Abstract
These NCCN Guidelines Insights focus on recent updates in the 2016 NCCN Guidelines for Non–Small Cell Lung Cancer (NSCLC; Versions 1–4). These NCCN Guidelines Insights will discuss new immunotherapeutic agents, such as nivolumab and pembrolizumab, for patients with metastatic NSCLC. For the 2016 update, the NCCN panel recommends immune checkpoint inhibitors as preferred agents (in the absence of contraindications) for second-line and beyond (subsequent) therapy in patients with metastatic NSCLC (both squamous and nonsquamous histologies). Nivolumab and pembrolizumab are preferred based on improved overall survival rates, higher response rates, longer duration of response, and fewer adverse events when compared with docetaxel therapy.
Overview
In 2016, an estimated 224,390 new cases (117,920 in men and 106,470 in women) of lung and bronchial cancer will be diagnosed in the United States, and 158,080 disease-related deaths (85,920 in men and 72,160 in women) are estimated to occur.1 Currently, most lung cancer is diagnosed clinically when patients present with symptoms, such as persistent cough, pain, and weight loss. Unfortunately, most patients are diagnosed when they already have advanced-stage disease. For earlier-stage disease, a significant relapse rate occurs even after multimodality therapy is used, when appropriate. Taking into account all stages at diagnosis, the 5-year survival rate for lung cancer is only 17.4%; the 5-year survival rate for those with stage IV (metastatic) disease at diagnosis is much lower (approximately 2%).2,3 Therefore, there is great interest in new treatment options for patients with metastatic non–small cell lung cancer (NSCLC). The complete version of the NCCN Guidelines for NSCLC addresses all aspects of disease management, including screening, diagnosis, evaluation, staging, treatment, surveillance, and therapy for recurrence and metastasis (to view the most recent version of these guidelines, visit NCCN.org). These guidelines are updated at least once a year by the NCCN NSCLC Panel; there were 8 updates between January 2015 and January 2016. The NCCN Guidelines for NSCLC were first published in 1996.4
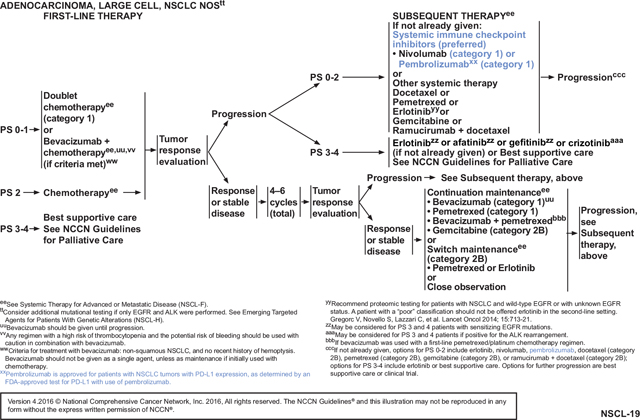
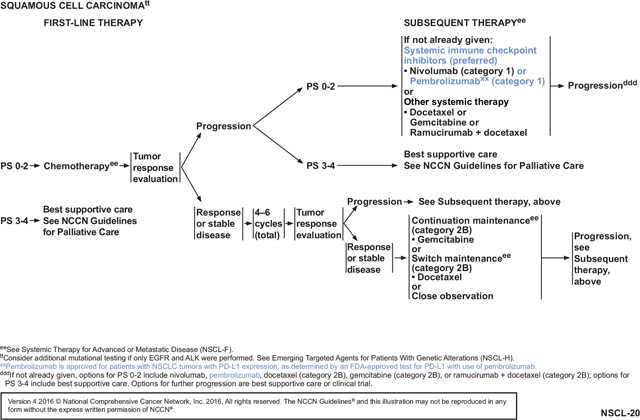
These NCCN Guidelines Insights will discuss the use of new immunotherapeutic agents, specifically nivolumab and pembrolizumab, for patients with metastatic NSCLC. For the 2016 updates (Versions 1–4), the NCCN panel added new recommendations for nivolumab (category 1) and pembrolizumab (category 1), which are immune checkpoint inhibitors, as second-line and beyond (subsequent) therapy for patients with metastatic nonsquamous and squamous NSCLC who have progressed on or after first-line platinum-based chemotherapy (see page NSCL-19 and NSCL-20, page 257 and above, respectively).5–10 For 2016 (Version 1), the NCCN panel recommended immune checkpoint inhibitors as preferred agents for subsequent therapy based on improved overall survival rates, higher response rates, longer duration of response, and fewer adverse events when compared with docetaxel in the second-line setting (see page NSCL-19 and NSCL-20, page 257 and above, respectively). Docetaxel was considered a standard second-line option and was often used for subsequent therapy in patients whose cancer had progressed on first-line pemetrexed-based regimens, had squamous cell NSCLC, or had renal impairment. Docetaxel was used as the control regimen in phase III clinical trials assessing nivolumab and pembrolizumab.6,9,10 Median overall survival is only approximatley 6 to 9 months when docetaxel is used for subsequent therapy.
The immune checkpoint inhibitors, nivolumab and pembrolizumab, are IgG4 monoclonal antibodies that target the programmed cell death protein 1 (PD-1) cell surface receptor. By blocking the interaction of PD-1 and programmed death-ligand 1 (PD-L1), treatment is hypothesized to improve antitumor immunity. The PD-1 pathway is one prominent inhibitory pathway associated with tumoral immune escape. T-cell activation is inhibited when PD-1 receptors on the surface of these cells engage with ligands present on the tumor cells, such as PD-L1.11–13 Nivolumab and pembrolizumab block the PD-1 receptor from engaging with its ligand, allowing cytotoxic T cells to be activated. These activated T cells can then recognize and kill tumor cells.12,14 Other immune checkpoint inhibitors that inhibit different parts of the PD-1 pathway are in clinical trials, notably atezolizumab and durvalumab, which target PD-L1.15–17 In addition, clinical trials are ongoing in which PD-1/PD-L1 antibodies are combined with CTLA-4 antibodies.
Although benefit from immune checkpoint inhibitors may manifest as objective responses or prolonged progression-free survival (PFS), in some cases, interpretation of benefit may be more complex than with traditional chemotherapies or targeted therapies. Pseudoprogression has been reported with immune checkpoint inhibitors, which is an increase in the size of existing lesions or the appearance of new lesions before later radiographic improvement occurs, usually within a few weeks. However, pseudoprogression is less common in patients with NSCLC than in those with melanoma. In patients with NSCLC, most radiographic progression with immune checkpoint inhibitors is likely to represent true progression. Early rescanning (eg, in the 4- to 6-week range) may help differentiate pseudoprogression from true progression. Pseudoprogression is differentiated by apparently paradoxical improvement in (or stable) symptoms despite “apparent” radiographic progression. Strong evidence of true progression is represented by further progression on rescanning or progression after an initial response. In equivocal cases, biopsy of an enlarging or new lesion may resolve the controversy. Despite the low rate of pseudoprogression, one striking thing about the immune checkpoint inhibitors has been recurring evidence of a greater effect on overall survival than on PFS, as manifested by consistently higher tails in the overall survival than in the PFS curves (eg, consistent with a potential long-term impact after treatment discontinuation, potentially including beneficial effects manifested across later lines of therapy).
Patients receiving traditional cytotoxic chemotherapy often have emetic and myelosuppressive adverse events, but these rarely occur with immune checkpoint inhibitors.18 Immune-related adverse events may occur with all anti–PD-1– and anti–PD-L1–directed antibodies.5,7,11,12,15,19–21 These adverse events may occur at any time during or after immunotherapy is complete. Immune-related adverse events include dermatologic, gastrointestinal, hepatic, pulmonary, and endocrine (including those requiring replacement therapy) events. Serious immune-related adverse events, such as pneumonitis (1%–3%), are rare. For patients with immune-related adverse events, in addition to withholding treatment, oral or intravenous high-dose corticosteroids should be administered based on the severity of the reaction.19,22 Immune checkpoint inhibitors should be discontinued for patients with severe or life-threatening pneumonitis or colitis, and should be withheld or discontinued for other severe or life-threatening immune-mediated adverse events when indicated (see prescribing information).
Factors associated with benefit from immune checkpoint inhibitors remain under investigation. Theoretically, in order for anti–PD-1– or PD-L1–directed monotherapy to result in a clinically beneficial tumoral immune response, several assumptions must be met, including (1) the presence of neoantigens in the tumor; (2) presentation of these antigens on the host’s specific major histocompatibility complex; (3) an appropriate proimmune environment (taking into account the appropriate presence and absence of other known immune-stimulatory and immune-inhibitory signals, respectively); and, finally, (4) use of the PD-1 axis as a dominant means of immune escape in that tumor in the patient.
Supporting these assumptions in NSCLC have been reports of increased response rates and/or prolonged PFS in association with greater smoking history, squamous versus nonsquamous histology, or greater nonsynonymous mutational burden. All of these factors would increase the chances of, at least, the first and second assumptions being met. However, perhaps the greatest data generated so far on a predictive biomarker for benefit from PD-1 axis inhibition relates to the expression level of PD-L1 on tumor biopsies as assessed by immunohistochemistry. It may seem surprising that only one of the previous assumptions in such a complex process would show any predictive value. Because PD-L1 levels are induced by gamma-interferon secreted by activated T lymphocytes, to some extent, the presence of PD-L1 indicates that all of the previous assumptions have already been met.
When considering PD-L1 as a biomarker, many different assays have been used, and the definition of positive (and the resulting percentage of tumors defined as positive) varies widely between assays. Although PD-L1 expression represents a means to enrich for benefit, it neither guarantees it nor excludes it, partly because (1) the previously described assumptions must be met; (2) PD-L1 levels represent a continuous variable; and (3) PD-L1 levels are inducible and may vary over both space and time within a tumor, between deposits of tumor within an individual, or in response to prior therapies.
By using different definitions of PD-L1 positivity or negativity, different assays (often used in association with specific drugs in the class) can significantly alter the response rate and benefit from the drug. More stringent definitions of PD-L1 positivity increase the apparent benefit from these drugs at the expense of shrinking the population considered eligible for treatment in a given setting. Less stringent definitions of PD-L1 positivity would include a greater percentage of patients for treatment in a given setting but in association with a lower degree of overall benefit. PD-L1 staining may be more valuable in determining the relative benefit of a PD-1/PD-L1–directed approach versus comparator treatment choices in first-line chemotherapy, in which more enrichment may be needed to rival the effectiveness of first-line platinum doublet chemotherapy. For any line of therapy, the degree of enrichment could significantly alter the health economics of the drug, making the preferred extent of enrichment potentially highly dependent on the payer situation that exists for any given patient or health care system.
Nivolumab
Metastatic Squamous NSCLC
The NCCN NSCLC Panel recommends (category 1) nivolumab as a preferred subsequent therapy for patients with metastatic squamous cell NSCLC whose disease has progressed on or after first-line chemotherapy based on data from a phase III randomized trial (CheckMate 017), FDA approval, and results of a phase II trial.6,7 In the CheckMate 017 trial, the median overall survival (the primary end point) was 9.2 months with nivolumab compared with 6.0 months for docetaxel (hazard ratio [HR] for death, 0.59; 95% CI, 0.44–0.79; P<.001).6 Patients had a response rate of 20% with nivolumab compared with 9% for docetaxel. PFS was also slightly improved with nivolumab (3.5 vs 2.8 months; P=.004). PD-L1 expression—as assessed by a proprietary PD-L1 assay using positivity definitions, which defined approximately 30% to 50% of the squamous population as positive—was not significantly associated with either PFS or overall survival benefit from nivolumab versus docetaxel. Fewer grade 3 to 4 adverse events were seen with nivolumab (7%) compared with docetaxel (55%). No patients died in the nivolumab arm, but 3 deaths occurred in the docetaxel arm.
Metastatic Nonsquamous NSCLC
The NCCN panel recommends nivolumab (category 1) as a preferred subsequent therapy for patients with metastatic nonsquamous NSCLC whose disease has progressed on or after platinum-based chemotherapy.5–7,9 For the 2016 update of the NCCN Guidelines (Version 1), the panel revised the recommendation for nivolumab to category 1 (from category 2A) based on a phase III randomized trial (CheckMate 057) and FDA approval of nivolumab for patients with metastatic nonsquamous NSCLC (see page NSCL-19, page 257). For patients receiving nivolumab, median overall survival (the primary end point) was 12.2 months compared with 9.4 months for docetaxel (HR, 0.73; 95% CI, 0.59–0.89; P=.002).9 Patients had a response rate of 19% with nivolumab compared with 12% for docetaxel. PFS was not improved with nivolumab (2.3 vs 4.2 months). The median duration of response was 17.2 months with nivolumab compared with 5.6 months for docetaxel. At 18 months, the overall survival rate was 39% (95% CI, 34%–45%) with nivolumab compared with 23% (95% CI, 19%–28%) with docetaxel. Fewer grade 3 to 5 adverse events were reported for nivolumab (10%) when compared with docetaxel (54%) in the CheckMate 057 trial. Subset analysis demonstrated that patients with sensitizing EGFR mutations whose disease progressed after first-line targeted agents were one of the few patient groups to derive little overall survival benefit from nivolumab (HR, 1.18; CI, 0.69–2.00), suggesting that such patients may benefit more from docetaxel. Insufficient data are available to recommend immune checkpoint inhibitors for patients whose disease progresses after first-line or second-line targeted agents.
PD-L1 expression was assessed by a nivolumab-associated proprietary PD-L1 assay (PD-L1 IHC 28–8 pharmDx test; Dako, Carpinteria, CA) using positivity definitions that defined approximately 40% to 50% of the nonsquamous population as positive (PD-L1 levels of ≥1% through ≥10%). The HR for overall survival benefit from nivolumab (vs docetaxel) was decreased by PD-L1 expression (from HR 0.73 to HR 0.4 in the most enriched group); these HRs retained statistical significance. In patients with tumors that did not have PD-L1 expression by this assay (<1% staining, representing approximately 47% of the nonsquamous NSCLC population in this study), only a nonsignificant trend was seen toward an improvement in overall survival for nivolumab versus docetaxel (HR, 0.9; 95% CI, 0.66–1.24). The response rate in PD-L1–negative cases, as defined by this assay, with nivolumab was also less than with docetaxel (9% vs 13%). However, the NCCN panel does not recommend PD-L1 testing using this assay for determining the use of nivolumab in the subsequent therapy nonsquamous setting, because the study met its primary end point in an unselected population and because nivolumab was associated with both a longer duration of response (when responses occurred) and with fewer side effects than docetaxel.
Pembrolizumab
The NCCN panel recommends pembrolizumab (category 1) as preferred subsequent therapy for patients with metastatic nonsquamous or squamous NSCLC whose disease has progressed after platinum-based chemotherapy when PD-L1 expression is present using the pembrolizumab-associated proprietary PD-L1 assay and the definition of positivity based on the single arm and later randomized KEYNOTE trials and recent FDA approval.8,10
A phase I trial (KEYNOTE-001) assessed the safety and efficacy of pembrolizumab at 10 mg/kg for patients with metastatic NSCLC and the effect of enrichment using a proprietary PD-L1 assay and definition of positivity.8 Among all patients, the response rate was 19%, the median duration of response was 12.5 months, PFS was 3.7 months, and median overall survival was 12.0 months. Patients with a PD-L1 “proportion score” of at least 50% (representing approximately 15% of NSCLC in this study) had a response rate of 45% and a PFS of 6.3 months, and overall survival was not reached. Less than 10% of patients had serious grade 3 or greater toxicity. Based on these data, the FDA approved pembrolizumab for subsequent therapy of metastatic NSCLC in tumors with PD-L1 expression equivalent to a proportion score of 50% using the same assay. The FDA has approved a companion diagnostic biomarker test (PD-L1 IHC 22C3 pharmDx kit) for assessing PD-L1 expression and determining which patients are eligible for pembrolizumab therapy.
For the 2016 NCCN Guidelines update (Version 4), the NCCN NSCLC Panel revised the recommendation for pembrolizumab to category 1 (from category 2A) as subsequent therapy for patients with metastatic nonsquamous or squamous NSCLC and PD-L1 expression based on a recent trial (see NSCL-19, NSCL-20, and NSCL-F 1 of 4, pages 257, 258, and 259, respectively).10 This phase II/III randomized trial (KEYNOTE-010) assessed pembrolizumab in patients with previously treated advanced NSCLC who were PD-L1–positive (≥1%); most were current or former smokers.10 There were 3 arms in this trial: pembrolizumab at 2 mg/kg, pembrolizumab at 10 mg/kg, and docetaxel at 75 mg/m2 every 3 weeks. The median overall survival was 10.4 months for the lower dose of pembrolizumab, 12.7 months for the higher dose, and 8.5 months for docetaxel. Overall survival was significantly longer for both doses of pembrolizumab compared with docetaxel (pembrolizumab, 2 mg/kg: HR, 0.71; 95% CI, 0.58–0.88; P=.0008) (pembrolizumab, 10 mg/kg: HR 0.61; CI, 0.49–0.75; P<.0001). For patients with at least 50% PD-L1 expression in tumor cells, overall survival benefit was associated with greater difference at either dose of pembrolizumab when compared with docetaxel (pembrolizumab at 2 mg/kg: 14.9 vs 8.2 months; HR, 0.54; 95% CI, 0.38–0.77; P=.0002 and pembrolizumab at 10 mg/kg: 17.3 vs 8.2 months; HR, 0.50; CI, 0.36–0.70; P<.0001). When compared with docetaxel, there were fewer grade 3 to 5 treatment-related adverse events at either dose of pembrolizumab (pembrolizumab at 2 mg/kg: 13% [43/339] of patients; pembrolizumab at 10 mg/kg: 16% [55/343]; and docetaxel: 35% [109/309]). As was seen with nivolumab, the group of patients with sensitizing EGFR mutations did not see the same degree of benefit with nivolumab as with docetaxel (HR, 0.88; CI, 0.45–1.7), suggesting that such patients may benefit more from docetaxel.
Although the single-arm KEYNOTE-001 trial supported using 10 mg/kg of pembrolizumab, the new randomized data also support using 2 mg/kg of intravenous pembrolizumab for subsequent therapy in patients. Whether these new data alter the preferred pembrolizumab-associated PD-L1 assay cut-point for defining positivity can be debated. Defining positivity using the higher (50%) proportion score by the proprietary PD-L1 assay (representing 28% of NSCLC within the KEYNOTE-010 study) was associated with a higher response rate with pembrolizumab than using the lower (>1%) proportion score (representing 66% of NSCLC within the KEYNOTE-010 study) at 30% versus 18%; the PFS HR was only significantly different from docetaxel in the higher enriched group (PFS HR, 0.88 vs 0.59). However, although the HR for overall survival trended toward a greater effect with more enrichment, the overall survival HRs were significantly different compared with docetaxel for both groups (overall survival HR, 0.71 [2 mg/kg ≥1% proportion score] and 0.61 [10 mg/kg ≥1% proportion score] vs 0.53 [pooled ≥50% proportion score]). The lower proportion score (≥1%) is now recommended in this setting based on these randomized data.
Phase III clinical trials are in progress assessing nivolumab, pembrolizumab, and atezolizumab as first-line monotherapy compared with standard platinum doublets (in all such studies, the patient populations are enriched for patients with PD-L1 expression). Numerous other clinical trials with these agents as monotherapy or in combination with other agents are also in progress.15
Summary
These NCCN Guidelines Insights focus on recent updates in the 2016 NCCN Guidelines for NSCLC (Versions 1–4), specifically the new immunotherapeutic agents, nivolumab and pembrolizumab, for patients with metastatic NSCLC. For the 2016 update (Version 1), the NCCN panel recommends immune checkpoint inhibitors as preferred agents for second-line therapy and beyond, which is termed subsequent therapy, for patients with metastatic nonsquamous or squamous NSCLC based on improved overall survival rates, higher response rates, longer duration of response, and fewer adverse events when compared with docetaxel. The NCCN panel revised the recommendation for nivolumab to category 1 preferred (from category 2A) based on the published data from a phase III randomized trial (CheckMate 057) for patients with metastatic nonsquamous NSCLC.9 The NCCN panel recommends (category 1) nivolumab as a preferred subsequent therapy for patients with metastatic squamous cell NSCLC whose disease has progressed on or after first-line chemotherapy based on data from a phase III randomized trial (Check-Mate 017).6 The NCCN panel also recommends pembrolizumab (category 1) as preferred subsequent therapy for patients with metastatic nonsquamous or squamous NSCLC and PD-L1 expression (using the ≥1% proportion score based assay conducted in these studies).8,10 For the 2016 NCCN Guidelines update (Version 4), the NCCN panel revised the recommendation for pembrolizumab to category 1 (from category 2A) based on the recent phase II/III trial (KEYNOTE-010).10 Immune-related adverse events may occur with nivolumab or pembrolizumab.19,23 For patients with immune-related adverse events, high-dose corticosteroids should be administered based on the severity of the reaction. Nivolumab or pembrolizumab should also be discontinued for patients with severe or life-threatening pneumonitis or colitis and should be withheld or discontinued for other severe or life-threatening immune-mediated adverse events when indicated (see prescribing information).
There are no data comparing the efficacy of pembrolizumab and nivolumab in patients with NSCLC, making it impossible to determine which drug is more efficacious. As data emerge about clinical factors or diagnostic tests that predict greater or lesser efficacy, it is likely that recommendations for use of nivolumab or pembrolizumab may become more refined. For instance, the efficacy data for patients with sensitizing EGFR mutation–positive NSCLC treated with pembrolizumab and nivolumab suggest that such patients are not the most likely group of patients to benefit from these immune checkpoint inhibitors.
Disclosure of Relevant Financial Relationships.
Editor:
Kerrin M. Green, MA, Assistant Managing Editor, JNCCN—Journal of the National Comprehensive Cancer Network Ms. Green has disclosed that she has no relevant financial relationships.
CE Planners:
Deborah J. Moonan, RN, BSN, Director, Continuing Education
Ms. Moonan has disclosed that she has no relevant financial relationships.
Ann Gianola, MA, Senior Manager, Continuing Education Accreditation and Program Operations
Ms. Gianola has disclosed that she has no relevant financial relationships.
Kristina M. Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations
Ms. Gregory has disclosed that she has no relevant financial relationships.
Rashmi Kumar, PhD, Senior Manager, Clinical Content, NCCN
Dr. Kumar has disclosed that she has no relevant financial relationships.
Individuals Who Provided Content Development and/or Authorship Assistance:
David S. Ettinger, MD, Panel Chair, has disclosed that he is a scientific advisor for ARIAD Pharmaceuticals, Inc.; Boehringer Ingelheim GmbH; Eli Lilly and Company; Genentech, Inc.; Helsinn Pharmaceutical; and EMD Serono. He receives consulting fees/honoraria from Bristol-Myers Squibb Company, and receives grant/research support from Golden Biotechnology Corporation.
Douglas E. Wood, MD, Panel Vice Chair, has disclosed that he receives grant/research support from is and is a scientific advisor for Spiration, Inc.
Hossein Borghaei, DO, MS, Panel Member, has disclosed that he is a scientific advisor for Bristol-Myers Squibb Company, Eli Lilly and Company, Boehringer Ingelheim GmbH, Pfizer Inc., Genentech, Inc., and Clovis Oncology; is a consultant for Bristol-Myers Squibb Company and Eli Lilly and Company; and receives research support from Millennium Pharmaceuticals, Inc.
David Ross Camidge, MD, PhD, Panel Member, has disclosed that he receives consulting fees from G1 Therapeutics, Inc.; ORION Clinical Services; ARIAD Pharmaceuticals, Inc.; Array BioPharma Inc.; Eli Lilly and Company; Novartis AG; Celgene Corporation; AbbVie Inc.; and Clovis Oncology.
Leora Horn, MD, MSc, Panel Member, has disclosed that she receives consulting fees/honoraria from Genentech, Inc. and Merck & Co., Inc.; receives research support from AstraZeneca Pharmaceuticals LP; is a scientific advisor for Xcovery, Bayer HealthCare, Bristol-Myers Squibb Company, and Boehringer Ingelheim GmbH; and is on a product/speakers’ bureau for Biodesix, Inc.
Karen Reckamp, MD, MS, Panel Member, has disclosed that she receives consulting fees/honoraria from Amgen Inc,. Astellas US LLC, Boehringer Ingelheim GmbH, Nektar Therapeutics, and ARIAD Pharmaceuticals, Inc.; she receives grant/research support from Boehringer Ingelheim GmbH, Bristol-Myers Squibb Company, Xcovery, Pfizer Inc., Eisai Inc., Adaptimmune LLC, Novartis Pharmaceuticals Corporation, ARIAD Pharmaceuticals, Inc., and Clovis Oncology.
Gregory J. Riely, MD, PhD, Panel Member, has disclosed that he receives consulting fees/honoraria from Genentech, Inc.
Kristina Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Miranda Hughes, PhD, Oncology Scientist/Senior Medical Writer, NCCN, has disclosed that she has no relevant financial relationships.
NCCN Categories of Evidence and Consensus.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.

Acknowledgments
This activity is supported by educational grants from AstraZeneca, Bayer Healthcare Pharmaceuticals Inc., Bristol-Myers Squibb, Clovis Oncology, Foundation Medicine, Genentech, Novartis Oncology, Otsuka America Pharmaceutical, Inc., Seattle Genetics, Inc., and Takeda Oncology; support provided by Actelion Pharmaceuticals US, Inc.; and by an independent educational grant from Astellas and Medivation, Inc.
Footnotes
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines® Insights highlight important changes to the NCCN Guidelines® recommendations from previous versions. Colored markings in the algorithm show changes and the discussion aims to further the understanding of these changes by summarizing salient portions of the NCCN Guideline Panel discussion, including the literature reviewed.
These NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding the content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their applications or use in any way.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. Bethesda, MD: National Cancer Institute; 2015. Available at: http://seer.cancer.gov/csr/1975_2012/. Accessed February 8, 2016. [Google Scholar]
- 3.SEER Cancer Statistics Factsheets: Lung and Bronchus Cancer. Bethesda, MD: National Cancer Institute. Available at: http://seer.cancer.gov/statfacts/html/lungb.html. Accessed February 8, 2016. [Google Scholar]
- 4.Ettinger DS, Cox JD, Ginsberg RJ, et al. NCCN Non-Small-Cell Lung Cancer Practice Guidelines. The National Comprehensive Cancer Network. Oncology (Williston Park) 1996;10:81–111. [PubMed] [Google Scholar]
- 5.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial [published online ahead of print December 18, 2015]. Lancet, doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol 2015;11:1307–1326. [DOI] [PubMed] [Google Scholar]
- 12.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribas A Releasing the brakes on cancer immunotherapy. N Engl J Med 2015;373:1490–1492. [DOI] [PubMed] [Google Scholar]
- 14.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustamante Alvarez JG, Gonzalez-Cao M, Karachaliou N, et al. Advances in immunotherapy for treatment of lung cancer. Cancer Biol Med 2015;12:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vansteenkiste J, Fehrenbacher L, Spira AI, et al. Atezolizumab monotherapy vs docetaxel in 2L/3L non-small cell lung cancer: primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR) [abstract]. Available at: http://www.europeancancercongress.org/Scientific-Programme/Abstract-search?abstractid=23093. Accessed February 8, 2016.
- 17.Besse B, Johnson ML, Janne PA, et al. Phase II trial (BIRCH) of atezolizumab as first-line or subsequent therapy for advanced PD-L1–selected non-small cell lung cancer (NSCLC) [abstract]. Available at: http://www.europeancancercongress.org/Scientific-Programme/Abstract-search?abstractid=23106. Accessed February 8, 2016.
- 18.Scarpace SL. Metastatic squamous cell non-small-cell lung cancer (NSCLC): disrupting the drug treatment paradigm with immunotherapies. Drugs Context 2015;4:212289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sgambato A, Casaluce F, Sacco PC, et al. Anti PD-1 and PDL-1 immunotherapy in the treatment of advanced non-small cell lung cancer (NSCLC): a review on toxicity profile and its management [published online ahead of print September 27, 2015]. Curr Drug Saf, in press. [DOI] [PubMed] [Google Scholar]
- 20.Chapman PB, D’Angelo SP, Wolchok JD. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N Engl J Med 2015;372:2073–2074. [DOI] [PubMed] [Google Scholar]
- 21.Kyi C, Hellmann MD, Wolchok JD, et al. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer 2014;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015;35:76–83. [DOI] [PubMed] [Google Scholar]
- 23.Khoja L, Butler MO, Kang SP, et al. Pembrolizumab. J Immunother Cancer 2015;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]


