Abstract
This study shows that gamma interferon (IFN-γ) and interleukin-4 (IL-4) cytokine responses are produced by peripheral blood cells in cattle infected with Mycobacterium bovis. The different kinetics of the IFN-γ and IL-4 responses to bovine tuberculin and to ESAT-6 following experimental intratracheal infection with M. bovis are described. An early increase in IFN-γ was observed that was maintained throughout the period studied. In contrast, the IL-4 response was delayed and confined to a peak of activity lasting 6 to 8 weeks. Interestingly, an experimental challenge of cattle with a lower dose of M. bovis which did not result in the development of lesions, positive DTH skin test, or substantial IFN-γ responses nevertheless generated strong specific IL-4 responses. Investigation of naturally infected M. bovis field reactors showed increased IFN-γ and IL-4 responses compared to uninfected cattle and that both of these cytokines were equally able to differentiate infected from uninfected animals. The magnitude of the M. bovis-induced IL-4 responses were found to be similar to the antigen-specific IL-4 responses of cattle infected with the parasitic nematode Onchocerca ochengi, further supporting the presence of this type 2 cytokine in bovine tuberculosis.
Tuberculosis is a major public health problem worldwide and a leading cause of mortality due to infectious disease (28). The emergence of drug-resistant strains of Mycobacterium tuberculosis (24) and the potential effects of the increasing prevalence of human immunodeficiency virus and AIDS (22) makes the search for both drug and vaccine solutions all the more pertinent. The incidence of Mycobacterium bovis tuberculosis in British cattle is also increasing, and the urgency for new and improved cattle vaccines and diagnostic aids has been clearly stated in a recent independent scientific review (16).
For the rational development of such reagents, it is important to assess the nature of the immune responses that occur following infection. In tuberculosis, much work has concentrated upon the two cytokines, gamma interferon (IFN-γ) and interleukin-4 (IL-4), and the inferred polarization of T-cell subsets. Mouse models of tuberculosis have been shown to display polarized T-cell responses, where IFN-γ is clearly an important cytokine in the control of the infection (12), largely due to its potent role in macrophage activation (7). Conversely, IL-4 has been reported to be associated with the development and progression of disease (14), although other studies using IL-4 gene-disrupted mice failed to support such a role for IL-4 (10, 23). It has also been postulated that the reactivation of latent tuberculosis involves a type-1-to-type-2 cytokine shift (13).
Cytokine responses in human tuberculosis appear to be more complex. Data reported from some studies have reflected those gathered from mouse models with a similar, if not quite so polarized, type-1-to-type-2 cytokine shift from mild through to advanced clinical disease (1, 8). However, others have observed an increase in serum IFN-γ (35) and in local IFN-γ and IL-4 responses of bronchoalveolar lavage cells (32, 34) in patients with active tuberculosis, suggesting the presence of both type 1 and type 2 cytokine responses during disease.
Opinions are similarly divided upon whether polarized T-cell responses occur against infectious agents in cattle. Brown et al. (3) described the presence of both IFN-γ and IL-4 mRNA in the T-cell clones of cattle infected with the parasitic helminth Fasciola hepatica. In contrast, bovine diarrhea virus in cattle has recently been shown to induce different T-cell subsets (CD4+ and CD8+) to produce IL-4 and IFN-γ proteins, respectively (29). In bovine tuberculosis, IFN-γ responses are well known to be produced after infection (9, 21, 39, 40), but IL-4 responses during infection have not yet been investigated.
This study describes the IL-4 and IFN-γ responses in the peripheral blood of cattle both experimentally and naturally infected with M. bovis. Cytokine responses to tuberculin purified protein derivative (PPD) and to a recombinant form of the immunodominant M. bovis antigen ESAT-6 (25, 26) were measured. The response kinetics of these two cytokines in experimentally infected cattle and the ability of both IFN-γ and IL-4 to differentiate between infected and uninfected animals are discussed.
MATERIALS AND METHODS
Mycobacterial antigens.
Tuberculin PPD preparations from M. bovis (PPD-M) and from Mycobacterium avium (PPD-A) were produced at the Veterinary Laboratories Agency (VLA) of Weybridge (VLA Weybridge) as described previously (36). Recombinant ESAT-6 (36) was kindly provided by Adam Whelan (VLA Weybridge).
Animals. (i) Experimental M. bovis infection.
Six female Friesian-Limousin cross-calves (CN1101, CN1104, CN1100, CN1104, CN1098, and CN1109) approximately 6 months old were obtained from a herd free of M. bovis infection, as defined by a history of negative skin test results. These animals were also found to be negative in an IFN-γ diagnostic assay for bovine tuberculosis. Two of the calves (CN1101 and CN1107) were infected with 6.6 × 103 CFU, two calves (CN1100 and CN1104) were infected with 6.6 × 104 CFU, and two calves were infected with 6.6 × 105 CFU M. bovis intratracheally according to the method of Buddle et al. (4). During the study, animals were housed in a high-security isolation unit under negative pressure, and expelled air was filtered. Two weeks prior to the postmortem point, the single comparative intradermal tuberculin skin test (SCITT) was performed on all animals as described previously (11). Euthanasia was carried out at 20 weeks postinfection by intravenous injection of sodium pentobarbitone, and a detailed postmortem analysis was performed as described below. For lymph nodes, each of the following types of lymph nodes (or lymph node chain) was removed aseptically: right and left lateral retropharyngeal, right and left medial retropharyngeal, right and left submandibular, right and left cervical superficial, right and left bronchial, cranial mediastinal chain, caudal mediastinal chain, and mesenteric. These lymph nodes were subsequently serially sliced (ca. 2 mm) with a scalpel and inspected. Samples from individual nodes and lesions were obtained for mycobacterial culture and histological examination. The remaining superficial and visceral lymph nodes were inspected in situ. For lungs, each lobe was serially sliced (ca. 5 mm) with a sharp knife. All slices were palpated and inspected. The respiratory airways were cut open as far into the lung parenchyma as possible and then inspected.
(ii) Naturally infected M. bovis field reactors.
Blood samples were obtained from 21 skin test-positive (SCITT+) cattle. Where gross lesions were visible, lesioned material was collected for microbiological culture. In the absence of visible lesions, a standard pool of lymph node material (cranial, bronchial mediastinal, and mesenteric lymph nodes) was collected at the abattoir for the bacterial culture which was used to confirm the presence of M. bovis.
(iii) Uninfected controls.
Thirteen cattle were obtained from farms free of M. bovis (confirmed as above by a history of negative skin test [SCITT−] results). These animals represented the control negative group for this investigation.
(iv) Experimental helminth infection.
Six Jersey bull calves reared off pasture, with no prior exposure to parasitic helminths, were infected with the filarial nematode Onchocerca ochengi. Infective larvae were produced in blackflies as described elsewhere (2) using cryopreserved microfilariae collected from cattle in northern Cameroon. Animals were infected at approximately 6 months of age and received 350 infective (third-stage) larvae, each by subcutaneous injection along the ventral midline. Patent infections (detected by the appearance of microfilariae in skin biopsies from the ventral midline) developed after 9 to 15 months. One uninfected control animal was used in the helminth study for longitudinal comparisons. Peripheral blood mononuclear cells (PBMC) culture supernatants from blood taken at the same time point as that for O. ochengi-infected cattle in our study were negative for both IFN-γ and IL-4 responses (data not shown).
PBMC culture.
PBMC culture supernatants were assessed for IL-4 and IFN-γ responses (field reactors, negative controls, and helminth-infected animals) or for IL-4 responses alone (experimental M. bovis-infected animals). PBMC were separated from heparinized venous blood over Histopaque 1077 (Sigma) and resuspended in culture medium (RPMI 1640 with Glutamax [Gibco] supplemented with 5% CPSR [serum replacement; Gibco], nonessential amino acids [Gibco], 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5 × 10−5 M 2-mercaptoethanol [Gibco]). Cells were resuspended to 2 × 106/ml, and 0.1 ml was added per well to 96-well flat-bottom microtiter plates (Nunc). Antigens were added, at 0.1 ml/well in triplicate (all at a 10-μg/ml final concentration). The cultures were incubated for 6 days at 37°C in 5% CO2. Supernatants (100 μl/well) were then harvested and assayed for IFN-γ and IL-4. PBMC from helminth (O. ochengi)-infected cattle, obtained 27 days postinfection, were cultured under similar conditions, except that the medium was supplemented with 10% fetal calf serum (FCS) in place of CPSR, parasite antigen (derived from a phosphate-buffered saline extract of adult worms) was used at 50 μg/ml, and cells were cultured for 3 days at 5 × 106/ml. The incubation times of PBMC from M. bovis and O. ochengi-infected cattle (6 and 3 days, respectively) for supernatants were set according to the optimal PBMC proliferation times determined for these two different experimental infection systems. Supernatants were then harvested and assessed for IFN-γ and IL-4. Data are shown for cytokine activity at 27 days postinfection when both IL-4 and IFN-γ were observed at peak response levels (S. Graham et al., unpublished data).
Whole-blood culture for IFN-γ.
IFN-γ production by experimentally infected animals was assessed using the whole-blood culture method described by Emery et al. (9). Peripheral whole blood was diluted 1:1 in culture medium (120 μl of blood plus 120 μl of medium per well in duplicate in 96-well flat-bottom tissue culture trays) in the presence or absence of antigen (10 μg/ml, final concentration) for 24 h. Supernatants were then harvested and assayed for IFN-γ.
Cytokine assays. (i) IFN-γ.
Whole-blood and PBMC neat supernatants were assessed for IFN-γ content using a commercially available antigen capture enzyme-linked immunosorbent assay (ELISA) kit, Bovigam (CSL, Ltd., Parkville, Victoria, Australia), according to the manufacturer's instructions. Results are expressed as changes in the optical density (ΔOD; i.e., the ΔOD at 450 nm [ΔOD450] is the mean OD of antigen-stimulated supernatants minus the mean OD of the control, unstimulated supernatants at 450 nm). A positive result was taken as an OD450 of antigen-stimulated supernatant that was greater than twice the OD450 of the control, unstimulated supernatant (36) and also greater than 0.2.
(ii) IL-4.
IL-4 activity in neat PBMC culture supernatants was measured by using a B-cell bioassay according to the method of Kuhnle et al. (17). Briefly, bovine B cells from a SCITT− animal were positively sorted from PBMC using the monoclonal antibody IL-A58 (immunoglobulin G2a [IgG2a]; courtesy of the Institute for Animal Health [IAH], Compton, Berkshire, United Kingdom) specific for bovine immunoglobulin light chains (38), which both labels and preactivates the B cells by cross-linking the surface immunoglobulin. PBMC were then incubated with rat anti-mouse IgG2(a+b)-coated microbeads before positive sorting using the MACS column separation system (Miltenyi Biotech, Surrey, United Kingdom). B cells (determined to be >97% pure by fluorescence-activated cell sorter analysis) were eluted, washed, and resuspended to 106/ml in tissue culture medium (RPMI 1640 with Glutamax [Gibco] supplemented with 10% FCS [Gibco], nonessential amino acids [Gibco], 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5 × 10−5 M 2-mercaptoethanol [Gibco]), and 100 μl was added per well to 96-well tissue culture trays with 50 μl of PBMC supernatants in duplicate. Plates were incubated for 24 h at 37°C in 5% CO2, pulsed with tritiated thymidine, and incubated for a further 24 h before harvesting. The results are presented as stimulation indices (SI), i.e., the mean counts per minute (cpm) of B-cell proliferation in the presence of antigen-stimulated supernatant divided by the mean cpm of B-cell proliferation in the presence of unstimulated control supernatant. A positive response was taken as an SI value of >3. The specificity of this bioassay for IL-4 has been previously determined by inhibition with a neutralizing monoclonal antibody (29). However, a neutralizing monoclonal antibody to IL-4 (IAH) was included in a number of the assays in this report and equally neutralized both the recombinant IL-4 (IAH) used as a positive control in all assays and the IL-4 activity present in the supernatants tested (data not shown).
RESULTS
Pathological findings of M. bovis-infected cattle. (i) Experimental infection.
Positive SCITT responses (ΔSCITT from 10 to 12 mm) were observed in four experimentally infected cattle (CN1100, CN1104, CN1098, and CN1109). These four animals had received infection doses of M. bovis of 6.6 × 104 or 6.6 × 105 CFU. At the postmortem point, all four animals presented with gross tuberculous lesions typical of natural bovine tuberculosis: CN1109 in the lateral and medial retropharyngeal, right submandibular, and bronchial lymph nodes; CN1098 in the medial retropharyngeal, bronchial, and mediastinal lymph nodes; CN1104 in the lateral and medial retropharyngeal, submandibular, bronchial, and mediastinal lymph nodes; and CN1100 in the lateral retropharyngeal, bronchial, mediastinal, and mesenteric lymph nodes and in the lungs. The lesions were histopathologically confirmed as tuberculous granulomas, and M. bovis was cultured from these lesions. The two animals (CN1101 and CN1107) that received the lowest dose (6.6 × 103 CFU) of M. bovis remained SCITT−. No lesions were observed in these animals, and M. bovis was not confirmed either by histopathology or by microbiological culture of tissues.
(ii) Field reactors.
Data concerning the naturally infected field reactor cattle are shown in Table 1. Visible lesions were observed in 17 of 21 animals, while 4 of 21 were classed as nonvisible-lesion reactors. However, M. bovis infection was confirmed in all animals by microbiological culture of M. bovis from tissues collected postmortem. Skin test response results (ΔSCITT) ranged from 2 to 42 mm.
TABLE 1.
M. bovis field reactorsa
| Animal no. | Presence of gross lesions | Δ SCITT response (mm) |
|---|---|---|
| 1 | VL | 19 |
| 2 | VL | 6 |
| 3 | VL | 5 |
| 4 | VL | 15 |
| 5 | VL | 16 |
| 6 | VL | 30 |
| 7 | VL | 8 |
| 8 | VL | 11 |
| 9 | VL | 13 |
| 10 | VL | 15 |
| 11 | VL | 42 |
| 12 | VL | 6 |
| 13 | VL | 22 |
| 14 | VL | 8 |
| 15 | VL | 31 |
| 16 | VL | 8 |
| 17 | VL | 23 |
| 18 | NVL | 11 |
| 19 | NVL | 11 |
| 20 | NVL | 5 |
| 21 | NVL | 2 |
A total of 21 cattle were included in the study. Of these, 17 cattle presented with visible lesions (VL) at the abbatoir, and 4 were classed as nonvisible-lesion (NVL) reactors. M. bovis infection was confirmed in all animals by microbiological culture. The ΔSCITT value shows the magnitude (in millimeters) by which the DTH response to PPD-M exceeded the DTH response to PPD-A.
IFN-γ and IL-4 responses following experimental infection with M. bovis.
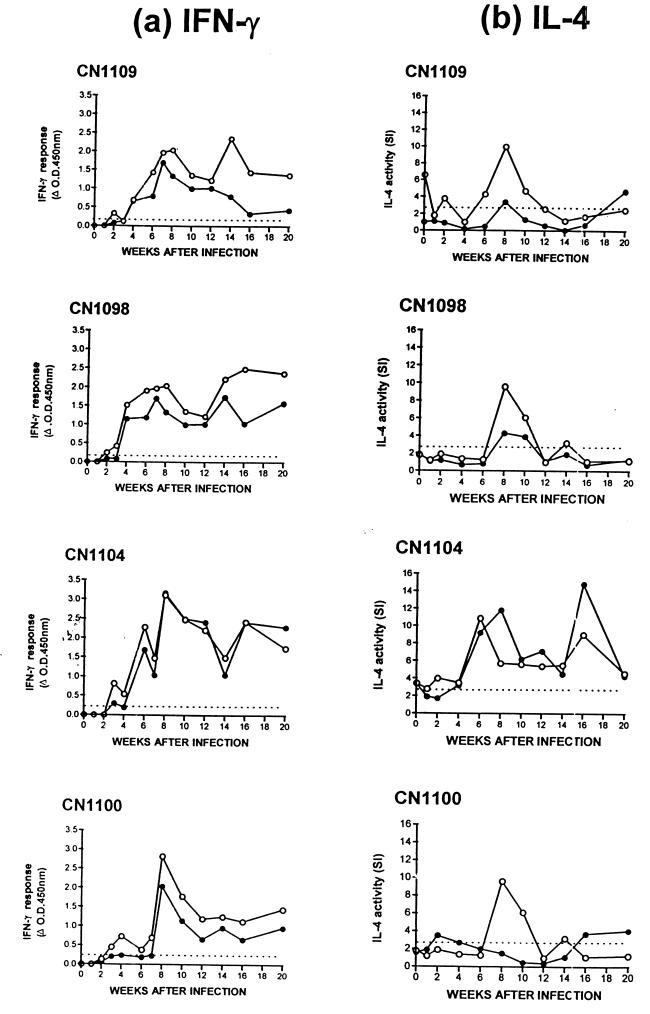
Figure 1 shows the IFN-γ and IL-4 responses induced by both PPD-M and ESAT-6 in the four SCITT+ experimentally infected cattle (CN1100, CN1104, CN1098, and CN1109). Increased IFN-γ and IL-4 responses were observed in all four animals, and cytokine responses to ESAT-6 largely reflected the responses to PPD-M, although they were slightly lower in most cases.
FIG. 1.
IFN-γ (a) and IL-4 (b) responses of four animals experimentally infected with either 6.6 × 104 CFU (CN1100 and CN1104) or 6.6 × 105 CFU (CN1098 and CN1109) of M. bovis. Responses to PPD-M (○) and ESAT-6 (●) are shown for each time point. Results are expressed as the ΔOD450 for IFN-γ and the SI of B-cell proliferation for IL-4. A positive IFN-γ response was taken as a mean OD value of antigen-stimulated supernatant that was greater than twice the mean OD of the control unstimulated supernatant and also >0.2 (the dotted line indicates an OD of 0.2). A positive IL-4 response was taken as an SI value of >3.0 (the dotted line indicates an SI of 3.0). Data for IFN-γ in Fig. 1a have been reproduced from Rhodes et al. (31).
An increased IFN-γ response to either PPD-M and/or ESAT-6 was observed in all four animals by 4 weeks postinfection, as previously described (31). In general, these responses continued to increase up to 6 to 8 weeks postinfection. Strong positive responses, with some variation over time, were then sustained throughout the period studied in all animals.
Increased IL-4 activity induced by tuberculin was detected slightly later than the IFN-γ responses, with a sharp rise occurring at around 6 to 8 weeks in all animals. In contrast to the IFN-γ responses, which were sustained during the period studied, IL-4 activity was contained within a distinct peak over a short period of time (6 to 8 weeks). A second peak of IL-4 activity was observed in response to ESAT-6 at 16 weeks in one animal only (CN1104), and this peak again lasted for approximately 6 weeks. The early peak of ESAT-6-induced IL-4 production (observed in CN1109, CN1098, and CN1104) was not observed in CN1100, where instead we observed ESAT-6-stimulated IL-4 production at 16 to 20 weeks postinfection.
IFN-γ and IL-4 responses in naturally infected field reactors.
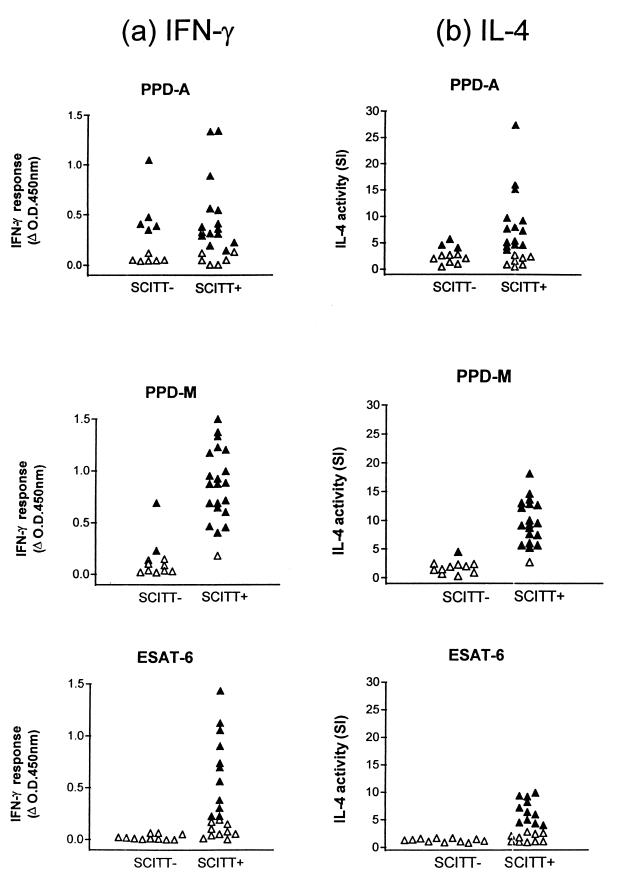
Figure 2 shows the IFN-γ and IL-4 responses observed in PBMC supernatants from naturally infected (SCITT+) and control uninfected (SCITT−) cattle following in vitro stimulation with PPD-A, PPD-M, and ESAT-6. The infected nonvisible-lesion animals were grouped together with the infected visible-lesion animals since there was no obvious difference between these groups on the basis of IFN-γ or IL-4 production.
FIG. 2.
IFN-γ (a) and IL-4 (b) responses of 21 naturally infected M. bovis field reactors (SCITT+) and 11 uninfected controls (SCITT−). Responses to PPD-A, PPD-M, and ESAT-6 are shown. Results are expressed as the ΔOD450 for IFN-γ and as SI values of B-cell proliferation for IL-4. A positive IFN-γ result was taken as a mean OD450 of the antigen-stimulated supernatant that was greater than twice the mean OD450 of the control unstimulated supernatant and also >0.2. A positive IL-4 result was taken as an SI value that was >3.0 (▴, positive responses; ▵, negative responses).
Of the infected (SCITT+) cattle, positive IFN-γ and IL-4 responses to PPD-A were identified in 15 and 14 of 21 cattle, respectively, while in the control uninfected (SCITT−) group 5 of 11 animals showed positive IFN-γ responses, and 3 of 11 showed positive IL-4 responses to PPD-A. In the responses to PPD-M, positive IFN-γ and IL-4 responses were observed in 20 of 21 SCITT+ cattle each, while in the SCITT− group only 2 and 1 of 11 animals gave positive IFN-γ and IL-4 responses, respectively. In all SCITT+ animals that gave positive cytokine responses, the IFN-γ or IL-4 response to PPD-M was always higher than the response to PPD-A in individual animals. This finding was in keeping with the normal method of diagnostic practice (i.e., responses to PPD-M to exceed responses to PPD-A). Of 21 SCITT+ cattle, 11 gave positive IFN-γ and IL-4 responses to ESAT-6, representing 55% of those animals that responded to PPD-M. None of the SCITT− cattle gave positive IFN-γ or IL-4 responses to ESAT-6. No correlations were observed between the magnitude of IFN-γ responses and the magnitude of IL-4 responses to individual antigens within the SCITT+ group or between the size of the SCITT response and the magnitude of the cytokine responses.
IFN-γ and IL-4 responses in low dose M. bovis-challenged calves.
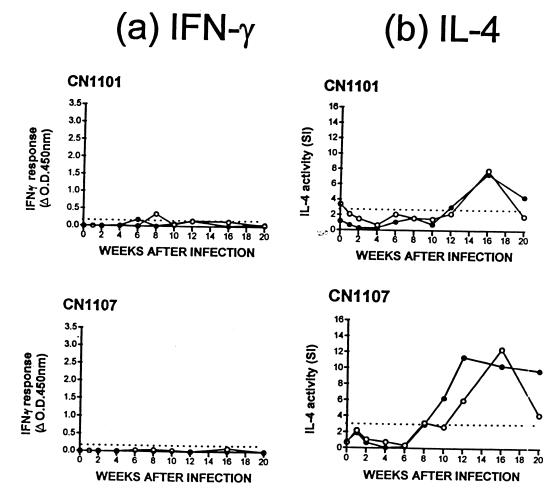
Figure 3 shows the IFN-γ and IL-4 responses of the two animals (CN1101 and CN1107) that were challenged intratracheally with the lowest dose (6.6 × 103 CFU) of M. bovis. These animals showed a negative SCITT test at 20 weeks postchallenge. No lesions were observed postmortem, and tissue samples removed for microbiological culture were negative for M. bovis. The results show that a weak and transitional positive IFN-γ response to PPD-M was observed in one animal (CN1101) at 8 weeks postchallenge. No other positive IFN-γ responses to any other antigens tested (MPB64, MPB70, MPB83, Ag85, hsp16.1, hsp65, hsp70, and 38kDa; data not shown) were recorded in these animals. In contrast, substantial IL-4 responses to both PPD-M and ESAT-6 were observed in both animals from 10 to 12 weeks postchallenge. IL-4 responses to PPD-M in both animals peaked at 16 weeks before a decline toward 20 weeks postchallenge. IL-4 responses to ESAT-6 again reflected those to PPD-M, except in animal CN1107, where the peak IL-4 activity occurred at 12 weeks, and this response was maintained over the 20 weeks of the experiment.
FIG. 3.
IFN-γ (a) and IL-4 (b) responses of two animals (CN1101 and CN1107) experimentally challenged with the lowest dose (6.6 × 103 CFU) of M. bovis. Responses to PPD-M (●) and ESAT-6 (●) are shown. Results are expressed as the ΔOD450 for IFN-γ and as the SI values of B-cell proliferation for IL-4. A positive IFN-γ response was taken as a mean OD value of antigen-stimulated supernatant that was greater than twice the mean OD of the control unstimulated supernatant and also >0.2 (the dotted line indicates an OD of 0.2). A positive IL-4 response was taken as an SI value of >3.0 (the dotted line indicates an SI of 3.0).
The peak of IL-4 activity in these two low-dose animals occurred at 5 to 8 weeks later than the peak IL-4 activity in SCITT+ cattle challenged with the higher doses of M. bovis. The magnitude of the IL-4 responses in all six experimental cattle, however, was equivalent regardless of M. bovis dose, i.e., the peak SI values in response to PPD-M were 7.9 (CN1101) and 12.4 (CN1107) in low-dose cattle versus 10.9 (CN1104), 6.3, (CN1100), 9.6 (CN1098), and 10.0 (CN1109) in higher-dose cattle.
Comparison of M. bovis-induced cytokines with those induced by a parasitic helminth.
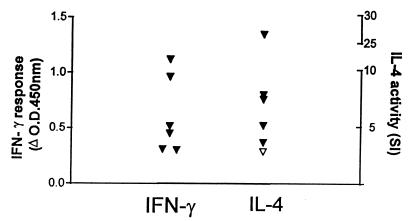
The IL-4 responses of M. bovis-infected cattle were compared to those observed in an IL-4-inducing bovine model of helminthic infection. We used the same B-cell bioassay to measure the helminth-specific IL-4 responses of cattle experimentally infected with the parasitic nematode O. ochengi. Figure 4 shows these helminth-specific IFN-γ and IL-4 responses at a single time point 27 days postinfection, when maximal cytokine responses were obtained (Graham et al., unpublished data).
FIG. 4.
Helminth-specific IFN-γ and IL-4 responses of six cattle experimentally infected with the parasitic nematode O. ochengi. Results are expressed as the ΔOD450 for IFN-γ and as the SI values of B-cell proliferation for IL-4. A positive IFN-γ result was taken as a mean OD value of antigen-stimulated supernatant that was greater than twice the mean OD value of control unstimulated supernatant and also >0.2. A positive IL-4 result was taken as an SI value of >3.0 (▾, positive responses; ▿, negative responses).
Positive IL-4 responses were observed in five of six cattle, and the SI values of these responses ranged from 3.6 to 26.5, with most being <10. These helminth-specific IL-4 responses therefore appeared to be similar in magnitude to the M. bovis-specific IL-4 responses measured above. However, supernatants from O. ochengi-infected cattle induced higher B-cell proliferation (measured in cpm) in the IL-4 bioassay than did supernatants from M. bovis-infected cattle (raw data not shown), and O. ochengi-infected animals also displayed higher spontaneous background IL-4 activity compared to M. bovis-infected animals (O. ochengi-infected animals: antigen-specific cpm of 23,347 to 62,776 versus background-spontaneous cpm of 1,252 to 17,631; M. bovis-infected animals: PPD-M-specific cpm of 3,927 to 26,423 versus background-spontaneous cpm of 512 to 2,916). Therefore, while our data indicate a comparable increase in the activity of IL-4-producing cells in helminth and mycobacterial infections following in vitro restimulation with specific antigen, the raw data suggest higher a background IL-4 activity in helminth-infected animals.
Levels of antigen-specific IFN-γ induced by M. bovis and O. ochengi infections were also comparable, with all 6 O. ochengi-infected animals generating positive antigen-specific IFN-γ responses (ΔOD450, 0.31 to 1.21) compared to 20 of 21 M. bovis-infected animals generating PPD-M-specific responses (ΔOD450, 0.46 to 1.56).
DISCUSSION
This study describes the presence of specific IL-4 responses in experimental and naturally acquired bovine tuberculosis and compares these IL-4 responses with the IFN-γ responses that are well known to occur during M. bovis infection (4, 9, 21, 25, 26, 30, 31, 30, 40). The antigens used in this study were the bovine and avian tuberculin preparations that are routinely used in the diagnosis of bovine tuberculosis, along with a recombinant form of ESAT-6. The ESAT-6 antigen occurs in pathogenic but not in most nonpathogenic mycobacteria (33), is a dominant antigen recognized by T cells following M. bovis infection of cattle (25, 26, 30, 31), and has shown potential in the development of more-specific diagnostic assays for bovine tuberculosis (5, 25, 36).
The IFN-γ test is a well-established method for the diagnosis of bovine tuberculosis (40) and has been used in parallel with the tuberculin skin test, with a sensitivity of 95.2% in the successful Australian Tuberculosis Eradication Campaign (39). The IFN-γ test has also been demonstrated to identify naturally infected animals that present as SCITT− (21). In our study of experimental M. bovis infection, IFN-γ responses to tuberculin and ESAT-6 were detected from 4 weeks postinfection, and these responses were sustained throughout the period studied. Positive IFN-γ responses were also observed in 20 of 21 naturally infected field reactor cattle.
IL-4 activity was measured using a sensitive bioassay. The specificity of this assay has been confirmed previously using a neutralizing monoclonal antibody (29). Using this assay we have demonstrated the presence of IL-4 activity in cattle both experimentally and naturally infected with M. bovis. IL-4 responses in experimentally infected animals were detected approximately 2 weeks after the onset of positive IFN-γ responses, peaked noticeably at 8 weeks postinfection, and then rapidly declined and generally remained low. One exception to this was animal CN1104, in which a second IL-4 peak was observed against ESAT-6 at 16 weeks.
The observed kinetics of IFN-γ and IL-4 responses in bovine tuberculosis allow for some comment on the possible mechanism of cytokine interplay that may be in operation. IFN-γ is a dominant proinflammatory cytokine during tuberculosis infection. One of the major roles of IFN-γ is to activate macrophages which then produce an array of proinflammatory cytokines, including IL-1β and TNF-α, both of which are thought to play an important part in the control of mycobacterial infection (15, 37). The delayed IL-4 response in experimental cattle relative to the IFN-γ response may be representative of an anti-inflammatory response which acts to dampen the IFN-γ-driven inflammation. Interestingly, in all animals a decrease in IFN-γ activity was preceded by a peak activity in IL-4. In support of an anti-inflammatory role for IL-4 in tuberculosis, recent reports by Mendez-Samperio et al. (19, 20) have demonstrated the downmodulation of mycobacterial antigen-induced IL-1β responses in the peripheral blood of BCG-vaccinated individuals by IL-4. Interestingly, the IL-4 responses observed in our experimentally infected cattle were not sustained and decreased rapidly, leaving the IFN-γ response in place at a time when tuberculous lesions would be developing. These results therefore demonstrate the development of tuberculous lesions in cattle in the presence of high IFN-γ and reduced or absent IL-4 responses and suggest that in cattle an unchecked inflammatory IFN-γ response may be contributing to the development of lesions.
Experimental cattle challenged with the lowest dose (6.6 × 103 CFU) of M. bovis did not show the characteristic IFN-γ responses seen in the higher-dose (6.6 × 104 or 6.6 × 105 CFU) cattle but did show equivalent IL-4 responses. Different cytokine responses to different doses of mycobacteria have been reported in mice infected with M. bovis BCG (27). In that case, however, the low-dose BCG was found to stimulate an exclusively type 1 response, while the higher dose induced a type 1-type 2 mixed response, which is the opposite of our findings in cattle. A possible interpretation for these observations is that the IL-4 response may be indicative of a cryptic infection following low-dose exposure that involves mainly local inflammatory responses. If this were true, then the measurement of specific IL-4 responses could provide additional information about the exposure of cattle to M. bovis.
The IL-4 responses observed in naturally infected field reactors distinguished infected from noninfected animals. For the limited number of clinical samples in this study, the sensitivity of the IL-4 assay was equivalent to that of the IFN-γ test in that the same animals gave positive responses in both tests (i.e., responses to PPD-M and ESAT-6). The number of positive IL-4 responses in the naturally infected animals was perhaps surprising, given the short sharp peak of IL-4 responses observed for experimentally infected cattle, and warrants further investigation.
Finally, M. bovis-specific IFN-γ and IL-4 responses were compared with helminth-specific cytokine responses. Parasitic helminths are particularly potent inducers of IL-4 (18), and experimental O. ochengi infection is known to induce both IFN-γ and IL-4 responses in cattle (Graham et al., unpublished data). The results suggest, first, a similar level of antigen-specific IFN-γ production induced by infection with either M. bovis or O. ochengi and, second, that the increased activity of IL-4-producing cells in either infection following restimulation with specific antigen in vitro was similar. However, there was a large disparity in the spontaneous release of IL-4 by unstimulated PBMC in these two very different infections of cattle, with high spontaneous release in O. ochengi-infected animals compared to a relatively low spontaneous release in M. bovis infected animals, indicating a higher overall IL-4 activity in helminth infection.
In summary, this study describes the production of both IFN-γ and IL-4 responses following the experimental infection of cattle with M. bovis and shows the distinct response kinetics of these two cytokines. Cytokine response measurements in naturally infected M. bovis field reactors showed that assays based on the detection of either IFN-γ or IL-4 responses were equally able to differentiate infected from uninfected animals. Finally, an interesting observation of positive IL-4 responses in low-dose M. bovis challenge animals that remained SCITT− suggests that the measurement of IL-4 could provide additional information of disease progression for use in investigations of the exposure to, and the immunopathology of, bovine tuberculosis.
ACKNOWLEDGMENTS
We thank Adam Whelan (VLA) for providing the recombinant protein ESAT-6 and D. Gavier-Widen (VLA) for conducting the postmortem analysis of M. bovis experimentally infected animals. We also thank the IAH, Compton, Berkshire, United Kingdom, for providing the monoclonal antibodies IL-A58 and CC-304 and the recombinant bovine IL-4.
This work was funded by the United Kingdom Ministry of Agriculture, Fisheries and Food.
REFERENCES
- 1.Baliko Z, Szereday L, Szekeres-Bartho J. Th2-biased immune responses in cases with active Mycobacterium tuberculosis infection and tuberculin anergy. FEMS Immunol Med Microbiol. 1998;22:199–204. doi: 10.1111/j.1574-695X.1998.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 2.Bianco A E, Ham P J, Townson S, Mustafa M B, Nelson G S. A semi-automated system of intrathoracic injection for the large-scale production of Onchocerca lienalis infective larvae. Trop Med Parasitol. 1989;40:57–64. [PubMed] [Google Scholar]
- 3.Brown W C, Davis W C, Dobbelaere D A E, Rice-Ficht A C. CD4+ T cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 profiles. Infect Immun. 1994;62:818–827. doi: 10.1128/iai.62.3.818-827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buddle B M, Keen D, Thomson A, Jowett G, Heslop J, deLisle G W, Stanford J L. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–16. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 5.Buddle B M, Parlane N A, Keen D L, Aldwell F E, Pollock J M, Lightbody K, Anderson P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin Diagn Lab Immunol. 1999;6:1–5. doi: 10.1128/cdli.6.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canals A, Zarlenga D S, Almeria S, Gasbarre L C. Cytokine profile induced by a primary infection with Ostertagia ostertagi in cattle. Vet Immunol Immunopathol. 1997;58:63–75. doi: 10.1016/s0165-2427(96)05775-3. [DOI] [PubMed] [Google Scholar]
- 7.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dlugovitzky D, Bay M L, Rateni L, Urizar L, Rondelli C F, Largacha C, Farroni M A, Molteni O, Bottasso O A. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol. 1999;492:210–217. doi: 10.1046/j.1365-3083.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 9.Emery D L, Duffy F H, Wood P R. An analysis of cellular proliferation and synthesis of lymphokines and specific antibody in vitro by leucocytes from immunized cattle. Vet Immunol Immunopathol. 1988;18:67–80. doi: 10.1016/0165-2427(88)90037-2. [DOI] [PubMed] [Google Scholar]
- 10.Erb K J, Kirman J, Delahunt B, Chen W, Le Gros G. IL-4, IL-5, and IL-10 are not required for the control of M. bovis-BCG infection in mice. Immunol Cell Biol. 1998;76:41–46. doi: 10.1046/j.1440-1711.1998.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.European Economic Community. EEC directive 80/219 EEC, amending directive 64/432 annex B. Offic J. 1980;L047:25–32. [Google Scholar]
- 12.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard A D, Zwilling B S. Reactivation of tuberculosis is associated with a shift from type 1 to type 2 cytokines. Clin Exp Immunol. 1999;115:428–434. doi: 10.1046/j.1365-2249.1999.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huygen K, Abramovicz D, Vandenbussche P, Jacobs F, DeBruyn J, Kentos A, Drowart A, Van Voornen J-P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindler V, Sappino A P, Grau G E, Piguet P F, Vassalli P. The inducing role of tumour necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–743. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 16.Krebs J R, Anderson R M, Clutton-Brock T, Young D, Morrison I, Donnely C. Bovine tuberculosis in cattle and badgers. Report to the Rt. Hon. Dr. Jack Cunningham M.P. 1997. MAFF Publications, London, England. [Google Scholar]
- 17.Kuhnle G, Collins R A, Scott J E, Keil G M. Bovine interleukin-2 and interleukin-4 expressed in recombinant bovine herpesvirus-1 are biologically active secreted glycoproteins. J Gen Virol. 1996;77:2231–2240. doi: 10.1099/0022-1317-77-9-2231. [DOI] [PubMed] [Google Scholar]
- 18.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 19.Mendez-Samperio P, Badillo-Flores A, Nunez-Vazquez A, Hernandez-Garay M. Interleukin-4 inhibits secretion of interleukin-1 beta in the response of human cells to mycobacterial heat shock proteins. Clin Diagn Lab Immunol. 1997;4:665–670. doi: 10.1128/cdli.4.6.665-670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez-Samperio P, Hernandez-Garay M, Badillo-Flores A, Nunez-Vazquez A. Down-modulation of mycobacterial-induced IL-1β production in human mononuclear cells by IL-4. Clin Exp Immunol. 1996;104:374–379. doi: 10.1046/j.1365-2249.1996.06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neill S D, Cassidy J, Hanna J, Mackie D P, Pollock J M, Clements A, Walton E, Bryson D G. Detection of Mycobacterium bovis infection in skin test-negative cattle with an assay for bovine interferon-gamma. Vet Rec. 1994;135:134–135. doi: 10.1136/vr.135.6.134. [DOI] [PubMed] [Google Scholar]
- 22.Nicoll A, Gill O N. The global impact of HIV infection and disease. Commun Dis Public Health. 1999;2:85–95. [PubMed] [Google Scholar]
- 23.North R J. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–58. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrini B, Hoffner S. Drug-resistant and multidrug-resistant tubercle bacilli. Int J Antimicrob Agents. 1999;13:93–97. doi: 10.1016/s0924-8579(99)00111-9. [DOI] [PubMed] [Google Scholar]
- 25.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 26.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Power C A, Wei G, Bretscher P A. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66:5743–5750. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raviglione M C, Dye C, Schmidt S, Kochi A. Assessment of world-wide tuberculosis control. Lancet. 1997;350:624–629. doi: 10.1016/s0140-6736(97)04146-9. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes S G, Cocksedge J M, Collins R A, Morrison W I. Differential cytokine responses of CD4+ and CD8+ T cells in response to bovine viral diarrhoea virus in cattle. J Gen Virol. 1999;80:1673–1679. doi: 10.1099/0022-1317-80-7-1673. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes S G, Buddle B M, Hewinson R G, Vordermeier H M. Bovine tuberculosis: immune responses in the peripheral blood and at the site of active disease. Immunology. 2000;99:195–202. doi: 10.1046/j.1365-2567.2000.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes S G, Gavier-Widen D, Buddle B M, Whelan A O, Singh M, Hewinson R G, Vordermeier H M. Antigenic specificity in experimental bovine tuberculosis. Infect Immun. 2000;68:2573–2578. doi: 10.1128/iai.68.5.2573-2578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somoskovi A, Zissel G, Zipfel P F, Ziegenhagen M W, Klaucke J, Haas H, Schlaak M, Muller-Quernheim J. Different cytokine patterns correlate with the extension of disease in pulmonary tuberculosis. Eur Cytokine Netw. 1999;10:135–141. [PubMed] [Google Scholar]
- 33.Sorensen A L, Nagai S, Houen G, Andersen P, Andersen A B. Purification and characterisation of a low molecular mass T cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–4618. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taha R A, Kotsimbos T C, Song Y-L, Menzies D, Hamid Q. IFN-γ and IL-12 are increased in active compared with inactive tuberculosis. Am J Respir Crit Care Med. 1997;155:1135–1139. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 35.Verbon A, Juffermans N, VanDeventer S J H, Speelman P, VanDeutekom H. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–113. doi: 10.1046/j.1365-2249.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vordermeier H M, Cockle P C, Whelan A O, Rhodes S, Palmer N, Bakker D, Hewinson R G. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6:675–682. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallis R S, Amir-Tahmasseb M, Ellner J J. Induction of interleukin-1 and tumour necrosis factor by mycobacterial proteins: the monocyte Western blot. Proc Natl Acad Sci USA. 1990;87:3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams D J L, Newson J, Naessens J. Quantitation of bovine immunoglobulin isotypes and allotypes using monoclonal antibodies. Vet Immunol Immunopathol. 1990;24:267–283. doi: 10.1016/0165-2427(90)90042-q. [DOI] [PubMed] [Google Scholar]
- 39.Wood P R, Corner L A, Rothel J S, Baldock C, Jones S L, Cousins D B, McCormick B S, Francis B R, Creeper J, Tweddle N E. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J. 1991;68:286–290. doi: 10.1111/j.1751-0813.1991.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 40.Wood P R, Corner L A, Plackett P. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of interferon-gamma. Res Vet Sci. 1990;49:46–49. [PubMed] [Google Scholar]