Background:
We sought to identify protein biomarkers of new-onset heart failure (HF) in 3 independent cohorts (HOMAGE cohort [Heart Omics and Ageing], ARIC study [Atherosclerosis Risk in Communities], and FHS [Framingham Heart Study]) and assess if and to what extent they improve HF risk prediction compared to clinical risk factors alone.
Methods:
A nested case-control design was used with cases (incident HF) and controls (without HF) matched on age and sex within each cohort. Plasma concentrations of 276 proteins were measured at baseline in ARIC (250 cases/250 controls), FHS (191/191), and HOMAGE cohort (562/871).
Results:
In single protein analysis, after adjusting for matching variables and clinical risk factors (and correcting for multiple testing), 62 proteins were associated with incident HF in ARIC, 16 in FHS, and 116 in HOMAGE cohort. Proteins associated with incident HF in all cohorts were BNP (brain natriuretic peptide), NT-proBNP (N-terminal pro-B-type natriuretic peptide), eukaryotic translation initiation factor 4E-BP1 (4E-binding protein 1), hepatocyte growth factor (HGF), Gal-9 (galectin-9), TGF-alpha (transforming growth factor alpha), THBS2 (thrombospondin-2), and U-PAR (urokinase plasminogen activator surface receptor). The increment in C-index for incident HF based on a multiprotein biomarker approach, in addition to clinical risk factors and NT-proBNP, was 11.1% (7.5%–14.7%) in ARIC, 5.9% (2.6%–9.2%) in FHS, and 7.5% (5.4%–9.5%) in HOMAGE cohort, all P<0.001), each of which was a larger increase than that for NT-proBNP on top of clinical risk factors. Complex network analysis revealed a number of overrepresented pathways related to inflammation (eg, tumor necrosis factor and interleukin) and remodeling (eg, extracellular matrix and apoptosis).
Conclusions:
A multiprotein biomarker approach improves prediction of incident HF when added to natriuretic peptides and clinical risk factors.
Keywords: biomarkers, cardiovascular diseases, heart failure, protein interaction maps, proteomics
What is New?
A multiprotein approach improved the prediction of new-onset heart failure.
The key proteins predicting heart are related to inflammatory (tumor necrosis factor and interleukins) and remodeling (extracellular matrix and apoptosis) pathways.
These results arise from 3 large international cohorts, thus ensuring a good external validity.
What are the Clinical Implications?
The predictive value of a combined approach using clinical and numerous biological biomarkers is excellent.
As predicting individuals at high risk for heart failure is the first step to a tailored intervention to prevent HF, this integrated approach opens up avenues for preventive strategies in a populational setting. These risk-stratification tools could aid in the design of preventive trials, seeking to decrease the incidence of heart failure with preserved ejection fraction.
The pathways identified as key in the prediction of heart failure (mostly related to inflammation and remodeling) could also highlight promising therapeutic approaches in preventive cardiology.
The pathways identified as key in the prediction of heart failure (mostly related to inflammation and remodeling) could also open therapeutic avenues in preventive cardiology.
The incidence of heart failure (HF) is increasing due to ageing of the population and associated increases in the prevalence of and duration of exposure to several risk factor for HF including hypertension, diabetes, and obesity.1–4 In the past several decades, important progress has been made in the treatment of patients with overt HF. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta blockers, and mineralocorticoid receptor antagonists have been shown to improve survival in patients with HF with reduced ejection fraction.5,6 More recently, modest advances have also been made in the treatment of patients with HF with preserved ejection fraction. For example, spironolactone now has a grade II indication according to recent guidelines.6 More recently, sodium/glucose cotransporter 2 inhibitors have been shown to improve prognosis regardless of HF type and have been proven to prevent HF onset in patients with diabetes.7 The best effective personalized strategies to prevent HF, however, should be further explored to enable HF prevention at a population level.
Circulating proteins, most notably NT-proBNP (N-terminal pro-B-type natriuretic peptide), have been studied as predictors of incident HF.8–11 The human plasma proteome is composed of proteins derived from multiple tissues and representing multiple biological pathways and can serve as a dynamic representation of the molecular states of diverse systems.12 High throughput, automated, high-sensitivity protein assays can now be applied to large cohorts and clinical trials to identify protein biomarker profiles of HF. Multiprotein biomarker strategies may aid in the characterization of high-risk individuals in whom earlier treatment and ongoing surveillance may be beneficial and may provide information that is not captured by traditional HF risk factors.
A recent attempt to predict HF using 80 proteins in community-based cohorts resulted in a sizeable increase in the C-index (10.1%; P=0.006) on top of HF clinical risk factors.11 The improvement in prediction from the circulating proteins biomarkers on top of natriuretic peptides, however, was a small and not significant (change in C-index 2.0%; P=0.40).11 Ho et al13 reported, based on findings in the FHS (Framingham Heart Study), that NT-proBNP, GDF-15 (growth/differentiation factor 15), CRP (C-reactive protein), and leptin (among 85 tested biomarkers) jointly predicted incident HF and were associated with a modest but statistically significant 3% improvement in the C-statistic on top of clinical risk factors (0.843–0.873; P<0.0001). We hypothesized, a priori, that a significantly larger panel of proteins, covering additional pathways and biological processes related to HF, would better identify individuals at high risk for new-onset HF and highlight pathways contributing to its occurrence.
To that end, we undertook a study of new-onset heart failure in 3 independent cohorts (the HOMAGE cohort [Heart Omics and Ageing], the ARIC study [Atherosclerosis in Communities], and the FHS) with the aim to identify protein biomarkers for the prediction of HF.
Methods
Data Availability
The data that support the findings of this study are available from N.G. and F.Z. upon reasonable request.
Study Populations
All studies were conducted in accordance with the Declaration of Helsinki and approved by each site ethics committees. All participants provided written informed consent.
ARIC Cohort
We used the data of the ARIC study, a longitudinal population-based cohort study of cardiovascular disease (CVD) and its risk factors ongoing since 1987. A detailed description of the ARIC study design and methods has been published elsewhere.14 The analysis reported herein was conducted among individuals who participated in ARIC visit 5 (age 69–88 years), conducted between June 1, 2011 and August 30, 2013.
Participants with HF history at the 5th visit were not considered eligible for this analysis. We performed a classical nested matched case-control study (ie, controls were study participants who did not develop HF up until the end of follow-up). Controls were matched within each cohorts on follow-up time, age, and sex.
The FHS Cohort
The FHS is a community-based longitudinal study that began in 1948. Residents of Framingham, Massachusetts were invited to participate in a study of the prevalence, incidence, and natural history of CVDs. Participants attend on-site clinical examinations and provide information on cardiovascular health through face-to-face interviews, self-administered questionnaires, physician examinations, and provide blood samples for laboratory tests. For the current study, the 7th examination FHS Offspring cohort examination, conducted from 1998 to 2001, was used. Information on CVDs diagnosis was abstracted from the Framingham Heart Study 3-physician verified medical record data file using December 31, 2016 as the deadline of update.
A total of 43 (1.22%) participants had a diagnosis of HF prior to their seventh examination date who were excluded from eligibility for this study. The remaining 3231 exam attendance included 265 participants who had a HF diagnosis following their baseline examination. Of these cases, 261 had blood samples for the measurement of plasma proteins and were considered as candidate cases in the subsequent matching procedure. We applied 1-to-1 match strategy and identified 211 age-sex matched controls using a nested matched case-case design in which individuals who developed HF were considered to be at-risk, that is, eligible to be selected as controls up until the time they became a case. We also excluded all case/control pairs for which the case was not hospitalized for HF (n=20 pairs). This design can also be referred to as a case-cohort design.
HOMAGE Cohort
The HOMAGE consortium (NCT02556450) is a European Union funded program that aims to identify and validate omics biomarkers associated with incident HF in order to potentially develop new and personalized preventive strategies. The HOMAGE consortium included 20 completed and ongoing studies conducted in 8 European countries that enrolled healthy subjects, patients with HF, and patients at high risk of CVD, all of which were pooled in a common database.15 From the HOMAGE population with >20 000 patients, we identified cohorts in whom individuals without a history of HF at baseline had been followed up until first hospitalization for HF. Participants from 2 suitable cohorts and 1 clinical trial population were identified: PREDICTOR16 (patients recruited from 2007 to 2010), HEALTH-ABC17 (recruited from 1997 to 1998), and PROSPER15,18 (recruited from 1997 and 1999). As in the FHS cohort, we then employed a nested matched case-cohort design in which individuals who developed HF were considered to be at-risk, that is, eligible to be selected as controls up until the time they became a case.19
The initial purpose of the HOMAGE project was to identify underlying mechanistic pathways that may be associated with a primary incident diagnosis of HF.20 The HOMAGE case-cohort study had 2 independent phases: discovery and replication. For the discovery phase, we selected 300 cases and 599 controls (1 case only had 1 match) randomly selected without replacement in a 1:2 proportion; because of 22 missing or poor-quality samples, the final match was 286 cases to 591 controls. For replication, we selected 315 cases and 315 controls randomly selected without replacement in a 1:1 proportion; because of 74 missing or poor-quality samples, the final match was 276 cases to 280 controls. Controls were matched within each cohorts on follow-up time, age, and sex. For the purpose of the current analysis, centered on risk prediction, we merged the datasets of the 2 phases (totalizing 562 cases and 871 controls).
Outcome
The outcome used in the HOMAGE, ARIC, and FHS studies was incident HF, which was defined as first hospitalization for HF as primary admission diagnosis (adjudicated by the investigators of the respective cohorts). NT-proBNP data were not required to ascertain the diagnosis, as most patients were included in an era during which natriuretic peptides were not yet standard of care.
Sample Handling
All sample shipments and sample data acquisition within the HOMAGE consortium (including the ARIC and FHS databases) are according to predefined standard operating procedures and material transfer agreements to maintain uniformity. Figure S1 shows the sample handling and storage per cohort and the sample flow until protein measurement at the TATAA-Biocenter (Gothenburg, Sweden) and OLINK Proteomics (Sweden). The cases and controls were separately identified and selected by the study statistician. All participants’ information was then removed and a randomly sorted list of patient/sample IDs for each cohort was sent to Maastricht University Medical Center. Aliquoting of the samples at Biobank Maastricht was performed using a multi-pipette in 1 run to reduce freeze/thaw cycles and batch effects. All samples were randomly aliquoted on each 96-well plate, independent of cohort allocation. The entire sampling handling/protein measurement was carried out fully blind to case-control status.
Assays and Studied Biomarkers
Baseline plasma samples were analyzed for protein biomarkers using the OLINK Proseek® Multiplex CVD II, CVD III, and inflammation panels for all cohorts. The HOMAGE analyses were carried out by the TATAA-Biocenter and by OLINK proteomics for the ARIC and FHS cohorts.
These panels were selected for the well-balanced inclusion of proteins with established associations with CV disease and HF (eg, BNP [brain natriuretic peptide], ST2 [suppression of tumorigenicity 2], and GDF-15 [growth and differentiation factor 15]) and others with less well-established associations (eg, TWEAK [tumor necrosis factor], PON3 [paraoxonase]; the full information on these panels is available at: https://www.olink.com/resources-support/document-download-center/). The assays use a proximity extension assay technology,21 where 92 oligonucleotide-labeled antibody probe pairs per panel are allowed to bind to their respective targets in the sample in 96-well plate format. When binding to their correct targets, they give rise to new DNA amplicons with each ID-barcoding their respective antigens. The amplicons are subsequently quantified using a Fluidigm BioMark HD real-time PCR platform. The OLINK platform provides log2-normalized protein expression data. A detailed description of the Olink technology is depicted in the Supplemental Material. For 9 proteins measured in both the inflammation panel and CVD panels, the one from CVD panels was used for further data analyses (the results for these pairs of proteins were strongly correlated ≥0.9). In addition, 15 proteins that were below the limit of detection (LOD) were not included in the analysis. The Olink® quality control samples are considered as flagged if they deviate more than 0.3 log2-normalized protein expression from the median of all samples in 1 of 2 control assays for incubation and detection. The LOD is defined by the 3 negative controls run on each plate and set to 3 SDs above the measured background.
In the ARIC and FHS cohorts, biomarkers with 80% or more values below the LOD were excluded from candidate variables. When the proportion of below LOD value was 80% or less, the below limit of detection values were replaced by the LOD.
A total of 247 protein biomarkers was assessed in the baseline samples of the 3 cohorts. The abbreviations, full names, and respective Olink® multiplex panels of the studied proteins are described in Table S1.
The assays were performed blinded to case/control status with cases and controls randomly distributed across plates. The proteomic results were then merged with the baseline data, which included the case-control status, matching variables, and the clinical risk factors.
Statistical Considerations
For the baseline clinical characteristics, continuous variables are expressed as means±SD or as medians (interquartile range) if the distribution was skewed and categorical variables as frequencies and percentages. Participants’ baseline characteristics were compared between cases and controls using Fisher exact test for categorical variables and t tests or nonparametric Wilcoxon tests for continuous variables, as appropriate.
Logistic regression models adjusting for the matching variables (age, sex, cohort, follow-up time), phase only for HOMAGE (discovery/replication), and relevant risk factor for HF were used to identify protein biomarkers associated with incident HF.22 The association with HF risk was assessed for each biomarker individually (mono-marker models). Relevant risk factors for HF were prespecified clinical risk factors previously found to represent the best clinical prognostic model for incident HF in the HOMAGE population23 (smoking, diabetes, history of coronary artery disease, serum creatinine, body mass index, systolic blood pressure, use of antihypertensive medication, heart rate) and FHS population (hypertension, history of atrial fibrillation, and ratio total/HDL cholesterol). In all 3 cohorts, we corrected for multiple testing using a false discovery rate of 5%, applying the Benjamini-Hochberg procedure.24
Because proteins expressions levels were log2-transformed, the odds ratio estimated the increase in odds of HF associated with a doubling of protein concentration.
To assess the added predictive value of biomarkers on top on routine HF predictors, we assessed the C-index of a baseline model including all the adjustment variables (matching variables, phase for HOMAGE, and the relevant risk factors described previously) in each cohort and assessed the increase in C-index when adding biomarkers individually (ie, 1 by 1) to the model.
In a subsequent analysis, several biomarkers were included in 1 unique model (multimarker model). Both BNP and NT-proBNP were measured but we kept only NT-proBNP in this multimarker model. To deal with the risk of multicollinearity, we used the variance inflation factor method within the logistic model. Biomarkers with the highest variance inflation factor were removed 1 by up until all variance inflation factor were 10 or less. One final model was fitted by cohort, and, as for the mono-marker models, the increase in C-index on top of routine HF predictors was assessed.
All statistical analysis were performed using R software (CRAN).
Network Analysis
The FHF-GKB resource, representing most available public knowledge about human protein-protein, protein-pathway and protein-Gene Ontology (GO) terms,25 was queried to extract overrepresented pathways and GO terms associated with statistically relevant biomarkers. For each pathway/GO term linked to 1 selected biomarker, overrepresentation was computed by a Fisher exact test with the whole FHF-GKB as annotation background. As previously, values were corrected for multiple testing using false discovery rate. Overrepresented terms were displayed with their associated biomarkers in Cytoscape.26 An additional step of clustering was performed for GO terms in order to obtain smaller and more understandable groups. This was done using the Community Clustering (GLay) method27,28 implemented in the Clustermaker2 Cytoscape app.29
Results
Characteristics of Study Participants
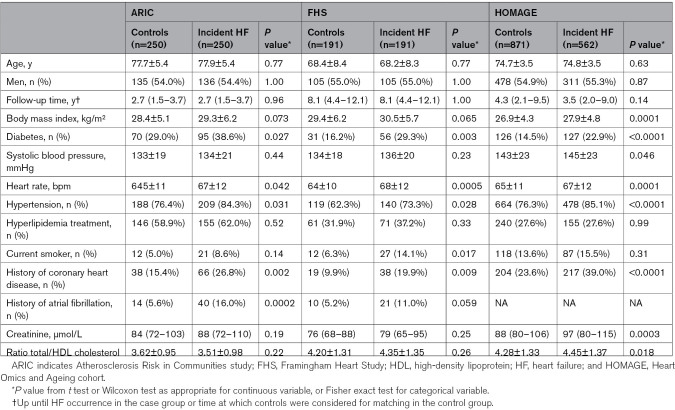
The clinical characteristics of the 3 contributing cohorts are provided in Table 1. Almost half of the participants were women. In the ARIC cohort, 136 participants (27.2%) were Black. The median (IQR) follow-up time was 2.7 (1.5–3.7) years in ARIC, 4.1 (2.1–9.2) years in HOMAGE, and 8.1 (4.5–12.1) years in FHS. FHS participants were approximately 10 years younger than participants in the other 2 cohorts.
Table 1.
Characteristics of the 3 Cohorts
Association of Individual Protein Biomarkers With Incident HF
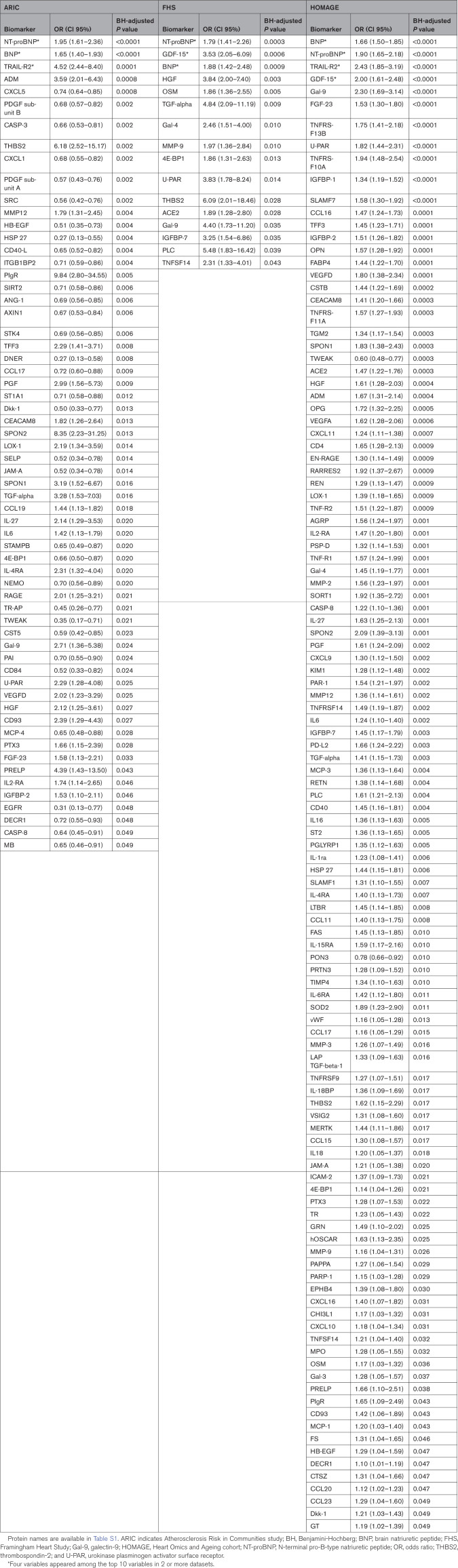
After adjusting for the matching variables and clinical HF risk factors, 142 proteins were individually associated with incident HF (correcting for multiple testing) in at least 1 cohort: 62 proteins in ARIC, 16 in FHS, and 116 in HOMAGE (Table 2; Table S2).
Table 2.
ORs and 95% CIs for the Biomarkers Associated (P<0.05) With Incident Heart Failure on Mono-Biomarker Analysis After Adjusting for Matching and Clinical Variables and Correction for Multiple Comparisons in Each Cohort
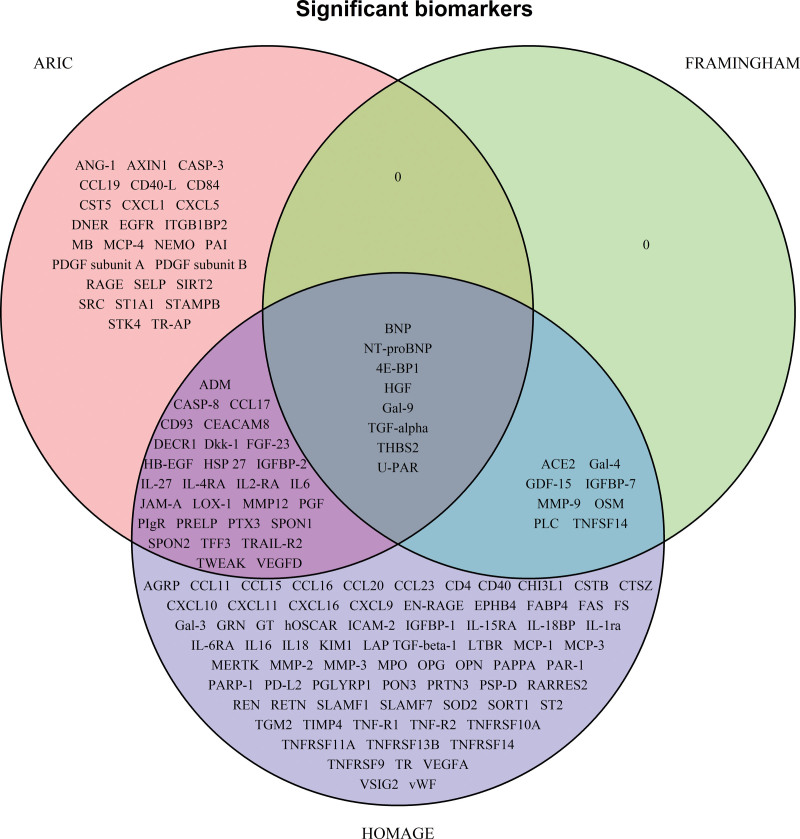
Proteins significantly associated with incident HF in all 3 cohorts were BNP, NT-proBNP, 4E-BP1 (4E-binding protein 1), HGF (hepatocyte growth factor), Gal-9 (galectin-9), TGF-alpha (transforming growth factor alpha), THBS2 (thrombospondin-2), and U-PAR (urokinase plasminogen activator surface receptor; Figure 1).
Figure 1.
Venn diagram with the significant biomarkers identified in each cohort. Protein names are available in Table S1.
In a sensitivity analysis performed in the ARIC cohort by adding Black race to the clinical HF risk factors, 44 proteins were individually associated with incident HF: 41 proteins already associated with incident HF in the previous results and 3 new proteins (MEPE [matrix extracellular phosphoglycoprotein], brother of CDO, and MMP-2 [matrix metalloproteinase-2]; Table S3).
Predictive Value of Individual Protein Biomarkers for Incident HF on Top of Clinical HF Risk Factors
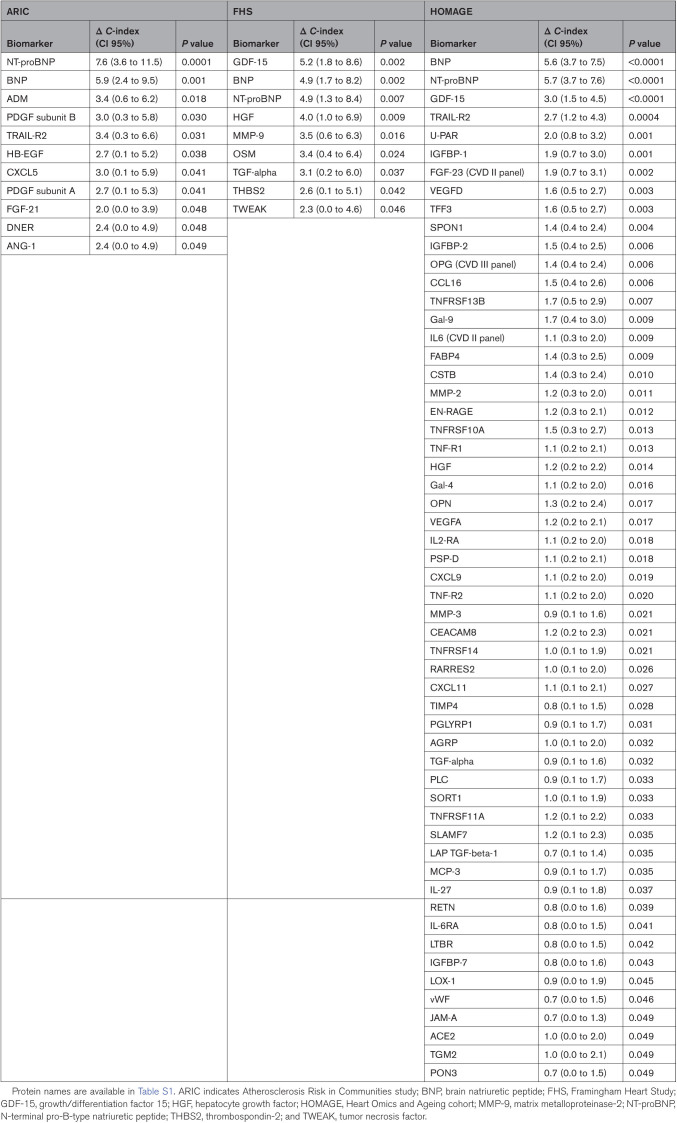
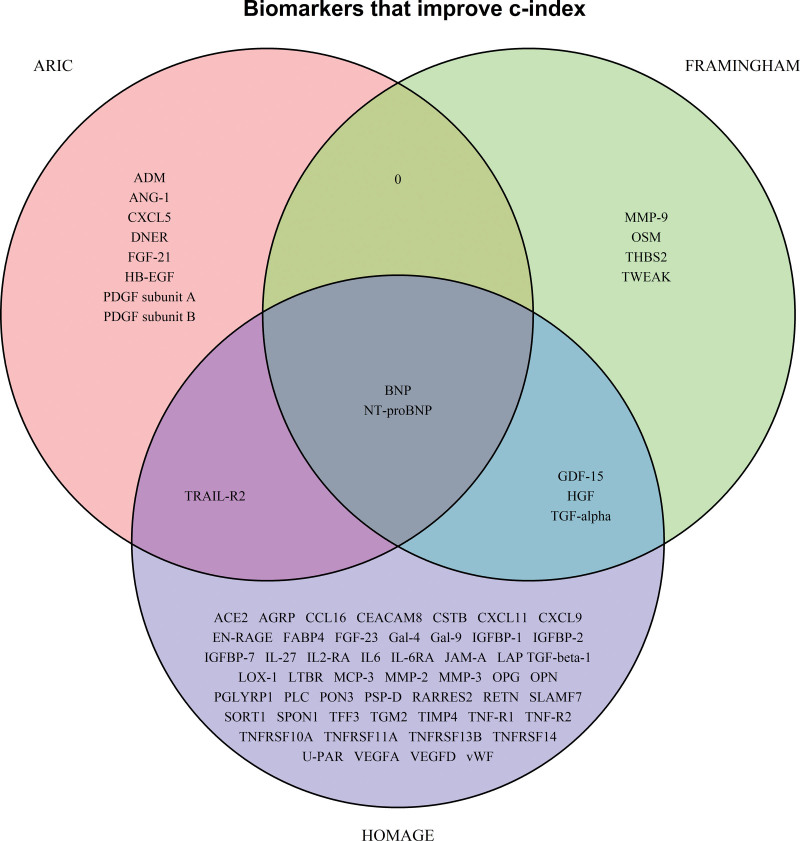
Sixty-eight proteins were found to improve HF prediction significantly (as assessed by a significant increase in the C-index) when considered individually on top of clinical HF risk factors in at least 1 cohort: 11 proteins in ARIC, 9 in FHS, and 56 in HOMAGE (Table 3; Table S4). Only BNP and NT-proBNP significantly improved prediction in all 3 cohorts (Figure 2).
Table 3.
Biomarkers That Improved the C-Index When Added to the Clinical Model
Figure 2.
Venn diagram for the biomarkers in each cohort that improved the C-index when added to the clinical model. Protein names are available in Table S1.
Predictive Value of a Protein Multimarker Approach for Incident HF Risk Prediction
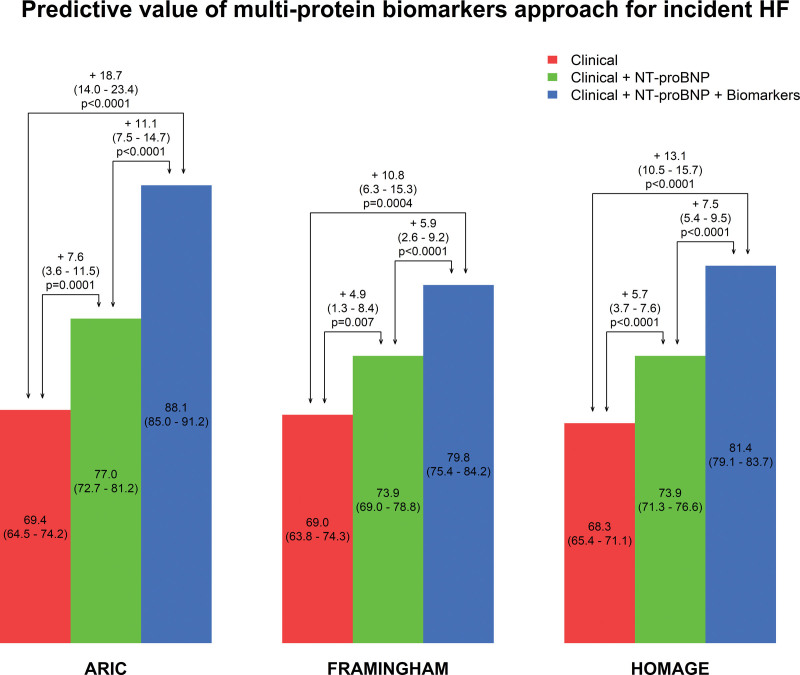
NT-proBNP significantly improved HF prediction on top of established clinical HF risk factors in all 3 cohorts (delta C-index=7.6% [95% CI, 3.6%–11.5%]) in ARIC, 4.9% (1.3%–8.4%) in FHS, and 5.7% (3.7%–7.6%) in HOMAGE (all P<0.01; Table 3).
The added predictive value for incident HF of a multimarker approach, on top of clinical HF risk factors and NT-proBNP was significant in all 3 cohorts (delta C-index=11.1% [7.5%–14.7%] in ARIC, 5.9% [2.6%–9.2%] in FHS, and 7.5% [5.4%–9.5%] in HOMAGE; all P<0.0001) and of larger magnitude than that of NT-proBNP when considered alone on top of routine HF risk factors (Figure 3).
Figure 3.
Predictive value of multiprotein biomarkers approach for incident heart failure (HF) in addition to routine clinical markers of incident heart failure in the 3 cohorts. Biomarkers retained in multivariable model in each cohort (including NT-proBNP [N-terminal pro-B-type natriuretic peptide]) included the following: ARIC (Atherosclerosis Risk in Communities study; P=55): all significant BMs, except BNP (brain natriuretic peptide)+AXIN1, CASP-3, PDGF subunit A, PDGF subunit B, SRC, STAMPB; FHS (Framingham Heart Study; P=15): all significant BMs, except BNP; HOMAGE (Heart Omics and Ageing cohort; P=109): all significant BMs, except BNP, EPHB4, IL-18BP, OSM, TNF-R1, TNF-R2, TNFRSF14. The clinical model included the following variables: age, sex, smoking, diabetes, history of coronary artery disease, serum creatinine, body mass index, systolic blood pressure, use of antihypertensive medication, heart rate, hypertension, history of atrial fibrillation, and ratio total/high-density lipoprotein cholesterol.
The total added predictive value of NT-proBNP and the multimarker approach was an increment in the C-index ranging from 10.8% (6.3%–15.3%) in FHS to 18.7% (14.0%–23.4%) in ARIC (Figure 3). Importantly, the achieved C-index (despite age and sex being part of the matching procedure) ranged from 79.8% in FHS to 88.1% in ARIC.
Network Analysis
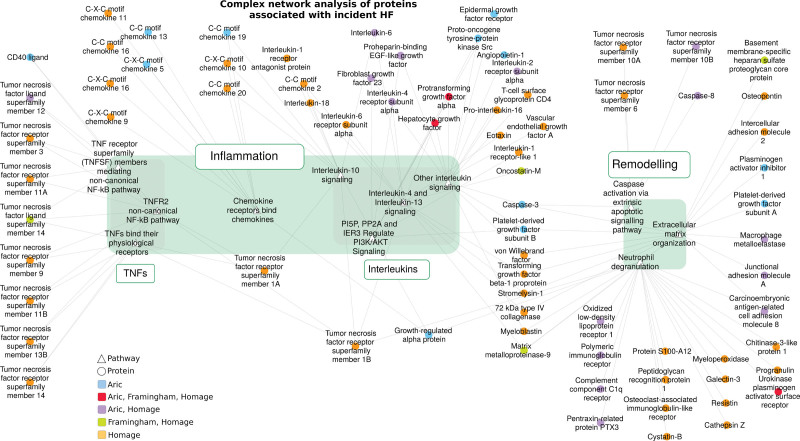
When including the 142 proteins significantly associated with HF risk in complex network analysis (Figure 4), several overrepresented pathways were identified as relevant, mostly related to inflammation and remodeling. Involved inflammatory pathways were mainly related to TNF and interleukin. Remodeling pathways were mainly related to extracellular matrix and apoptosis.
Figure 4.
Complex network analysis of significant protein biomarkers. Overrepresented pathways are symbolized by white triangles and biomarkers are ellipses. Biomarker color depends on the study where it was identified. Protein names are available in Table S1.
When further narrowing the analysis to over-represented GO terms (Supplemental Material), we identified 9 mechanistic clusters: matrix metalloproteinase (MMP)/U-PAR, GDF-15/interleukin-4, apoptotic mechanisms, endocytosis/phagocytosis mechanisms, chemokines, regulation of inflammation and inflammation effectors, TNF/ nuclear factor-kappa B/TNF-related apoptosis-inducing ligand, TNF/interleukin-12 and interleukin-related mechanisms.
Discussion
We identified 142 protein biomarkers of incident HF in at least 1 of the 3 independent cohorts (ARIC, FHS, and HOMAGE). When we tested these proteomic biomarkers in a multimarker approach, we found that they significantly and substantially improved the prediction of incident HF on top of clinical HF risk factors in all 3 cohorts (C-index increases all >10%); the improvement in prediction for the protein multi-biomarkers was appreciable after accounting for HF risk factors and NT-proBNP (C-index increases all ≥5.9%). The proteins identified as significantly associated with HF varied across cohorts, but they were all related to inflammatory (eg, TNF and interleukins) and remodeling pathways (eg, extracellular matrix and apoptosis) in complex network analysis. In addition, we identified 9 mechanistic clusters related to these proteins, mostly related to inflammatory pathways.
Most previous biomarker studies of incident HF used single proteins or oligo-marker approaches. The natriuretic peptides have emerged as the most consistent protein predictors of risk,5 and the results of our analyses confirm their importance in HF prediction. Some oligo-biomarkers strategies have been shown to improve HF risk prediction, such as the LIPID HF risk-prediction model (BNP, cystatin C, D-dimer, high-sensitivity CRP, and high-sensitivity troponin I), with an increment in C-index of 4% on top of clinical risk factor.30 Stenemo et al11 used data from 2 community-based cohorts from Uppsala, Sweden (mean age 70 and 78 years) and reported that the use of 18 proteins (out of 80 measured proteins) resulted in a sizeable increase in C-index (10.1%; P=0.006) on top of established HF risk factors. However, the incremental value of the protein biomarkers on top of clinical HF risk factors and natriuretic peptides was modest and not significant (change in C-index 2.0%; P=0.40).11 Based on findings in the FHS, Ho et al13 reported that NT-proBNP, GDF-15, CRP, and leptin (among 85 tested biomarkers) jointly predicted incident HF and were associated with a modest but statistically significant 3% improvement in the C-statistic on top of clinical risk factors (0.843–0.873; P<0.0001). This analysis, however, did not separately adjust for natriuretic peptides. We show that in all 3 cohorts there was a significant increase in C-index from the incorporation of multiple proteins on top of routine HF risk factors (whether excluding or including NT-proBNP in the models). In addition, we show that the increase in prediction arising from NT-proBNP on top of clinical HF risk factors was substantial (increment in C-index ranging from 4.9 to 7.6 in the 3 cohorts), but smaller than the increment arising from the further addition of multiple proteins on top of NT-proBNP (increment in C-index 5.9 to 11.1 in the 3 cohorts; Figure 3).
In the study by Stenemo et al,11 9 proteins (GDF-15, TIM-1 [T-cell immunoglobulin and mucin domain 1], TNF-related apoptosis-inducing ligand-R2, SPON1 [spondin-1], MMP-12, FS [follistatin], U-PAR, osteoprotegerin [OPG], and ST2) were associated with incident HF after adjusting for established HF risk factors. Among these 9 protein biomarkers, the only 1 not measured in our study was TIM-1 Of these previously reported proteins, only U-PAR was significantly associated with incident HF in each of our cohorts. GDF-15 was significantly associated with incident HF in FHS and HOMAGE, whereas TNF-related apoptosis-inducing ligand-R2, SPON1, and MMP-12 were significantly associated with incident HF in ARIC and HOMAGE. ST2 and FS were significantly associated with HF only in HOMAGE.
When including the HF-associated proteins in complex network analysis, all were related to inflammatory and remodeling pathways. This finding emphasizes the central importance of these pathways in HF pathogenesis, as emphasized previously in relation to HF with preserved ejection fraction.31 In addition, when considering GO terms complex network analysis, a limited number of mechanistic clusters were highlighted, mostly related to inflammatory processes. These network results strongly emphasize the central importance of inflammation in HF.
The results presented herein expand the knowledge derived from a previous work from the HOMAGE project. For the purpose of the current analysis, we merged the datasets of the 2 phases of the previously reported HOMAGE study20 (totalizing 562 cases and 871 controls) and conducted the same analysis in 3 large case-control studies: HOMAGE (merged), ARIC and FHS. This new analysis provides an important increase in the level of external validity achieve by our results, as they arise from both American and European settings. From this analysis that maximizes external validity, 8 proteins (BNP, NT-proBNP, 4E-BP1, HGF, Gal-9, TGF-alpha, THBS2, U-PAR) appeared as the most replicable biomarkers predicting incident HF in all cohorts. This top list of biomarkers, in light of the strength of the combined analysis we report herein, should be considered in future biomarker studies aiming to predict incident HF. Importantly, among this list of 8 proteins, 4E-BP1, HGF, TGF-alpha, THBS2, we not identified in our previous report as significant predictors of incident HF,20 probably because of lower power compared with this new multi-cohort analysis. In addition, the current analysis also expands our mechanistic understanding of the biological pathways underlying the pathogenesis of HF. In our earlier analysis, we reported pathways related to inflammation, apoptosis, vascular function, matrix remodeling, blood pressure control, and metabolism.20 With the additional data we report herein, we are able to further refine biological mechanisms an focus on a limited number of biological processes, namely inflammation (through tumor necrosis factor and interleukins) and remodeling (involving extracellular matrix and apoptosis). This may help guide future therapeutic development to target these key biological pathways in order to best prevent HF.
Perspectives
The total predictive value achieved using routine clinical and biological predictors is excellent (C-index ranging from 80% to 90%) despite cases and controls being matched on age and sex, that is, thus decreasing the C-index by removing these important variables from the model. This suggests an excellent total predictive value of an integrated approach based on numerous biological biomarkers and clinical variables. Adequately predicting individuals at high risk for HF is the prerequisite of tailored interventions to prevent the onset of HF in a populational setting.
Identifying protein biomarkers of incident HF could have a major impact on our understanding of HF pathogenesis. Identifying pathway involved in the transition to HF with preserved ejection fraction in specific clinical phenotypes would considerably improve our understanding of this disease and could help tailor therapeutic interventions targeting specific biological processes relevant to specific clinical settings. In addition, improving the prediction of incident HF could pave the way for the design of preventive trials.
Preventive trials are of uttermost importance as the therapeutic management of HF remains imperfect, especially for HF with preserved ejection fraction. We believe that this new avenue in biomarker research is likely to greatly extend in the next few years and could have a major impact on preventive cardiology.
Limitations
Several limitations should be highlighted in the present study. First, this is an observational case-control study, hence causality cannot be ascertained.
Natriuretic peptides were not routinely used to ascertain HF diagnosis in most patients considered in this analysis as it was not a standard of care in the era during which study participants were recruited. However, our definition required being hospitalized for HF, which may be sufficiently stringent to ensure patients did have HF despite not considering natriuretic peptides.
The mean age of patients included in this analysis ranged from 68 to 78 years according to the considered cohort. Our results consequently apply to the development of HF in fairly old population and do not provide insight on the development of specific causes of HF encountered usually/more frequently earlier in life such as peripartum, stress induced or tachycardia related cardiomyopathy.
We did not use large unbiased screens but rather preselected protein biomarkers based on mechanistic hypotheses and previous literature. The Olink panels used herein were mostly biomarkers related to cardiovascular and inflammatory diseases. We cannot consequently ascertain we did not miss important biomarkers not included in these panels.
Whether the biomarkers identified as associated with incident HF are causally involved in the development of HF and could serve as therapeutic targets is uncertain. It is possible that many biomarkers are actually bystander variables predicting HF incident because they are related to underlying causes of HF. The causality between these circulating biomarkers and HF genesis is to be further explored in animal/mechanistic models.
We did not have access to the left ventricular ejection fraction at the time of hospitalization; therefore, we cannot assess the potential value of these biomarkers in distinguishing progression to HF with reduced ejection fraction from HF with preserved ejection fraction and the HF cause.
The proteomics assay does not provide standard concentration units, making comparisons with clinically applied cutoffs difficult.
Most of the cohorts used in this analysis included a vast majority of Whites. Our results consequently do not improve our understanding of biological mechanisms involved in HF onset in other races.
Conclusions
In 3 large cohorts, a multiprotein approach improved HF prediction. The proteins identified as significantly associated with HF varied across cohorts but were all related to inflammatory (eg, TNF and interleukins) and remodeling (eg, extracellular matrix and apoptosis) pathways in complex network analysis. We identified 9 key mechanistic clusters related to these proteins, mostly related to inflammatory pathways, which further emphasizes the pivotal role of inflammation in the pathogenesis of HF.
Article Information
Sources of Funding
The research leading to these results has received funding from the European Union Commission’s Seventh Framework Programme under grant agreement 305507 (HOMAGE [Heart Omics in Ageing consortium]). The authors acknowledge the support from the Netherlands Cardiovascular Research Initiative, an initiative with the support of the Dutch Heart Foundation CVON2016-Early HFPEF, and CVON 2017, ShePREDICTS.
Disclosures
Drs Ferreira, Girerd, Rossignol, and Zannad are supported by the French National Research Agency Fighting Heart Failure (ANR-15-RHU-0004), by the French PIA project Lorraine Université d’Excellence GEENAGE (ANR-15-IDEX-04-LUE) programs, and the Contrat de Plan Etat Région Lorraine and FEDER IT2MP. Dr Girerd reports honoraria from Novartis, AstraZeneka, Boehringer, and Vifor. Dr Rossignol received personal fees (consulting) from Idorsia and G3P, honoraria from AstraZeneca, Bayer, CVRx, Fresenius, Grunenthal, Novartis, NovoNordisk, Servier, StealthPeptides, Ablative Solutions, Corvidia, Relypsa, and Vifor Fresenius Medical Care Renal Pharma, outside the submitted work, and is the cofounder of CardioRenal. Dr Zannad reports steering committee personal fees from Applied Therapeutics, Bayer, Boehringer, Boston Scientific, Novartis, Janssen, and CVRx, advisory board personal fees from AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, and Merck, stock options at G3Pharmaceutical, and being the founder of CardioRenal and CVCT. Dr Mebazaa received honoraria for lectures from Roche and Abbott, consultation fees from Sanofi and Servier, and research grants from Adrenomed and Sphyngotec. Dr Ballantyne is the coinventor on a provisional patent (patent 61721475) entitled Biomarkers to Improve Prediction of Heart Failure Risk filed by Roche and Baylor College of Medicine on their behalf. Dr Ballantyne has received grant support from Abbott Diagnostics and Roche Diagnostics and is a consultant for Roche Diagnostics and Abbott Diagnostics. The other authors report no conflicts.
Supplemental Material
Tables S1–S3
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 4E-BP1
- 4E-binding protein 1
- ARIC
- Atherosclerosis in Communities study
- BNP
- brain natriuretic peptide
- CRP
- C-reactive protein
- CVD
- cardiovascular disease
- FHS
- Framingham Heart Study
- FS
- follistatin
- Gal-9
- galectin-9
- GO
- gene ontology
- HF
- heart failure
- HOMAGE
- Heart Omics and Ageing cohort
- LOD
- limit of detection
- MEPE
- matrix extracellular phosphoglycoprotein
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- PON3
- paraoxonase
- SPON1
- spondin-1
- TGF-alpha
- transforming growth factor alpha
- THBS2
- thrombospondin-2
- TIM-1
- T-cell immunoglobulin and mucin domain 1
- TWEAK
- tumor necrosis factor
- U-PAR
- urokinase plasminogen activator surface receptor
N. Girerd and D. Levy contributed equally.
For Sources of Funding and Disclosures, see page 440.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.122.009694.
Contributor Information
Daniel Levy, Email: levyd@nhlbi.nih.gov.
Kevin Duarte, Email: k.duarte@chru-nancy.fr.
Joao Pedro Ferreira, Email: jp7ferreira@hotmail.com.
Christie Ballantyne, Email: cmb@bcm.edu.
Timothy Collier, Email: Timothy.Collier@lshtm.ac.uk.
Anne Pizard, Email: anne.pizard@inserm.fr.
Jens Björkman, Email: Jens.bjorkman@tataa.com.
Javed Butler, Email: javed.butler@bswhealth.org.
Andrew Clark, Email: a.l.clark@hull.ac.uk.
John G. Cleland, Email: j.cleland@imperial.ac.uk.
Christian Delles, Email: Christian.Delles@glasgow.ac.uk.
Javier Diez, Email: jadimar@unav.es.
Arantxa González, Email: amiqueo@unav.es.
Mark Hazebroek, Email: mark.hazebroek@mumc.nl.
Jennifer Ho, Email: jho@bidmc.harvard.edu.
Anne-Cécile Huby, Email: annececilehuby@yahoo.fr.
Shih-Jen Hwang, Email: Hwangs2@nhlbi.nih.gov.
Roberto Latini, Email: roberto.latini@marionegri.it.
Beatrice Mariottoni, Email: beatrice.m91@virgilio.it.
Alexandre Mebazaa, Email: alexandre.mebazaa@aphp.fr.
Pierpaolo Pellicori, Email: pierpaolo.pellicori@glasgow.ac.uk.
Naveed Sattar, Email: naveed.sattar@glasgow.ac.uk.
Peter Sever, Email: p.sever@imperial.ac.uk.
Jan A. Staessen, Email: jan.staessen@appremed.org.
Job Verdonschot, Email: job.verdonschot@mumc.nl.
Stephane Heymans, Email: s.heymans@maastrichtuniversity.nl.
Patrick Rossignol, Email: p.rossignol@chu-nancy.fr.
Faiez Zannad, Email: f.zannad@chru-nancy.fr.
References
- 1.McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart. 2000;83:596–602. doi: 10.1136/heart.83.5.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrie M, McMurray J. Changes in notions about heart failure. Lancet. 2001;358:432–434. doi: 10.1016/S0140-6736(01)05664-1 [DOI] [PubMed] [Google Scholar]
- 3.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–580. doi: 10.1016/s0140-6736(17)32520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manzano L, Babalis D, Roughton M, Shibata M, Anker SD, Ghio S, van Veldhuisen DJ, Cohen-Solal A, Coats AJ, Poole-Wilson PPA, et al. Predictors of clinical outcomes in elderly patients with heart failure. Eur J Heart Fail. 2011;13:528–536. doi: 10.1093/eurjhf/hfr030 [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, et al. ; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. ; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borne Y, Persson M, Melander O, Smith JG, Engstrom G. Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur J Heart Fail. 2014;16:377–383. doi: 10.1002/ejhf.49 [DOI] [PubMed] [Google Scholar]
- 10.Echouffo-Tcheugui JB, Greene SJ, Papadimitriou L, Zannad F, Yancy CW, Gheorghiade M, Butler J. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail. 2015;8:438–447. doi: 10.1161/CIRCHEARTFAILURE.114.001896 [DOI] [PubMed] [Google Scholar]
- 11.Stenemo M, Nowak C, Byberg L, Sundström J, Giedraitis V, Lind L, Ingelsson E, Fall T, Ärnlöv J. Circulating proteins as predictors of incident heart failure in the elderly. Eur J Heart Fail. 2017;20:55–62. doi: 10.1002/ejhf.980 [DOI] [PubMed] [Google Scholar]
- 12.Smith JG, Gerszten RE. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation. 2017;135:1651–1664. doi: 10.1161/CIRCULATIONAHA.116.025446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, Hwang S-J, Massaro JM, Larson MG, Levy D. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc. 2018;7:e008108. doi: 10.1161/JAHA.117.008108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. doi: 10.1093/oxfordjournals.aje.a115184 [PubMed] [Google Scholar]
- 15.Jacobs L, Thijs L, Jin Y, Zannad F, Mebazaa A, Rouet P, Pinet F, Bauters C, Pieske B, Tomaschitz A, et al. Heart “omics” in AGEing (HOMAGE): design, research objectives and characteristics of the common database. J Biomed Res. 2014;28:349–359. doi: 10.7555/JBR.28.20140045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, Cesaroni G, Masson S, Cacciatore G, Colivicchi F, Uguccioni M, et al. ; PREDICTOR Study Group. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in Central Italy. Eur J Heart Fail. 2012;14:718–729. doi: 10.1093/eurjhf/hfs052 [DOI] [PubMed] [Google Scholar]
- 17.Beavers KM, Hsu FC, Houston DK, Beavers DP, Harris TB, Hue TF, Kim LJ, Koster A, Penninx BW, Simonsick EM, et al. ; Health ABC Study. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013;68:617–623. doi: 10.1093/gerona/gls213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, Ford I, Jukema JW, Hyland M, Gaw A, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999;84:1192–1197. doi: 10.1016/s0002-9149(99)00533-0 [DOI] [PubMed] [Google Scholar]
- 19.Essebag V, Genest J, Jr, Suissa S, Pilote L. The nested case-control study in cardiology. Am Heart J. 2003;146:581–590. doi: 10.1016/S0002-8703(03)00512-X [DOI] [PubMed] [Google Scholar]
- 20.Ferreira JP, Verdonschot J, Collier T, Wang P, Pizard A, Bär C, Björkman J, Boccanelli A, Butler J, Clark A, et al. Proteomic bioprofiles and mechanistic pathways of progression to heart failure. Circ Heart Fail. 2019;12:e005897. doi: 10.1161/CIRCHEARTFAILURE.118.005897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. doi: 10.1093/nar/gkr424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang W-Y, Zhang Z-Y, Latini R, Masson S, Agabiti N, Sever P, et al. ; Heart “OMics” in AGEing (HOMAGE) investigators. Risk for incident heart failure: a subject-level meta-analysis from the Heart “OMics” in AGEing (HOMAGE) study. J Am Heart Assoc. 2017;6:e005231. doi: 10.1161/JAHA.116.005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green GH, Diggle PJ. On the operational characteristics of the Benjamini and Hochberg False Discovery Rate procedure. Stat Appl Genet Mol Biol. 2007;6:Article27. doi: 10.2202/1544-6115.1302 [DOI] [PubMed] [Google Scholar]
- 25.Girerd N, Bresso E, Devignes MD, Rossignol P. Insulin-like growth factor binding protein 2: a prognostic biomarker for heart failure hardly redundant with natriuretic peptides. Int J Cardiol. 2020;300:252–254. doi: 10.1016/j.ijcard.2019.11.100 [DOI] [PubMed] [Google Scholar]
- 26.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113 [DOI] [PubMed] [Google Scholar]
- 28.Su G, Kuchinsky A, Morris JH, States DJ, Meng F. GLay: community structure analysis of biological networks. Bioinformatics. 2010;26:3135–3137. doi: 10.1093/bioinformatics/btq596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris JH, Apeltsin L, Newman AM, Baumbach J, Wittkop T, Su G, Bader GD, Ferrin TE. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinf. 2011;12:436. doi: 10.1186/1471-2105-12-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Driscoll A, Barnes EH, Blankenberg S, Colquhoun DM, Hunt D, Nestel PJ, Stewart RA, West MJ, White HD, Simes J, et al. Predictors of incident heart failure in patients after an acute coronary syndrome: the LIPID heart failure risk-prediction model. Int J Cardiol. 2017;248:361–368. doi: 10.1016/j.ijcard.2017.06.098 [DOI] [PubMed] [Google Scholar]
- 31.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from N.G. and F.Z. upon reasonable request.