INTRODUCTION
Pancreatic cancer remains one of the most lethal diseases worldwide. Although surgical resection combined with multimodality therapy affords a significant survival advantage in some patients, the vast majority of patients present with locally advanced (LA) or metastatic disease for which palliation is the only option. For those patients who are candidates for resection, surgical options include a pancreaticoduodenectomy (PD), distal or left pancreatectomy (LP), or total pancreatectomy (TP), depending on the location of tumor within the pancreas.
Although surgical resection was initially associated with significant perioperative mortality, advances in surgical technique and perioperative care have reduced the mortality to the low single digits in high-volume centers.1,2 In addition, improvements in preoperative imaging modalities have enabled better determination of the extent of disease and have thus allowed for better operative planning and patient selection as well as better standardization of treatment regimens. Nevertheless, perioperative morbidity remains a significant problem and can often result in inadequate administration of appropriate adjuvant therapy. However, even in those patients who undergo successful surgical resection and appropriate adjuvant therapy, 5-year survival rates remain low, ranging from 5% to 15%.3
In this review, the current state of surgical management of resectable and borderline-resectable (BR) pancreatic cancer is discussed, focusing on both the technical aspects and the common postoperative complications and their management.
DETERMINING RESECTABILITY
Whether a pancreatic tumor is amenable to surgical intervention is defined by the probability of achieving microscopically negative margins (R0) at the time of resection. Numerous studies have demonstrated a strong correlation between R0 resections and decreased recurrence rates and improved overall survival (OS).4–12 Accordingly, margin status remains one of the most important predictors of long-term survival in pancreatic cancer.4,6,13 The status of the resection margin is often cited as a significant predictor of patient outcomes following PD, with the median survival of patients with microscopically positive (R1) resection significantly decreased as compared with those undergoing R0 resection.10,14,15 However, this is not without some controversy, as some studies do not indicate significant differences in survival between these 2 groups when controlled for other prognostic factors.10,14,15 Overall, in patients undergoing PD for resectable disease, there is wide variance in the reported R1 resection rates with those rates varying from 20 to more than 80%.10,14,15 These differences represent differences not only in patient selection and operative technique but also in the identification and analysis of the specimen by pathologists.
Although dependent on what type of resection being performed, margins during pancreatic resection typically include the following:
- Transection margins
- Pancreatic neck margin
- Common bile duct (CBD)/hepatic duct (CHD) margin
- Distal stomach/proximal duodenum
- Circumferential margins
- Anterior pancreatic margin
- Posterior pancreatic margin
- Superior mesenteric artery (SMA) margin
- Superior mesenteric vein (SMV) margin
- Radial CBD/CHD margin
Importantly, the transection margins can generally be extended if intraoperative analysis (usually by frozen section) suggests disease involvement. Circumferential margins are less clearly defined, and extending these margins is more difficult.
Unfortunately, there are no clear consensus guidelines addressing the pathologic analysis of PD specimens, including the number or types of margins, inking practices, or even diagnostic criteria for positivity. For example, in European studies, tumor found within 1 mm of the resection is defined as R1 resection, whereas in the United States, tumor must be present at the margin.10,14 Moreover, the inflammation, atrophy, and fibrosis associated with pancreatic cancer, particularly in the setting of prior pancreatitis or neoadjuvant chemoradiation therapy, make the analyses of margins more difficult. Careful multicolor inking of the specimen with extensive sampling and thin axial slices as well as adoption of the European definition of R1 resection has been show to lead to more accurate R1 margin rates.10,14,16,17 In fact, when careful pathologic analysis is performed, R1 resection rates tend to be higher and the discrepancy between the survival of patients undergoing R0 and R1 resections are more pronounced.10,14,15 Current College of American Pathologists guidelines are vague and recommend “inking the posterior surface of the pancreas and submission of sections through the tumor at its closest approach to this surface as well as the retroperitoneal (uncinate) margin.”18
In determining resectability, the main barrier to achieving R0 resection remains the relationship of the tumor to the mesenteric vasculature. The goal of preoperative staging and imaging, therefore, is not only to evaluate for metastatic disease but also to delineate the relationship of the relevant vasculature structures to the tumor, including the common hepatic artery (CHA), gastroduodenal artery (GDA), SMA, celiac axis, SMV, portal vein (PV), and the SMV-PV confluence. Preoperative staging and imaging enable the patient’s tumor to be appropriated into 1 of 4 categories: resectable, BR, LA, or metastatic. One of the major obstacles in determining resectability of pancreas cancer has been the lack of a unified definition of these categories. Several institutions and agencies have published definitions; however, significant variation persists.
Resectable Disease
In general, upfront resectable disease is defined as
Tumor without contact with the celiac artery (CA), hepatic artery (HA), SMA, or SMV, suggesting a high likelihood that margin-negative resection can be achieved without preoperative therapy (Fig. 1A)
Fig. 1.
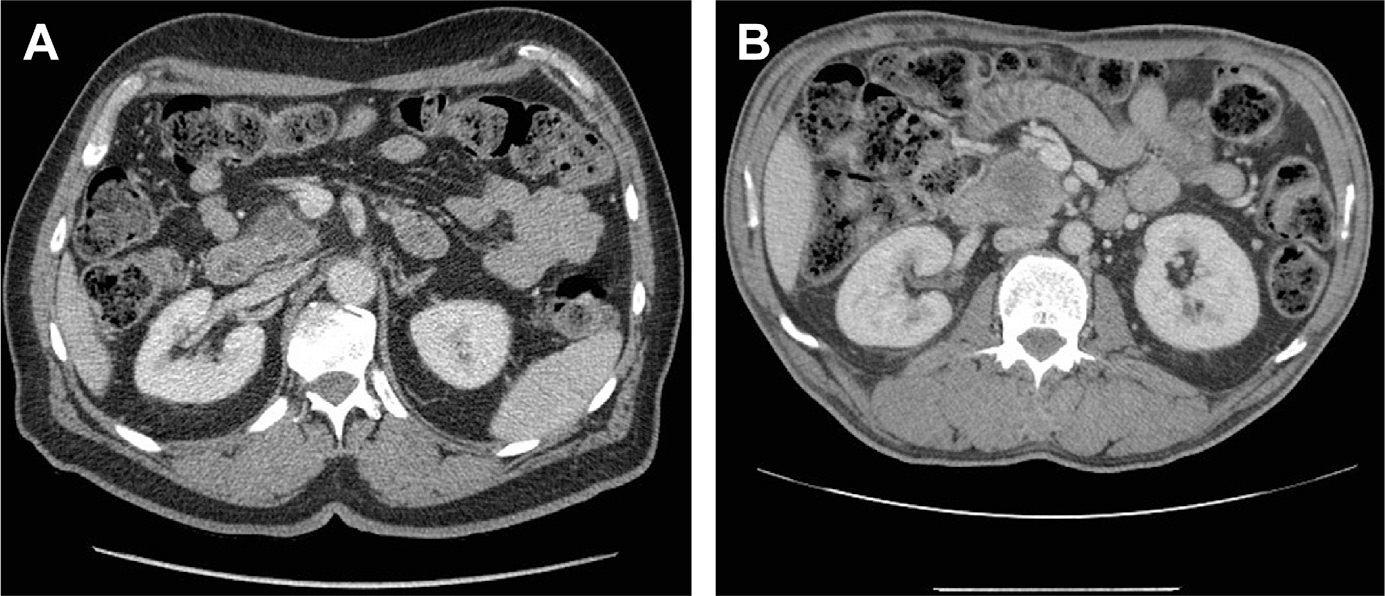
(A) Cross-sectional imaging of a resectable pancreatic adenocarcinoma with preservation of a fat plane between the tumor and the SMV and SMA. (B) Resectable pancreatic adenocarcinoma with less than 180° involvement of the SMV but with a preserved fat plane between the tumor and SMA.
With improvements in surgical technique and vascular resection, some groups also include tumors with limited involvement of the SMV-PV confluence in which an R0 resection is still possible, albeit with vascular resection and reconstruction in the resectable category (Fig. 1B). For example, the MD Anderson Cancer Center (MDACC) definition deems tumors resectable if there is abutment of the SMV-PV with patent vessels.19 The National Cancer Center Network (NCCN) also allows for 180°contact or less of the SMV-PV but only in the absence of any vein contour irregularity.20
Locally Advanced Disease
The definition of LA disease also varies, although in general, LA characterizes patients in whom the likelihood of attempting to resect the tumor even after treatment with systemic or locoregional therapy to allow a margin-negative resection is essentially minimal. Although several varying definitions exist, essentially tumors with extensive vascular involvement especially arterial involvement are deemed LA (Fig. 2, Table 1).
Fig. 2.
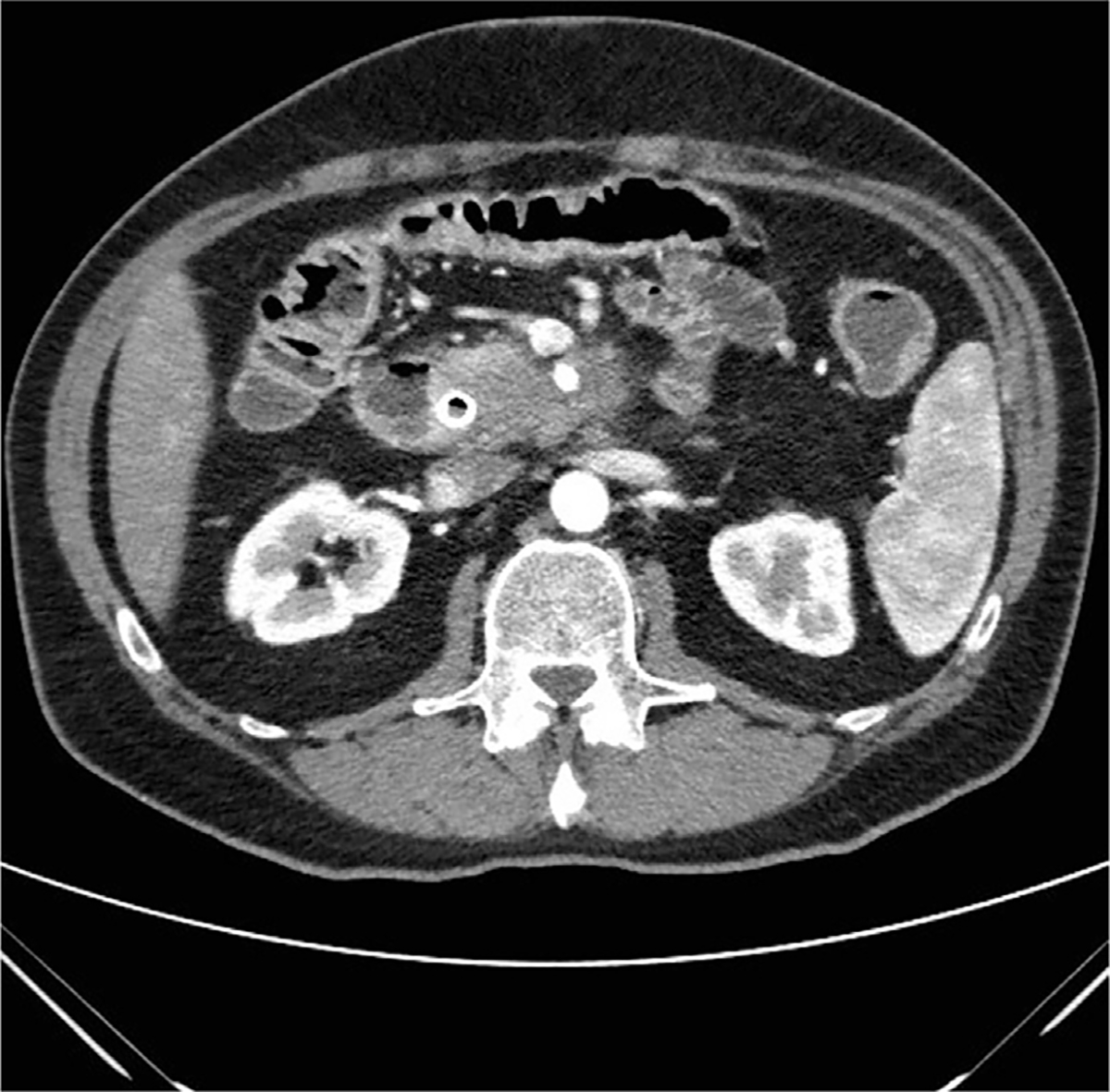
Cross-sectional imaging of an LA pancreatic cancer with greater than 180° involvement of the SMA.
Table 1.
Definitions of locally advanced pancreatic cancer
| Vessel Involved | MDACC | AHPBA/SSO/SSAT | NCCN/ISGPS |
|---|---|---|---|
| SMA | Encasement | Encasement | Contact >180° or contact with first jejunal SMA branch |
| CHA | Encasement—unable to reconstruct | Encasement with extension to CA | Contact with extension to CA or bifurcation |
| Celiac axis | Encasement | Abutment or encasement | Contact >180° |
| SMV-PV confluence | Occluded—unable to reconstruct | Occluded—unable to reconstruct | Unable to reconstruct |
Borderline Resectable Disease
The original term marginally resectable was coined to identify patients at high risk of a macroscopically positive (R2) resection with upfront surgery.21 The term BR pancreas cancer was subsequently adopted by the NCCN in 2006 to describe patients who might benefit from neoadjuvant therapy in order to reduce the likelihood of margin positivity.20 Unfortunately, what defines BR disease remains an area of significant controversy.
Over the past decade, several different classification schemes have been published to describe which patients are considered BR pancreatic cancer, including consensus statements and guidelines from the NCCN; the International Study Group of Pancreatic Surgery (ISGPS); MDACC; Americas Hepato-Pancreato-Biliary Association/Society of Surgical Oncology (AHPBA/SSO); the Society for Surgery of the Alimentary Tract (SSAT); and the Moffitt Cancer Center19,20,22 (Fig. 3, Table 2).
Fig. 3.
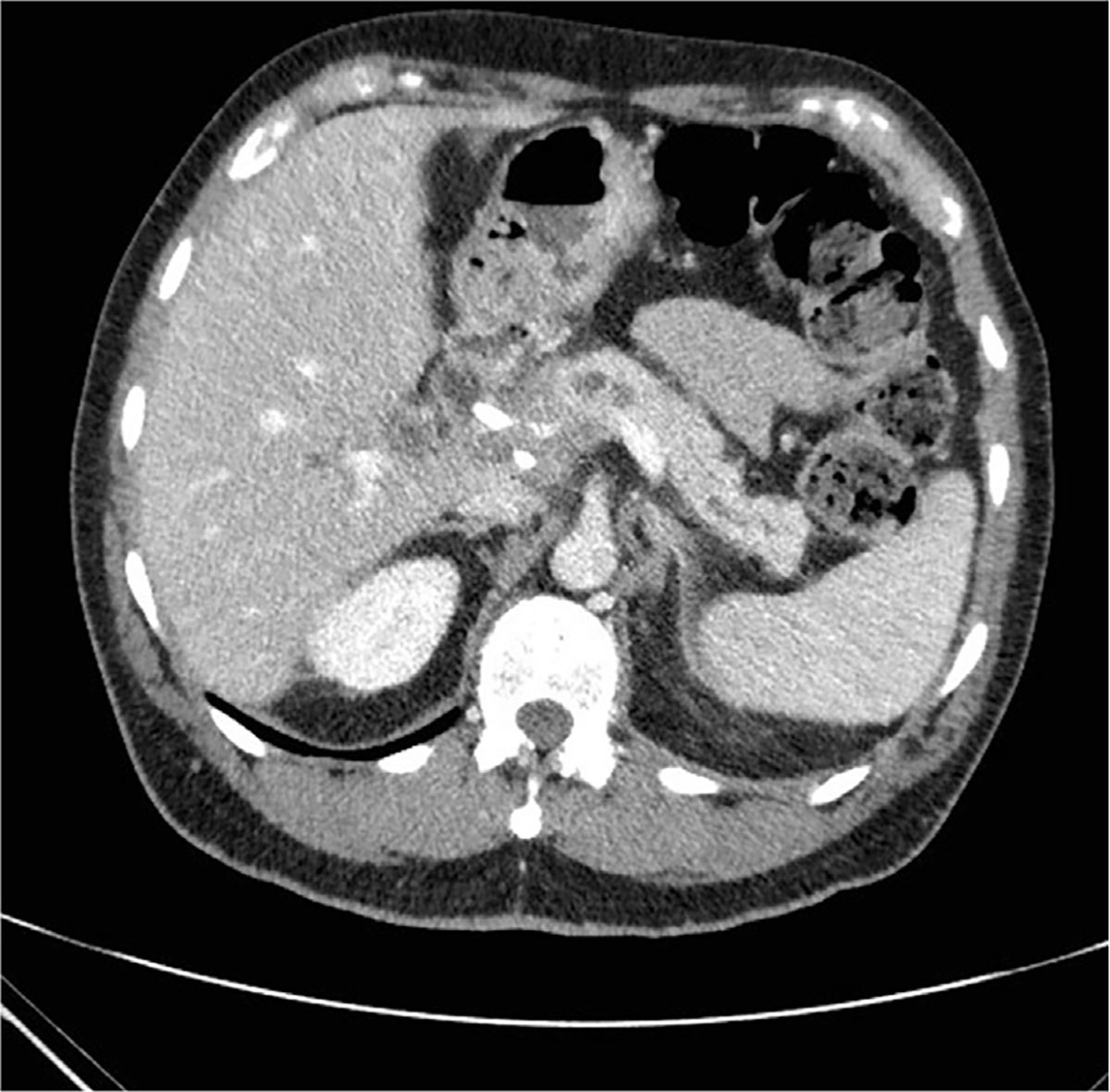
Cross-sectional imaging of a BR pancreatic adenocarcinoma with encasement of the SMV, but with preservation of a fat plane between the tumor and SMA.
Table 2.
Definitions of borderline resectable pancreatic cancer
| Vessel Involved | MDACC | AHPBA/SSO/SSAT | NCCN/ISGPS | Moffitt |
|---|---|---|---|---|
| SMA | Abutment | Abutment | Abutment | Abutment |
| CHA | Abutment or short segment encasement | Abutment or short segment encasement | Abutment without extension to celiac or HA bifurcation | Abutment or short segment encasement |
| Celiac axis | Abutment | No abutment or encasement | No contact | Not specified |
| SMV-PV confluence | Short-segment occlusion amenable to reconstruction | Abutment, encasement, or occlusion amenable to reconstruction | Abutment or encasement amenable to reconstruction | Abutment or encasement amenable to resection |
Despite what would seem to be rather objective criteria, all of these definitions are somewhat subjective and are continually evolving. In the recent Alliance Trial (A021101), a multi-institutional single-armed trial designed to evaluate toxicity and feasibility of neoadjuvant chemotherapy and chemoradiation for BR pancreatic cancer, for example, the investigators advocated for an easily reproducible definition based on more objective data obtained from computed tomographic (CT) imaging, avoiding subjective terms like abutment or encasement. The Alliance definition consisted of the following: (1) an interface between the tumor and SMV-PV 180° or greater of the vein wall circumference; (2) short-segment occlusion of the SMV-PV with normal vein above and below the obstruction amenable to resection and reconstruction; (3) short-segment interface of any degree between tumor and HA with normal artery proximal and distal to the interface amenable to arterial resection and reconstruction; and (4) interface between the SMA and CA measuring less than 180 of the circumference of the artery.23
DIAGNOSTIC WORKUP
Imaging Modalities
Imaging modalities including CT, MRI, and endoscopic ultrasound (EUS) are the mainstay in the diagnosis and staging of pancreatic cancer, and advances in these techniques have dramatically improved the preoperative determination of resectability for pancreatic cancer. Specifically, the development of multi-detector-row CT allows for high-resolution scan as well as 3-dimensional (3D) reconstructions; this, in addition to a “pancreas protocol CT,” which includes 3 phases (arterial phase, pancreatic parenchymal phase, and venous washout phase), has given physicians the ability to analyze the relationship of the pancreatic tumor to important vascular structures discussed above. CT is also effective in detecting lymphadenopathy and peritoneal and liver metastases. Enhanced MRI (with gadolinium or pancreatic tumor, excluding mangafodipir trisodium) is also effective in detecting local extension and vascular involvement. It is often superior to CT in detecting small liver lesions. Magnetic resonance cholangiopancreatography has the added benefit of noninvasively evaluating biliary and pancreatic ducts. Finally, EUS is highly sensitive for the detection of small tumors, surpassing both CT and MRI in this regard, as well as lymph node (LN) metastases and vascular invasion.24–26 In addition, EUS provides the safest avenue for pathologic confirmation of disease, which is crucial for those patients undergoing neoadjuvant therapy.26
The Role for Diagnostic Laparoscopy
Although CT and MRI are highly sensitive for detection of LA as well as distant metastatic disease, small liver metastases and peritoneal disease may be undetectable even with high-quality axial imaging. As a result, many surgeons advocate the use of diagnostic laparoscopy before attempted resection, especially among patients with findings suspicious for advanced disease on imaging, such as ascites, indeterminate liver lesions, or peritoneal or omental thickening. Staging laparoscopy is not as useful for determining resectability of LA disease because tumor involvement of the vasculature, specifically the SMA, which is not easily detected by laparoscopy.27
A Cochrane database review of the use of diagnostic laparoscopy demonstrated a decreased laparotomy and aborted resection rate from 40% with CT alone to 17% with CT combined with diagnostic laparoscopy.28 If metastatic disease is detected, staging laparoscopy not only decreases hospital length of stay but also allows patients to initiate chemotherapy significantly earlier than those undergoing exploratory laparotomy.29–31
An adjunct to laparoscopy includes peritoneal washings. Notably, positive peritoneal cytology occurs in 7% to 30% of potentially resectable cases, and those patients have similar outcomes to those with metastatic tumor burden; thus, they are generally not considered candidates for radical resection.32,33 By NCCN standards, positive washings are considered stage IV disease.32,34 The use of peritoneal washings for patients with pancreatic cancer, however, is not universally used.
VASCULAR RESECTION DURING PANCREATICODUODENECTOMY
Although historically abandoned due to poor outcomes, vascular resection, particularly venous resection, has now gained widespread acceptance in the resection of pancreatic cancer in selected patients. There is near universal agreement, however, that aggressive vascular resections for patients with BR disease should only be performed in patients with favorable tumor biology who have received neoadjuvant therapy before resection and by surgeons experienced in these procedures.
Ideally, the need for vascular resection is determined preoperatively, although this is not always possible. Signs of vascular involvement on cross-sectional imaging include proximity of tumor, loss of the fat plane or interface between a given vessel and tumor, and narrowing or impingement of venous structures (see Fig. 1). Narrowing of vein is highly specific, but not sensitive, for tumor involvement. Arterial involvement is more easily defined by CT preoperatively given the nerve plexus and fat plane normally surrounding an artery35 (see Fig. 2).
However, more than 40% of tumors ultimately requiring vascular resection and reconstruction did not clearly demonstrate findings suggestive of vascular resection on preoperative imaging,36–39 again underscoring the importance of centralization of pancreatic cancer care to high-volume centers in which surgeons are experienced and facile with vascular reconstruction or to medium volume centers at which a vascular surgeon is available to assist with resection and reconstruction.40,41 Importantly, one study indicated that the cross-sectional imaging before neoadjuvant therapy was more predictive of vascular involvement than imaging performed after completion of preoperative therapy. The investigators found initial imaging to be 98% predictive of vascular involvement compared with only 25% after neoadjuvant therapy.42
Venous Resections
Resection of the SMV, PV, or SMV-PV confluence is not uncommon at high-volume centers. Reports indicate that an average of 30% (7%–80%) of PDs performed at major medical centers now include venous resection and reconstruction.36,43–45 Perioperative morbidity and mortality associated with venous resection and reconstruction are similar to PDs performed without any venous construction, with short-term mortality ranging from 4% to 12% and complications ranging from 17% to 100%.46–48 Venous resection and reconstruction can vary in extent, including the following:
Partial lateral venorrhaphy with transverse closure
Resection with vein patch
Interposition graft with either autologous or prosthetic vein graft
Patch reconstruction with autologous vein is standard practice; however, both cadaveric allografts and xenografts (porcine peritoneum, bovine pericardium) have also been used.49 The autologous vein of choice for interposition grafts is the internal jugular vein or greater saphenous vein, but other conduits, including the superficial femoral, left renal, and inferior mesenteric veins (IMVs), have also been used.50,51 The risk of prosthetic graft in a potentially contaminated operative field with risk of pancreatic fistula is significant; thus, prosthetic grafts are not recommended but are sometimes required if suitable autologous vein is not available.49,52 Notably, up to 5- to 7-cm gaps can be primarily anastomosed with division of splenic vein (SV), mobilization of the right colon, hepatic flexure, and root of the mesentery and full mobilization of the liver, often obviating interposition graft.52–54
Splenic Vein Ligation and Sinistral Hypertension
As noted, division of the SV to allow for additional mobilization enabling an end-to-end anastomosis and eliminating need for interposition graft is commonly practiced. In addition, when tumor involvement includes the area of confluence of SV and SMV, the SV is often ligated and divided. However, division of the SV has the theoretic risk of sinistral or left-sided portal hypertension. Some studies have demonstrated increased frequency of sinistral hypertension as defined by an increase in spleen volume or decrease in platelet count. In addition, there are case reports of hypertensive gastropathy with gastric varices and gastrointestinal (GI) bleed following SV ligation during PD.55,56 Any theoretic risk of SV thrombosis and sinistral hypertension can be avoided by preservation of not only the short gastric vessels but also the SV-IMV confluence, both of which would allow for decompression of the SV to the systemic circulation. In addition, reanastomosis in an end-to-end fashion of SV to IMV, use of an interposition graft, or even a distal splenorenal shunt, mesocaval shunt, or IMV to right gastroepiploic vein anastomosis may be performed.55
The incidence and clinical significance of this theoretic complication have been called into question by several studies. In one study, only a very limited number of patients in whom the SV had been ligated actually developed clinically significant splenomegaly.57 The reasons for this are likely many. For one, survival following PD may not be long enough for this process to manifest itself. In addition, following ligation of the SV, venous blood can re-enter the systemic circulation via the esophageal veins or right colic tributaries.55 Finally, tumor involvement of the SV or confluence can cause relative stenosis, and collateral vessels may already be well developed at the time of PD.55
Arterial Resections
Although venous resection is not uncommon, consensus guidelines still consider extensive tumor involvement of major arteries (SMA, celiac, and common hepatic arteries) to be unresectable. In a recent meta-analysis, patients undergoing arterial reconstruction were associated with increased operative times, higher morbidity rate, including vascular and nonvascular complications, and a higher rate of reoperation, as well as an increased mortality rate.36 Patients who underwent combined venous and arterial resection had worse outcomes when compared with patients undergoing venous reconstruction alone. In addition, the 1-, 3-, and 5-year survival rates of PD with arterial resection were worse than PD alone and to PD with venous reconstruction.46 It should be noted, however, that this meta-analysis included a heterogeneous patient population over an extended time period (1977–2010), during which significant improvements in patient outcomes as well as advancements in neoadjuvant therapy regimens took place.35,46,58
At the current time, greater than 180° abutment, or encasement of the SMA, is considered a contraindication to resection. There has, however, been limited experience but acceptable outcomes achieved in young, otherwise healthy individuals who underwent extended resection for LA disease involving the CHA or celiac axes after completion of neoadjuvant chemotherapy at high-volume centers.58,59 In nearly all patients who require arterial resections, venous resections are also required, further increasing the complexity and potential morbidity of the resection.
Postoperative Anticoagulation
Although the risk of mesenteric or PV thrombosis may be increased in patients undergoing resection for malignancy, it appears to be particularly increased when vascular reconstruction is performed.60 It is important that the anastomoses or graft is not twisted or kinked in any way, and all venous anastomoses should allow for dilation and avoid a “waist” at the suture line once normal venous flow is re-established.52
A recent review article in 2014 found a rate of thrombosis of 25% with reconstruction.52 Thrombosis can range from partial occlusion of the SMV or a lesser tributary to the more feared complication of early or late PV thrombosis. The area and extent of resection and reconstruction almost certainly influence venous thrombosis rates.61 Rates of thrombosis with polytetrafluoroethylene grafts are consistently higher than interposition grafts using autologous vein; most studies report patency rates of approximately 90% for autologous vein conduit.49,52,62
Patients may be asymptomatic or, rarely, may develop severe peritonitis secondary to acute mesenteric ischemia. Early PV thrombosis is associated with a 30% to 40% mortality rate. Although early thrombosis is deadly, patients with delayed PV thrombosis following PD with vascular reconstruction have survival rates greater than those without thrombosis presumably because of interval development of collateral flow.63
Intraoperative systemic anticoagulation during reconstruction is not routinely used. Some surgeons advocate its routine use, whereas others advocate its use when the SMA is formally clamped for an extended period; there are no data to support or refute its use. Data supporting the use of postoperative anticoagulation for patients undergoing vascular reconstruction during PD likewise are sparse. Most protocols are based on surgeon preference. A recent review article in 2014 summarized the available data; however, given the wide variation in practice in the primary articles (heparin and warfarin vs aspirin), no firm conclusions could be drawn.52 A more guided prospective multi-institutional research trial would be necessary to define best practice standards with regards to anticoagulation for venous reconstruction.49 Current practice at the authors’ institution involves the use of low-dose aspirin postoperatively for all PDs requiring vascular reconstruction.
ABERRANT VASCULAR ANATOMY AND CELIAC STENOSIS
As many as 20% to 50% of patients exhibit aberrant vascular anatomy. The variants most critical for the surgeon during PD include an accessory or replaced right hepatic artery (RRHA) and replaced common hepatic artery (RCHA) because they typically course through an area predisposed to tumor involvement. Although these aberrancies may add to the complexity of the operation, it is not clear whether these variants affect resectability. Although traditionally these abnormalities may have been discovered in the operating room, modern multiphase imaging techniques nearly always identify these abnormalities preoperatively and allow for proper operative planning.
Replaced Right and Common Hepatic Arteries
The most common variant overall is an RRHA, which branches from the SMA, and is seen in 11% to 20% of individuals. It typically courses behind or through the pancreatic head, predisposing it to tumor involvement especially with posteriorly located malignancies.64 Fortunately, an RRHA can often be preserved with careful dissection even with tumor nearby. If division is required, the primary concern is preservation of blood flow to the CBD, which could lead to failure of the bilioenteric anastomosis or delayed anastomotic stenosis.64,65 Given this, some argue that it is important to attempt to reconstruct if the replaced RHA must be sacrificed (either primarily or using an autologous vein graft), although this may be technically difficult given its small diameter.66,67 A second anatomic variant of concern to the surgeon performing PD is the rarer RCHA, in which the CHA originates from the SMA. Fortunately, this variant occurs only in 0.4% to 4.5% of population.64 If the replaced CHA has an intrapancreatic course, reconstruction is usually necessary.64
Celiac Artery Stenosis
Celiac artery stenosis is most often secondary to intrinsic disease (plaque) or extrinsic forces secondary to either nodal disease burden, median arcuate ligament syndrome, or a tumor-associated fibroinflammatory process. Celiac stenosis is encountered in up to 5% of all pancreaticoduodenectomies.68,69 Most patients are asymptomatic due to the propensity for extensive collateralization; the CHA receives retrograde flow via the GDA or dorsal pancreatic artery coming off the SMA, or via the Arc of Buhler, which is a direct connection between SMA and CHA. Elimination of collateral flow by division of the GDA during PD in the setting of celiac stenosis has theoretic and observed implications for compromised flow to liver, stomach, and spleen.68,69
Preoperatively, CT imaging has low sensitivity for detecting clinically significant celiac stenosis but significant collateralization or an unusually large GDA raises suspicion.68 Given this low sensitivity, it should be routine practice during PD to assess flow within the HA by temporarily occluding flow from the GDA before division.70 Significant stenosis from atherosclerosis that is identified preoperatively can be managed with angioplasty and stent, but the long-term outcome is not clear.70,71 Intraoperatively, if blood flow in the proper HA becomes significantly reduced following ligation of the GDA, it is recommended that restoration of flow be performed. In cases with eccentric compression, division of the median arcuate ligament is successful in up to 70% of cases.72–74 If that is not successful or if reduced blood flow is due to other reasons, an autologous graft from the aorta to the proper HA is recommended.
LYMPHADENECTOMY IN PANCREATIC CANCER
A large proportion of pancreatectomy specimens contain positive nodes, ranging from 50% to 80%.75–77 Lymphatic spread is thought to begin with peripancreatic nodes either by direct invasion or via lymphatic channels, then spreading to SMA and CHA nodes, and finally, to para-aortic nodes.
LN involvement is a significant negative prognostic factor. The 5-year actuarial survival of patients with node positive disease is 5%, as compared with 10% survival among patients with node-negative disease.75–77 Local recurrence rates are also significantly higher in those with LN-positive disease. Survival is increased dramatically with N0 resection, and, within N0 resections, there is increased survival with increased nodal sampling. In addition, for N1 disease, the LN ratio (positive LN/resected LN) is critical, with ratios of 0 to 0.2 having more favorable outcomes than 0.2 to 0.4,76,78,79 underscoring the responsibility of both surgeon and pathologist to provide an adequate nodal assessment at the time of PD. At least 12 to 15 nodes are recommended for adequate analysis.75 Similar to pathologic analysis of specimen margins, however, the quality of LN evaluation within a given surgical specimen varies significantly.80
It is not known which groups of nodes offer the most prognostic impact, or whether nodal invasion of peripancreatic LNs, likely by direct invasion, has the same prognostic implication as true lymphatic dissemination into nodal groups further from the primary mass.81,82 In one study, peripancreatic nodal invasion only had survival rates similar to those without nodal invasion, whereas, in others, direct invasion had similar prognostic implications as true lymphatic spread.81,82 In general, however, patients with involvement of a single nodal group have better outcomes than involvement of multiple nodal groups.83
Extended Lymphadenectomy
Although the prognostic impact of overt nodal spread is rarely disputed, there was, for some time, significant debate as to whether aggressive nodal dissection at the time of PD to attain locoregional control of disease truly affected outcomes.84
The need for definition of a standard nodal dissection was addressed at the 2013 ISGPS consensus meeting. The standard lymphadenectomy for PD should include the following:
The peripancreatic tissue to the right of SMA and proper HA—specifically nodal stations 5, 6, 8a (proper HA), 12b, 12c, 13a, 13b (posterior surface), 14a (right lateral SMA), 14b (right lateral SMA), 17a, 17b (anterior surface) as defined by the Japanese Pancreas Society75
Anything beyond this is considered an extended lymphadenectomy.75
Several meta-analyses have revealed no improvement in survival with extended lymphadenectomy, as extraregional LN involvement should be considered metastatic disease. In addition, more extensive dissections are associated with higher rates of postoperative complications, including delayed gastric emptying (DGE), increased weight loss, and diarrhea thought to be secondary to autonomic disruption.85–87
PYLORUS-SPARING VERSUS STANDARD PANCREATICODUODENECTOMY
The pylorus-preserving PD (PPPD) was first introduced in 1944 by Watson,88 and interest was reignited in 1980 by Traverso and Longmire.89 This procedure differs from the standard PD by sparing the pylorus and the proximal 1 to 3 cm of duodenum in an effort to prevent the dumping, bile reflux gastritis, and marginal ulceration associated with the traditional standard PD reconstruction that includes an antrectomy. A 2014 meta-analysis showed no difference in disease outcomes, morbidity, mortality, or OS with pylorus-preserving as compared with standard PD.90,91 Furthermore, a randomized controlled trial showed a significant increase in DGE with PPPD as compared with standard PD.92 The decision as to the type of reconstruction is therefore largely based on surgeon preference.
THE ROLE FOR MINIMALLY INVASIVE SURGERY
PD requires intricate dissection deep within the retroperitoneum of tumor that is often adherent to major vessels. Once the specimen is resected, a series of complex anastomoses must be completed. The complexity inherent to this procedure resulted in the late acceptance of minimally invasive approaches as compared with other major abdominal operations. The minimally invasive approach to PD was first introduced by Gagner and Pomp93 in 1994 for a patient with chronic pancreatitis. Since that time, laparoscopic, laparoscopic hand-assisted, and laparoscopic robot-assisted pancreaticoduodenectomies have all been completed with success, even including vascular resection.94,95 Robot-assisted minimally invasive surgery has several distinct advantages over pure laparoscopic techniques because it gives the surgeon improved dexterity and better visualization given stereoscopic 3D optic capabilities.96 Laparoscopic PD is rarely performed outside of specialized institutions now, but laparoscopic robot-assisted PD remains a viable alternative to the conventional open approach.
Available data demonstrate that there is no difference in morbidity, mortality, or major complications of pancreatic fistula and DGE in minimally invasive as compared with open PD. The rate of conversion to an open procedure is approximately 10% to 15%, and there is a steep learning curve.96 In addition, there is associated increased cost, longer operating room (OR) time, and higher reoperation rates.96 Benefits may include decreased estimated blood loss (EBL), lower hospital length of stay, and a trend toward starting adjuvant therapy earlier.97 Little has been written about the overall cost-effectiveness of robot-assisted or laparoscopic-only PD, but one study proposes that the higher upfront cost of equipment, training, and personnel may be balanced by reduced hospital length of stay.98
Regarding oncologic outcomes, in theory, the use of high-resolution cameras might enable better visualization and finer dissection and may lead to higher R0 resection rates, especially in borderline tumors following neoadjuvant therapy. This theory has not yet been demonstrated in the literature.96 In addition, there is no significant improvement in LN dissection; the number of nodes obtained during minimally invasive procedures as compared with open PD is similar.96,99 It should be noted that the interpretation of any data regarding oncologic outcomes in minimally invasive PD is potentially biased, because those patients with low American Society of Anesthesiologists performance status, with large tumors, or at high risk for R1/R2 resection are generally not offered a minimally invasive procedure.100 In addition, no data on long-term oncologic outcomes are available. Prospective randomized controlled trials with patients with comparable disease states with long-term follow-up are needed before these minimally invasive techniques are universally recommended; however, because of the low numbers of truly surgically resectable patients, this is likely not feasible. (See Deepa Magge, Amer Zureikat, Melissa Hogg, et al: Minimally Invasive Approaches to Pancreatic Surgery, in this issue.)
COMPLICATIONS FOLLOWING PANCREATICODUODENECTOMY
Although mortality rates after PD remain low (1%–2%) in experienced centers, morbidity rates remain high (40%–50%), even among high-volume centers.3 Postoperative complications increase hospital length of stay and cost, worsen patient outcomes, and often delay adjuvant therapy plans. Specifically, postoperative complications significantly delay the initiation of adjuvant chemotherapy, decrease the percentage of patients undergoing adjuvant multimodality therapy, and decrease the intensity of the adjuvant therapy tolerated.101–103
Delayed Gastric Emptying
The most common complication following PD is DGE with prevalence ranging from 20% to 40%. Although DGE is a transient phenomenon generally resolving in a few days to weeks following the operation, the impact of DGE should not be underestimated. It greatly increases length of hospital stay and readmission rates following PD as well as patient satisfaction and quality of life.
The cause remains unclear, but is generally thought to be a result of the major perturbations in the normal anatomy and physiology of the upper GI tract inherent to PD. Relative gastric antropyloric ischemia, vagal dysfunction, motilin deficiency resulting from duodenal resection, and peripancreatic inflammation have all been implicated.67,104–106
The diagnosis of DGE is largely clinical. In 2007, the ISGPS compiled a classification system for DGE based on nasogastric tube output and time to regular diet107 (Table 3). Several risk factors for DGE have been identified and include the following108–114:
Pylorus-preserving PD
Retrocolic gastrojejunostomy
Diabetes
Presence of pancreatic fistula
Increased OR times
Increased body mass index
Other postoperative complications
Table 3.
International Study Group of Pancreatic Surgery classification scheme for delayed gastric emptying
| Characteristic | Grade A | Grade B | Grade C |
|---|---|---|---|
| Nasogastric tube required | 4–7 d or reinsertion after postoperative day (POD) 3 | 8–14 d or reinsertion after POD 7 | >14 d or reinsertion after POD 14 |
| Unable to tolerate food at: | POD 7 | POD 14 | POD 21 |
| Gastric distention/vomiting | Yes | Yes | Yes |
| Use of prokinetics | Yes | Yes | Yes |
The use of octreotide in the perioperative period was also found to be an independent risk factor for DGE.112 If these risk factors are identified preoperatively or intraoperatively, consideration should be given to enteral access; high-risk patients should undergo nasojejunal postanastomotic feeding tube or surgical jejunostomy placement at the time of the resection.
Treatment of DGE is largely based on dietary modification and the use of promotility agents, such as erythromycin, metoclopramide, and domperidone.105,115 Erythromycin is generally seen as safe, inexpensive, and more effective than either domperidone or metoclopramide but has some important side effects and drug interactions.116 Gastric stimulators are often used to treat idiopathic or diabetic gastroparesis but are of no significant utility in the treatment of DGE following PD. Symptoms are managed with antiemetics, generally in combination with promotility agents.
Pancreatic Fistula
Postoperative pancreatic fistula is also a common complication following PD, affecting 25% to 40% of patients and often leading to abscess formation, hemorrhage, or DGE. It is also the most common cause of mortality. Unfortunately, all attempts thus far to prevent pancreatic fistula have largely been unsuccessful.117 A classification scheme for pancreatic fistulas has been formulated by the ISGPS/International Study Group of Pancreatic Fistula based on severity and need for intervention (Table 4).
Table 4.
International Study Group of Pancreatic Surgery classification scheme for pancreatic fistula
| Characteristic | Grade A | Grade B | Grade C |
|---|---|---|---|
| Signs of systemic illness | No | Yes | Yes |
| Treatment required? | No | Yes | Yes |
| CT or ultrasound evidence | No | Yes | Yes |
| Persistent drainage (>3 wk) | No | Yes | Yes |
| Signs of infection | No | Yes | Yes |
| Readmission | No | Yes | Yes |
| Sepsis | No | No | Yes |
| Reoperation | No | No | Yes |
| Death | No | No | Yes |
Major risk factors include a “soft pancreas,” which generally indicates that the gland has preserved exocrine function, which would lead to more effluent from ducts. It is also technically more difficult to place sutures in a soft gland. Ducts smaller than 3 mm in diameter are also associated with higher leak rates. Not surprisingly, pancreatic fistula is more commonly seen following resection of ampullary, duodenal, or islet cell tumors in which pancreatic gland function is normal and ductal dilatation is absent, as compared with adenocarcinoma or chronic pancreatitis, which are associated with more atrophic, “harder” glands and chronically dilated ducts. Increased intraoperative blood loss has also been shown to augment the risk of developing a fistula.117
The pancreaticojejunostomy can be performed in an end-to-end or end-to-side manner with duct-to-mucosal anastomosis versus an invagination technique. In some studies, direct anastomosis of duct-to-mucosa as compared with invagination technique is associated with lower leak rate, but this has not been recapitulated.118 There are some scant data suggesting that pancreaticogastrostomy may have a more favorable leak rate than pancreaticojejunostomy, but this has not been confirmed in randomized controlled trials.119
The use of fibrin glue at the anastomosis was evaluated in both randomized controlled trials and in systematic reviews and was found to result in no significant change in postoperative pancreatic fistula.120 Thus, no intraoperative modifications have resulted in clearly demonstrable improvements in pancreatic fistula rates.
In theory, decreasing pancreatic secretion/effluent should decrease fistula severity and/or duration. Various somatostatin analogues have been trialed, including octreotide, which in European centers was effective in reducing fistula rates. A Cochrane Review in 2012 confirmed a decrease in overall pancreatic fistula rate with postoperative somatostatin therapy; however, there were no differences in rates of clinically significant fistulas, no change in reoperation rates, no increased rate of fistula closure, and most importantly, no change in mortality.121 Other somatostatin analogues with longer half-lives and broader and stronger binding profiles are in various stages of development and have shown promise.122 Specifically, pasireotide has recently been shown in randomized controlled trials to decrease clinically significant pancreatic fistula rates (ISGPS grade 2 or higher) by almost 50% for pancreatectomies, including those with dilated or normal pancreatic ducts.122 Although these data are very convincing, its generalized use, however, is still lacking—perhaps due to cost.
Hemorrhage
Postoperative hemorrhage is the third most common severe complication following PD. The most common sites of hemorrhage include the GDA stump, tributaries of the PV or SMV, branches of HA or SMA, the cut surface of the pancreas, suture lines, and the gallbladder fossa.123 The ISGPS has devised a classification scheme for postoperative hemorrhage based on time of onset in addition to location and severity of bleed (Table 5). Early-onset hemorrhage (postoperative days 1–5) is most likely secondary to technical failure or postoperative coagulopathy, whereas late-onset (after postoperative day 5) bleeds most often result from the erosion of peripancreatic vessels or pseudoaneurysm formation due to pancreatic leak or intra-abdominal abscess.123 Severity can be mild or severe with transfusion requirement or need for reoperation or an interventional radiologic procedure. Overall, treatment algorithms differ for early versus delayed postpancreatectomy hemorrhage. Severe early bleeding into the peritoneal cavity warrants relaparotomy for ligation of the bleeding vessel, whereas bleeding into the GI tract (likely from gastrojejunal or enteroenteroanastomosis) may be amenable to endoscopic intervention. Late-onset hemorrhage can be addressed with angiography with coiling/embolization/stenting if technically feasible provided that the patient is hemodynamically stable with appropriate resuscitation and lesion is visualized. If these conditions are not met, relaparotomy is again warranted.124,125
Table 5.
International Study Group of Pancreatic Surgery classification scheme for postoperative hemorrhage following pancreatectomy
| Characteristic | Grade A | Grade B | Grade C |
|---|---|---|---|
| Time of onset | Early | Early/late | Late |
| Site | Intraluminal or extraluminal | Intraluminal or extraluminal | Intraluminal or extraluminal |
| Clinical impact | Mild | Severe early/mild late | Severe |
| Therapeutic consequences | No | Yes | Yes |
PALLIATIVE OPTIONS
As mentioned earlier, only 20% of patients present with resectable disease; the remainder of patients unfortunately present with LA (40%) or metastatic disease (40%). Therapy for LA and metastatic disease focuses on palliative systemic therapy, with median survival of 9 to 11 months.126 Although these patients have no options for potential cure, there remains a possibility for palliative surgery for the treatment of symptoms caused by their disease, including obstructive jaundice, gastric outlet obstruction, and intractable pain. Patients who are candidates for minimally invasive options available for palliation may benefit from a shorter recovery period, even more important given their expected short life expectancy.38
In general, obstructive jaundice is best addressed with endoscopic stent placement. Expandable metal stents offer the best long-term patency and lowest complication rate. Surgical approaches for biliary obstruction not amenable to endoscopic therapy include biliary bypass procedures such as hepaticojejunostomies or choledochojejunostomies. Although a cholecystojejunostomy is less frequently used, it is an attractive option in patients with tumors at least 1 cm away from the cystic duct/hepatic duct junction because it can often be performed using minimally invasive techniques.127,128
In contrast to obstructive jaundice, endoscopic approaches to the treatment of gastric outlet obstruction have been largely unsuccessful. Duodenal stents are associated with significant risk of migration, perforation, or biliary obstruction and require significant changes in diet that impact quality of life.129 Although often used in patients with a life expectancy of only a few months, surgical gastrojejunostomy, either open or minimally invasive, is preferred in those expected to live more than 2 months.129,130 Studies have shown that gastrojejunostomy for palliation is best performed in a retrocolic position in an isoperistaltic configuration with the anastomosis below the mesocolon to prevent stricture.131 Prophylactic gastrojejunostomy performed in the setting of exploratory laparoscopy/laparotomy and discovery of unresectable disease with high risk of obstruction generally does not lead to increased morbidity and mortality.131 (See Christopher Wolfgang, Katherine E. Poruk: Palliative Management of Unresectable Pancreatic Cancer, in this issue, for further details of palliative management of pancreatic diseases.)
Pancreatic cancer, particularly LA disease, is particularly painful because of involvement of the celiac nerve plexus. In the treatment of intractable abdominal/back pain, all standard pharmacologic options should be exhausted before any attempts at additional intervention are made. Potential invasive therapies include ethanol injection or ablation of the celiac plexus. These procedures can be performed percutaneously, endoscopically, laparoscopically, or in an open fashion at the time of exploration. There are no significant benefits of neurolysis over pharmacologic agents, however, and any manipulation of the nerve plexus can result in significant morbidity, including debilitating diarrhea, significant orthostasis, and even spinal cord hematomas.132–134
Notably, there is no role for palliative resection of the primary mass. There has, however, been some interest recently in tumor ablation either through thermal damage (radiofrequency ablation, high-intensity focused ultrasound, cryoablation, and microwave ablation), or nonthermal techniques (irreversible electroporation, stereotactic body radiation therapy, photodynamic therapy, and 125I seeding).126 Nonthermal techniques have garnered significant attention as the “heat sink” associated with thermal techniques is especially problematic given nearby structures, such as the duodenum, biliary tree, and mesenteric vasculature.135 Importantly, all have been associated with some relief of pain.126,135 To date, however, there are no prospective data comparing ablative therapies with standard of care in terms of safety or even efficacy; most of the analyses thus far have been single-institution studies. Most available data, however, suggest that these strategies may be more efficacious in combination with other therapies (chemoradiation and others).135
SUMMARY
For pancreatic cancer, successful surgical resection offers the only chance for cure. It is possible in a small minority of patients, however, even with successful resection; recurrence rates remain high, and OS is less than 25%. Over the past several years, advances in imaging and diagnostic modalities have allowed better characterization and selection of patients that are potentially resectable as well as BR and LA in an attempt to standardize resectional, neoadjuvant, and palliative therapeutic strategies. Response to neoadjuvant therapy has resulted in a higher number of patients eligible for resection. In addition, a better understanding of the biology of disease has allowed us to better define the factors important for improved outcomes.
Although mortality rates in high-volume centers are low, morbidity rates after resection remain significant. A thorough understanding of the factors related to these complications is crucial to minimize these events and allow for the expeditious use of adjuvant therapy. It is hoped that further understanding of the biology of this lethal disease will lead to the development of novel targeted therapies and a subsequent increase in the number of patients eligible for resection as well as improved long-term survival.
KEY POINTS.
Despite successful surgical resection, recurrence rates remain high and overall survival isless than 20%.
Advances in cross-sectional imaging and diagnostic modalities such as endoscopic ultrasound have allowed better characterization and selection of patients that will benefit from upfront surgical resection versus neoadjuvant therapy to improve the probability of achieving microscopically negative margins (R0).
Margin-negative resection is possible in the setting of vascular involvement in borderline resectable and selected locally advanced patients after neoadjuvant therapy with vascular resection and/or reconstruction.
Although mortality rates in high-volume centers are low, morbidity rates after resection remain significant, and efforts to minimize these complications are important to allow for the expeditious use of adjuvant therapy.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998;228(3):429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999;125(3):250–6. [PubMed] [Google Scholar]
- 3.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB 2008;10(1):58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10(9):1199–210 [discussion: 1210–1]. [DOI] [PubMed] [Google Scholar]
- 5.Moon HJ, An JY, Heo JS, et al. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas 2006;32(1):37–43. [DOI] [PubMed] [Google Scholar]
- 6.Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer 2004;40(4):549–58. [DOI] [PubMed] [Google Scholar]
- 7.Han SS, Jang JY, Kim SW, et al. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas 2006;32(3):271–5. [DOI] [PubMed] [Google Scholar]
- 8.Jarufe NP, Coldham C, Mayer AD, et al. Favourable prognostic factors in a large UK experience of adenocarcinoma of the head of the pancreas and periampullary region. Dig Surg 2004;21(3):202–9. [DOI] [PubMed] [Google Scholar]
- 9.Westgaard A, Tafjord S, Farstad IN, et al. Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer 2008;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbeke CS. Resection margins and R1 rates in pancreatic cancer–are we there yet? Histopathology 2008;52(7):787–96. [DOI] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221(6):721–31 [discussion: 731–3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatima J, Schnelldorfer T, Barton J, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg 2010;145(2):167–72. [DOI] [PubMed] [Google Scholar]
- 13.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4(6):567–79. [DOI] [PubMed] [Google Scholar]
- 14.Verbeke CS. Resection margins in pancreatic cancer: are we entering a new era? HPB 2014;16(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB 2009;11(4):282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15(6):1651–60. [DOI] [PubMed] [Google Scholar]
- 17.Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009;55(3):277–83. [DOI] [PubMed] [Google Scholar]
- 18.Washington K, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. Guidelines of the College of American Pathologists. 2015. [Google Scholar]
- 19.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13(8):1035–46. [DOI] [PubMed] [Google Scholar]
- 20.TME a. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma. 2nd edition. National Comprehensive Cancer Network. [Google Scholar]
- 21.Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5(1):27–35. [DOI] [PubMed] [Google Scholar]
- 22.Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009;16(7):1751–6. [DOI] [PubMed] [Google Scholar]
- 23.Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20(8):2787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 1994;190(3):745–51. [DOI] [PubMed] [Google Scholar]
- 25.Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol 1998;170(5):1315–22. [DOI] [PubMed] [Google Scholar]
- 26.Miura F, Takada T, Amano H, et al. Diagnosis of pancreatic cancer. HPB 2006;8(5):337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennig R, Tempia-Caliera AA, Hartel M, et al. Staging laparoscopy and its indications in pancreatic cancer patients. Dig Surg 2002;19(6):484–8. [DOI] [PubMed] [Google Scholar]
- 28.Allen VB, Gurusamy KS, Takwoingi Y, et al. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 2013;(11):CD009323. [DOI] [PubMed] [Google Scholar]
- 29.Beenen E, van Roest MH, Sieders E, et al. Staging laparoscopy in patients scheduled for pancreaticoduodenectomy minimizes hospitalization in the remaining life time when metastatic carcinoma is found. Eur J Surg Oncol 2014;40(8):989–94. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto D, Chikamoto A, Sakata K, et al. Staging laparoscopy leads to rapid induction of chemotherapy for unresectable pancreatobiliary cancers. Asian J Endosc Surg 2015;8(1):59–62. [DOI] [PubMed] [Google Scholar]
- 31.Velanovich V, Wollner I, Ajlouni M. Staging laparoscopy promotes increased utilization of postoperative therapy for unresectable intra-abdominal malignancies. J Gastrointest Surg 2000;4(5):542–6. [DOI] [PubMed] [Google Scholar]
- 32.Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg 2006;10(10):1347–53. [DOI] [PubMed] [Google Scholar]
- 33.Clark CJ, Traverso LW. Positive peritoneal lavage cytology is a predictor of worse survival in locally advanced pancreatic cancer. Am J Surg 2010;199(5): 657–62. [DOI] [PubMed] [Google Scholar]
- 34.Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010;8(9):972–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christians K, Evans DB. Pancreaticoduodenectomy and vascular resection: persistent controversy and current recommendations. Ann Surg Oncol 2009;16(4):789–91. [DOI] [PubMed] [Google Scholar]
- 36.Porembka MR, Hawkins WG, Linehan DC, et al. Radiologic and intraoperative detection of need for mesenteric vein resection in patients with adenocarcinoma of the head of the pancreas. HPB 2011;13(9):633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Zeng MS, Zhou KR, et al. Pancreatic adenocarcinoma: the different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomogr 2005;29(2):170–5. [DOI] [PubMed] [Google Scholar]
- 38.Buchs NC, Chilcott M, Poletti PA, et al. Vascular invasion in pancreatic cancer: imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol 2010;16(7):818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vollmer CM, Drebin JA, Middleton WD, et al. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg 2002;235(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marangoni G, O’Sullivan A, Faraj W, et al. Pancreatectomy with synchronous vascular resection–an argument in favour. Surgeon 2012;10(2):102–6. [DOI] [PubMed] [Google Scholar]
- 41.Turley RS, Peterson K, Barbas AS, et al. Vascular surgery collaboration during pancreaticoduodenectomy with vascular reconstruction. Ann Vasc Surg 2012;26(5):685–92. [DOI] [PubMed] [Google Scholar]
- 42.Valls C, Andia E, Sanchez A, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 2002;178(4):821–6. [DOI] [PubMed] [Google Scholar]
- 43.Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg 2006;93(6):662–73. [DOI] [PubMed] [Google Scholar]
- 44.Ramacciato G, Mercantini P, Petrucciani N, et al. Does portal-superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma? Ann Surg Oncol 2009;16(4):817–25. [DOI] [PubMed] [Google Scholar]
- 45.Chua TC, Saxena A. Extended pancreaticoduodenectomy with vascular resection for pancreatic cancer: a systematic review. J Gastrointest Surg 2010;14(9): 1442–52. [DOI] [PubMed] [Google Scholar]
- 46.Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254(6):882–93. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Zhang Z, Liu Y, et al. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J Surg 2012;36(4):884–91. [DOI] [PubMed] [Google Scholar]
- 48.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8(8):935–49 [discussion: 949–50]. [DOI] [PubMed] [Google Scholar]
- 49.Krepline AN, Christians KK, Duelge K, et al. Patency rates of portal vein/superior mesenteric vein reconstruction after pancreatectomy for pancreatic cancer. J Gastrointest Surg 2014;18(11):2016–25. [DOI] [PubMed] [Google Scholar]
- 50.Lee DY, Mitchell EL, Jones MA, et al. Techniques and results of portal vein/superior mesenteric vein reconstruction using femoral and saphenous vein during pancreaticoduodenectomy. J Vasc Surg 2010;51(3):662–6. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Yoshidome H, Kimura F, et al. Renal function is well maintained after use of left renal vein graft for vascular reconstruction in hepatobiliarypancreatic surgery. J Am Coll Surg 2006;202(1):87–92. [DOI] [PubMed] [Google Scholar]
- 52.Chandrasegaram MD, Eslick GD, Lee W, et al. Anticoagulation policy after venous resection with a pancreatectomy: a systematic review. HPB 2014;16(8):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang F, Arianayagam R, Gill A, et al. Grafts for mesenterico-portal vein resections can be avoided during pancreatoduodenectomy. J Am Coll Surg 2012;215(4):569–79. [DOI] [PubMed] [Google Scholar]
- 54.Fujisaki S, Tomita R, Fukuzawa M. Utility of mobilization of the right colon and the root of the mesentery for avoiding vein grafting during reconstruction of the portal vein. J Am Coll Surg 2001;193(5):576–8. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira N, Oussoultzoglou E, Fuchshuber P, et al. Splenic vein-inferior mesenteric vein anastomosis to lessen left-sided portal hypertension after pancreaticoduodenectomy with concomitant vascular resection. Arch Surg 2011;146(12):1375–81. [DOI] [PubMed] [Google Scholar]
- 56.Ono Y, Matsueda K, Koga R, et al. Sinistral portal hypertension after pancreaticoduodenectomy with splenic vein ligation. Br J Surg 2015;102(3):219–28. [DOI] [PubMed] [Google Scholar]
- 57.Strasberg SM, Bhalla S, Sanchez LA, et al. Pattern of venous collateral development after splenic vein occlusion in an extended Whipple procedure: comparison with collateral vein pattern in cases of sinistral portal hypertension. J Gastrointest Surg 2011;15(11):2070–9. [DOI] [PubMed] [Google Scholar]
- 58.Christians KK, Pilgrim CH, Tsai S, et al. Arterial resection at the time of pancreatectomy for cancer. Surgery 2014;155(5):919–26. [DOI] [PubMed] [Google Scholar]
- 59.Amano R, Kimura K, Nakata B, et al. Pancreatectomy with major arterial resection after neoadjuvant chemoradiotherapy gemcitabine and S-1 and concurrent radiotherapy for locally advanced unresectable pancreatic cancer. Surgery 2015;158(1):191–200. [DOI] [PubMed] [Google Scholar]
- 60.Kang MJ, Jang JY, Chang YR, et al. Portal vein patency after pancreatoduodenectomy for periampullary cancer. Br J Surg 2015;102(1):77–84. [DOI] [PubMed] [Google Scholar]
- 61.Ouaissi M, Sielezneff I, Pirro N, et al. Therapeutic anticoagulant does not modify thromboses rate vein after venous reconstruction following pancreaticoduodenectomy. Gastroenterol Res Pract 2008;2008:896320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu CK, Farnell MB, Nguyen JH, et al. Prosthetic graft reconstruction after portal vein resection in pancreaticoduodenectomy: a multicenter analysis. J Am Coll Surg 2010;211(3):316–24. [DOI] [PubMed] [Google Scholar]
- 63.Sgroi MD, Narayan RR, Lane JS, et al. Vascular reconstruction plays an important role in the treatment of pancreatic adenocarcinoma. J Vasc Surg 2015;61(2):475–80. [DOI] [PubMed] [Google Scholar]
- 64.Shukla PJ, Barreto SG, Kulkarni A, et al. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol 2010;17(1):186–93. [DOI] [PubMed] [Google Scholar]
- 65.Traverso LW, Freeny PC. Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg 1989;55(7):421–6. [PubMed] [Google Scholar]
- 66.Allendorf JD, Bellemare S. Reconstruction of the replaced right hepatic artery at the time of pancreaticoduodenectomy. J Gastrointest Surg 2009;13(3):555–7. [DOI] [PubMed] [Google Scholar]
- 67.Kim DK, Hindenburg AA, Sharma SK, et al. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Ann Surg Oncol 2005;12(3):222–7. [DOI] [PubMed] [Google Scholar]
- 68.Sakorafas GH, Sarr MG, Peros G. Celiac artery stenosis: an underappreciated and unpleasant surprise in patients undergoing pancreaticoduodenectomy. J Am Coll Surg 2008;206(2):349–56. [DOI] [PubMed] [Google Scholar]
- 69.Bong JJ, Karanjia ND, Menezes N, et al. Total gastric necrosis due to aberrant arterial anatomy and retrograde blood flow in the gastroduodenal artery: a complication following pancreaticoduodenectomy. HPB 2007;9(6):466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai EC. Vascular resection and reconstruction at pancreatico-duodenectomy: technical issues. Hepatobiliary Pancreat Dis Int 2012;11(3):234–42. [DOI] [PubMed] [Google Scholar]
- 71.Pallisera A, Morales R, Ramia JM. Tricks and tips in pancreatoduodenectomy. World J Gastrointest Oncol 2014;6(9):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nara S, Sakamoto Y, Shimada K, et al. Arterial reconstruction during pancreatoduodenectomy in patients with celiac axis stenosis–utility of Doppler ultrasonography. World J Surg 2005;29(7):885–9. [DOI] [PubMed] [Google Scholar]
- 73.Farma JM, Hoffman JP. Nonneoplastic celiac axis occlusion in patients undergoing pancreaticoduodenectomy. Am J Surg 2007;193(3):341–4 [discussion: 344]. [DOI] [PubMed] [Google Scholar]
- 74.Kurosaki I, Hatakeyama K, Nihei KE, et al. Celiac axis stenosis in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg 2004;11(2):119–24. [DOI] [PubMed] [Google Scholar]
- 75.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156(3):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15(1):165–74. [DOI] [PubMed] [Google Scholar]
- 77.Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009;13(7):1337–44. [DOI] [PubMed] [Google Scholar]
- 78.Sierzega M, Popiela T, Kulig J, et al. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas 2006;33(3):240–5. [DOI] [PubMed] [Google Scholar]
- 79.Zhan HX, Xu JW, Wang L, et al. Lymph node ratio is an independent prognostic factor for patients after resection of pancreatic cancer. World J Surg Oncol 2015;13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adsay NV, Basturk O, Altinel D, et al. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol 2009;22(1): 107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pai RK, Beck AH, Mitchem J, et al. Pattern of lymph node involvement and prognosis in pancreatic adenocarcinoma: direct lymph node invasion has similar survival to node-negative disease. Am J Surg Pathol 2011;35(2):228–34. [DOI] [PubMed] [Google Scholar]
- 82.Konstantinidis IT, Deshpande V, Zheng H, et al. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J Gastrointest Surg 2010;14(2):261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 2012;147(8):753–60. [DOI] [PubMed] [Google Scholar]
- 84.Rupp CC, Linehan DC. Extended lymphadenectomy in the surgery of pancreatic adenocarcinoma and its relation to quality improvement issues. J Surg Oncol 2009;99(4):207–14. [DOI] [PubMed] [Google Scholar]
- 85.Peparini N Mesopancreas: a boundless structure, namely the rationale for dissection of the paraaortic area in pancreaticoduodenectomy for pancreatic head carcinoma. World J Gastroenterol 2015;21(10):2865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iqbal N, Lovegrove RE, Tilney HS, et al. A comparison of pancreaticoduodenectomy with extended pancreaticoduodenectomy: a meta-analysis of 1909 patients. Eur J Surg Oncol 2009;35(1):79–86. [DOI] [PubMed] [Google Scholar]
- 87.Michalski CW, Kleeff J, Wente MN, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg 2007;94(3):265–73. [DOI] [PubMed] [Google Scholar]
- 88.Watson K. Carcinoma of ampulla of Vater successful radical resection. Br J Surg 1944;31(124):368–73. [Google Scholar]
- 89.Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978;146(6):959–62. [PubMed] [Google Scholar]
- 90.Diener MK, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2011;(5):CD006053. [DOI] [PubMed] [Google Scholar]
- 91.Diener MK, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2014;(11):CD006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawai M, Tani M, Hirono S, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg 2011;253(3):495–501. [DOI] [PubMed] [Google Scholar]
- 93.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8(5):408–10. [DOI] [PubMed] [Google Scholar]
- 94.Addeo P, Calabrese DP, Bachellier P. Robotic pancreaticoduodenectomy: fad or the future? Adv Robot Autom 2012;2168–9695. [Google Scholar]
- 95.Giulianotti PC, Addeo P, Buchs NC, et al. Early experience with robotic total pancreatectomy. Pancreas 2011;40(2):311–3. [DOI] [PubMed] [Google Scholar]
- 96.Cirocchi R, Partelli S, Coratti A, et al. Current status of robotic distal pancreatectomy: a systematic review. Surg Oncol 2013;22(3):201–7. [DOI] [PubMed] [Google Scholar]
- 97.Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy–a comparative study. Int J Surg 2012;10(9):475–9. [DOI] [PubMed] [Google Scholar]
- 98.Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148(4):814–23. [DOI] [PubMed] [Google Scholar]
- 99.Palanivelu C, Jani K, Senthilnathan P, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 2007;205(2):222–30. [DOI] [PubMed] [Google Scholar]
- 100.Zeh HJ, Zureikat AH, Secrest A, et al. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol 2012;19(3): 864–70. [DOI] [PubMed] [Google Scholar]
- 101.Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21(9):2873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy afterdefinitive surgery forductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 2014;32(6):504–12. [DOI] [PubMed] [Google Scholar]
- 103.Gnerlich JL, Posner MC. More harm than good? Ann Surg Oncol 2014;21(9): 2817–9. [DOI] [PubMed] [Google Scholar]
- 104.Naritomi G, Tanaka M, Matsunaga H, et al. Pancreatic head resection with and without preservation of the duodenum: different postoperative gastric motility. Surgery 1996;120(5):831–7. [DOI] [PubMed] [Google Scholar]
- 105.Tran TC, van Lanschot JJ, Bruno MJ, et al. Functional changes after pancreatoduodenectomy: diagnosis and treatment. Pancreatology 2009;9(6):729–37. [DOI] [PubMed] [Google Scholar]
- 106.Courvoisier T, Donatini G, Faure JP, et al. Primary versus secondary delayed gastric emptying (DGE) grades B and C of the International Study Group of Pancreatic Surgery after pancreatoduodenectomy: a retrospective analysis on a group of 132 patients. Updates Surg 2015;67(3):305–9. [DOI] [PubMed] [Google Scholar]
- 107.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142(5):761–8. [DOI] [PubMed] [Google Scholar]
- 108.El Nakeeb A, Askr W, Mahdy Y, et al. Delayed gastric emptying after pancreaticoduodenectomy. Risk factors, predictors of severity and outcome. A single center experience of 588 cases. J Gastrointest Surg 2015;19(6):1093–100. [DOI] [PubMed] [Google Scholar]
- 109.Hartel M, Wente MN, Hinz U, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg 2005;140(11):1094–9. [DOI] [PubMed] [Google Scholar]
- 110.Bell R, Pandanaboyana S, Shah N, et al. Meta-analysis of antecolic versus retrocolic gastric reconstruction after a pylorus-preserving pancreatoduodenectomy. HPB 2015;17(3):202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su AP, Cao SS, Zhang Y, et al. Does antecolic reconstruction for duodenojejunostomy improve delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? A systematic review and meta-analysis. World J Gastroenterol 2012;18(43):6315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robinson JR, Marincola P, Shelton J, et al. Peri-operative risk factors for delayed gastric emptying after a pancreaticoduodenectomy. HPB 2015;17(6):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qu H, Sun GR, Zhou SQ, et al. Clinical risk factors of delayed gastric emptying in patients after pancreaticoduodenectomy: a systematic review and meta-analysis. Eur J Surg Oncol 2013;39(3):213–23. [DOI] [PubMed] [Google Scholar]
- 114.Hu HL, Zhou XD, Zhang Q, et al. Factors influencing delayed gastric emptying after pancreaticoduodenectomy—a meta-analysis. Hepatogastroenterology 2014;61(134):1539–45. [PubMed] [Google Scholar]
- 115.Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebocontrolled trial. Ann Surg 1993;218(3):229–37 [discussion: 237–8]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion 1999;60(5):422–7. [DOI] [PubMed] [Google Scholar]
- 117.Machado NO. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol 2012;2012: 602478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bassi C, Falconi M, Molinari E, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery 2003;134(5):766–71. [DOI] [PubMed] [Google Scholar]
- 119.Tewari M, Hazrah P, Kumar V, et al. Options of restorative pancreaticoenteric anastomosis following pancreaticoduodenectomy: a review. Surg Oncol 2010;19(1):17–26. [DOI] [PubMed] [Google Scholar]
- 120.Orci LA, Oldani G, Berney T, et al. Systematic review and meta-analysis of fibrin sealants for patients undergoing pancreatic resection. HPB 2014;16(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gurusamy KS, Koti R, Fusai G, et al. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev 2010;(2):CD008370. [DOI] [PubMed] [Google Scholar]
- 122.Allen PJ, Gonen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med 2014;370(21):2014–22. [DOI] [PubMed] [Google Scholar]
- 123.Rajarathinam G, Kannan DG, Vimalraj V, et al. Post pancreaticoduodenectomy haemorrhage: outcome prediction based on new ISGPS Clinical severity grading. HPB 2008;10(5):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yekebas EF, Wolfram L, Cataldegirmen G, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 2007;246(2):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Limongelli P, Khorsandi SE, Pai M, et al. Management of delayed postoperative hemorrhage after pancreaticoduodenectomy: a meta-analysis. Arch Surg 2008;143(10):1001–7 [discussion: 1007]. [DOI] [PubMed] [Google Scholar]
- 126.Rombouts SJ, Vogel JA, van Santvoort HC, et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg 2015;102(3):182–93. [DOI] [PubMed] [Google Scholar]
- 127.Gouma DJ, Busch OR, Van Gulik TM. Pancreatic carcinoma: palliative surgical and endoscopic treatment. HPB 2006;8(5):369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tarnasky PR, England RE, Lail LM, et al. Cystic duct patency in malignant obstructive jaundice. An ERCP-based study relevant to the role of laparoscopic cholecystojejunostomy. Ann Surg 1995;221(3):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc 2010;71(3):490–9. [DOI] [PubMed] [Google Scholar]
- 130.Nagaraja V, Eslick GD, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction—a systematic review and meta-analysis of randomized and non-randomized trials. J Gastrointest Oncol 2014;5(2):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gurusamy KS, Kumar S, Davidson BR. Prophylactic gastrojejunostomy for unresectable periampullary carcinoma. Cochrane Database Syst Rev 2013;(2):CD008533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaufman M, Singh G, Das S, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol 2010;44(2):127–34. [DOI] [PubMed] [Google Scholar]
- 133.de Leon-Casasola OA. Critical evaluation of chemical neurolysis of the sympathetic axis for cancer pain. Cancer Control 2000;7(2):142–8. [DOI] [PubMed] [Google Scholar]
- 134.Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004;9(5):571–91. [DOI] [PubMed] [Google Scholar]
- 135.Ierardi AM, Lucchina N, Petrillo M, et al. Systematic review of minimally invasive ablation treatment for locally advanced pancreatic cancer. Radiol Med 2014;119(7):483–98. [DOI] [PubMed] [Google Scholar]


