Abstract
Background
Molecular brain tumor classification using DNA methylation profiling has revealed that the methylation-class of pleomorphic xanthoastrocytoma (mcPXA) comprised a substantial portion of divergent initial diagnoses, which had been established based on histology alone. This study aimed to characterize the survival outcome in patients with mcPXAs—in light of the diverse selected treatment regimes.
Methods
A retrospective cohort of adult mcPXAs were analyzed in regard to their progression-free survival following surgical resection and postoperative radiotherapy. Radiotherapy treatment plans were correlated with follow-up images to characterize the pattern of relapse. Treatment toxicities and molecular tumor characteristics were further analyzed.
Results
Divergent initial histological diagnoses were encountered in 40.7%. There was no significant difference in local progression-free (PFS) and overall survival (OS) following gross total or subtotal resection. Postoperative radiotherapy was completed in 81% (22/27) following surgical intervention. Local PFS was 54.4% (95% CI: 35.3–84.0%) and OS was 81.3% (95% CI: 63.8–100%) after 3 years following postoperative radiotherapy. Initial relapses post-radiotherapy were primarily located in the previous tumor location and/or the planning target volume (PTV) (12/13). All patients in our cohort demonstrated the prognostically favorable pTERT-wildtype mcPXA.
Conclusion
Our study demonstrated that adult patients with mcPXAs display a worse progression-free survival compared to the reported WHO grade 2 PXAs. Future matched-pair analyses are required with a non-irradiated cohort to elucidate the benefit of postoperative radiotherapy in adult patients with mcPXAs.
Keywords: DNA methylation profiling, glioma, molecular diagnostics, Pleomorphic xanthoastrocytoma, radiotherapy
Pleomorphic xanthoastrocytoma (PXA) represents a rare astrocytic tumor with varied histological features and was initially described in 1979 as a distinct entity.1 Until the current update of the WHO classification of central nervous system tumors in 2021, PXAs were previously classified as WHO grade II or III. Pleomorphic xanthoastrocytoma generally occur in supratentorial regions, often displaying leptomeningeal involvement. Median age at diagnosis was reported to be around 20.5 years.2 While the prognostic value of extent of resection is still ambiguous, a number of studies indicate an association between gross total resection and improved survival.1–5 Further, the effect of adjuvant radiotherapy (RT) was explored in smaller case series: while some studies tentatively suggest an improvement in local tumor control following postoperative RT,4,6 the same benefit could not be definitively demonstrated in other PXA populations.7,8 In 2015, Ida et al2,5 published a series comprising 74 patients, with 5-year recurrence-free survival rates of 70.9% for WHO grade II, and 48.9% for grade III tumors. The 5-year overall survival was estimated at 90.4% and 57.1% for WHO grade II and III, respectively.5
However, DNA methylation profiling revealed a considerable molecular heterogeneity within histologically diagnosed PXAs (histPXA).2,9 In 2022, Ebrahimi et al9 performed a comprehensive molecular analysis on 144 histologically—(histPXA) and 220 methylation-defined PXAs (mcPXA). The initial histological diagnoses were correspondent with the methylation-class in only 56.3% (81/144), while 31.9% (46/144) were classified into other molecular subgroups, with glioblastoma constituting the most frequent mismatch. Similarly, the mcPXA cohort comprised tumors of diverse initial diagnoses, which had been based on histology only (eg, glioblastoma, ganglioglioma, etc.). Further survival analyses have demonstrated the presence of pTERT mutations to be associated with unfavorable prognosis among mcPXA, while 5-year overall survival estimate in pTERT-wildtype was reported be approximately 75%.9 Further, employing WHO grading criteria to mcPXAs failed to separate mcPXAs into subgroups of distinct survival. Interestingly, the discrepancy between conventional WHO grading of histologically-diagnosed and mcPXAs was explained by the influx of tumors which qualified for glioblastoma on one end of the malignancy spectrum and low-grade gliomas on the other end.2,3
To date, comprehensive analyses focusing on the progression-free and overall survival following RT in this enigmatic tumor entity are still missing, mainly attributed to the rarity and the molecular heterogeneity of PXAs.2 In this present study, clinical treatment and follow-up data were acquired and investigated to assess survival outcome of 27 adult patients with PXAs as defined by DNA methylation profiling (mcPXAs).
Methods
DNA Methylation Profiling and Genomic Sequencing of mcPXAs
DNA was extracted from FFPE tumor material using a Maxwell system (Promega, Fitchburg, WI, USA) and the Maxwell 16 FFPE Plus LEV DNA Purification Kit, according to the manufacturer’s guidelines. DNA concentration was determined via Invitrogen Qubit dsDNA BR Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) and FLUOstar Omega Microplate Reader (BMG Labtech GmbH, Ortenberg, Germany). Genome-wide DNA methylation profiles were previously generated using the Illumina Infinium HumanMethylation450 (450k) and MethylationEPIC (EPIC) array according to the manufacturer’s guidelines (Illumina, San Diego, USA) at the Genomics and Proteomics Core Facility of the DKFZ (Heidelberg, Germany), according to the manufacturer’s guidelines (Illumina, San Diego, USA). To perform unsupervised dimension reduction, the remaining probes were used to calculate the 1-variance weighted Pearson correlation between the samples. The resulting distance matrix was used as input for t-SNE analysis (t-Distributed Stochastic Neighbor Embedding; Rtsne package), as previously described.10,11 To perform unsupervised hierarchical clustering, the 10 000 probes with highest standard deviation were selected to calculate the Euclidean distance between samples, followed by applying Wards linkage method for sample clustering. Further, the principal output of the Classifier is a list of the predicted class membership probabilities for every class currently included (v12.5; https://www.molecularneuropathology.org/mnp/classifiers), which are referred to as the “calibrated” Classifier scores. The sum of all calibrated scores of all included methylation classes combined will add up to 1. For example, as all calibrated scores add up to 1, a calibrated score of 0.9 for the class of mcPXAs implies that all the remaining brain tumor classes add to 0.1 in such a classifiable tumor.
Patients with methylation-class PXA (mcPXA) with a calibrated score of >0.9 or distinct classification in t-SNE (t-distributed stochastic neighboring embedding) were included in the study cohort.
Genomic alterations of the genes with reported relevance for gliomas (eg, CDK4, CDK6, CDKN2A/B, EGFR, MDM4, MET, MYC, MYCN, NF1, NF2, PDGFRA, PPM1D, PTEN and RB1) were further analyzed.9,12 Targeted Sanger sequencing of BRAF, IDH and pTERT was conducted in cases with sufficient tumor DNA. All computational analyses were performed in R version 3.4.1 (R Development Core Team, 2018), as previously described.11,13 The study was approved by the Independent Ethics Committee (EC) of the Medical Faculty Heidelberg (S-293/2022).
Definition of mcPXA and Clinical Patient Characteristics
Clinical patient characteristics (eg, age at diagnosis, date of diagnosis, sex), tumor characteristics (location, size, WHO grade, molecular features) and the course of treatment (incl. surgical resection, radiotherapy, chemotherapy) were obtained from patients with a calibrated score of >0.9 or distinct classification in t-SNE (t-distributed stochastic neighboring embedding) to the mcPXA, using the database of the Department of Radiation Oncology, University Hospital Heidelberg and the Heidelberg Institute for Radiation Oncology (HIRO). Survival data was obtained from the national registration office (national cancer registry). Patients were carefully followed with routine clinical examinations and follow-up magnetic resonance imaging (MRI) as per standard institutional guidelines to collect treatment response and toxicity data.
Planning and Treatment Features
Patients were immobilized with custom thermoplastic masks and treatment planning simulation scans were obtained, including computed tomography (CT) as well as cranial MRI (cMRI). Gross tumor volume (GTV) included the macroscopic tumor and/or resection cavity. For the clinical target volume (CTV), a safety margin was applied while adding all available information from MRI sequences and surgical reports to account for suspected microscopic tumor spread and simultaneously respecting anatomic boundaries. An isotropic margin of 3–5 mm was used for creation of the planning target volume (PTV) to account for geometric uncertainties and physical inaccuracies of the treatment technique. Treatment planning followed the principle of as low as reasonably achievable (ALARA) and was according to the constraints of ICRU report 50 and 62 as well as normal tissue constraints according to QUANTEC and Emami et al.14
Radiotherapy was applied in 1.8–3.0 Gy single doses over 5–6 fractions per week. Photon radiotherapy was applied with 3D-conformal radiotherapy (3DCRT) or intensity-modulated radiotherapy (IMRT). Proton radiotherapy (PRT) was applied using active raster-scanning technique and a constant relative biological effectiveness (RBE) factor of 1.1. One patient received photon radiotherapy with 50 Gy and carbon ion radiotherapy with 18 Gy (RBE) in 6 fractions. Carbon ion radiotherapy planning was performed using the treatment planning software PT-Planning including biologic plan optimization. Biologically effective dose distributions were calculated using the local effect model I (LEM-1) and an a/β ratio = 2, as used for glioblastoma WHO 4.15
Survival Analysis and Statistical Considerations
Extent of surgical resection was evaluated based on intra- or post-operative MRI or surgical reports (in 6 cases) and was classified as gross total resection (GTR) when no nodular residual tumor was visible, subtotal resection or biopsy. For analysis of the prognostic impact of the extent of resection, subtotal resection and biopsy cases were grouped together (STR).
Local progression-free survival following surgical resection was selected as primary endpoint. Local progression-free survival (l-PFS) was defined from the surgical resection until tumor progression within the surgical cavity and/or the PTV of the radiation plan. Tumor progression was defined according to the criteria presented by the Response Assessment in Neuro-Oncology Working Group (RANO).16 Available radiation treatment plans were correlated with all available clinical follow-up MR-imaging to evaluate the location of initial relapses (infield vs outfield). Overall survival was determined from the date of initial diagnosis until death. Patients, who became lost to follow-up were censored at the date of the last follow-up examination to define overall and local progression-free survival. Overall survival (OS) and local progression-free survival (l-PFS) were calculated via Kaplan–Meier analysis.
Toxicity Analysis and Statistical Considerations
Toxicity was classified according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.00 8–12 weeks after RT (acute toxicity) or at the last follow-up (late toxicity) (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). Fisher’s exact test was applied for independent group comparison. P-value < .05 was considered significant.
Radiation induced contrast enhancement (RICE) was defined as new post-treatment contrast enhancement on cMRI in surrounding brain tissue within the 80% isodose line analogous to RANO criteria during the follow-up period.17 Cases were evaluated using all available cMRIs, radiation treatment plans, and medical records that reflect time course and concurrent therapies.
Results
Clinical Patient Characteristics and Diverse Initial Histological Diagnoses
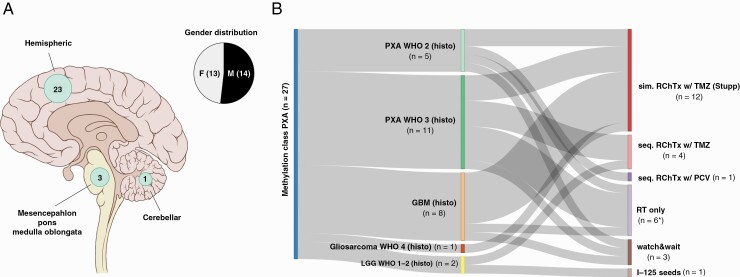
The study cohort comprised 27 patients with molecularly-diagnosed PXAs, comprising 10 patients identified by a calibrated score of >0.9 using DNA methylation profiling alone. Additional 17 cases—with a calibrated score below 0.9—were identified using t-SNE analysis and further integrated morphological-molecular diagnostics, including histological appearance, BRAF V600E mutation or/and loss of CDKNA2/B (Supplementary Figure 3). Single cases demonstrated a low tumor cell content before DNA extraction, which may result in a calibrated score below 0.9. Age at diagnoses ranged from 16 to 85 years, with no gender predilection (male-to-female ratio, 14:13). For one patient diagnosed at the age of 16, radiotherapy was administered after the patient reaching adulthood (>18 years). Methylation-class PXAs (mcPXA) were most frequently encountered in the cerebral hemispheres (23/27) (Figure 1A). In total, the initial diagnosis of PXA WHO grade II or III was established in 59.3% (16/27), with support of the DNA methylation classifier in 9/16 cases. However, various diagnoses different to PXA were designated to 11 patients, with glioblastoma WHO grade IV representing the most frequent alternative histological diagnosis (n = 8). Single cases were initially diagnosed as gliosarcoma WHO grade IV, ganglioglioma WHO grade I or pilocytic astrocytoma WHO grade I (Figure 1B). Roman numerals were utilized for cases diagnosed before the current WHO 2021 CNS tumor classification.
Figure 1.
Clinical patient characteristics, initial histological diagnoses and subsequent treatment regimens in the study cohort. (A) Tumor location and gender distribution, initial histological diagnoses and (B) subsequent treatment regimens are shown, with numbers in brackets indicating group size. *Radiotherapy was aborted in one case. PXA, pleomorphic xanthoastrocytoma; LGG, low-grade glioma; sim. RChTx w/ TMZ, simultaneous radiochemotherapy with temozolomide; seq. RChTx w/ TMZ, sequential radiochemotherapy with temozolomide; RT, radiotherapy.
Treatment Regimens in mcPXA
Surgical tumor resection was typically performed as primary treatment, with gross total (GTR) in 13/27 and subtotal resections (or biopsies) (STR) in 14/27 cases. Postoperative (external beam) radiotherapy was planned in 85.2% (23/27), and completed in 81.5% (22/27), as part of the first-line treatment: most frequently with concomitant and adjuvant temozolomide in 44.4% (12/27), including 6 cases which were initially diagnosed as glioblastoma WHO grade 4. Sequential chemoradiotherapy with temozolomide in 14.8% (4/27) or procarbazine, lomustine, and vincristine (PCV) in 3.4% (1/27), and radiotherapy alone in 22.2% (6/27) of the cases was planned. A median dose of 60.0 Gy (range: 50.4–68.0 Gy) was prescribed in the first-line treatment. Radioactive iodine-125 seeds were implanted in one patient during surgery (1/27). Further, a watch-and-wait strategy was applied in the remaining 3 mcPXA-patients (11.1%, 3/27); these patients received radiotherapy during the course of disease after tumor recurrence.
If the initial diagnosis of PXA WHO grade II or III had been directly established following surgical resection, the selected postoperative treatment concepts comprised radiotherapy (n = 14) with concomitant and adjuvant temozolomide (5/14), sequential chemoradiotherapy with temozolomide (3/14) or PCV (1/14), radiotherapy alone (5/14) and watch-and-wait (n = 2) (Figure 1B). However, postoperative radiotherapy was aborted in one patient (PXA-P09) due to the patients’ general state. For irradiated patients with PXA, a median dose of 54 Gy (range: 54.0–60.0 Gy) was administered for WHO grade II, and 59.4 Gy (range: 59.4–68.0 Gy) for WHO grade III tumors (Figure 2A). Further details regarding patient, radiotherapy modalities and treatment characteristics are listed in Table 1.
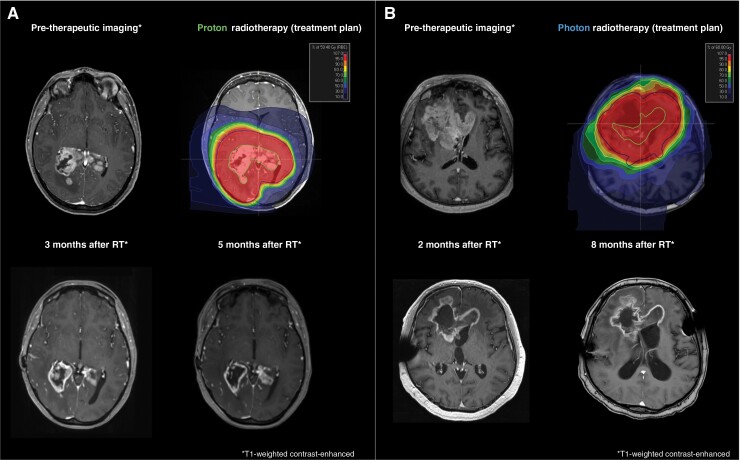
Figure 2.
Preoperative and post-therapeutic MR-imaging and radiotherapy treatment plans. (A) Proton therapy with a total dose of 59.4 Gy (RBE) in 33 fractions was administered following tumor biopsy. Follow-up imaging demonstrated a partial response in 3- and 5-months following radiotherapy, according to RANO guidelines. (B) Photon radiotherapy with 60 Gy in 30 fractions was applied following subtotal resection. Follow-up imaging demonstrated a stable disease in 2- and 8-months following radiotherapy, according to RANO guidelines. PTVs are depicted in blue, CTVs in red and GTVs in green.
Table 1.
Clinical patient characteristics and radiotherapy concepts
| Patients | Overall cohort |
|---|---|
| Gender | n = 27 [%] |
| Female | 14 [51.8] |
| Male | 13 [48.2] |
| Age at initial diagnosis* (n = 27) | |
| Median | 41 |
| Minimum–maximum | 16–85 |
| Extent of resection (n = 27) | |
| Gross total resection | 13 [48.1] |
| Subtotal resection | 9 [33.3] |
| Biopsy | 5 [18.5] |
| Initial histological diagnosis | |
| PXA WHO 2 | 5 [18.5] |
| PXA WHO 3 | 11 [40.7] |
| GBM WHO 4 | 8 [29.6] |
| Gliosarcoma WHO 4 | 1 [3.7] |
| Ganglioglioma WHO 2 | 1 [3.7] |
| Pilocytic astrocytoma WHO 1 | 1 [3.7] |
| Postoperative radiotherapy (completed) | |
| No | 5 [18.5] |
| Yes | 22 [81.5] |
| Total dose in Gy/Gy (RBE) | |
| Median | 60 |
| Minimum–maximum | 50.4–68 |
| Single dose | 1.8–3.0 |
| RT modality (n = 22) | |
| Photons | 11 [40.7] |
| Protons | 8 [29.6] |
| Bimodal: photons and protons | 2 [9.1] |
| Bimodal: photons and C12-ion | 1 [4.5] |
| Chemotherapy (n = 17) | |
| Temozolomide (Stupp protocol) | 12 [44.4] |
| Temozolomide (sequential) | 4 [14.8] |
| PCV | 1 [3.7] |
| Time diagnosis until radiotherapy start (months) | |
| Median | 1.5 |
| Minimum–maximum | 0–14 |
| PTV volume (ml) | |
| Median | 129.03 |
| Minimum–maximum | 60.7–400.1 |
| Median total dose in Gy/Gy (RBE) in PXA WHO 2–3 and GBM | |
| PXA WHO 2 | 54 |
| PXA WHO 3 | 59.4 |
| GBM WHO 4 | 60 |
If not otherwise visible, absolute and relative frequencies were shown. Relative frequencies are based on the available data and exclude missings.
Abbreviations: Gy RBE, Gray relative biological effectiveness; PCV, procarbazine, lomustine (CCNU), and vincristine.
*Preselected cohort with an age of >16.
Treatment Toxicities
No acute treatment toxicities exceeding CTCAE grade II were reported during radiotherapy or within 3 months after completion of the treatment. RICE was encountered in three patients following subtotal resection/biopsy and postoperative proton radiotherapy (3/8). In one patient (mcPXA-P19), the RICE was initially treated with high-dose dexamethasone and further escalated after 4 weeks using bevacizumab (biweekly 4 cycles, 7.5mg/m2 body surface), with regressive FLAIR-hyperintensity as adequate treatment response (Supplementary Figure 1). Further late toxicities are listed in Supplementary Table 1.
Progression-free Survival Following Gross Total (GTR) and Subtotal Resection (STR) in mcPXA
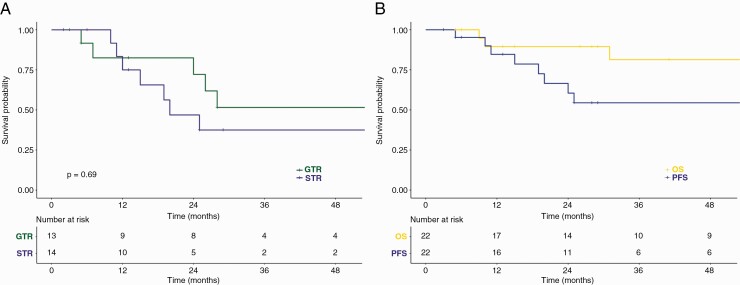
Median follow-up time was 31 months (range: 2–471 months). There was no significant difference (P = .69) in local progression-free survival after 3 years with 51.6% (95% CI: 28.2–93.4%) following GTR and 37.5% following subtotal resections or biopsy (STR; 95% CI: 17.4–80.7%) (Figure 3A).
Figure 3.
(A) Progression-free survival following gross total (GTR) and subtotal resection (STR), and (B) following radiotherapy via Kaplan–Meier analysis. OS, overall survival.
Local Progression-free Survival and Overall Survival Following Postoperative Radiotherapy
For patients following postoperative (external beam) radiotherapy (n = 22), local progression-free survival (3y-lPFS) was estimated at 54.4% (95% CI: 35.3–84.0%) and overall survival (3y-OS) at 81.3% (95% CI: 63.8–100%) after 3 years (Figure 3B). Initial relapses following radiotherapy were primarily located in the previous tumor location and/or the planning target volume (PTV) (12/13). Only one patient (PXA-P18) presented an out-of-field recurrence at initial relapse.
Further, the differences in initial diagnoses (GBM WHO IV vs PXA WHO II–III) had no significant prognostic value in terms of progression-free survival, with a 3y-lPFS in mcPXA with the initial diagnosis of GBM of 57.1% (95% CI: 30.1–100%) and 43.9% (95% CI: 21.7–88.7%, P = .53) in mcPXA with the initial diagnosis of PXA WHO II–III (Supplementary Figure 2).
BRAF V600E Mutations and Homozygous Deletion of CDKN2A/B Represent Recurrent Genomic Alterations in mcPXA
BRAF status was tested in 24/27 cases, with 75% (18/24) exhibiting a BRAF V600E mutation. Homozygous deletion of the CDKNA2/B gene was demonstrated in 85.7% (18/21) of all tested samples, while one mcPXA (PXA-PUB-04) displayed a heterozygous deletion. Mutations in pTERT (0/13) or IDH1/2 (0/24) were absent in the study cohort. Loss of nuclear ATRX expression was found in 14.3% (2/14). MGMT promotor status was analyzed in 21 samples: an unmethylated MGMT promotor (57.1%, 12/21) was more frequently encountered compared to methylated status (33.3%, 7/21) (Supplementary Figure 3). MGMT promoter status was inconclusive in 2/21 cases. There was no association between the molecular characteristics (eg, BRAF V600E mutation, homozygous deletion of CDKN2A/B) and clinical prognosis.
Discussion
The present study provides the largest, molecularly-homogenous cohort of PXAs as defined by DNA methylation with comprehensive data on treatment (eg, extent of surgical resection, postoperative radiotherapy), pattern of recurrence, survival data and toxicity analysis following radiotherapy.
If the diagnosis of PXA had been established as initial diagnosis following surgery, postoperative radiotherapy was initiated in 87.5% (14/16), typically with 50-60 Gy in 1.8–2.0 Gy daily fractions, as suggested by the EANO guidelines on diffuse gliomas of adulthood.18,19 Notably, a wide variety of chemoradiotherapy regimes were applied in the treatment of mcPXAs, as utilized for oligodendroglioma WHO grade 2–3, or IDH-mutant astrocytoma WHO grade 2–4.2,18,19 However, the role of postoperative radiotherapy remains enigmatic to date, particularly without comprehensive molecular characterization in previously reported “PXA” cohorts.2
The prognostic value of GTR was indicated in previous case studies.1–5 In 2014, Ida et al5 reported 5-year estimates of recurrence-free survival of 84.9% and 45.4% following GTR (n = 29) and STR/biopsy (n = 24), respectively. There was no clear advantage of GTR in terms of progression-free survival (n = 13, 3y-PFS in GTR: 51.6%) as compared to subtotal resection/biopsy (n = 14, 3y-PFS: 37.5%, P = .69), possibly owing to the small number of patients in each cohort. In general, the clinical outcome of mcPXAs following surgical resection and postoperative radiotherapy (3-year local-PFS 54.4% and 3y-OS at 81.3%) was intermediate between the reports in the current WHO classification for grade 2 and 3 tumors.2 Further, Ida et al5 suggested a 5-year recurrence-free survival rate of 70.9% for patients with grade II and 48.9% for grade III tumors in a histologically-diagnosed cohort. The aforementioned discrepancies may be explained by the divergence between histologically- and molecularly-diagnosed PXAs, and the reported inferior outcome in adults compared to pediatric patients.2,5 There was no significant difference in terms of the 3-year l-PFS and OS between the group of patients, which were initially diagnosed as “GBM WHO grade IV” and patients with the initial histological diagnoses of PXA (Supplementary Figure 2). No significant difference in l-PFS was observed in patients initially diagnosed as WHO grade II or III, which could have been expected in light of the small cohort sizes—thus, the value of conventional histopathological grading in mcPXAs remains unclear.
While PXAs are known to disseminate along the whole central nervous system during the course of disease,2 the first relapses were mostly encountered in the primary tumor region of mcPXAs, emphasizing the importance of intensifying local tumor therapy to achieve local control. On the contrary, RICE occurred in 3/8 cases following STR/biopsy and proton radiotherapy. The rate of RICE was slightly higher compared to previous reports, which indicate that RICE was found in approximately 25% of all adult patients with low-grade glioma following proton radiotherapy.20,21 Further analyses on the ideal radiation dose and treatment modality in larger cohorts are required in the future.
The presence of canonical pTERT mutations were reported to be associated with an unfavorable prognosis among mcPXAs in terms of overall survival, with a 3-y OS of approximately 90% in TERT-wildtype and 50% in TERT-mutated mcPXAs.9 Notably, only prognostically favorable pTERT-wildtype mcPXAs were encountered in the study cohort. The clinical outcome in adult mcPXAs resembles WHO grade 3 IDH-mutant, 1p/19q non-co-deleted anaplastic gliomas, as presented in the CATNON-trial.22–25 In recent years, targeted therapies using BRAF/MEK inhibitors (vemurafenib and trametinib) were explored in BRAF V600E-mutated anaplastic PXAs in single patients, with temporary tumor control (range: 2–10 months).26,27
In summary, our study analysis showed that adult patients with mcPXA display a poorer prognosis than previously reported for PXA grade 2. Thus, future matched-pair analysis with a non-irradiated cohort are required to identify patients at risk (eg, TERT-mutated mcPXAs) and to elucidate the benefit of radiotherapy in adult patients with mcPXAs.
Supplementary Material
Acknowledgments
For excellent technical support and expertise we thank the Genomics and Proteomics Core Facility (GPCF) and Omics, IT and Data Management Core Facility (ODCF) of the German Cancer Research Center (DKFZ).
Contributor Information
Maximilian Deng, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany.
Felix Hinz, Department of Neuropathology, Heidelberg University Hospital and CCU Neuropathology, German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Semi Harrabi, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany.
Dominik Sturm, Hopp Children’s Cancer Center Heidelberg (KiTZ), Heidelberg, Germany; Division of Pediatric Glioma Research, German Cancer Research Center (DKFZ), Heidelberg, Germany; Department of Pediatric Oncology, Hematology, Immunology and Pulmonology, University Hospital Heidelberg, Heidelberg, Germany.
Martin Sill, Hopp Children’s Cancer Center Heidelberg (KiTZ), Heidelberg, Germany; Division of Pediatric Neurooncology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Andrey Korshunov, Department of Neuropathology, Heidelberg University Hospital and CCU Neuropathology, German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany; Hopp Children’s Cancer Center Heidelberg (KiTZ), Heidelberg, Germany.
Tanja Eichkorn, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany.
Juliane Hörner-Rieber, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Clinical Cooperation Unit Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Klaus Herfarth, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany.
Christine Jungk, Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany.
Andreas Unterberg, Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany.
Stefan Pfister, Hopp Children’s Cancer Center Heidelberg (KiTZ), Heidelberg, Germany; Department of Pediatric Oncology, Hematology, Immunology and Pulmonology, University Hospital Heidelberg, Heidelberg, Germany; Division of Pediatric Neurooncology, German Cancer Consortium (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
Wolfgang Wick, National Center for Tumor Diseases (NCT), Heidelberg, Germany; Clinical Cooperation Unit Neurooncology, German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany; Department of Neurology, Heidelberg University Hospital, Heidelberg, Germany.
Andreas von Deimling, Department of Neuropathology, Heidelberg University Hospital and CCU Neuropathology, German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany.
David Jones, Hopp Children’s Cancer Center Heidelberg (KiTZ), Heidelberg, Germany; Division of Pediatric Glioma Research, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Jürgen Debus, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Clinical Cooperation Unit Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Felix Sahm, Department of Neuropathology, Heidelberg University Hospital and CCU Neuropathology, German Consortium for Translational Cancer Research (DKTK), German Cancer Research Center (DKFZ), Heidelberg, Germany; Hopp Children’s Cancer Center Heidelberg (KiTZ), Heidelberg, Germany.
Laila König, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany; Heidelberg Institute of Radiation Oncology (HIRO), Heidelberg, Germany; National Center for Tumor Diseases (NCT), Heidelberg, Germany; Heidelberg Ion-Beam Therapy Center (HIT), Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany.
Funding
M.D. received research funding from the medical faculty of the Heidelberg University. F.S. received research funding from the Else Fresenius Stiftung EKFS (Grant Nos. 2015_A_60 and 2017_EKES.24), the German Cancer Aid (Grant No. 70112956), and the Hertie Foundation (Hertie Network of Excellence in Clinical Neuroscience).
Conflict of Interest
The authors declare no conflicts of interest.
Authorship statement
Conceived the strategy: M.D., D.T.J., L.K., F.S., J.D.; supervised the project: M.D., D.T.J., L.K., F.S., J.D.; performed the analysis: M.D., F.H., D.T.J., L.K., F.S., M.S., C.J.; provided material, clinical and methodological expertise: S.H., D.S., M.S., A.K., T.E., J. H.-R., K.H., C.J., A.U., S.M.P., W.W., A.v.D.; wrote the manuscript: M.D., D.T.J., J.D., F.S., L.K. All authors have seen and approved the manuscript.
References
- 1. Kepes JJ, Rubinstein LJ, Eng LF. Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer. 1979; 44(5):1839–1852. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Wesseling P, et al. WHO Classification of Tumors of the Central Nervous System (2021). Vol 6. 5th ed. Lyon (France): International Agency for Research of Cancer (IARC); 2021. [Google Scholar]
- 3. Dono A, Lopez-Rivera V, Chandra A, et al. Predictors of outcome in pleomorphic xanthoastrocytoma. Neurooncol Pract. 2021; 8(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim S, Kim JH, Kim SA, et al. Prognostic factors and therapeutic outcomes in 22 patients with pleomorphic xanthoastrocytoma. J Korean Neurosurg Soc. 2013; 53(5):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ida CM, Rodriguez FJ, Burger PC, et al. Pleomorphic xanthoastrocytoma: natural history and long-term follow-up. Brain Pathol. 2015; 25(5):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koga T, Morita A, Maruyama K, et al. Long-term control of disseminated pleomorphic xanthoastrocytoma with anaplastic features by means of stereotactic irradiation. Neuro Oncol. 2009; 11(4):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marton E, Feletti A, Orvieto E, Longatti P. Malignant progression in pleomorphic xanthoastrocytoma: personal experience and review of the literature. J Neurol Sci. 2007; 252(2):144–153. [DOI] [PubMed] [Google Scholar]
- 8. Pahapill PA, Ramsay DA, Del Maestro RF. Pleomorphic xanthoastrocytoma: case report and analysis of the literature concerning the efficacy of resection and the significance of necrosis. Neurosurgery. 1996; 38(4):822–828; discussion 828–829. [PubMed] [Google Scholar]
- 9. Ebrahimi A, Korshunov A, Reifenberger G, et al. Pleomorphic xanthoastrocytoma is a heterogeneous entity with pTERT mutations prognosticating shorter survival. Acta Neuropathol Commun. 2022; 10(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018; 555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Capper D, Stichel D, Sahm F, et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018; 136(2):181–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153–166. [DOI] [PubMed] [Google Scholar]
- 13. Sahm F, Schrimpf D, Stichel D, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 14. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. [DOI] [PubMed] [Google Scholar]
- 15. Grun R, Friedrich T, Elsasser T, et al. Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys Med Biol. 2012; 57(22):7261–7274. [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 17. Chukwueke UN, Wen PY. Use of the response assessment in neuro-oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019; 8(1):CNS28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021; 18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weller M, van den Bent M, Tonn JC, et al. European association for neuro-oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017; 18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 20. Eichkorn T, Bauer J, Bahn E, et al. Radiation-induced contrast enhancement following proton radiotherapy for low-grade glioma depends on tumor characteristics and is rarer in children than adults. Radiother Oncol. 2022;172:54–64. [DOI] [PubMed] [Google Scholar]
- 21. Harrabi SB, von Nettelbladt B, Gudden C, et al. Radiation induced contrast enhancement after proton beam therapy in patients with low grade glioma—How safe are protons? Radiother Oncol. 2022;167:211–218. [DOI] [PubMed] [Google Scholar]
- 22. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017; 390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021; 22(6):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 25. Wick W, Roth P, Hartmann C, et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hussain F, Horbinski CM, Chmura SJ, Yamini B, Lukas RV. Response to BRAF/MEK inhibition after progression with BRAF inhibition in a patient with anaplastic pleomorphic xanthoastrocytoma. Neurologist. 2018;23(5):163–166. [DOI] [PubMed] [Google Scholar]
- 27. Migliorini D, Aguiar D, Vargas MI, Lobrinus A, Dietrich PY. BRAF/MEK double blockade in refractory anaplastic pleomorphic xanthoastrocytoma. Neurology. 2017; 88(13):1291–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.