Abstract
Recent evidence suggests that certain periodontal pathogens preferentially stimulate T cells expressing specific variable regions on the β chain (Vβ) of the T-cell receptor, which may indicate the presence of a superantigen. Superantigens are microbial proteins that activate large numbers of CD4+ T cells in a Vβ-specific manner. The purpose of this study was to determine whether Prevotella intermedia, a putative periodontal pathogen, activates populations of specific Vβ on CD4+ T cells. Among the bacterial strains tested, P. intermedia strain 17, a clinical isolate, induced the strongest proliferative response in peripheral blood mononuclear cells. Antibodies raised against whole cells of this organism blocked the proliferative activity. P. intermedia-induced proliferation was T-cell specific and required the presence of antigen-presenting cells. Flow cytometric analysis showed that CD4+ T-cell subsets expressing Vβ8, Vβ12, and Vβ17 expanded in response to P. intermedia strain 17. The ability of P. intermedia to stimulate CD4+-T-cell proliferation was further supported by the production profiles of key T-cell cytokines, gamma interferon and interleukin-2. The data collectively suggest that certain strains of P. intermedia can activate Vβ-specific T cells in a manner similar to that of other known microbial superantigens.
Prevotella intermedia, a gram-negative, black-pigmented, obligate anaerobic rod, has been implicated in many forms of human periodontal disease, including chronic periodontitis (26, 27, 38), early-onset periodontitis (28, 36), acute necrotizing ulcerative gingivitis (4, 18), and pregnancy gingivitis (14, 23). Studies have shown that P. intermedia is associated with periodontal breakdown in type I diabetics (40) and is frequently encountered in periodontal lesions or abscesses associated with destructive disease (5, 7, 9, 10, 25, 33, 35, 36, 39). Thus, these studies provide strong clinical support for a role for P. intermedia in the development of periodontal diseases.
It is generally accepted that periodontal disease is primarily a localized disease caused by the dynamic interactions between the inflammatory bacterial agents and the host's immunopathological reactions, resulting in the destruction of connective tissues supporting the teeth (2, 16, 22). In this regard, there are reports suggesting that lipopolysaccharides (LPS) (11, 30, 31) and other surface components (12, 21, 29) isolated from P. intermedia are capable of inducing inflammatory lymphokines, which may contribute to the pathological inflammatory mechanisms that are operative in periodontal disease. Recently, there has been evidence to suggest that some periodontopathic organisms may be involved in the activation of T cells expressing specific variable regions on the β chain (Vβ) of T-cell receptors (TCRs) (8, 19, 20, 37). One study showed that up to 50% of all gingival T cells express unique subsets of Vβ TCRs in most periodontitis patients but not in healthy subjects (37). Using peripheral blood mononuclear cells (PBMC) cocultured for 48 h with heat-inactivated periodontal bacteria, including Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and P. intermedia, Mathur et al. (19) showed that P. intermedia, but not the other tested bacteria, increases the number of T cells expressing Vαβ2, Vβ5, and Vβ6 regardless of donor disease status. These results suggest that certain periodontal bacteria can activate T cells in a Vβ-specific manner, thus providing the evidence for the possible involvement of superantigens in human periodontal diseases (19, 20, 37). The purpose of the present study was to test whether some clinical strains of P. intermedia were capable of inducing T-cell responses and, if so, whether the activation was Vβ specific.
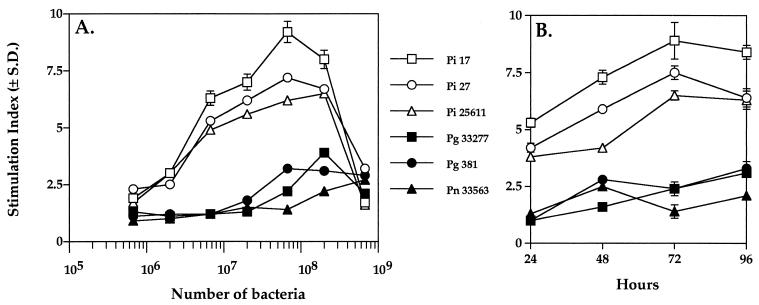
Initial studies were performed by coculturing PBMC of healthy individuals with paraformaldehyde-treated periodontal bacterial strains for 96 h. Prevotella strains and P. gingivalis were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% yeast extract, 0.075% cysteine, and 5 μg of hemin and menadione (Sigma Chemical Co., St. Louis, Mo.) per ml in an anaerobic chamber (Vacuum Atmospheres, Hawthorne, Calif.) with an atmosphere of 5% CO2, 10% H2, and 85% N2 as described previously (1, 6). Escherichia coli MC1061 was grown in Luria-Bertani medium consisting of Bacto tryptone (10 g/liter), Bacto yeast extract (5 g/liter), and NaCl (10 g/liter) under aerobic conditions (6). Overnight cultures were washed three times in phosphate-buffered saline, pH 7.2 (PBS), and fixed in 2% paraformaldehyde in PBS at 4°C. After overnight fixation, bacteria were washed three times in PBS and adjusted to make stocks with a concentration of 2.0 × 109 cells/ml. PBMC were isolated as described in the legend for Fig. 1. As shown in Fig. 1A, P. intermedia strains 17, 27, and ATCC 25611 induced strong proliferative responses in PBMC cultures, as indicated by the cellular uptake of [3H]thymidine. The optimal concentration of Prevotella strains to stimulate proliferation in PBMC was 6.7 × 107 cells/ml. Modest proliferative responses were observed with paraformaldehyde-treated P. gingivalis strains 381 and ATCC 33277 and Prevotella nigrescens ATCC 33563 (Fig. 1A). On the other hand, E. coli MC1061 and medium alone did not induce any proliferative responses (data not shown). Thus, P. intermedia strains caused significant proliferation of human PBMC.
FIG. 1.
Proliferation of human PBMC in response to Prevotella and other strains of bacteria. PBMC were isolated from peripheral blood of healthy volunteers by Ficoll-Hypaque centrifugation (34). PBMC (2 × 105 cells) were cultured in RPMI 1640 medium containing 5% fetal bovine serum in the presence of paraformaldehyde-treated bacteria. Microtiter plates were used for cultures, and experiments were carried out in quadruplicate. (A) Paraformaldehyde-treated P. intermedia (Pi) strains 17, 27, and ATCC 25611, P. gingivalis (Pg) strains 33277 and 381, and P. nigrescens (Pn) strain ATCC 33563 at different cell concentrations were cocultured with PBMC for 90 h. [3H]thymidine was added (1 μCi/well; Amersham, Indianapolis, Ind.), and cells were harvested onto filter paper at 96 h of culture using a model M12 cell harvester (Brandel, Gaithersburg, Md.). Radioactivity associated with the cells was quantified using a Beckman model LS3801 beta scintillation counter. The stimulation index was calculated by dividing the mean experimental counts per minute by the mean unstimulated counts per minute. The mean counts per minute (± standard deviation [S.D.]) from unstimulated cultures was 287 ± 24. (B) PBMC were cultured with paraformaldehyde-treated bacteria (6.7 × 107 cells/ml) for various times under the culture conditions described above. Six hours prior to harvest, cells were pulsed with [3H]thymidine. PBMC were harvested at 24, 48, 72, and 96 h. The data are representative of three experiments, each performed in triplicate.
Further, a time course study showed that, among the periodontopathic bacteria tested for stimulation of PBMC, P. intermedia strains at 6.7 × 107 cells/ml (the optimal concentration) generated a significant and rapid proliferative response within 24 h, while P. gingivalis and P. nigrescens at similar concentrations required 96 h of coculturing to reach similar levels of response (Fig. 1B). Among the Prevotella strains tested, P. intermedia strain 17 induced the greatest proliferation at each 24-h interval (Fig. 1B). Further, studies were performed using antisera generated against whole cells and against the isolated fimbriae of P. intermedia strain 17 (6, 17). As seen in Table 1, immunoglobulin G (IgG) to whole cells blocked the proliferative activity of P. intermedia strain 17, whereas antifimbria antibodies (IgG) had no effect. The results suggest that a membrane component(s) on the bacterium may be involved in the activation of human PBMC.
TABLE 1.
Proliferation of PBMC in response to P. intermedia strain 17 is blocked by antisera generated against the whole bacterium
| Culturea | Affinity-purified IgGb | Mean cpm ± SDc |
|---|---|---|
| PBMC alone | 352 ± 42 | |
| PBMC alone | Antifimbria | 408 ± 54 |
| PBMC alone | Anti-whole cell | 414 ± 38 |
| PBMC + strain 17 | 6,214 ± 297 | |
| PBMC + strain 17 | Antifimbria | 6,583 ± 349 |
| PBMC + strain 17 | Anti-whole cell | 612 ± 88 |
| PBMC + SEA | 16,289 ± 1,312 | |
| PBMC + SEA | Antifimbria | 17,366 ± 1,658 |
| PBMC + SEA | Anti-whole cell | 16,004 ± 1,299 |
PBMC were cultured in the presence of either strain 17 (6.7 × 107 cells/ml) or the bacterial superantigen SEA (0.5 μg/ml). Proliferation was measured after 96 h.
SEA and strain 17 were preincubated with either antifimbria (86 μg/ml) or anti-whole cell (142 μg/ml) IgG for 45 min prior to addition to PBMC. Preimmune sera tested at 1:100 dilution did not have an effect.
Proliferation was measured by the incorporation of [3H]thymidine, as described in the legend for Fig. 1. The data are representative of three experiments, each performed in triplicate.
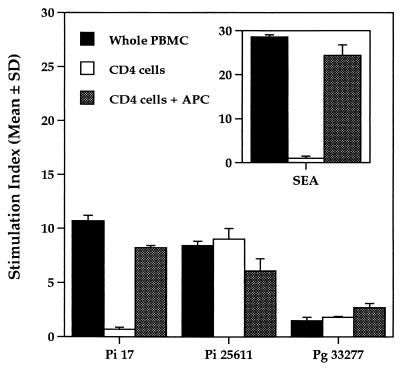
To determine whether the stimulatory effects of P. intermedia and other periodontal bacteria required the presence of antigen-presenting cells (APC), CD4+ T cells were isolated from PBMC for further studies. In these experiments, paraformaldehyde-treated bacterial whole cells (6.7 × 107 cells/ml) were incubated with whole PBMC alone, CD4+ T cells alone, or CD4+ T cells cultured in the presence of paraformaldehyde-treated PBMC (APC). Proliferation was measured after 96 h. As shown in Fig. 2, cultures of whole PBMC proliferated in response to the P. intermedia strains tested. However, cultures of purified CD4+ T cells did not respond to P. intermedia strain 17. Proliferation in response to P. intermedia strain 17 occurred in CD4+-cell cultures reconstituted with inactivated APC, indicating that T cells were the cells that responded to P. intermedia strain 17 and that APC were required for proliferation. These results are different from those obtained with P. intermedia ATCC 25611, where proliferative responses of CD4+ T cells took place in either the presence or the absence of APC. P. gingivalis showed patterns of proliferative responses similar to those of P. intermedia ATCC 25611, although the degree of proliferation was much lower. No proliferation was observed in inactivated PBMC (containing both the CD4+ T cells and APC) when cocultured with various bacterial whole cells (data not shown). The results indicate that proliferation in response to P. intermedia strain 17 requires the presence of APC without the antigen uptake and processing steps and that the responding cells are CD4+ T cells. These results were similar to the proliferation of purified CD4+ T cells in response to the bacterial superantigen staphylococcal enterotoxin A (SEA) (15), which occurred only in the presence of APC (Fig. 2, inset). Thus, P. intermedia strain 17 is capable of inducing CD4+-T-cell proliferation in a manner similar to that of known superantigens.
FIG. 2.
P. intermedia-induced proliferation is T-cell specific and requires the presence of APC. Cultures consisted of whole PBMC, CD4+ T cells, and CD4+ T cells and APC. CD4+ T cells were isolated from whole PBMC using CD4+-T-cell Cellect columns (Cytovax, Edmondton, Alberta, Canada). APC consisted of whole PBMC that were inactivated by treatment with 2% paraformaldehyde and washed extensively to remove residual paraformaldehyde. Cells were cultured in the presence of bacteria for 96 h as described in the legend for Fig. 1. (Inset) Cultures were stimulated with 0.5 μg of the known bacterial superantigen SEA per ml. The data are representative of three experiments, each performed in triplicate. The mean counts per minute (± standard deviation [SD]) from unstimulated cultures was 301 ± 16. Pi, P. intermedia; Pg, P. gingivalis.
It has been well demonstrated that bacterial proteins possessing superantigenic activity preferentially stimulate T cells expressing distinctive Vβ TCRs (3). In this regard, we were interested in determining whether the stimulation of CD4+-T-cell proliferation by these periodontal bacteria was Vβ specific. Initial screening was performed using a simple amplification procedure to detect Vβ expansion (32), and a panel of monoclonal antibodies was selected for use in the subsequent cytometric analysis. Vβ expression of stimulated PBMC after coculture with different bacteria for 72 h was determined by flow cytometric analysis (FACSort flow cytometer; Becton Dickinson, San Jose, Calif.) using a fluorescein-labeled, CD4+-T-cell-specific monoclonal antibody (Becton Dickinson) and a panel of rhodamine-labeled, anti-Vβ antibodies (Becton Dickinson). The percentages of T cells expressing each Vβ were determined for PBMC incubated in medium alone (unstimulated controls) and compared with the Vβ expression following coculturing with each tested organism. Flow cytometric analysis data showed that CD4+ T-cell subsets expressing Vβ8, Vβ12, and Vβ17 expanded in response to P. intermedia strain 17, compared to unstimulated controls (Table 2). The percentages of T cells expressing Vβ8, Vβ12, and Vβ17 were at least twice those of unstimulated controls. These results suggest that a superantigen is associated with this periodontal pathogen, which activates T cells expressing a particular Vβ chain. With the exception of the proliferation of Vβ12 CD4+ T cells in response to P. intermedia ATCC 25611, there was no significant increase in CD4+ T cells expressing Vβ TCRs in samples stimulated with other tested organisms.
TABLE 2.
Fluorescence-activated cell sorter analysis of CD4+ Vβ T-cell subsets induced by P. intermediaa
| Culture | % of cells expressing:
|
||||
|---|---|---|---|---|---|
| Vβ1 | Vβ8 | Vβ12 | Vβ17 | Vβ22 | |
| PBMC alone | 3.1 ± 0.1 | 2.4 ± 0.1 | 2.8 ± 0.2 | 3.5 ± 0.1 | 4.0 ± 0.2 |
| PBMC + strain 17 | 2.7 ± 0.1 | 4.3 ± 0.0 | 6.2 ± 0.1 | 7.4 ± 0.1 | 4.2 ± 0.2 |
| PBMC + strain 25611 | 2.8 ± 0.0 | 2.5 ± 0.1 | 5.4 ± 0.2 | 3.2 ± 0.2 | 3.0 ± 0.1 |
| PBMC + E. coli | 3.0 ± 0.1 | 2.2 ± 0.1 | 2.4 ± 0.1 | 3.1 ± 0.2 | 4.6 ± 0.3 |
PBMC were cultured with medium or in the presence of the bacteria indicated for 72 h as described in the legend for Fig. 3. Cells were washed, resuspended in FACS buffer (PBS containing 5% fetal bovine serum and 0.1% sodium azide), and adjusted to 107 cells/ml. Cells were then stained with fluorescein-labeled anti-CD4 for 1 h. After being washed in FACS buffer, cells were stained with rhodamine-labeled anti-Vβ antibodies. Cells were washed in FACS buffer, and Vβ subsets of CD4+ T cells were quantified using a Becton Dickinson FACSort. Values shown are the percentages of CD4+ T cells that double-stained for the indicated Vβ. The data are representative of three experiments, each with a different donor.
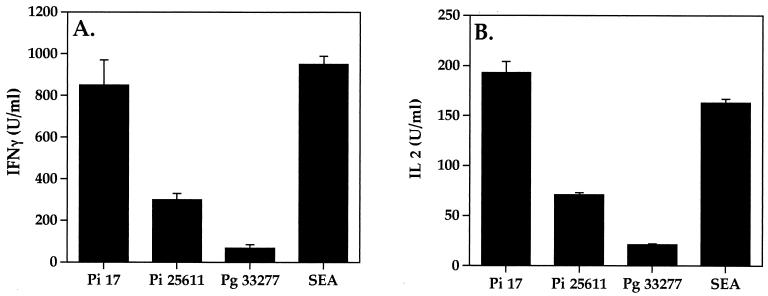
The ability of P. intermedia to stimulate CD4+-T-cell proliferation was further supported by the profiles of cytokine production. Culture supernatants from PBMC stimulated with periodontal bacteria and E. coli were collected in a time course study and tested for cytokine production using functional assays for interleukin-2 (IL-2) and gamma interferon (IFN-γ) (34). As shown in Fig. 3, P. intermedia strongly induced the production of IL-2 and IFN-γ. Since these cytokines are known to be produced by CD4+ Th1 cells, the results further substantiate that P. intermedia can activate CD4+ T cells. In addition, similar profiles were observed for another CD4+-T-cell cytokine, tumor necrosis factor (data not shown).
FIG. 3.
P. intermedia induces the production of the CD4+-T-cell cytokines IFN-γ and IL-2. Whole PBMC (5 × 106 cells/ml) were cocultured in 24-well plates with bacteria (6.7 × 107 cells/ml) in a final volume of 0.3 ml. SEA was used at 0.5 μg/ml. Supernatants were collected at 72 h and assayed for biological activity of IFN-γ and IL-2. IFN-γ antiviral activity was assessed on human WISH cells using a cytopathic-effect reduction assay with vesicular stomatitis virus (34). The antiviral activity was determined to be IFN-γ by neutralization with antibodies to human IFN-γ. IL-2 titers were determined using the IL-2-dependent cell line CTLL (34). The data are representative of three experiments, each performed in triplicate. Pi, P. intermedia; Pg, P. gingivalis.
Thus, similar to other known bacterial superantigens, stimulation by P. intermedia induced proliferation of Vβ-specific T-cells in an APC-dependent manner and caused the production of key T-cell cytokines (IL-2, IFN-γ, and tumor necrosis factor). The results strongly suggest that P. intermedia possesses superantigenic activity and corroborate the earlier studies of Mathur et al. (19), which showed that among the three periodontal pathogens tested, only P. intermedia stimulates the expression of Vβ subsets. While coculture of T cells with P. intermedia resulted in increases in the percentage of cells expressing Vαβ2, Vβ5, or Vβ6 as demonstrated by Mathur et al. (19), different Vβ subsets (Vβ8, Vβ12, and Vβ17) were preferentially expanded by the P. intermedia strains tested in this study. The differences observed may be attributable to the use of different strains of P. intermedia in the two studies. Similarly, it has been shown that Vβ expression varied in diseased gingival tissues obtained from patients. It was speculated that the differences observed may be due to the specific bacteria present at the sample site (20).
In this study, strain 17 appeared to be the most potent stimulator among the P. intermedia strains tested to induce proliferation of Vβ-specific T cells, suggesting the existence of functional heterogeneity among these strains in their ability to stimulate proliferation of T cells. P. intermedia ATCC 25611 seems to have a mitogenic property (Fig. 2) as well as a possible superantigenic component (Table 2). Such functional heterogeneity was also observed among the Prevotella strains that were tested earlier for their ability to adhere to erythrocytes (17) and to invade human oral epithelial cells (6). In these experiments, P. intermedia strain 17 had the strongest hemagglutinating activity and emerged as the only strain that is invasive. Antibodies raised against the P. intermedia strain 17 whole cell and against its isolated fimbriae inhibited both hemagglutination and invasion. The results suggest that the fimbriae are involved in promoting adhesion to erythrocytes (17) and invasion of epithelial cells by P. intermedia 17 (6). On the other hand, in this study antibodies raised against isolated fimbriae of P. intermedia 17 did not inhibit the proliferation of T cells, while the anti-P. intermedia 17 whole cell IgG was effective in inhibiting the proliferation of T cells induced by these bacterial cells. Consistent with this observation was the fact that isolated fimbriae (up to 230 μg/ml) of P. intermedia failed to stimulate the proliferation of PBMC (data not shown). We have also examined the stimulatory effects of LPS isolated from Prevotella strains on CD4+ T cells. Up to 300 μg of LPS per ml did not stimulate proliferation of these CD4+ T cells (data not shown). Recently, several novel cell surface-associated components that can induce cytokine activities, including a 55-kDa surface protein, have been isolated from P. intermedia (12, 21). While neither the fimbriae nor LPS stimulated the proliferation of T cells, results for the inhibition of stimulation by IgG against P. intermedia whole cells, together with the fact that fixed bacteria were used as stimulators in this study, strongly suggest that a certain cell surface-associated component(s) is likely to be responsible for the stimulatory effects observed with P. intermedia. In addition, the fact that the IgG used was generated against formalin-treated whole cells and was inhibitory suggests that the component(s) is cell associated. Whether this component(s) is protein in nature like some of the other immune reactive surface-associated components isolated from P. intermedia remains to be determined. However, inhibition by anti-whole cell IgG suggests that the component(s) is protein in nature, since IgG is generally produced in response to protein antigens, and is unlike the T-cell-independent antigens that are LPS or polysaccharide in nature and that induce only IgM responses. Work is in progress to identify the cell surface-associated component(s) of P. intermedia that has superantigenic activity.
Immune responses to microbial factors that contribute to the pathogenesis of periodontal diseases are complex. It has been suggested that periodontal diseases may be associated with imbalances in the regulation of immune responses (20). In this study, which is consistent with an earlier study (19), we demonstrated that certain clinical strains of P. intermedia have the ability to preferentially activate the expression of CD4+ T cells. Recent studies have shown that P. gingivalis, another periodontal pathogen, also causes the expansion of the population of Vβ-specific T cells (8, 37). We were unable to detect such expansions, which may be attributable to the types of strains that were used and/or whether the T cells used were antigen specific. Together, these findings strongly suggest the involvement of T cells in the disease process. The overall activation of selective subsets of Vβ-specific CD4+ T cells has significant implications in the pathogenesis of periodontal diseases. For example, the activation and expansion of large numbers of T cells may result in the production of large quantities of regulatory and effector cytokines, which could overwhelm the normal immune functions of the host (24, 37). Massive activation may also lead to clonal deletion of T cells expressing certain Vβ subsets, resulting in the dilution of the antigen-specific response that could be protective against microbial infection (13, 19, 37).
Acknowledgments
This work was supported by Public Health Service grant DE 05429 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Beem J E, Nesbitt W E, Leung K-P. Identification of hemolytic activity in Prevotella intermedia. Oral Microbiol Immunol. 1998;13:97–105. doi: 10.1111/j.1399-302x.1998.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 2.Çelenligil H, Kansu E, Ruacan S, Eratalay K, Çaglayan G. In situ characterization of gingival mononuclear cells in rapidly progressive periodontitis. J Periodontol. 1993;64:120–127. doi: 10.1902/jop.1993.64.2.120. [DOI] [PubMed] [Google Scholar]
- 3.Choi Y, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung C P, Nisengard R J, Slots J, Genco R J. Bacterial IgG and IgM antibody titers in acute necrotizing ulcerative gingivitis. J Periodontol. 1983;54:557–562. doi: 10.1902/jop.1983.54.9.557. [DOI] [PubMed] [Google Scholar]
- 5.Dahlén G G. Black-pigmented gram-negative anaerobes in periodontitis. FEMS Immunol Med Microbiol. 1993;6:181–192. doi: 10.1111/j.1574-695X.1993.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 6.Dorn B R, Leung K-P, Progulske-Fox A. Invasion of human oral epithelial cells by Prevotella intermedia. Infect Immun. 1998;66:6054–6057. doi: 10.1128/iai.66.12.6054-6057.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzink J L, Gibbons R J, Childs III W C, Socransky S S. The predominant cultivable microbiota of crevicular epithelial cells. Oral Microbiol Immunol. 1988;4:1–5. doi: 10.1111/j.1399-302x.1989.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 8.Gemmell E, Grieco D A, Cullinan M P, Westerman B, Seymour G J. Antigen-specific T-cell receptor V beta expression in Porphyromonas gingivalis-specific T-cell lines. Oral Microbiol Immunol. 1998;13:335–361. doi: 10.1111/j.1399-302x.1998.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 9.Haffajee A D, Socransky S S, Dzink J L, Taubman M A, Ebersole J L, Smith D J. Clinical, microbiological and immunological features of subjects with destructive periodontal diseases. J Clin Periodontol. 1988;15:240–246. doi: 10.1111/j.1600-051x.1988.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 10.Hafström C A, Wikström M B, Renvert S N, Dahlén G G. Effect of treatment on some periodontopathogens and their antibody levels in periodontal abscesses. J Periodontol. 1994;65:1022–1028. doi: 10.1902/jop.1994.65.11.1022. [DOI] [PubMed] [Google Scholar]
- 11.Hamada S, Takada H, Ogawa T, Fujiwara T, Mihara J. Lipopolysaccharides of oral anaerobes associated with chronic inflammation: chemical and immunomodulating properties. Int Rev Immunol. 1990;6:247–261. doi: 10.3109/08830189009056635. [DOI] [PubMed] [Google Scholar]
- 12.Iki K, Kawahara K, Sawamura S, Arakaki R, Sakuta T, Sugiyama A, Tamura H, Sueda T, Hamada S, Takada H. A novel component different from endotoxin extracted from Prevotella intermedia ATCC 25611 activates lymphoid cells from C3H/HeJ mice and gingival fibroblasts from humans. Infect Immun. 1997;65:4531–4538. doi: 10.1128/iai.65.11.4531-4538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imberti L, Sottini A, Bettinardi A, Puoti M, Primi D. Selective depletion in HIV infection of T cells that bear specific T cell receptor Vβ sequences. Science. 1991;254:860–862. doi: 10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- 14.Kornman K S, Loesche W J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15:111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 15.Kotzin B L, Leung D Y, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 16.Lehner T. Cellular aspects of host responses. Cellular immunity in periodontal disease: an overview. In: Genco R J, Mergenhagen S E, editors. Host-parasite interactions in periodontal diseases. Washington, D.C.: American Society for Microbiology; 1982. pp. 202–216. [Google Scholar]
- 17.Leung K-P, Fukushima H, Nesbitt W E, Clark W B. Prevotella intermedia fimbriae mediate hemagglutination. Oral Microbiol Immunol. 1996;11:42–50. doi: 10.1111/j.1399-302x.1996.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 18.Loesche W J, Syed S A, Laughon B E, Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982;53:223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- 19.Mathur A, Michalowicz B, Yang C, Aeppli D. Influence of periodontal bacteria and disease status on V beta expression in T cells. J Periodontal Res. 1995;30:369–373. doi: 10.1111/j.1600-0765.1995.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 20.Mathur A, Michalowicz B S. Cell-mediated immune system regulation in periodontal diseases. Crit Rev Oral Biol Med. 1997;8:76–89. doi: 10.1177/10454411970080010401. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita K, Nagaoka S, Arakaki R, Kawabata Y, Iki K, Kawagoe M, Takada H. Immunobiological activities of a 55-kilodalton cell surface protein of Prevotella intermedia ATCC 25611. Infect Immun. 1994;62:2459–2469. doi: 10.1128/iai.62.6.2459-2469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page R C. Lymphoid cell responsiveness and human periodontitis. In: Genco R J, Mergenhagen S E, editors. Host-parasite interactions in periodontal diseases. Washington, D.C.: American Society for Microbiology; 1982. pp. 217–224. [Google Scholar]
- 23.Raber-Durlacher J E, van Steenbergen T J, Van der Velden U, de Graaff J, Abraham-Inpijn L. Experimental gingivitis during pregnancy and post-partum: clinical, endocrinological, and microbiological aspects. J Clin Periodontol. 1994;21:549–558. doi: 10.1111/j.1600-051x.1994.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 24.Scherer M T, Ignatowicz L, Winslow G M, Kappler J W, Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 25.Slots J, Bragd L, Wikström M, Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 26.Slots J, Genco R J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 27.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel C A, Hayduk S E, Minah G E, Krywolap G N. Black pigmented Bacteroides from clinically characterized periodontal sites. J Periodontal Res. 1979;14:376–382. doi: 10.1111/j.1600-0765.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 29.Takada H, Iki K, Sakuta T, Sugiyama A, Sawamura S, Hamada S. Lipopolysaccharides of oral black pigmented bacteria and periodontal diseases. A novel immunomodulator different from endotoxin was extracted from Prevotella intermedia ATCC 25611 with hot phenol-water. Prog Clin Biol Res. 1995;392:59–68. [PubMed] [Google Scholar]
- 30.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun. 1992;60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanabe T, Torres B A, Subramaniam P S, Johnson H M. Vβ activation by HIV Nef protein: detection by a simple amplification procedure. Biochem Biophys Res Commun. 1997;230:509–513. doi: 10.1006/bbrc.1996.5991. [DOI] [PubMed] [Google Scholar]
- 33.Tanner A C R, Haffer C, Bratthall G T, Visconti R A, Socransky S S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979;6:278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 34.Torres B A, Tanabe T, Johnson H M. Characterization of Nef-induced CD4 T cell proliferation. Biochem Biophys Res Commun. 1996;225:54–61. doi: 10.1006/bbrc.1996.1130. [DOI] [PubMed] [Google Scholar]
- 35.van der Weijden M, Timmerman F, Reijerse E, Wolffe G N, van Winkelhoff A J, van der Velden U. The prevalence of A. actinomycetemcomitans, P. gingivalis and P. intermedia in selected subjects with periodontitis. J Clin Periodontol. 1994;21:583–588. doi: 10.1111/j.1600-051x.1994.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 36.White D, Mayrand D. Association of oral Bacteroides with gingivitis and adult periodontitis. J Periodontal Res. 1981;16:259–265. doi: 10.1111/j.1600-0765.1981.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 37.Zadeh H H, Kreutzer D L. Evidence for involvement of superantigens in human periodontal diseases: skewed expression of T cell receptor variable regions by gingival T cells. Oral Microbiol Immunol. 1996;11:88–95. doi: 10.1111/j.1399-302x.1996.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 38.Zambon J J. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 39.Zambon J J, Reynolds H S, Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981;32:198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zambon J J, Reynolds M, Fisher J G, Sclossmann M, Dunford R, Genco R J. Microbiological and immunological studies of adult periodontitis in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1988;59:23–31. doi: 10.1902/jop.1988.59.1.23. [DOI] [PubMed] [Google Scholar]