Abstract
In this study, a method to both qualitatively and quantitively analyze the components of Oryeong-san (ORS), which is composed of five herbal medicines (Alisma orientale Juzepzuk, Polyporus umbellatus Fries, Atractylodes japonica Koidzumi, Poria cocos Wolf, and Cinnamomum cassia Presl) and is prescribed in traditional Oriental medicine practices, was established for the first time. First, ORS components were profiled using ultra-high-performance liquid chromatography/quadrupole Orbitrap mass spectrometry, and 19 compounds were clearly identified via comparison against reference standard compounds. Subsequently, a quantitative method based on ultra-high-performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry was established to simultaneously measure the identified compounds. Nineteen compounds were accurately quantified using the multiple-reaction-monitoring mode and used to analyze the sample; we confirmed that coumarin was the most abundant compound. The method was validated, achieving good linearity (R2 ≤ 0.9991), recovery (RSD, 0.11–3.15%), and precision (RSD, 0.35–9.44%). The results suggest that this method offers a strategy for accurately and effectively determining the components of ORS, and it can be used for quality assessment and management.
Keywords: Oryeong-san, UHPLC-Q-Orbitrap-MS, UPLC-TQ-MS/MS, quality control
1. Introduction
Oryeong-san (ORS; also known as Wulingsan in China and Gorei-san in Japan) is a traditional Korean prescription manufactured in Sanghanron; it is composed of five herbal medicines (Alisma orientale Juzepzuk, Polyporus umbellatus Fries, Atractylodes japonica Koidzumi, Poria cocos Wolf, and Cinnamomum cassia Presl at the ratio 5:3:3:3:2) [1,2]. ORS is prescribed to promote diuresis, reduce edema, and improve water metabolism in the body [3,4]. In addition, it is widely used in the treatment of renal diseases, and has been reported to improve renal functioning through antihypertensive and antidiabetic effects [5,6,7]. Clinical studies have also reported that ORS is effective in preventing calcium oxalate nephrolithiasis [8]. In addition, ORS was found to reduce gastrointestinal adverse reactions in patients taking selective serotonin reuptake inhibitors (SSRIs), and it was proven to reduce hematoma in patients with chronic subdural hematoma (CSDH) [9,10]. Traditional Oriental medicines (TOMs), which include prescriptions such as ORS, are widely used to prevent diseases, owing to their efficacy and low toxicity [11]. When evaluating the quality of TOMs, the components of various herbal medicines are selected as evaluation indicators; however, these approaches primarily focus on individual herbal medicines. If prescriptions are composed of two or more herbal medicines, their chemical properties may differ from those of a single herbal medicine; this makes it difficult to accurately reflect their characteristics in prescription quality evaluations [12,13,14].
According to Korean Pharmacopoeia [15], the quality control of each herbal medicine and ORS except C. cassia is mainly managed by thin-layer chromatographic analysis. The five individual herbal medicine components in ORS have been reported as follows: triterpenoids (e.g., alisol A) from A. orientale [16], steroids (e.g., polyporusterone A) from P. umbellatus [17], sesquiterpenoids (e.g., atractyloside A) from A. japonica [18], triterpenoids (e.g., 16α-hydroxytrametenolic acid) from P. cocos [19], coumarins (e.g., coumarin), and flavonoids (e.g., procyanidin B1) from C. cassia [20]. In addition, studies have simultaneously determined the components of ORS using high-performance liquid chromatography and liquid chromatography–mass spectrometry (LC-MS) for ORS quality control [1,21,22]. However, these studies are limited to the quantitative analysis of several major components or the screening-based qualitative analysis of all components. Therefore, it is necessary to establish an appropriate analytical method that can simultaneously identify components and determine their contents to facilitate accurate quality control of the ORS.
LC-MS methods are widely used to identify and characterize the chemical compositions of various TOMs, as well as their related preparations [23,24,25]. These methods can facilitate comprehensive chemical profiling; in particular, mass spectrometry using an Orbitrap analyzer offers ion information with low mass errors, thereby facilitating rapid component identification [14]. In addition, triple-quadrupole mass spectrometry (TQ-MS/MS) using multiple-reaction monitoring (MRM) is a highly sensitive and powerful quantitative method offering high throughput [26,27].
Therefore, in this study, an analysis method based upon ultra-high-performance liquid chromatography/quadrupole Orbitrap mass spectrometry (UHPLC-Q-Orbitrap-MS) was established to identify the components of ORS, and 19 compounds were identified. For the simultaneous quantitative analysis of the identified compounds, an ultra-performance liquid chromatography coupled with a triple-quadrupole tandem mass spectrometry (UPLC-TQ-MS/MS) analysis method using the MRM mode was established and applied for content analysis.
2. Results and Discussion
2.1. Identification of Compounds in ORS via UHPLC-Q-Orbitrap-MS
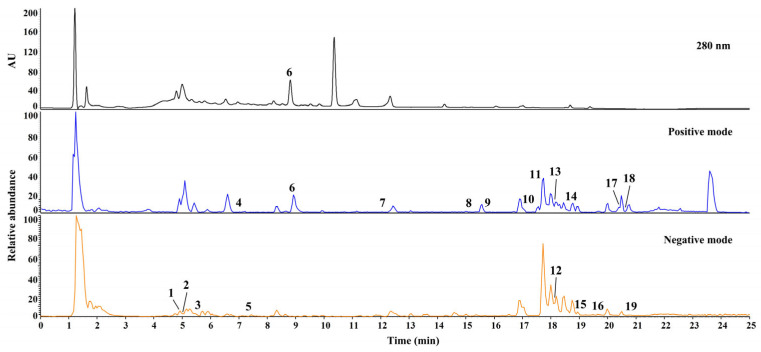
Qualitative analysis was performed to identify the chemical compounds in ORS; to this end, UHPLC-Q-Orbitrap-MS was used. The chemical compounds in ORS were identified by comparing the retention times and mass spectra of the reference standard compounds, and 19 compounds were identified. The base peak chromatograms of the ORS in both positive and negative ion modes are shown in Figure 1, and detailed information regarding the identified compounds is listed in Table 1. Injection peak was found around 1.5 min, and this peak may be composed of the various eluents of sugars, amino acids, and so on (Figure 1). The apparent peak at 10.4 min, which were only found in UV chromatogram, was not sufficient to identify a certain phytochemical with low intensity and less MS2 fragmentation pattern (Figure 1, upper panel). In this regard, the quantitative analysis of ORS was performed except for the above-mentioned peaks.
Figure 1.
UHPLC-Q-Orbitrap-MS base peak ion chromatograms of ORS extract. The ID numbers of the various types of phytochemicals are listed in Table 1.
Table 1.
Characterization of chemical constituents of ORS using UHPLC-Q-Orbitrap-MS.
| No. | Identification | Rt (min) |
Formula | Adduct | Predicted (m/z) |
Measured (m/z) |
Error (ppm) |
MS/MS (m/z) |
|---|---|---|---|---|---|---|---|---|
| 1 | Atractyloside A | 4.95 | C21H36O10 | [M + HCO2]− | 493.2290 | 493.2293 | −0.02 | 447.2245, 285.1714, 89.0229 |
| 2 | Procyanidin B1 | 5.05 | C30H26O12 | [M − H]− | 577.1351 | 577.1352 | −0.34 | 407.0771, 289.0720 125.0230 |
| 3 | Procyanidin B2 | 5.62 | C30H26O12 | [M − H]− | 577.1351 | 577.1351 | −0.44 | 407.0774, 289.0721 125.0231 |
| 4 | Umbelliferone | 7.12 | C9H6O3 | [M + H]+ | 163.0390 | 163.0388 | −0.84 | 163.0388 |
| 5 | Rosavin | 7.40 | C20H28O10 | [M + HCO2]− | 473.1664 | 473.1661 | −1.23 | 293.0878, 89.0228 |
| 6 | Coumarin | 9.09 | C9H6O2 | [M + H]+ | 147.0441 | 147.0440 | −0.44 | 147.0439, 103.0546 |
| 7 | Polyporusterone A | 12.02 | C28H46O6 | [M + H]+ | 479.3365 | 479.3365 | −0.40 | 95.0861 |
| 8 | Alisol C | 15.16 | C30H46O5 | [M + H]+ | 487.3418 | 487.3417 | −0.18 | 415.2840 |
| 9 | Atractylenolide III | 15.79 | C15H20O3 | [M + H]+ | 249.1485 | 249.1483 | −0.88 | 231.1379, 163.0753 |
| 10 | Alisol C 23-acetate | 17.07 | C32H48O6 | [M + H]+ | 529.3524 | 529.3526 | 0.44 | 451.3205 |
| 11 | Atractylenolide II | 17.69 | C15H20O2 | [M + H]+ | 233.1536 | 233.1535 | −0.57 | 233.1535, 215.1431 187.1481, 151.0754 |
| 12 | Alisol A | 18.12 | C30H50O5 | [M + HCO2]− | 535.3640 | 535.3643 | −0.01 | 471.3499 |
| 13 | 16α-Hydroxytrametenolic acid | 18.13 | C30H48O4 | [M + H]+ | 473.3625 | 473.3625 | −0.03 | 437.3433, 295.2415 |
| 14 | Atractylenolide I | 18.79 | C15H18O2 | [M + H]+ | 231.1380 | 231.1379 | −0.27 | 231.1379 |
| 15 | Alisol A 24-acetate | 18.93 | C32H52O6 | [M + HCO2]− | 577.3746 | 577.3748 | −0.02 | 169.0408, 59.0122 |
| 16 | Alisol B | 19.82 | C30H48O4 | [M + HCO2]− | 517.3535 | 517.3535 | −0.48 | 241.4872, 100.0714 |
| 17 | 3-O-Acetyl-16α-hydroxytrametenolic acid | 20.42 | C32H50O5 | [M + H]+ | 515.3731 | 515.3730 | −0.09 | 437.3416, 295.2421 133.0860, 89.0603 |
| 18 | Alisol B 23-acetate | 20.62 | C32H50O5 | [M + H]+ | 515.3731 | 515.3729 | −0.33 | 339.2672, 151.1116 97.0653 |
| 19 | Pachymic acid | 20.82 | C33H52O5 | [M − H]− | 527.3742 | 527.3740 | −0.90 | 527.3741 |
Among the chemical compounds identified in ORS, 16 compounds were identical to those reported in previous studies; these are as follows: alisol A, alisol A 24-acetate, alisol B, alisol B 23-acetate, alisol C, and alisol C 23-acetate from A. orientale; polyporusterone A from P. umbellatus; atractyloside A, atractylenolide Ⅰ, atractylenolide Ⅱ, and atractylenolide Ⅲ from A. japonica; 16α-hydroxytrametenolic acid, 3-O-acetyl-16α- hydroxytrametenolic acid, and pachymic acid from P. cocos; and procyanidin B2 and coumarin from C. cassia [5,22,28]. As such, some studies on the pharmacological activities of the compounds identified in ORS have been reported. It has been reported that procyanidin B1, procyanidin B2, and rosavin have antioxidant activities, and coumarin and polyporusterone A have various activities, such as anti-inflammatory, antioxidant, and anticancer activities [17,29,30,31]. Atractyloside A, a compound of A. japonica, and other compounds were also found to have anti-inflammatory activity [18]. In addition, studies have reported that pachymic acid and other compounds that are components of P. cocos, and alisol A and other compounds of A. orientale, have anti-inflammatory and anticancer effects [32,33].
2.2. Quantitative Analysis of Compounds in ORS Using UPLC-TQ-MS/MS
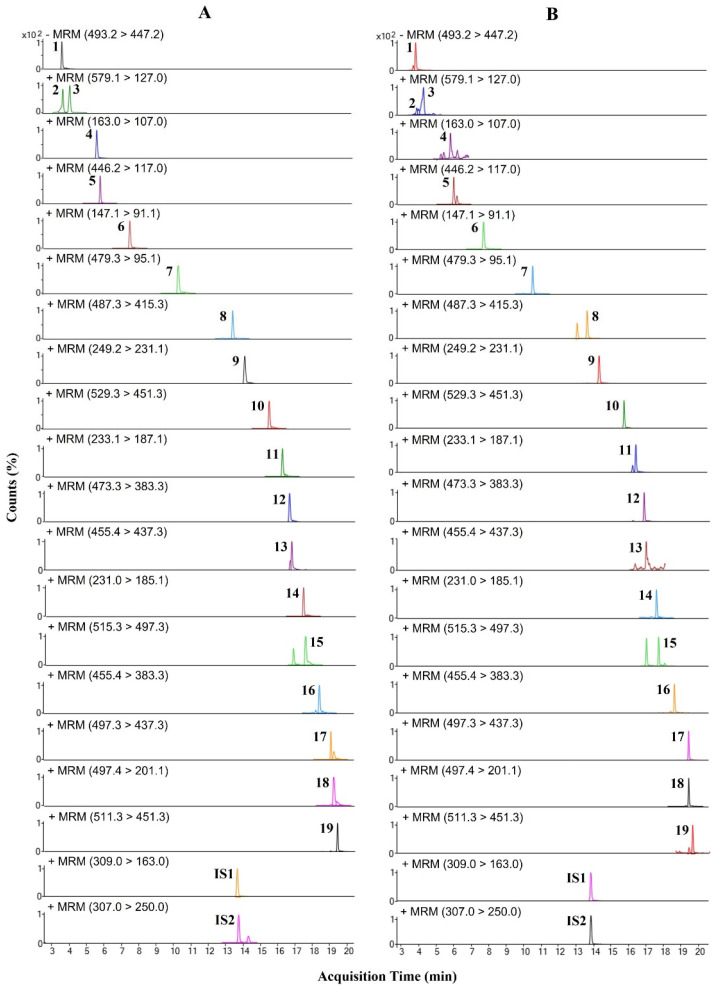
The UPLC-TQ-MS/MS method (in the MRM mode) was used to quantify the compounds in ORS, and 19 compounds were simultaneously detected within 20 min. The MRM mode allows for highly specific and sensitive analyses [34]. A single standard solution of each analyte was injected to investigate the ion pairs (consisting of precursor and product ions in both positive and negative ion modes). Atractyloside A was determined in the negative ion mode, and all remaining compounds were determined in the positive ion mode. For all analytes (including the internal standard, IS), the MRM parameters (including the selected MRM pairs and collision energies) were optimized (the details are listed in Table 2). The chromatograms of the 19 compounds and IS, as obtained in the MRM mode, are shown in Figure 2.
Table 2.
Optimized MRM parameters of the 19 compounds in the UPLC-TQ-MS/MS.
| No. | Compound | Rt (min) |
MW | MRM Transition (m/z) |
Collision Energy (V) |
|---|---|---|---|---|---|
| 1 | Atractyloside A | 3.63 | 448.5 | 493.2 → 447.2 | 14 |
| 2 | Procyanidin B1 | 3.68 | 578.5 | 579.1 → 127.0 | 30 |
| 3 | Procyanidin B2 | 4.09 | 578.5 | 579.1 → 127.0 | 30 |
| 4 | Umbelliferone | 5.67 | 162.1 | 163.0 → 107.0 | 22 |
| 5 | Rosavin | 5.84 | 428.4 | 446.2 → 117.0 | 14 |
| 6 | Coumarin | 7.55 | 146.1 | 147.1 → 91.1 | 26 |
| 7 | Polyporusterone A | 10.34 | 478.7 | 479.3 → 95.1 | 30 |
| 8 | Alisol C | 13.48 | 486.7 | 487.3 → 415.3 | 18 |
| 9 | Atractylenolide III | 14.18 | 248.3 | 249.2 → 231.1 | 10 |
| 10 | Alisol C 23-acetate | 15.59 | 528.7 | 529.3 → 451.3 | 18 |
| 11 | Atractylenolide II | 16.28 | 232.3 | 233.1 → 187.1 | 14 |
| 12 | Alisol A | 16.62 | 490.7 | 473.3 → 383.3 | 10 |
| 13 | 16α-Hydroxytrametenolic acid | 16.71 | 472.7 | 455.4 → 437.3 | 18 |
| 14 | Atractylenolide I | 17.40 | 230.3 | 231.0 → 185.1 | 18 |
| 15 | Alisol A 24-acetate | 17.50 | 532.8 | 515.3 → 497.3 | 10 |
| 16 | Alisol B | 18.41 | 472.7 | 455.4 → 383.3 | 10 |
| 17 | 3-O-Acetyl-16α-hydroxytrametenolic acid | 19.06 | 514.7 | 497.3 → 437.3 | 18 |
| 18 | Alisol B 23-acetate | 19.24 | 514.7 | 497.4 → 201.1 | 22 |
| 19 | Pachymic acid | 19.45 | 528.8 | 511.3 → 451.3 | 18 |
| IS1 | Warfarin | 13.64 | 307.1 | 309.0 → 163.0 | 14 |
| IS2 | Warfarin | 13.64 | 307.1 | 307.0 → 250.0 | 22 |
Figure 2.
UPLC-TQ-MS/MS chromatograms in MRM mode for (A) mixed-reference solution and (B) ORS extract.
The same precursor ion (m/z 579.1) was selected for procyanidins B1 and B2, and a characteristic fragment ion was produced at m/z 127.0; this was selected as the product ion for each compound [35]. Umbelliferone and coumarin generated precursor ions in the form of [M + H]+ at m/z 163.0 and m/z 147.1, respectively; both compounds formed product ions in the form of [M + H − 2CO]+. Umbelliferone formed a product ion at m/z 107.0, and coumarin formed a product ion at m/z 91.1 as a result of the loss of both CO2 (m/z 44) and C (m/z 12) [36,37,38]. The precursor ions of alisol A and alisol A 24-acetate were formed at m/z 473.3 and m/z 515.3, respectively, in the form of [M + H − H2O]+; however, in the case of the product ion, alisol A was formed at m/z 383.3 in the form of [M + H − H2O − C4H10O2]+, and alisol A 24-acetate was formed at m/z 497.3 in the form of [M + H − 2H2O]+. Similarly, alisol B produced a precursor ion at m/z 455.4 in the form of [M + H − H2O]+ and a product ion at m/z 383.3 in the form of [M + H − H2O − C4H8O]+. In addition, the precursor ions of alisol C and alisol C 23-acetate were produced in the form of [M + H]+ at m/z 487.3 and m/z 529.3, respectively, and alisol C formed a product ion in the form of [M + H − C4H8O]+ at m/z 415.3. For alisol C 23-acetate, the loss of HAc at C-23 and the loss of H2O (18 Da) occurred simultaneously to generate a product ion in the form of [M + H − Hac − H2O]+ at m/z 451.3 [39,40]. The lactone components, atractylenolide I, II, and III, produced precursor ions in the form of [M + H]+ at m/z 231.0, m/z 233.1, and m/z 249.2, respectively. Atractylenolide I and atractylenolide II produced product ions in the form of [M + H − H2O − CO]+ at m/z 185.1 and m/z 187.1, respectively, via the simultaneous loss of H2O and CO groups. Unlike the other two compounds, atractylenolide III formed a product ion in the form of [M + H − H2O]+ at m/z 231.1, owing to the loss of H2O molecules [41,42]. 16α-hydroxytrametenolic acid and 3-O-acetyl-16α-hydroxytrametenolic acid generated precursor ions at m/z 455.4 and m/z 497.3, respectively, in the form of [M + H − H2O]+; both compounds formed product ions at m/z 437.3: 16α-hydroxytrametenolic acid in the form of [M + H − 2H2O]+ and 3-O-acetyl-16α-hydroxytrametenolic acid in the form of [M + H − H2O − CH3COOH]+, respectively [43]. Similarly, pachymic acid generated a precursor ion in the form of [M + H − 2H2O]+ at m/z 511.3 and then formed a product ion in the form of [M + H − H2O − CH3COOH]+ at m/z 451.3, owing to the loss of the AcOH moiety [44].
2.3. Method Validation
Calibration curves for all analytes were plotted against the concentrations of the standard solutions, and the linearity of the analytical method was evaluated using the correlation coefficient of each calibration curve. The correlation coefficient of the calibration curve exhibited good linearity (>0.9991) within the test range. The lower limit of quantitation (LLOQ) was within the range of 0.01–0.20 ng/mL for all compounds. Therefore, it was confirmed that the established method provides a sensitive quantitative analysis of ORS compounds. Detailed information for the regression equation, correlation coefficient (R2), linear range, and LLOQ is listed in Table 3.
Table 3.
Calibration curves, linear range, and lower limit of quantification (LLOQ) for the 19 compounds.
| No. | Compound | Calibration Curves | R2 | Linear Range (ng/mL) |
LLOQ (ng/mL) |
|---|---|---|---|---|---|
| 1 | Atractyloside A | y = 1.5739x + 0.0418 | 0.9995 | 0.10–25.00 | 0.10 |
| 2 | Procyanidin B1 | y = 0.2186x − 0.0094 | 0.9991 | 0.10–12.50 | 0.10 |
| 3 | Procyanidin B2 | y = 0.2277x − 0.0083 | 0.9994 | 0.10–12.50 | 0.10 |
| 4 | Umbelliferone | y = 3.7074x − 0.0012 | 0.9996 | 0.01–3.13 | 0.01 |
| 5 | Rosavin | y = 2.4065x − 0.0108 | 0.9993 | 0.02–3.13 | 0.02 |
| 6 | Coumarin | y = 3.9901x + 0.1995 | 0.9994 | 0.10–50.00 | 0.10 |
| 7 | Polyporusterone A | y = 0.9290x − 0.0015 | 0.9998 | 0.10–25.00 | 0.10 |
| 8 | Alisol C | y = 1.2274x − 0.0099 | 0.9993 | 0.05–6.25 | 0.05 |
| 9 | Atractylenolide III | y = 2.9593x − 0.0390 | 0.9996 | 0.10–25.00 | 0.10 |
| 10 | Alisol C 23-acetate | y = 5.9883x + 0.0233 | 0.9992 | 0.01–3.13 | 0.01 |
| 11 | Atractylenolide II | y = 7.2963x + 0.0235 | 0.9995 | 0.01–3.13 | 0.01 |
| 12 | Alisol A | y = 1.4905x − 0.0296 | 0.9995 | 0.10–25.00 | 0.10 |
| 13 | 16α-Hydroxytrametenolic acid | y = 0.6745x − 0.0043 | 0.9991 | 0.02–3.13 | 0.02 |
| 14 | Atractylenolide I | y = 3.8661x + 0.0091 | 0.9993 | 0.01–3.13 | 0.01 |
| 15 | Alisol A 24-acetate | y = 1.2214x − 0.0693 | 0.9993 | 0.20–25.00 | 0.20 |
| 16 | Alisol B | y = 0.2495x − 0.0075 | 0.9992 | 0.20–25.00 | 0.20 |
| 17 | 3-O-Acetyl-16α-hydroxytrametenolic acid | y = 0.5743x − 0.0036 | 0.9994 | 0.02–3.13 | 0.02 |
| 18 | Alisol B 23-acetate | y = 0.5967x + 0.0013 | 0.9992 | 0.05–12.50 | 0.05 |
| 19 | Pachymic acid | y = 1.1861x − 0.0156 | 0.9991 | 0.05–6.25 | 0.05 |
Recovery tests were performed by spiking mixed standard solutions of three different concentrations with a known quantity of the sample. As shown in Table 4, the recovery values of the 19 compounds were 89.32–110.32%, and the relative standard deviation (RSD) was less than 3.15, indicating that the accuracy of the quantification method was good.
Table 4.
Recovery of the 19 compounds in ORS.
| No. | Compound | Spiked Concentration (ng/mL) |
Measured Concentration (ng/mL) |
Recovery (%) |
RSD (%) |
|---|---|---|---|---|---|
| 1 | Atractyloside A | 10.671 | 10.160 | 95.21 | 1.09 |
| 4.421 | 4.349 | 98.39 | 0.90 | ||
| 2.858 | 2.891 | 101.17 | 1.67 | ||
| 2 | Procyanidin B1 | 4.351 | 3.998 | 91.89 | 1.18 |
| 1.226 | 1.245 | 101.60 | 1.29 | ||
| 0.445 | 0.468 | 105.29 | 1.13 | ||
| 3 | Procyanidin B2 | 4.458 | 3.982 | 89.32 | 0.91 |
| 1.333 | 1.255 | 94.11 | 2.38 | ||
| 0.552 | 0.550 | 99.60 | 1.33 | ||
| 4 | Umbelliferone | 1.051 | 1.011 | 96.20 | 0.81 |
| 0.270 | 0.272 | 100.93 | 1.30 | ||
| 0.075 | 0.069 | 92.96 | 1.73 | ||
| 5 | Rosavin | 1.223 | 1.215 | 99.39 | 1.09 |
| 0.442 | 0.438 | 99.09 | 0.76 | ||
| 0.246 | 0.238 | 96.82 | 1.64 | ||
| 6 | Coumarin | 11.404 | 10.354 | 90.79 | 0.97 |
| 5.154 | 5.686 | 110.32 | 0.47 | ||
| 3.592 | 3.713 | 103.37 | 0.91 | ||
| 7 | Polyporusterone A | 8.429 | 7.874 | 93.41 | 1.02 |
| 2.179 | 2.154 | 98.86 | 0.66 | ||
| 0.617 | 0.568 | 92.02 | 0.61 | ||
| 8 | Alisol C | 2.322 | 2.281 | 98.22 | 1.20 |
| 0.759 | 0.756 | 99.55 | 1.25 | ||
| 0.369 | 0.363 | 98.44 | 0.63 | ||
| 9 | Atractylenolide III | 10.491 | 10.006 | 95.37 | 0.83 |
| 4.241 | 4.164 | 98.19 | 1.08 | ||
| 2.678 | 2.629 | 98.14 | 0.70 | ||
| 10 | Alisol C 23-acetate | 2.312 | 2.359 | 102.03 | 0.56 |
| 1.531 | 1.590 | 103.90 | 0.73 | ||
| 1.335 | 1.456 | 109.02 | 0.99 | ||
| 11 | Atractylenolide II | 1.482 | 1.463 | 98.71 | 2.57 |
| 0.700 | 0.716 | 102.23 | 1.98 | ||
| 0.505 | 0.537 | 106.31 | 3.15 | ||
| 12 | Alisol A | 8.927 | 8.475 | 94.94 | 0.44 |
| 2.677 | 2.521 | 94.21 | 0.51 | ||
| 1.114 | 1.050 | 94.28 | 0.87 | ||
| 13 | 16α-Hydroxytrametenolic acid | 1.067 | 1.058 | 99.18 | 1.32 |
| 0.286 | 0.280 | 97.95 | 1.53 | ||
| 0.091 | 0.084 | 92.76 | 3.07 | ||
| 14 | Atractylenolide I | 1.115 | 1.036 | 92.90 | 1.81 |
| 0.334 | 0.334 | 99.95 | 1.47 | ||
| 0.139 | 0.146 | 105.17 | 0.48 | ||
| 15 | Alisol A 24-acetate | 8.635 | 7.766 | 89.94 | 0.11 |
| 2.385 | 2.214 | 92.84 | 0.65 | ||
| 0.822 | 0.776 | 94.43 | 0.43 | ||
| 16 | Alisol B | 8.966 | 8.620 | 96.14 | 1.17 |
| 2.716 | 2.517 | 92.67 | 1.71 | ||
| 1.153 | 1.055 | 91.51 | 2.49 | ||
| 17 | 3-O-Acetyl-16α-hydroxytrametenolic acid | 1.094 | 1.069 | 97.66 | 1.06 |
| 0.313 | 0.308 | 98.29 | 0.65 | ||
| 0.118 | 0.107 | 90.68 | 2.20 | ||
| 18 | Alisol B 23-acetate | 6.990 | 6.709 | 95.98 | 1.05 |
| 3.865 | 3.890 | 100.65 | 1.56 | ||
| 3.084 | 3.171 | 102.83 | 1.30 | ||
| 19 | Pachymic acid | 2.186 | 2.092 | 95.68 | 1.36 |
| 0.624 | 0.603 | 96.66 | 1.11 | ||
| 0.233 | 0.227 | 97.36 | 2.88 |
The precision and accuracy were validated by mixing standard solutions at three concentrations, as shown in Table 5. Intra- and inter-day tests were performed by analyzing a sample prepared six times within the same day and over three consecutive days, respectively. The precision of the method was expressed in terms of the RSD; the intra-day value was less than 7.32%, the inter-day value was less than 9.44%, and the accuracy of the intra- and inter-day values varies as 89.17–112.34% and 88.86–110.20%, respectively.
Table 5.
Precision and accuracy of the 19 compounds in ORS.
| No. | Compound | Concentration (ng/mL) |
Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|---|
| Precision (%) |
Accuracy (%) |
Precision (%) |
Accuracy (%) |
|||
| 1 | Atractyloside A | 16.67 | 1.39 | 105.13 | 3.01 | 102.02 |
| 4.17 | 2.46 | 105.61 | 3.97 | 101.06 | ||
| 1.04 | 2.42 | 108.63 | 7.03 | 101.57 | ||
| 2 | Procyanidin B1 | 8.33 | 1.73 | 96.24 | 2.53 | 96.19 |
| 2.08 | 7.32 | 91.29 | 0.40 | 90.99 | ||
| 0.52 | 1.72 | 94.25 | 9.44 | 97.56 | ||
| 3 | Procyanidin B2 | 8.33 | 1.43 | 96.55 | 1.99 | 98.25 |
| 2.08 | 1.52 | 89.43 | 1.82 | 88.86 | ||
| 0.52 | 4.31 | 92.04 | 0.97 | 91.15 | ||
| 4 | Umbelliferone | 2.08 | 1.14 | 104.94 | 1.01 | 106.14 |
| 0.52 | 0.77 | 102.78 | 2.25 | 105.50 | ||
| 0.13 | 0.87 | 95.94 | 4.41 | 95.53 | ||
| 5 | Rosavin | 2.08 | 0.72 | 96.17 | 4.09 | 98.06 |
| 0.52 | 1.35 | 95.38 | 2.95 | 96.38 | ||
| 0.13 | 0.98 | 99.75 | 3.51 | 98.32 | ||
| 6 | Coumarin | 16.67 | 0.44 | 103.09 | 3.35 | 107.23 |
| 4.17 | 1.52 | 108.42 | 1.66 | 107.39 | ||
| 1.04 | 0.95 | 108.28 | 2.40 | 106.47 | ||
| 7 | Polyporusterone A | 16.67 | 0.65 | 107.98 | 6.53 | 105.20 |
| 4.17 | 0.59 | 104.15 | 5.62 | 102.74 | ||
| 1.04 | 0.97 | 93.65 | 3.49 | 97.52 | ||
| 8 | Alisol C | 4.17 | 1.23 | 101.40 | 0.35 | 101.56 |
| 1.04 | 1.36 | 104.58 | 1.65 | 103.01 | ||
| 0.26 | 1.28 | 104.09 | 4.13 | 103.30 | ||
| 9 | Atractylenolide III | 16.67 | 1.36 | 109.94 | 2.78 | 106.52 |
| 4.17 | 0.77 | 106.08 | 1.32 | 104.49 | ||
| 1.04 | 0.64 | 90.99 | 1.70 | 92.68 | ||
| 10 | Alisol C 23-acetate | 2.08 | 0.93 | 108.62 | 6.55 | 104.88 |
| 0.52 | 0.55 | 108.14 | 3.27 | 106.80 | ||
| 0.13 | 1.14 | 98.57 | 1.79 | 99.63 | ||
| 11 | Atractylenolide II | 2.08 | 1.33 | 109.62 | 3.45 | 106.47 |
| 0.52 | 3.25 | 112.34 | 2.87 | 110.20 | ||
| 0.13 | 2.62 | 99.07 | 2.11 | 98.06 | ||
| 12 | Alisol A | 16.67 | 0.85 | 106.82 | 3.93 | 103.79 |
| 4.17 | 0.96 | 102.06 | 4.29 | 99.09 | ||
| 1.04 | 0.94 | 92.37 | 2.65 | 94.02 | ||
| 13 | 16α-Hydroxytrametenolic acid | 2.08 | 2.29 | 100.72 | 9.09 | 101.95 |
| 0.52 | 2.67 | 89.17 | 6.92 | 93.24 | ||
| 0.13 | 3.91 | 98.91 | 3.27 | 97.03 | ||
| 14 | Atractylenolide I | 2.08 | 0.62 | 107.66 | 1.80 | 105.46 |
| 0.52 | 1.14 | 109.94 | 4.81 | 107.22 | ||
| 0.13 | 2.57 | 93.70 | 1.52 | 95.15 | ||
| 15 | Alisol A 24-acetate | 16.67 | 0.76 | 111.72 | 6.84 | 108.29 |
| 4.17 | 0.83 | 99.21 | 5.50 | 97.96 | ||
| 1.04 | 1.52 | 93.60 | 3.05 | 94.06 | ||
| 16 | Alisol B | 16.67 | 1.48 | 109.18 | 5.11 | 106.96 |
| 4.17 | 1.33 | 101.20 | 4.18 | 99.03 | ||
| 1.04 | 1.23 | 94.37 | 1.12 | 93.73 | ||
| 17 | 3-O-Acetyl-16α-hydroxytrametenolic acid | 2.08 | 1.16 | 93.66 | 3.90 | 96.65 |
| 0.52 | 1.75 | 101.84 | 5.62 | 95.74 | ||
| 0.13 | 1.09 | 100.28 | 2.29 | 98.15 | ||
| 18 | Alisol B 23-acetate | 8.33 | 1.06 | 110.55 | 7.93 | 106.71 |
| 2.08 | 3.74 | 111.31 | 8.95 | 106.38 | ||
| 0.52 | 2.38 | 92.11 | 1.83 | 93.99 | ||
| 19 | Pachymic acid | 4.17 | 1.56 | 95.04 | 7.22 | 98.00 |
| 1.04 | 0.98 | 94.44 | 3.02 | 92.49 | ||
| 0.26 | 1.04 | 95.46 | 2.37 | 98.11 | ||
These results indicate that the established analytical method based on UPLC-TQ-MS/MS can accurately and efficiently quantify the 19 constituent compounds of ORS.
2.4. Sample Analysis
The established UPLC-TQ-MS/MS method was used to simultaneously determine 19 compounds in 3 batches of ORS. The contents of all compounds, as obtained from the calibration curves calculated via the IS method, are shown in Table 6.
Table 6.
Contents of the 19 compounds in 3 batches of ORS.
| No. | Compound | ORS-1 | ORS-2 | ORS-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (ng/g) |
SD | CV (%) |
Mean (ng/g) |
SD | CV (%) |
Mean (ng/g) |
SD | CV (%) |
||
| 1 | Atractyloside A | 3.697 | 0.057 | 1.549 | 3.511 | 0.060 | 1.722 | 3.699 | 0.043 | 1.171 |
| 2 | Procyanidin B1 | 0.265 | 0.004 | 1.320 | 0.263 | 0.001 | 0.349 | 0.264 | 0.004 | 1.514 |
| 3 | Procyanidin B2 | 0.550 | 0.013 | 2.396 | 0.525 | 0.010 | 1.933 | 0.554 | 0.020 | 3.542 |
| 4 | Umbelliferone | 0.022 | 0.000 | 2.100 | 0.023 | 0.001 | 2.503 | 0.023 | 0.001 | 2.341 |
| 5 | Rosavin | 0.276 | 0.004 | 1.530 | 0.264 | 0.006 | 2.403 | 0.280 | 0.001 | 0.226 |
| 6 | Coumarin | 7.611 | 0.066 | 0.873 | 7.391 | 0.100 | 1.348 | 7.683 | 0.082 | 1.070 |
| 7 | Polyporusterone A | 0.239 | 0.004 | 1.528 | 0.231 | 0.003 | 1.222 | 0.242 | 0.004 | 1.574 |
| 8 | Alisol C | 0.395 | 0.004 | 1.112 | 0.382 | 0.007 | 1.843 | 0.400 | 0.006 | 1.390 |
| 9 | Atractylenolide III | 3.208 | 0.090 | 2.811 | 3.117 | 0.050 | 1.601 | 3.277 | 0.056 | 1.701 |
| 10 | Alisol C 23-acetate | 2.540 | 0.016 | 0.645 | 2.437 | 0.015 | 0.606 | 2.573 | 0.028 | 1.079 |
| 11 | Atractylenolide II | 0.749 | 0.009 | 1.256 | 0.726 | 0.015 | 2.056 | 0.770 | 0.014 | 1.857 |
| 12 | Alisol A | 1.027 | 0.013 | 1.226 | 0.994 | 0.009 | 0.871 | 1.045 | 0.014 | 1.353 |
| 13 | 16α-Hydroxytrametenolic acid | 0.070 | 0.003 | 3.847 | 0.068 | 0.003 | 4.264 | 0.070 | 0.003 | 4.123 |
| 14 | Atractylenolide I | 0.108 | 0.003 | 2.979 | 0.104 | 0.002 | 1.737 | 0.108 | 0.004 | 3.866 |
| 15 | Alisol A 24-acetate | 0.689 | 0.004 | 0.539 | 0.680 | 0.008 | 1.150 | 0.694 | 0.005 | 0.690 |
| 16 | Alisol B | 1.120 | 0.036 | 3.225 | 1.078 | 0.018 | 1.647 | 1.134 | 0.024 | 2.095 |
| 17 | 3-O-Acetyl-16α-hydroxytrametenolic acid | 0.102 | 0.001 | 1.420 | 0.099 | 0.002 | 1.923 | 0.101 | 0.005 | 4.674 |
| 18 | Alisol B 23-acetate | 4.406 | 0.092 | 2.084 | 4.181 | 0.226 | 5.403 | 4.433 | 0.071 | 1.602 |
| 19 | Pachymic acid | 0.212 | 0.003 | 1.316 | 0.206 | 0.005 | 2.328 | 0.215 | 0.004 | 2.037 |
Among the 19 compounds measured, the coumarin content was highest (at 7.391–7.683 ng/g) in the 3 batches of ORS; these results resemble those of previously reported studies [2]. In addition to coumarin, the contents of alisol B 23-acetate, atractyloside A, and atractylenolide III were higher in ORS than in the other compounds. These four compounds are derived from C. cassia, A. orientale, and A. japonica (three of the single-herb medicines constituting ORS) and they have been reported as major compounds in previous studies into these individual herbal medicines [18,20,45]. The UPLC-TQ-MS/MS method established in our study was successfully applied to simultaneously quantify the 19 compounds, demonstrating the method’s suitability for component analysis of ORS.
3. Materials and Methods
3.1. Materials and Reagents
The reference standards used in this study were atractyloside A, procyanidin_B1, procyanidin B2, umbelliferon, rosavin, coumarin, alisol C, atractylenolide III, alisol C 23-acetate, atractylenolide II, alisol A, 16α-hydroxytrametenolic acid, atractylenolide I, alisol A 24-acetate, alisol B, 3-O-acetyl-16α-hydroxytrametenolic acid, alisol B 23-acetate, and pachymic acid; these were purchased from ChemFaces (Wuhan, China). Polyporusterone A was purchased from Chem-Norm Biotech (Wuhan, China), and warfarin, an IS, was purchased from Sigma-Aldrich (St. Louis, MO, USA). All reference standards were used with a purity of 98% or higher. MS-grade methanol, acetonitrile, water, and formic acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The five herbal medicines (A. orientale, P. umbellatus, A. japonica, P. cocos, and P. cocos) were purchased from Kwangmyungdang Pharmaceutical (Ulsan, Republic of Korea). Each raw herbal medicine was deposited at the KM Convergence Research Division of the Korea Institute of Oriental Medicine (specimen No. TDC-01, TDC-04, and TDC-06–08).
3.2. Preparation of ORS
ORS extracts were prepared using the method described in a previous study [46]. In total, 5 herbal medicines were combined in the ratios shown in Table 7, and 10 times their total weight of water was added, followed by reflux extraction at 100 °C for 3 h. The extracted water was then filtered and concentrated under reduced pressure using a rotary evaporator. The concentrated aqueous extract was freeze-dried, and the obtained powder sample (yield: 20.3%) was used for analyses.
Table 7.
Composition of ORS.
| Scientific Name | Scientific Name | Weight Ratio |
|---|---|---|
| Alismatis Rhizoma | Alisma orientale Juzepzuk | 5.0 |
| Polyporus | Polyporus umbellatus Fries | 3.0 |
| Atractylodis Rhizoma Alba | Atractylodes japonica Koidzumi | 3.0 |
| Poria Sclerotium | Poria cocos Wolf | 3.0 |
| Cinnamomi Cortex | Cinnamomum cassia Presl | 2.0 |
3.3. Preparation of Standard and Sample Solutions
Nineteen standard compounds and one IS (warfarin) were individually dissolved in methanol to prepare stock solutions. The stock solutions were prepared by mixing aliquots for each solution. Working solutions containing 19 compounds were diluted in methanol to prepare a set of appropriate concentrations. The IS concentration was kept consistent in each sample at 5 ng/mL. Quality control (QC) samples were used for method validation and prepared at high, medium, and low concentrations using the method described above. ORS powder (20 mg) was extracted using methanol in an ultrasonic bath for 30 min. The extract was centrifuged at 12,500 rpm for 15 min and analyzed.
3.4. UHPLC-Q-Orbitrap-MS Conditions for Qualitative Analysis
The UHPLC-Q-Orbitrap-MS method was performed on a Dionex UltiMate 3000 system equipped with a Thermo Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The analytical conditions for determining the chemical compounds in the ORS matched those of previously reported analytical methods [46]. In brief, the separation was performed on an C18 column (100 × 2.1 mm, 1.7 µm, Acquity BEH C18, Waters, Milford, MA, USA), and a mobile phase (a gradient mixture of 0.1% formic acid in water (A) and acetonitrile (B)) was used. All samples were analyzed in the positive and negative ion conversion modes, and the mass scan was performed in the range 100–1500 m/z. Full scan and MS/MS scan data were acquired at resolutions of 70,000 full width at half maximum and 17,500 in both positive and negative modes, respectively. Xcalibur v.3.0 (Thermo Fisher Scientific, Waltham, MA, USA) was used to acquire and analyze the data.
3.5. UPLC-TQ-MS/MS Conditions for Quantitative Analysis
Quantitative analyses were conducted using an Agilent 1290 Infinity II system interfaced with an Agilent 6495C triple-quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Ionization was performed using a jet-stream electrospray ionization source. The operating conditions for chromatographic separation and mass spectrometric detection were determined using previously reported methods [46]. MRM was performed to quantify the 19 ORS compounds, and the MRM transitions and collision energy values were optimized for each compound (Table 2). All MRM data were acquired and processed using the Agilent MassHunter workstation quantitative analysis software (version 10.1).
3.6. Method Validation of Quantitative Analysis
The UPLC-TQ-MS/MS method used to quantitatively analyze the 19 ORS compounds was validated using linearity, recovery, precision, and accuracy parameters [47]. Linearity was evaluated by constructing a calibration curve for each compound, using the peak area ratio between the analyte concentration and IS. The LLOQ was defined as the lowest concentration in the standard curve that could be measured with acceptable accuracy and precision, and the limit of quantification (LOQ) was determined at a signal to noise (S/N) ratio of 10. To investigate the recovery, different concentrations (high, medium, and low) for each compound were added to the ORS samples. Recovery was evaluated by comparing the spiked and detected quantities of each analyte. Intra- and inter-day variations were performed to evaluate the method precision. QC samples were prepared at three concentration levels, and precision was determined using the relative standard deviation calculated from the measured concentrations. To evaluate intra-day precision, QC samples were measured six times within one day; to evaluate inter-day precision, the same samples were measured on three consecutive days.
4. Conclusions
In this study, the qualitative and quantitative analysis methods UHPLC-Q-Orbitrap-MS and UPLC-TQ-MS/MS, respectively, were applied to comprehensively analyze ORS components. Chemical profiling of ORS was performed using the developed UHPLC-Q-Orbitrap-MS analysis method, and 19 compounds were identified via comparison with reference standard compounds. The 19 identified compounds were simultaneously quantified within 20 min using an established MRM-mode quantitative analysis method; this was successfully applied for practical sample analysis. These results facilitate the qualitative and quantitative analysis of ORS constituents, in turn facilitating the accurate chemical identification and simultaneous determination of compounds. It also aids in the routine analysis of ORS and the identification of biologically active substances, suggesting that it can be effectively applied for overall quality control.
Author Contributions
Conceptualization, Y.-H.H.; investigation, S.J., A.L. and Y.-H.H.; writing—original draft preparation, S.J.; writing—review and editing, S.J. and Y.-H.H.; funding acquisition, Y.-H.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Korea Institute of Oriental Medicine (grant number: KSN2212020).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lee J., Weon J.B., Lee B., Yun B.-R., Eom M.R., Ma C.J. Simultaneous determination of six components in the traditional herbal medicine ‘Oryeongsan’ by HPLC-DAD and LC-MS/MS. Nat. Prod. Sci. 2013;19:20–27. [Google Scholar]
- 2.Lee M.Y., Seo C.S., Kim J.Y., Shin H.K. Genotoxicity evaluation of Oryeong-san water extract using in vitro and in vivo tests. BMC Complement. Altern. Med. 2015;15:273. doi: 10.1186/s12906-015-0804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He L., Rong X., Jiang J.M., Liu P.Q., Li Y. Amelioration of anti-cancer agent adriamycin-induced nephrotic syndrome in rats by Wulingsan (Gorei-San), a blended traditional Chinese herbal medicine. Food Chem. Toxicol. 2008;46:1452–1460. doi: 10.1016/j.fct.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.-H., Shin H.-K. Analysis of biological experiment on Oryeong-san (Wuling-san) J. Intern. Korean Med. 2012;33:69–82. [Google Scholar]
- 5.Kiga C., Goto H., Sakurai H., Hayashi K., Hikiami H., Shimada Y., Saiki I. Effects of traditional Japanese (Kampo) medicines (orengedokuto, goreisan and shichimotsukokato) on the onset of stroke and expression patterns of plasma proteins in spontaneously hypertensive stroke-prone rats. J. Trad. Med. 2008;25:125–132. [Google Scholar]
- 6.Kim S.J., Leem H.H., Nam W.H., Son S.M., Choi H.M., Kim M.J., Kim J.O., Lee H.D. Effect of anti-inflammation on Oryeong-san formulation for Mix extract tablet. J. Physiol. Pathol. Korean Med. 2020;34:348–354. doi: 10.15188/kjopp.2020.12.34.6.348. [DOI] [Google Scholar]
- 7.Liu I.M., Tzeng T.F., Liou S.S., Chang C.J. The amelioration of streptozotocin diabetes-induced renal damage by Wu-Ling-San (Hoelen Five Herb Formula), a traditional Chinese prescription. J. Ethnopharmacol. 2009;124:211–218. doi: 10.1016/j.jep.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Lin E., Ho L., Lin M.S., Huang M.H., Chen W.C. Wu-Ling-San formula prophylaxis against recurrent calcium oxalate nephrolithiasis—A prospective randomized controlled trial. Afr. J. Tradit. Complement. Altern. Med. 2013;10:199–209. doi: 10.4314/ajtcam.v10i5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada K., Yagi G., Kanba S. Effectiveness of Gorei-san (TJ-17) for treatment of SSRI-induced nausea and dyspepsia: Preliminary observations. Clin. Neuropharmacol. 2003;26:112–114. doi: 10.1097/00002826-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nakao J., Marushima A., Fujita K., Fujimori H., Mashiko R., Kamezaki T., Ishikawa E. Conservative Treatment of Chronic Subdural Hematoma with Gorei-san. Neurol. Med. Chir. 2023;63:31–36. doi: 10.2176/jns-nmc.2022-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D., Lin S., Xu W., Huang M., Chu J., Xiao F., Lin J., Peng J. Qualitative and quantitative analysis of the major constituents in Shexiang Tongxin dropping pill by HPLC-Q-TOF-MS/MS and UPLC-QqQ-MS/MS. Molecules. 2015;20:18597–18619. doi: 10.3390/molecules201018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Yang Y., Shi Y.P. Simultaneous quantification of twelve active components in Yiqing granule by ultra-performance liquid chromatography: Application to quality control study. Biomed. Chromatogr. 2011;25:1045–1053. doi: 10.1002/bmc.1569. [DOI] [PubMed] [Google Scholar]
- 13.Du K., Yang J., Yang L., Wang Z., Wang R., Shi Y. Chemical profiling and marker characterization of Huangqin decoction prepared with three types of peony root by liquid chromatography with electrospray ionization mass spectrometry. J. Sep. Sci. 2020;43:2558–2570. doi: 10.1002/jssc.201901305. [DOI] [PubMed] [Google Scholar]
- 14.Wang D.D., Liang J., Yang W.Z., Hou J.J., Yang M., Da J., Wang Y., Jiang B.H., Liu X., Wu W.Y., et al. HPLC/qTOF-MS-oriented characteristic components data set and chemometric analysis for the holistic quality control of complex TCM preparations: Niuhuang Shangqing pill as an example. J. Pharm. Biomed. Anal. 2014;89:130–141. doi: 10.1016/j.jpba.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Food and Drug Safety of the Republic of Korea . The Korean Pharmacopoeia. 12th ed. The KFDA Notification; Cheongju, Republic of Korea: 2020. [Google Scholar]
- 16.Tian T., Chen H., Zhao Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014;158:373–387. doi: 10.1016/j.jep.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y.Y. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and quality control of Polyporus umbellatus (Pers.) Fries: A review. J. Ethnopharmacol. 2013;149:35–48. doi: 10.1016/j.jep.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Xu S., Qi X., Liu Y., Liu Y., Lv X., Sun J., Cai Q. UPLC-MS/MS of Atractylenolide I, Atractylenolide II, Atractylenolide III, and Atractyloside A in Rat Plasma after Oral Administration of Raw and Wheat Bran-Processed Atractylodis Rhizoma. Molecules. 2018;23:3234. doi: 10.3390/molecules23123234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou Y.T., Long F., Wu C.Y., Zhou J., Zhang W., Xu J.D., Zhang Y.Q., Li S.L. A dereplication strategy for identifying triterpene acid analogues in Poria cocos by comparing predicted and acquired UPLC-ESI-QTOF-MS/MS data. Phytochem. Anal. 2019;30:292–310. doi: 10.1002/pca.2813. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y., Li Y., Sun A., Liu X. Chemical compound identification and antibacterial activity evaluation of cinnamon extracts obtained by subcritical n-butane and ethanol extraction. Food Sci. Nutr. 2019;7:2186–2193. doi: 10.1002/fsn3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo C.-S., Shin H.-K. Simultaneous determination of cinnamaldehyde and coumarin in Oryeong-san using HPLC with Photodiode array detector. Herb. Formula Sci. 2010;18:251–257. [Google Scholar]
- 22.Xiao S., Hao C., Ai N., Luo K., Wen X., Wang S., Fan X. Deciphering the differentiations of traditional Chinese medicine analogous formulae by parallel liquid chromatography-mass spectrometry coupled with microplate-based assays. Anal. Methods. 2014;6:9283–9290. doi: 10.1039/C4AY01972E. [DOI] [Google Scholar]
- 23.He F., Wang C.J., Xie Y., Cheng C.S., Liu Z.Q., Liu L., Zhou H. Simultaneous quantification of nine aconitum alkaloids in Aconiti Lateralis Radix Praeparata and related products using UHPLC-QQQ-MS/MS. Sci. Rep. 2017;7:13023. doi: 10.1038/s41598-017-13499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji Z., Jiang Y., Lin H., Ren W., Lin L., Guo H., Huang J., Li Y. Global identification and quantitative analysis of representative components of Xin-Nao-Kang Capsule, a traditional Chinese medicinal formula, by UHPLC-Q-TOF-MS and UHPLC-TQ-MS. J. Pharm. Biomed. Anal. 2021;198:114002. doi: 10.1016/j.jpba.2021.114002. [DOI] [PubMed] [Google Scholar]
- 25.Tian T., Xu X., Li X., Zhang W., Lu H. Precision-characterization and quantitative determination of main compounds in Si-Ni-San with UHPLC-MS/MS based targeted-profiling method. J. Pharm. Biomed. Anal. 2021;194:113816. doi: 10.1016/j.jpba.2020.113816. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Z., Wang X., Wang J., Liu C., Pan J. Simultaneous determination of eight flavonoids in Sedum sarmentosum Bunge from different areas by UHPLC with triple quadrupole MS/MS. Biomed. Chromatogr. 2019;33:e4601. doi: 10.1002/bmc.4601. [DOI] [PubMed] [Google Scholar]
- 27.Li X.Y., Xu J.D., Zhou S.S., Kong M., Xu Y.Y., Zou Y.T., Tang Y., Zhou L., Xu M.Z., Xu J., et al. Time segment scanning-based quasi-multiple reaction monitoring mode by ultra-performance liquid chromatography coupled with quadrupole/time-of-flight mass spectrometry for quantitative determination of herbal medicines: Moutan Cortex, a case study. J. Chromatogr. A. 2018;1581–1582:33–42. doi: 10.1016/j.chroma.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.K., Lee S. Drug-likeness and Oral bioavailability for Chemical compounds of Medicinal Materials Constituting Oryeong-san. Korea J. Herbol. 2018;33:19–37. [Google Scholar]
- 29.Yang H., Tuo X., Wang L., Tundis R., Portillo M.P., Simal-Gandara J., Deng J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021;111:114–127. doi: 10.1016/j.tifs.2021.02.063. [DOI] [Google Scholar]
- 30.Zhou W., Chen K., Lu Q., Luo Y., Zhang C., Zheng Y., Sha W. The protective effect of rosavin from Rhodiola rosea on radiation-induced intestinal injury. Chem. Biodivers. 2020;17:e2000652. doi: 10.1002/cbdv.202000652. [DOI] [PubMed] [Google Scholar]
- 31.Stefanachi A., Leonetti F., Pisani L., Catto M., Carotti A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules. 2018;23:250. doi: 10.3390/molecules23020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ríos J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011;77:681–691. doi: 10.1055/s-0030-1270823. [DOI] [PubMed] [Google Scholar]
- 33.Wang P., Song T., Shi R., He M., Wang R., Lv J., Jiang M. Triterpenoids from Alisma species: Phytochemistry, structure modification, and bioactivities. Front. Chem. 2020;8:363. doi: 10.3389/fchem.2020.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q.L., Chen Y.J., Zhou S.S., Yip K.M., Xu J., Chen H.B., Zhao Z.Z. Laser microdissection hyphenated with high performance gel permeation chromatography-charged aerosol detector and ultra performance liquid chromatography-triple quadrupole mass spectrometry for histochemical analysis of polysaccharides in herbal medicine: Ginseng, a case study. Int. J. Biol. Macromol. 2018;107:332–342. doi: 10.1016/j.ijbiomac.2017.08.162. [DOI] [PubMed] [Google Scholar]
- 35.Deshaies S., Sommerer N., Garcia F., Mouls L., Saucier C. UHPLC-Q-Orbitrap/MS2 identification of (+)-Catechin oxidation reaction dimeric products in red wines and grape seed extracts. Food Chem. 2022;382:132505. doi: 10.1016/j.foodchem.2022.132505. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y., Han C., Liu B., Lin Z., Zhou X., Wang C., Zhu Z. Determination of vanillin, ethyl vanillin, and coumarin in infant formula by liquid chromatography-quadrupole linear ion trap mass spectrometry. J. Dairy Sci. 2014;97:679–686. doi: 10.3168/jds.2013-7308. [DOI] [PubMed] [Google Scholar]
- 37.Yang W., Ye M., Liu M., Kong D., Shi R., Shi X., Zhang K., Wang Q., Lantong Z. A practical strategy for the characterization of coumarins in Radix Glehniae by liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J. Chromatogr. A. 2010;1217:4587–4600. doi: 10.1016/j.chroma.2010.04.076. [DOI] [PubMed] [Google Scholar]
- 38.Zheng G., Liu M., Chao Y., Yang Y., Zhang D., Tao Y., Zhang J., Zeng C., Wei M. Identification of lipophilic components in Citri Reticulatae Pericarpium cultivars by supercritical CO2 fluid extraction with ultrahigh-performance liquid chromatography-Q Exactive Orbitrap tandem mass spectrometry. J. Sep. Sci. 2020;43:3421–3440. doi: 10.1002/jssc.202000490. [DOI] [PubMed] [Google Scholar]
- 39.Tai Y., Zou F., Zhang Q., Wang J., Rao R., Xie R., Wu S., Chu K., Xu W., Li X., et al. Quantitative analysis of eight triterpenoids and two sesquiterpenoids in Rhizoma alismatis by using UPLC-ESI/APCI-MS/MS and its application to optimisation of best harvest time and crude processing temperature. J. Anal. Methods Chem. 2019;2019:8320171. doi: 10.1155/2019/8320171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao W., Huang X., Li X., Zhang F., Chen S., Ye M., Huang M., Xu W., Wu S. Qualitative and quantitative analysis of major triterpenoids in Alismatis rhizoma by high performance liquid chromatography/diode-array detector/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. Molecules. 2015;20:13958–13981. doi: 10.3390/molecules200813958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L., Qi J., Chang Y.X., Zhu D., Yu B. Identification and determination of the major constituents in Traditional Chinese Medicinal formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS. J. Pharm. Biomed. Anal. 2009;50:127–137. doi: 10.1016/j.jpba.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y.Y., Guan S.H., Tang R.N., Tao S.J., Guo D.A. Simultaneous determination of atractylenolide II and atractylenolide III by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of Atractylodes macrocephala rhizoma extract. Biomed. Chromatogr. 2012;26:1386–1392. doi: 10.1002/bmc.2709. [DOI] [PubMed] [Google Scholar]
- 43.Wu L.F., Wang K.F., Mao X., Liang W.Y., Chen W.J., Li S., Qi Q., Cui Y.P., Zhang L.Z. Screening and analysis of the potential bioactive components of Poria cocos (Schw.) wolf by HPLC and HPLC-MS(n) with the aid of chemometrics. Molecules. 2016;21:227. doi: 10.3390/molecules21020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang H., Liu J., Wang Y., Chen L., Liu H., Wang Z., Wang B. Screening the Q-markers of TCMs from RA rat plasma using UHPLC-QTOF/MS technique for the comprehensive evaluation of Wu-Wei-Wen-Tong Capsule. J. Mass Spectrom. 2021;56:e4711. doi: 10.1002/jms.4711. [DOI] [PubMed] [Google Scholar]
- 45.Shu Z., Pu J., Chen L., Zhang Y., Rahman K., Qin L., Zheng C. Alisma orientale: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. Am. J. Chin. Med. 2016;44:227–251. doi: 10.1142/S0192415X16500142. [DOI] [PubMed] [Google Scholar]
- 46.Jang S., Lee A., Hwang Y.H. Qualitative profiling and quantitative analysis of major constituents in Jinmu-tang by UHPLC-Q-Orbitrap-MS and UPLC-TQ-MS/MS. Molecules. 2022;27:7887. doi: 10.3390/molecules27227887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Center for Biologics Evaluation and Research (CBER) Guidance for Industry. ICH; Rockville, MD, USA: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.