Abstract
The possibility of maternal in utero modulation of the innate and/or adaptive immune responses of uninfected newborns from Trypanosoma cruzi-infected mothers was investigated by studying the capacity of their whole blood cells to produce cytokines in response to T. cruzi lysate or lipopolysaccharide-plus-phytohemagglutinin (LPS-PHA) stimulation. Cells of such newborns occasionally released gamma interferon (IFN-γ) and no interleukin-2 (IL-2) and IL-4 upon specific stimulation, while their mothers responded by the production of IFN-γ, IL-2, and IL-4. Infection in mothers was also associated with a hyperactivation of maternal cells and also, strikingly, of cells of their uninfected neonates, since their release of proinflammatory (IL-1β, IL-6, and tumor necrosis factor alpha [TNF-α]) as well as of anti-inflammatory (IL-10 and soluble TNF receptor) cytokines or factors was upregulated in the presence of LPS-PHA and/or parasite lysate. These results show that T. cruzi infection in mothers induces profound perturbations in the cytokine response of their uninfected neonates. Such maternal influence on neonatal innate immunity might contribute to limit the occurrence and severity of congenital infection.
Maternal-fetal transmission of Trypanosoma cruzi, the protozoan parasite agent of Chagas' disease in Latin America, occurs in 2 to 12% of pregnancy in chronically infected mothers, inducing severe disease and significant mortality (4, 7). The factors enabling the vertical transmission to occur, as well as those allowing the vast majority of babies of infected mothers to remain uninfected, are not entirely known. Maternal-fetal transfer of antigens might influence the capacity of the progeny to respond to infection through modulating the fetal immune system (11, 12, 19, 22, 28, 39). However, there is little information on innate immunity of neonates in the case of maternal infection, whereas monocyte activation plays a central role in controlling infection (20) as well as in maintaining pregnancy (36). To better understand the maternal-fetal immunological relationship in human T. cruzi infection, we explored the capacity of uninfected neonates and their infected mothers to produce type 1 (gamma interferon [IFN-γ] and interleukin-2 [IL-2]) and type 2 (IL-4 and IL-5) cytokines, as well as proinflammatory (IL-1β, IL-6, and tumor necrosis factor alpha [TNF-α]) and anti-inflammatory (IL-10 and soluble TNF receptor [sTNFR]) factors.
Seventeen asymptomatic mothers chronically infected with T. cruzi and 58 uninfected mothers from the Maternity German Urquidi (Universidad Mayor de San Simon [UMSS]), Cochabamba, Bolivia, and their uninfected neonates were enrolled in this study. All newborns were delivered at term. Cases of known pathology of newborns or mothers during pregnancy, twins, or cesarean-born babies were excluded. Cross-reactive protein levels in maternal and cord plasma, determined by routine immunoturbidimetry, were within the normal ranges (means ± standard errors of the means [SEM]): 1.30 ± 0.17 and 1.19 ± 0.32 mg/dl for uninfected and infected mothers, respectively, and below the detection limit of 0.2 mg/dl for neonates). This study was approved by the scientific/ethic committees of UMSS and Free University of Brussels (ULB), and informed written consent of the mothers was obtained before blood collection.
Cord and maternal blood were collected just after delivery in endotoxin-free heparinized tubes (Becton Dickinson, MLS SA, Menin, Belgium) and immediately used for stimulation of whole blood cells (WBC) and study of cytokine production. Cord blood (10 ml) was also collected on EDTA disodium salt (Sigma, St. Louis, Mo.), added to 10 ml of 6 M guanidine hypochloride (Sigma), and kept at room temperature until use for T. cruzi-specific PCR.
T. cruzi trypomastigotes and amastigotes (Tehuantepec strain) were obtained from culture medium of infected 3T3 fibroblasts as previously described (18). After three washings in RPMI 1640 medium, they were submitted to eight cycles of freezing-thawing under sterile conditions. The parasite lysate was stored at −70°C until use for cell stimulation. The prepared batch of T. cruzi lysate contained no detectable endotoxin (<1 pg/107 lysed parasites), as determined by the Limulus amebocyte lysate assay (BioWhittaker Europe, Verviers, Belgium). Lipopolysaccharide (LPS; from Escherichia coli O111B4) and phytohemagglutinin (PHA) were purchased from Sigma.
Maternal infection was assessed using the classical parasite-specific serological tests of enzyme-linked immunosorbent assay (ELISA), hemagglutination, and immunofluorescence (9). T. cruzi-specific immunoglobulin M (IgM) antibodies were not detected in maternal plasma by ELISA, indicating that mothers were in the chronic form of Chagas' disease. T. cruzi congenital infection in newborns was sought in cord blood by (i) direct analysis of parasites by microscopic examination of buffy coat of blood collected in four heparinized microhematocrit tubes (each of 75 μl) (23), (ii) T. cruzi-specific PCR, performed with the primers S35 (5′-AAA TAA TGT ACG GG(T/G) GAG ATG CAT GA-3′) and S36 (5′-GGG TTC GAT TGG GGT TGG TGT-3′) (42) as described by Centurion-Lara et al. (13), and (iii) detection of T. cruzi-specific IgM and IgA antibodies by specific ELISA. These techniques allowed us to detect one congenitally infected newborn in 18 seropositive mothers (5.6%), who was not included in this study.
The WBC stimulation test was performed as previously reported (15), with minor modifications. Tenfold-diluted blood was incubated in polypropylene tubes (Falcon) either without stimulating agents or in the presence of either LPS (10 ng/ml) plus PHA (5 μg/ml) or T. cruzi lysate (106 lysed parasites/ml). After 24 or 72 h of incubation at 37°C in a 5% CO2 atmosphere, the samples were centrifuged and the supernatants were collected and kept at −70°C. Cytokine levels were determined using the following commercially available ELISAs: for IL-2, IFN-γ, and TNF-α (detecting free and soluble TNFR-bound TNF-α), EASIA from Medgenix, BioSource Europe, Nivelles, Belgium; for IL-4 and IL-10, ultrasensitive assays from Biosource, Nivelles, Belgium; for IL-1β, standard and antibody pairs from Genzyme, R&D Systems Europe, Abigdon, Oxon, United Kingdom; and for IL-5, Duoset Genzyme. Antibodies and standards kindly given by W. Buurman (Department of Surgery, University of Limburg, Maastricht, The Netherlands) were used for sTNFR type 1 (sTNFR1), sTNFR2, and IL-6 determinations, performed as previously described (16, 26, 38). The detection limits of ELISAs were as follows: for IL-1β and IL-2, 10 pg/ml; for IL-4, 0.07 pg/ml; for IL-5, 8 pg/ml; for IL-10, 0.20 pg/ml; for IFN-γ, 1.5 pg/ml; for IL-6, TNF-α, sTNFR1, and sTNFR2, 20 pg/ml. Results after WBC stimulation are expressed as the differences between the cytokine levels of stimulated and unstimulated cell samples (supernatants of 1/10-diluted blood) at the same incubation time, without further correction by the dilution factor. Results are expressed as arithmetic means ± SEM of all individual patients tested in each group. The Mann-Whitney-Wilcoxon U test was used for comparison between groups.
(i) Cells of T. cruzi-infected mothers product IFN-γ, IL-2, and IL-4 in response to parasite stimulation, whereas neonate cells occasionally release IFN-γ.
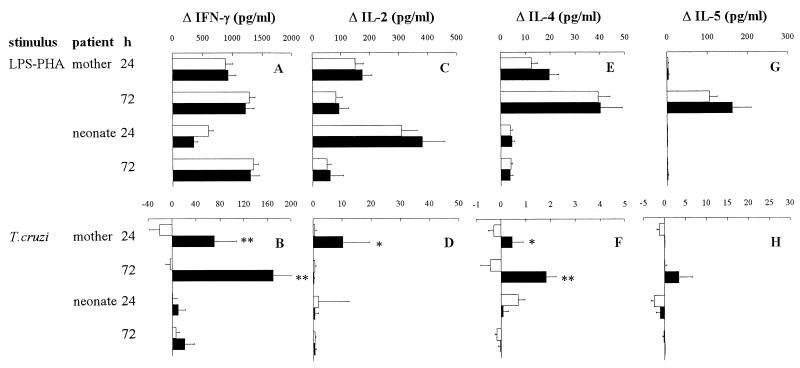
Whatever the patient group (infected or uninfected mothers and their neonates), IFN-γ, IL-2, IL-4, and IL-5 were not spontaneously released by blood cells or were released only at similar background levels in all patient groups. Mitogenic stimulation with LPS-PHA showed that maternal and neonatal cells were able to produce the type 1 cytokines IL-2 and IFN-γ, with no significant difference according to maternal infection status (Fig. 1A and C). Though neonates are generally considered poor IFN-γ producers (45), in this study newborn WBC were as able as WBC from the mothers to produce significant amounts of IFN-γ and IL-2, although with slightly different kinetics: newborns were more prone to produce IL-2 at 24 h than their mothers, whereas IFN-γ release was delayed. By contrast, but in agreement with previous work (46), the type 2 cytokines IL-4 and IL-5 were not produced by neonates from both groups in the presence of mitogens, whereas their mothers did produce these cytokines (Fig. 1E and G).
FIG. 1.
Production of type 1 and type 2 cytokines by WBC from T. cruzi-infected (■) or uninfected (□) mothers and their newborns. WBC were stimulated or not for 24 or 72 h with LPS (10 ng/ml) plus PHA (5 μg/ml) (A, C, E, and G) or a lysate of T. cruzi (106 lysed parasites/ml) (B, D, F, and H). Numbers of individuals in each group ranged from 26 to 43 for uninfected mothers, 11 to 16 for infected mothers, 25 to 47 for neonates from uninfected mothers, and 7 to 13 for uninfected neonates from infected mothers. Results (mean ± SEM) are expressed as the differences between levels obtained for stimulated and unstimulated cells. The Mann-Whitney-Wilcoxon U test was used for statistical comparisons between infected and uninfected mothers and between their newborns (∗, P < 0.05; ∗∗, P < 0.005).
As expected, mothers chronically infected with T. cruzi harbored parasite-specific memory T cells, since their WBC released lymphocyte-derived cytokines (IFN-γ, IL-2, and IL-4 but not IL-5 [Fig. 1B, D, F, and H]) when incubated with T. cruzi antigens. Moreover, the production of both type 1 and type 2 cytokines suggests that the adaptive response to the parasite in mothers is not polarized, in agreement with previous reports on human and experimental Chagas' disease (17, 47). Since maternal-fetal transfer of parasite antigens, present in maternal blood (1) or released from parasites present in the placenta (unpublished data), is likely in the case of maternal T. cruzi infection and could lead to lymphocyte priming (29), we also investigated the production of lymphocytic cytokines in uninfected neonates from infected mothers upon specific stimulation of WBC with T. cruzi lysate. However, except for significant IFN-γ production by one neonate at 24 h (100 pg/ml) and another at 72 h (170 pg/ml), no type 1 or 2 cytokines were detected (Fig. 1B, D, F, and H). This could be related to (i) a low release of such cytokines and/or cytokine consumption by cell receptors, thereby preventing their detection in ELISA (5); (ii) the presence of parasite-specific antibodies, transferred from mother into fetal blood, disturbing antigen binding to antigen-presenting cells and the subsequent response by newborn T cells (40); or (iii) immunosuppression and/or apoptosis of lymphocytes induced by the parasitic molecules added in the WBC culture (25, 30).
(ii) Maternal T. cruzi infection upregulates the capacity of maternal and neonate cells to produce proinflammatory cytokines.
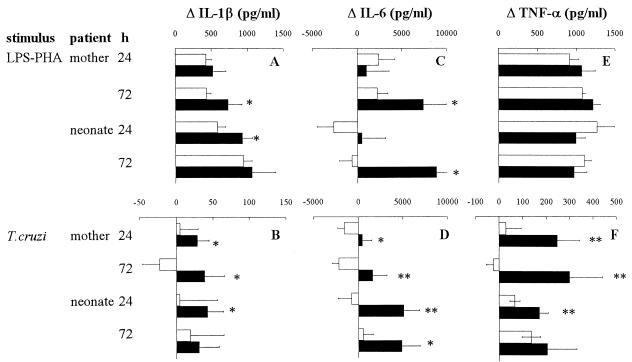
In contrast to lymphocytic cytokines, IL-1β, IL-6, and TNF-α were spontaneously released by blood cells from mothers as well as their neonates (data not shown), likely reflecting the monocytic activation normally associated with pregnancy and delivery (3, 37). As shown in Fig. 2A, C, and E, such spontaneous inflammatory cytokine release was enhanced in mothers and neonates upon in vitro stimulation with LPS-PHA (except for IL-6 in neonates of uninfected mothers). Moreover, IL-6, and to a lesser extent IL-1β, levels were still higher in infected mothers and also, surprisingly, in their uninfected newborns. The parasite lysate strongly stimulated the production of TNF-α and IL-6, and slightly that of IL-1β, in both chagasic mothers and their neonates (Fig. 2B, D, and F). A detailed analysis of individual results indicated that a simultaneous higher production of these cytokines was observed in 58% of newborns. T. cruzi infection in mothers could easily explain their higher capacity to produce inflammatory cytokines. Indeed, although PHA-activated lymphocytes could be the source of IL-6, the simultaneous overproduction of three inflammatory cytokines suggests a monocytic origin. Different mechanisms could account for monocytic activation in infected mothers: (i) in vivo monocyte priming, known to occur during T. cruzi infection (8), strengthening the direct effect of parasite molecules supporting proinflammatory activities (2, 10, 21, 35) and present in the parasite lysate used to activate cells; (ii) monocytic activation due to IFN-γ released in vitro by specific lymphocytes after recognition of T. cruzi antigens; or (iv) proinflammatory cytokine release resulting from a cross-linking of FcR on monocytes and/or NK cells (6, 14, 27) by immune complexes formed by the T. cruzi-specific antibodies present in the blood of infected mothers and the parasite lysate added in vitro.
FIG. 2.
Production of proinflammatory cytokines by WBC from T. cruzi-infected (■) or uninfected (□) mothers and their newborns. WBC were stimulated or not for 24 or 72 h with LPS (10 ng/ml) plus PHA (5 μg/ml) (A, C, and E) or a lysate of T. cruzi (106 lysed parasites/ml) (B, D, and F). Numbers of individuals in each group ranged from 23 to 42 for uninfected mothers, 7 to 13 for infected mothers, 16 to 46 for neonates from uninfected mothers, and 8 to 15 for uninfected neonates from infected mothers. Results (mean ± SEM) are expressed as the difference between levels obtained for stimulated and unstimulated cells. The Mann-Whitney-Wilcoxon U test was used for statistical comparisons between infected and uninfected mothers and between their newborns (∗, P < 0.05; ∗∗, P < 0.005).
It is puzzling to observe such overproduction of inflammatory cytokines also in uninfected neonates from infected mothers. This did not result from an increase of monocyte concentration, since we have verified that their numbers are similar in both groups of neonates (202 ± 42 and 230 ± 23 monocytes/mm3 for newborn of infected and uninfected mothers, respectively), strongly suggesting that their monocytes are hyperactivated as in their mothers. Although the monocytic origin of proinflammatory cytokines has still to be confirmed, this is, as far as we know, the first indication of a maternal modulation of neonatal innate immunity in human infection. This agrees with our previous observations regarding experimental Chagas' disease showing that both fetuses and offspring of infected mice display increased TNF-α gene transcription and/or production (34). The origin of neonatal (and probably fetal) monocytic hyperactivation remains unknown. Mechanisms similar to those mentioned above for mothers might also function in neonates, following maternal-fetal transfer of shed T. cruzi molecules or antibodies. In vivo IFN-γ-dependent monocyte activation seems unlikely since this cytokine was hardly detectable in the supernatants of neonate cells. The maternal induction of such neonatal cell activation raises the question of its contribution in the control of congenital infection, since the neonates displaying such activation were uninfected (as verified by parasitological, PCR, and antibody detection). It is tempting to hypothesize that such newborns could be protected against an eventual vertical transmission of parasites, by a synergy between maternally transferred antibodies and activated monocytes, previously shown to be effective in the in vitro and in vivo killing of parasites (32, 33, 44).
(iii) Maternal T. cruzi infection upregulates the capacity of maternal and newborn cells to produce anti-inflammatory factors.
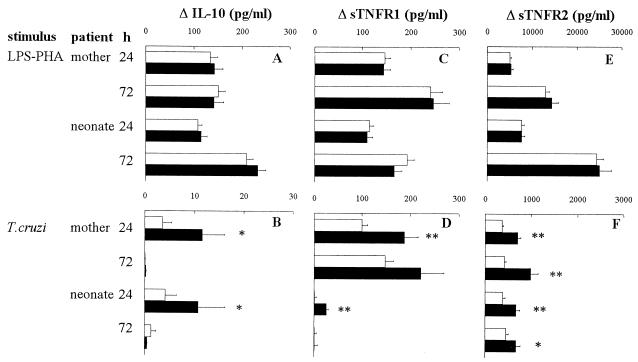
The effect of maternal infection on proinflammatory cytokines prompted us to also investigate the production of the potent anti-inflammatory factor IL-10 (41), as well as the sTNFR1 and sTNFR2, previously shown to play an essential role in modulating TNF bioactivity (31), particularly in experimental Chagas' disease (43). Though only traces of IL-10 could be found in supernatants of unstimulated cells from newborns and mothers (data not shown), LPS-PHA stimulated IL-10 production by WBC from all patient groups (Fig. 3A), whatever the maternal infection status. By contrast, maternal infection was associated with a higher reactivity of both mother and newborn WBC to parasite lysate, which produced two- to threefold more IL-10 after 24 h of culture than the control couples (Fig. 3B). Infection status did not modify the WBC release of both sTNFRs, either spontaneously (data not shown) or after incubation with LPS-PHA (Fig. 3C and E). By contrast, T. cruzi components induced a higher release of both sTNFRs in infected mothers and their newborns (Fig. 3D and F). The overproduction of IL-10 and sTNFR by cells incubated with the parasite lysate was observed in the same newborns from infected mothers as those who responded upon proinflammatory cytokine production.
FIG. 3.
Production of anti-inflammatory cytokine and factors by WBC from T. cruzi-infected (■) or uninfected (□) mothers and their newborns. WBC were stimulated or not for 24 or 72 h with LPS (10 ng/ml) plus PHA (5 μg/ml) (A, C, and E) or a lysate of T. cruzi (106 lysed parasites/ml) (B, D, and F). Numbers of individuals in each group ranged from 22 to 41 for uninfected mothers, 9 to 15 for infected mothers, 26 to 43 for neonates from uninfected mothers, and 8 to 13 for uninfected neonates from infected mothers. Results (mean ± SEM) are expressed as the difference between levels obtained in stimulated and unstimulated cells. The Mann-Whitney-Wilcoxon U test was used for statistical comparisons between infected and uninfected mothers and between their newborns (*, P < 0.05; **, P < 0.005).
The in vivo triggering of anti-inflammatory factors might protect fetal or neonatal tissues against the harmful effects generated by the intense inflammatory reaction that would occur in the case of congenital infection. Such homeostatic regulation might also contribute to avoidance of the fetal growth retardation associated with overproduction of TNF (24). Indeed, sTNFR/TNF molar ratios (calculated as previously described [43]) were similar in neonates of infected and uninfected mothers (data not shown). This suggests that TNF bioactivity remains similar in control and infected groups of mothers or neonates and could explain why neonates from infected mothers displayed normal birth weights (3,438 ± 154 and 3,293 ± 48 g for newborns from infected and uninfected mothers, respectively).
In conclusion, a potent state of cell activation is induced in uninfected neonates (and probably fetuses) from T. cruzi-infected mothers, leading to the simultaneous production of pro- and anti-inflammatory factors in the presence of parasite antigens. Such maternal influence on neonatal innate immunity might have protective effects, limiting the occurrence and outcome of congenital infections.
Acknowledgments
We thank Mildreth Castro (CUMETROP, UMSS, Cochabamba, Bolivia) and Antonio Pardo, Amilcar Mercado, and Jaime Vargas (Maternity German Urquidi, Cochabamba, Bolivia) for the management of patients, Jean-Marie Boeynaems (Erasmus Hospital, ULB, Brussels, Belgium) for CRP quantification, Corine Liesnard, Françoise Brancart, and Laurent Debaisieux (Erasmus Hospital, ULB) for help with PCR assays, and Wim Buurman (Department of Surgery, Faculty II, University of Limburg, Maastricht, The Netherlands) for providing reagents for some ELISAs.
This work was supported by grants from Fonds National de la Recherche Scientifique (FNRS), Centre de Recherche Inter-Universitaire en Vaccinologie (CRIV), and Action de Recherche Concertée de la Communauté Française de Belgique (ARC).
REFERENCES
- 1.Affranchino J L, Ibanez C F, Luquetti A O, Rassi A, Reyes M B, Macina R, Aslund L, Pettersson U, Frasch A C. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989;34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 2.Almeida I C, Camargo M M, Procopio D O, Silva L S, Mehlert A, Travassos L R, Gazzinelli R T, Ferguson M A. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austgulen R, Lien E, Liabakk N B, Jacobsen G, Arntzen K J. Increased levels of cytokines and cytokine activity modifiers in normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1994;57:149–155. doi: 10.1016/0028-2243(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 4.Azogue E, La Fuente C, Darras C. Congenital Chagas' disease in Bolivia: epidemiological aspects and pathological findings. Trans R Soc Trop Med Hyg. 1985;79:176–180. doi: 10.1016/0035-9203(85)90328-1. [DOI] [PubMed] [Google Scholar]
- 5.Baroja M L, Ceuppens J L. More exact quantification of interleukin-2 production by addition of anti-Tac monoclonal antibody to cultures of stimulated lymphocytes. J Immunol Methods. 1987;98:267–270. doi: 10.1016/0022-1759(87)90014-7. [DOI] [PubMed] [Google Scholar]
- 6.Berger S, Ballo H, Stutte H J. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur J Immunol. 1996;26:1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 7.Bittencourt A L. American trypanosomiasis (Chagas' disease) In: MacLeod C, editor. Parasitic infections in pregnancy and the newborn. Oxford, United Kingdom: Oxford Medical Publications; 1988. pp. 62–86. [Google Scholar]
- 8.Brener Z, Gazzinelli R T. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 9.Breniere S F, Carrasco R, Miguez H, Lemesre J L, Carlier Y. Comparisons of immunological tests for serodiagnosis of Chagas disease in Bolivian patients. Trop Geogr Med. 1985;37:231–238. [PubMed] [Google Scholar]
- 10.Camargo M M, Almeida I C, Pereira M E, Ferguson M A, Travassos L R, Gazzinelli R T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 11.Camus D, Carlier Y, Bina J C, Borojevic R, Prata A, Capron A. Sensitization to Schistosoma mansoni antigen in uninfected children born to infected mothers. J Infect Dis. 1976;134:405–408. doi: 10.1093/infdis/134.4.405. [DOI] [PubMed] [Google Scholar]
- 12.Carlier Y, Truyens C. Influence of maternal infection on offspring resistance towards parasites. Parasitol Today. 1999;11:94–99. doi: 10.1016/0169-4758(95)80165-0. [DOI] [PubMed] [Google Scholar]
- 13.Centurion-Lara A, Barrett L, Van Voorhis W C. Quantitation of parasitemia by competitive polymerase chain reaction amplification of parasite kDNA minicircles during chronic infection with Trypanosoma cruzi. J Infect Dis. 1994;170:1334–1339. doi: 10.1093/infdis/170.5.1334. [DOI] [PubMed] [Google Scholar]
- 14.Debets J M, Van de Winkel J G, Ceuppens J L, Dieteren I E, Buurman W A. Cross-linking of both Fc gamma RI and Fc gamma RII induces secretion of tumor necrosis factor by human monocytes, requiring high affinity Fc-Fc gamma R interactions. Functional activation of Fc gamma. J Immunol. 1990;144:1304–1310. [PubMed] [Google Scholar]
- 15.De Groote D, Zangerle P F, Gevaert Y, Fassotte M F, Beguin Y, Noizat P-F, Pirenne J, Gathy R, Lopez M, Dehart I, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–248. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 16.Dentener M A, Bazil V, Von Asmuth E J, Ceska M, Buurman W A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 17.Dutra W O, Gollob K J, Pinto Dias J C, Gazzinelli G, Correa Oliveira R, Coffman R L, Carvalho Parra J F. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 18.el Bouhdidi A, Truyens C, Rivera M T, Bazin H, Carlier Y. Trypanosoma cruzi infection in mice induces a polyisotypic hypergammaglobulinaemia and parasite-specific response involving high IgG2a concentrations and highly avid IgG1 antibodies. Parasite Immunol. 1994;16:69–76. doi: 10.1111/j.1365-3024.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 19.Elson L H, Days A, Calvopina M, Paredes W, Araujo E, Guderian R H, Bradley J E, Nutman T B. In utero exposure to Onchocerca volvulus: relationship to subsequent infection intensity and cellular immune responsiveness. Infect Immun. 1996;64:5061–5065. doi: 10.1128/iai.64.12.5061-5065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fearon D T. Seeking wisdom in innate immunity. Nature. 1997;388:323–324. doi: 10.1038/40967. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Gomez R, Esteban S, Gomez-Corvera R, Zoulika K, Ouaissi A. Trypanosoma cruzi: Tc52 released protein-induced increased expression of nitric oxide synthase and nitric oxide production by macrophages. J Immunol. 1998;160:3471–3479. [PubMed] [Google Scholar]
- 22.Fievet N, Ringwald P, Bickii J, Dubois B, Maubert B, Le Hesran J Y, Cot M, Deloron P. Malaria cellular immune responses in neonates from Cameroon. Parasite Immunol. 1996;18:483–490. doi: 10.1046/j.1365-3024.1996.d01-19.x. [DOI] [PubMed] [Google Scholar]
- 23.Freilij H, Muller L, Gonzalez Cappa S M. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J Clin Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried M, Muga R O, Misore A O, Duffy P E. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 25.Kierszenbaum F, de Diego J L, Fresno M, Sztein M B. Inhibitory effects of the Trypanosoma cruzi membrane glycoprotein AGC10 on the expression of IL-2 receptor chains and secretion of cytokines by subpopulations of activated human T lymphocytes. Eur J Immunol. 1999;29:1684–1691. doi: 10.1002/(SICI)1521-4141(199905)29:05<1684::AID-IMMU1684>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Leeuwenberg J F, Jeunhomme T M, Buurman W A. Slow release of soluble TNF receptors by monocytes in vitro. J Immunol. 1994;152:4036–4043. [PubMed] [Google Scholar]
- 27.Leite de Moraes M C, Dy M. Natural killer T cells: a potent cytokine-producing cell population. Eur Cytokine Netw. 1997;8:229–237. [PubMed] [Google Scholar]
- 28.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma J H, Kazura J W, King C L. Helminth- and Bacillus Calmette Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–6848. [PubMed] [Google Scholar]
- 29.Neves S F, Eloi-Santos S, Ramos R, Rigueirinho S, Gazzinelli G, Correa-Oliveira R. In utero sensitization in Chagas' disease leads to altered lymphocyte phenotypic patterns in the newborn cord blood mononuclear cells. Parasite Immunol. 1999;21:631–639. doi: 10.1046/j.1365-3024.1999.00262.x. [DOI] [PubMed] [Google Scholar]
- 30.Nunes M P, Andrade R M, Lopes M F, DosReis G A. Activation-induced T cell death exacerbates Trypanosoma cruzi replication in macrophages cocultured with CD4+ T lymphocytes from infected hosts. J Immunol. 1998;160:1313–1319. [PubMed] [Google Scholar]
- 31.Olsson I, Gatanaga T, Gullberg U, Lantz M, Granger G A. Tumour necrosis factor (TNF) binding proteins (soluble TNF receptor forms) with possible roles in inflammation and malignancy. Eur Cytokine Netw. 1993;4:169–180. [PubMed] [Google Scholar]
- 32.Plata F, Garcia Pons F, Wietzerbin J. Immune resistance to Trypanosoma cruzi: synergy of specific antibodies and recombinant interferon gamma in vivo. Ann Inst Pasteur Immunol. 1987;138:397–415. doi: 10.1016/s0769-2625(87)80051-x. [DOI] [PubMed] [Google Scholar]
- 33.Plata F, Wietzerbin J, Pons F G, Falcoff E, Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984;14:930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- 34.Rivera M T, Marques de Araujo S, Lucas R, Deman J, Truyens C, Defresne M P, de Baetselier P, Carlier Y. High tumor necrosis factor alpha (TNF-α) production in Trypanosoma cruzi-infected pregnant mice and increased TNF-α gene transcription in their offspring. Infect Immun. 1995;63:591–595. doi: 10.1128/iai.63.2.591-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saavedra E, Herrera M, Gao W, Uemura H, Pereira M A. The Trypanosoma cruzi trans-sialidase, through its COOH-terminal tandem repeat, upregulates interleukin 6 secretion in normal human intestinal microvascular endothelial cells and peripheral blood mononuclear cells. J Exp Med. 1999;190:1825–1836. doi: 10.1084/jem.190.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20:114–118. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 37.Sarandakou A, Giannaki G, Malamitsi-Puchner A, Rizos D, Hourdaki E, Protonotariou E, Phocas I. Inflammatory cytokines in newborn infants. Mediators Inflamm. 1998;7:309–312. doi: 10.1080/09629359890811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schols A M, Buurman W A, Staal van den Brekel A J, Dentener M A, Wouters E F. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–824. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shearer G M, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 40.Siegrist C A. Vaccination in the neonatal period and early infancy. Int Rev Immunol. 2000;19:195–219. doi: 10.3109/08830180009088505. [DOI] [PubMed] [Google Scholar]
- 41.Stordeur P, Goldman M. Interleukin-10 as a regulatory cytokine induced by cellular stress: molecular aspects. Int Rev Immunol. 1998;16:501–522. doi: 10.3109/08830189809043006. [DOI] [PubMed] [Google Scholar]
- 42.Sturm N R, Degrave W, Morel C, Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989;33:205–214. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- 43.Truyens C, Torrico F, Lucas R, de Baetselier P, Buurman W A, Carlier Y. The endogenous balance of soluble tumor necrosis factor receptors and tumor necrosis factor modulates cachexia and mortality in mice acutely infected with Trypanosoma cruzi. Infect Immun. 1999;67:5579–5586. doi: 10.1128/iai.67.11.5579-5586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umekita L F, Mota I. How are antibodies involved in the protective mechanism of susceptible mice infected with T. cruzi? Braz J Med Biol Res. 2000;33:253–258. doi: 10.1590/s0100-879x2000000300001. [DOI] [PubMed] [Google Scholar]
- 45.Wilson C B, Penix L, Weaver W M, Melvin A, Lewis D B. Ontogeny of T lymphocyte function in the neonate. Am J Reprod Immunol. 1992;28:132–135. doi: 10.1111/j.1600-0897.1992.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang L P, Byun D G, Demeure C E, Vezzio N, Delespesse G. Default development of cloned human naive CD4 T cells into interleukin-4- and interleukin-5-producing effector cells. Eur J Immunol. 1995;25:3517–3520. doi: 10.1002/eji.1830251247. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Tarleton R L. Characterization of cytokine production in murine Trypanosoma cruzi infection by in situ immunocytochemistry: lack of association between susceptibility and type 2 cytokine production. Eur J Immunol. 1996;26:102–109. doi: 10.1002/eji.1830260116. [DOI] [PubMed] [Google Scholar]