Abstract
Staphylococcus aureus frequently colonizes the airways of patients with compromised airway defenses (e.g., cystic fibrosis [CF] patients) for extended periods. Persistent and relapsing infections may be related to live S. aureus bacteria actively residing inside epithelial cells. In this study, we infected a respiratory epithelial cell line, which was derived from a CF patient, with S. aureus RN6390. Internalization of S. aureus was found to be time and dose dependent and could be blocked by cytochalasin D. Transmission electron microscopy revealed that internalized bacteria resided within endocytic vacuoles without any evidence of lysosomal fusion in a 24-h period. The results of internalization experiments and time-lapse fluorescence microscopy of epithelial cells infected with green fluorescent S. aureus indicate that, after an initial lag period of 7 to 9 h, intracellular bacteria began to replicate, with three to five divisions in a 24-h period, leading to apoptosis of infected cells. Induction of apoptosis required bacterial internalization and is associated with intracellular replication. The slow and gradual replication of S. aureus inside epithelial cells hints at the role of host factors or signals in bacterial growth and further suggests possible cross talk between host cells and S. aureus.
Respiratory infections with Staphylococcus aureus are common in patients with compromised airway defenses (e.g., cystic fibrosis [CF] and hospitalized patients) (5, 25). Despite the prevalence of these infections, the mechanism by which S. aureus colonizes the respiratory tract of susceptible patients is not well defined. In the case of CF, it has been suggested that inactive defensins due to elevated salt concentrations in the surface fluid of respiratory epithelium (24) and imbalances in fluid flow across the epithelium (14) lead to progressively thickened mucus and impaired mucociliary clearance, thereby promoting persistent colonization and, quite frequently, infections. Additionally, asialoglycolipid (asialo-GM1), which is exposed in increased numbers on the surfaces of CF epithelial cells, has been postulated to serve as a receptor for CF pathogens such as S. aureus (12). Furthermore, the development of bacterial biofilms as sessile communities with inherent antimicrobial resistance on the surfaces of airway tissues also plays a role in the development of chronic lung diseases (8).
S. aureus is second to Pseudomonas aeruginosa as one of the most common pathogens isolated from the respiratory tracts of CF patients (5). Contrary to P. aeruginosa infection, persistent S. aureus infection starts in early infancy, often preceding chronic infections with P. aeruginosa (1, 26). It has been hypothesized that persistent S. aureus infections in diseases other than CF may be related to internalization of the pathogen by host cells, thereby creating a protected environment where the bacteria are shielded against host defenses and antimicrobial therapy (3, 10, 19). The persistence of S. aureus inside epithelial cells may also signal possible cross talk between the microorganism and the epithelial cell. In particular, recent studies have shown that both bovine mammary epithelial cells and human endothelial cells internalize S. aureus and subsequently undergo apoptosis (3, 19). However, the intracellular fate of S. aureus (i.e., dead or alive) is not clear from these studies.
Apoptosis is an innate cell suicide mechanism that plays a role in homeostasis in multicellular organisms. In contrast to necrosis, however, apoptosis is accompanied by little inflammatory response. Increasing numbers of bacterial pathogens have been found to utilize an assortment of virulence factors to interact with key components of the cell death program (29); these interactions, leading to eventual apoptosis, may be necessary to subvert normal host defenses (27).
In this study, we constructed a derivative of S. aureus strain RN6390 containing a plasmid expressing a green fluorescent protein (GFP) variant (excitation maxima at 488 nm) that is amenable to fluorescence microscopy. Using green fluorescent S. aureus, we demonstrated clearly that internalized S. aureus is not a passive bystander but rather replicates actively inside pulmonary epithelial cells and induces apoptosis. Compared to extracellular growth (cell division every 20 min), intracellular replication was indolent (three to five divisions in 24 h) and did not begin until 6 to 7 h after internalization. The gradual replication of S. aureus hints at the role of host factors or signals on intracellular bacterial growth. This finding, coupled with electron microscopy data that show that intracellular bacteria at 24 h after infection remained in vacuoles without evidence of lysosomal fusion, is concordant with possible interactions between intracellular S. aureus and host cells. As we have consistently found intracellular replication to precede apoptosis, it is conceivable that S. aureus, by virtue of its internalization and replication within pulmonary epithelial cells, could contribute to persistent pulmonary infections in susceptible patients.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus RN6390 (20), a laboratory strain that has been shown to be virulent in several animal models (4, 6), was chosen for the internalization experiments. An overnight culture grown at 37°C with aeration in brain heart infusion (Difco) was washed twice in phosphate-buffered saline (PBS), vortexed, passed through a 5-μm-pore-size filter, diluted to an optical density at 650 nm of 0.4 (∼108 CFU/ml), and resuspended in invasion medium (growth medium without antibiotics, antimycotics, or fetal bovine serum). To visualize bacteria in contact with epithelial cells, we used S. aureus RN6390 containing the plasmid pALC1743, a pSK236-derived shuttle plasmid carrying an active S. aureus promoter (RNAIII promoter) which drives the expression of GFPuvr, a derivative of GFPuv that is optimized for prokaryotic expression (Clontech, Palo Alto, Calif.). RNAIII promoter was chosen because of its detectable activity in the log phase and maximal activity in the stationary phase. GFPuvr was constructed by introducing an S65T mutation into gfpuv with the Quick Change mutagenesis kit (Stratagene, La Jolla, Calif.). The mutated gene (gfpuvr) was cloned into the shuttle vector pSK236, which contains an upstream RNAIII promoter fragment, to yield pALC1743. The alteration in excitation maxima from 395 to 488 nm was verified by spectrofluorometry as described previously (7). For invasion experiments with heat-killed S. aureus, bacteria were incubated for 1 h at 55°C and used in invasion assays as described below.
Cell culture of the CF cell line CFT-1 and the complementary cell line CFT-1-LCFSN.
CFT-1, a papilloma virus-immortalized tracheal epithelial cell line derived from a CF tissue donor homozygous for the ΔF508 mutation of the CF transmembrane regulator (CFTR) gene (28) and its complemented counterpart with the wild-type human CFTR, CFT-1-LCFSN, were used for our experiments. The growth medium was Dulbecco's modified Eagle's medium Nut Mix F-12 Ham (Cellgro; Mediatech) plus 10% fetal bovine serum (HyClone Laboratories), supplemented with the following (all from Sigma): 5 μg of insulin/ml, 3.7 μg of endothelial cell growth supplement/ml, 25 ng of epidermal growth factor/ml, 3 × 10−8 M triiodothyronine, 10−6 M hydrocortisone, 5 μg of transferrin/ml, and 10 ng of cholera toxin/ml. This medium also contained an antibiotic-antimycotic solution containing 100 U of penicillin G/ml, 25 μg of amphotericin B, and 100 μg of streptomycin/ml (Cellgro; Mediatech). Prior to use, cells were seeded at 6 × 104 cells/well in 24-well tissue culture plates (Costar) and grown for 3 days at 37°C with 5% CO2 to confluency (approximately 2 × 105 cells/well) in medium containing no antibiotics or antimycotics.
Internalization assay.
Internalization of S. aureus by the airway epithelial cells was determined as described previously (13). Briefly, epithelial cells were infected with bacteria diluted in invasion medium. After various incubation periods, the medium was replaced by medium containing 100 μg of gentamicin (Sigma)/ml to kill extracellular bacteria. After additional incubation, infected cells were washed thrice with PBS to remove gentamicin, treated with 200 μl of 0.25% trypsin–0.1% EDTA in Hanks balanced salt solution (Cellgro; Mediatech) for 5 min, and lysed with 800 μl of 0.025% Triton X-100 (Sigma) in water. Cell lysates were diluted, plated in triplicate on brain heart infusion agar, and incubated overnight at 37°C, and the numbers of CFU per milliliter were determined.
DIC, phase-contrast, and epifluorescence microscopy.
The internalization experiments were performed in borosilicate chamber slides (Labtek chambered coverglass; Nalge Nunc International). Microscopy was performed with an Olympus IX-70 equipped with 40×/0.6 numerical aperture (phase) and 100×/1.35 numerical aperture differential interference contrast (DIC) objectives. For epifluorescence, we used a 150-W xenon lamp (Optiquip) and standard filter sets. Images were acquired with a cooled charged coupled device camera (Hamamatsu Orca) using in-house software.
To serially observe intracellular replication of S. aureus in airway epithelial cells, the medium was buffered with 20 mM HEPES (pH 7.4), and infected cells on the microscope stage were kept at 37°C. To ensure that these internalized green fluorescent bacteria are intracellular, we stained the epithelial cells with chicken anti-protein A antibody (1:3,000) (Accurate Chemicals) to detect extracellular bacteria, followed by a second antibody conjugated to tetramethyl rhodamine isocyanate (TRITC).
TEM.
For transmission electron microscopy (TEM) analysis, the internalization experiment was performed with cells seeded on Thermanox coverslips (Nalge Nunc International). At different times after infection, the monolayers were washed with PBS, fixed in 3% glutaraldehyde, and postfixed in 1% osmium tetroxide. Samples were serially dehydrated in alcohol and embedded in Epon. Sections were stained with uranyl acetate and lead citrate and viewed in a transmission electron microscope (JEM 100CX; JEOL).
Inhibition assays.
For inhibition experiments, cells were treated with cytochalasin D (Sigma) (0.5 μg/ml) or colchicine (Sigma) (10 or 40 μg/ml) for 30 min prior to the internalization assay and subsequently during the 2-h infection period. Cycloheximide (Sigma) (20 μg/ml) was added 30 min prior to the infection period and remained throughout the experiment. To inhibit RNA transcription of intracellular bacteria, rifampin (0.018 μg/ml), which readily permeates cells, was added to the culture 2 h postinfection.
Assessment of apoptosis by DNA laddering and Cy3-labeled annexin V staining.
DNA of infected and noninfected cell monolayers was extracted with phenol-chloroform and ethanol precipitated as described in reference ;29;. DNA fragments were resolved in a 2% agarose gel and stained with ethidium bromide. To confirm apoptosis, infected cells were stained with Cy3-labeled annexin V (Sigma), according to the manufacturer's directions, or with propidium iodide (PI) and were visualized with an Olympus inverted microscope.
RESULTS
Internalization of S. aureus by CFT-1 and LCFSN cells.
We used CFT-1 cells and the complemented counterpart, LCFSN cells, for our internalization experiments. Pilot data revealed that S. aureus was internalized by both cell lines in a time- and dose-dependent manner (data not shown). Since both cell lines yielded similar internalization results at higher inocula (106 to 107 CFU/ml) (multiplicity of infection [MOI], 5:1 to 50:1), we decided to focus on the CF cell line, CFT-1, for the ensuing studies with 1 × 106 to 5 × 106 CFU/ml as the inoculum for epithelial cells (∼2 × 105 cells) for a 1-h infection period, followed by gentamicin treatment to kill extracellular bacteria.
To determine whether the cytoskeleton of epithelial cells is involved in bacterial uptake, epithelial cells were treated with cytochalasin D (to inhibit actin polymerization) or with colchicine (to inhibit microtubule formation) for 30 min prior to and during infection. Internalization of S. aureus was blocked by cytochalasin D at 0.5 μg/ml [mean internalization ± standard deviation, 0.7% ± 0.25%, versus 9.94% ± 3.29% for the untreated control at 5 h after infection] and colchicine at 40 μg/ml (3.35% ± 1.88% for the treated sample at 5 h after infection). Cycloheximide treatment (20 μg/ml) did not influence the uptake of S. aureus (data not shown). These data suggest that actin polymerization plays a major role in bacterial internalization but that the uptake process does not require de novo protein synthesis in epithelial cells.
To examine if the internalization process requires live bacteria, we heat killed S. aureus at 55°C for 1 h prior to the internalization assay. TEM revealed that heat-killed bacteria were also effectively internalized, indicating that internalization is not dependent on metabolically active organisms (data not shown).
Replication of intracellular S. aureus. (i) Internalization, with determination of CFU/ml.
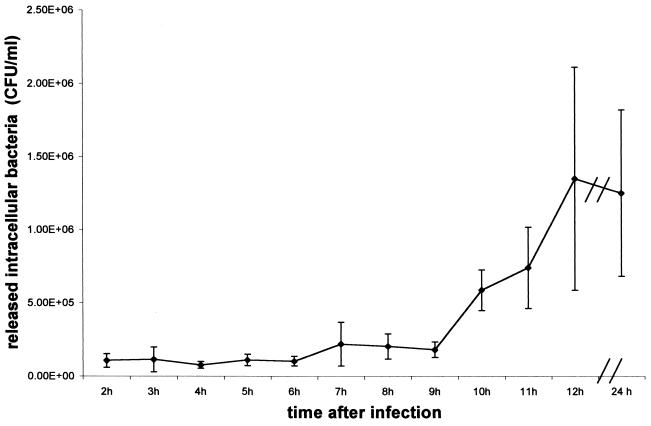
To determine if intracellular replication occurs, we enumerated hourly the number of intracellular bacteria in triplicate in 24-well plates beginning 2 h after infection, using 106 CFU/ml as the inoculum. As shown in Fig. 1, the number of intracellular bacteria did not change significantly until ∼7 to 9 h after infection, at which time the number of intracellular bacteria noticeably increased, indicating initiation of replication. As a negative control, we found that a protein A-deficient mutant of S. aureus invaded the CFT-1 cells poorly.
FIG. 1.
Study of intracellular replication. The internalization experiment was performed as described in Materials and Methods. Cells were infected for 1 h before gentamicin application and were lysed hourly to enumerate intracellular bacteria. Results are presented as means ± standard deviations (error bars) from one representative experiment repeated three times in triplicate.
(ii) TEM studies.
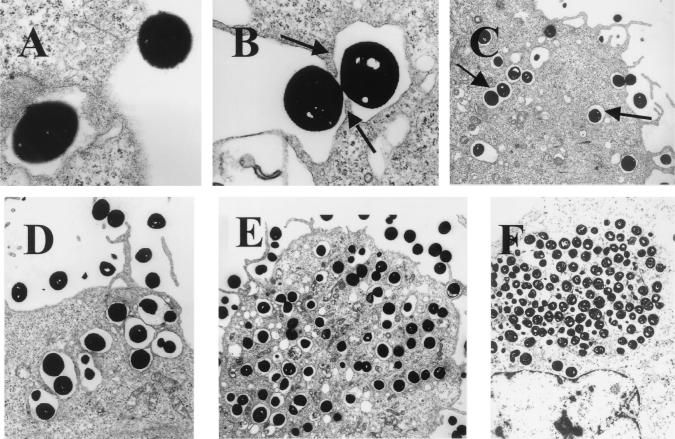
To facilitate visualization with TEM, we infected epithelial cells with a large inoculum (108/ml; MOI, 1:500). Electron microscopy revealed that endocytosis occurred within 5 min after infection (Fig. 2A). As shown in Fig. 2B, pseudopodia engulfing a bacterium were observed, suggesting a zipper-like mechanism of internalization. Bacteria were uniformly found in vacuoles inside the cells (Fig. 2C, D, and E), with one or two bacteria per vacuole. Increasing numbers of intracellular bacteria could be observed as the incubation period lengthened. At 24 h after infection, some cells were filled with intracellular bacteria (Fig. 2F). We also performed similar studies with heat-killed bacteria. Some vacuoles containing heat-killed bacteria (at 24 h after infection) were found to fuse with lysosomes, whereas vacuoles from epithelial cells infected with live bacteria did not (data not shown).
FIG. 2.
Electron micrographs of epithelial cells infected with S. aureus. Epithelial cells were infected with S. aureus at an MOI of 1:500 and fixed at various time points. (A) Immediately after infection (magnification, ×16,000); (B) 5 min after infection (magnification, ×8,300); (C) 15 min after infection (magnification, ×3,300); (D) 30 min after infection (magnification, ×3,300); (E) 1 h after infection (magnification, ×3,300); (F) 24 h after infection (magnification, ×3,300).
(iii) Fluorescence microscopy.
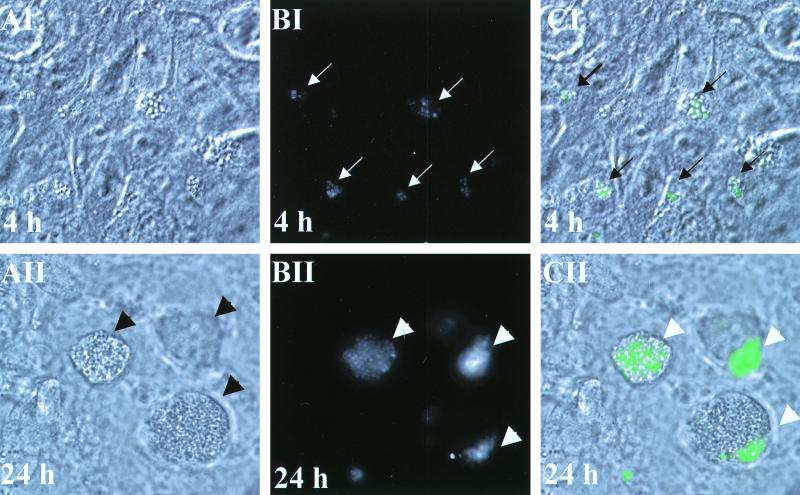
To analyze bacteria directly inside living cells, we infected cells with S. aureus RN6390 expressing GFPuvr. At 4 h after infection, morphologically normal cells (Fig. 3AI) similar to the noninfected control (not shown) were observed. However, green fluorescent bacteria (Fig. 3BI, in gray scale) in association with several cells were clearly visible when DIC and fluorescence images were merged (Fig. 3CI). At 24 h after infection, dead cells, appearing rounded and granulated, were found to be detached from the bottom of the well (Fig. 3AII). This cellular morphology is consistent with that of apoptosis (29). The noninfected control also demonstrated occasional rounded and detached cells, but to a much lesser extent (not shown). Heat-killed RN6390, like the noninfected control, also did not induce significant apoptosis. Combining DIC (Fig. 3AII) and fluorescence microscopy (Fig. 3BII), revealed that many apoptotic cells were filled with green fluorescent bacteria (Fig. 3CII).
FIG. 3.
Analysis of living epithelial cells infected with GFPuvr-expressing S. aureus RN6390. Cells were infected for 4 h (panels AI, BI, and CI) or 24 h (panels AII, BII, and CII). (AI and AII) DIC; magnification, ×92. (BI and BII) Fluorescence microscopy (in gray scale). (CI and CII) Merged images of DIC and fluorescence microscopy. The arrows point to fluorescent colonies.
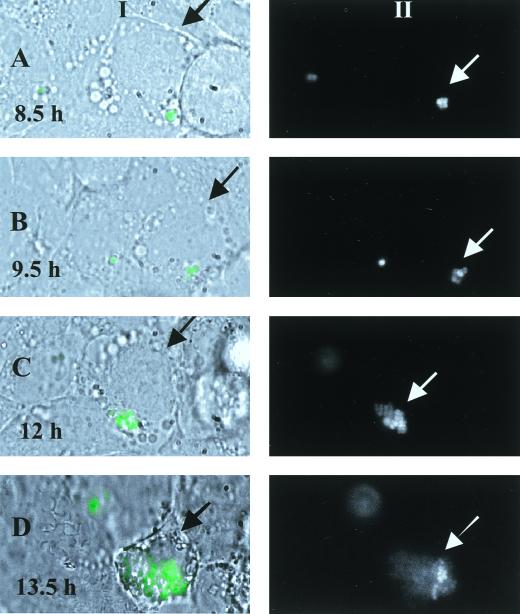
To follow the replication of intracellular S. aureus more closely, we monitored the replication of GFPuvr-expressing bacteria inside a single living cell for 14 h. As displayed in Fig. 4, replication of intracellular bacteria in this particular cell started at 9 h after infection (Fig. 4A), with increasing bacterial numbers as the incubation time lengthened (Fig. 4C and D). Phase-contrast images revealed that the morphology of the infected cell did not change until the 12th to 13th h after infection, at which time the cell began to round up with single blebbings (Fig. 4CI), proceeding to more pronounced rounding, blebbings, and subsequent detachment of the cell at 13.5 h (Fig. 4DI) as described elsewhere for apoptotic cells (27).
FIG. 4.
Intracellular replication of GFPuvr-expressing S. aureus inside a single epithelial cell. Cells were infected for 1 h before gentamicin application and then monitored under a microscope kept at 37°C at 30-min intervals beginning at 7 h postinfection up to 14 h. (A) Fluorescence microscopy merged with phase contrast (I) at 8.5 h; black arrows point to the monitored epithelial cell, while white arrows indicate fluorescent bacteria on a gray-scale image (II). The white color indicates saturation of the gray-scale image for green fluorescence (maximum intensity). (B, C, and D) Images at 9.5, 12, and 13.5 h.
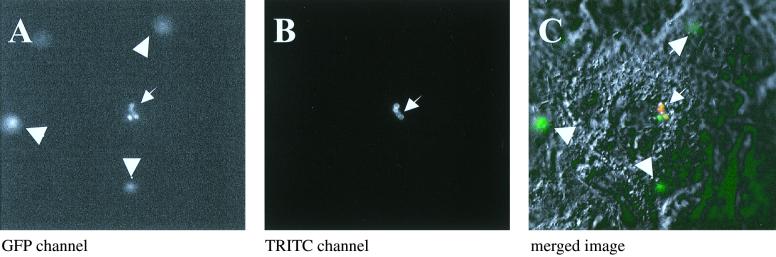
To ensure that most of the green fluorescent bacteria were intracellular, we stained extracellular S. aureus with chicken anti-protein A antibody followed by anti-chicken antibody conjugated to TRITC. Only a very few green fluorescent bacteria were stained red (TRITC), suggesting that most of the bacteria were intracellular (Fig. 5).
FIG. 5.
Staining of extracellular bacteria with anti-protein A antibody. At 4 h after infection of CFT-1 cells with GFPuvr-expressing S. aureus (A, GFP channel in gray scale), the cells were probed with chicken anti-protein A antibody, followed by a TRITC-labeled second antibody to label bacteria that were not internalized (B, TRITC channel). (C) Merged image of phase contrast and both channels.
Apoptosis induced in CFT-1 airway epithelial cells by infection with S. aureus, as assessed by DNA fragmentation.
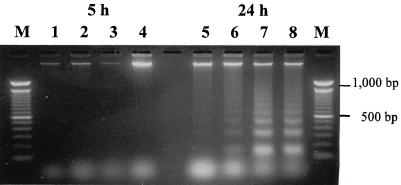
A hallmark of apoptosis is the characteristic DNA laddering upon gel electrophoresis. In early infection (i.e., 5 h after infection), extracted DNA from infected cells did not exhibit any fragmentation, even when the bacterial inoculum was increased to 107 CFU/ml (Fig. 6, lanes 2 through 4). At 24 h after infection, DNA laddering characteristic of apoptotic cells was apparent (Fig. 6, lanes 6 through 8). In contrast, similar fragmentation did not occur in noninfected controls. Remarkably, DNA fragmentation was not observed in infected cells in which internalization of S. aureus had been blocked by cytochalasin D. Likewise, cells infected with heat-killed bacteria or cells infected with live bacteria but treated with rifampin to inhibit bacterial transcription did not manifest any DNA fragmentation (data not shown).
FIG. 6.
DNA fragmentation in CFT-1 cells following infection with increasing inocula of S. aureus RN6390. At indicated times after infection, epithelial cell DNA was extracted, separated in a 2% agarose gel, and stained with ethidium bromide. Lane M, 100-bp ladder; lanes 1 and 5, control cells; lanes 2 and 6, 1 × 105 CFU/ml (MOI, 0.5:1); lanes 3 and 7, 1 × 106 CFU/ml (MOI, 5:1); lanes 4 and 8, 1 × 107 CFU/ml (MOI, 50:1).
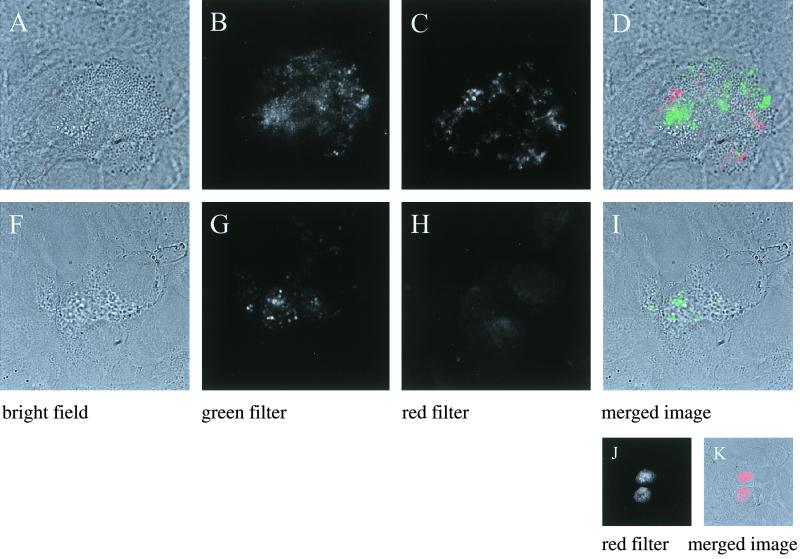
As annexin V is an early marker of apoptosis, we also stained infected cells and noninfected controls with Cy3-labeled annexin V, using PI as an indicator of necrosis, since this dye is excluded in early apoptotic cells. In correlation with the DNA fragmentation data, there was no staining with annexin V in either infected or control cells at 4 h after infection. However, at 24 h after infection, cells with GFPuvr-expressing bacteria were stained with Cy3-labeled annexin V but excluded PI (Fig. 7). These results, together with the DNA laddering data, demonstrated that cells infected with S. aureus became apoptotic.
FIG. 7.
Assessment of apoptosis in pulmonary epithelial cells by staining with Cy3-labeled annexin V (A to D) or PI (F to I). Morphologically altered cells with GFPuvr-expressing S. aureus (B) in infected monolayers were stained with Cy3-labeled annexin V (C). A merged image of bright field and green and red (Cy-3) channels is presented in panel D. A morphologically comparable but infected epithelial cell (F through I) excluded PI. (H) PI channel. As positive controls, necrotic epithelial cells were stained with PI (J and K).
DISCUSSION
Epithelial cells, which have an intact host defense mechanism, are normally efficient in keeping the respiratory tract sterile in healthy individuals. However, in compromised individuals such as CF patients, a breach of the innate immune system (2) leads to chronic and persistent pulmonary infections with S. aureus (5). Although S. aureus has been classically described as an exclusively extracellular pathogen, there is growing evidence that S. aureus may be internalized into epithelial cells (3, 10, 11, 17, 19). However, because of technical limitations in visualizing live intracellular bacteria, it was not possible to determine in these antecedent studies whether the bacteria are actively replicating or are merely intracellular bystanders (i.e., acting like latex particles). Alternatively, with a large infecting inoculum, the recovered organisms may represent adherent but extracellular colonies that are not killed efficiently by gentamicin.
From the bacteria's perspective, there are obvious advantages in maintaining an intracellular location, since the microorganisms will be able to avoid many host defense mechanisms as well as to shield themselves from extracellular antibiotics. Importantly, indolent but gradual intracellular replication may minimize acute inflammatory responses from the host. Additionally, a successful sojourn inside nonphagocytic cells (e.g., pulmonary epithelial cells) would likely entail subversion of the host signals in the endocytic pathway (e.g., for fusion with the lysosome), thus implying cross talk between S. aureus and host cells. For these reasons, we wanted to demonstrate intracellular replication of green fluorescent S. aureus, using pulmonary epithelial cells as a model.
Our studies demonstrate that S. aureus was efficiently internalized by CF airway epithelial cells as well as the complemented counterpart in a time- and dose-dependent fashion. Consistent with the results of Pier et al. (21), we did not observe differences in internalization between the CF cell line CFT-1 and the complemented cell line LCFSN at challenge inocula of 106 to 108 CFU/ml, indicating that S. aureus internalization is not mediated by CFTR. Contrary to the study by Imundo et al. (12), who found increased S. aureus adherence to CF cell lines compared with controls, our studies compared internalization and not adherence. Interestingly, Sinha et al. (23) recently showed that S. aureus internalization by several cell lines, including a kidney cell line, endothelial cells, and primary fibroblasts, was mediated by the binding of the bacterial fibronectin-binding receptor to cellular fibronectin. Whether the fibronectin-binding protein of S. aureus mediates bacterial internalization via the α5β1-integrin fibronectin receptor on airway epithelial cells remains to be determined.
Bacterial uptake, as revealed by electron microscopy, is probably facilitated by the formation of pseudopodia which engulf the organism. The close contact of the bacterium with the host membrane resembles a zipper-like mechanism, as has been described for the internalization of Yersinia. Accordingly, Yersinia binds mammalian adhesion receptors via a surface protein (invasin). In contrast, a trigger-type mechanism is used by Salmonella species to induce internalization by injecting bacterial proteins into the host cell cytosol (9).
Contrary to the classical view of S. aureus as an extracellular pathogen, we found that S. aureus replicates actively inside pulmonary epithelial cells. Although other gram-positive bacteria such as group A and group B streptococci have been shown to be internalized by respiratory epithelial cells, the intracellular fate of these organisms (i.e., replication versus degradation within the endosome) has not been defined (16, 22). In this study, the replication of intracellular S. aureus was confirmed by several experimental techniques, including (i) internalization experiments with hourly bacterial enumeration, (ii) TEM studies showing higher numbers of intracellular bacteria at 24 h after infection than at 1 h, and (iii) monitoring living cells infected with GFPuvr-expressing S. aureus by time-lapse video and directly observing initiation of intracellular replication at ∼9 h after infection. The combination of these experimental approaches clearly demonstrated the replication of S. aureus inside pulmonary epithelial cells. The observation that intracellular replication initiated after a lag phase of 7 to 9 h emphasizes the putative role of host signals or factors in intracellular bacterial growth. Alternatively, S. aureus bacteria may have to adapt to the intracellular milieu, with the eventual delay of de novo protein synthesis and DNA replication.
In our TEM study, we consistently observed that live S. aureus internalized into pulmonary epithelial cells remained in vacuoles without any evidence of lysosomal fusion even 24 h after infection. In contrast, some vacuoles containing heat-killed bacteria revealed evidence of lysosomal fusion. This observation is consistent with our replication data, indicating that there may be cross talk between intracellular S. aureus and pulmonary epithelial cells. However, a previous study by Lowy and colleagues (18) found no difference in lysosomal fusion between viable and UV-killed S. aureus that had been internalized into cultured human endothelial cells. The difference in results may be due to cell lines (endothelial versus pulmonary epithelial cells) and/or killing methods (UV versus heat).
We also demonstrated that infected airway epithelial cells revealed evidence of apoptosis, with typical DNA laddering, alteration of cellular morphology, and annexin V staining occurring at 24 h but not at 4 h after infection. Like human intestinal epithelial cells infected with invasive intestinal pathogens (15), airway epithelial cells undergo apoptosis late in response to S. aureus internalization and replication. The colocalization of green fluorescent bacteria inside apoptotic cells in these experiments suggests that bacterial replication precedes and conceivably induces apoptosis. Time-lapse monitoring of single infected living cells confirmed that intracellular bacterial replication indeed preceded gross morphological changes typical of apoptosis (e.g., apoptotic bodies). Apoptosis was not induced in cells where internalization was blocked by cytochalasin D or in cells infected with heat-killed bacteria. In addition, treatment of intracellular bacteria with rifampin, a bacteriostatic antibiotic that readily enters mammalian cells, prevented the induction of apoptosis. We also found that Cowan I, an S. aureus laboratory isolate that was internalized but failed to replicate, did not induce apoptosis in pulmonary epithelial cells; likewise, a protein A mutant of S. aureus was poorly internalized and hence did not lead to apoptosis (unpublished data). Taken together, these data imply that internalization and replication of metabolically active bacteria are associated with the commitment of apoptosis in respiratory epithelial cells.
Several factors, including reduced bacteriocidal activity of airway surface fluid (24) and/or increased availability of asialo-GM1 receptors on epithelial cells (12), contribute to persistent infections with S. aureus in susceptible (e.g., CF) patients. It now appears that internalization and replication of S. aureus in pulmonary epithelial cells, with ensuing apoptosis, may be important factors contributing to the pathogenesis of persistent S. aureus infections in patients with compromised airway defense mechanisms. The triggering of apoptosis may be a host response to reduce or inhibit bacterial proliferation, since these cells will be sloughed off or phagocytosed by scavenging macrophages (27). However, the bacteria released from the apoptotic cells may usurp this defense mechanism by initiating a new round of host cell infection. Further studies are needed to understand the molecular mechanisms by which S. aureus replicates intracellularly and induces apoptosis. The indolent replication of S. aureus also serves to emphasize the importance of the intracellular growth requirement and the effect of host factors on bacterial replication. Understanding these phenomena will help identify ways to augment the host immune system to combat this important pathogen.
ACKNOWLEDGMENTS
We thank W. Hellmann for her excellent assistance with the TEM, J. R. Yankaskas for providing the CFT-1 and LCFSN-CFT-1 cell lines, and M. Roggiani for fruitful discussions and critical review of the manuscript.
B.C.K. was funded by the Medical Faculty of the University of Münster, IMF grant Ka-1-6-I/98-45. M.G. is a W.M. Keck fellow. M.G. and S.M.S. were supported by the Molecular Biophotonics Laboratory (Hamamatsu City, Japan) and American Cancer Society grant RPG9817701CDD (to S.M.S.). G.K. was supported by NIH grant AI22616, and A.L.C. was supported partially by NIH grant AI47441.
REFERENCES
- 1.Abman S H, Ogle J W, Harbeck R J, Butler-Simon N, Hammond K B, Accurso F J. Early bacteriologic, immunologic and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J Pediatr. 1991;119:211–217. doi: 10.1016/s0022-3476(05)80729-2. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Weiner D J, Wilson J M. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;365:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns J L, Emerson J, Stapp J R, Yim D L, Krzewinski J, Louden L, Ramsey B W, Clausen C R. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 6.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar− agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Nast C C, Bayer A S. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect Immun. 1998;66:5988–5993. doi: 10.1128/iai.66.12.5988-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 9.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamill R J, Vann J M, Proctor R A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986;54:833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 12.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci USA. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isberg R R, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by E. coli K12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 14.Jiang C, Finkbeiner W E, Widdicombe J H, McCray P B, Jr, Miller S S. Altered fluid transport across airway epithelium in cystic fibrosis. Science. 1993;262:424–427. doi: 10.1126/science.8211164. [DOI] [PubMed] [Google Scholar]
- 15.Kim J M, Eckmann L, Savidge T C, Lowe D C, Witthoeft T, Kagnoff M F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaPenta D, Rubens C E, Chi E Y, Cleary P P. Group A streptococci efficiently invades human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–12119. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowy F. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 18.Lowy F D, Fant J, Higgins L L, Ogawa S K, Hatcher V B. Staphylococcus aureus-human endothelial cells interactions. J Ultrastruct Mol Struct Res. 1988;98:137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- 19.Menzies B E, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66:5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novick R P. The staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH; 1990. pp. 1–40. [Google Scholar]
- 21.Pier G B, Grout M, Zaida T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubens C E, Smith S, Hulse M, Chi E Y, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha B, Francois P, Vaudaux P, Foti M, Hartford O M, Foster T J, Lew D P, Hermann M, Krause K H. Fibronectin binding protein acts as S. aureus invasin via fibronectin bridging to integrin α5 β1. Cell Microbiol. 1999;1:101–118. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith J J, Travid S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 25.Touchie C, Marrie T J. Respiratory tract infections. In: Crossley K B, Archer G L, editors. The staphylococci in human diseases. London, England: Churchill Livingstone; 1997. pp. 475–492. [Google Scholar]
- 26.Weaver L T, Green M R, Nicholson K, Mills J, Heeley M E, Kuzemko J A, Austin S, Gregory G A, Dux A E W, David J A. Prognosis in cystic fibrosis treated with continuous flucloxacillin from the neonatal period. Arch Dis Child. 1994;70:84–89. doi: 10.1136/adc.70.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinrauch Y, Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Yankaskas J R, Haizlip J E, Conrad M, Koval D, Lazarowski E, Paradiso A M, Reinhart C A J, Sarkaki B, Schlegel R, Boucher R C. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–C1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 29.Zychlinsky A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]