Abstract
In situ-perfused rat livers were infused with a single dose of 1.5 × 107 radiolabeled cells of Leptospira interrogans serovar icterohaemorrhagiae, the agent of leptospirosis, or with Borrelia burgdorferi IRS, the agent of Lyme disease. Significant (P < 0.0001) differences in the liver uptake of L. interrogans and of B. burgdorferi were observed, the uptakes being 37.4% ± 2.3% for L. interrogans and 60.5% ± 3.1% for B. burgdorferi. Leptospires, in contrast to borreliae, were recovered from the livers when liver samples were cultured in growth medium. Leptospires but not borreliae were recovered in bile within 30 min of infusion. The association of leptospires and borreliae with reticuloendothelial cells of the liver was demonstrated by immunohistochemistry. Leptospires and borreliae were found to be associated with vimentin-positive cells and not with desmin-positive cells. Few leptospires but no borreliae were also seen associated with vimentin- and desmin-negative cells, suggesting the presence of leptospires outside the sinusoidal spaces, in the liver parenchyma.
The spirochetes Borrelia burgdorferi and Leptospira interrogans are the etiological agents of Lyme disease and leptospirosis, respectively. Despite substantial biological differences among human spirochetes, all spirochetoses share a spirochetemic phase during the early stage of infection (6, 17, 20, 22). Leptospires can cause hepatitis in humans. This will result in microscopic alteration in the liver, including swelling of parenchymal cells, disruption of the liver cord, enlargement of Kupffer cells, and bile stasis in biliary canaliculi (2). In Lyme disease, liver function test abnormalities are common but mild and are most often not associated with symptoms (12). Lyme disease presenting as hepatitis and jaundice has been also reported (8). The involvement of the hepatic reticuloendothelial system (RES) in host defense by phagocytosis and killing of blood-borne spirochetes has been previously demonstrated in animals. Studies by us (18) have shown significant differences in the rat liver uptake of borreliae causing Lyme disease and of borreliae involved in relapsing fevers and have also indicated that B. burgdorferi is efficiently taken up by hepatic macrophages in the absence of serum factors. Electron microscopy studies by Faine (10) showed that in experimentally infected mice leptospires are found almost entirely in Kupffer cells and also interstitially between or in parenchymal liver cells.
The perfused liver has been used several times in the past few decades to study bacterial hepatic phagocytosis (5, 13, 15). We therefore used such a technique to evaluate the uptake and killing of leptospires in comparison with borreliae by the rat hepatic RES in the elimination of circulating bacteria. Although the mouse is a widely used model for experimental infections with borreliae, this study was performed with rats, since they are more suitable for liver perfusion. The applicability of rats for experimental studies on borreliae has also been shown previously (4, 11). It is also well known that rats are carriers of leptospires (21).
Bacterial strains, culture conditions, and labeling.
The following spirochetal strains were used: B. burgdorferi IRS (ATCC 35211) and L. interrogans serovar icterohaemorrhagiae (a gift of M. Fabbi, Istituto Zooprofilattico Sperimentale, Pavia, Italy). Borreliae were cultured in Barbour-Stoenner-Kelly (BSK) II medium at 34°C, as previously reported (19), whereas leptospires were grown in liquid Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (9) at 30°C under aerobic conditions to a density of ca. 108 bacteria per ml and counted in a Petroff-Hausser counting chamber. When required, bacteria were grown in the presence of 1 μCi of 14C-labeled amino acid mixture (>50 mCi/ml; Amersham Co., Amersham, United Kingdom) per ml for 96 h, washed three times with Krebs-Ringer solution (see below), counted in a Petroff-Hausser chamber, resuspended in Krebs-Ringer solution at a concentration of 1.5 × 106 or 5 × 107 motile organisms per ml, and used within 1 h for rat liver perfusion experiments.
Liver perfusion.
Male Sprague-Dawley rats (180 to 220 g [body weight]) were used as liver donors. Food was withdrawn the evening before the experiment, and water was available ad libitum. The technique of rat liver perfusion has been already described (1) and was performed according to the method of Mortimore (16). Briefly, the animals were anesthetized intraperitoneally with pentobarbital sodium (50 mg/kg, given intraperitoneally), and the livers were perfused through the portal vein, the effluent being collected from the inferior vena cava immediately above the sovrahepatic veins. The perfusate was Krebs-Ringer bicarbonate solution containing glucose (5.55 mmol/liter), with bovine serum albumin (3% [wt/vol]) (fraction V; Sigma Chemical Co., St. Louis, Mo.). Taurocholate (sodium salt; Sigma Co.) at 0.5 mM was added to maintain the enterohepatic circulation of bile acids and bile flow. The complete blanching of all liver lobes indicated satisfactory perfusion. Oxygenation was done with O2 + CO2 (95/5 [vol/vol]) using Silastic tubing (Dow-Corning, Midland, Mich.). The temperature and pH of the perfusate leaving the liver were monitored throughout the experiment. The perfusate flow was established at a value of 2.2 to 2.9 ml/min/g of liver. The portal vein pressure was constant at 12-cm of water; no significant change in the levels of aspartate aminotransferase (12.0 ± 2.0 IU/liter) was observed throughout the experiment, and the pH of the effluent from the liver ranged between 7.36 and 7.42. During each perfusion, one dose of 1.5 × 107 bacteria in 10 ml of Krebs-Ringer solution was infused into the portal vein. The effluent from the livers was collected at 1-min intervals over 30 min in preweighted tubes immediately after infusion. At the end of each experiment, radioactivity was measured by applying directly 100 μl of each outflow sample to cellulose acetate membrane filters (0.45-μm [pore size]) and counted after addition of Ready-Solv EP (Beckman Instruments, Inc., Fullerton, Calif.) using a Beckman LS 1801 liquid scintillation analyzer. The cumulative radioactivity throughout the experiment represented the bacteria escaping the liver uptake. A 30-min period was chosen in order to rule out bacterial efflux from the liver in later times. When required, one dose of 5 × 108 labeled bacteria was infused, and spirochetes were searched for in bile by cannulation of the common bile duct with a PE-10 catheter (PE-10 Tubing; Clay Adams, Parsippany, N.J.). All animals were given humane care in compliance with institutional guidelines according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The experiments were approved by the Ethical Committee of the University of Bologna (prot.16745).
Expression of the results of liver uptake of spirochetes.
The hepatic uptake was expressed as a percentage of the administered bacteria and calculated as follows: 100 − the percentage outflow. The statistical analysis was performed using the Student t test for unpaired data.
Immunohistochemistry.
Liver specimens, obtained at the end of the perfusions, were snap frozen in isopentane precooled in liquid nitrogen and stored at −70°C. Immunofluorescence and double-immunofluorescence techniques were performed on serial 5-mm frozen sections as previously described (3). In brief, sections were air dried 1 h and then fixed for 5 min with cold acetone, washed with phosphate-buffered saline (PBS), and incubated in a moist chamber with primary and secondary reagents for 30 min. Each incubation was followed by 3- to 5-min PBS washes. In double immunofluorescence, a 1:100-diluted convalescent-phase serum from a patient suffering from leptospirosis (the serum was a gift of M. A. Santos, INSA, Porto, Portugal) or mouse immune ascitic fluid anti-B. burgdorferi (7) was first applied, followed by rabbit immunoglobulins to human immunoglobulin G or to mouse fluorescein isothiocyanate (FITC)-conjugated immunoglobulin (Dako, Copenhagen, Denmark) diluted 1:30. Monoclonal antibodies to vimentin and desmin (Dako) were then diluted 1:20, followed by treatment with tetramethyl rhodamine isothiocyanate (TRITC)-conjugated rabbit anti-mouse immunoglobulin (Dako), diluted 1:20 in PBS, to identify the total sinusoidal mesenchymal cell and sinusoidal stellate (Ito) cell subpopulations (3, 23), respectively. Sections were mounted in PBS-glycerol and observed with a Zeiss Axioskop microscope (Oberkochen, Germany) with epi-illumination at different wavelengths to evaluate the FITC- or TRITC-conjugated reagents.
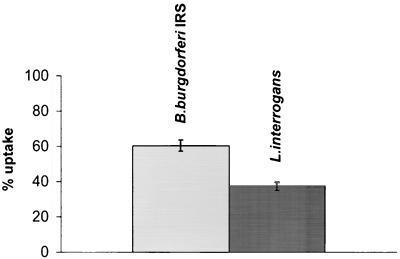
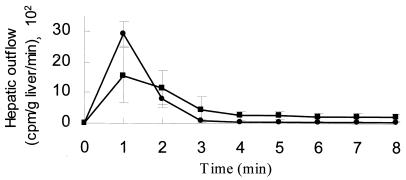
The motility and viability of 14C-labeled spirochetes in Krebs-Ringer solution were preliminarily evaluated. Bacteria suspended in Krebs-Ringer solution for 1, 2, and 4 h were studied by dark-field microscopy and by culturing. No reduction in the number of motile organisms or viable bacteria in comparison with the controls suspended in BSK II (borreliae) or in EMJH medium (leptospires) was observed. The results of the uptake of radiolabeled leptospires and borreliae after infusion in the perfused rat liver, which had previously been washed free of blood, are reported in Fig. 1. A single dose of 1.5 × 107 14C-labeled bacteria (150,000 ± 30,000 cpm) was infused into the portal vein, and the effluent from the sovrahepatic veins was counted for radioactivity. The liver uptake, expressed as the percentage of radioactivity retained by the liver, was 37.4% ± 2.3% for L. interrogans and 60.5% ± 3.1% for B. burgdorferi IRS. The difference in liver uptake was statistically significant (P < 0.0001), suggesting that L. interrogans escaped uptake by the liver RES to a significantly higher extent than did the agent of Lyme disease. The kinetics of the radioactivity appearing in the liver effluents after portal infusion of borreliae and leptospires are given in Fig. 2. Bacteria appeared in the effluent immediately after infusion, peaked, and then decreased, leveling off and then disappearing from the perfusate within 7 to 8 min of infusion. No tail in the radioactivity was observed, showing that no back diffusion from the liver was present. Under the present experimental conditions, the hepatic uptake was far from saturation, since an increase in spirochete load to the liver by 100-fold (2.5 × 109) reduced the hepatic uptake only by 10-fold (data not reported).
FIG. 1.
Uptake of radiolabeled spirochetes (percentage of the infused dose) by the liver. A single dose of 1.5 × 107 14C-labeled bacteria (150,000 ± 30,000 cpm) was infused into the portal vein, and the effluent from the sovrahepatic veins was counted for radioactivity. The data shown are the means ± the standard deviations. The differences in liver uptake for L. interrogans and B. burgdorferi IRS were statistically significant (P < 0.0001).
FIG. 2.
Time course of the kinetic display of the radioactivity (means ± the standard deviations) appearing in the effluent after infusion of 1.5 × 107 labeled bacteria (150,000 ± 30,000 cpm). Symbols: ■, B. burgdorferi IRS; ●, L. interrogans.
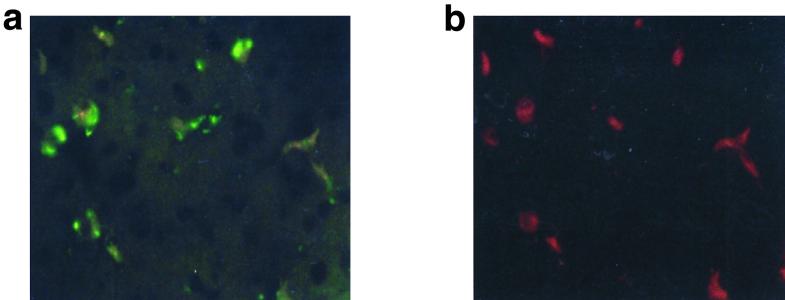
In order to evaluate the recovery of infused bacteria, the liver specimens were washed and either homogenized in a mortar or simply minced and subsequently cultured in EMJH or BSKII medium for the isolation of leptospires and borreliae, respectively. Borreliae were not recovered after 14 days of incubation both of homogenized and of minced liver samples in BSK II medium, showing an efficient uptake and killing of bacteria by the cells of the RES. On the other hand, leptospires could partially escape uptake and killing by the cells of the RES since they were reisolated in EMJH medium from minced liver samples (6.6 × 106 leptospires/g of liver) after 7 days of incubation. Leptospires were not reisolated from liver homogenates, very likely due to the toxic effect of bile on leptospires (14) during the homogenization process. In order to evaluate whether the high ability of leptospires to escape the hepatic sinusoidal uptake could also allow the passage of bacteria across cells lining sinusoids and hence a passage through the interspaces between the hepatocytes up to the bile canaliculus into bile, we looked for the presence of leptospires and borreliae in bile. Five rats were infused with a single dose of 5 × 108 14C-labeled leptospires (7,000,000 ± 100,000 cpm), whereas five rats were infused with the same amount of heat-inactivated (56°C for 1 h) 14C-labeled leptospires. A volume of 250 to 300 μl of bile was obtained from each rat during the 30-min collection period: 150 μl of bile was counted for radioactivity, aliquots (10 μl) were used for counting leptospires by dark-field microscopy, and 100 μl of bile was inoculated into growth medium for isolation purposes. Rats infused with living leptospires demonstrated the presence both of radioactivity (300 ± 45 cpm/150 μl) and of partially motile leptospires (1.5 × 103 bacteria/10 μl) in the bile. However, it was not possible to isolate bacteria in EMJH medium, very likely due to the toxic effect of undiluted bile on leptospires (14). When heat-inactivated, 14C-labeled leptospires (n = 5 × 108) were infused, bacteria were not observed in bile samples by microscopy examination, nor was significant radioactivity detected (50 ± 10 cpm/150 μl). Bile samples similarly obtained from rats (n = 4) injected with B. burgdorferi IRS (5 × 108 14C-labeled bacteria) were negative as determined by dark-field examination, by culture in BSK II medium, and by the detection of radioactivity (60 ± 15 cpm/150 μl). Since leptospires were already present in the bile as early as 30 min after infusion, the high motility and thinness of the bacteria seem to be the likely pathogenic mechanism that allowed leptospires to escape uptake by reticuloendothelial cells and hence to penetrate the endothelial lining of the liver sinusoids, reaching the spaces between liver parenchymal cells (14). In order to evaluate the association of leptospires and borreliae with reticuloendothelial cells of the liver, histological sections of the liver taken 30 min after infusion were used for double-staining experiments with rhodamine-labeled antivimentin or antidesmin antibodies for the identification of liver nonparenchymal cells, with leptospires and borreliae being revealed by the indirect immunofluorescence technique with FITC-conjugated antibodies. Only the vimentin-positive cells were associated with leptospires (Fig. 3) or borreliae (not shown), whereas the desmin-positive cells were not (not shown). Since antivimentin antibodies stain total sinusoidal cells, i.e., Kupffer, stellate (Ito), and endothelial cells, while only the sinusoidal stellate (Ito) cells (23) are identified also by antidesmin antibodies, we concluded that spirochetes were associated with Kupffer and/or endothelial cells and not with stellate cells, which are fat-storing cells and have no phagocytic activity. Few leptospires, but no borreliae, were also seen associated with vimentin- and desmin-negative cells (data not shown), suggesting the presence of leptospires outside the sinusoidal spaces, in the liver parenchyma (10). In conclusion, the results obtained by in vivo studies showed that leptospires could avoid uptake by the liver RES to a significantly higher extent than did borreliae and could also penetrate the endothelial lining of the liver sinusoids and reach biliary canaliculi. The different compositions and structures of the surfaces of leptospires and borreliae, as well as the motility and thinness of leptospires, may explain the different sinusoidal uptake of spirochetes by the liver.
FIG. 3.
Immunohistochemical localization of L. interrogans in rat liver sections subjected to double immunofluorescence. Thirty minutes after perfusion, the liver was fixed and liver sections were stained with human antibodies specific for leptospires followed by the addition of FITC-labeled anti-human antibodies. Afterwards, the section was probed with TRITC-labeled antivimentin monoclonal antibodies. (a) Spirochetal antigens were stained green in the cytoplasm of infected cells by FITC. (b) The same preparation shown in panel a observed at different wavelengths to evaluate TRITC reagents: the FITC-positive cells were stained red by TRITC. The results demonstrate that leptospires are associated with vimentin-positive cells.
Acknowledgments
This study was partially supported by MURST grant “Cofinanziamento 98.”
REFERENCES
- 1.Aldini R, Roda A, Simoni P, Lenzi P, Roda E. Uptake of bile acids by perfused rat liver: evidence of a structure-activity relationship. Hepatology. 1988;10:840–845. doi: 10.1002/hep.1840100515. [DOI] [PubMed] [Google Scholar]
- 2.Arean V M. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil's disease) Am J Pathol. 1962;40:393–399. [PMC free article] [PubMed] [Google Scholar]
- 3.Ballardini G, Groff P, Badiali De Giorgi L, Shuppan D, Bianchi F B. Ito cell heterogeneity: desmin negative Ito cells in normal rat liver. Hepatology. 1994;19:440–446. [PubMed] [Google Scholar]
- 4.Barthold S W, Moody K D, Terwilliger J A, Duray P H, Jacoby R O, Steere A C. Experimental Lyme arthritis of rats infected with Borrelia burgdorferi. J Infect Dis. 1988;157:842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre P F, Oxsman E. Phagocytosis and intracellular disposition of viable bacteria by the isolated perfused rat liver. J Reticuloendothel Soc. 1965;2:313–325. [PubMed] [Google Scholar]
- 6.Burgdorfer W. The diagnosis of the relapsing fevers. In: Johnson R C, editor. The biology of parasitic spirochetes. New York, N.Y: Academic Press, Inc.; 1976. pp. 225–234. [Google Scholar]
- 7.Cevenini R, Sambri V, Massaria F, Franchini R, D'Antuono A, Borda G, Negosanti M. Surface immunofluorescence assay for diagnosis of Lyme disease. J Clin Microbiol. 1992;30:2456–2461. doi: 10.1128/jcm.30.9.2456-2461.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards K S, Kanengiser S, Li K I, Glassman M. Lyme disease presenting as hepatitis and jaundice in a child. Pediatr Infect Dis J. 1990;9:592–593. doi: 10.1097/00006454-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Ellighausen H C, McCullough W G. Nutrition of Leptospira pomona and growth of 13 other serotypes: fractionation of oleic albumin complex and a medium of bovine albumin and polysorbate 80. Am J Vet Res. 1965;26:45–51. [PubMed] [Google Scholar]
- 10.Faine S. Reticuloendothelial phagocytosis of virulent leptospires. Am J Vet Res. 1964;25:830–835. [PubMed] [Google Scholar]
- 11.Galbe J L, Guy E, Zapatero J M, Peerschke E I B, Benach J L. Vascular clearance of B. burgdorferi in rats. Microb Pathog. 1993;14:187–202. doi: 10.1006/mpat.1993.1019. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz H W, Dworkin B, Forseter G, Nadelman R B, Connolly C, Luciano B B, Nowakowski J, Obrien T A, Calmann M, Wormser G P. Liver function in early Lyme disease. Hepatology. 1996;23:1412–1417. doi: 10.1002/hep.510230617. [DOI] [PubMed] [Google Scholar]
- 13.Klein A, Zhadkewich M, Margolick J, Winkelstein J, Bulkley G. Quantitative discrimination of hepatic reticuloendothelial clearance and phagocytic killing. J Leukoc Biol. 1994;55:248–252. doi: 10.1002/jlb.55.2.248. [DOI] [PubMed] [Google Scholar]
- 14.Miller N G, Wilson R B. Electron microscopy of the liver of the hamster during acute and chronic leptospirosis. Am J Vet Res. 1966;17:1071–1081. [PubMed] [Google Scholar]
- 15.Moon R J, Vrable A, Broka J A. In situ separation of bacterial trapping and killing functions of the perfused liver. Infect Immun. 1975;12:411–418. doi: 10.1128/iai.12.2.411-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortimore G E. Effect of insulin on potassium transfer in isolated rat liver. Am J Physiol. 1961;200:1315–1319. doi: 10.1152/ajplegacy.1961.200.6.1315. [DOI] [PubMed] [Google Scholar]
- 17.Musher D M. Syphilis. In: Gorbach S L, Bartlett J G, Blacklow N R, editors. Infectious diseases. W. B. Philadelphia, Pa: Saunders; 1992. pp. 822–828. [Google Scholar]
- 18.Sambri V, Aldini R, Massaria F, Montagnani M, Casanova S, Cevenini R. Uptake and killing of Lyme disease and relapsing fever borreliae in the perfused rat liver and by isolated Kupffer cells. Infect Immun. 1996;64:1858–1861. doi: 10.1128/iai.64.5.1858-1861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambri V, Stefanelli C, Rossoni C, La Placa M, Jr, Cevenini R. Acylated proteins in Borrelia hermsii, Borrelia parkeri, Borrelia anserina, and Borrelia coriaceae. Appl Environ Microbiol. 1993;59:3938–3940. doi: 10.1128/aem.59.11.3938-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shotts E B. Laboratory diagnosis of leptospirosis. In: Johnson R C, editor. The biology of parasitic spirochetes. New York, N.Y: Academic Press, Inc.; 1976. pp. 209–223. [Google Scholar]
- 21.Thiermann A B. The Norway rat as selective chronic carrier of Leptospira icterohaemorrhagiae. J Wildl Dis. 1981;17:39–43. doi: 10.7589/0090-3558-17.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Wilske B, Preac-Mursic V. Microbial diagnosis of Lyme borreliosis. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer-Verlag; 1993. pp. 267–299. [Google Scholar]
- 23.Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells) Hepatology. 1984;5:683–692. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]