Abstract
Neisseria gonorrhoeae is a strict human pathogen that is, primarily, transmitted by close sexual contact with an infected individual. Gonococcal infection of the male urogenital tract has been well studied in experimental human models and in urethral cell culture systems. Recent studies, using tissue culture cell systems, have suggested a role for the cervical epithelium in gonococcal infection of females; however, the nature of gonococcal infection of the normal uterine cervix remains controversial. To address this enigma, we have developed two primary human cervical epithelial cell systems from surgical biopsies. Gonococcal infection studies and electron microscopy show that N. gonorrhoeae is capable of infecting and invading both the endo- and the ectocervix. Invasion was found to occur primarily in an actin-dependent manner, but it does not appear to require de novo protein synthesis by either the bacterium or the host cervical cell. Membrane ruffles appear to be induced in response to gonococci. Consistent with membrane ruffling, gonococci were found residing within macropinosomes, and a concentrated accumulation of actin-associated proteins was observed to occur in response to gonococcal infection. Electron microscopy of clinically derived cervical biopsies show that lamellipodia formation and cytoskeletal changes, suggestive of membrane ruffles, also occur in the cervical epithelium of women with naturally acquired gonococcal cervicitis. These studies demonstrate the ability of N. gonorrhoeae to infect and invade both the endo- and the ectocervix of the normal uterine cervix. Gonococcal induced ruffling is a novel finding and may be unique to the cervical epithelium.
Neisseria gonorrhoeae remains one of the leading causes of sexually transmitted disease. Gonorrhea persists in the general population, in part, because of the presence of asymptomatic carriers who unknowingly transmit gonococci to another individual via close sexual contact. The lower-genital-tract, asymptomatic, carriage rate of N. gonorrhoeae in females has been estimated to be between 25 and 80% of infected individuals (24). Asymptomatic infection of the lower female genital tract can, eventually, become acute. This can result in an ascending infection of the uterus and fallopian tubes, thereby causing an acute pelvic inflammatory disease that, if left untreated, can lead to infertility or ectopic pregnancies (21, 33).
Our laboratory previously reported the use of primary urethral tissue culture systems to study gonococcal infection as it pertains to the male urethra (23). Christodoulides et al. (3) recently described the use of primary human endometrial cells to study gonococcus-host interactions as they pertain to the female endometrium. Primary cell culture systems confer several advantages over the use of immortalized and malignant tissue culture cell lines in that gonococcal-host interactions can be simulated in vitro in an environment that closely mimics that environment encountered by the bacterium in vivo. Cellular immortalization and continued laboratory passage of cell lines can alter their protein expression patterns (8, 16, 25, 27, 29, 30, 31, 35, 47, 49, 51, 52, 54). Therefore, it is not clear if cell lines are truly representative of the in vivo cellular environment encountered by the gonococcus within the female genital tract.
During the 1940s, Harkness provided the framework for our current understanding of gonococcal pathogenesis as it pertains to the female genital tract. Harkness concluded, through clinical observations and light microscopic studies of tissue derived from patients naturally infected with gonococci, that N. gonorrhoeae was incapable of invading the stratified squamous epithelium of the ectocervix (22). He also concluded that progressive infection occurred as the result of colonization of the endocervical columnar epithelium with subsequent transmigration through the intercellular junctions to the subepithelial tissues or lymphatic vessels (22). Evans (11) performed electron microscopic examination of biopsies derived from the cervical squamocolumnar junction of patients infected with N. gonorrhoeae. These studies implied that N. gonorrhoeae could infect the stratified squamous epithelium. Although these studies suggested a role for the cervical epithelium in female gonococcal infection, the gonococcal pathogenesis of the cervical epithelium remains unclear. In order to elucidate the mechanism(s) associated with gonococcal infection of the cervical epithelium, we have developed two primary cell culture systems derived from ectocervical and endocervical tissues. These studies demonstrate the ability of N. gonorrhoeae to invade both the endo- and the ectocervical epithelium by several mechanisms. Our studies indicate that the cervical epithelium may serve as a primary site of gonococcal infection in females.
MATERIALS AND METHODS
Bacteria.
N. gonorrhoeae strains 1291, 1291-green (1291 expressing green fluorescent protein and to be described elsewhere; the plasmid pLES98 was a gift from V. Clark), FA1090, MS11-A (a gift from M. So), and MS11mkC were used in these infection studies. N. gonorrhoeae strains 1291, FA1090, MS11-A, and MS11mkC are clinically isolated gonococci. N. gonorrhoeae FA1090 is a serum-resistant, genital isolate from a patient with disseminated gonococcal infection, while N. gonorrhoeae strain 1291 is a urethral isolate from a male patient with gonococcal urethritis. MS11mkC is a hyperinfectious gonococcal variant that was isolated from a male patient who was experimentally infected with MS11mkA. These strains are P+ and Opa+. Strains 1291, 1291-green, MS11-A, and MS11mkC contain the pathogenicity island recently described by Dillard (7).
Development of primary cervical cell culture systems.
Surgical biopsies were obtained from 30 premenopausal women undergoing hysterectomy at the University of Iowa Hospitals and Clinics (Iowa City, Iowa). Endocervical (proximal to the cervical os) and ectocervical (distal to the cervical os) tissue biopsies were obtained in 4- to 6-mm2 sections and further subdivided into 2- to 3-mm2 sections. Sectioned tissues were rinsed twice for 10 min in Hanks balanced salt solution (HBSS) supplemented with 1% amphotericin B (Fungizone; Irvine Scientific, Santa Ana, Calif.) and 1% penicillin (100 U/ml)-streptomycin (1 mg/ml). The tissue was placed with the epithelium downward on polystyrene, 35-mm tissue culture dishes (Falcon; Becton Dickinson, Franklin Lakes, N.J.). Tissue explants were incubated in filtered airway medium (1 part Dulbecco modified Eagle medium, 1 part Ham's F-12, 5% fetal calf serum (FCS), 1% nonessential amino acids (Sigma-Aldrich, St. Louis, Mo.), 1% penicillin-streptomycin, and insulin (10 μg/ml). After 48 h, airway medium was replaced with keratinocyte growth medium 2 (KGM-2) Bullet Kit (Clonetics, San Diego, Calif.). KGM-2 was replaced every 2 to 3 days until near confluence was obtained (1 to 2 weeks), at which time the cells were passaged as outlined below. Although variability exists among tissue samples, this process allows for an average of three passages of cell growth to fresh tissue culture dishes from a single tissue explant prior to fibroblast development, at which time tissue explants were discarded.
Cell passage.
At near-confluent growth the cells were passaged by a 5-min, 37°C incubation in HBSS–0.25% trypsin–0.1% EDTA. Cell suspensions were collected and centrifuged at 5,000 rpm for 5 min. The resulting cell pellet was rinsed in HBSS, resuspended in KGM-2, and used to seed glass, eight-well chamber slides (Nalge Nunc International, Naperville, Ill.) or human, placental collagen-coated, 12-mm glass coverslips previously placed in 24-well tissue culture dishes (Falcon). Cells were seeded to transwell membrane systems (Biocoat Cell Environments; Becton Dickinson, Bedford, Mass.) to allow for polarized cell growth. Primary cervical cells were maintained in KGM-2 until near confluence was again obtained, at which time they were infected with N. gonorrhoeae as outlined below. Where applicable, cellular polarity was determined as an electrical resistance greater than 2 KΩ/cm2 as measured across the cell monolayer. Infected and uninfected (i.e., control) cervical cell-harboring membranes (from transwell systems) were subsequently subdivided into equal sections. Sections to be used for scanning electron microscopy (SEM) were processed while attached to the well apparatus so that the cellular orientation would be maintained. Remaining sections were removed from the well structure and subsequently processed independently for either confocal, transmission electron, or bright-field light microscopy.
Infection of the primary cells.
N. gonorrhoeae cells allowed to grow overnight (37°C, 5% CO2) on GC-IsoVitaleX agar plates were harvested using a sterile swab and resuspended in sterile saline. Culture density was determined spectrophotometrically, where an optical density of 1 at 600 nm was equivalent to 109 bacteria ml−1 of cell culture. Bacterial cells were then diluted to a concentration of 107 bacteria ml−1 in KGM-2 lacking gentamicin and used to infect 105 primary cervical cells (maintained as outlined above). Gonococcal infection was allowed to progress for variable time periods, after which the infection was stopped by the removal of the infection medium, rinsing infected cervical cells with phosphate-buffered saline (PBS), and cell fixation. Samples to be used in laser scanning confocal microscopy (LSCM) or differential-interference contrast (DIC) analyses were immunolabeled directly following fixation. SEM, transmission electron microscopy (TEM), and bright-field light microscopy (BFLM) samples were further processed by graded ethanol dehydration and resin (TEM) or paraffin (BFLM) embedment. Embedded samples were sectioned and immunolabeled as noted. Where indicated, the infection medium was harvested from the cervical cell monolayer and reused to infect fresh, uninfected cell cultures, which were subsequently processed for SEM analysis.
Invasion assays in the presence of inhibitors of cytoskeletal motility and protein synthesis.
Cervical cells were passed to 12-mm collagen-coated coverslips as outlined above. Prior to infection with N. gonorrhoeae 1291 wild-type cells, primary cell cultures were left untreated, or they were preincubated with 300 nM wortmannin (Sigma), 1 μM cytochalasin D (Sigma), or 400 mM EGTA (Amresco, Solon, Ohio) for 2 h, 30 min, or 30 min, respectively, or else they were pretreated with 100 μg of nocodazole (Calbiochem-Novabiochem Corp., La Jolla, Calif.) per ml for 1 h at 4°C, followed by a 30-min incubation at 37°C. The requirement for de novo protein synthesis, either by the bacteria or by the primary cervical cells, was tested by pretreatment (30 min, 37°C) of the bacterial cultures or cervical cell monolayers with 4 μg of chloramphenicol (Sigma) per ml or 25 μM cycloheximide (Calbiochem-Novabiochem Corp.), respectively. All chemical reagents used were maintained in the infection medium throughout the course of the infection. Trypan blue exclusion revealed no significant toxicity to the primary cervical cells at the indicated concentrations for each of the chemical reagents used. Infection was allowed to progress at 37°C and 5% CO2 for 1.5 h, after which the medium was removed, and the cells were rinsed with PBS and then incubated with KGM-2 containing 100 μg of gentamicin per ml to kill extracellular bacteria. Postincubation the cervical cells were lysed with 0.5% saponin to release invasive bacteria. The percent invasion was determined as a function of the original inoculum and the number of colonies formed with subsequent plating of the cellular lysate. Kruskal-Wallis analysis of variance was used to determine the statistical significance of the calculated percent invasion for each of the cytoskeletal motility inhibitors used with respect to the untreated, infected cell cultures.
Microscopy.
Samples were processed for LSCM, SEM, or TEM as previously described (28). Samples to be analyzed by BFLM were paraffin embedded using an automated tissue processor (RMC 1530 Paraffin Tissue Processor; RMC, Tucson, Ariz.), cut into thick (1-μm) sections, and mounted onto glass microscope slides. Immunolabeling of infected and uninfected cervical cells for TEM analysis was performed using the monoclonal antibody 2C3, which specifically recognizes the H.8 gonococcal surface protein, or the antigonococcal porin monoclonal antibody, 3H1 (a gift from M. Blake), in conjunction with a polyclonal antibody to filamentous (F) actin. Secondary labeling proceeded with the use of 30- and 10-nm colloidal gold-beaded antibody conjugates (Amersham Pharmacia Biotech, Piscataway, N.J.) to the bacterium- and actin-specific antibodies, respectively. B. A. Evans generously provided clinical biopsies used in TEM analysis. The samples were viewed with an H-7000 Hitachi Transmission Electron Microscope (Hitachi Corp., Mountain View, Calif.).
Primary antibodies used for LSCM or DIC microscopy were as follows: anti-cytokeratin 8.12 (Sigma), -cytokeratin 4 (Sigma), -talin (Sigma), -vinculin (Sigma), -α-actinin (Sigma), -myosin (Sigma), -ezrin (Santa Cruz Biotechnology, Santa Cruz, Calif.), -CD66 (Dako, Carpinteria, Calif.), and -CD46 (Santa Cruz Biotechnology) and 2C3. Immunolabeling of cervical cell monolayers with anti-cytokeratin, -talin, -vinculin, -myosin, -ezrin, and -α-actinin occurred subsequent to a 15-min incubation in 0.2% Triton X-100 to allow cervical cells to become permeable. Where indicated, counterstaining occurred at room temperature for 6 min. The counterstains used were specific for nucleic acids and consisted of YOYO-1 (Molecular Probes, Eugene, Oreg.) or ethidium bromide. Samples were viewed using the Bio-Rad MRC-1024 or the Zeiss 510 Laser Scanning Confocal viewing systems.
Cervical tissue biopsies (obtained as outlined above) to be used for LSCM cytokeratin analysis were processed (within 1 to 2 h of obtaining the tissue specimen) for cyrosectioning by a 30-min incubation in 1% paraformaldehyde, followed by infiltration with 30% sucrose prior to embedment in Tissue-Tek O.C.T. compound (Sakura Finetek USA, Inc., Torrance, Calif.) and sectioning (6 to 8 nm). Frozen sections were allowed to stand at room temperature for 1 h prior to immunolabeling with the indicated anti-cytokeratin antibody. A fluorescein isothiocyanate (FITC)-conjugated secondary antibody was applied, and tissues were subsequently counterstained with ethidium bromide (0.5 ng/ml, 6 min).
Cervical cells passaged to 12-mm coverslips were used to assay for gonococcus-induced macropinocytosis. Cervical cell monolayers were infected with 1291-green for variable time periods in the presence of 1 mg of tetramethyl rhodamine isothiocyanate (TRITC)-dextran (Mr, 150,000) per ml. Infection was stopped by the removal of the infection medium. Infected monolayers were extensively washed prior to fixation with 2% paraformaldehyde. Coverslips were mounted onto glass microscope slides and viewed using the Bio-Rad MRC-1024 Laser Scanning Confocal viewing system.
Slides prepared for BFLM were hematoxylin-eosin stained using a standard protocol and then viewed with a Leitz Diaplan microscope with an Optronics Engineering viewing system. SEM analysis was performed using an H-4000 Hitachi Scanning Electron Microscope (Hitachi).
All of the microscopes used for these studies are located at the University of Iowa Central Microscopy Research Facility (Iowa City, Iowa).
RESULTS
Characterization of primary human endocervical epithelial cells.
Primary cervical epithelial cells were allowed to grow as described above. Epithelial cells could be seen extending from the cervical explants within 2 to 3 days from the start of the cultures. Growth radiated from the tissue foci in a contiguous monolayer, and confluence was observed within 10 to 14 days. Transfer of endocervix-derived cells to transwell membrane systems resulted in polarized cell growth, as determined by an electrical resistance greater than 2 KΩ/cm2 as measured across the cell monolayer.
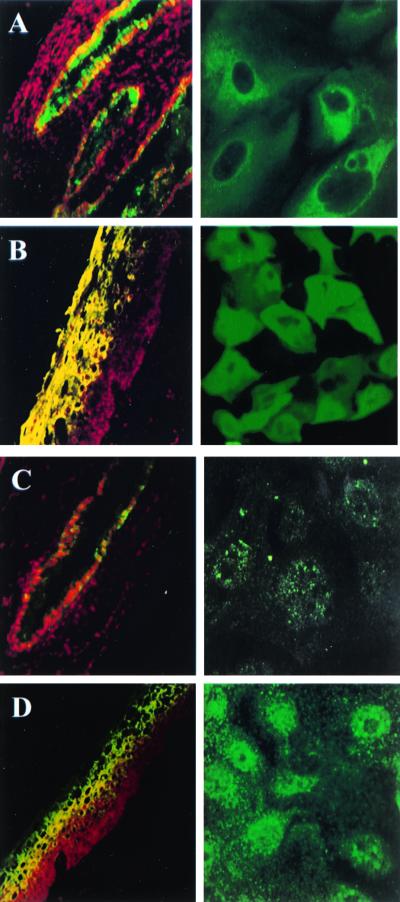
The cytokeratin expression pattern of the normal human uterine cervix has been well characterized. LSCM was used to determine the cytokeratin expression pattern of the primary cervical cell monolayers with respect to the tissue from which they were derived. Sectioned tissue biopsies (obtained from the endo- and ectocervix) and the cervix-derived cell monolayers were immunohistochemically examined with antibodies to cytokeratins 4, 13, 15, and 16. The results of these studies can be seen in Fig. 1. Immunolabeling of ectocervical tissue sections revealed that expression of cytokeratin 4 was modest at the intermediate epithelial layers and intense at the superficial ectocervical epithelium. Labeling with anti-cytokeratin 8.12, which specifically recognizes cytokeratins 13, 15, and 16, occurred throughout the thickness of the ectocervical tissue with the exception of the suprabasal layer in which label was not detected. Similar results were obtained for the ectocervix-derived cell monolayers, which labeled positively for cytokeratins 4, 13, 15, and 16. In contrast, a small subpopulation of endocervical cells were visible when the sectioned tissue biopsy was immunolabeled with the anti-cytokeratin 4 antibody, but vivid labeling occurred with the use of anti-cytokeratin 8.12. Immunolabeling of the endocervix-derived cell monolayers yielded consistent results in that the endocervix-derived cells labeled poorly with the anti-cytokeratin 4 antibody and intensely with anti-cytokeratin 8.12. The specific cytokeratin staining character of the endo- and ectocervical tissue was, therefore, retained in the primary cell monolayers (Fig. 1).
FIG. 1.
LSCM demonstrates that the characteristic cytokeratin-staining pattern of the tissue biopsies has been retained in the respective primary cervical epithelial cell cultures. Sectioned tissue biopsies (left column, ×20 magnification) and primary cervical epithelial cell monolayers (right column, oil immersion, ×60 magnification) were incubated with an FITC-conjugated antibody to the noted specific cytokeratin. Ethidium bromide was used to counterstain the tissue sections. (A) As can be seen, endocervical cells were labeled intensely with antibody 8.12, which is specific for type I cytokeratins 13, 15, and 16. (C) The labeling of endocervical cells with an antibody specific for type II cytokeratin 4 was considerably less intense, and it was not uniformly distributed. Ectocervical cells were labeled positive with an antibody specific for cytokeratins 13, 15, and 16 (B) and cytokeratin 4 (D).
LSCM analysis of sectioned tissue biopsies and cervix-derived cell monolayers demonstrated the expression of CD66 and CD46 in both the endo- and the ectocervix (data not shown).
N. gonorrhoeae infection of primary cervical epithelial cells.
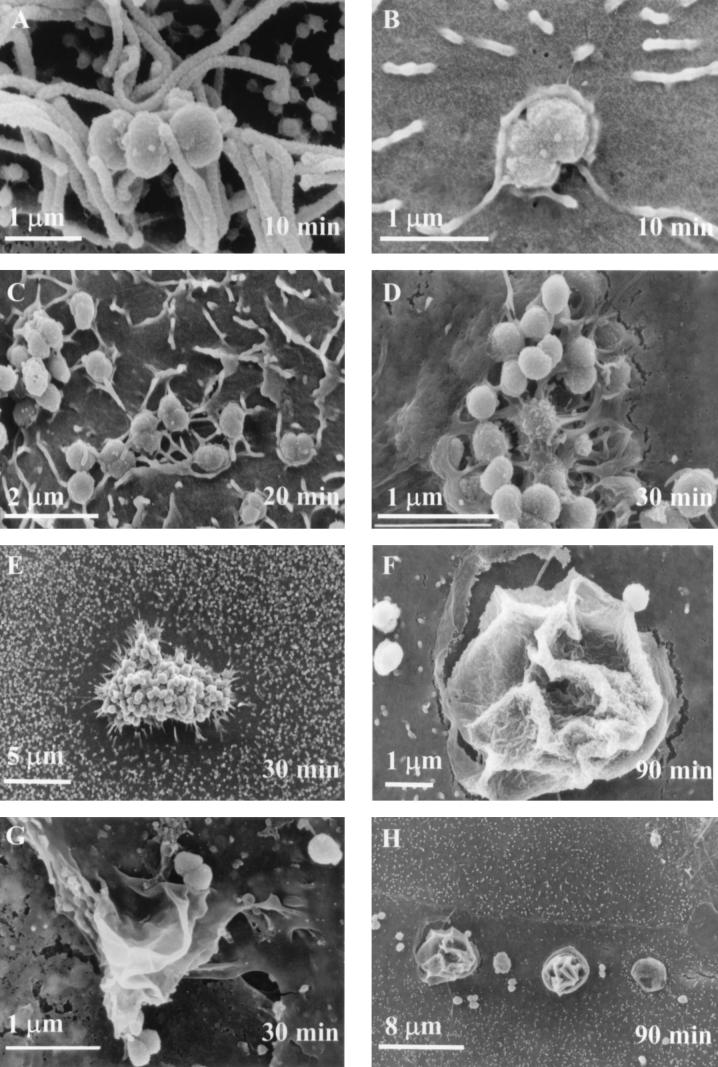
SEM analysis of N. gonorrhoeae 1291-, MS11-A-, MS11mkC-, and FA1090-infected polarized and nonpolarized cells showed that bacteria could adhere to both types of primary cervical cells. Electron micrographs representative of data obtained are shown in Fig. 2 and 3. Bacteria were found distributed across the monolayer surface. The interaction of the bacteria with the cervical cell surface appeared to occur by multiple mechanisms. At approximately 10 min postinfection, gonococci could be found associated with the cervical cell membrane both dependent (Fig. 2A) and independent (Fig. 2B) of microvilli. Small tufts of microvilli were associated with bacteria on some cervical cells. Gonococci associated with the cervical cells independent of microvilli appeared to be entering the cervical cell by an endocytic process. At approximately 20 and 30 min postinfection, filopodia and lamellipodia formation was readily observed (Fig. 2C) and bacteria appeared to be undergoing internalization (Fig. 2D). Additionally, a visible smoothing of the cervical cell membrane was evident around the periphery of some sites of bacterial infection (Fig. 2E). By 60 min postinfection, the filopodia and lamellipodia became less prominent. Large membrane ruffles (Fig. 2F and G) became prominent at about 90 min postinfection of cervical cells. Membrane ruffles were abutting and contiguous with gonococci. Generally, ruffles could be readily identified by a smoothing of the cervical cell membrane that encircled the ruffle (Fig. 2H). At 3 h post-gonococcal infection, membrane ruffles and bacteria associated with microvilli were still evident. Perturbations of the cell membrane that were reminiscent of ruffles were also evident. The delay observed in the onset of ruffling suggested that time was required either by the gonococcus or by the cervical cell to produce and/or release a factor(s) that facilitated the ruffling process. To test this idea, we performed infection studies in which media and bacteria from endo- and ectocervical infections were collected at various time points and reused to infect uninfected cervical cell monolayers. We found that ruffling could be induced to occur at approximately 30 min post-gonococcal infection in both primary cell systems when uninfected cervical cells were infected with a primed infection inoculum derived from 1-h (ectocervical) (Fig. 2G) and 90-min (endocervical) infections.
FIG. 2.
SEM analysis shows the predominant changes that occur in the cervical cell membrane over the course of a 3-h infection as the result of cervical-cell–gonococcus interactions. At early phases of infection (0 to 60 min) N. gonorrhoeae could be found on the surface of endocervical cells either associated with microvilli (A) or undergoing an endocytic process (B). Bacteria appear to be entering the cell in a phagosome; however, rudimentary lamellipodia or microvilli could also account for the apparent membrane perturbations. As the infection process continued, microvilli appeared to acquire directionality. (C) Filopodia and/or lamellipodia became evident after 30 min postinfection. (D) Bacteria appeared to be in the process of internalization. (E) Loss of microvilli with a smoothing of the cervical cell membrane around the periphery of some sites of gonococcal infection also became evident at approximately 30 min postinfection. Membrane ruffles (F, endocervical; G, ectocervical) appeared at 60 min, and they became prevalent at 90 min postinfection. (G) Ruffles could be induced to occur at approximately 30 min postinfection with use of a primed infection inoculum (see Materials and Methods). (H) A visible smoothing of the cervical cell membrane encircling membrane ruffles can be seen. By 3 h postinfection large ruffles could readily be observed.
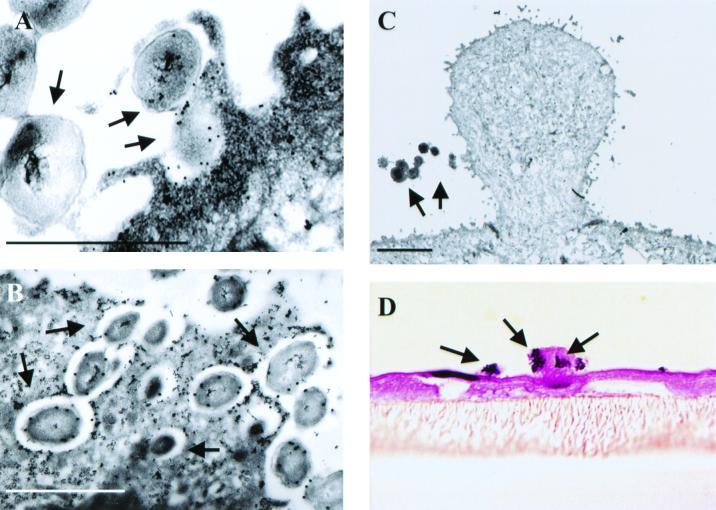
FIG. 3.
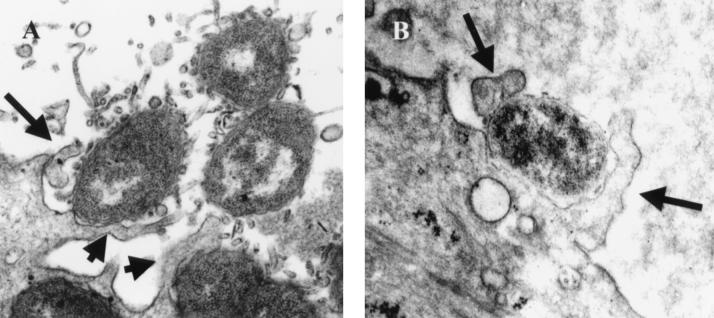
BFLM and immuno-TEM studies demonstrate ruffling of the endocervical surface and invasion of the primary endocervical epithelial cells at 90 min and 3 h postinfection. For TEM analysis, bacteria were labeled with an antibody specific to the gonococcal surface protein, H.8; cervical cells were labeled with a polyclonal antibody to actin. Then, 30- and 10-nm gold bead-antibody conjugates were used to label the bacterium- and host-specific primary antibodies, respectively. (A) Membrane protrusions can be seen that are labeled with actin and that are encompassing gonococci at 90 min after the onset of infection. (B) Bacteria can also be seen entering the endocervical cell as individual entities in actin-lined, spacious vacuoles. (C and D) Large membrane ruffles can be seen associating with gonococci at 3 h postinfection. For BFLM (panel D), thick (1-μm) paraffin sections of endocervical cells were stained with hematoxylin and eosin. Bacteria appear purple; the cervical cell membrane appears pink. Arrows denote bacteria. Bar, 2 μm.
BFLM and TEM analysis of polarized endocervical cells infected with N. gonorrhoeae confirmed the observation made by SEM analysis (Fig. 3). Actin-filled membrane protrusions were readily observed encompassing gonococci (Fig. 3A) at 90 min and 3 h postinfection. Clusters of bacteria were found breaching the superficial cervical epithelial layer; however, bacteria entered the cervical cells as single entities, with each bacterium being surrounded by its own actin-lined vacuole (Fig. 3B). Consistent with SEM analysis, gonococcus-associated membrane ruffles were readily observed at 3 h postinfection by both high (TEM, Fig. 3C)- and low (BFLM, Fig. 3D)-powered magnification with microscopy. TEM analysis revealed that, within the host cell cytoplasm, bacterium-containing vacuoles appeared to coalesce prior to bacterial exocytosis to the subepithelial space. TEM analysis of Epon-embedded, clinically derived cervical biopsies from women naturally infected with gonococci revealed similar processes (Fig. 4). Large membrane protrusions (indicative of ruffles) (Fig. 4) and smaller, less-organized membrane structures (Fig. 4A) were readily observed. Gonococci were again observed to enter the cervical cells as single entities in spacious vacuoles (data not shown).
FIG. 4.
TEM studies of cervical biopsies from women with gonococcal cervicitis. The panels show evidence that extensive cytoskeletal rearrangements and membrane changes, which are suggestive of ruffling, occur during naturally acquired gonococcal infection. The large arrows highlight structures resembling lamellipodia. Extensive cytoskeletal rearrangement is also visible in proximity to the bacteria in panel A and may be suggestive of filopodia or microvilli extensions that are similar to those seen in Fig. 3.
Primary cell monolayers infected with gonococci in the presence of a TRITC-conjugated dextran, which would be excluded by nonmacropinocytic cellular events, demonstrated that, upon invasion, gonococci reside within macropinosomes (data not shown).
LSCM analysis of infection studies performed using polarized endocervical cells and ectocervical cell monolayers suggested colocalization of CD66 and CD46 with gonococci. With extended infection (i.e., 6 h) clustering of CD46 molecules, which was not observed to occur at earlier time points in the infection, became prevalent in response to gonococci (data not shown).
Cytoskeletal changes occur in cervical cells with gonococcal infection.
Immunolabeling of N. gonorrhoeae-infected primary cells with antibody conjugates to actin-associated proteins confirmed that changes of the cervical cell cytoskeletal network were occurring (Fig. 5). Antibodies to talin, vinculin, ezrin, myosin, and α-actinin demonstrated a focused accumulation of these proteins, in membrane projections, at 10 min postinfection with gonococci. Membrane projections were also observed to colocalize with gonococci. This effect was most pronounced with the use of vinculin (Fig. 5) and ezrin; however, a modest accumulation of talin and α-actinin was also observed to occur. Immunolabeled projections were not observed upon analysis of uninfected cervical cells.
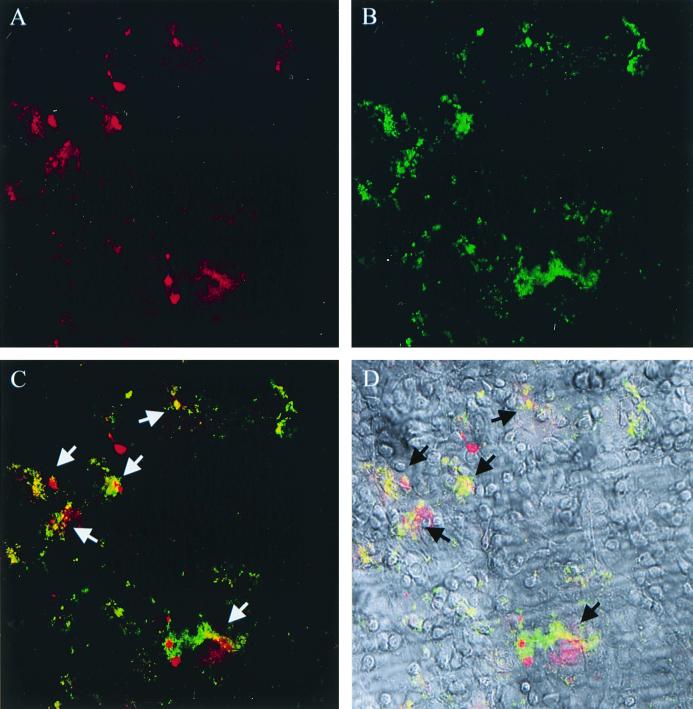
FIG. 5.
DIC and LSCM analysis demonstrates colocalization of N. gonorrhoeae 1291-green with a concentrated accumulation of the actin-associated protein, vinculin. In panel A, vinculin has been immunolabeled with a TRITC-conjugated antibody and is visible as a red fluorescence (A); in panel B, bacteria have been transformed with GFP and are visible as a green fluorescence. (C) In a merged image of panels A and B, arrows denote colocalization of bacteria (green) with vinculin (red), which is visualized as a yellow-orange because of the combined signal of the individual fluorophores. (D) Merged LSCM and DIC image (of the ectocervical cells). Similar results were seen with endocervical cells and for the actin-associated proteins ezrin and myosin, but the focal accumulation of α-actinin and talin was less pronounced. No accumulation of actin-associated proteins was observed in uninfected (control) cervical epithelial cells. Magnification, ×20.
Gonococcal invasion of cervical cells occurs primarily in an actin-dependent manner and does not require de novo protein synthesis.
Standard gentamicin resistance assays performed with endo- and ectocervix-derived cells confirmed results obtained by BFLM and TEM analysis and the invasive nature of gonococci with respect to both the endo- and the ectocervix. Gonococci were found to invade endocervix-derived cells at a proportion of 1.57% (Table 1). A slightly higher percentage (2.70%) was observed to occur with gonococcal invasion of the ectocervix-derived cells (Table 1). The inclusion of wortmannin, cytochalasin D, and EGTA in the invasion assay prohibited bacterial entry into both cell types (Table 1). Pretreatment of primary cervical cell monolayers with the microtubule-specific depolymerizing agent nocodazole resulted in an approximate 67% decrease in gonococcal invasion (Table 1). Chloramphenicol and cycloheximide, which inhibit gonococcal and eukaryotic cell protein synthesis, respectively, did not inhibit gonococcal invasion of the primary cervical cell monolayers (Table 1).
TABLE 1.
Percent invasion of N. gonorrhoeae 1291 in primary cervical cells
| Cell treatment | Endocervical cells
|
Ectocervical cells
|
||||
|---|---|---|---|---|---|---|
| Mean % invasiona | Variance of the mean | Pb | Mean % invasiona | Variance of the mean | Pb | |
| None | 1.5517 | 0.3030 | NAc | 2.6953 | 1.3569 | NA |
| Cytochalasin D | 0.0358 | 0.0186 | 0.05 | 0.0233 | 0.0046 | 0.025 |
| Wortmannin | 0.0177 | 0.0180 | 0.05 | 0.0260 | 0.0158 | 0.025 |
| EGTA | 0.0431 | 0.0087 | 0.05 | 0.0303 | 0.0052 | 0.025 |
| Nocodazole | NDd | ND | ND | 0.9000 | 0.7906 | 0.25 |
| Cycloheximide | ND | ND | ND | 2.5601 | 1.8816 | 0.75 |
| Chloramphenicol | ND | ND | ND | 2.6688 | 1.9590 | 0.50 |
The mean is the average percentage of at least three trials in which the percent invasion was determined as a function of the original inoculum and the subsequent CFU.
P values given were determined using a Kruskal-Wallis κ-sample test of the percent gonococcal invasion determined for each cellular treatment applied to primary cervical cells in comparison to the percent gonococcal invasion of untreated, primary cervical cells.
NA, not applicable.
ND, not determined.
DISCUSSION
We have described primary human ecto- and endocervical epithelial cell models whose cytokeratin, CD66, and CD46 profiles are identical to the tissue from which they were derived. Confocal and electron microscopic analysis of primary, human, cervical cells infected with N. gonorrhoeae 1291, FA1090, and MS11 have demonstrated the ability of gonococci to adhere to and to induce cytoskeletal changes within both of these cell systems. Bacteria were found to associate with the primary cervical cells by more than one mechanism, as evidenced by microvillus-dependent and -independent modes of bacterial attachment. Membrane perturbations resulted in the formation of membrane ruffles, which became prominent by 90 min postinfection and after which ruffles remained readily observable. Ruffling could be induced to occur at 30 min post-gonococcal infection in both primary cell systems when uninfected cervical cells were infected with a primed infection inoculum; however, de novo protein synthesis was not required to prime the infection process for invasion. Actin-associated proteins were also observed to accumulate in response to gonococcal infection. Gonococci were found to be internalized within the cervical cells in actin-lined spacious vacuoles.
The ability of gonococci to attach to the endocervical epithelium is well accepted. In contrast, attachment to the stratified squamous epithelium of the ectocervix and to transitional cells of the cervical squamocolumnar junction (10, 11) remains controversial. Studies conducted in vitro with our primary cell culture systems demonstrated gonococcal adherence to both the endo- and the ectocervix. Considerable anatomical variation exists in the length of the squamocolumnar transition zone of the cervix (14). Additionally, to a variable measure, columnar epithelium may overlap the stratified squamous epithelium (of the ectocervix) at the transition zone. This may, in part, account for the controversy associated with gonococcal attachment to the cervical epithelium. Cervical biopsies used in the studies described herein were obtained from sites distinct from the transformation zone, i.e., >0.5 cm from the squamocolumnar junction. Of the 30 cervical specimens used to generate primary cell cultures for use in these studies, all have supported gonococcal adherence with minimal variability. Gonococcal adherence has, to date, primarily been associated with microvilli formation; however, we found gonococci associated with the cervical epithelium both dependent and independent of microvilli.
Attachment is not synonymous with tissue damage or with the initiation of a diseased state; it is a discrete event from phagocytic internalization, i.e., invasion. Four general mechanisms of bacterial invasion of host cells have been proposed to occur: receptor-mediated endocytosis (44), microtubule-dependent endocytosis (37, 41, 48), zippering (19, 20), and triggering (9, 12, 36, 42, 58). Several eukaryotic cell surface molecules have been proposed to serve as receptors for gonococcal invasion (reviewed in references 6, 9, 26, 32, 34, 38, 39, and 40). In fallopian tube organ culture gonococcal invasion has been proposed to occur in a manner reminiscent of “zipper-type” phagocytosis (9, 32, 53).
The observation that gonococci appear to induce membrane ruffling is a novel finding. Ruffling is the result of a complex interaction that occurs between a bacterium and a host cell and is associated with a triggering mechanism (48) that leads to macropinocytosis (1, 15, 17, 56). Infection of our primary cell culture systems resulted in ruffling of both the endo- and the ectocervix-derived cells. Ruffling was evident in the endocervical cells as convoluted spheres, whereas ruffling of the ectocervical cells was observed to occur as long, ribbon-like folds. The characteristic structural morphology of endo- and ectocervix-associated ruffles appeared to be specific for each of their respective cell types; hence, we have described the ruffles found on the ectocervical cells as ribbons.
Salmonella and Shigella spp. have been shown to induce membrane ruffling in a contact-dependent manner in which a (highly conserved) type III secretion system (TTSS) allows for the secretion of numerous effector proteins that initiate the cellular response required for the observed cytoskeletal rearrangements (13, 45, 57). A TTSS has not been described for N. gonorrhoeae. A search of the N. gonorrhoeae strain FA1090 genome database (University of Oklahoma Advanced Center for Genome Technology) for the possible existence of Salmonella and Shigella TTSS and effector protein homologs yielded no significant matches to ruffling-associated proteins. Dillard (7) recently described the existence of a pathogenicity island in N. gonorrhoeae strain MS11, which encodes a secretion system. This pathogenicity island is also present in N. gonorrhoeae 1291, but it is absent in N. gonorrhoeae FA1090. This pathogenicity island (and its encoded secretion system) may therefore share homology with Salmonella and Shigella TTSS and effector proteins; however, these data are currently unavailable.
Ruffling and subsequent invasion by Salmonella and Shigella spp. show an actin dependence but occur independent of microtubules. It has previously been demonstrated that gonococcal invasion of tissue culture cell lines is dependent upon microtubules and a functional actin cytoskeleton (2, 18, 43). Using standard gentamicin resistance assays, we examined endo- and ectocervical cells to determine if these primary cells displayed a microtubule or actin dependence for gonococcal invasion. Cytochalasin D, wortmannin, and EGTA brought invasion levels down to (essentially) zero in both cell systems, suggesting that gonococcal entry is dependent upon actin rearrangements. TEM analysis of N. gonorrhoeae-infected polarized cervical cells supported a role for actin in the gonococcal invasion process in that actin-filled ruffles and large, spacious, actin-lined vacuoles encompassed invading gonococci. The latter finding is in contrast to the study by Grassmé et al. (18), who demonstrated that gonococcal association with actin was transient. In multiple experiments using cervical cell monolayers derived from different patients, invasion was not found to be significantly inhibited when primary cervical cells were pretreated with nocodazole to disrupt microtubules.
A concentrated accumulation of actin-associated proteins has been demonstrated to occur in response to membrane ruffling (5, 13, 50). To our knowledge, the role of actin-associated proteins in gonococcal infection has not been examined. We found that in response to gonococcal invasion a concentrated accumulation of predominantly ezrin and vinculin occurs in a manner analogous to Shigella infections. A modest accumulation of talin and α-actinin also was observed during gonococcal infection of cervical cells. Additionally, although myosin was observed to accumulate in response to, and colocalize with, gonococci at 5 and 10 min postinfection, myosin was also observed to be fairly diffuse throughout some of the infected cervical cells. This may reflect the relative abundance of this protein in comparison to the other actin-associated proteins that were examined. Alternatively, the observed myosin distribution may be indicative of the initiation of a concurrent change occurring in the actin cytoskeleton, or it is possible that gonococci elicit only a minimal recruitment of myosin upon ruffle induction.
The host cell surface molecule exploited by Salmonella spp. to initiate ruffling has, to date, not been elucidated. The Shigella protein complex of IpaB/C/D has been shown to bind the fibronectin receptor, integrin α5β1 (58). The predominant accumulation of ezrin and vinculin in N. gonorrhoeae-infected primary cervical cells and the ability of these actin-associated proteins to directly interact with integrin molecules to initiate cellular responses (4, 46) make integrin molecules attractive candidates as potential gonococcal receptors that serve to initiate gonococcus-induced ruffling. Studies using the larynx carcinoma cell line, HEp-2, have demonstrated that gonococcal binding of fibronectin results in coligation of heparan sulfate proteoglycan to gonococcal Opa proteins and subsequent binding to the α5β1 integrin (40). Ruffling was not observed to occur in these cells, suggesting that gonococcal induction of ruffles may be unique to the cervical epithelium. Investigation of male primary urethral cells has shown that some gonococci can enter these cells by focal macropinocytosis, but no evidence of ruffling was seen (H. A. Harvey and M. K. Zenni, unpublished data). This would suggest that perhaps a cell surface molecule unique to the cervical epithelium may be involved in ruffle induction and that gonococci invoke membrane ruffles by a mechanism distinct from that observed for Shigella strains. Salmonella and Shigella strains share many common characteristics with respect to their ability to induce membrane ruffles; however, they each also display ruffling characteristics that are unique to their genus.
Through coevolution with their exclusive human hosts the pathogenic Neisseria have developed several mechanisms by which they successfully persist in the general population. Previous studies of N. gonorrhoeae have demonstrated the ability of these organisms to invade eukaryotic cells by receptor-mediated endocytosis, microtubule-dependent endocytosis, and zippering. We have described yet another mechanism by which gonococci are able to exploit their human host. Ruffling, via a triggering mechanism, has not been observed to occur in male primary urethral cells, tissue culture cell lines, or fallopian tube organ culture, nor has ruffling been described to occur with Neisseria meningiditis infections. Ruffling of primary cervical cells, which is induced with gonococcal infection, therefore, is a novel finding. Future studies will concentrate on identifying the bacterial and host factors involved in gonococcal induction of ruffling in the hope that identification of these factors will aid in the delineation of the gonococcal infection process as it pertains to the female genital tract.
ACKNOWLEDGMENTS
We thank Meg Ketterer and Hillery Harvey for their assistance in development of the primary cell culture systems and Peter Giardina for constructing the 1291-green used in these studies. We also thank the staff at the University of Iowa Central Microscopy Research Facility.
This work was funded by NIH grants 5T32HL07638, AI45728, AI43924, and AI45424.
REFERENCES
- 1.Alpuche-Aranda C M, Racoosin E L, Swanson J A, Miller S I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessen D, Gotschlich E C. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986;54:154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christodoulides M, Everson J S, Liu B L, Lambden P R, Watt P J, Thomas E J, Heckels J E. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol Microbiol. 2000;35:32–43. doi: 10.1046/j.1365-2958.2000.01694.x. [DOI] [PubMed] [Google Scholar]
- 4.Clarke M, Spudich J A. Non-muscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- 5.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehio C, Gray-Owen S D, Meyer T F. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 7.Dillard J. A variable pathogenicity island associated with disseminated gonococcal infection. Milwaukee, WI: Sixth Annual Midwest Microbial Pathogenesis Meeting. Midwest Microbial Pathogenesis Group; 1999. [Google Scholar]
- 8.DiPaolo J A, Popescu N C, Alvarez L, Woodworth C D. Cellular and molecular alterations in human epithelial cells transformed by recombinant human papillomavirus DNA. Crit Rev Oncog. 1993;4:337–360. [PubMed] [Google Scholar]
- 9.Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- 10.Draper D L, Donegan E A, James J F, Sweet R L, Brooks G F. Scanning electron microscopy of attachment of Neisseria gonorrhoeae colony phenotypes to surfaces of human genital epithelia. Am J Obstet Gynecol. 1980;138:818–826. doi: 10.1016/s0002-9378(16)32743-0. [DOI] [PubMed] [Google Scholar]
- 11.Evans B A. Ultrastructure study of cervical gonorrhea. J Infect Dis. 1977;136:248–255. doi: 10.1093/infdis/136.2.248. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay B B, Ruschkowski S. Cytoskeletal rearrangements accompany Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 14.Fluhmann C F. The squamocolumnar transitional zone of the cervix uteri. Obstet Gynecol. 1959;14:133–148. [PubMed] [Google Scholar]
- 15.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 16.Gadzar A F, Kurvari V, Virmani A, Gollahon L, Sakaguchi M, Westerfield M, Kodagoda D, Stasny V, Cunningham H T, Wistuba I I, Tomlinson G, Tonk V, Ashfaq R, Leitch A M, Minna J D, Shay J W. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-del Portillo F, Finlay B B. Salmonella invasion of nonphagocytic cells induces formation of macropinosomnes in the host cell. Infect Immun. 1994;62:4641–4645. doi: 10.1128/iai.62.10.4641-4645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassmé H U C, Ireland R M, van Putten J P M. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin F M, Jr, Griffin J A, Silverstein S C. Studies on the mechanisms of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin F M, Jr, Griffin J A, Leider J E, Silverstein S C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handsfield H H. Neisseria gonorrhoeae. In: Mandell G L, Douglas R G Jr, Bennett J E, editors. Principles and practice of infectious disease. 3rd ed. New York, N.Y: Churchill Livingstone; 1990. pp. 1613–1631. [Google Scholar]
- 22.Harkness A H. The pathology of gonorrhoea. Br J Vener Dis. 1948;24:137–147. [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey H A, Ketterer M R, Preston A, Lubaroff D, Williams R, Apicella M A. Ultrastructure analysis of primary human urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect Immun. 1997;65:2420–2427. doi: 10.1128/iai.65.6.2420-2427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hook E W, III, Handsfield H H. Gonococcal infections in the adult. In: Holmes K K, Mårdh P-A, Sparling P F, Lemon S M, Stamm W E, Piot P, Wasserheit J N, editors. Sexually transmitted diseases. 3rd ed. New York, N.Y: McGraw-Hill; 1999. pp. 451–466. [Google Scholar]
- 25.Iglesias M, Plowman G D, Woodworth C D. Interleukin-6 and interleukin-6 soluble receptor regulate proliferation of normal, human papillomavirus-immortalized, and carcinoma-derived cervical cells in vitro. Am J Pathol. 1995;146:944–952. [PMC free article] [PubMed] [Google Scholar]
- 26.Jerse A E, Jerse R F. Adhesion and invasion by the pathogenic Neisseria. Trends Microbiol. 1997;5:217–221. doi: 10.1016/s0966-842x(97)01056-1. [DOI] [PubMed] [Google Scholar]
- 27.Kaur P, McDougall J K. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988;62:1917–1924. doi: 10.1128/jvi.62.6.1917-1924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketterer M R, Shao J Q, Hornick D B, Buscher B, Bandi V K, Apicella M A. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect Immun. 1999;67:4161–4170. doi: 10.1128/iai.67.8.4161-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo T, Mihara K, Inoue Y, Namba M. Two-dimensional electrophoretic studies on down-regulated intracellular transferring in human fibroblasts immortalized by treatment with either 4-nitroquinoline 1-oxide or 60Co gamma rays. Electrophoresis. 1996;17:1638–1642. doi: 10.1002/elps.1150171026. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Lei Z M, Lojun S, Rao Ch V, Satyaswaroop P G, Day T G. Increased expression of luteinizing hormone/human chorionic gonadotropin receptor gene in human endometrial carcinomas. J Clin Endocrinol Metab. 1994;79:1483–1491. doi: 10.1210/jcem.79.5.7962347. [DOI] [PubMed] [Google Scholar]
- 31.Maitra A, Wistuba I I, Virmani A K, Sakaguchi M, Park I, Stucky A, Milchgrub S, Gibbons D, Minna J D, Gazdar A F. Enrichment of epithelial cells for molecular studies. Nat Med. 1999;5:459–463. doi: 10.1038/7458. [DOI] [PubMed] [Google Scholar]
- 32.McGee Z A, Stephens D S, Hoffman L, Schlech III W F, Horn R G. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev Infect Dis. 1983;5:S708–S714. doi: 10.1093/clinids/5.supplement_4.s708. [DOI] [PubMed] [Google Scholar]
- 33.McNeely S G. Gonococcal infections in women. Sex Transm Dis. 1989;16:467–478. [Google Scholar]
- 34.Meyer T F. Pathogenic neisseriae: complexity of pathogen-host cell interplay. Clin Infect Dis. 1999;28:433–441. doi: 10.1086/515160. [DOI] [PubMed] [Google Scholar]
- 35.Moll R, Franke W W, Schiller D L. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 36.Moulder J W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 38.Nassif X, Pujol C, Morand P, Eugène E. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol Microbiol. 1999;32:1124–1132. doi: 10.1046/j.1365-2958.1999.01416.x. [DOI] [PubMed] [Google Scholar]
- 39.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naumann M, Rudel T, Meyer T F. Host cell interactions and signaling with Neisseria gonorrhoeae. Curr Opin Microbiol. 1999;2:62–70. doi: 10.1016/s1369-5274(99)80011-3. [DOI] [PubMed] [Google Scholar]
- 41.Oelschlager T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–88. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 43.Richardson W P, Sadoff J C. Induced engulfment of Neisseria gonorrhoeae by tissue culture cells. Infect Immun. 1998;56:2512–2514. doi: 10.1128/iai.56.9.2512-2514.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson M S. The role of clathrin, adaptors, and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 45.Rosqvist R, Håkansson S, Forsberg Å, Wolf-Watz H. Functional conservaton of the secretion and translocation machinery for the virulence proteins of yersiniae, salmonellae, and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt A, Hall M N. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- 47.Seya T, Hara T, Matsumoto M, Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990;145:238–245. [PubMed] [Google Scholar]
- 48.Silverstein S C, Steinman R M, Cohn Z A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- 49.Sizemore N, Rorke E A. Human papillomavirus 16 immortalization of normal ectocervical epithelial cells alters retinoic acid regulation of cell growth and epidermal growth factor receptor expression. Cancer Res. 1993;53:4511–4517. [PubMed] [Google Scholar]
- 50.Skoudy A, Tran Van Nhieu G, Mantis N, Arpin M, Mounier J, Gounon P, Sansonetti P. A functional role for ezrin during Shigella flexneri entry into epithelial cells. J Cell Sci. 1999;112:2059–2068. doi: 10.1242/jcs.112.13.2059. [DOI] [PubMed] [Google Scholar]
- 51.Smedts F, Ramaekers F, Robben H, Pruszczynski M, van Muijen G, Lane B, Leigh I, Vooijs P. Changing patterns of keratin expression during progression of cervical intraepithelial neoplasia. Am J Pathol. 1990;136:657–668. [PMC free article] [PubMed] [Google Scholar]
- 52.Smedts F, Ramaekers F, Troyanovsky S, Pruszczynski M, Link M, Lane B, Leigh I, Schijf C, Vooijs P. Keratin expression in cervical cancer. Am J Pathol. 1992;141:497–511. [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens D S. Gonococcal and meningococcal pathogenesis as defined by human cell, cell culture, and organ culture assays. Clin Microbiol Rev. 1989;2:S104–S111. doi: 10.1128/cmr.2.suppl.s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Q, Tsutsumi K, Yokoyama M, Pater M M, Pater A. In vivo cytokeratin-expression pattern of stratified squamous epithelium from human papillomavirus-type-16-immortalized ectocervical and foreskin keratinocytes. Int J Cancer. 1993;54:656–662. doi: 10.1002/ijc.2910540422. [DOI] [PubMed] [Google Scholar]
- 55.Swanson J A, Baer S C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- 56.Swanson J A, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 57.Tran Van Mhieu G, Sansonetti P J. Mechanisms of Shigella entry into epithelial cells. Curr Opin Microbiol. 1999;2:51–55. doi: 10.1016/s1369-5274(99)80009-5. [DOI] [PubMed] [Google Scholar]
- 58.Watarai M, Funato S, Sasakawa C. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J Exp Med. 1996;183:991–999. doi: 10.1084/jem.183.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]