Abstract
In developing organs, the regulation of cell proliferation and patterning of cell fates is coordinated. How this coordination is achieved, however, is unknown. In the developing Drosophila wing, both cell proliferation and patterning require the secreted morphogen Wingless (Wg) at the dorsoventral compartment boundary (reviewed in ref. 1). Late in wing development, Wg also induces a zone of non-proliferating cells at the dorsoventral boundary. This zone gives rise to sensory bristles of the adult wing margin2,3. Here we investigate how Wg coordinates the cell cycle with patterning by studying the regulation of this growth arrest. We show that Wg, in conjunction with Notch, induces arrest in both the G1 and G2 phases of the cell cycle in separate subdomains of the zone of non-proliferating cells. Wg induces G2 arrest in two subdomains by inducing the proneural genes achaete and scute, which downregulate the mitosis-inducing phosphatase String (Cdc25)4. Notch activity creates a third domain by preventing arrest at G2 in wg-expressing cells, resulting in their arrest in G1.
The zone of non-proliferating cells (ZNC) is composed of three subdomains, each about four cells wide. Cells in the central domain express wg (Fig. 1a)5. This central domain is flanked by dorsal and ventral domains which, in the anterior compartment, express the proneural transcription factors Achaete (Ac) and Scute (Sc) (Fig. 2d)6,7. Cells of the ZNC stop proliferating 30 h before most other cells in the disc but re-enter the cell cycle for two or three divisions after pupariation2,8. The arrest is seen by an absence of cells in S phase or mitosis (Fig. 1b, f). The domain architecture of the ZNC is suggested by the expression patterns of string (stg) and of the G2 cyclins A (CycA) and B (CycB)9. In the anterior compartment, cells in the dorsal and ventral domains do not express stg messenger RNA but accumulate high levels of G2 cyclins in the cytoplasm (Fig. 1d, e). As Stg is required for mitosis4 and Stg and the G2 cyclins are degraded at cell division9, these patterns are indicative of arrest in G2. In contrast, in the central domain CycA and CycB proteins are undetectable (Fig. 1d), but stg mRNA is expressed (Fig. 1e). This indicates that these central cells may be arrested in G1. To confirm the phases in which cells of the different subdomains are arrested, we induced expression of the mitotic inducer Stg or of the S-phase inducer cyclin E (CycE)10. Within an hour after inducing stg expression, cells in the anterior dorsal and ventral domains entered mitosis (Fig. 1g). This indicates that these two domains become arrested in G2 because of an absence of stg. Induction of cycE led to entry into S phase only in the central domain in the anterior ZNC, but in the posterior ZNC most cells entered S phase (Fig. 1h). We conclude that the anterior central domain and the entire posterior ZNC are arrested in G1, whereas the dorsal and ventral domains of the anterior compartment are arrested in G2 (Fig. 1j).
Figure 1.
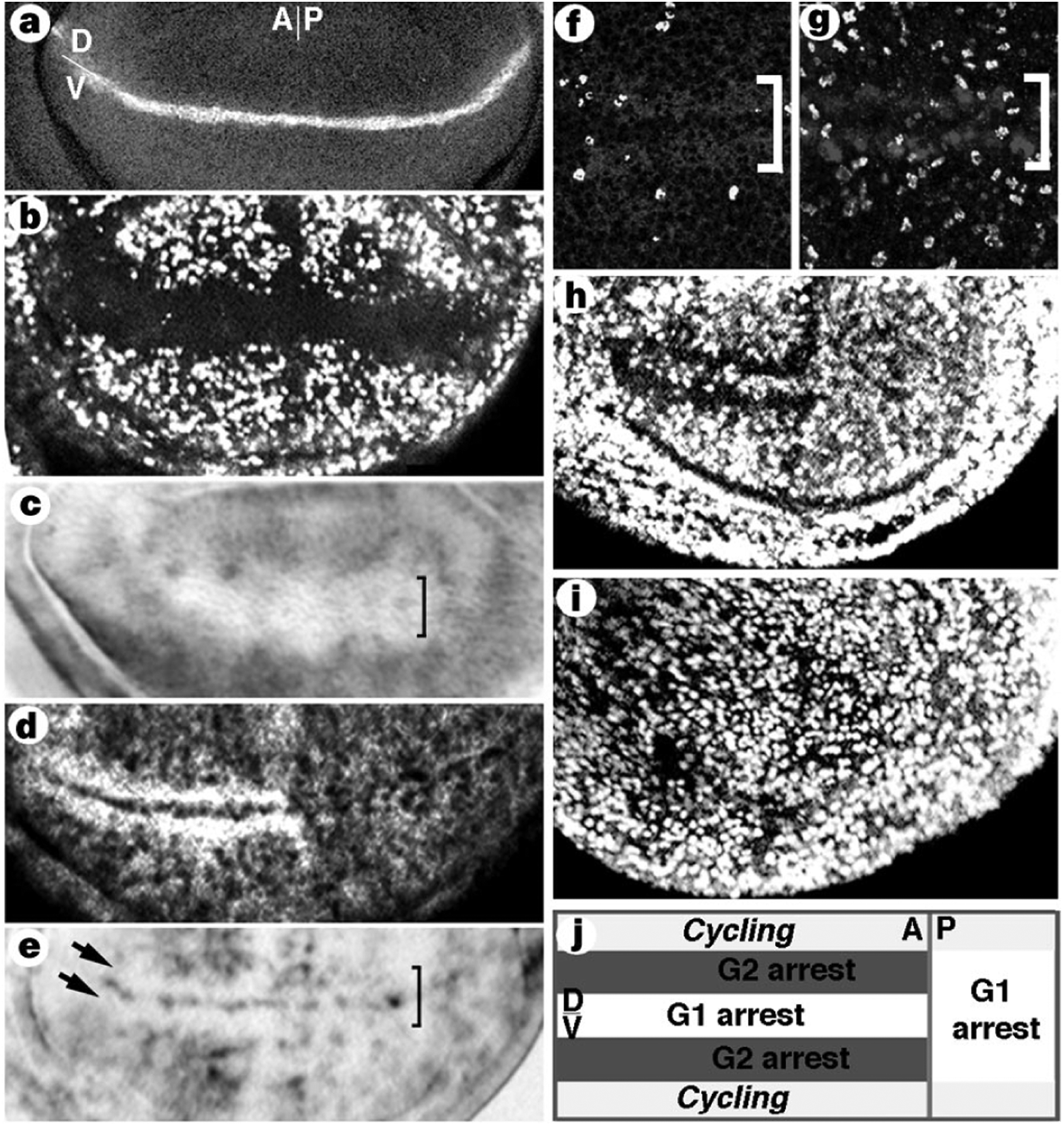
The ZNC contains cells arrested in G1 or G2 phase. a, Anti-Wg staining, D/V, dorsoventral boundary; A/P, anterioposterior boundary. All discs are orientated similarly. b, The ZNC does not incorporate BrdU. c, RNR-2 mRNA is absent from the ZNC. d, CycA accumulates to high levels in the two anterior domains. CycB accumulates similarly. e, stg mRNA is absent (arrows) from anterior dorsal and ventral cells of the ZNC, but present in posterior and anterior central cells. f, Wild-type ZNC, stained for mitotic cells. g, stg overproduction induces mitoses in the dorsal and ventral anterior domains. The ZNC is marked by brackets, mitotic cells are white, and the dorsal and ventral domains are light grey in f, g. h, cycE overproduction induces BrdU incorporation in all posterior cells of the ZNC, but only in the central domain in the anterior compartment. i, BrdU labelling showing that simultaneous induction of stg and cycE bypasses both G1 and G2 arrests. j, Summary of cell-cycle arrests in the ZNC.
Figure 2.
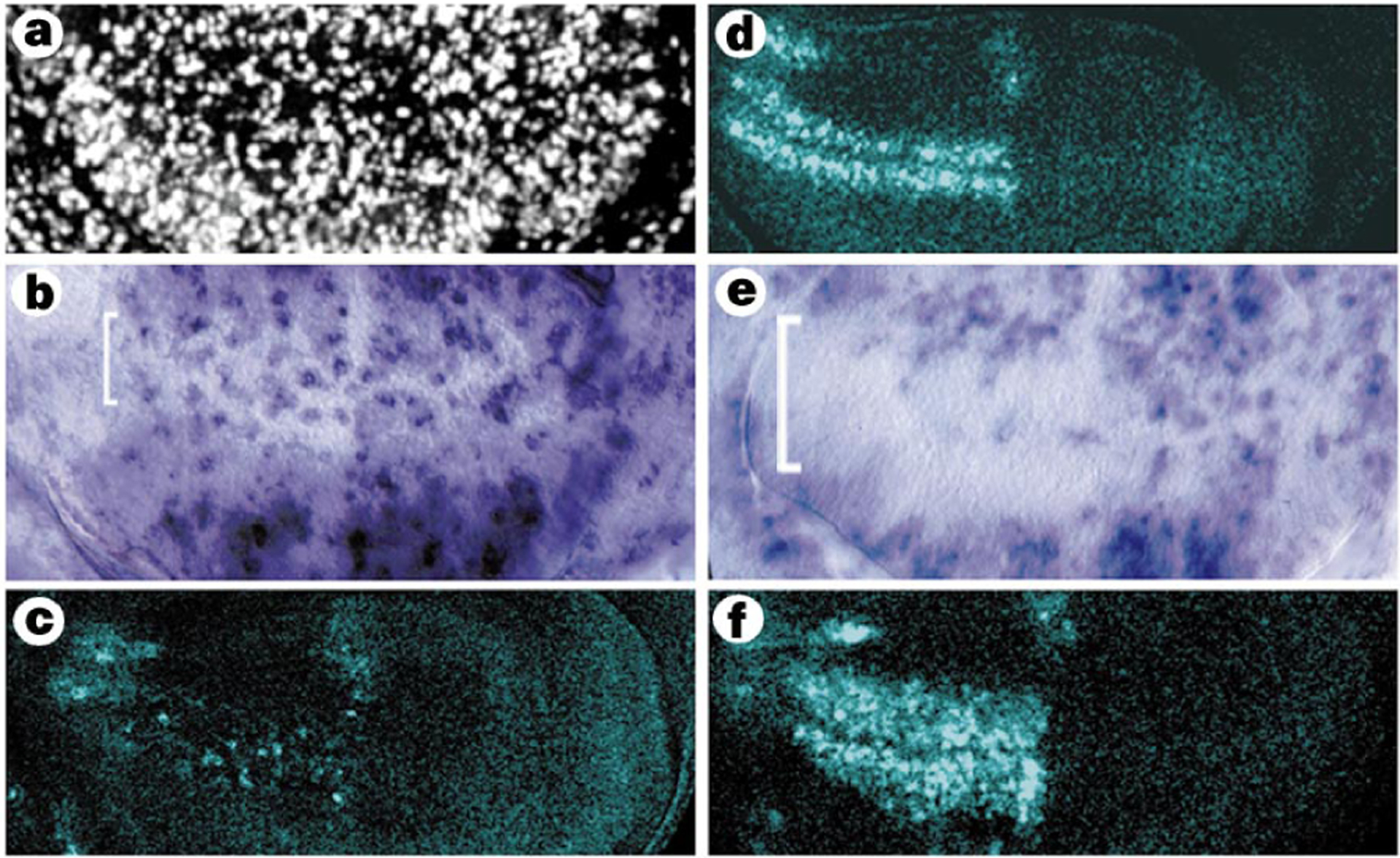
Wg regulates stg in the G2-arrested cells. Sections are orientated as in Fig.1. a, Ectopic expression of dominant–negative dTCF (dTCFΔN) causes loss of arrest and BrdU incorporation. Use of the temperature-sensitive wg allele at the restrictive temperature produced identical results. dTCFΔN results in stg expression where it is normally repressed (b, bracket) and causes loss of ac expression (c). d, Wild-type ac expression. e, Ectopic Wg expression in all three domains of the ZNC widens the domain of stg repression (bracket). f, ac expression is widened dorsally and ventrally by overproduction of Wg.
Several observations indicate that the G1 arrest may be due to inactivation of dE2F, a factor required to activate the transcription of genes needed for DNA replication11. First, although dE2F is expressed throughout the ZNC (data not shown), its activity appears to be downregulated because one of its targets, the ribo-nucleotide reductase-2 gene (RNR-2)11, is not expressed there (Fig. 1c). Second, forced expression of dE2F bypassed the G1 arrest and also induced expression of RNR-2 in the ZNC (data not shown). CycE is also normally present throughout the ZNC (data not shown) but does not promote S-phase progression, indicating that repression of CycE activity may also have a role in the G1 arrest.
How does Wg activity regulate the G1 and G2 arrests in the ZNC? Wg activates transcription of target genes through its signal-transducing proteins, Armadillo/[H9252]-catenin (Arm) and dTCF/Lef-1 (reviewed in ref. 12). We eliminated Wg activity either by using a temperature-sensitive wg allele or by expressing a dominant-negative form of dTCF throughout the ZNC. Loss of wg function during the last 24–30 h of disc development abolished both the G1 and G2 arrests (Fig. 2a) and allowed stg expression in the anterior dorsal and ventral domains (Fig. 2b). To determine how Wg normally represses stg in these domains, we broadened the effects of wg by expressing Wg or a constitutively active form of Arm in all three domains of the ZNC. Both treatments caused more cells to lose stg expression and arrest in G2, including many central domain cells which usually express stg (Fig. 2e). Ectopic Wg expression also expanded the G2 arrest dorsally and ventrally, indicating that the dorsal and ventral limits of the ZNC reflect a specific concentration threshold of Wg signalling.
Four observations suggested that the proneural genes ac and sc regulated the G2 arrest of the ZNC. First, ac and sc are expressed in the stg-negative, G2-arrested cells, but not in G1-arrested cells (Fig. 2d). Second, loss of Wg activity results in loss of expression of ac and sc (Fig. 2c)3 and causes expression of stg (Fig. 2b). Third, ectopic Wg or activated Arm expands the domain of stg-negative, Ac-positive cells (Fig. 2e, f). Fourth, expression of ac and sc in the ZNC is extinguished just before re-entry into the cell cycle after pupariation7,13. We therefore examined stg expression in the ZNC of discs from animals lacking both ac and sc function. Although the ZNC of these discs did not incorporate bromodeoxyuridine (BrdU) (Fig. 3a)14, many cells expressed stg mRNA, indicating that the ZNC was probably exclusively G1-arrested (data not shown). This was confirmed by overproduction of cycE in the ac- and sc-null discs; this led to S-phase entry throughout the ZNC (Fig. 3b). Thus, in the absence of ac and sc function, all cells in the ZNC arrested in G1. Conversely, forced expression of Sc throughout the ZNC resulted in loss of stg expression and cytoplasmic accumulation of CycA, indicating G2 arrest, in both posterior and anterior compartments (Fig. 3c, d). Together, these results indicate that Wg controls the G2 arrest in the ZNC by inducing expression of ac and sc, which in turn downregulate stg.
Figure 3.
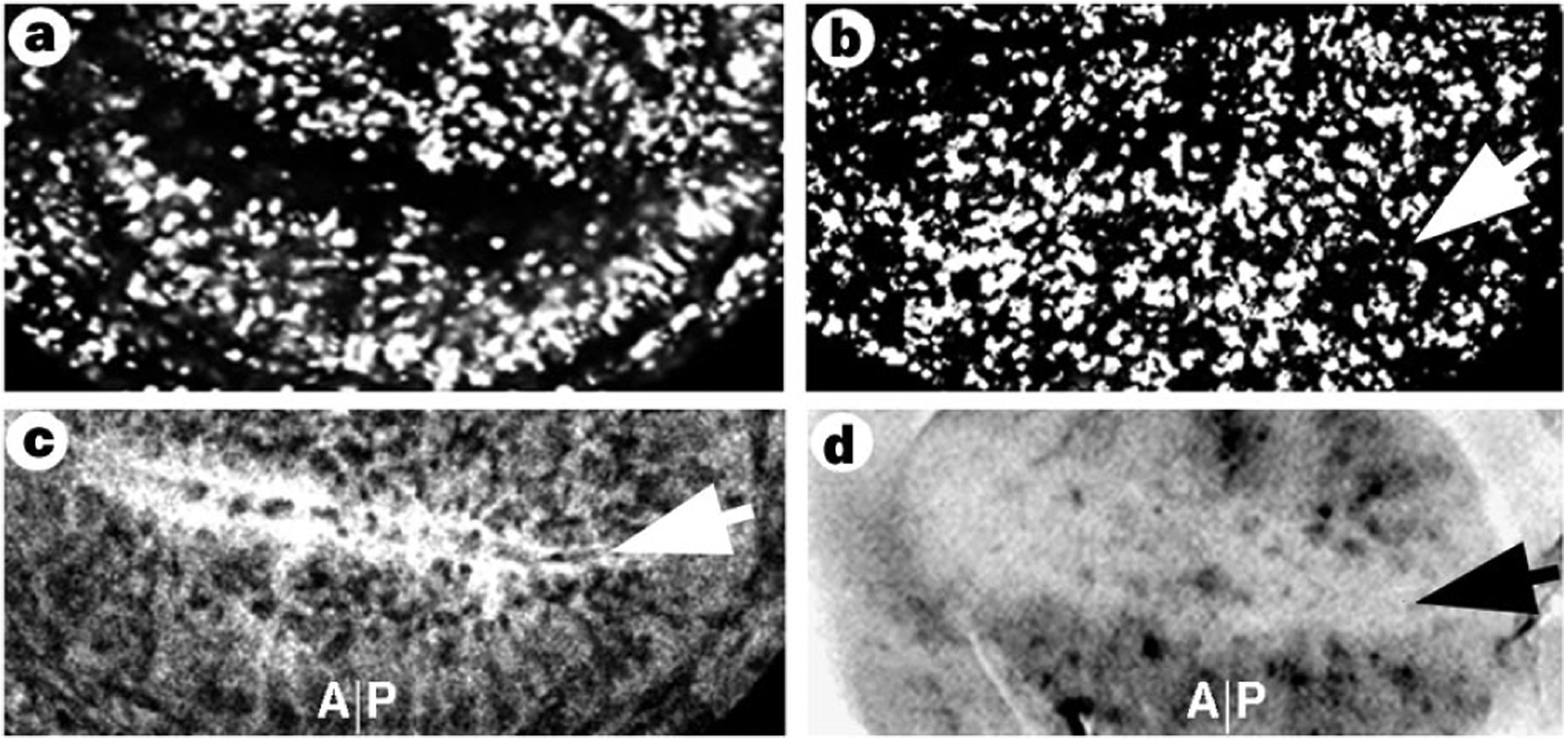
Ac and Sc repress stg transcription. Sections are orientated as in Fig.1. a, BrdU labelling of sc10.1 discs shows a ZNC. b, Overproduction of CycE in sc10.1 discs allows BrdU incorporation throughout the ZNC (arrow), indicating that this ZNC consisted previously of only G1-arrested cells. c, d, Overproduction of Sc arrests both anterior and posterior cells of the ZNC in G2. c, Scute causes CycA to accumulate in both anterior and posterior cells (arrow; compare with Fig. 1d). d, stg mRNA is repressed in both anterior and posterior cells of the ZNC after overproduction of Scute (arrow; compare with Fig.1e).
Notch is highly activated in the wg-expressing cells of the ZNC15 and is required for wg expression there16,17. To determine whether Notch also regulates the cell-cycle arrests in the ZNC, we generated clones of cells that lacked the activity of Suppressor of Hairless (Su(H)), a transcription factor that transduces the Notch signal18. When such clones were located in the central domain of the ZNC, they lost expression of wg (Fig. 4a)19 and many cells entered S phase (Fig. 4b). However, when cells within anterior clones were located adjacent to wild-type cells that expressed Wg, they accumulated CycA (Fig. 4c) and did not incorporate BrdU, indicating G2 arrest. Cells lacking Su(H) activity located a distance from wg-expressing cells did not accumulate CycA, and neither did posterior clones. Thus, cells lacking the ability to transduce Notch signalling either entered S phase or, if anterior and adjacent to Wg, they arrested in G2.
Figure 4.
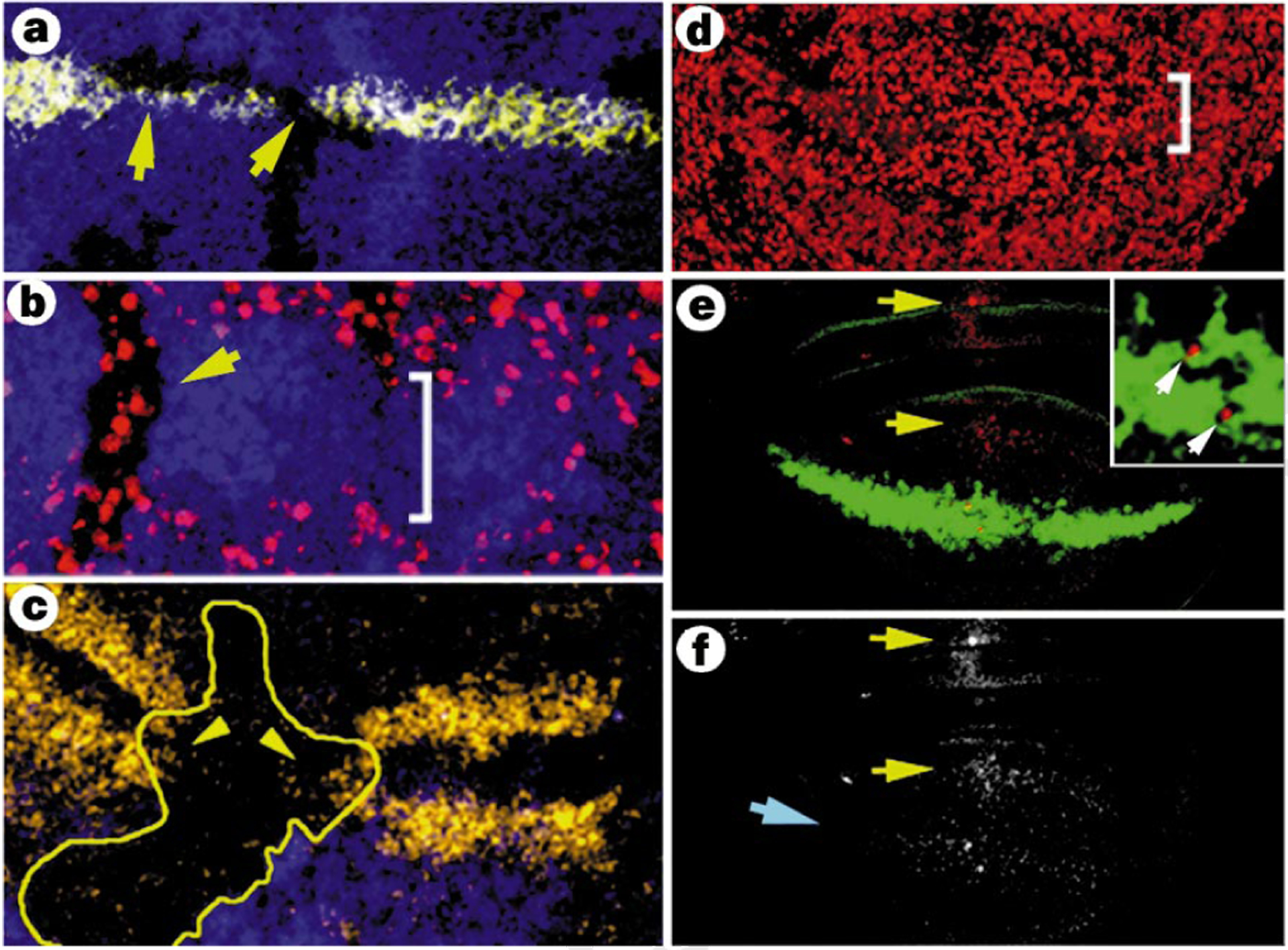
Notch activity prevents G2 arrest. a, Disc stained for Wg protein (yellow); Wg is not expressed in clones lacking Su(H) activity (arrows). b, BrdU incorporation (red) in Su(H)-deficient clones (lacking blue staining). c, CycA expression (yellow) in a Su(H)-deficient clone (inside the outline) in the ZNC. Only Su(H)-deficient cells adjacent to Wg-expressing cells (outside the clone) accumulate CycA (arrowheads). d, Overproduction of cycE in discs expressing activated Notch (NΔE) leads to BrdU incorporation (red) throughout the ZNC. e, Anti-Ac staining, showing expression outside the ZNC (red; arrows), but not where NΔE is expressed (green). Insert, magnification of two Ac-positive cells in the ZNC, showing no overlap with NΔE expression. f, Single-channel image of disc in e showing lack of ac expression in the ZNC (blue arrow; compare with Fig. 2d).
To test whether G1 arrest requires Notch activity, we expressed a constitutively active form of Notch throughout the ZNC. In these discs the ZNC was still present. However, stg was expressed and CycA did not accumulate in anterior cells, indicating that although the cells were not cycling, activated Notch prevented the G2 arrest (not shown). Overproduction of cycE in discs expressing activated Notch led to S phase throughout the ZNC (compare Figs 4d, 1h), confirming that cells in the ZNC had been entirely G1-arrested when activated Notch was expressed alone. In addition, activated Notch severely reduced expression of Ac in the ZNC (Fig. 4e, f). As ac and sc are required for the G2 arrest, we conclude that expression of activated Notch prevents G2 arrest by blocking expression of Ac and, presumably, Sc. These results indicate that expression of Ac and Sc, and G2 arrest, in the central domain is normally prevented by the high level of endogenous Notch activity there. To test whether Notch directly regulates the G1 arrest, we constructed discs lacking Wg activity, but expressing activated Notch in the ZNC. These discs did not form a ZNC at all (data not shown). Thus, in the absence of Wg activity, Notch is not sufficient to induce a G1 arrest.
Our observations lead to a model of how signalling by Wg controls a bipartite cell-cycle arrest in the wing disc (Fig. 5). Late in disc development, Wg protein emanating from the dorsoventral boundary inhibits dE2F activity throughout the ZNC region, promoting a G1 arrest. In the anterior dorsal and ventral domains, Wg also induces expression of Ac and Sc, leading to repression of stg transcription and therefore to G2 arrest in these cells. The stg promoter region contains putative Ac/Sc-binding sites, indicating that these basic helix–loop–helix proteins could repress stg transcription directly (L.A.J. and B.A.E., unpublished data). Our results indicate that Notch controls neither the G1 nor G2 arrest directly, but that it generates diversity within the ZNC in two ways. First, Notch indirectly promotes both arrests by maintaining wg expression16,19. Second, Notch blocks the G2 arrest in the central domain by inhibiting ac/sc expression. This function of Notch is also essential to inhibit the acquisition of neural fates20. Thus, one mechanism that patterning signals such as Wg use to control cell proliferation is the induction of target genes, for example ac and sc, that regulate both the cell cycle and cell-fate specification. Intriguingly, the dorsoventral-boundary region of the developing vertebrate spinal cord also contains a ZNC, and the corresponding region in Xenopus expresses the ac/sc homologue Xash-3 (C. Queva and K. Sharma, personal communications)21. Thus, the insect and vertebrate cell-cycle arrests may be produced by similar mechanisms. The presence of a constriction at the ZNC in the spinal cord indicates that zones of non-proliferating cells may act to constrain tissue growth and provides a mechanism for morphogenesis.
Figure 5.
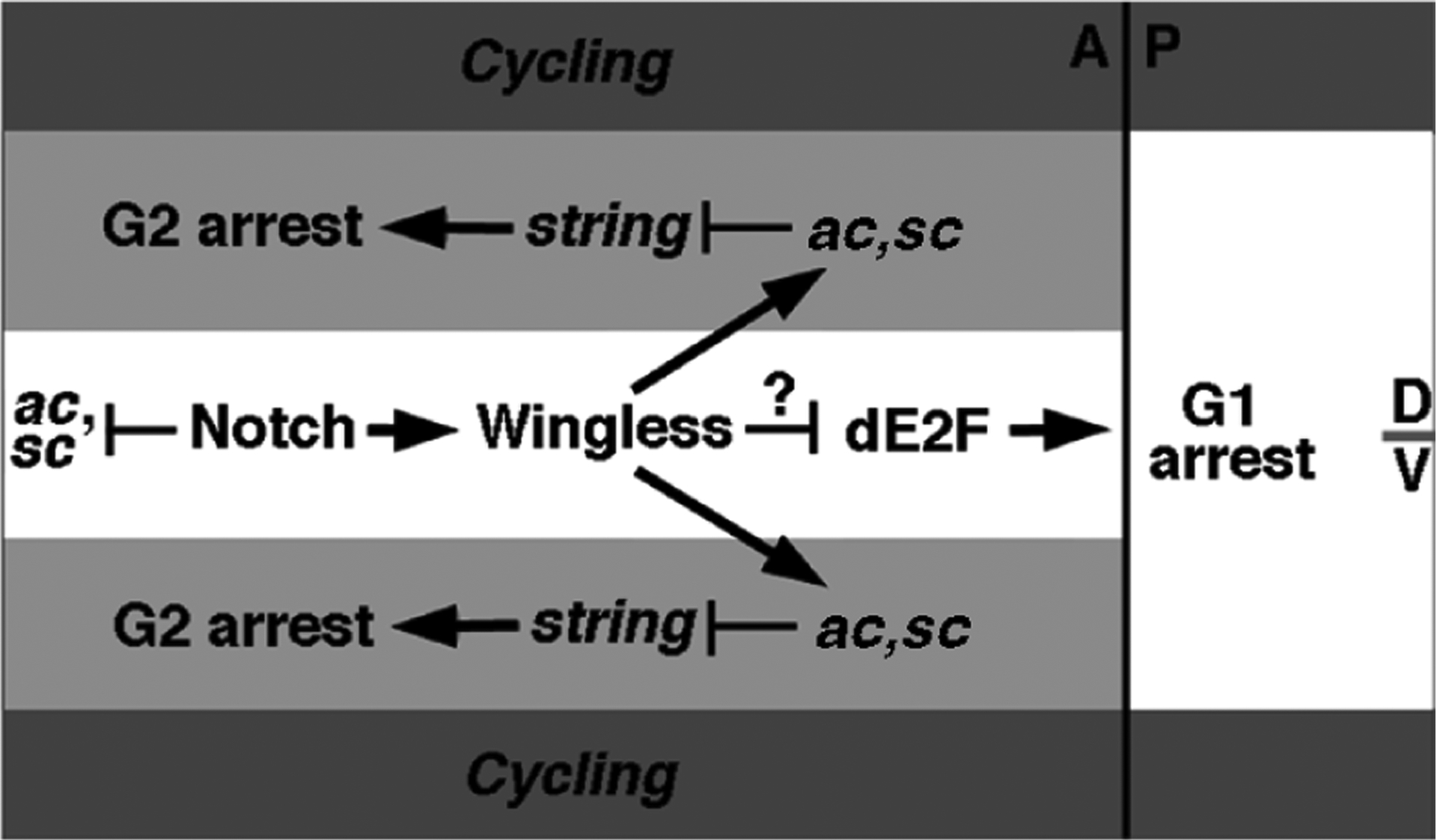
Model of cell-cycle arrests in the ZNC. Wingless induces a G2 arrest in dorsal and ventral anterior cells by inducing ac and sc, which repress string expression (light grey domains). At the same time Wingless inactivates dE2F throughout the ZNC, leading to G1 arrest in the posterior and anterior central domains (white domains). Notch promotes both G1 and G2 arrests by sustaining Wingless expression, and also creates the anterior domain architecture by blocking ac and sc expression in Wingless-expressing cells. A, anterior; P, posterior; D, dorsal; V, ventral.
Methods
Mutant stocks.
We removed both Ac and Sc functions using the sc10.1 allele22. In some experiments Wg activity was reduced using the temperature-sensitive allele wgIL114 (ref. 23) at 25 [H11034]C. wgcx4 is a null allele of wg24.
Immunocytochemistry.
BrdU labelling and immunocytochemistry were performed as described25. A protocol for in situ hybridization is available on request.
Transgenes and construction of recombinant strains.
We used the Gal4/ UAS (upstream activation sequence) system26 to express transgenes specifically in the ZNC. Gal4C96 (ref. 27) is expressed in the wing disc from approximately 80 h of development, before ZNC formation, and is expressed in cells in all three domains. The following UAS transgenes were expressed: dominant–negative dTCF28, activated Arm29, Wg (gift from J.-P. Vincent), Scute (gift from C. Doe), CycE (gift from C. Lehner), dE2F, Stg, or activated Notch (NΔE; gift from E. Giniger). We also used heat-shock transgenes (HS) of CycE, Stg, or dE2F. Larvae were heat-shocked at 37 [H11034]C for 1 h, and discs were dissected following a 1–2 h recovery period. To eliminate Wg function in discs expressing activated Notch, we constructed w; wgcx4/UASdTCFDN; Gal4C96/UAS NDE animals. To express CycE in animals with activated Notch, HS-CycE was recombined onto the NDE chromosome to make w; UAS-NDE, HS-CycE/ Gal4C96 animals. CycE was overproduced in the ZNC of sc10.1 animals with sc10.1/Y; Gal4C96/UAS-CycE.
Mitotic recombination.
Clones of cells lacking Su(H) activity were generated by mitotic recombination induced with the Flp/FRTsystem30 in HSFlp; FRT40A armlacZ (or HS-π-myc)/FRT40A Su(H)SF8 larvae during the second instar.
Acknowledgements.
We thank T. Neufeld, A. Bejsovec, M. Peifer, J-P. Vincent, C. Doe, S. Blair, S. Carroll, R. Nusse, S. Parkhurst, H. Richardson, C. Lehner, G. Boulianne and E. Giniger for gifts of flies or antibodies, C. Queva and K. Sharma for communicating unpublished results, and R. Kopan, M. Schubiger, S. Blair, J. Overbaugh, J. Priess, M. Emerman, J. Roberts and members of the Edgar lab for discussions and comments on the manuscript. L.A.J. is supported by a grant from the NIH. B.A.E. receives support from the NIH, and is a Rita Allen and a Lucille P. Markey Scholar.
References
- 1.Serrano N & O’Farrell PH Limb morphogenesis: connections between patterning and growth. Curr. Biol 7, 186–195 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brochta DA & Bryant PJ A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature 313, 138–141 (1985). [DOI] [PubMed] [Google Scholar]
- 3.Phillips RG & Whittle JR wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development 118, 427–438 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Edgar BA & O’Farrell PH The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell 62, 469–480 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair SS Mechanisms of compartment formation: evidence that non-proliferating cells do not play a critical role in defining the D/V lineage restriction in the developing wing of Drosophila. Development 119, 339–351 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Cubas P, de Celis JF, Campuzano S & Modolell J Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Gene Dev. 5, 996–1008 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Skeath JB & Carroll SB Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 5, 984–995 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Hartenstein V & Posakony JW Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107, 389–405 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Lehner CF & O’Farrell PH The roles of Drosophila cyclins A and B in mitotic control. Cell 61, 535–547 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoblich JA et al. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77, 107–120 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Duronio RJ, O’Farrell PH, Xie JE, Brook A & Dyson N The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev. 9, 1445–1455 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Nusse R A versatile transcriptional effector of Wingless signaling. Cell 89, 321–323 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Romani S, Campuzano S, Macagno ER & Modolell J Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 3, 997–1007 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Usui K & Kimura K-I Sensory mother cells are selected from among mitotically quiescent clusters of cells in the wing disc of Drosophila. Development 116, 601–610 (1992). [Google Scholar]
- 15.de Celis JF, Garcia-Bellido A & Bray SJ Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122, 359–369 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Couso JP, Knust E & Martinez AA Serrate and Wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr. Biol 5, 1437–1448 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Rulifson EJ & Blair SS Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121, 2813–2824 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Schweisguth F Suppressor of Hairless is required for signal reception during lateral inhibition in the Drosophila pupal notum. Development 121, 1875–1884 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Neumann CJ & Cohen SM A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122, 3477–3485 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Kopan R & Turner DL The Notch pathway: democracy and aristocracy in the selection of cell fate. Curr. Opin. Neurobiol 6, 594–601 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman K, Shih J, Bars J, Collazo A & Anderson DJ Xash-3, a novel Xenopus achaete-scute homolog, provides an early marker of planar neural induction and position along the mediolateral axis of the neural plate. Development 119, 221–232 (1993). [DOI] [PubMed] [Google Scholar]
- 22.Campuzano S et al. Molecular genetics of the achaete-scute complex of D. melanogaster. Cell 40, 327–338 (1985). [DOI] [PubMed] [Google Scholar]
- 23.Bejsovec A & Martinez AA Roles of wingless in patterning the larval epidermis of Drosophila. Development 113, 471–485 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Baker NE Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 6, 1765–1773 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston LA & Schubiger G Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development 122, 3519–3529 (1996). [DOI] [PubMed] [Google Scholar]
- 26.Brand AH & Perrimon N Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Gustafson K & Boulianne GL Distinct expression patterns detected within individual tissues by the Ga14 enhancer trap technique. Genome 39, 174–182 (1996). [DOI] [PubMed] [Google Scholar]
- 28.van de Wetering M et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Pai LM et al. Drosophila alpha-catenin and E-cadherin bind to distinct regions of Drosophila Armadillo. J. Cell Biol 271, 32411–32420 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Xu T & Rubin GM Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993). [DOI] [PubMed] [Google Scholar]


