Abstract
Allium is a common functional vegetable with edible and medicinal value. Allium plants have a special spicy taste, so they are often used as food and seasoning in people’s diets. As a functional food, Allium also has abundant biological activities, some of which are used as drugs to treat diseases. By consuming Allium on a daily basis, people can receive active compounds of natural origin, thereby improving their health status and reducing the likelihood of disease. Steroidal saponins are important secondary metabolites of Allium, which are formed by the steroidal aglycone group and sugar. Steroidal saponins have various physiological activities, such as hypoglycemic, antiplatelet aggregation, anti-inflammatory, antitumor, antimicrobial, and enzyme activity inhibition, which is one of the key reasons why Allium has such significant health benefits. The structural diversity and rich biological activities of steroidal saponins make Allium important plants for both food and medicine. In this paper, the chemical structures, biological activities, and structure–activity relationships of steroidal saponins isolated from Allium are reviewed, and the biosynthetic pathways of some key compounds are proposed as well, to provide a molecular reference basis based on secondary metabolites for the health value of Allium.
Keywords: Allium, nutritious vegetable, health benefits, steroidal saponins, biological activity
1. Introduction
Supplementing nutrition from the diet is a guarantee for human bodies to maintain health. Allium plays an indispensable role in people’s diets, whether it is directly eaten as a vegetable or pickled condiments. The unique taste of natural Allium enhances people’s appetite, and its abundant biological activities bring people health and nutrition. A. sativum (garlic), for example, is a nutritious vegetable that is widely used as a condiment throughout the world. Fresh garlic bulbs contain about 65% water, 28% carbohydrates, 2.3% organic sulfur compounds, 2% protein, and 1.2% free amino acids (e.g., arginine, glycine, and cystine). Garlic contains 146 kcal/100 g of edible parts. It is also a rich source of vitamin C. There are 10–78.8 mg of this vitamin in 100 g of the edible parts of the product. Garlic also contains minerals—relatively high amounts of potassium, iron, and phosphorus (373–1367 mg, 1.5–13 mg, and 88–522 mg, respectively, in 100 g of the product) [1]. Allium plants usually have medicinal value, which can prevent and treat diseases. A. sativum is believed to have antibacterial, antioxidant, hypotension, and other effects and is used to treat influenza and hypertension.
Allium is a widespread perennial herbaceous plant and is one of the largest monocotyledonous genera. There are various species of Allium. However, the classification of Allium is still uncertain. Scholars generally believe that Allium belongs to Liliaceae in the broad sense at present. The classic Chinese works Flora Reipublicae Popularis Sinicae, Flora of China, and Higher Plants of China all follow Engler’s view of the plant classification system to deal with the systematic position of Allium and have assigned it to the broad family Liliaceae. The Angiosperm Phylogeny Group (APG) system of plant classification is a modern system of plant taxonomy. In APG III, the genus Allium belongs to the family Alliaceae under the family Amaryllidaceae, with more than 500 species, which is the largest genus in the family.
Allium plants are mostly distributed in temperate climates of the Northern Hemisphere, except for a few species occurring in Chile (e.g., A. juncifolium), Brazil (e.g., A. sellovianum), and tropical Africa (e.g., A. spathaceum and A. dregeanum). Allium plants are perennial bulb plants, with a few species such as A. fistulosum and A. ampeloprasum developing thickened leaf bases rather than forming bulbs. Allium plants are important cash crops, often with edible or medicinal value. For example, leeks (A. tuberosum), garlic (A. sativum), scallions (A. fistulosum), and onions (A. cepa) are common seasoning vegetables, while leek seeds and roots are used for medicinal purposes. Some species are also used as ornamental flowers, such as A. cristophii and A. giganteum. Allium plants usually have a peculiarly irritating odor due to the organosulfur compounds they produce. The main secondary metabolites of Allium plants are steroidal saponins. In addition, they also produce polysaccharides, proteins, phenolics, and other components. Steroidal saponins have been shown to have a variety of important biological effects and are thought to be one of the key reasons why Allium has such significant health benefits.
Steroidal saponins are a class of oligoglycosides, with spirostanes as the basic skeleton bonded to sugars, converted through the MVA or MEP pathway, and they are widely distributed in monocotyledonous plants, such as Liliaceae, Dioscoreaceae, and Agave, and less in dicotyledonous plants. As steroidal sapogenins are the raw materials for the synthesis of steroidal contraceptives and hormonal drugs, scholars have been studying steroidal saponin components in depth and have gradually found that most of them have good biological activities, such as hypoglycemic, antithrombotic, anti-inflammatory, antitumor, antimicrobial, and immune function-enhancing effects. By incorporating Allium into our diet, we can potentially reduce the risk of chronic diseases and improve our overall health.
A large number of steroidal saponins with favorable biological activities were isolated from Allium, but systematic and comprehensive comparison and biological activity mechanism studies are still lacking. A summary of the chemical structure of these compounds is necessary because it provides important information about their functional groups, stereochemistry, and sugar-linking sequence, which is essential for understanding their biological activities and potential therapeutic functions. At the same time, understanding their structure helps to identify and isolate these compounds from natural plant sources, as well as synthesize analogs for structure–activity relationship studies, thereby identifying key structural features of their biological activities and designing more effective and selective compounds. In addition, a summary of the biosynthetic pathways of these compounds will provide insight into the key enzymes and intermediates involved in the biosynthesis of these compounds, which will help optimize the production of steroidal saponins for medical and industrial applications. Therefore, this paper reviews the chemical compounds, biological activities, and structure–activity relationships of steroidal saponins from Allium reported in the literature and proposes the biosynthetic pathways of some key compounds to further explore the health value and therapeutic function of Allium vegetables from the molecular level of secondary metabolites.
2. Chemical Structures of Allium Steroidal Saponins
Steroidal saponins of Allium can be classified into three major categories according to the existence of E and F rings: furostane (F ring cleavage), spirostane (E/F rings into spiral rings), and cholestane (with C-17 side chains rather than oxygenated spiral rings) saponins, in addition to a few special glycoside types of steroidal saponins. The A/B ring of steroidal saponins is cis or trans (5-β and 5-α), the B/C ring is trans, the C/D ring is trans, and the D/E ring is cis. The C-10 and C-13 positions are connected to β-CH3, the C-20 is connected to α-CH3, and the C-25 position has two configurations of R and S. Steroidal saponins are commonly attached to straight chain sugar chains or branched sugar chains at C-3, and the types of saccharides are commonly glucose, galactose, rhamnose, xylose, and arabinose. The number of saccharides attached to steroidal saponins from Allium varies from one to five. All steroidal saponins isolated from Allium reported in the literature are shown in Table 1.
Table 1.
Steroidal saponins/sapogenins isolated from Allium plants reported in the literature.
| No. | Common Name | Structure Name | Species | Parts | References |
|---|---|---|---|---|---|
| 1 | proto-eruboside-B | 26-O-β-glucopyranosyl 22-hydroxy-25(R)-5α-furostane-3β,6β-26-triol 3-O-β-glucopyranosyl-(1→2)-[β-glucopyranosyl-(1→3)]-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. sativum L | bulbs | [2] |
| 2 | ampeloside Bf1 | (25R)-26-O-β-glucopyranosyl-22-hydroxy-5α-furostane-2α,3β,6β,26-tetraol-3-O-β-glucopyranosyl-(1→3)-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] |
| 3 | ampeloside Bf2 | (25R)-26-O-β-glucopyranosyl-22-hydroxy-5α-furostane-2α,3β,6β,26-tetraol-3-O-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] |
| 4 | sativoside-B1 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5α-furostane-3β,6β,26-triol 3-O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. sativum L | bulbs | [4] |
| 5 | proto-desgalactotigonin | A. sativum L | bulbs and roots | [4] | |
| 6 | sativoside-R1 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5α-furostane-3β,26-diol 3-O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyde | A. sativum L | roots | [4] |
| 7 | 22-O-methyl-26-O-β-D-glucopyranosyl-(25R)-5α-furostane-2α,3β,6β,22ξ,26-pentol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| A. ostrowskianum | bulbs | [5] | |||
| A. giganteum | bulbs | [6] | |||
| 8 | 26-O-β-D-glucopyranosyl-(25S)-5α-furostane-2α,3β,6β,22ξ,26-pentaol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| A. ostrowskianum | bulbs | [5] | |||
| 9 | 26-O-β-D-glucopyranosyl-(25R)-5α-furostan-2α,3β,6β,22ξ,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 10 | 26-O-β-D-glucopyranosyl-(25S)-5α-furostan-2α,3β,6β,22ξ,26-pentol 3-O-β-D-glucopyranosyl-(l→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 11 | macrostemonoside J | 26-O-β-D-glucopyranosyl 2β,3β,22,26-tetrahydroxy-25(R)-5β-furostan 3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [8] |
| 12 | 3-O-benzoyl-22-O-methyl-26-O-β-D-glucopyranosyl-(25R)-5α-furostane-2α,3β,5α,6β,22ξ,26-hexol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [6] | |
| 13 | 3-O-acetyl-22-O-methyl-26-O-β-D-glucopyranosyl-(25R)-5α-furostane-2α,3β,5α,6β,22ξ,26-hexol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [6] | |
| 14 | (25R)-26-O-β-D-glucopyranosyl-22-O-methyl-5α-furostane-2α,3β,5,6β,22ξ-pentol 2-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 15 | elburzensosides A1 | furost-2α,3β,5α,6β,22α-pentol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 16 | elburzensosides A2 | furost-2α,3β,5α,6β,22β-pentol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 17 | elburzensosides B1 | furost-2α,3β,5α,6β,22α-pentol 3-O-[β-D-glucopyranosyl-(1→4)-O-β-D-glucopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 18 | elburzensosides B2 | furost-2α,3β,5α,6β,22β-pentol 3-O-[β-D-glucopyranosyl-(1→4)-O-β-D-glucopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 19 | elburzensosides C1 | furost-2α,3β,5α,22α-tetrol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 20 | elburzensosides C2 | furost-2α,3β,5α,22β-tetrol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 21 | elburzensosides D1 | furost-2α,3β,5α,22α-tetrol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 22 | elburzensosides D2 | furost-2α,3β,5α,22β-tetrol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 23 | 26-O-β-D-glucopyranosyl-(25R)-3β,22ξ,26-trihydroxyl-5α-furostane 3-O-β-chacotrioside | A. tuberosum Rottler | seeds | [11] | |
| 24 | 26-O-β-D-glucopyranosyl-(25S)-3β,5β,6α,22ξ,26-pentahydroxyl-5β-furostane 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | A. tuberosum Rottler | seeds | [11] | |
| 25 | hirtifolioside A1 | furost-2α,3β,22α-triol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl]-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 26 | hirtifolioside A2 | furost-2α,3β,22β-triol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl]-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 27 | hirtifolioside C1 | (25R)-5α-furostane-2α,3β,22α,26-tetraol-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| A. chinense G. Don | bulbs | [13] | |||
| 28 | hirtifolioside C2 | furost-2α,3β,22β-triol 26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 29 | minutoside A | (25R)-furost-2α,3β,6β,22α,26-pentaol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. minutiflorum Regel | bulbs | [14] |
| 30 | minutoside C | (25R)-furost-2α,3β,5α,6β,22α,26-esaol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. minutiflorum Regel | bulbs | [14] |
| 31 | macrostemonoside P | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furostane-1β,3β, 26-triol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| 32 | macrostemonoside Q | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-1α,2β,3β, 26-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| 33 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furostane-3β, 26-diol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] | |
| 34 | macrostemonoside R | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-furostane-2α,3β,26-triol-3-O-β-D-glucopyranosyl (1→2)-[β-D-glucopyranosyl (1→3)]-β-D-glucopyranosyl (1→4)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| A. chinense G. Don | bulbs | [16] | |||
| 35 | macrostemonoside B | A. macrostemon Bunge | bulbs | [15] | |
| 36 | macrostemonoside M | (25R)-22-hydroxy-5β-furostane-1β,2β,3β,6α-tetraol-26-O-β-D-glucopyranoside | A. macrostemon Bunge | bulbs | [17] |
| 37 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,12β,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2) [β-D-glucopyranosyl (1 →3)]-β-D-glucopyranosyl (1 →4)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [18] | |
| 38 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,12α,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2) [β-D-glucopyranosyl (1→3)]-β-D-glucopyranosyl (1 →4) -β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [18] | |
| 39 | (25R)-26-O-β-D-glucopyranosyl-5β-furostane-3β,12α,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [18] | |
| 40 | 26-O-β-D-glucopyranosyl-(25R)-5α-furostan-2α,3β,22α,26-tetraol 3-O-β-D-glucopyranosyl-(1→2)[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. rotundum | inflorescences and flower stalks | [19] | |
| 41 | voghieroside A1 | furosta-2α,3β,5α,22α,26-pentol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 42 | voghieroside A2 | furosta-2α,3β,5α,22β,26-pentol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 43 | voghieroside B1 | furosta-2α,3β,5α,22α,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 44 | voghieroside B2 | furosta-2α,3β,5α,22β,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 45 | voghieroside C1 | furosta-2α,3β,6β,22α,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl -26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 46 | voghieroside C2 | furosta-2α,3β,6β,22β,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl -26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 47 | voghieroside D1 | furosta-2α,3β,22α,26-tetrol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 48 | voghieroside D2 | furosta-2α,3β,22β,26-tetrol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 49 | voghieroside E1 | furosta-2α,3β,22α,26-tetrol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 50 | voghieroside E2 | furosta-2α,3β,22β,26-tetrol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 51 | persicoside D1 | furosta-2α,3β,22ξ,26-tetraol 3-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-β-D-glucopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 52 | persicoside D2 | furosta-2α,3β,22ξ,26-tetraol 3-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-β-D-glucopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 53 | leucofuranoside A | 26-O-β-D-glucopyranosyl-(25R)-5α-furostane-3β,6β-diol-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. leucanthum | flowers | [22] |
| 54 | (25R)-26-O-β-D-glucopyranosyl-5α-furost-3-β,26-didyroxy-3-O-{O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [16] | |
| 55 | tomatoside A | A. chinense G. Don | bulbs | [16] | |
| 56 | macorstemonoside C | A. chinense G. Don | bulbs | [16] | |
| 57 | (25R)-26-O- β -D-glucopyranosyl-5α -furostane-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | A. chinense G. Don | bulbs | [13] | |
| 58 | dichotomin | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3β, 26-diol-3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 59 | parisaponin | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3β, 26-diol-3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-arabinofuranosyl-(1→4)]-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 60 | persicoside C1 | furosta-1β,3β,22ξ,26-tetraol 5-en 1-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-α-L-rhamnopyranosyl (1→2)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 61 | persicoside C2 | furosta-1β,3β,22ξ,26-tetraol 5-en 1-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-α-L-rhamnopyranosyl (1→2)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 62 | ceposide A1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 63 | ceposide A2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 64 | ceposide C1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 65 | ceposide C2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 66 | tropeoside A1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 67 | tropeoside A2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 68 | tropeoside B1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 69 | tropeoside B2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 70 | ascalonicoside A1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 71 | ascalonicoside A2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 72 | deltoside | (25R)-furost-5-en-3β,22α,26-triol 26-O-β-D-glucopyranosyl-3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→4)]-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] |
| 73 | karatavioside G | (25R)-26-[(O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl)oxy]-2α-hydroxy-22α-methoxyfurost-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 74 | allimacroside D | (25R)-26-O-β-D-glucopyranosyl-5-enefurostan-2α,3β,22α,26-tetraol-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 75 | tuberoside F | 26-O-β-D-glucopyranosyl-(25S,20R)-20-O-methyl-5α-furost-22(23)-en-2α,3β,20,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 76 | tuberoside G | 26-O-β-D-glucopyranosyl-(25S,20R)-5α-furost-22(23)-en-2α,3β,20,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L- rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 77 | tuberoside H | 26-O-β-D-glucopyranosyl-(25S,20S)-5α-furost-22(23)-en-2α,3β,20,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 78 | tuberoside I | 26-O-β-D-glucopyranosyl-(25S,20S)-5α-furost-22(23)-en-3β,20,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 79 | macrostemonoside L | 26-O-β-D-glucopyranosyl 2β,3β,26-trihydroxy-25(R)-5β-furostan-20(22)-ene 3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [8] |
| 80 | tuberoside A | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-2α,3β,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranoside | A. tuberosum | seeds | [28] |
| 81 | tuberoside B | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-2α,3β,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [28] |
| 82 | tuberoside C | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-2α,3β,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside | A. tuberosum | seeds | [28] |
| 83 | tuberoside R | 26-O-β-D-glucopyranosyl-(25S)-5β-furost-20(22)-ene-2β,3β, 5, 26-tetraol 3-O-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 84 | tuberoside S | 26-O-β-D-glucopyranosyl-(25S)-5β-furost-20(22)-ene-3β,26-diol 3-O-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 85 | tuberoside T | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-3β, 26-diol 3-O-α-L-rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 86 | hirtifolioside B | furost-20(22)-ene-2α,3β-diol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl]-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 87 | macrostemonoside E | A. macrostemon Bunge | bulbs | [17] | |
| 88 | macrostemonoside G | 26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-25(27)-ene-3β,12β,26-triol 3-O-β-D-glucopyranosyl(1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [30] |
| 89 | macrostemonoside O | 26-O-β-D-glucopyranosyl-22-hydroxy-5-β-furost-25 (27)-ene-3β, 26-diol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| 90 | macrostemonoside N | 22-hydroxy-5β-furost-25-(27)-ene-1β,2β,3β,6α-tetraol-26-O-β-D-glucopyranoside | A. macrostemon Bunge | bulbs | [17] |
| 91 | 26-O-β-D-glucopyranosyl-5α-furost-25 (27)-ene-3β,12β,22,26-tetraol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [31] | |
| 92 | allimacroside E | 26-O-β-D-glucopyranosyl-20β-methoxyl-25(R)-furostan-5,22(23)-dien-3β,26-diol-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 93 | 26-O-β-D-glucopyranosyl-5β-furost-20 (22)-25 (27)-dien-3β,12β,26-triol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [31] | |
| 94 | ascalonicoside C | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5α-furost-2-one-3β,5,6β, 26-tetraol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 95 | ascalonicoside D | (25R)-26-O-β-D-glucopyranosyl-22-methoxy-5α-furost-2-one-3β,5,6β, 26-tetraol- 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 96 | chinenoside I | 26-O-β-D-glucopyranosyl 3β,22,26-tridyroxy-25(R)-5α-furostan-6-one 3-O-β-D-xylopyranosyl(1→4)-[α-L-arabinopyranosyl (1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [32] |
| 97 | 26-O-β-D-glucopyranosyl 3β,22α,26-trihydroxy-25(R)-5α-furostan-6-one | A. chinense G. Don | bulbs | [16] | |
| 98 | 26-O-β-D-glucopyranosyl 3β,22α,26-trihydroxy-25(R)-5α-furostan-6-one 3-O-β-D-glucopyranoside | A. chinense G. Don | bulbs | [16] | |
| 99 | 26-O-β-D-glucopyranosyl 3β,22,26-tridyroxy 25(R)-5α-furostan-6-one 3-O-α-L-arabinopyranosyl(1→6)-β-D-glucopyranoside | A. chinense G. Don | bulbs | [16] | |
| 100 | (25R)-6-ketone-26-O-β-D-glucopyranosyl-5α-furostane-3β,22α,26-triol-3-O-α-L-xylopyranosyl-(1→ 4)-β-D-glucopyranoside | A. chinense G. Don | bulbs | [13] | |
| 101 | (25R)-6-ketone-5α -furostane-3β,22α,24β,26-tetraol-3-O-β-D-xylopyranosy-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [13] | |
| 102 | chinenoside II | 26-O-β-glucopyranosyl 3β,26-dihydroxy-(25R)-5α-furost-20(22)-en-6-one 3-O-β-xylopyranosyl-(1→4)-[α-arbinopyranosyl(1→6)]-β-glucopyranoside | A. chinense G. Don | bulbs | [33] |
| 103 | chinenoside III | 26-O-β-glucopyranosyl 3β,26-dihydroxy-(25R)-5α-furost-20(22)-en-6-one 3-O-α-arabinopyranosyl(1→6)-β-glucopyranoside | A. chinense G. Don | bulbs | [33] |
| 104 | chinenoside IV | 26-O-β-glucopyranosyl-3β,26-dihydroxy-23-hydroxymethyl-25(R)-5α-furost-20(22)-en-6-one 3-O-β-xylopyranosyl(1→4)-[α-arabinopyranosyl(1→6)]-β-glucopyranoside | A. chinense G. Don | bulbs | [34] |
| 105 | chinenoside V | 26-O-β-glucopyranosyl-3β,26-dihydroxy-23-hydroxymethyl-25(R)-5α-furost-20(22)-en-6-one 3-O-α-arabinopyranosyl(1→6)-β-glucopyranoside | A. chinense G. Don | bulbs | [34] |
| 106 | 26-O-β-D-glucopyranosyl 3β,26-dihydroxy-25(R)-5α-furostan-20(22)-en-6-one | A. chinense G. Don | bulbs | [16] | |
| 107 | macrostemonoside I | 26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-25(27)-ene12-one-3β,26-diol 3-O-β-D-glucopyranosyl(1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [30] |
| 108 | agigenin 3-O-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] | |
| 109 | ampeloside Bs1 | agigenin 3-O-β-glucopyranosyl-(1→3)-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] |
| A. sativum L. var. Voghiera | bulbs | [20] | |||
| 110 | desgalactotigonin | A. sativum L | roots | [4] | |
| 111 | F-gitonin | (25R)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. sativum L | roots | [4] |
| A. ostrowskianum | bulbs | [5] | |||
| A. jesdianum | bulbs | [35] | |||
| A. porrum L | bulbs | [36] | |||
| flowers | [37] | ||||
| A. cyrillii | bulbs | [38] | |||
| 112 | sativoside-R2 | tigogenin 3-O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyde | A. sativum L | roots | [4] |
| 113 | (24S,25R)-5α-spirostan-2α,3β,5α,6β,24-pentaol 24-O-β-D-glucopyranoside | A. giganteum | bulbs | [39] | |
| 114 | aginoside | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. giganteum | bulbs | [39] |
| A. albopilosum | bulbs | [5] | |||
| A. ostrowskianum | bulbs | [5] | |||
| A. schubertii | bulbs | [7] | |||
| A. macleanii | bulbs | [40] | |||
| A. ampeloprasum L | bulbs | [41] | |||
| A. jesdianum | bulbs | [35] | |||
| A. leucanthum | flowers | [42] | |||
| A. nigrum L | bulbs | [43] | |||
| root–bulb basal stem | [44] | ||||
| A. porrum L | flowers | [37] | |||
| 115 | (25R)-5α-spirostan-2α,3β,5α,6α-tetraol 2-O-β-D-glucopyranoside | A. aflatunense | bulbs | [39] | |
| 116 | alliogenin | (25R)-5α-spirostan-2α,3β,5α,6β-tetraol | A. giganteum | bulbs | [45] |
| A. albopilosum | bulbs | [5] | |||
| A. karataviense | bulbs | [9] | |||
| A. minutiflorum Regel | bulbs | [14] | |||
| 117 | (25R)-3-O-acetyl-5α-spirostan-2α,3β,5α,6β-tetraol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [45] | |
| A. albopilosum | bulbs | [5] | |||
| A. karataviense | bulbs | [9] | |||
| 118 | (25R)-5α-spirostan-2α,3β,5α,6β-tetraol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [45] | |
| A. albopilosum | bulbs | [5] | |||
| A. macleanii | bulbs | [40] | |||
| A. karataviense | bulbs | [9] | |||
| 119 | (25R)-3-O-benzoyl-5α-spirostan-2α,3β,5α,6β-tetraol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [45] | |
| A. macleanii | bulbs | [40] | |||
| A. karataviense | bulbs | [9] | |||
| 120 | (25R)-5α-spirostane-2α,3β,6β-triol 3-O-(O-β-D-glucopyranosyl-(1→2)-O-[3-O-acetyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| 121 | (25S)-5α-spirostane-2α,3β,6β-triol 3-O-(O-β-D-glucopyranosyl-(1→2)-O-[3-O-acetyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| 122 | (25R)-2-O-[(S)-3-hydroxy-3-methylglutaroyl]-5α-spirostane-2α,3β,6β-triol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| 123 | turoside A | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] |
| A. nigrum L | bulbs | [43] | |||
| root–bulb basal stem | [44] | ||||
| 124 | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| A. macleanii | bulbs | [40] | |||
| 125 | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 126 | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[3-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 127 | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[3-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 128 | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-(3S)-3-hydroxy-3-methylglutaroyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| A. giganteum | bulbs | [6] | |||
| A. nigrum L | bulbs | [43] | |||
| 129 | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-(3S)-3-hydroxy-3-methylglutaroyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| A. nigrum L | bulbs | [43] | |||
| 130 | 3-O-acetyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [6] | |
| 131 | methyl ester of (25R)-5α-spirostane-2α,3β,6β-triol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. macleanii | bulbs | [40] | |
| 132 | tigogenin 3-O-{O-α-L-rhamnopyranosyl-(1→2)-O-β-D-xylopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. macleanii | bulbs | [40] | |
| 133 | macrostemonoside A | (25R)-5α-spirostan-3β-ol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyraoside} | A. chinense G. Don | bulbs | [46] |
| A. macrostemon Bunge | bulbs | [47] | |||
| 134 | (25S)-5α-spirostan-3β-ol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyraoside} | A. chinense G. Don | bulbs | [46] | |
| 135 | (25R)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| A. sativum L. var. Voghiera | bulbs | [20] | |||
| 136 | (25S)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 137 | (25R)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 138 | (25S)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 139 | agigenin | spirostan-2α, 3β, 6β–triol | A. porrum L | bulbs | [48] |
| flowers | [37] | ||||
| 140 | neoagigenin | A. porrum L | bulbs | [48] | |
| A. minutiflorum Regel | bulbs | [14] | |||
| 141 | porrigenin A | (25R)-5α-spirostan-2β,3β,6β-triol | A. porrum L | bulbs | [48] |
| 142 | neoporrigenin A | (25S)-5α-spirostan-2β,3β,6β-triol | A. porrum L | bulbs | [48] |
| 143 | yayoisaponin A | agigenin 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum L | bulbs | [41] |
| 144 | yayoisaponin C | agigenin 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum L | bulbs | [41] |
| 145 | timosaponin A III | sarsasapogenin 3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [49] |
| 146 | macrostemonoside D | tigogenin 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-(6-acetyl-β-D-glucopyranosyl)-(1→4)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [49] |
| 147 | neomacrostemonoside D | neotigogenin 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-(6-acetyl-β-D-glucopyranosyl)-(1→4)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [49] |
| 148 | alliogenin 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. karataviense | bulbs | [9] | |
| 149 | (25R)-3-O-(2-hydroxybutyryl)-5α-spirostane-2α,3β,5,6β-tetrol 2-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 150 | (24S,25S)-3-O-benzoyl-5α-spirostane-2α,3β,5,6β,24-pentol 2-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 151 | (24S,25S)-5α-spirostane-2α,3β,5,6β,24-pentol 2,24-di-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 152 | (24S,25S)-3-O-benzoyl-5α-spirostane-2α,3β,5,6β,24-pentol 2,24-di-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 153 | (24S,25S)-5α-spirostane-2α,3β,5,6β,24-pentol 2-O-β-D-glucopyranosyl 24-O-{O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 154 | (25R)-5α-spirostan-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| 155 | (25R)-5α-spirostan-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| A. rotundum | inflorescences and flower stalks | [19] | |||
| 156 | (25R)-5α-spirostan-3β,6β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| A. leucanthum | flowers | [42] | |||
| 157 | (25R)-5α-spirostan-3β,6β-diol 3-O-{O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| 158 | tuberoside D | (25S)-5α-spirostane-2α,3β‚-diol 3-O-α-L-rhamnopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-O-β-D-glucopyranoside | A. tuberosum | seeds | [51] |
| 159 | tuberoside E | (25S)-5α-spirostan-2α,3β-diol 3-O-β-D-glucopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]O-β-D-glucopyranoside | A. tuberosum | seeds | [51] |
| 160 | (25S)-spirostane-3β,5β,6α-triol 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | A. tuberosum | seeds | [52] | |
| 161 | (25S)-5β-spirostane-3β,6α-diol (25epi-ruizgenin) 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | A. tuberosum | seeds | [52] | |
| 162 | tuberoside J | (25R)-5α-spirostan-2α,3β,27-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. tuberosum | seeds | [53] |
| 163 | tuberoside K | (25R)-5α-spirostan-2α,3β,27-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [53] |
| 164 | tuberoside L | 27-O-β-D-glucopyranosyl-(25R)-5α-spirostan-2α,3β,27-triol 3-O-α-D-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [53] |
| 165 | tuberoside | (2α,3β,5α,25S)-2,3,27-trihydroxyspirostane 3-O-α-L-rhamnopyranoyl-(1→2)-O-[α-L-rhamnopyranoyl-(1→4)]-β-D-glucopyranoside | A. tuberosum Rottl. ex Spreng | seeds | [54] |
| 166 | tuberoside N | (25S)-5β-spirostan-2β,3β-diol 3-O-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 167 | tuberoside O | (25S)-5β-spirostan-2β,3β,5-triol 3-O-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| roots | [55] | ||||
| 168 | tuberoside P | (25S)-5β-spirostan-2β,3β, 5-triol 3-O-α-L-rhamnopyranosyl (1→4)-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 169 | tuberoside Q | (24S,25S)-5β-spirostan-2β,3β,5,24-tetraol 3-O-α-L-rhamnopyranosyl (1→4)-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 170 | agapanthagenin | A. elburzense | bulbs | [10] | |
| 171 | hirtifolioside D | spirostan-2α,3β,6β-triol 3-O-β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 172 | agapanthagenin 3-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] | |
| 173 | atroviolacegenin | (25R)-5α-spirostan-2α,3β,6β,27-tetrol | A. atroviolaceum | flowers | [56] |
| 174 | atroviolaceoside | (25R)-5α-spirostan-2α,3β,6β,27-tetrol 3-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. atroviolaceum | flowers | [56] |
| 175 | minutoside B | (25S)-spirostan-2α,3β,6β-triol 3-O-β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. minutiflorum Regel | bulbs | [14] |
| 176 | eruboside B | β-chlorogenin 3-O-β-glucopyranosyl(1→2)-[β-glucopyranosyl-(1→3)]-β-glucopyranosyl(1→4)-β-galactopyranoside | A. leucanthum | flowers | [42] |
| A. sativum L | bulbs | [2] | |||
| 177 | leucospiroside A | (25R)-5α-spirostane-2α,3β,6β-triol 3-O-β-glucopyranosyl-(1→3)-β-glucopyranosyl-(1→2)-[β-glucopyranosyl-(1→3)]-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. leucanthum | flowers | [42] |
| A. ampeloprasum var. porrum | bulbs | [57] | |||
| 178 | (25R)-5α-spirostane-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. leucanthum | flowers | [42] | |
| 179 | (25R)-5α-spirostane-3β,6β-diol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. leucanthum | flowers | [42] | |
| 180 | tuberoside A | (24S, 25S)-5β-spirostan-2β,3β,24-triol 3-O-a-L-rhamnopyranoyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum Rottl. ex Spreng | seeds | [58] |
| 181 | (3β,5α,6β,25R)-6-[(β-D-glucopyranosyl)oxy]spirostan-3-yl O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-β-D-galactopyranoside | A. ampeloprasum var. porrum | bulbs | [59] | |
| 182 | nigroside A1 | 25(R)-5α-spirostan-2α,3β,6β-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. nigrum L | bulbs | [43] |
| 183 | nigroside A2 | 25(S)-5α-spirostan-2α,3β,6β-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. nigrum L | bulbs | [43] |
| 184 | nigroside B1 | 25(R)-5α-spirostan-2α,3β,6β-trio 1-2-O-[β-D-glucopyranosyl]-3-O-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 185 | nigroside B2 | 25(S)-5α-spirostan-2α,3β,6β-trio 1-2-O-[β-D-glucopyranosyl]-3-O-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 186 | β-D-glucopyranosyl-(1→2)-[4-O-(3-hydroxy-3-methylglutaryl)-β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-(1→3)-(25R)-5α-spirostan-2α,3β-diol | A. cyrillii | bulbs | [38] | |
| 187 | persicoside A | (25S)-spirostan-2α,3β,6β-triol 3-O-[β-D-glucopyranosyl-(1→3)] [β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 188 | persicoside B | (25S)-spirostan-2α,3β,6β-triol 3-O-[β-D-xylopyranosyl-(1→3)] [α-L-rhamnopyranosyl(1→2)]-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 189 | (25R)-5α-spirostan-3β,11aα-diol 3-O-β-D-glucopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→4)]-β-D-galactopyranoside | A. schoenoprasum | whole plants | [24] | |
| 190 | tuberoside B | (24S,25S)-5β-spirostan-2α,3β,5,24-tetraol 3-O-α-L-rhamnopyranoyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum Rottl. ex Spreng | seeds | [60] |
| 191 | tuberosine A | (25S)-5β-spirostan-2β,3β-diol 3-O-β-D-glucopyranoside | A. tuberosum | roots | [55] |
| 192 | tuberosine B | (25S)-5β-spirostan-2β,3β,19-triol 3-O-β-D-glucopyranoside | A. tuberosum | roots | [55] |
| 193 | tuberosine C | (25S)-5β-spirostan-2β,3β-diol 3-O-α-L-rhamnopyranoyl-(1→4)-O-β-D-glucopyranoside | A. tuberosum | roots | [55] |
| 194 | 25(S)-schidigera-saponin D5 | A. tuberosum | roots | [55] | |
| 195 | shatavarin IV | A. tuberosum | roots | [55] | |
| 196 | karatavioside I | (24S,25S)-24-[(O-β-D-glucopyranosyl(1→2)-β-D-glucopyranosyl)oxy]-2α,5α,6β-trihydroxyspirostan-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 197 | neogitogenin | A. chinense G. Don | bulbs | [61] | |
| 198 | (25R)-5α-spirostan-3β-yl-3-O-acetyl-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [61] | |
| 199 | (25S)-5α-spirostane-3β-ol-3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 200 | allimacroside B | (24S,25S)-24-[(β-D-glucopyranosyl)oxy]-5α-spirostan-3β-yl-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 201 | yayoisaponin A/alliporin | (2α, 3β, 6β, 25R)-2,6-dihydroxyspirostan-3-yl β-D-glucopyranosyl-(1→3)-β-D-glucopranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl]-(1→4)-β-D-galactopyranoside | A. porrum L | flowers | [37] |
| 202 | alliospiroside A | (25S)-3β-hydroxyspirost-5-en-1β-yl-2-O-(6-deoxy-α-L-mannopyranosyl)-α-L-arabinopyranoside | A. cepa L | collective fruit | [62] |
| A. cepa L. Aggregatum group | roots | [63] | |||
| 203 | alliospiroside B | A. cepa L | collective fruit | [62] | |
| 204 | alliospiroside D | A. cepa L | collective fruit | [62] | |
| 205 | (25R)-spirost-5-en-3β-ol (diosgenin) 3-O-{O-α-L-rhamnopyranosyl-(1→2)-O-[O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyanoside} | A. senescens | bulbs | [40] | |
| 206 | diosgenin 3-O-{O-α-L-rhamnopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside} | A. senescens | bulbs | [40] | |
| 207 | dioscin | A. ampeloprasum L | bulbs | [41] | |
| 208 | (25R)-spirost-5-ene-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. karataviense | bulbs | [9] | |
| 209 | β-chacotriosyl lilagenin | A. tuberosum | seeds | [52] | |
| 210 | (20S,25S)-spirost-5-en-3β,12β,21-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 211 | (20S,25S)-spirost-5-en-3β,11α,21-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 212 | diosgenin 3-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 213 | deltonin | diosgenin 3-O-β-D-glucopyranosyl-(1→ 4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] |
| 214 | karatavioside H | (24S,25S)-24-[(O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl) oxy]-2α-hydroxyspirost-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 215 | allimacroside C | (24S,25S)-24-[(β-D-glucopyranosyl)oxy]-spirost-5-ene-3β,24-diol-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 216 | 5α-spirostane 25(27)-ene-2α,3β-diol-3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 217 | porrigenin B | (25R)-2-oxo-5α-spirostan-3β,6β-diol | A. porrum L | bulbs | [48] |
| 218 | neoporrigenin B | (25S)-2-oxo-5α-spirostan-3β,6β-diol | A. porrum L | bulbs | [48] |
| 219 | yayoisaponin B | porrigenin B 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum L | bulbs | [41] |
| 220 | (3β,5α,6β,25R)-3-{(O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-O-[O-β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl)oxy}-6-hydroxyspirostan-2-one | A. ampeloprasum var. porrum | bulbs | [64] | |
| 221 | 3-keto umbilicagenin A | (25R)-3-keto-spirostan-2α,5α,6β-triol | A. umbilicatum Boiss | flowers | [65] |
| 222 | 3-keto umbilicagenin B | (25R)-3-keto-spirostan-2α,5α-diol | A. umbilicatum Boiss | flowers | [65] |
| 223 | anzurogenin A | 2α,3β,5β-trihydroxy-(25R)-spirostan-6-one | A. suvorovii and A. stipitatum | fruit | [66] |
| 224 | anzuroside | (24S, 25S)-2α,3β,5,24-tetrahydroxy-5β-spirostan-6-one 24-O-β-D-glucopyranoside | A. suvorovii and A. stipitatum | fruits | [67] |
| 225 | anzurogenin C | (24S, 25S)-2α,3β,5,24-tetrahydroxy-5β-spirostan-6-one | A. suvorovii and A. stipitatum | fruits | [67] |
| A. chinense G. Don | bulbs | [49] | |||
| 226 | (25R)-3β-hydroxy-5α-spirostan-6-one (laxogenin) 3-O-{O-α-L-arabinopyranosyl-(1→6)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 227 | laxogenin 3-O-{O-(2-O-acetyl-α-L-arabinopyranosyl)-(1→6)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 228 | xiebai-saponin I | laxogenin 3-O-{O-β-D-xylopyranosyl-(1→4)-O-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [46] |
| 229 | chinenosideVI | (25R)-24-O-β-D-glucopyranosyl-3β,24β-dihydroxy-5α-spirost 3-O-α-arabinopyranosyl-(1→6)-β-D-glucopyranoside | A. chinense G. Don | bulbs | [49] |
| 230 | laxogenin 3-O-β-D-glucopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [49] | |
| 231 | laxogenin | A. chinense G. Don | bulbs | [68] | |
| 232 | laxogenin 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→4)]-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 233 | laxogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 234 | laxogenin 3-O-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 235 | laxogenin 3-O-{β-D-xylopyranosyl-(1→4)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 236 | (25R)-5α-spirostan 3-O-{O-(4-O-acetyl-α-L-arabinopyranosyl)-(1→6)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 237 | (25R)-3β-hydroxy-5β-spirostan-6-one 3-O-β-D-xylopyranosyl(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 238 | (25R)-3β-hydroxy-5α-spirostan-6-one 3-O-{[O-β-D-glucopyranosyl-(1→3)-O-β-D-xylopyranosyl]-(1→4)-O-[α-L-arabinopyranosyl-(1→6)]}-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 239 | (25S)-3β,24β-dihydroxy-5α-spirostan-6-one 3-O-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 240 | (25S)-24-O-β-D-glucopyranosyl-3β,24β-dihydroxy-5α-spirostan-6-one | A. chinense G. Don | bulbs | [61] | |
| 241 | 12-keto-porrigenin | (25R)-5α-spirostan-3β, 6β-diol-12-one | A. porrum L | [69] | |
| 242 | (25S)-5α-spirostan-3β, 6β-diol-12-one | A. porrum L | [69] | ||
| 243 | (25R)-3β,6β‚dihydroxy-5α-spirostan-12-one-3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [36] | |
| 244 | (25R)-5α-spirostan-3β,6β-diol-12-one 3-O-β-D-glucopyranosyl-(1→2)-[β-D-fucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. porrum L | bulbs | [70] | |
| 245 | porrigenin C | A. porrum L | [71] | ||
| 246 | neoporrigenin C | A. porrum L | [71] | ||
| 247 | (25R)-3β,6β-dihydroxy-5α-spirostan-2,12-dione-3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [36] | |
| 248 | (25R)-5α-spirostane-3β,6β-diol-2,12-dione 3-O-{β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β -D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [72] | |
| 249 | (25R)-spirost-4-ene-3-one-2-ol | A. fistulosum L | seeds | [73] | |
| 250 | (25R)-spirost-1,4-diene-3-one-2,6-diol | A. fistulosum L | seeds | [73] | |
| 251 | (25R)-spirost-1,4-diene-3-one-2-ol | A. fistulosum L | seeds | [73] | |
| 252 | (25R)-19-norspirosta-1,3,5 (10)-triene-4-methyl-2-ol | A. fistulosum L | seeds | [73] | |
| 253 | anzurogenin B | 2α,5α-epoxy-(25R)-spirostan-3β,6β-diol | A. suvorovii and A. stipitatum | fruit | [74] |
| 254 | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol 1-O-α-L-rhamnopyranoside 16-O-{O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside} | A. albopilosum | bulbs | [5] | |
| 255 | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol 16-O-{O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside} | A. ostrowskianum | bulbs | [5] | |
| 256 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol 1,3-di-O,O’-α-L-rhamnopyranoside 16-O-β-D-glucopyranoside | A. macleanii | bulbs | [40] | |
| 257 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol 1,16-di-O-β-D-glucopyranoside | A. jesdianum | bulbs | [35] | |
| 258 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol 1-O-α-L-rhamnopyranosyl 16-O-β-D-glucopyranoside | A. jesdianum | bulbs | [35] | |
| 259 | 22S-cholest-5-ene-1β,3β,16β,22-tetrol 1-O-α-L-rhamnopyranosyl 16-O-β-D-galactopyranoside | A. porrum L | bulbs | [36] | |
| 260 | 22S-cholest-5-ene-1β,3β,16β,22-tetrol 1-O-[O-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranoside] 16-O-β-D-galactopyranoside | A. porrum L | bulbs | [36] | |
| 261 | tuberoside U | 16-O-β-D-glucopyranosyl-(22S,25S)-cholest-5-ene-3β,16β, 22, 26-tetraol 3-O-α-L-rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 262 | nigroside C | (22S)-cholest-5-ene-1β,3β,16β, 22-tetraol 1-O-[α-L-rhamnopyranosyl] 16-O-α-L-rhamnopyranosyl-(1→3)-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 263 | nigroside D | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol 16-O-α-L-rhamnopyranosyl-(1→3)-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 264 | persicoside E | (22S)-cholesta-1β,3β,16β,22β-tetraol 5-en 1-O-α-L-rhamnopyranosyl 16-O-α-L-rhamnopyranosyl (1→2)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 265 | karatavioside J | (22S)-16β-[(β-D-glucopyranosyl)oxy]-22-hydroxycholest-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→ 4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 266 | karatavioside K | (22S)-16β-[(O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl)oxy]-22-hydroxycholest-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 267 | 1β,3β,16β-trihydroxy-5α-cholestan-22-one 1-O-α-L-rhamnopyranoside 16-O-{O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside} | A. albopilosum | bulbs | [5] | |
| 268 | 1β,3β,16β-trihydroxycholest-5-en-22-one 1-O-α-L-rhamnopyranoside 16-O-{O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside} | A. albopilosum | bulbs | [5] | |
| 269 | 2,3-seco-porrigenin | (25R)-5α-2,3-secospirostan-2,3-dioic acid-6β-hydroxy-3,6-γ-lactone | A. porrum L | [69] | |
| 270 | (25S)-5α-2,3-secospirostan-2,3-dioic acid-6β-hydroxy-3,6-γ-lactone | A. porrum L | [69] | ||
| 271 | 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl 3β,5β,6α,16β-tetrahydroxypregnane 16-(5-O-β-D-glucopyranoyl-4(S)-methyl-5-hydroxypentanoic acid) ester | A. tuberosum Rottler | seeds | [11] | |
| 272 | 5α-cholano-22,16-lactone-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | A. chinense G. Don | bulbs | [13] | |
| 273 | 6-ketone-5α-cholano-22,16-lactone-3-O-β-D-6-xylopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [13] | |
| 274 | allimacroside A | pregna-5,16-dien-3β-ol-20-one-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-Dglucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
2.1. Furostane Saponins/Sapogenins
Furostane saponins usually have a saturated sapogenin, and its F ring is split. Some furostane saponins form double bonds at C-5(6), C-20(22), C-22(23), and C-25(27), or carbonyl groups at C-2, C-6, and C-12. The C-22 position of furostane saponins has α and β configurations. The sugar chains are often attached to C-3, C-26, C-2, and C-1. C-26 is mostly attached to monosaccharides and a few to disaccharides, in particular, C-26 of compound 73 isolated from the bulbs of A. karataviense is linked to trisaccharide [25]. The chemical structures of furostane saponins isolated from Allium in recent years are shown in Figure 1.
Figure 1.
Furostane saponins/sapogenins isolated from Allium plants in recent years.
2.2. Spirostane Saponins/Sapogenins
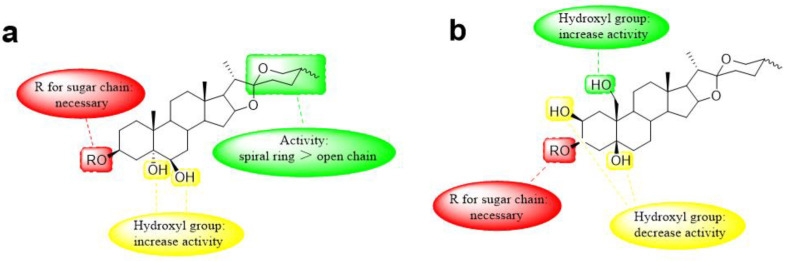
Most spirostane saponins have a saturated spirostane skeleton, except that some saponins form carbonyl groups at C-2, C-3, C-6, C-12, or double bonds at C-5(6) and C-25(27). Notably, the A ring of compound 252 is aromatized, compound 249 forms a cyclohexenone structure at the A ring, compounds 250 and 251 form a cyclohexadienone structure at the A ring [73], and compound 253 forms an oxygen bridge between C-2 and C-5 [74]. The sugar moiety is commonly linked to C-2, C-3, and C-24, while compound 164 forms a glycosidic bond at C-27 [53], and compound 181 forms a glycosidic bond at C-6 [59]. The chemical structures of spirostane saponins isolated from Allium in recent years are shown in Figure 2.
Figure 2.
Spirostane saponins/sapogenins isolated from Allium plants in recent years.
2.3. Cholestane Saponins/Sapogenins
Cholestane saponins, also known as open-chain saponins, usually have double bonds at C-5(6) and are oxidized at C-1, C-3, C-16, and C-22, except that compound 267 has no double bond at C-5(6) [5], and the C-1 of compounds 261, 265, and 266 are not oxidized [25,29]. This class of compounds usually forms glycosidic bonds at C-1, C-3, and C-16. The chemical structures of cholestane saponins isolated from Allium in recent years are shown in Figure 3.
Figure 3.
Cholestane saponins isolated from Allium plants in recent years.
2.4. Other Types of Steroidal Saponins/Sapogenins
In addition to the three main types mentioned above, there are some steroidal saponins with rare skeletons. Compounds 271 and 274 are derived from pregnane [11,26]. Compounds 269 and 270 have an open A-ring and form a lactone ring at C-5 and C-6 [69]. Compounds 272 and 273 form a lactone ring structure at the E ring [13]. The chemical structures of other types of saponins isolated from Allium in recent years are shown in Figure 4.
Figure 4.
Other types of saponins/sapogenins isolated from Allium plants in recent years.
3. Biological Activities of Allium Steroidal Saponins
Now it is generally believed that Allium exerts good biological activities due to the presence of organosulfur compounds and steroidal saponins. However, sulfur-containing compounds are much less stable than steroidal saponins, so it is of certain significance to study the biological activities of steroidal saponins of Allium. Modern pharmacological studies have demonstrated the hypoglycemic, antiplatelet aggregation, anti-inflammatory and other activities of these components through in vitro and in vivo experiments, confirming the medicinal value of Allium.
3.1. Hypoglycemic Effect
Visfatin is an insulin-mimetic adipocytokine that acts synergistically with insulin to enhance glucose uptake in vivo and in vitro and to inhibit the breakdown of liver glycogen into glucose, with insulin resistance-reducing and antidiabetic effects. Compound 133 isolated from A. macrostemon Bunge can increase the transcription of visfatin partly through the p38 MAPK pathway in differentiated 3T3-L1 adipocytes, which in turn increases the mRNA expression of visfatin and enhances the synthesis and secretion of the visfatin protein, thus having a hypoglycemic effect [47].
3.2. Antiplatelet Aggregation Effect
Adenosine diphosphate (ADP) can cause platelet aggregation through ADP receptors on platelet membranes, leading to thrombosis. Thrombus often causes angina pectoris, myocardial infarction, cerebral infarction, pulmonary embolism, and other acute diseases. A. macrostemon Bunge is a common traditional Chinese medicine used to treat cardiovascular diseases in China, as well as a common edible vegetable, and experiments have shown that the compound 88 isolated from this plant can inhibit platelet aggregation induced by ADP in vitro with an IC50 of 0.871 mM, confirming the plausibility of the pharmacological effect of this plant [30].
3.3. Gastroprotective Effect
Compounds 177 and 181 from the bulbs of A. ampeloprasum var. porrum, a Brazilian vegetable, have been proven to have anti-gastric ulcerative effects [57,59]. They may show protective properties on gastric cells by interfering with the ulcerogenesis mechanism, protecting the gastric mucosa, and reducing gastric congestion caused by acidified ethanol-induced acute gastric injury.
3.4. Immune Adjuvant Effect
Immune adjuvants are non-specific immune-enhancing substances that are injected into the body in advance or simultaneously with antigens and enhance the response of the body to the antigen or change the type of response. The immune adjuvant effect of compound 220, which was isolated from A. ampeloprasum L. var. porrum, was higher than that of commercial adjuvants Freund’s Complete Adjuvant (FCA) and Freund’s Incomplete Adjuvant (FIA) in mice with ovalbumin (OVA) as an antigen by the delayed-type hypersensitivity (DTH) method [64]. The immune adjuvant effect of this compound may be due to its amphiphilic structure with hydrophilic sugar chains and lipophilic glycosides, which form adjuvant–antigen complexes and enhance antigen delivery to antigen-presenting cells for processing.
3.5. Anti-Inflammatory Effect
Ten compounds from the bulbs of A. chinense were evaluated for their anti-inflammatory effects by inhibiting NO production induced by lipopolysaccharide (LPS) in RAW 264.7 cells, and it was found that the NO inhibitory activity of steroidal saponins was related to both aglycones and sugar chains [16]. Compounds 34 and 54 showed significant anti-inflammatory effects with IC50 values of 2.01 ± 1.40 μM and 2.49 ± 1.54 μM, respectively. The compounds 225 and 230, also from the bulbs of A. chinense showed anti-inflammatory activity with IC50 values of 32.20 ± 0.65 μM and 34.33 ± 5.04 μM [61]. Comparison with other compounds isolated in the same period showed that the A/B ring was active in trans and inactive in cis. The carbonyl group at C-6 increased the activity, while the hydroxylation at C-24 made the activity disappear, and the sugar chain at C-3 also affected the anti-inflammatory activity.
The anti-inflammatory effects of compounds 177 and 181 from the bulbs of A. ampeloprasum var. porrum were evaluated using an in vivo model of acute inflammation formed by foot swelling caused by carrageenan gum, and both compounds showed significant anti-inflammatory effects by rapidly controlling both stages of inflammation [57,59]. PCR was used to detect the inhibitory effects of compounds 37, 38, and 39 from the bulbs of A. macrostemon Bunge on the expression of CD40 ligand (CD40L) on the surface of platelet membranes activated by ADP stimulation [18]. Compounds 37 and 38 were able to significantly inhibit CD40L expression in a dose-dependent manner, indicating that they could be used as CD40L inhibitors for the treatment of platelet inflammation.
Figure 5 presents the effect of the structure–activity relationship of steroidal saponins on the anti-inflammatory effect.
Figure 5.
Effect of structure–activity relationship of steroidal saponins on anti-inflammatory effect.
3.6. Cytotoxicity and Antitumor Effects
Studies have shown that some steroidal saponins from Allium are cytotoxic to tumor cells and may serve as drug candidates to treat cancer. Currently, it has been reported that steroidal saponins have significant antitumor activity on more than twenty types of tumor cells, such as A549 (human lung cancer cells), AGS (human gastric gland cancer cells), CNE-1 (human nasopharyngeal cancer cells), DLD-1 (human colorectal cancer cells), HeLa (human cervical cancer cells), HepG2 (human liver cancer cells), HT-29 (human colon cancer cells), MCF-7 (human breast cancer cells), etc.
The cytotoxicity of steroidal saponins is influenced by both aglycone and sugar chains. Compounds 111, 155, and 247 of spirostane saponins and compounds 259 and 260 of cholestane saponins from the bulbs of A. porrum L. exhibited strong cytotoxicity against both J-774 (IC50 3.7, 2.1, 5.8, 4.6, and 4.0 μg/mL, respectively) and WEHI-164 cells (IC50 4.8, 1.9, 4.3, 5.8, and 5.4 μg/mL, respectively), although a lack of activity of cholestane saponin was previously reported in the literature [36]. Compounds 111, 114, and 201 from the flowers of A. porrum L. were cytotoxic to mouse peritoneal cells, with an IC50 ranging from 5.70 to 11.13 μM [37]. Compounds 133, 134, 137, 138, 198, 199, and 216 from the bulbs of A. chinense exhibited significant cytotoxicity against HepG2, A549, SPC-A-1 (human lung adenocarcinoma cells), CNE-1, and MGC80-3 (human gastric adenocarcinoma cells), with an IC50 ranging from 1.43 to 22.32 μM [61]. Compound 199 was not cytotoxic to MRC-5 (human embryonic lung fibroblasts), and its antitumor effects were selective. Comparison with other compounds isolated at the same time reveals that the configuration of H at C-5 and the selective acetylation of the hydroxyl group of sugar significantly affected the cytotoxicity. Beyond that, the polar oxygen-containing group (C=O) at C-6 of the B ring and the hydroxyl group at C-24 of the F ring led to a decrease in antitumor activity. Compounds 34, 54, 55, and 56, also from the bulbs of A. chinense, showed significant inhibitory effects on HepG2,2 A549, SPC-A-1, MGC80-3, MDA-MB-231(human breast cancer cells), SW620 (human colon cancer cell), and CNE-1, with IC50 values ranging from 1.76 to 22.83 μM [16]. Compound 54 also exhibited selective inhibitory effects and showed no cytotoxicity to MRC-5, and it could exert antitumor effects by inducing HepG2 cell cycle arrest and apoptosis. The relationship between steroidal saponins’ cytotoxicity on normal cells and the pharmacological activities, such as anti-inflammation and immune enhancement, deserves more in-depth study.
Figure 6 presents the effect of the structure–activity relationship of steroidal saponins on cytotoxicity.
Figure 6.
Effect of structure–activity relationship of steroidal saponins on cytotoxicity.
3.7. Antimicrobial Effect
Steroidal saponins usually have the effect of inhibiting fungi and bacteria. Several studies have investigated the antifungal effect of steroidal saponins isolated from Allium. The reported fungal species include Aspergillus niger, Alternaria alternata, Alternaria porri, Botrytis cinerea, Candida albicans, Fusarium solani, Fusarium oxysporum, Fusarium oxysporum f. sp. lycopersici, Mortierella ramanniana, Penicillium italicum, Pythium ultimum, Rhizoctonia solani, Trichoderma harzianum, Trichoderma harzianum (strains P1), and Trichoderma harzianum (strain T39). Compared to fungi, bacteria are usually less sensitive to saponin components, and fewer species of bacteria have been reported to be inhibited by steroidal saponins from Allium, including Bacillus subtilis, Escherichia coli, etc.
Compounds 29, 30, 116, 140, and 175 from the bulbs of Allium minutiflorum Regel inhibited ten soil-borne fungal pathogens, including Fusarium oxysporum, Fusarium oxysporum f. sp. lycopersici, Fusarium solani, and biocontrol fungi, but not against bacteria such as Xanthomonas campestris pv. Campestris, Agrobacterium tumefaciens, and Streptomyces turgidiscabies [14]. Compound 175, with a high content in bulbs (83.5 mg/kg), showed the strongest antifungal activity. A structure–activity relationship analysis revealed that the spirostane skeleton was more active than the furostane skeleton, and the hydroxyl group at C-5 of the furostane saponins promoted antifungal activity. Compounds 187 and 188 from the seeds of Allium ampeloprasum subsp. Persicum showed antifungal activity against Penicillium italicum, Aspergillus niger, and Trichoderma harzianum, while other steroidal saponins isolated at the same time showed no antifungal activity, indicating that the spirostane skeleton was more active than the furostane and cholestane skeletons [21]. This also confirms that the hydroxyl group at C-6 could enhance the antifungal activity.
Six spirostane saponins from the roots of Allium tuberosum were evaluated for their antibacterial effects against Bacillus subtilis and Escherichia coli, and the results showed that compound 192 exhibited good antibacterial activity (MIC 64 μg/mL, both), compounds 194 (MIC 16 and 32 μg/mL, respectively) and 195 (MIC 16 μg/mL, both) showed potent antibacterial activity, while compounds 167, 191, and 193 showed almost no bacterial inhibitory effect (MIC > 128 μg/mL) [55]. A structure–activity relationship analysis revealed that the sugar at C-3 and the hydroxyl group at C-19 enhanced the antibacterial activity, while the hydroxyl groups at C-2 and C-5 attenuated the antibacterial activity.
Figure 7 presents the effects of steroidal saponins on antifungal and antibacterial activities, respectively.
Figure 7.
Effect of structure–activity relationship of steroidal saponins on antimicrobial effect. (a) Effect of structure–activity relationship on antifungal activity. (b) Effect of structure–activity relationship on antibacterial activity.
3.8. Enzyme Activity Inhibition Effect
Na,K-ATPase is responsible for the active transport of Na+ and K+ in cells, and drugs that inhibit Na,K-ATPase activity can be used to treat diseases associated with ion active transport disorders for which the transporter enzyme is responsible. Fourteen compounds from the collective fruit of A. karataviense Rgl and A. cepa L were tested for their inhibition of Na,K-ATPase activity [62]. Compounds 202, 203, and 204 had the strongest enzyme inhibitory activity, with IC50 values of 1.0 × 10−5 M, 3.4 × 10−5 M, and 8.8 × 10−5 M. Furthermore, compounds 202 and 203 had non-competitive inhibition, and compound 204 had competitive inhibition. The results of the structure–activity analysis show that the hydroxyl group at C-24 of the F ring decreased the inhibitory activity, the carbonyl group at C-6 of the steroid skeleton slightly enhanced the inhibitory activity, and when the F ring was opened and the sugar was connected to the hydroxyl group at C-25, the inhibitory activity was weakened, and even enzyme activity was promoted. In addition, compounds 133 and 134 from the bulbs of A. chinense also inhibited Na, K-ATPase, with an IC50 of 4 × 10−5 M [46].
cAMP phosphodiesterases are enzymes that catabolize cyclic adenosine acids under the activation of calmodulin bound to Ca2+. The inhibition of cAMP phosphodiesterase increases intracellular cAMP content, which enhances myocardial contraction, dilates peripheral blood vessels, and improves heart failure. Compounds 133, 134, 226, 227, and 228 from the bulbs of A. chinense significantly reduced the activity of cAMP phosphodiesterase, especially compound 227, with an IC50 of 3.3 × 10−5 M, which is comparable to the positive drug papaverine (IC50 3.0 × 10−5 M) [46].
The compounds 7, 12, 13, 114, 117, 128, and 130 from the bulbs of A. giganteum can significantly inhibit the activity of cAMP phosphodiesterase [6]. A structure–activity relationship analysis revealed that the inhibition activity was enhanced when the (S)-3-hydroxy-3-methylglutaroyl (HMG group) was attached to the hydroxyl group at C-4 of xylose. Furostane steroidal saponins 7, 12, and 13 exhibited stronger activity than the corresponding spirostane steroidal saponins 114 and 117, which may be related to the multiple hydroxyl groups in their A and B rings.
3.9. Antispasmodic Effect
Compounds 27, 28, and 172 isolated from the flowers of A. hirtifolium Boiss and compounds 15, 16, 19, 20, and 170 isolated from the bulbs of A. elburzense were tested for their antispasmodic activity by using the histamine-induced contractions of isolated ileum of guinea-pig as a model [12]. Compounds 19, 20, and 170 exhibited the strongest inhibitory contractile activity. A structure–activity relationship analysis showed that the antispasmodic activity can be enhanced by the hydroxyl group at C-5 and glucose at C-26, while it can be attenuated by the hydroxyl group at C-6 and glucose at C-3.
3.10. Cardiomyocyte Regulation Effect
Calcium ions play an important role in the regulation of cell physiological function. Under normal physiological conditions, the intracellular calcium levels are relatively stable and in dynamic regulation; otherwise, it may lead to cell damage or apoptosis. Compounds 33, 35, 36, and 90 isolated from A. macrostemon further increased KCl-induced [Ca2+]i increase in guinea pig cardiomyocytes, suggesting that it may enhance myocardial function [17]. In contrast, compound 87 inhibited the KCl-induced [Ca2+]i increase, suggesting its potential for the treatment of heart failure.
3.11. Nerve Cell Protection Effect
The antioxidant activity was investigated using the CCK-8 method for six compounds isolated from A. chinense G. Don, and hydrogen peroxide was selected to induce a neuronal oxidative damage model in PC12 cells [13]. Compared to five other compounds isolated simultaneously, compound 57 had a significant protective effect on neuronal cell injury induced by hydrogen peroxide in a dose-dependent manner, which may be related to its H at C-22 and the higher amounts of glucose and galactose in the sugar chain.
3.12. Hemolysis Effect
Steroidal saponins can bind to cholesterol on the erythrocyte membrane to form insoluble complexes, disrupting the osmotic pressure of the cell membrane and inducing hemolysis. Usually, spirostane saponins have stronger hemolytic activity due to a higher affinity with cholesterol. Compounds 177, 181, and 220 isolated from the bulbs of A. ampeloprasum var. porrum, have all shown hemolytic ability, with HD50 values of 20 μg/mL, 6.5 μg/mL, and 13 μg/mL, respectively [57,59,64]. Their hemolysis is on the same order of magnitude as that of the reference commercial saponin QS-21 isolated from Quillaja saponaria (HD50 5 μg/mL).
4. Proposed Biosynthetic Pathways of Allium Steroidal Saponins
The biosynthetic pathway of steroid saponins is acetyl coenzyme A through the mevalonate (MVA) pathway or pyruvate through the methylerythritol 4-phosphate (MEP) pathway to produce farnesyl pyrophosphate (FPP), which is then catalyzed by squalene synthase (SQS) to produce squalene, and by squalene epoxidase (SQE) to produce 2, 3-oxidized squalene, and then catalyzed by cycloatenol synthase (CAS) to produce cycloatenol, which is the precursor of steroid compounds. The cycloatenol further synthesizes cholestanol, which is then hydroxylated at C-22, C-26, and C-16 to form semi-ketal structure compounds, which in turn synthesizes furostane saponin. The glycosidic bond at C-26 of furostane saponin is easily hydrolyzed, and after hydrolysis, it cyclizes into a spiral ketal structure to synthesize spirostane saponin.
Cholestane compounds 259 and 260 can be produced by the glycosylation of 1,16,22-trihydroxycholestrol. Pregnane compound 274 can be synthesized from cholesterol by multi-step oxidative breakage to produce pregnenolone, which in turn synthesizes compound 274 by glycosylation. Compound 271 can be synthesized from furostane compound 24 by oxidative breakage at C-22, and compound 272 can be synthesized from furostane compound 35 by a similar mechanism. The oxygen bridge of the A-ring of spirostane compound 253 can be synthesized by the dehydration cyclization of compound 116. Compound 269 can be synthesized from compound 141 by oxidation at C-2 and C-3, and then C-3 is dehydrated with C-6 to form a lactone ring. The proposed biosynthetic pathways of Allium steroidal saponin are shown in Figure 8.
Figure 8.
Proposed biosynthetic pathways of Allium steroidal saponins (MVA: mevalonate; MEP: methylerythritol 4-phosphate).
5. Conclusions
In recent decades, as common functional vegetables, a wide variety of Allium plants have been extensively studied for their important edible and medicinal values. Undoubtedly, their secondary metabolite, a steroidal saponin, is one of the most important chemical bases for the healthcare functions of Allium. In this paper, we have summarized the chemical constituents, biological activities, and structure–activity relationships of steroidal saponins isolated from Allium and have proposed the biosynthetic pathways of some key compounds to clarify the molecular basis of the rich health function of Allium as a vegetable and condiment from the perspective of secondary metabolites. In addition, we have explored the positive role of Allium in the prevention and treatment of diseases more comprehensively.
Author Contributions
Conceptualization, H.W., J.W. and J.S.; writing—original draft preparation, H.W. and A.D.; writing—review and editing, H.W. and Q.Z.; supervision, J.W. and J.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS), grant number 2022-I2M-1-017.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Omar S.H., Al-Wabel N.A. Organosulfur Compounds and Possible Mechanism of Garlic in Cancer. Saudi Pharm. J. 2010;18:51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuura H., Ushiroguchi T., Itakura Y., Hayashi N., Fuwa T. A Furostanol Glycoside from Garlic, Bulbs of Allium sativum L. Chem. Pharm. Bull. 1988;36:3659–3663. doi: 10.1248/cpb.36.3659. [DOI] [PubMed] [Google Scholar]
- 3.Morita T., Ushiroguchi T., Hayashi N., Matsuura H., Itakura Y., Fuwa T. Steroidal Saponins from Elephant Garlic, Bulbs of Allium ampeloprasum L. Chem. Pharm. Bull. 1988;36:3480–3486. doi: 10.1248/cpb.36.3480. [DOI] [PubMed] [Google Scholar]
- 4.Matsuura H., Ushiroguchi T., Itakura Y., Fuwa T. Further Studies on Steroidal Glycosides from Bulbs, Roots and Leaves of Allium sativum L. Chem. Pharm. Bull. 1989;37:2741–2743. doi: 10.1248/cpb.37.2741. [DOI] [Google Scholar]
- 5.Mimaki Y., Kawashima K., Kanmoto T., Sashida Y. Steroidal Glycosides from Allium albopilosum and A. ostrowskianum. Phytochemistry. 1993;34:799–805. doi: 10.1016/0031-9422(93)85362-U. [DOI] [PubMed] [Google Scholar]
- 6.Mimaki Y., Nikaido T., Matsumoto K., Sashida Y., Ohmoto T. New Steroidal Saponins from the Bulbs of Allium giganteum Exhibiting Potent Inhibition of CAMP Phosphodiesterase Activity. Chem. Pharm. Bull. 1994;42:710–714. doi: 10.1248/cpb.42.710. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima K., Mimaki Y., Sashida Y. Steroidal Saponins from the Bulbs of Allium schubertii. Phytochemistry. 1993;32:1267–1272. doi: 10.1016/S0031-9422(00)95103-3. [DOI] [PubMed] [Google Scholar]
- 8.Peng J., Yao X., Okada Y., Okuyama T. Further Studies on New Furostanol Saponins from the Bulbs of Allium macrostemon. Chem. Pharm. Bull. 1994;42:2180–2182. doi: 10.1248/cpb.42.2180. [DOI] [PubMed] [Google Scholar]
- 9.Mimaki Y., Kuroda M., Fukasawa T., Sashida Y. Steroidal Saponins from the Bulbs of Allium karataviense. Chem. Pharm. Bull. 1999;47:738–743. doi: 10.1248/cpb.47.738. [DOI] [PubMed] [Google Scholar]
- 10.Barile E., Zolfaghari B., Sajjadi S.E., Lanzotti V. Saponins of Allium elburzense. J. Nat. Prod. 2004;67:2037–2042. doi: 10.1021/np0497752. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda T., Tsumagari H., Okawa M., Nohara T. Pregnane- and Furostane-Type Oligoglycosides from the Seeds of Allium tuberosum. Chem. Pharm. Bull. 2004;52:142–145. doi: 10.1248/cpb.52.142. [DOI] [PubMed] [Google Scholar]
- 12.Barile E., Capasso R., Izzo A.A., Lanzotti V., Sajjadi S.E., Zolfaghari B. Structure-Activity Relationships for Saponins from Allium hirtifolium and Allium elburzense and Their Antispasmodic Activity. Planta Med. 2005;71:1010–1018. doi: 10.1055/s-2005-873134. [DOI] [PubMed] [Google Scholar]
- 13.Yiran Y., Hua Y., Jianghong Y., Zhiheng S., Yu Z., Xueqing F., Xuwen L., Yongri J. Chemical Constituents of New Steroidal Saponins from Allium chinense G. Don. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ. 2021;42:1742–1753. doi: 10.7503/cjcu20200905. [DOI] [Google Scholar]
- 14.Barile E., Bonanomi G., Antignani V., Zolfaghari B., Sajjadi S.E., Scala F., Lanzotti V. Saponins from Allium minutiflorum with Antifungal Activity. Phytochemistry. 2007;68:596–603. doi: 10.1016/j.phytochem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Chen H., Wang G., Wang N., Yang M., Wang Z., Wang X., Yao X. New Furostanol Saponins from the Bulbs of Allium macrostemon Bunge and Their Cytotoxic Activity. Pharmazie. 2007;62:544–548. doi: 10.1691/ph.2007.7.6725. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Yi X., Xiang L., Huang Y., Wang Z., He X. Furostanol Saponins from Chinese Onion Induce G2/M Cell-Cycle Arrest and Apoptosis through Mitochondria-Mediate Pathway in HepG2 Cells. Steroids. 2019;148:11–18. doi: 10.1016/j.steroids.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Chen H.-F., Wang N.-L., Sun H.-L., Yang B.-F., Yao X.-S. Novel Furostanol Saponins from the Bulbs of Allium macrostemon B. and Their Bioactivity on [Ca2+]i Increase Induced by KCl. J. Asian Nat. Prod. Res. 2006;8:21–28. doi: 10.1080/10286020500172533. [DOI] [PubMed] [Google Scholar]
- 18.Chen H., Ou W., Wang G., Wang N., Zhang L., Yao X. New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets. Molecules. 2010;15:4589–4598. doi: 10.3390/molecules15074589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maisashvili M.R., Kuchukhidze D.K., Kikoladze V.S., Gvazava L.N. Steroidal Glycosides of Gitogenin from Allium rotundum. Chem. Nat. Compd. 2012;48:86–90. doi: 10.1007/s10600-012-0164-x. [DOI] [Google Scholar]
- 20.Lanzotti V., Barile E., Antignani V., Bonanomi G., Scala F. Antifungal Saponins from Bulbs of Garlic, Allium sativum L. Var. Voghiera. Phytochemistry. 2012;78:126–134. doi: 10.1016/j.phytochem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Sadeghi M., Zolfaghari B., Senatore M., Lanzotti V. Spirostane, Furostane and Cholestane Saponins from Persian Leek with Antifungal Activity. Food Chem. 2013;141:1512–1521. doi: 10.1016/j.foodchem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Mskhiladze L., Chincharadze D., Mshvildadze V., Pichette A., Frederich M., Ollivier E., Elias R. Steroidal Glycosides from the Flowers of Allium leucanthum. Chem. Nat. Compd. 2015;51:900–904. doi: 10.1007/s10600-015-1444-z. [DOI] [Google Scholar]
- 23.Kang L.-P., Liu Z.-J., Zhang L., Tan D.-W., Zhao Y., Zhao Y., Chen H.-B., Ma B.-P. New Furostanol Saponins from Allium ascalonicum L. Magn. Reson. Chem. 2007;45:725–733. doi: 10.1002/mrc.2037. [DOI] [PubMed] [Google Scholar]
- 24.Timité G., Mitaine-Offer A.-C., Miyamoto T., Tanaka C., Mirjolet J.-F., Duchamp O., Lacaille-Dubois M.-A. Structure and Cytotoxicity of Steroidal Glycosides from Allium schoenoprasum. Phytochemistry. 2013;88:61–66. doi: 10.1016/j.phytochem.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda M., Ori K., Takayama H., Sakagami H., Mimaki Y. Karataviosides G–K, Five New Bisdesmosidic Steroidal Glycosides from the Bulbs of Allium karataviense. Steroids. 2015;93:96–104. doi: 10.1016/j.steroids.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y.S., Suh W.S., Park K.J., Choi S.U., Lee K.R. Allimacrosides A–E, New Steroidal Glycosides from Allium macrostemon Bunge. Steroids. 2017;118:41–46. doi: 10.1016/j.steroids.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Sang S., Mao S., Lao A., Chen Z., Ho C.-T. Four New Steroidal Saponins from the Seeds of Allium tuberosum. J. Agric. Food Chem. 2001;49:1475–1478. doi: 10.1021/jf001062b. [DOI] [PubMed] [Google Scholar]
- 28.Sang S., Lao A., Wang H., Chen Z. Furostanol Saponins from Allium tuberosum. Phytochemistry. 1999;52:1611–1615. doi: 10.1016/S0031-9422(99)00312-X. [DOI] [Google Scholar]
- 29.Sang S., Mao S., Lao A., Chen Z., Ho C.-T. New Steroid Saponins from the Seeds of Allium tuberosum L. Food Chem. 2003;83:499–506. doi: 10.1016/S0308-8146(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 30.Peng J., Yao X., Kobayashi H., Ma C. Novel Furostanol Glycosides from Allium macrostemon. Planta Med. 1995;61:58–61. doi: 10.1055/s-2006-958000. [DOI] [PubMed] [Google Scholar]
- 31.Chen H.-F., Wang G.-H., Luo Q., Wang N.-L., Yao X.-S. Two New Steroidal Saponins from Allium macrostemon Bunge and Their Cytotoxity on Different Cancer Cell Lines. Molecules. 2009;14:2246–2253. doi: 10.3390/molecules14062246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuura H., Ushiroguchi T., Itakura Y., Fuwa T. A Furostanol Glycoside from Allium chinense G. DON. Chem. Pharm. Bull. 1989;37:1390–1391. doi: 10.1248/cpb.37.1390. [DOI] [Google Scholar]
- 33.Peng J.-P., Yao X.-S., Tezuka Y., Kikuchi T. Furostanol Glycosides from Bulbs of Allium chinense. Phytochemistry. 1996;41:283–285. doi: 10.1016/0031-9422(95)00553-6. [DOI] [PubMed] [Google Scholar]
- 34.Peng J., Yao X., Tezuka Y., Kikuchi T., Narui T. New Furostanol Glycosides, Chinenoside IV and V, from Allium chinense. Planta Med. 1996;62:465–468. doi: 10.1055/s-2006-957941. [DOI] [PubMed] [Google Scholar]
- 35.Mimaki Y., Kuroda M., Fukasawa T., Sashida Y. Steroidal Glycosides from the Bulbs of Allium jesdianum. J. Nat. Prod. 1999;62:194–197. doi: 10.1021/np980346b. [DOI] [PubMed] [Google Scholar]
- 36.Fattorusso E., Lanzotti V., Taglialatela-Scafati O., Di Rosa M., Ianaro A. Cytotoxic Saponins from Bulbs of Allium porrum L. J. Agric. Food Chem. 2000;48:3455–3462. doi: 10.1021/jf000331v. [DOI] [PubMed] [Google Scholar]
- 37.Harmatha J., Buděšínský M., Zídek Z., Kmoníčková E. Spirostanol Saponins from Flowers of Allium porrum and Related Compounds Indicating Cytotoxic Activity and Affecting Nitric Oxide Production Inhibitory Effect in Peritoneal Macrophages. Molecules. 2021;26:6533. doi: 10.3390/molecules26216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolkacheva N.V., Shashkov A.S., Chirva V.Y. Steroidal Glycosides from Allium cyrillii Bulbs. Chem. Nat. Compd. 2012;48:272–275. doi: 10.1007/s10600-012-0219-z. [DOI] [Google Scholar]
- 39.Kawashima K., Mimaki Y., Sashida Y. Steroidal Saponins from Allium giganteum and A. aflatunense. Phytochemistry. 1991;30:3063–3067. doi: 10.1016/S0031-9422(00)98253-0. [DOI] [Google Scholar]
- 40.Inoue T., Mimaki Y., Sashida Y., Nishino A., Satomi Y., Nishino H. Steroidal Glycosides from Allium macleanii and A. senescens, and Their Inhibitory Activity on Tumour Promoter-Induced Phospholipid Metabolism of Hela Cells. Phytochemistry. 1995;40:521–525. doi: 10.1016/0031-9422(95)00223-T. [DOI] [PubMed] [Google Scholar]
- 41.Sata N., Matsunaga S., Fusetani N., Nishikawa H., Takamura S., Saito T. New Antifungal and Cytotoxic Steroidal Saponins from the Bulbs of an Elephant Garlic Mutant. Biosci. Biotechnol. Biochem. 1998;62:1904–1911. doi: 10.1271/bbb.62.1904. [DOI] [PubMed] [Google Scholar]
- 42.Mskhiladze L., Legault J., Lavoie S., Mshvildadze V., Kuchukhidze J., Elias R., Pichette A. Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum. Molecules. 2008;13:2925–2934. doi: 10.3390/molecules13122925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jabrane A., Ben Jannet H., Miyamoto T., Mirjolet J.-F., Duchamp O., Harzallah-Skhiri F., Lacaille-Dubois M.-A. Spirostane and Cholestane Glycosides from the Bulbs of Allium nigrum L. Food Chem. 2011;125:447–455. doi: 10.1016/j.foodchem.2010.09.028. [DOI] [Google Scholar]
- 44.Mostafa A., Sudisha J., El-Sayed M., Ito S., Ikeda T., Yamauchi N., Shigyo M. Aginoside Saponin, a Potent Antifungal Compound, and Secondary Metabolite Analyses from Allium nigrum L. Phytochem. Lett. 2013;6:274–280. doi: 10.1016/j.phytol.2013.03.001. [DOI] [Google Scholar]
- 45.Sashida Y., Kawashima K., Mimaki Y. Novel Polyhydroxylated Steroidal Saponins from Allium giganteum. Chem. Pharm. Bull. 1991;39:698–703. doi: 10.1248/cpb.39.698. [DOI] [Google Scholar]
- 46.Kuroda M., Mimaki Y., Kameyama A., Sashida Y., Nikaido T. Steroidal Saponins from Allium chinense and Their Inhibitory Activities on Cyclic AMP Phosphodiesterase and Na+K+ ATPase. Phytochemistry. 1995;40:1071–1076. doi: 10.1016/0031-9422(95)00423-5. [DOI] [PubMed] [Google Scholar]
- 47.Zhou H., Yang X., Wang N., Zhang Y., Cai G. Macrostemonoside A Promotes Visfatin Expression in 3T3-L1 Cells. Biol. Pharm. Bull. 2007;30:279–283. doi: 10.1248/bpb.30.279. [DOI] [PubMed] [Google Scholar]
- 48.Carotenuto A., Fattorusso E., Lanzotti V., Magno S., De Feo V., Carnuccio R., D’Acquisto F. Porrigenins A and B, Novel Cytotoxic and Antiproliferative Sapogenins Isolated from Allium porrum. J. Nat. Prod. 1997;60:1003–1007. doi: 10.1021/np960657r. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Y., Wang N.-L., Yao X.-S., Kitanaka S. Steroidal Saponins from the Bulbs of Allium chinense. Stud. Plant. Sci. 1999;6:212–219. doi: 10.1016/S0928-3420(99)80029-9. [DOI] [Google Scholar]
- 50.Carotenuto A., Fattorusso E., Lanzotti V., Magno S. Spirostanol Saponins of Allium porrum L. Phytochemistry. 1999;51:1077–1082. doi: 10.1016/S0031-9422(98)00712-2. [DOI] [PubMed] [Google Scholar]
- 51.Sang S., Lao A., Wang H., Chen Z. Two New Spirostanol Saponins from Allium tuberosum. J. Nat. Prod. 1999;62:1028–1029. doi: 10.1021/np980486l. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda T., Tsumagari H., Nohara T. Steroidal Oligoglycosides from the Seeds of Allium tuberosum. Chem. Pharm. Bull. 2000;48:362–365. doi: 10.1248/cpb.48.362. [DOI] [PubMed] [Google Scholar]
- 53.Sang S., Zou M., Xia Z., Lao A., Chen Z., Ho C.-T. New Spirostanol Saponins from Chinese Chives (Allium tuberosum) J. Agric. Food Chem. 2001;49:4780–4783. doi: 10.1021/jf010529v. [DOI] [PubMed] [Google Scholar]
- 54.Zou Z.-M., Yu D.-Q., Cong P.-Z. A Steroidal Saponin from the Seeds of Allium tuberosum. Phytochemistry. 2001;57:1219–1222. doi: 10.1016/S0031-9422(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 55.Fang Y.-S., Cai L., Li Y., Wang J.-P., Xiao H., Ding Z.-T. Spirostanol Steroids from the Roots of Allium tuberosum. Steroids. 2015;100:1–4. doi: 10.1016/j.steroids.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 56.Zolfaghari B., Barile E., Capasso R., Izzo A.A., Sajjadi S.E., Lanzotti V. The Sapogenin Atroviolacegenin and Its Diglycoside Atroviolaceoside from Allium atroviolaceum. J. Nat. Prod. 2006;69:191–195. doi: 10.1021/np0503350. [DOI] [PubMed] [Google Scholar]
- 57.Adão C.R., da Silva B.P., Parente J.P. A New Steroidal Saponin from Allium ampeloprasum Var. porrum with Antiinflammatory and Gastroprotective Effects. Phytochem. Lett. 2011;4:306–310. doi: 10.1016/j.phytol.2011.05.009. [DOI] [Google Scholar]
- 58.Hu G., Mao R., Ma Z. A New Steroidal Saponin from the Seeds of Allium tuberosum. Food Chem. 2009;113:1066–1068. doi: 10.1016/j.foodchem.2008.08.061. [DOI] [Google Scholar]
- 59.Adão C.R., da Silva B.P., Parente J.P. A New Steroidal Saponin with Antiinflammatory and Antiulcerogenic Properties from the Bulbs of Allium ampeloprasum Var. porrum. Fitoterapia. 2011;82:1175–1180. doi: 10.1016/j.fitote.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Hu G., Lu Y., Yu W., Ding Q., Yang Q., Zhou J., Ma Z. A Steroidal Saponin from the Seeds of Allium tuberosum. Chem. Nat. Compd. 2014;49:1082–1086. doi: 10.1007/s10600-014-0825-z. [DOI] [Google Scholar]
- 61.Wang Y., Li C., Wang L., Huang W., He X. Spirostanol Saponins from Chinese Onion (Allium chinense) Exert Pronounced Anti-Inflammatory and Anti-Proliferative Activities. J. Funct. Foods. 2016;25:208–219. doi: 10.1016/j.jff.2016.06.005. [DOI] [Google Scholar]
- 62.Mirsalikhova N.M., Kravets S.S., Sokolova S.F., Abubakirov N.K. Inhibition of Highly Purified Porcine Kidney Na,K-ATPase by Steroid Glycosides of the Spirostan and Furostan Series and a Study of Structure-Activity Relationships. Chem. Nat. Compd. 1993;29:490–497. doi: 10.1007/BF00630576. [DOI] [Google Scholar]
- 63.Abdelrahman M., Mahmoud H.Y.A.H., El-Sayed M., Tanaka S., Tran L.S. Isolation and Characterization of Cepa2, a Natural Alliospiroside A, from Shallot (Allium cepa L. Aggregatum Group) with Anticancer Activity. Plant Physiol. Biochem. 2017;116:167–173. doi: 10.1016/j.plaphy.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Adão C.R., Pereira da Silva B., Tinoco L.W., Parente J.P. Haemolytic Activity and Immunological Adjuvant Effect of a New Steroidal Saponin from Allium ampeloprasum Var. porrum. Chem. Biodivers. 2012;9:58–67. doi: 10.1002/cbdv.201100005. [DOI] [PubMed] [Google Scholar]
- 65.Sadeghi M., Zolfaghari B., Troiano R., Lanzotti V. 3-Keto Umbilicagenin A and B, New Sapogenins from Allium umbilicatum Boiss. Fitoterapia. 2015;102:198–202. doi: 10.1016/j.fitote.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Vollerner Y.S., Kravits S.D., Shashkov A.S., Gorovits M.B., Abubakirov N.K. Steroids of the Spirostan and Furostan Series from Plants of the Genus Allium. Structure of Anzurogenin A from Allium suvorovii and A. stipitatum. Chem. Nat. Compd. 2004;24:58–62. doi: 10.1007/BF00597575. [DOI] [Google Scholar]
- 67.Vollerner Y.S., Kravets S.D., Shashkov A.S., Gorovits M.B., Abubakirov N.K. Steroids of the Spirostan and Furostan Series from Plants of the Genus Allium. XXVI. Structure of Anzurogenin and Anzuroside from the Collective Fruits of Allium suvorovii and Allium stipitatum. Chem. Nat. Compd. 1989;25:431–435. doi: 10.1007/BF00597651. [DOI] [Google Scholar]
- 68.Baba M., Matsuda M.O., Kishi N., Okada Y., Shibata S., Peng J., Yao S.-S., Nishino H., Okuyama T. Saponins Isolated from Allium Chinense G. DON and Antitumor-Promoting Activities of Isoliquiritigenin and Laxogenin from the Same Drug. Biol. Pharm. Bull. 2000;23:660–662. doi: 10.1248/bpb.23.660. [DOI] [PubMed] [Google Scholar]
- 69.Carotenuto A., Fattorusso E., Lanzotti V., Magno S., Carnuccio R., D’Acquisto F. 12-Keto-Porrigenin and the Unique 2,3-Seco-Porrigenin, New Antiproliferative Sapogenins from Allium porrum. Tetrahedron. 1997;53:3401–3406. doi: 10.1016/S0040-4020(97)00062-8. [DOI] [PubMed] [Google Scholar]
- 70.Gvazava L.N., Skhirtladze A.V. Steroidal Saponin from Allium porrum. Chem. Nat. Compd. 2017;53:1093–1095. doi: 10.1007/s10600-017-2208-8. [DOI] [Google Scholar]
- 71.Fattorusso E., Lanzotti V., Magno S., Taglialatela-Scafati O. Sapogenins of Allium porrum L. J. Agric. Food Chem. 1998;46:4904–4908. doi: 10.1021/jf980849n. [DOI] [Google Scholar]
- 72.Gvazava L.N., Skhirtladze A.V. Steroidal Glycoside from Allium porrum. Chem. Nat. Compd. 2018;54:487–489. doi: 10.1007/s10600-018-2385-0. [DOI] [Google Scholar]
- 73.Lai W., Yang Y.B., Li X., Sun L.N., Wu Z.J., Chen W.S. New Steroidal Sapogenins from the Acid Hydrolysis Product of the Whole Glycoside Mixture of Welsh Onion Seeds. Chin. Chem. Lett. 2012;23:193–196. doi: 10.1016/j.cclet.2011.11.014. [DOI] [Google Scholar]
- 74.Vollerner Y.S., Kravets S.D., Shashkov A.S., Gorovits M.B., Abubakirov N.K. Steroids of the Spirostan and Furostan Series from Plants of the Genus Allium. XXV. Structure of Anzurogenin B from Allium Suvorovii and Allium Stipitatum. Chem. Nat. Compd. 1988;24:183–186. doi: 10.1007/BF00596746. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.