Abstract
Strains of the periodontal pathogen Actinobacillus actinomycetemcomitans are variable with respect to display of phosphorylcholine (PC)-bearing antigens. We have examined strains of A. actinomycetemcomitans with and without PC to assess their ability to invade endothelial cells via the receptor for platelet-activating factor (PAF). Results of antibiotic protection assays indicate that PC-bearing A. actinomycetemcomitans invade human vascular endothelial cells by a mechanism inhibitable by CV3988, a PAF receptor antagonist, and by PAF itself. The invasive phenotype was verified by transmission electron microscopy. A PC-deficient strain of this organism was not invasive. This property, in addition to the established ability of A. actinomycetemcomitans to invade epithelial cells, may provide this organism with access to the systemic circulation. The ability of PC-bearing oral bacteria to access the circulation may also explain the elevated levels of anti-PC antibody in serum found in patients with periodontitis.
Phosphorylcholine (PC) has been detected on a number of pathogenic prokaryotes, including Streptococcus pneumoniae and other gram-positive bacteria such as other streptococci, Bacillus spp., Clostridium spp., and other bacilli, as well as the gram-negative species Haemophilus influenzae (5). All of these prokaryotes contain choline within structural molecules, within either teichoic acids, lipoteichoic acids, or lipopolysaccharide (LPS). The function of such PC in pathogenesis is largely unknown for most species. However, specific examples exist that implicate PC as a virulence factor. The prototypical bacterial species containing PC is S. pneumoniae, which incorporates choline from culture media into PC in its teichoic acid and lipoteichoic acid (9). It has been shown that PC may mediate invasiveness of S. pneumoniae in the lung (1, 10, 11) and the brain (7) by permitting access of this bacterium to the receptor for platelet-activating factor (PAF) on endothelial cells. In addition, it has been suggested that PC contributes to the persistence of H. influenzae in the human respiratory tract (12). A genetic locus required for PC metabolism in S. pneumoniae has been identified which contains genes similar to a homologous locus in H. influenzae; mutation of some of these genes leads to decreased virulence of S. pneumoniae (13).
Recent studies of the oral flora and the respiratory tract flora have identified additional species which have structural molecules bearing PC (3, 4, 6); these molecules have invariably been shown to contain PC by specific reactivity with monoclonal antibodies or myeloma proteins which react only with PC. Studies performed in our laboratories (8) and an extensive survey of plaque bacteria by Gmur and coworkers (4) indicate that a significant proportion of supragingival and subgingival plaque bacteria react with TEPC-15, an immunoglobulin A myeloma protein with specificity for PC. Although the importance of PC as an antigen in oral bacteria has not been established, it has been proposed that it is a virulence factor of S. pneumoniae (10, 11). This idea is supported by the fact that PAF, which contains PC, is mimicked by virulent strains of S. pneumoniae, which access the circulatory system by binding to the PAF receptor on endothelial cells, invading these cells, and transmigrating through the endothelium into the bloodstream.
It is thought that induction of anti-PC is mainly due to exposure to S. pneumoniae. However, our recent findings indicate that patients with periodontal attachment loss (in all disease categories) have higher levels of anti-PC than healthy patients (8). The implication is that the oral flora is likely a source of immunogen for generation of anti-PC. These studies further show that about 40% of plaque bacteria react with TEPC-15 and thus likely contain PC. Given these observations, it is reasonable to hypothesize that some oral bacterial species behave like S. pneumoniae, gaining access to the circulatory system by binding to the PAF receptor on endothelial cells and inducing elevated levels of antibody to PC.
Identification of strains of Actinobacillus actinomycetemcomitans bearing PC.
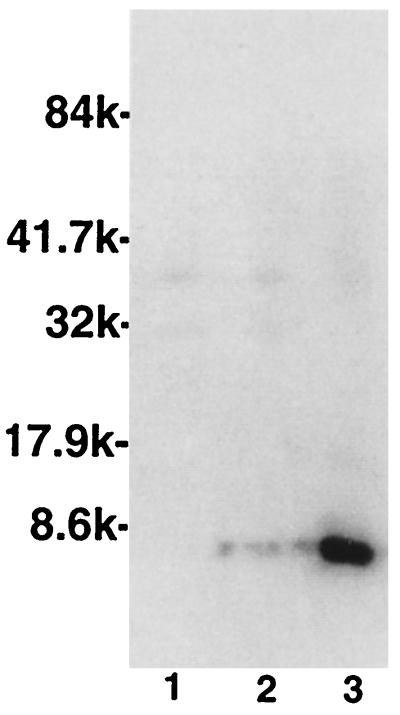
A. actinomycetemcomitans is a gram-negative rod that is associated particularly with early-onset periodontal diseases. This species has a wide array of virulence factors, among which is its ability to invade epithelial cells (2). We identified strains of A. actinomycetemcomitans bearing the PC epitope using two methods. First, the uptake of [3H]choline from culture media was measured as an indicator of the relative incorporation of choline into PC in structural molecules (8). Bacterial cultures were grown to log phase in brain heart infusion medium (Difco Laboratories, Detroit, Mich.) containing 1.5 μCi of [3H]choline chloride (New England Nuclear Life Science Products, Boston, Mass.)/ml. Cultures were washed three times with phosphate-buffered saline and resuspended in the same buffer to an optical density of 1.0 at 650 nm. Following the addition of 5 ml of scintillation cocktail (3270B; Research Products International Corp., Mount Prospect, Ill.) to 1 ml of washed bacterial suspension, the samples were subjected to scintillation counting. Data are reported as mean counts per minute for two experiments. As seen in Table 1, consistent with the data reported by Gmur et al., one of four tested strains of A. actinomycetemcomitans incorporated significantly greater amounts of choline than the other strains; this amount of incorporation was approximately 10% of that seen with a PC-positive strain of S. pneumoniae. Next, we sought to demonstrate PC-bearing antigens on these strains. We treated cultures of A. actinomycetemcomitans with sodium dodecyl sulfate to elute LPS from the bacterial surface and examined these antigens on immunoblots that were probed with TEPC-15 or with MOPC-315, an immunoglobulin A myeloma protein with specificity for 2,4-dinitrophenol (isotype control) as previously described (8). As shown in Fig. 1, A. actinomycetemcomitans D045D-40 demonstrated a pattern typical of that seen for H. influenzae LPS following reaction with anti-PC, where specific TEPC-15-reactive antigens are found below 8,000 kDa. Further verification of the presence of PC-bearing surface antigens was sought by performing immunofluoresence microscopy with TEPC-15 as the primary antibody; strain D045D-40 demonstrated weak positive reactivity, whereas strain DB03A-42 was unreactive. In all assays, both whole cells and eluted antigens failed to react with MOPC-315 in control experiments (data not shown). Thus, some strains of A. actinomycetemcomitans contain structural molecules bearing PC antigens.
TABLE 1.
Incorporation of [3H]choline by strains of A. actinomycetemcomitans and S. pneumoniae 39937
| Organism | Mean 3H cpm |
|---|---|
| A. actinomycetemcomitans serotype a (SUNY Buffalo 122) | 350 |
| A. actinomycetemcomitans serotype b (VPI DB03A-42) | 476 |
| A. actinomycetemcomitans serotype c (SUNY Buffalo 360) | 232 |
| A. actinomycetemcomitans serotype b (D045D-40 [clinical isolate]) | 20,675 |
| S. pneumoniae (39937) | 262,289 |
FIG. 1.
Western blot analysis of TEPC-15-reactive antigens of A. actinomycetemcomitans DB03A-42 (lane 1), A. actinomycetemcomitans DR03D-03A (lane 2), and A. actinomycetemcomitans D045D-40 (lane 3). Molecular weights, in thousands (k), are on the left.
Invasion of HUVEC by A. actinomycetemcomitans.
Our previous data indicated that higher levels of anti-PC antibody in serum are present in patients with periodontitis than in unaffected controls (8). Thus, we asked how these bacteria might induce an antibody response against PC. One potential mechanism was that PC-bearing oral bacteria can gain access to the immune system, and perhaps the general circulation, via interaction with the PAF receptor on endothelial cells. To test this hypothesis, A. actinomycetemcomitans (at an optical density of 0.9 at 650 nm, 100 μl/well) was incubated with monolayers of human vascular endothelial cells (HUVEC) for 4 h as described by Ring and coworkers (7). Following incubation, the monolayers were washed and some cultures were treated with gentamicin (50 μg/ml, 2 h) to kill bacteria external to the HUVEC. Subsequently, the HUVEC were lysed and bacteria were plated to enumerate either total cell-associated bacteria or ingested bacteria alone. In some experiments, cultures were treated with CV3988, a synthetic competitive inhibitor of the PAF receptor, to block access of the PC-bearing bacteria to the PAF receptor. This compound was shown to have no influence on the viability of A. actinomycetemcomitans strains. Table 2 shows typical results of several experiments in which PC-positive A. actinomycetemcomitans (D045D-40) was incubated with HUVEC that had been pretreated with a PAF receptor antagonist and subsequently treated with gentamicin. The table shows the mean CFU remaining associated with the HUVEC following treatment with gentamicin to kill externalized cells. The percentage of cells internalized was calculated by dividing the CFU remaining after gentamicin treatment by the total cells associated with HUVEC prior to gentamicin treatment. The data indicate that the bacteria were internalized and that the PAF receptor antagonist inhibited internalization. In contrast, experiments with a PC-negative strain of A. actinomycetemcomitans (DB03A-42) indicated that this strain was not internalized (0.00%) by HUVEC. Previously, Cundell and coworkers (1) observed that invasion of HUVEC by S. pneumoniae was enhanced by cytokines such as tumor necrosis factor. We observed that A. actinomycetemcomitans readily invaded HUVEC without a great deal of modulation by previous treatment with mediators (data not shown).
TABLE 2.
Internalization of A. actinomycetemcomitans by HUVEC
| A. actinomycetemcomitans strain | CV3988 concn (μM) | No. of CFU/ml (mean ± SD)
|
% of CFU
|
||
|---|---|---|---|---|---|
| Bound + internalized | Internalizeda | Compared to controlb | Internalizedc | ||
| D045D-40 | 0 | 3,250,000 ± 926,427 | 36,650 ± 5,400 | 1.13 | |
| 1 | 2,580,000 ± 473,568 | 28,400 ± 2,861 | 77 | 1.1 | |
| 25 | 1,870,000 ± 441,362 | 6,350 ± 1,204 | 17 | 0.34 | |
| 50 | 1,745,000 ± 634,849 | 1,655 ± 375 | 5 | 0.09 | |
| DB03A-42 | 0 | 33,500 ± 15,264 | 0 | 0 | 0 |
Cultures of HUVEC containing A. actinomycetemcomitans were washed and treated with gentamicin. Subsequently, HUVEC were lysed and bacteria were plated to determine the number of CFU remaining.
Control cultures contained no CV3988.
CFU remaining following gentamicin treatment/CFU without gentamicin treatment × 100.
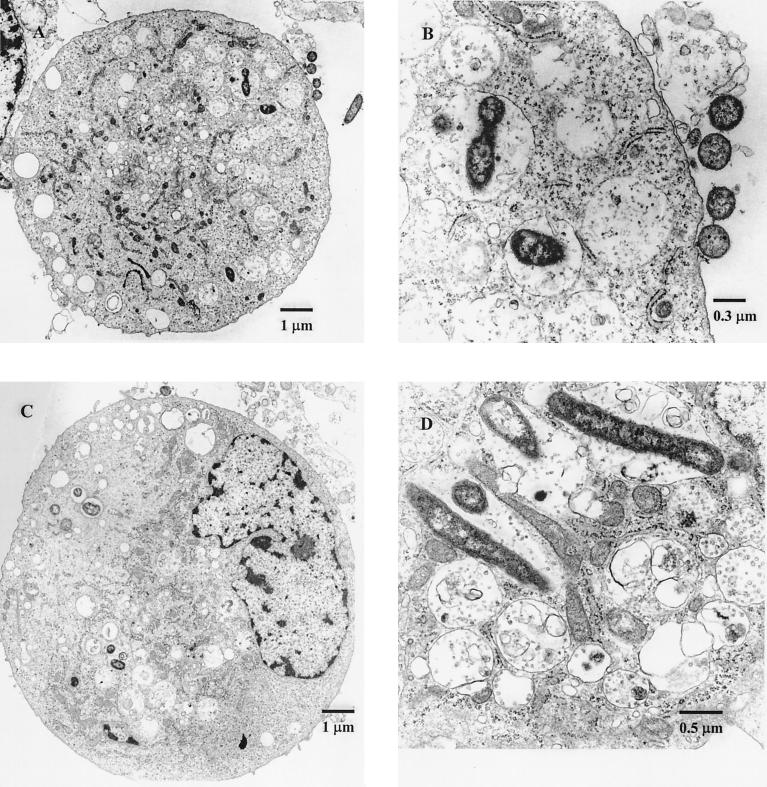
We examined HUVEC from the above experiment to ensure that A. actinomycetemcomitans was internalized within the endothelial cells. The electron micrographs in Fig. 2 indicate that this was in fact the case, showing that the bacteria do bear the invasive phenotype. An evaluation of microscopic sections from 300 individual cells revealed that approximately 93% of microscopically intact HUVEC had at least one internalized bacterial cell following interaction with A. actinomycetemcomitans. Interestingly, many more bacterial cells were found within the HUVEC than was anticipated from the results of the antibiotic protection assays, indicating that, as seen with S. pneumoniae, a significant proportion of bacteria may be killed within endothelial cells. Alternatively, A. actinomycetemcomitans may multiply within the HUVEC with subsequent bacterial death, which would account for the apparent discrepancy between viable cell counts and the observed level of invasion.
FIG. 2.
Transmission electron micrographs demonstrating A. actinomycetemcomitans within HUVEC. (A) A. actinomycetemcomitans within HUVEC (magnification, ×8,100; original magnification, ×9,000); (B) Detail of panel A (magnification, ×38,900; original magnification, ×43,200); (C) HUVEC containing A. actinomycetemcomitans (magnification, ×8,100; original magnification, ×9,000); (D) HUVEC with internalized A. actinomycetemcomitans (magnification, ×38,900; original magnification, ×43,200).
The results demonstrate that A. actinomycetemcomitans likely invades endothelial cells via a mechanism dependent upon the engagement of the PAF receptor by bacterial PC. Extensive previous data indicate that this species invades epithelial cells via a mechanism independent of this receptor (2). In addition to the well-established ability of A. actinomycetemcomitans to invade epithelial cells, we propose that PC-positive members of this species can also gain access to the circulation through intact oral tissues. This activity may be a model for invasive activity of other PC-bearing oral bacteria that otherwise gain access to underlying connective tissues, perhaps as a result of denudation and ulceration of the pocket epithelium consequent to periodontal infections.
Acknowledgments
This work was supported by grant DE10703 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 2.Fives-Taylor P M, Meyer D H, Mintz K P, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology. 2000;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie S H, McWhinney P H, Patel S, Raynes J G, McAdam K P, Whiley R A, Hardie J M. Species of alpha-hemolytic streptococci possessing a C-polysaccharide phosphorylcholine-containing antigen. Infect Immun. 1993;61:3076–3077. doi: 10.1128/iai.61.7.3076-3077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gmur R, Thurnheer T, Guggenheim B. Dominant cross-reactive antibodies generated during the response to a variety of oral bacterial species detect phosphorylcholine. J Dent Res. 1999;78:77–85. doi: 10.1177/00220345990780011201. [DOI] [PubMed] [Google Scholar]
- 5.Harnett W, Harnett M M. Phosphorylcholine: friend or foe of the immune system? Immunol Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 6.Kolberg J, Hoiby E A, Jantzen E. Detection of the phosphorylcholine epitope in streptococci, Haemophilus and pathogenic Neisseriae by immunoblotting. Microb Pathog. 1997;22:321–329. doi: 10.1006/mpat.1996.0114. [DOI] [PubMed] [Google Scholar]
- 7.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenkein H A, Gunsolley J C, Best A M, Harrison M T, Hahn C L, Wu J, Tew J G. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect Immun. 1999;67:4814–4818. doi: 10.1128/iai.67.9.4814-4818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967;157:694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]
- 10.Tuomanen E I. Molecular and cellular mechanisms of pneumococcal meningitis. Ann N Y Acad Sci. 1996;797:42–52. doi: 10.1111/j.1749-6632.1996.tb52948.x. [DOI] [PubMed] [Google Scholar]
- 11.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 12.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J-R, Idanpaan-Heikkila I, Fischer W, Tuomanen E I. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol Microbiol. 1999;31:1477–1488. doi: 10.1046/j.1365-2958.1999.01291.x. [DOI] [PubMed] [Google Scholar]