Abstract
Background:
Hemodynamic-guided heart failure management is a superior strategy to prevent decompensation leading to hospitalization compared with traditional clinical methods. It remains unstudied if hemodynamic-guided care is effective across severities of comorbid renal insufficiency or if this strategy impacts renal function over time.
Methods:
In the CardioMEMS US PAS (Post-Approval Study), heart failure hospitalizations were compared from 1 year before and after pulmonary artery sensor implantation in 1200 patients with New York Heart Association class III symptoms and a previous hospitalization. Hospitalization rates were evaluated in all patients grouped into baseline estimated glomerular filtration rate (eGFR) quartiles. Chronic kidney disease progression was evaluated in patients with renal function follow-up data (n=911).
Results:
Patients with stage 2 or greater chronic kidney disease at baseline exceeded 80%. Heart failure hospitalization risk was lower in all eGFR quartiles ranging from a hazard ratio of 0.35 (0.27–0.46; P<0.0001) in patients with eGFR >65 mL/min per 1.73 m2 to 0.53 (0.45–0.62; P<0.0001) in patients with eGFR ≤37 mL/min per 1.73 m2. Renal function was preserved or improved in most patients. Survival was different between quartiles and lower in quartiles with more advanced chronic kidney disease.
Conclusions:
Hemodynamic-guided heart failure management using remotely obtained pulmonary artery pressures is associated with lower hospitalization rates and general preservation of renal function in all eGFR quartiles or chronic kidney disease stages.
Keywords: diuretics, heart failure, hemodynamics, hospitalization, renal insufficiency
What is New?
Many drugs and devices for patients with heart failure benefit those with normal or mildly impaired renal function but are either unstudied or appear to lose effectiveness with increasing severity of chronic kidney disease. The current study found that adjustments in medical therapy, particularly up and down titration of diuretics, based on ambulatory pulmonary artery pressure measurements safely and effectively reduced heart failure hospitalizations even in patients with advanced renal impairment. Benefits of hemodynamic-guided management were not associated with progressive renal dysfunction allowing personalized intravascular volume control in this complex group of patients.
What are the Clinical Implications?
Long-term volume management in patients with heart failure and comorbid renal insufficiency is a complicated balance between avoiding intravascular volume overload leading to acute decompensation while simultaneously avoiding worsening renal function. Based on the current results, remote adjustments of diuretic dosing based on daily cardiac filling pressure measurements from a permanently implanted pulmonary artery sensor was directly associated with lower decompensation events without inducing further renal injury or chronic kidney disease progression. This approach provides a novel and effective remote management solution to improve clinical outcomes in patients with heart failure and comorbid chronic kidney disease.
Management of congestion in patients with heart failure (HF) and comorbid chronic kidney disease (CKD) remains a major challenge for health care providers.1,2 Underlying CKD, which is prevalent in patients with HF, increases mortality risk and complicates volume management. Effective diuretic titration to avoid worsening congestion, without adversely impacting renal function, is essential in these patients.3,4 Previous remote HF management strategies, relying on symptom detection or other markers, have failed to consistently reduce HF hospitalization rates, in general, and many trials excluded patients with CKD.5,6 In contrast, a large body of evidence has accumulated over the past 2 decades supporting the concept that elevation of cardiac filling pressures precedes congestive symptoms by weeks and remote hemodynamic-guided HF management consistently reduces decompensation events.7–9
The CardioMEMS US PAS (Post-Approval Study)10 was a prospective internal control design open-label registry evaluating the generalizability of hemodynamic-guided care first demonstrated in the CHAMPION trial (CardioMEMS Heart Sensor Allows Monitoring of Pressures to Improve Outcomes in NYHA Class III Heart Failure Patients). Both CHAMPION and PAS included patients with CKD with estimated glomerular filtration rates (eGFR) >24 mL/min per 1.73 m2. What remains unstudied is whether the comorbidity of CKD influences the effectiveness of hemodynamic-guided HF management and whether this management strategy impacts progression of renal dysfunction over time.
Reported here are 3 retrospective analyses performed in the PAS to determine if hemodynamic-guided management reduces HF hospitalizations in patients with comorbid CKD, if hemodynamic-guided HF management worsened CKD progression and if mortality was higher in patients with more advanced CKD compared with individuals with preserved renal function.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The CardioMEMS US PAS (URL: https://www.clinicaltrials.gov; Unique identifier: NCT02279888) was a multi-center, prospective, open-label, single-arm study evaluating the use of pulmonary artery (PA) pressure-guided management in a postmarket, real-world setting.10 In brief, the study enrolled 1200 patients at 104 US clinical sites and followed patients for 2 years. Patients were included if they had persistent New York Heart Association class III symptoms and a HF hospitalization in the 12 months prior to enrollment, regardless of the underlying ejection fraction. The study was conducted according to Good Clinical Practice guidelines, and Institutional Review Board approval was obtained at each study site. All participants provided written informed consent and were followed for 24 months or until they exited the study. Follow-up visits were conducted at 1, 6, 12, 18, and 24 months post sensor implant. The PAS compared HF hospitalization rates for the 12 months before CardioMEMS implantation with hospitalization rates following implant. All hospitalizations were adjudicated by an independent Clinical Events Committee.
Patients with a glomerular filtration rate (GFR) below 25 mL/min per 1.73 m2 or requiring dialysis within 2 weeks of the baseline visit were excluded from the study. eGFR was calculated using the Modification of Diet in Renal Disease equation. Collection of eGFR, serum creatinine, and blood urea nitrogen data was required at baseline screening in order to satisfy inclusion and exclusion criteria. Collection of renal function data at follow-up visits was optional and available in 911 patients.
All patients with baseline eGFR information (n=1200) were included in the efficacy evaluation comparing HF hospitalization rates 12 months prior to implant to the following 12 months along with mortality in groups defined by eGFR quartiles using baseline renal function data. The resulting quartiles are unique to the population. All patients received maximally tolerated guideline recommended medical therapies, when appropriate, and baseline medication use is reported in those with left ventricular ejection fraction ≤40% (heart failure reduced ejection fraction) or >40% (heart failure preserved ejection fraction). At the time of this study, there were no consensus medication recommendations for patients with left ventricular ejection fraction >40% except for diuretics to control volume.
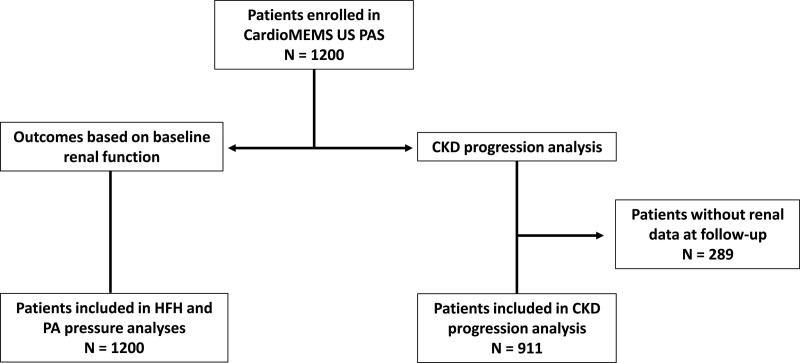
Patients with renal function information in the first 12 months of the follow-up period comprised the study cohort to examine the impact of hemodynamic-guided HF care on renal function progression (n=911). Results in these patients were examined based on the Kidney Disease Improving Global Outcomes classification of CKD stages11 in order to determine progression of renal dysfunction over time. Figure 1 illustrates flow of patients from baseline implantation to groups used to evaluate the study’s questions.
Figure 1.
Composition of cohorts used to examine heart failure outcomes and renal progression hypotheses.
Heart Failure Hospitalization Rates and Baseline Renal Function
All patients (n=1200) were stratified into quartiles using baseline eGFR as follows: eGFR >65 mL/min per 1.73 m2 (Q1), eGFR >50 to ≤65 mL/min per 1.73 m2 (Q2), eGFR >37 to ≤50 mL/min per 1.73 m2 (Q3), and eGFR ≤37 mL/min per 1.73 m2 (Q4). HF hospitalization rates, survival, diuretic changes, and PA pressure (PAP) changes were calculated for each quartile and between quartile comparisons were made. Hospitalization rates during the 12 months before enrollment were compared with the 12 months after implantation for each eGFR quartile.
Chronic Kidney Disease Progression in Patients With Hemodynamic-Guided HF Care
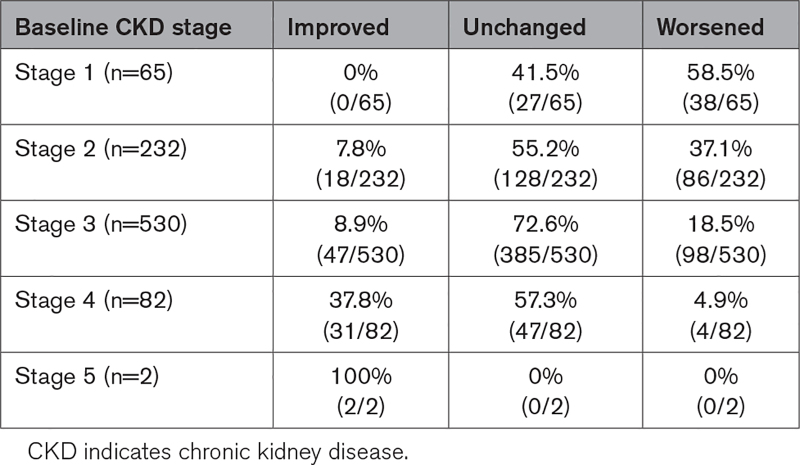
CKD stages were used to examine possible progressive renal dysfunction in patients with follow-up eGFR information (n=911; Figure 1). Patients were stratified for this analysis using the Kidney Disease Improving Global Outcomes CKD classification system11 to objectively characterize severity of renal dysfunction (eGFR, mL/min/1.73 m2): stage 1: eGFR >90, stage 2: eGFR 60 to 89, stage 3: eGFR 30 to 59, stage 4: eGFR 15 to 29, and Stage 5: <15. Renal function change was defined as improved if the CKD stage changed by at least 1 less severe level, unchanged if the stage did not change, or worsened if the CKD stage changed by at least 1 more severe level during follow-up.
Changes in PAPs and Diuretic Dosing
Changes from baseline in PA diastolic pressures over 1 year were examined in each eGFR quartile and for the entire population. Additionally, changes in PA diastolic pressures were examined in each CKD progression cohort (improved, unchanged, and worsened). Loop diuretic doses were normalized to furosemide equivalents as previously described12–14 and were compared within the CKD stage cohort at 3-month, 6-month, and 12-month timepoints.
Statistical Methods
Heart failure hospitalization (HFH) rates in the 12 months before implant were compared the 12 months after using the Andersen-Gill model to account for recurrent events, with censoring at the time of death, ventricular assist device implantation, or cardiac transplantation. Annualized HF hospitalization rates and mortality were compared between each GFR quartile (n=1200). Crude 12-month mortality rates were estimated using Kaplan-Meier survival analysis and stratified by GFR quartiles. Changes in PAPs and change in renal function over time were compared in the improved, unchanged, and worsened groups using a χ2 test of independence to assess for an association between variables. All P values were generated using t tests for continuous variables and χ2 tests for categorical variables.
Results
Baseline Demographics
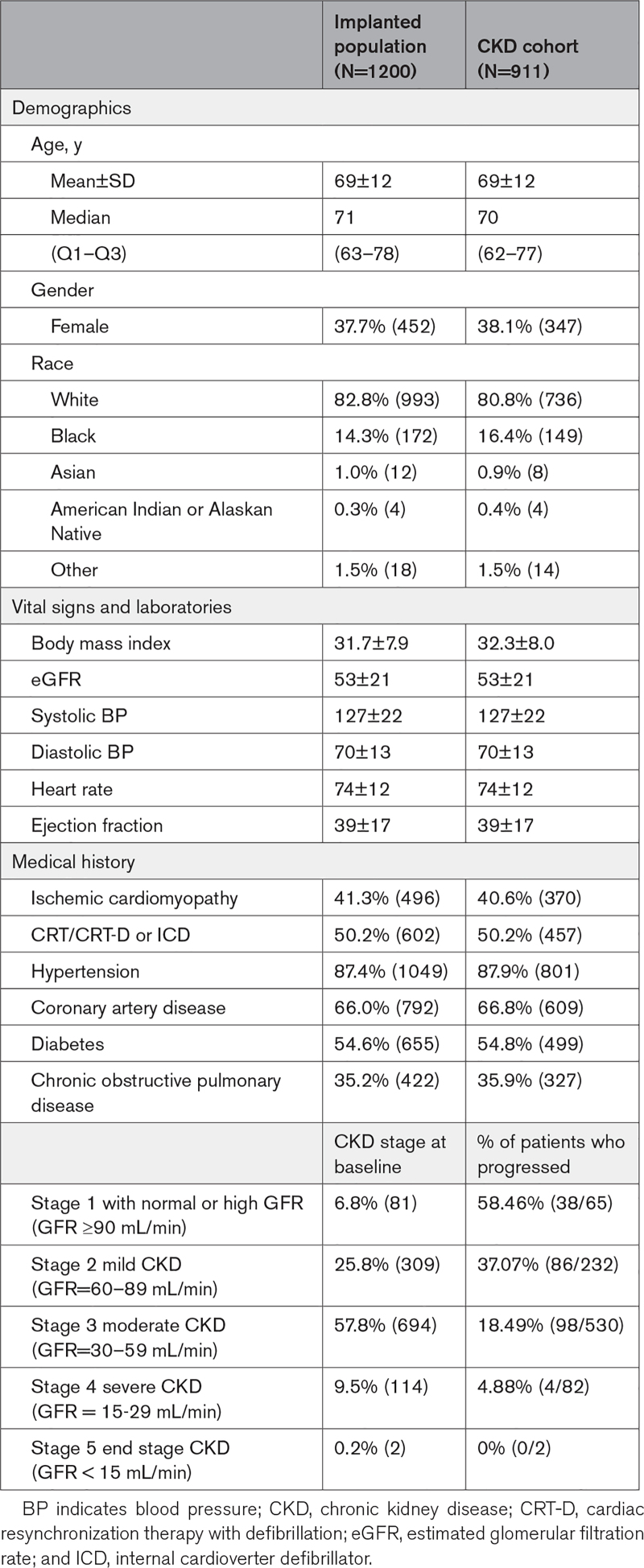
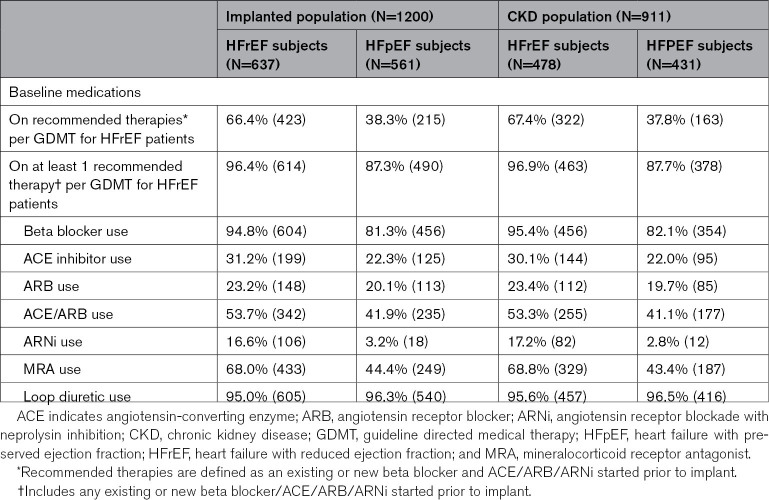
A full description of the baseline demographics of the CardioMEMS US PAS cohort is published.7 As shown in Table 1, 6.8% of patients had normal baseline eGFR (≥90 mL/min per 1.73 m2), while 25.8% had stage 2 CKD, 57.8% with stage 3 CKD, and 9.5% with Stage 4 CKD. Table 2 lists the medication use at enrollment of the entire cohort with baseline eGFR information (n=1200) and the subgroup with follow-up renal function data (n=911) separated by ejection fraction. Ninety-five percent of patients with heart failure reduced ejection fraction in the CKD subgroup received beta-blocker therapy at enrollment and 82% of patients with heart failure preserved ejection fraction were on beta-blockers. Angiotensin intervention was used in 54% of the entire heart failure reduced ejection fraction population and similar use in patients with follow-up renal function data. Sacubitril/valsartan use was low as clinical availability of this drug was introduced during the PAS. Finally, mineralocorticoid antagonism was used in 68% of the patients enrolled. The lower use of angiotensin intervention may relate to the observation that 93% of the enrolled subjects had CKD stage 2 or greater CKD at baseline (Table 1). The protocol did not enforce compliance to guideline medical therapy recommendations for patients with diabetes, thus the low utilization of renin-angiotensin-aldosterone system antagonists likely represents usual care for patients with combined heart failure, diabetes, and renal insufficiency. All guideline directed medical therapies for patients with heart failure reduced ejection fraction were required to qualify for the CardioMEMS US PAS, unless the investigator provided evidence of intolerance to omitted medications.
Table 1.
Subject Demographics for the Chronic Kidney Disease Progression Cohort
Table 2.
Baseline Medication Use
Two hundred eighty-nine (289) patients had no follow-up renal function data leaving 911 subjects available for the renal progression portion of this study (Figure 1). Patients who did not have follow-up renal function data (n=289) were slightly older (71 versus 69 in CKD cohort; P=0.003), were more likely to be White (89% versus 81% in CKD cohort; P<0.001), and had a lower body mass index (30 versus 32 in CKD cohort; P<0.0001) than patients in the CKD cohort. Demographics of the patients available for the renal progression study are noted in Table 1. Of note, 55% of subjects in the progression cohort had diabetes and 88% had hypertension.
Clinical Outcomes of Hemodynamic-Guided Care in Patients With CKD
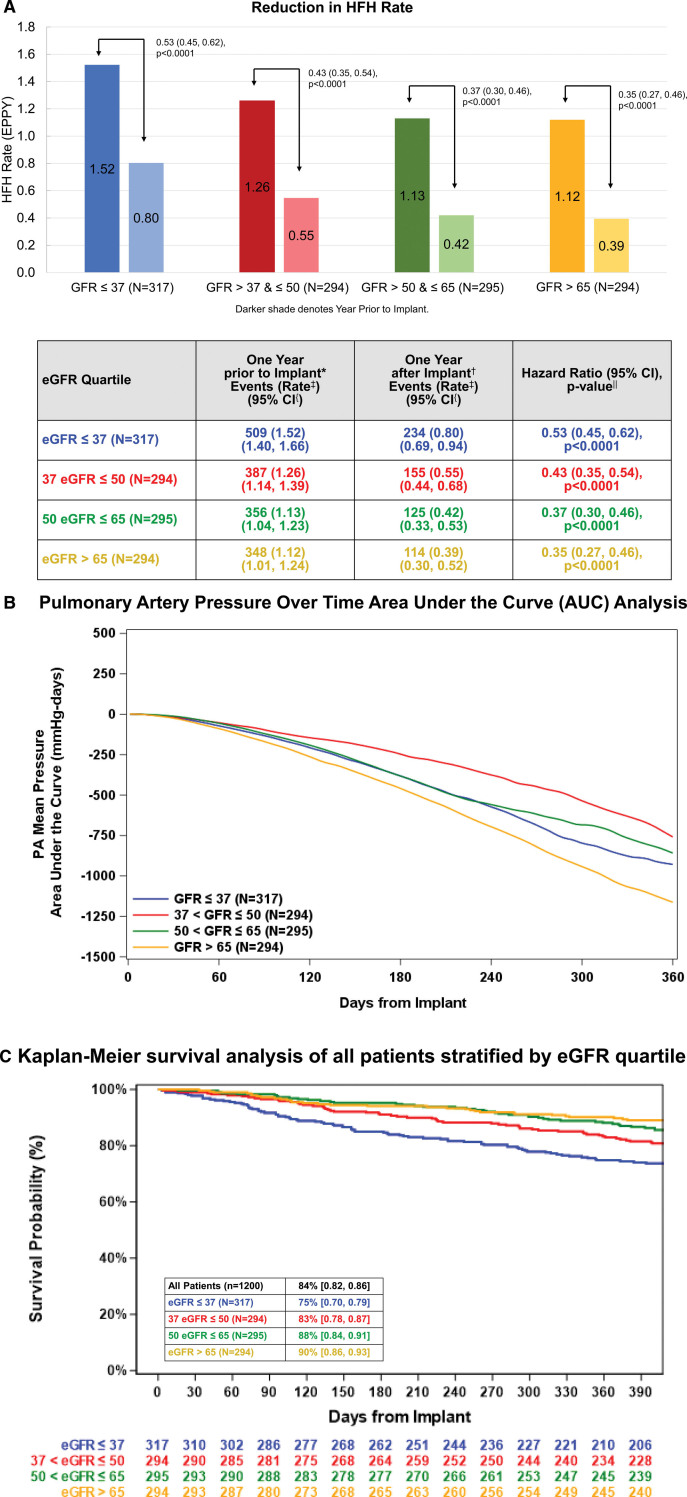
HFH rates were lower in the 12 months following sensor implant compared with 12 months before enrollment in each of the eGFR quartiles (Figure 2A). Lower HF hospitalization rates were associated with lower PAPs over time, measured as an area under the curve analysis (Figure 2B). PAPs were lower after 12 months of hemodynamic-guided care in all eGFR quartiles. Overall, 12-month survival was 84% (95% CI, 0.82–0.86). One-year survival was lowest at 75% (95% CI, 0.70–0.79) in the lowest eGFR quartile and highest at 90% (95% CI, 0.86–0.93) in patients with eGFR >65 at baseline (Figure 2C).
Figure 2.
Outcomes across eGFR quartiles. A, Heart failure hospitalization (HFH) rates 12 months before implantation compared with 12 months after implantation based on estimated glomerular filtration rate (eGFR) quartiles (n=1200). Change in pulmonary artery (PA) pressures over time using an area under the curve analysis, based on eGFR quartiles in the entire population of 1200 enrolled patients (B). C, Depicts Kaplan-Meier survival analysis of all patients stratified by eGFR quartile at baseline. Kaplan-Meier survival expressed as % (95% CI). EPPY indicates events per patient year. *Includes all Clinical Events Committee (CEC) adjudicated heart failure hospitalizations with an admission date on the date of implant and through 390 days prior to the date of implant. †Includes all CEC adjudicated heart failure hospitalizations with an admission date after the implant procedure discharge date through 390 days after the date of implant. ‡Hospitalization rate is an annualized rate estimated from the Andersen-Gill model. {95% CI on annualized hospitalization rate from the Andersen-Gill model. ‖Hazard ratio, 95% CI, and P value estimated from the Andersen-Gill model with robust sandwich estimates.
CKD Stage Progression
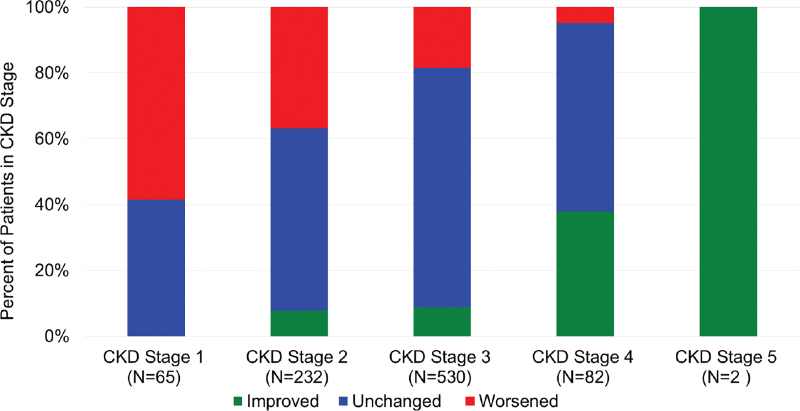
Two hundred twenty-six (25%) of the renal progression cohort worsened at least 1 CKD stage in the 12-month follow-up of the study (Table 3; Figure 3). Renal function progression occurred mostly in patients with stage 1 or 2 CKD at baseline (Figure 3). Patients with stage 3 to 5 tended to remain unchanged, but the most patients with renal function improvement started the study with CKD stage 3 or 4 disease (78 of 96 patients with improved CKD, 81%). Of the remaining patients in this cohort, 64% had no underlying renal function change and 11% improved at least 1 CKD stage. No patient developed end-stage renal disease or required dialysis during follow-up. χ2 test of independence found no evidence of a relationship between a CKD stage progression and change in PAPs over time.
Table 3.
Percentage and Numbers of Patients With Improved CKD Stage Defined as Movement of at Least One Level Lower Over Time, Unchanged and Worsened Renal Function (at Least One Level Higher Stage Over Time)
Figure 3.
Chronic kidney disease (CKD) stage change over the 12-month follow-up period in patients based on their baseline CKD stage. Improvement was defined as a change of at least on stage lower than baseline and worsened was defined as a change that was at least 1 stage higher than baseline.
Medication Changes
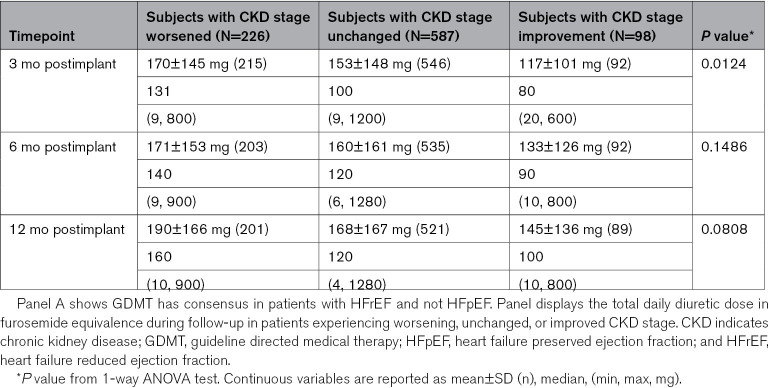
Diuretic dosing was the most frequently changed medical intervention in the study, consistent with previous trials examining hemodynamic-guided HF management. Both up and down titration of loop and thiazide diuretics occurred in ≈80% of population. Patients with CKD worsening of at least 1 stage were prescribed higher daily diuretic doses after 3 months persisting to the 12-month follow-up (Table 4). Furosemide equivalent daily dosing at 12 months in the subjects experiencing renal function worsening averaged 190±166 mg with a range of 10 to 900 mg daily. This dose was higher than those with unchanged CKD stage (168±167 mg, 4–1280 mg range) and those with improved CKD stage (145±136 mg, 10–800 mg range). Changes in nitrates and hydralazine were more common in patients with higher CKD stages and, conversely, the percentage of patients with changes in angiotensin intervention, including ACE (angiotensin-converting enzyme) inhibitor, angiotensin receptor blocker, or ARNi (angiotensin receptor blockade with neprolysin inhibition) dosing was highest in stage 2 CKD compared with stage 3 or 4. Rates of changes in GDMT overall were small likely due to the inclusion criteria requiring patients to already be on maximally tolerated doses.
Table 4.
Medication Use at Baseline and Diuretic Changes Throughout 12 Months of Study Follow-Up
Discussion
These retrospective subgroup analyses from the CardioMEMS US PAS address important clinical questions concerning the safety and clinical impact of hemodynamic-guided HF management in patients with comorbid chronic kidney disease. Importantly, 85% of patients enrolled in this study had CKD stage 2 or greater at baseline, underscoring the prevalence of comorbid kidney disease in patients with symptomatic heart failure.15 Patients with all stages of CKD experienced less HF hospitalizations in the 12 months following PA sensor implant compared with the 12 months before implant, which was associated with a significant lowering of PAPs. The observed reduction in HF hospitalization rate was similar to the entire cohort and with other internal control studies recently published. Lower hospitalization rates were seen across all quartiles of GFR including a 47% reduction within the lowest GFR quartile patients. These findings support the contention that hemodynamic monitoring remains effective in patients with comorbid CKD.
Renal function remained stable in most patients in this study, even in patients with stage 5 CKD. Patients experiencing worsening of CKD stage tended to be in stage 1 or 2 at baseline and most individuals with improved CKD status started the study in stage 3 or 4 disease. No patient required dialysis or advanced to end-stage renal disease during the 12-month follow-up period analyzed for this examination. The findings of this study support the hypothesis that hemodynamic-guided care in patients with CKD effectively lowers hospitalization rates without worsening renal function over time. Interestingly, patients who worsened from CKD stage 1 or 2 were prescribed higher daily diuretic dosing compared with the improved or unchanged group. HFH rates and PAPs were lower in this group suggesting that restoring optimal volume guided by PAPs may have unmasked the true degree of renal dysfunction in this population. This speculation cannot be evaluated in the current data set as renal function evaluation shortly after sensor implantation was not required and is not available.
Sustained PA reduction occurred across all quartiles of GFR in this analysis, but pressures and renal function changes were independent variables. A growing body of evidence supports the contention that higher PAPs are associated with increased risk of HFH events and strategies to reduce PAP to goal targets can effectively improve HF outcomes.16 Diuretics remain the cornerstone of therapy to improve congestion and lower pulmonary pressures in chronic HF patients, and yet commonly these therapies are decreased or under-utilized in patients with concomitant CKD. Despite data suggesting that increases in serum creatinine occur more frequently in patients receiving high dose diuretics, there is significant evidence that this does not necessarily portend worsened outcomes.17,18 In fact, persistent clinical or subclinical congestion remains a strong predictor of poor outcomes including HFH, particularly in patients with worsening renal function.18–20 Identifying subclinical congestion in these patients remains difficult, as significant discrepancy exists between standard assessment such as clinical weights with that of true euvolemia and fluid balance.21 Therefore, a strategy to guide diuretic adjustments using PAP sensor data, with focused changes particularly during early detection of pulmonary pressures outside of goal range, continues to be mechanistically sound, and notably safe in patients with combined CKD and HF.
Survival was lower in patients with increasing CKD stage, consistent with previously reported epidemiologic data from patients with HF and CKD. Both all-cause mortality and cardiovascular mortality were higher with lower levels of eGFR.11 Large meta-analyses have shown that up to 55% of patients with HF may have CKD stage 3 or higher, which was confirmed in this population of New York Heart Association class III previously hospitalized patients.22 Strategies to modify risk of death in patients with combined HF and advanced CKD remain limited. Further insight is needed to ascertain whether earlier intervention with PAP sensor guided HF treatment can impact survival in this population.
Limitations
The observations reported here must be interpreted within the limitations of the current study design. All prospective, historical control, open-label studies have the potential to overestimate effectiveness. This may arise due a change in non-PAP related disease management at enrollment, inaccurate assessment of preenrollment HF hospitalizations and the lack of a concomitant control group to which outcomes can be compared. Therefore, the exact magnitude of HF hospitalization reduction must be interpreted with caution. Prospective historical control studies are useful to understand potential generalizability of a clinical innovation proved effective in a strict, placebo-controlled trial. Hospitalization reductions are consistently seen in randomized controlled trials and real-world registries, which supports generalizability of this management strategy.
In addition, a relatively low percentage of patients in this study were prescribed renin-angiotensin-aldosterone system antagonists, especially in light of the prevalence of comorbid diabetes (>50%). It is possible that mortality rates may have been influenced by low renin-angiotensin-aldosterone system antagonist use.
Conclusions
In summary, hemodynamic-guided HF management in patients with concomitant CKD was associated with lower 12-month hospitalization rates compared with the 12 months prior to enrollment in all eGFR quartiles. These beneficial HF outcomes were temporally associated with reduced PAPs over time, which was predominately achieved by increasing doses of loop diuretics. Renal function remained relatively stable in most patients and worsened in those with baseline CKD stage 1 or 2. Patients with the most advanced CKD tended to have the most opportunity for renal function improvement over time. Hemodynamic-guided HF management remains effective and safe in patients with comorbid CKD.
Article Information
Sources of Funding
The United States CardioMEMS Post-Approval Study (NCT02279888) was funded by Abbott.
Disclosures
Dr Raval received research support from Abbott. Dr Valika received research support from Abbott. C. Williams is an Abbott employee. Dr Brett is an Abbott employee; Dr Adamson is an Abbott employee and shareholder. Dr Costanzo received research support from Abbott.
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- ARNi
- angiontensin receptor neprilysin inhibitor
- CHAMPION
- CardioMEMS Heart Sensor Allows Monitoring of Pressures to Improve Outcomes in NYHA Class III Heart Failure Patients
- CKD
- chronic kidney disease
- eGFR
- estimated glomerular filtration rate
- GDMT
- guideline-directed medical therapy
- HF
- heart failure
- HFH
- heart failure hospitalization
- PA
- pulmonary artery
- PAS
- Post-Approval Study
For Sources of Funding and Disclosures, see page 411.
Contributor Information
Ali Valika, Email: ali.valika@aah.org.
Philip B. Adamson, Email: philip.adamson@abbott.com.
Christopher Williams, Email: philip.adamson@abbott.com.
Marie-Elena Brett, Email: marieelena.brett@abbott.com.
Maria Rosa Costanzo, Email: maria_rosa_costanzo@msn.com.
References
- 1.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4:387–399. doi: 10.1016/j.hfc.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J; ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 3.Al-Naher A, Wright D, Devonald MAJ, Pirmohamed M. Renal function monitoring in heart failure - what is the optimal frequency? A narrative review. Br J Clin Pharmacol. 2018;84:5–17. doi: 10.1111/bcp.13434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the medicare population. J Card Fail. 2004;10:467–472. doi: 10.1016/j.cardfail.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, Marco T, Escarce JJ, Evangelista LS, Hanna B, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition -- Heart Failure (BEAT-HF) randomized clinical trial. JAMA Internal Medicine. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, et al. ; DOT-HF Investigators. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042 [DOI] [PubMed] [Google Scholar]
- 7.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Abraham WT, Smart FW, Stevnson LW, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910 [DOI] [PubMed] [Google Scholar]
- 8.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, et al. ; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 9.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0 [DOI] [PubMed] [Google Scholar]
- 10.Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, et al. ; CardioMEMS Post-Approval Study Investigators. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS post-approval study. Circ Heart Fail. 2020;13:e006863. doi: 10.1161/CIRCHEARTFAILURE.119.006863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt K-U. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 12.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WHW. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–270. doi: 10.1161/CIRCHEARTFAILURE.113.000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–189. doi: 10.1038/ki.1984.153 [DOI] [PubMed] [Google Scholar]
- 14.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:60190222–60190609. doi: 10.1016/0009-9236(95)90222-8 [DOI] [PubMed] [Google Scholar]
- 15.Costanzo MR. The cardiorenal syndrome in heart failure. Heart Fail Clin. 2020;16:81–97. doi: 10.1016/j.hfc.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Stevenson LW, Zile M, Bennett TD, Kueffer FJ, Jessup ML, Adamson P, Abraham WT, Manda V, Bourge RC. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail. 2010;3:580–587. doi: 10.1161/CIRCHEARTFAILURE.109.923300 [DOI] [PubMed] [Google Scholar]
- 17.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WHW, Testani JM. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. 2016;22:753–760. doi: 10.1016/j.cardfail.2016.06.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413 [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;258:185–191. doi: 10.1016/j.ijcard.2018.01.067 [DOI] [PubMed] [Google Scholar]
- 21.Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, Coca SG, Tang WHW. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med. 2015;128:776–83.e4. doi: 10.1016/j.amjmed.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A, Gotsman I, Whalley G, Earle Poppe KK, et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new chronic kidney disease-epidemiology collaboration group formula. Circ Heart Fail. 2012;5:309–314. doi: 10.1161/CIRCHEARTFAILURE.111.966242 [DOI] [PubMed] [Google Scholar]