Abstract
Staphylococcus aureus Newman with an insertion mutation in clfB, the gene encoding clumping factor B, only marginally decreased infection rate (P > 0.05) in rats with experimental endocarditis. In contrast, clfB complementation on a multicopy plasmid significantly increased infectivity (P < 0.05) over the deleted mutants. Although clfB could affect endovascular infection, its importance in experimental endocarditis was limited.
Staphylococcus aureus is a major cause of endovascular infections, including both native valve and prosthetic valve endocarditis (13). One reason for this association is its peculiar tropism for damaged endothelia. This is thought to occur via ligand-adhesin interactions between host proteins recovering endovascular injuries and prosthetic materials and staphylococcal surface determinants (7, 8, 10–12, 16, 22, 24).
Fibrinogen/fibrin is the most abundant host protein in endothelial lesions (6). It is a 340-kDa hexamer composed of 2α-, 2β-, and 2γ-chains (18). Staphylococci express several fibrinogen-binding proteins on their surface (1, 3, 19, 20). Among those, clumping factor A (ClfA) is responsible for typical S. aureus clumping in plasma and was shown to promote both adherence to fibrinogen-coated surfaces in vitro (4, 14, 24) and endocarditis in experimental animal models in vivo (16).
Recently, a new clumping factor, ClfB, was described (17). ClfA and ClfB have a similar molecular organization and a great deal of sequence similarity. However, their fibrinogen-binding domain (the A domain) is only 26% identical (17) and interacts with different parts of fibrinogen, i.e., the γ-chain for ClfA and both the α- and β-chains for ClfB (15, 17). Moreover, the clfA and clfB genes are differentially regulated, clfA being expressed throughout bacterial growth, whereas clfB is expressed only during the early logarithmic phase (14, 17). This raises the question of whether ClfA and ClfB might act in synergy to help cells attach more firmly to fibrinogen-coated surfaces during the bacterial growth cycle.
In the present experiments, this hypothesis was tested in rats with catheter-induced aortic vegetations. Specifically, we determined the ability of isogenic clfA and clfB single and double mutants, as well as of their clfB-complemented derivatives, to induce valve infection using a previously described experimental design (16). The bacterial strains used and their clumping phenotypes are listed in Table 1. Microorganisms were grown on blood agar plates or in tryptic soy broth (Difco Laboratories, Detroit, Mich.) with aeration at 37°C. For both clumping determination and inoculum preparation, overnight broth cultures of bacteria were diluted 1:100 in fresh medium and grown to the early logarithmic phase, at an optical density at 620 nm of 0.2, corresponding to ca. 108 CFU/ml. Mutant strains were grown on antibiotic-supplemented medium containing either 2 μg of erythromycin per ml (for strain DU5852), 2 μg of tetracycline per ml (for strains DU5943 and DU5944), or 5 μg of chloramphenicol per ml (for the clfB-complemented strains DU5943-pCU1 and DU5944-pCU1). The presence of antibiotics did not alter the clumping phenotype in vitro. After inoculation, the rats did not receive antibiotics. Catheter-induced aortic vegetations were produced in rats as previously described (9). Groups of animals were inoculated 24 h after catheterization by intravenous injection of 0.5 ml of saline containing increasing numbers of organisms. Bacterial inocula were prepared from cultures in the early logarithmic growth phase. Bacterial aggregation was negligible, as determined by phase-contrast microscopy. Animals were sacrificed 12 h after bacterial challenge, and quantitative vegetation, blood, and spleen cultures were performed (16). Statistical differences comparing the rates of valve infections were evaluated by the χ2 test with the Yates correction. Differences between median bacterial densities in infected tissues were analyzed by the Kruskal-Wallis one-way analysis of variance on ranks with Dunn's method for multiple comparisons.
TABLE 1.
Bacterial strains used in this study
| S. aureus strain | Clf phenotype | Clumping titera | Reference |
|---|---|---|---|
| Newman | ClfA+ ClfB+ | <1 | 14 |
| DU5852 | ClfA− ClfB+ | 8 | 14 |
| DU5943 | ClfA+ ClfB− | 8 | 17 |
| DU5944 | ClfA− ClfB− | 250 | 17 |
| DU5943(pCU1-clfB+) | ClfA+ ClfB+ (complemented) | <1 | 17 |
| DU5944(pCU1-clfB+) | ClfA− ClfB+ (complemented) | 4 | 17 |
Clumping titer (in micrograms per milliliter) was determined in early exponential phase (optical density at 620 nm of 0.2) as a function of the fibrinogen concentration in microtiter plates as described previously (17).
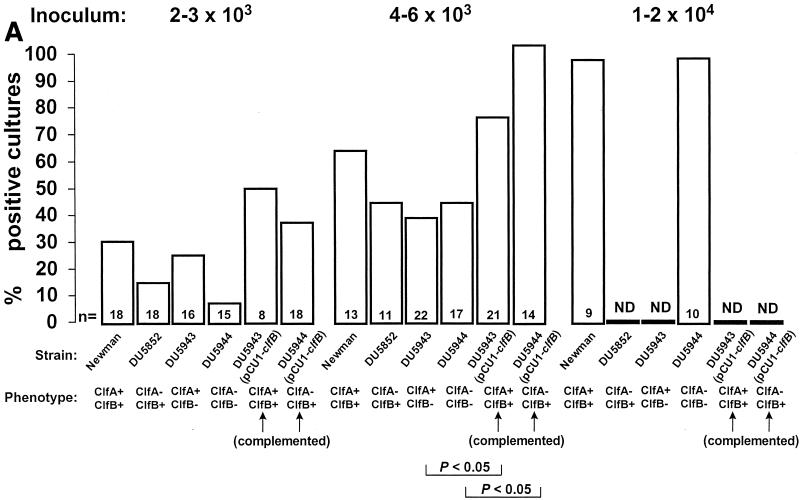
Figure 1 depicts the titration of infectivity with the various organisms. As in previous experiments, the rate of infection was inoculum dependent (16). At inocula producing endocarditis in 30 to 60% of rats with the parent strain (left and middle panels), both ClfA- and ClfB-defective mutants tended to produce less endocarditis than the parent organism, but this was not significant (P > 0.05). Moreover, the ClfA/ClfB-negative double mutant was not less infective than either single mutant alone. In contrast, complementation of the defective mutants with the wild-type clfB gene on a multicopy plasmid doubled the rate of endocarditis compared to that with the defective bacteria. This was already a trend at the lowest inoculum (left panel), but became statistically significant (P < 0.05) in rats challenged with larger inocula (middle panel). Finally, when greater bacterial numbers were injected, all the valves became infected whether the parent or the double mutant was used.
FIG. 1.
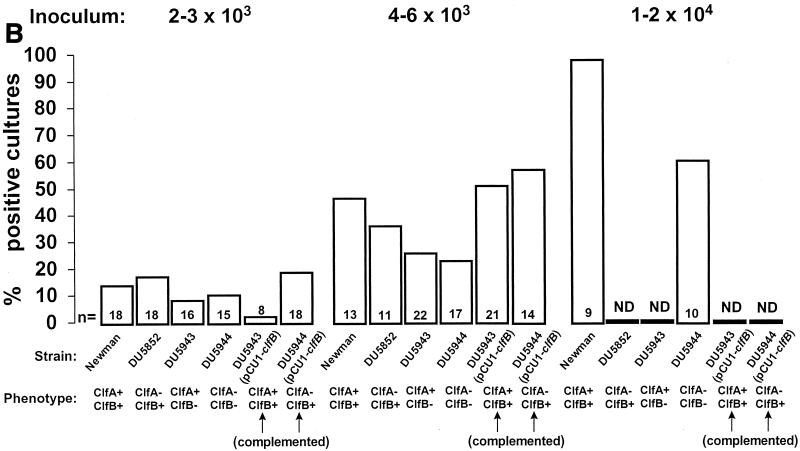
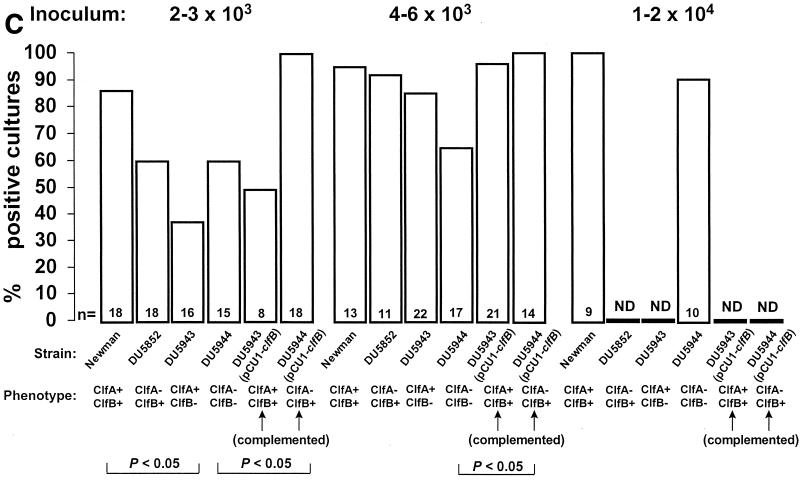
Infectivity titration of the various test organisms described in Table 1 in the rat model of experimental endocarditis. The figure shows a compilation of seven separate experiments. The rats were challenged with bacterial inocula of gradually increasing sizes, producing rates of endocarditis of ca. 30 and 60% with parent strain S. aureus Newman as well as with an inoculum four times larger than the 60% infectious dose. The columns indicate the percentage of positive vegetations (A), blood cultures (B), and spleen cultures (C). The number of animals per group (n) and the ClfA and ClfB phenotypes are indicated at the bottom of the columns. ND, not determined. Statistical differences were evaluated by the χ2 test with the Yates correction.
Figure 1 also presents the pattern of positive blood and spleen cultures. Blood cultures were positive only in rats with infected vegetations. Spleen cultures were more often positive, especially at higher inocula. This was indicative that the animals had been appropriately inoculated and that spleen colonization was not dependent on valve infection. This is a common observation in the early-induction phase of experimental endocarditis. After intravenous inoculation of small animals with large inocula (104 CFU), circulating bacteria rapidly (within minutes) concentrate in the spleen, where they are slowly eradicated over the next 12 to 24 h (16; unpublished observation). Thus, spleen colonization at 12 h is indicative of inoculation, whereas vegetation infection is indicative of endocarditis. One supplementary question was whether the difference in infectivity between the various mutants might be related to their intrinsic ability to grow in the vegetation milieu. Table 2 indicates that this was an unlikely explanation. Indeed, all the rats that developed endocarditis had similar vegetation bacterial densities at the time of sacrifice, irrespective of the infecting strain. Thus, all the test organisms had grown at similar rates after valve colonization. Similar observations were also made in the spleen, indicating that growth of the mutants was unaffected in this organ as well (Table 2). Importantly, the infections were not due to revertants, because all the bacteria recovered from organs had retained their mutation-related antibiotic resistance markers.
TABLE 2.
Bacterial densities in vegetations and spleens of rats that developed experimental endocarditis after challenge with inoculum sizes equal to the 60% infections dose of the parent strain Newman
| S. aureus strain | Clf phenotype | Median (25%–75%) bacterial density in infected tissues (CFU/g)
|
|
|---|---|---|---|
| Vegetations | Spleens | ||
| Newman | ClfA+ ClfB+ | 7.44 (5.34–8.10) | 3.51 (2.91–4.02) |
| DU5852 | ClfA− ClfB+ | 6.93 (6.31–7.90) | 2.80 (2.63–4.63) |
| DU5943 | ClfA+ ClfB− | 7.90 (7.37–8.21) | 3.66 (2.61–4.62) |
| DU5944 | ClfA− ClfB− | 7.72 (7.40–7.96) | 3.25 (2.62–4.45) |
| DU5943(pCU1-clfB+) | ClfA+ ClfB+ (complemented) | 7.26 (5.09–7.86) | 2.96 (2.49–4.61) |
| DU5944(pCU1-clfB+) | ClfA− ClfB+ (complemented) | 6.58 (4.62–7.40) | 3.46 (3.12–4.61) |
Both ClfA and ClfB are surface adhesins mediating attachment of S. aureus to fibrinogen/fibrin (14, 17). ClfA has already been shown to play a role in the virulence of S. aureus in rats with experimental endocarditis (16). However, its role was detected within a limited window of inoculum sizes. The present study indicates that all the clumping factor-defective mutants tended to be less infective than the parent, confirming their generally lower ability to induce endocarditis. However, while the effect of ClfB was only a trend in defective mutants, it was clearly observed by the increased infectivity of the complemented derivatives. Such results were to be expected from the nature of the bacterial constructs. ClfB mutants were affected in a surface protein that was only transiently expressed during early bacterial growth (17). As a result, its contribution to infection might be difficult to highlight in the complex staphylococcal background. In the complemented mutant, on the other hand, ClfB is constitutively expressed on a multicopy plasmid, and thus more of the adhesin was expressed (17). In this case, production of ClfB significantly increased infectivity over that of the defective mutants, and the mutant even tended to be more infective than the parent.
Thus, while ClfB increased infectivity in the overexpressing mutants, the problem resides now in understanding the articulation of the native determinant with the rest of the S. aureus pathogenic armamentarium. Indeed, this bacterium produces several additional fibrinogen-binding proteins, including coagulase (1), fibrinogen-binding protein (FbpA) (3), extracellular fibrinogen-binding protein (Efb) (19), and extracellular adherence protein (Eap) (21). Among these, at least Efb has been involved in the pathogenesis of experimental wound infection (19). Moreover, these determinants may be differentially regulated both at the gene expression level, by global regulators such as agr (accessory gene regulator) and sar (staphylococcal accessory regulator) (2), and at the functional level, for instance, by the local concentration of Ca2+ (17, 18). Finally, vascular lesions contain not only fibrinogen/fibrin, but also platelet and extracellular matrix proteins of the host, to which S. aureus possesses multiple additional adhesins, encompassed by the acronym MSCRAMM (microbial surface components recognizing adhesive matrix molecules) (22). In this intricate context, the intrinsic contribution of wild-type ClfB to induction of experimental endocarditis remains unclear, since ClfB-negative mutants were only slightly affected in infectivity.
In conclusion, the ability of S. aureus to bind fibrinogen contributed to endovascular infection. However, while both ClfA- and ClfB-defective mutants were impaired in fibrinogen binding, they could still colonize and infect damaged valves when large bacterial inocula were used. It is likely that many individual components of the staphylococcal surface take part in this process, and future efforts should aim at better understanding the synchronization and interplay of all these determinants. Site-specific mutagenesis and complementation is an essential step in this comprehension. However, complementary techniques will also be needed. These include the determination of gene expression in infected tissues (5) and the recently proposed adoptive pathogenesis, in which pathogenic genes are expressed in a surrogate bacterium to study their individual contribution to disease outside of the staphylococcal background (23; P. Stutzmann, I. Caldelari, P. Francioli, P. Vaudaux, T. J. Foster, D. McDevitt, and P. Moreillon, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-62, 1996).
Acknowledgments
This work was supported by grant 32-47099-96 from the Swiss National Funds for Scientific Research and by a Wellcome Trust project grant (number 052320).
We thank Marlyse Giddey and Jacques Vouillamoz for outstanding technical support.
REFERENCES
- 1.Boden M K, Flock J I. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb Pathog. 1992;12:289–298. doi: 10.1016/0882-4010(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 2.Cheung A I, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar-lagr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Investig. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung A I, Projan S J, Edelstein R E, Fischetti V A. Cloning, expression, and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect Immun. 1995;63:1914–1920. doi: 10.1128/iai.63.5.1914-1920.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung A I, Fischetti V A. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J Infect Dis. 1990;161:1177–1186. doi: 10.1093/infdis/161.6.1177. [DOI] [PubMed] [Google Scholar]
- 5.Cheung A L, Nast C C, Bayer A S. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect Immun. 1998;66:5988–5993. doi: 10.1128/iai.66.12.5988-5993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972;53:44–89. [PMC free article] [PubMed] [Google Scholar]
- 7.Flock J I, Hienz S A, Heimdahl A, Schennings T. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect Immun. 1996;64:1876–1878. doi: 10.1128/iai.64.5.1876-1878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.François P, Vaudaux P, Foster T J, Lew D P. Host-bacteria interactions in foreign body infections. Infect Control Hosp Epidemiol. 1996;17:514–520. doi: 10.1086/647358. [DOI] [PubMed] [Google Scholar]
- 9.Heraief E, Glauser M P, Freedman L. Natural history of aortic valve endocarditis in rats. Infect Immun. 1982;37:127–131. doi: 10.1128/iai.37.1.127-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann M, Vaudaux P E, Pittet D, Auckenthaler R, Lew P D, Schumacher Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 11.Hienz S A, Schennings T, Heimdahl A, Flock J I. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J Infect Dis. 1996;174:83–88. doi: 10.1093/infdis/174.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Kuypers J M, Proctor R A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 14.McDevitt D, François P, Vaudaux P, Fostr T. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 15.McDevitt D, Nanavaty T, House-Pompeo K, Bell E C, Turner N, McIntire L, Foster T, Höök M. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem. 1997;247:416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 16.Moreillon P, Entenza J M, Francioli P, McDewitt D, Foster T J, François P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni Eidhin D, Perkins S, François P, Vaudaux P, Höök M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell D P, Nanavaty T, McDewitt D, Gurusiddappa S, Höök M, Foster T J. The fibrinogen-binding MSCRAMM (clumping factor) of Staphylococcus aureus has a Ca2+-dependent inhibitory site. J Biol Chem. 1998;273:6821–6829. doi: 10.1074/jbc.273.12.6821. [DOI] [PubMed] [Google Scholar]
- 19.Palma M, Nozohoor S, Schennings T, Heimdahl A, Flock J I. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect Immun. 1996;64:5284–5289. doi: 10.1128/iai.64.12.5284-5289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma M, Wade D, Flock M, Flock J I. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J Biol Chem. 1998;273:13177–13181. doi: 10.1074/jbc.273.21.13177. [DOI] [PubMed] [Google Scholar]
- 21.Palma M, Haggar A, Flock J I. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J Bacteriol. 1999;181:2840–2845. doi: 10.1128/jb.181.9.2840-2845.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patti J M, McGavin M J, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 23.Que Y A, Haefliger J A, Francioli P, Moreillon P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect Immun. 2000;68:3516–3522. doi: 10.1128/iai.68.6.3516-3522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaudaux P, François P, Proctor R A, McDewitt D, Foster T J, Albrecht R M, Lew D P, Wabers H, Cooper S L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]