acscatal.2c00813. [DOI] [Google Scholar]
- 18.Tischler O., Bokányi Z., Novák Z. Activation of C–H Activation: The Beneficial Effect of Catalytic Amount of Triaryl Boranes on Palladium-Catalyzed C–H Activation. Organometallics. 2016;35:741–746. doi: 10.1021/acs.organomet.5b01017. [DOI] [Google Scholar]
- 19.Anguille S., Brunet J.-J., Chu N.C., Diallo O., Pages C., Vincendeau S. Platinum-Catalyzed Formation of Quinolines from Anilines. Aliphatic α-C−H Activation of Alkylamines and Aromatic ortho-C−H Activation of Anilines. Organometallics. 2006;25:2943–2948. doi: 10.1021/om058047m. [DOI] [Google Scholar]
- 20.Li W., Wang R., Li Z., Chen J., Zhang Y., Lv N. Convergent synthesis of triarylamines via Ni-catalyzed dual C(sp2)–H amination from benzamides with benzohydroxamic acids. Chem. Commun. 2023;59:4360–4363. doi: 10.1039/D3CC00165B. [DOI] [PubMed] [Google Scholar]
- 21.Nan J., Ren X., Yan Q., Liu S., Wang J., Ma Y., Szostak M. Hypervalent iodine-promoted twofold oxidative coupling of amines with amides and thioamides: Chemoselective pathway to oxazoles and thiazoles. Chem. Sci. 2023;14:3338–3345. doi: 10.1039/D3SC00301A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalyani D., Sanford M.S. Regioselectivity in Palladium-Catalyzed C−H Activation/Oxygenation Reactions. Org. Lett. 2005;7:4149–4152. doi: 10.1021/ol051486x. [DOI] [PubMed] [Google Scholar]
- 23.Leigh K.B. Going Complex for Easy C–H Activation. Vol. 98. C&EN Global Enterprise; New York, NY, USA: 2020. p. 8. [Google Scholar]
- 24.Bhanja R., Bera S.K., Mal P. Regioselective synthesis of phenanthridine-fused quinazolinones using a 9-mesityl-10-methylacridinium perchlorate photocatalyst. Chem. Commun. 2023;59:4455–4458. doi: 10.1039/D3CC00537B. [DOI] [PubMed] [Google Scholar]
- 25.Dias G.G., Paz E.R.S., Kadooca J.Y., Sabino A.A., Cury L.A., Torikai K., de Simone C.A., Fantuzzi F., da Silva Júnior E.N. Rhodium(III)-Catalyzed C–H/N–H Alkyne Annulation of Nonsymmetric 2-Aryl (Benz)imidazole Derivatives: Photophysical and Mechanistic Insights. J. Org. Chem. 2021;86:264–278. doi: 10.1021/acs.joc.0c02054. [DOI] [PubMed] [Google Scholar]
- 26.Gong T.-J., Xiao B., Liu Z.-J., Wan J., Xu J., Luo D.-F., Fu Y., Liu L. Rhodium-Catalyzed Selective C–H Activation/Olefination of Phenol Carbamates. Org. Lett. 2011;13:3235–3237. doi: 10.1021/ol201140q. [DOI] [PubMed] [Google Scholar]
- 27.Wang S., Zhu C., Ning L., Li D., Feng X., Dong S. Regioselective C–H alkylation of anisoles with olefins by cationic imidazolin-2-iminato scandium(iii) alkyl complexes. Chem. Sci. 2023;14:3132–3139. doi: 10.1039/D2SC06725K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart D.R., Alsabeh P., Kuhn M., Fagnou K. Rhodium(III)-Catalyzed Arene and Alkene C−H Bond Functionalization Leading to Indoles and Pyrroles. J. Am. Chem. Soc. 2010;132:18326–18339. doi: 10.1021/ja1082624. [DOI] [PubMed] [Google Scholar]
- 29.Green K.D., Pang A.H., Thamban Chandrika N., Garzan A., Baughn A.D., Tsodikov O.V., Garneau-Tsodikova S. Discovery and Optimization of 6-(1-Substituted pyrrole-2-yl)-s-triazine Containing Compounds as Antibacterial Agents. ACS Infect. Dis. 2022;8:757–767. doi: 10.1021/acsinfecdis.1c00450. [DOI] [PubMed] [Google Scholar]
- 30.Kumari P., Kaur S., Kaur J., Bhatti R., Singh P. Modification of the lead molecule: Tryptophan and piperidine appended triazines reversing inflammation and hyeperalgesia in rats. Bioorg. Med. Chem. 2020;28:115246. doi: 10.1016/j.bmc.2019.115246. [DOI] [PubMed] [Google Scholar]
- 31.Kaur S., Kumari P., Singh G., Bhatti R., Singh P. Design and Synthesis of Aza-/Oxa Heterocycle-Based Conjugates as Novel Anti-Inflammatory Agents Targeting Cyclooxygenase-2. ACS Omega. 2018;3:5825–5845. doi: 10.1021/acsomega.8b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanoli S.T., Ramzan M., Hassan A., Sadiq A., Jan M.S., Khan F.A., Ullah F., Ahmad H., Bibi M., Mahmood T., et al. Design, synthesis and bioevaluation of tricyclic fused ring system as dual binding site acetylcholinesterase inhibitors. Bioorg. Chem. 2019;83:336–347. doi: 10.1016/j.bioorg.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 33.Padilla-Salinas R., Sun L., Anderson R., Yang X., Zhang S., Chen Z.J., Yin H. Discovery of Small-Molecule Cyclic GMP-AMP Synthase Inhibitors. J. Org. Chem. 2020;85:1579–1600. doi: 10.1021/acs.joc.9b02666. [DOI] [PubMed] [Google Scholar]
- 34.Song C., Wang T., Yu T., Cui D.-M., Zhang C. 2,4-Diamino-1,3,5-triazine-enabled Cu-catalyzed direct sulfonamidation of aromatic C–H bonds. Org. Biomol. Chem. 2017;15:7212–7217. doi: 10.1039/C7OB01872J. [DOI] [PubMed] [Google Scholar]
- 35.Zeng M., Xie Z.P., Cui D.-M., Zhang C. Ruthenium-catalyzed synthesis of 1,3,5-triazin-2(1H)-ones and dihydro[1,3,5]triazino[1,2-a]benzimidazoles from alcohols and guanides. New J. Chem. 2018;42:11905–11907. doi: 10.1039/C8NJ02035C. [DOI] [Google Scholar]
- 36.Zeng M., Xie Z.P., Cui D.-M., Zhang C. Ruthenium-catalyzed synthesis of arylethyl 1,3,5-triazines from arylallyl alcohols and biguanides. Org. Biomol. Chem. 2018;16:6140–6145. doi: 10.1039/C8OB01397G. [DOI] [PubMed] [Google Scholar]
- 37.Zeng M., Wang T., Cui D.-M., Zhang C. Ruthenium-catalyzed synthesis of tri-substituted 1,3,5-triazines from alcohols and biguanides. New J. Chem. 2016;40:8225–8228. doi: 10.1039/C6NJ01620K. [DOI] [Google Scholar]
- 38.Zeng M., Yuan Z.-X., Wen L.-F., Jiang D., Lu H., Liu W., Dai J., Zeng S.-x. The copper-catalyzed oxidation of arylmethyl triazines with H2O toward the oxidant-free synthesis of aroyl triazines. Org. Biomol. Chem. 2022;20:5406–5411. doi: 10.1039/D2OB00582D. [DOI] [PubMed] [Google Scholar]
- 39.Zeng M., Liu Y.-X., Zheng J.-H., Zhao L., Zhu Q.-h., Jiang D., Ling Y., Liu W., Zeng S.-x. Direct α-methylenation of triazines to terminal olefins with DMA. New J. Chem. 2022;46:20065–20068. doi: 10.1039/D2NJ04417J. [DOI] [Google Scholar]
- 40.Ackermann L., Lygin A.V., Hofmann N. Ruthenium-Catalyzed Oxidative Annulation by Cleavage of C–H/N–H Bonds. Angew. Chem. Int. Ed. 2011;50:6379–6382. doi: 10.1002/anie.201101943. [DOI] [PubMed] [Google Scholar]
- 41.Ackermann L., Lygin A.V. Cationic Ruthenium(II) Catalysts for Oxidative C–H/N–H Bond Functionalizations of Anilines with Removable Directing Group: Synthesis of Indoles in Water. Org. Lett. 2012;14:764–767. doi: 10.1021/ol203309y. [DOI] [PubMed] [Google Scholar]
- 42.Xu F., Li Y.-J., Huang C., Xu H.-C. Ruthenium-Catalyzed Electrochemical Dehydrogenative Alkyne Annulation. ACS Catalysis. 2018;8:3820–3824. doi: 10.1021/acscatal.8b00373. [DOI] [Google Scholar]
- 43.Allu S. Ruthenium-Catalyzed Oxidative Annulation of 6-Anilinopurines with Alkynes via C-H Activation: Synthesis of Indole-Substituted Purines/Purine Nucleosides. Adv. Syn. Catal. 2015;357:2665–2680. doi: 10.1002/adsc.201500314. [DOI] [Google Scholar]
- 44.Song W., Ackermann L. Nickel-catalyzed Alkyne Annulation by Anilines: Versatile Indole Synthesis by C–H/N–H Functionalization. Chem. Commun. 2013;49:6638–6640. doi: 10.1039/c3cc43915a. [DOI] [PubMed] [Google Scholar]
- 45.Su C.W., Zeng M., Zhang C., Cui D.-M. Ruthenium Catalyzed Divergent Alkylation and Olefination of Methyl 1,3,5-Triazines with Alcohols. Eur. J. Org. Chem. 2020;2020:4942–4949. doi: 10.1002/ejoc.202000781. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.