Abstract
Wastewater-based monitoring of SARS-CoV-2 has become a promising and useful tool in tracking the potential spread or dynamics of the virus. Its recording can be used to predict how the potential number of infections in a population will develop. Recent studies have shown that the use of passive samplers is also suitable for the detection of SARS-CoV-2 genome copies (GC) in wastewater. They can be used at any site, provide timely data and may collect SARS-CoV-2 GC missed by traditional sampling methods. Therefore, the aim of this study was to evaluate the suitability of passive samplers for the detection of SARS-CoV-2 GC in wastewater in the long-term at two different scales. Polyethylene-based plastic passive samplers were deployed at the city-scale level of Leipzig at 13 different locations, with samples being taken from March 2021 to August 2022. At the smaller city district level, three types of passive samplers (cotton-cloth, unravelled polypropylene plastic rope and polyethylene-based plastic strips) were used and sampled on a weekly basis from March to August 2022. The results are discussed in relation to wastewater samples taken at the individual passive sampling point. Our results show that passive samplers can indicate at a city-scale level an accurate level of positive infections in the population (positive-rate: 86 %). On a small-scale level, the use of passive samplers was also feasible and effective to detect SARS-CoV-2 GC easily and cost-effectively, mirroring a similar trend to that at a city-scale level. Thus, this study demonstrated that passive samplers provide reproducible SARS-CoV-2 GC signals from wastewater and a time-integrated measurement of the sampled matrix with greater sensitivity compared to wastewater. We thus recommend the use of passive samplers as an alternative method for wastewater-based epidemiology. Passive samplers can in particular be considered for a better estimation of infections compared to incidence levels.
Keywords: SARS-CoV-2, COVID-19, Monitoring, Passive sampling, Wastewater, Wastewater-based epidemiology
Graphical abstract
1. Introduction
COVID-19 is a disease that was first reported in December 2019 and is caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2). SARS-CoV-2 has been detected in both, respiratory and gastrointestinal tracts (Xiao et al., 2020). Genetic fragments of the virus have also been found in faeces and urine samples of symptomatic, as well as asymptomatic, individuals (Ahmed et al., 2020a; Gupta et al., 2020; Lee et al., 2020; Tian et al., 2020; Wu et al., 2020b), eventually ending up in wastewater and, finally, in municipal wastewater treatment plants (WWTP) (Crank et al., 2022).
SARS-CoV-2 GC was quantified in raw wastewater (Kitajima et al., 2020; Peccia et al., 2020) as well as in sludge (Balboa et al., 2021), days before positive cases were clinically reported (Medema et al., 2020), thereby potentially serving as an early warning tool. This led scientists, public health agencies, and governments worldwide to focus on wastewater-based epidemiology (WBE) (Gawlik et al., 2021) for the detection of pathogens of concern (e.g. SARS-CoV-2) in wastewater.
The detection of SARS-CoV-2 GC included e.g., the monitoring of wastewater treatment plants of different sizes in the Czech Republic (Mlejnkova et al., 2020), in different catchment areas in Australia and Spain (Ahmed et al., 2020a; Guerrero-Latorre et al., 2022), on a nationwide scale in the Netherlands, where the National Institute for Public Health and the Environment is analysing wastewater samples from over 300 WWTP, serving approximately 17 million people, at 55 locations representing approximately 12.5 million people in the USA (Duvallet et al., 2022) or in 81 cities in Turkey (Kocamemi et al., 2020).
Broad screening surveys for human based diseases are often logistically costly, economically inefficient, resource onerous, and, when using PCR, always provide only a snapshot of the actual situation (Bivins et al., 2021). However, the actual potentially infected proportion of the population is a key parameter for epidemiological assessment. Its recording is crucial in predicting how potential number of infections in a population will develop and how they will change in response to interventions.
While progress has been made in the diagnosis of the disease, there is a lack of tools that help capture the prevalence of potential infections for larger populations. Most studies consider wastewater samples from large WWTPs at the city-scale level, however the collection of representative, standardised samples is still challenging (George et al., 2022).
Over the last two years, the monitoring of SARS-CoV-2 in wastewater has become a promising and useful tool in tracking the potential spread or dynamics of the virus at city- to suburban-scale levels. In general, autosamplers are used to collect continuous samples over a specific time interval (e.g. 24 h) since SARS-CoV-2 GC in wastewater are extremely variable during the day depending on time, dilution and sewer length (Curtis et al., 2021; Habtewold et al., 2022). Furthermore, autosamplers are not always available (e.g., acquisition and maintenance costs, unsecure sites, safety concerns) and processing the liquid is time-consuming (Habtewold et al., 2022; Kitajima et al., 2020; Schang et al., 2021). Therefore, the application and development of practical and inexpensive tools, such as passive samplers, may be crucial for WBE monitoring in general and the detection of SARS-CoV-2 GC in wastewater.
Passive samplers, made from stripes of polyethylene have been used for decades in sewer systems by municipal WWTPs in Germany (German term: ‘Sielhautsammler’) to identify illegal wastewater discharges containing heavy metals (Boës and Caspary, 1987). The biofilm laid on the plastic passive sampler consists of microorganisms (bacteria and fungi), which are capable of absorbing and accumulating pollutants from the wastewater (Vincent-Hubert et al., 2017), and depending on the microbial composition, often have a ‘greasy and soapy’ consistency. This biofilm can then be harvested and analysed (Horning and Sbieschni, 1991), usually for organic or inorganic contaminants. Recently, the capture and detection of SARS-CoV-2 GC using nylon nets was described by (Vincent-Hubert et al., 2022).
It has been shown that the use of passive samplers is also suitable for the detection of SARS-CoV-2 GC in wastewater (Bivins et al., 2022; Jones et al., 2022). They can be used at any site, provide timely data and may collect SARS-CoV-2 GC missed by traditional sampling methods (Habtewold et al., 2022). Furthermore, the great advantage is that it is a cost-effective tool and can be easily deployed at various locations in the city's sewer catchment and/or can target and monitor specific small-scale locations such as districts or individual buildings (Acer et al., 2022; Hayes et al., 2021). However, the application of passive samplers for wastewater-based monitoring of SARS-CoV-2 is still highly underrepresented.
We searched the ‘Web of Science’ (January 2023) to identify relevant articles combining “wastewater”, “SARS-CoV-2” and “passive sampler”. We identified 11 articles, whereas the combination of “wastewater” and “SARS-CoV-2” only, resulted in 860 identified articles. For instance, the detection of SARS-CoV-2 GC at low prevalence levels was demonstrated by Schang et al. (2021), Habtewold et al. (2022) and Hayes et al. (2021), although experimental trials were only carried out for short periods, that is, 22 days, 48 h and 96 h respectively. Furthermore, various materials for passive samplers were used such as cotton buds or gauze pads (Habtewold et al., 2022), Moore swabs (Rafiee et al., 2021), cheesecloth, cellulose sponge and electronegative membranes (Hayes et al., 2021). Hayes et al. (2022) describes the adsorption of SARS-CoV-2 GC on granular activated carbon as a media for passive samplers and Vincent-Hubert et al. (2022) Zetapor and nylon membranes. Corchis-Scott et al. (2021), Liu et al. (2022) and Wilson et al. (2022) concluded that passive sampling had a better sensitivity over wastewater monitoring results in general and was even able to detect single COVID-19 cases at a household level.
Therefore, the overall goal of this study was to evaluate the suitability of passive samplers for the detection of SARS-CoV-2 GC in wastewater over the long-term, at two different scales: city and district-scale level, thus enabling the identification of potential ‘hot spots’ (targeted locations).
The specific aims were to: (i) carry out a field-scale test of passive samplers across the city of Leipzig (city-scale), Germany, for a period of 16 months and; (ii) to carry out a field-scale test of three different passive sampling materials at district-scale level in the city of Leipzig, for a period of 5 months, for monitoring SARS-CoV-2 in wastewater. (iii) It is, furthermore, briefly discussed why it is difficult to link the results to the reported case numbers (7-day incidence) in general used by the authorities.
2. Material and methods
2.1. Composite wastewater sampling and passive samplers at city-scale level
The city of Leipzig, Germany, is equipped with a combined sewer system. Samples of the wastewater were taken at the municipal WWTP plant in Leipzig (Klärwerk Rosental), serving approximately 600,000 inhabitants and were then put in relation to the passive samplers. An automatic sampler collected, on average, three individual 24 h composite samples per week at the inflow of the WWTP. In total, 217 wastewater samples were taken and analysed for SARS-CoV-2 GC over the period of March 2021 until the end of August 2022. The data obtained from a SARS-CoV-2 monitoring campaign is still in progress.
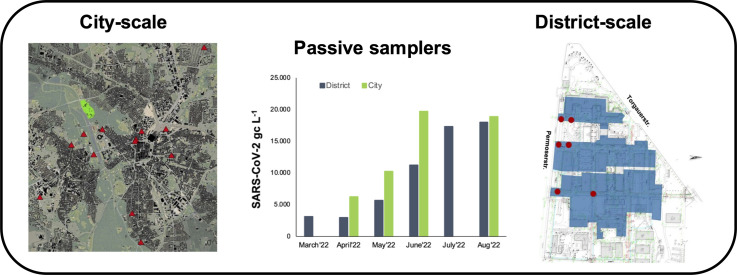
Polyethylene-based plastic passive samplers were deployed in the catchment area of the municipal WWTP plant in Leipzig at 13 different sub-catchment locations (city-scale; see Fig. 1 ) and samples were taken in March, May, June, July, August and September 2021; and February, April, May, June, July and August 2022. With our sampling strategy we wanted to cover the entire city area of Leipzig. However, concerning the sampling we were dependent on the municipal water authority of Leipzig and in order to realize a simultaneous sampling of all sampling points every 4 weeks, not >13 were logistically feasible.
Fig. 1.
Polyethylene-based plastic passive samplers used for the detection of SARS-CoV-2 GC in wastewater (left and middle photos), and the location of the 13 passive samplers (red) across the city of Leipzig. Green: Location of the WWTP.
As shown in Fig. 1, the polyethylene-based passive samplers consisted of eight clustered 1.5 m long polyethylene plastic strips. The overall sampling number as well as location differed every time (see Table A1). Overall, 92 samples from passive samplers were provided to us from the city-scale level monitoring by the Leipzig municipal water utilities (Kommunale Wasserwerke Leipzig GmbH): March 2021: n = 9; May 2021: n = 7, June 2021: n = 8; July 2021: n = 7; August 2021: n = 9; September 2021: n = 5; February 2022: n = 8; April 2022: n = 6; May 2022: n = 12, June 2022: n = 12, and August 2022: n = 9.
All wastewater samples, along with the samples on polyethylene-based passive samplers, were stored at 4 °C on the day of collection and SARS-CoV-2 GC was extracted during the next day.
The average ambient air temperature of the calendar week (CW) during sampling is listed in the Supplementary section.
2.2. Wastewater sampling and passive samplers at district-scale level
The experimental setup at a district-scale level (e.g., micro-catchment) was used at the campus of the Helmholtz-Centre for Environmental Research – UFZ in Leipzig, Germany (Fig. A1). Approximately 700 employees work at the Centre; however, due to Covid restrictions and working from home regulations, significantly fewer employees were present during the experimental trial.
The campus has an internal network of a combined sewer, all wastewater flows towards a main sewer line at ‘Permoser street’ (Fig. A1) and wastewater samples (n = 3 per sampling) were taken as grab samples in the morning during the peak-flow.
Three types of passive samplers were used to investigate differences in biofilm growth and possible effects on SARS-CoV-2 GC detection. These types were the polyethylene-based passive samplers as described above, one made from cotton and one from an unravelled polypropylene plastic rope (Fig. 2 ). For the cotton passive samplers, two 10 cm wide and 1.5 m long cotton-cloths were used. For the rope passive samplers, an unravelled 1.5 m plastic rope was used (Fig. 2).
Fig. 2.
Passive samplers used for the detection of SARS-CoV-2 GC in wastewater at district-scale level. From left: polyethylene-based plastic sampler, cotton-cloth sampler and unravelled polypropylene plastic rope sampler.
Three replicates of each passive sampler type were placed and distributed in the wastewater stream at six locations in street 2 (S2), street 3 (S3) and street 4 (S4) of the campus (Fig. A1). In order to prevent any clogging and ragging of the sewer line, the polyethylene-based passive samplers were placed in the manholes closest to ‘Permoser street’. The cotton and the unravelled plastic rope were placed together in the same wastewater stream in the manhole closest to the location of the polyethylene-based passive samplers.
Samples were taken on a weekly basis from March 2022 until August 2022. Since there was not always enough material present, the overall sampling number differed each time. Overall, 197 samples from the district-scale level were analysed: wastewater: n = 58; polyethylene-based passive sampler: n = 39; cotton passive sampler: n = 43 and unravelled rope passive sampler: n = 57 (see Table A2).
All wastewater, along with samples from passive samplers, were stored at 4 °C on the day of collection, and SARS-CoV-2 GC was extracted during the next day.
To evaluate the decay of SARS-CoV-2 GC on passive samples, SARS-CoV-2 GC was analysed 0, 12, 24, 48, 72, 96, 148, 172, 196, 220, 244 and 288 h after sampling. Samples were stored at a temperature of 13 °C (average temperature of wastewater). Furthermore, the total suspended solid content of the wastewater at S2–S4 was determined according to DIN EN 872.
2.3. RNA extraction from wastewater and passive samplers
Viral components were precipitated as described previously (Dumke et al., 2021). Briefly, 45 mL of wastewater samples or 25 mL of biofilm samples, mixed with 25 mL TM Buffer (50 mM Tris HCl, 10 mM Magnesium Sulphate at pH 7.5), were vortexed for 20 min at maximum speed and then centrifuged for 15 min at 3000 g. The supernatant was carefully taken (40 mL) and 10 % (w/v) PEG (Carl Roth, Karsruhe, Germany) (MW 8000)/2.25 % NaCl (Th. Geyer, Renningen, Germany) were added to the samples and mixed head-over-head until additives were completely dissolved. Samples were then centrifuged at 12000 g for 1 h. The supernatant was carefully removed and pellets were suspended in 500 μL TM buffer. PM1 buffer (1.3 mL), (Qiagen, Hilden, Germany) was immediately added to lyse viral particles and inactivate nucleases. Samples were stored at −20 °C until nucleic acid extraction.
Total nucleic acids were extracted using a RNeasy Microbiome Kit (Qiagen) as described by the manufacturer.
SARS-CoV-2 RNA was detected and quantified using a SARS-CoV-2 specific RT-qPCR targeting part of the E gene as described previously (Corman et al., 2020). A standard curve was generated to calculate SARS-CoV-2 copy numbers from RT-qPCR cT-values using eight observations in three independent experiments (in vitro transcribed RNA molecule numbers between 3.125 and 1 million). RT-qPCR efficiency was 110 %, R2 value was 0.9702, the linear dynamic range was between 12.5 and 1 million copy numbers (R2 = 0.9982). Limit of detection was 2.53 and limit of quantification was 7.69.
2.4. Data analysis
Mean Ct values measured in wastewater and passive samplers were converted into genome copies, based on an in-house SARS-CoV-2 standard with known GC numbers. Data on the incidence as well as the number of RT-qPCR tests carried out and positive RT-qPCR tests were provided by the ‘Robert Koch Institute (RKI)’ (www.rki.de), which is the German federal government agency and research institute responsible for disease control and prevention.
Data were analysed using the statistical program R, Version 4.2.1 (R Core Team, 2022). Distributions of data were tested for normality and homogeneity, and are presented as arithmetic means ± standard errors (SE) in the figures as box plots. If necessary, results were evaluated statistically using analysis of variance (ANOVA) followed by a Tukey's post hoc test. Where the assumptions of the model were not fulfilled, a Box-Cox transformation was applied and effects were regarded as significant if p ≤ 0.05.
3. Results and discussion
3.1. Detection of SARS-CoV-2 in passive samplers and wastewater at a city-scale level
So far, only three other studies have considered passive samplers in larger catchments for the detection of SARS-CoV-2 GC in wastewater (Ahmed et al., 2022; Li et al., 2022; Schang et al., 2021). For the first time, passive samplers were now used at various locations at a city-scale level over several months to monitor SARS-CoV-2 in wastewater. All results were discussed in relation to SARS-CoV-2 GC detected directly in the wastewater at the municipal WWTP.
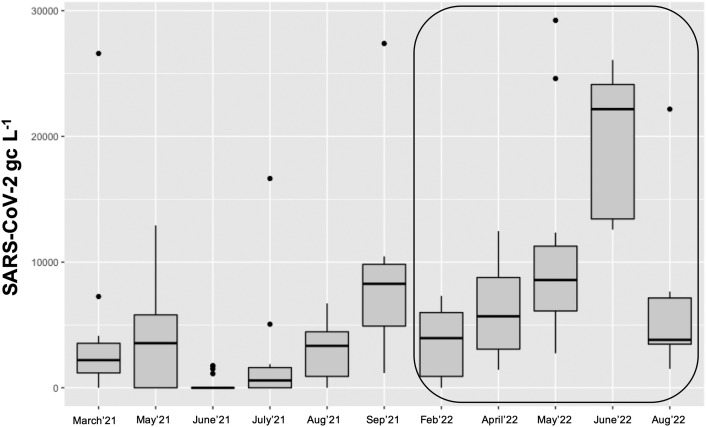
Of the 92 passive samples taken across the city of Leipzig, about 86 % were positive for SARS-CoV-2 in the period from March 2021 until August 2022. Even with low reported incidence levels during June to August 2021 (monthly incidence level of 28 in June, 7 in July and 15 in August) SARS-CoV-2 GC were detected (Fig. 3 ). The data shows that it is also possible to detect the presence of the virus in the population based on passive samplers for wastewater monitoring on a larger scale. Some outliers are present, however, significant differences (p ≤ 0.05) between the months were detected (Fig. 3), and there was a slightly higher positive rate compared to wastewater (82 %). We therefore postulate that passive samplers in wastewater can indicate a more realistic level of infections in the population than measured directly in wastewater, however at this stage only retrospectively.
Fig. 3.
Detection of SARS-CoV-2 GC in passive samplers in wastewater as aggregate data across the city of Leipzig in 2021 and 2022 (framed). Solid line, boxes, and whiskers: median, IQR and 1.5 × IQR, respectively.
Additionally, samples were taken at various location across the city of Leipzig (Fig. 1) and not at only one sampling point, as is the case for the wastewater monitoring. However, the downside is that, with passive samplers at a city scale level, we were not able to increase the sampling frequency, due to logistic reasons (e.g., blocking roads to obtain the sample) and therefore could only retrieve samples every 4 weeks from the 13 sampling points.
A significant variation (p ≤ 0.05) of SARS-CoV-2 GC between sampling points of the passive samplers was measured, indicating the identification of potential ‘hot spots’ of community transmission in local areas or catchments is possible. However, the catchment areas of the 13 sampling locations in Leipzig are huge, ranging from 1.3 to 18.8 km2, similar in size to small villages, and therefore, also due to logistic reasons, it was not possible to narrow further down the ‘hot spots’ to smaller catchments, such as individual household blocks. Thus, further analyses at smaller scales are necessary.
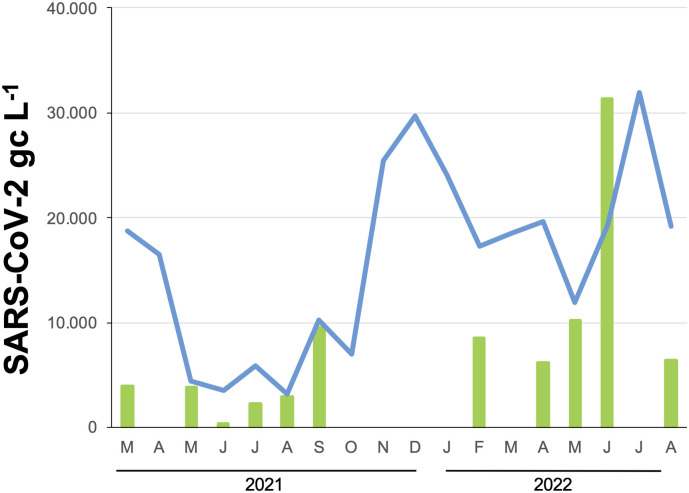
In the following, we aligned the passive sampling data at city scale level with the wastewater data from the municipal WWTP described above. The data significantly correlate (R2 = 0.75, p ≤ 0.05), however, we can see that trends between months are more precise in the data from passive samplers than in those from the wastewater data and the inflection point in the curve, such as seen in May, is not visible (Fig. 4 ). SARS-CoV-2 GC passive samplers gradually increased from June 2021 until August 2021, however SARS-CoV-2 GC in wastewater decreased again in August 2021 (Fig. 4). Similar results were found from February until June 2022, where SARS-CoV-2 GC in wastewater decreased in May. From January to May 2022, when the Delta virus variant was being replaced with the Omicron virus variant (Paton et al., 2022), passive samplers seemed to be unaffected by this change and seemed to be more sensitive compared to 24 h composite wastewater samples.
Fig. 4.
Comparison of detected SARS-CoV-2 GC in passive samplers (green) across the city of Leipzig and in wastewater (blue) at the municipal treatment plant in Leipzig for 2021 and 2022, presented as aggregate data. Passive samplers were not sampled in the months April, Oct.-Dez. 2021 and March and July 2022.
Overall, the results show that passive samplers can detect SARS-CoV-2 GC more consistently in wastewater. This is probably because of the larger volume of sewage passing the passive sampler compared to that which can be collected by 24 h composite wastewater samples. It, therefore, can provide a time-integrated measurement of the sampled matrix and greater sensitivity (Bivins et al., 2022; Li et al., 2022), but over a much shorter time.
In our study, we assumed that SARS-CoV-2 GC in wastewater reflects a more realistic level of infections in the population and that it can also be detected by other sampling procedures such as passive samplers as discussed above. However, to date the health authorities usually rely on the 7-day incidence, which is then put in relation to results from wastewater for instance. This in our opinion highly underestimates the real infection rate in the population and certainly can give a completely wrong impression. Since the results of the passive samplers are validated against the results of the composite wastewater monitoring, thus to prove this assumption, SARS-CoV-2 GC in wastewater are discussed briefly in the following.
Among the 217 wastewater samples taken at the municipal WWTP, approximately 82 % were positive for SARS-CoV-2 GC in the 16 months from March 2021 to August 2022.
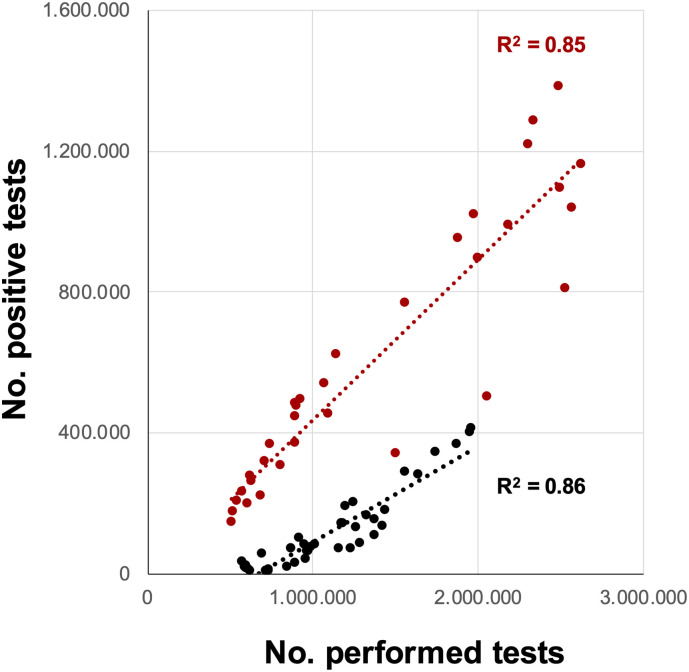
It has been shown that a clear quantification and correlation of SARS-CoV-2 GC in wastewater with reported case numbers (7-day incidence) is difficult; this has also been reported by Wu et al. (2020a), who noted that SARS-CoV-2 in wastewater may not accurately reflect the incidence levels, while there might be an age dependence between the viral load in wastewater and the number of confirmed cases (Omori et al., 2021). A trend is detectable, as also found by Ho et al. (2022). However, particularly in the period January to May 2022, when the Delta variant was being replaced by the Omicron variant (Paton et al., 2022), this trend was no longer visible and did not follow the 7-day incidence. When calculating the 7-day incidence, only the average daily number of newly detected COVID-19 cases over a period of 7 days is taken into account. However, the overall number of tests carried out in the population, to normalize the data, is not considered. As shown in Fig. 5 , the overall number of RT-qPCR tests and number of positive RT-qPCR tests are highly correlated (2021: R2 = 0.86; 2022: R2 = 0.85). More tests result in higher detection rates, that means the likelihood to identify SARS-CoV-2 positive individuals was increased. At the same time, the risk to obtain false positives also increases (Braunstein et al., 2021). Surprisingly, the two years 2021 and 2022 are clearly clustered away from each other, probably due to a change of the sensitivity of the RT-qPCR tests (e.g. new chemistry).
Fig. 5.
Relationship between the weekly number of performed RT-qPCR tests carried out and the number of positive RT-qPCR tests from March 2021 to August 2022, separated for the year 2021 (black) and 2022 (red). Data on the number of RT-qPCR tests carried out and positive RT-qPCR tests were retrieved from the ‘Robert Koch Institute (RKI)’ (www.rki.de), which is the German federal government agency and research institute responsible for disease control and prevention.
For the municipal wastewater treatment plant in Leipzig, a relationship between the weekly number of positive tests per 100,000 tests in wastewater, and the detected SARS-CoV-2 GC in wastewater is also visible (Fig. A2), clearly showing how changes in dominant virus variant shifts the relationship between wastewater measurements and clinical – likely due to shedding distribution/rates. Therefore, due to the importance of the overall number of tests carried out, SARS-CoV-2 GC from March 2021 until August 2022 were correlated to the number of positive tests per 100,000 tests carried out (Fig. A3). This relationship is more pronounced and, as stated before, a further indication that the SARS-CoV-2 GC in wastewater does indeed reflect a more realistic level of potential infections in the community.
Since the official incidence levels are highly dependent on the number of tests carried out, it does not accurately represent a realistic infection level of SARS-CoV-2 in the population, and correlations with SARS-CoV-2 GC in wastewater are unlikely or often just a coincidence also depending on the number of samples taken. Nevertheless, our observation of SARS-CoV-2 GC in wastewater in the long-term, with over 200 analyses, show that WBE for SARS-CoV-2 can be considered as a wastewater early-warning system as stated, for example, by Rossmann et al. (2021) and Rossmann et al. (2022), however at this stage only retrospectively. Wastewater-based monitoring of SARS-CoV-2 makes sense as a complementary and cheaper method compared to individual, and often unnecessary, testing in the community.
3.2. Detection of SARS-CoV-2 in passive samplers at a district-scale level
Since it was not possible to narrow down further potential ‘hot spots’ of SARS-CoV-2 infections at city-scale level to a specific community/district, due to logistical reasons as described above, we carried out a second experiment with passive samplers on district-scale level. The experiment was set up at the campus of the Helmholtz-Centre for Environmental Research – UFZ, with an internal network of a combined sewer and approximately 700 employees. It is important to note is that due to Covid restriction and working from home regulations, fewer employees were present during the experimental trial.
An initial comparison of the three investigated small catchment areas showed that the detected total suspended solid content in the wastewater was clearly higher at S2 (Fig. A4). This is in accordance with the observations during sampling, since the wastewater at S2 only originates from office buildings, whereas the catchment of S3 and S4 include offices, laboratories, and garages. We assumed that, therefore, the chances were greater of finding higher SARS-CoV-2 GC levels at S2. However, no significant differences in SARS-CoV-2 GC between the three micro-catchment areas (S2, S3 and S4) were found. Contrary, Hayes et al. (2022) described in both field and isotherm experiments, equilibrium behavior and viral recovery of passive samplers to be associated with total suspended solid concentrations, highlighting when high TSS concentrations are apparent, viral quantification may appear low or even absent, regardless of the viral concentration.
Overall, 197 samples, made up of 58 wastewater and 139 samples from passive samplers, were analysed and it was possible to detect SARS-CoV-2 GC. Some 37 % of the samples of the wastewater, 31 % of the cotton-cloth samples, 52 % of the unravelled rope samples and 61 % of the polyethylene-based samples tested positive for SARS-CoV-2 (Table A2). Individual number of samples differed, because on a weekly basis, not always enough material could be removed from the passive samplers.
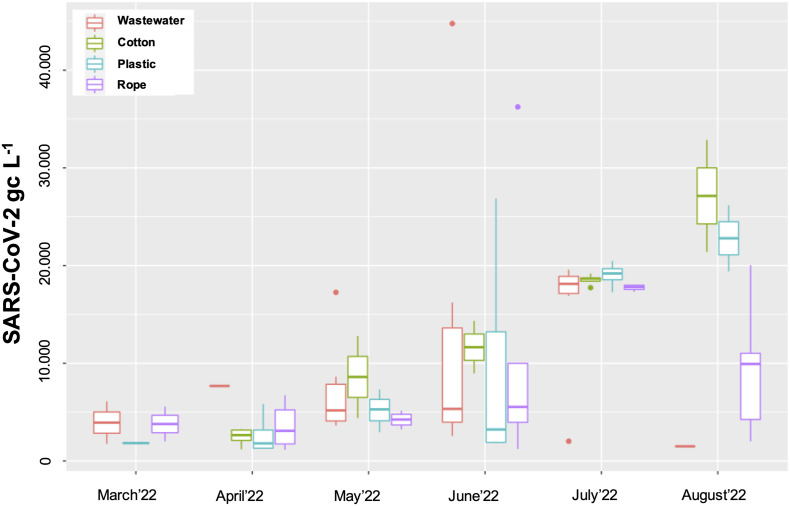
On a monthly basis, small variations between samples, especially in June and August 2022, were visible and there was no significant difference between SARS-CoV-2 GC in passive samplers and wastewater (Fig. 6 ). Overall, a monthly increase of SARS-CoV-2 GC was detected between March and July 2022, with a slight decrease in August 2022. SARS-CoV-2 GC was detected more frequently in passive samplers (plastic: 65 %; cotton-cloth: 72 %; unravelled rope: 95 %) than in the wastewater (37 %). However, this could also be because grab samples of the wastewater were taken, and it was more likely to miss peaks of flushes in the wastewater.
Fig. 6.
Detection of SARS-CoV-2 GC in wastewater as well as in passive samplers made of three different materials: cotton-cloth; plastic and unravelled rope in wastewater at district level in 2022. Solid line, boxes, and whiskers: median, IQR and 1.5 × IQR, respectively.
From a practical viewpoint, retrieving samples was easiest from the polyethylene-based passive samplers, however, samples could not be taken every week. The cotton-cloth was saturated with wastewater and small particles accumulated in the cotton, and could be taken on a weekly basis, however, the material was vulnerable to decay. Finally, a significant amount of suspended solid substance accumulated in the unravelled rope, and it was sometimes difficult to retrieve material for analysis.
More information on the stability of SARS-CoV-2 GC on passive samplers is needed. While some studies have reported that the persistence of SARS-CoV-2 is up to 50 days at temperatures of 4–6 °C (Silverman and Boehm, 2020), 1–2 days at 20° (Bivins et al., 2020), others have reported a decay of only several hours at temperatures of 4, 10 and 35 °C (Weidhaas et al., 2021). In a small side experiment, to evaluate the decay of SARS-CoV-2 GC on passive samples, we found that even after 196 h after sampling, positive signals were still detectable in our samples.
The dilution of SARS-CoV-2 GC signals in wastewater has been reported by Ahmed et al. (2020b), Liu et al. (2022) and Li et al. (2022), who saw that signals were significantly reduced and dilution was the dominant factor influencing the fate of SARS-CoV-2 GC. We can also confirm these results. If sampling took place the day following a rain event with >3.2 L m−2, no SARS-CoV-2 GC could be detected. This was the case for, in total, four sampling events.
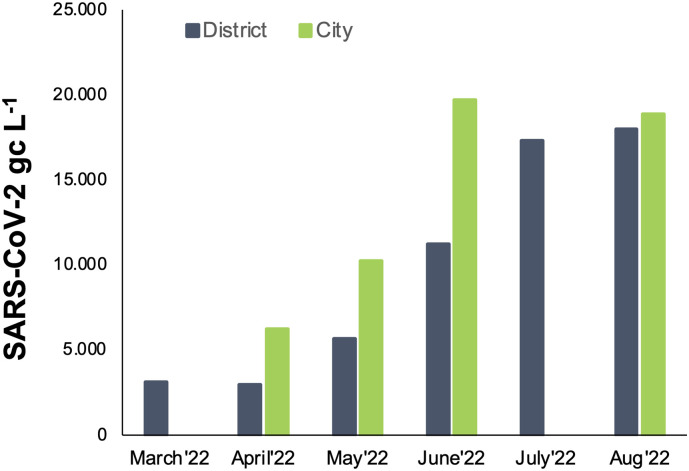
The comparison of the results from passive samplers taken at district-scale level with the ones from city-scale level, showed that the district-scale level results are similar, although on a slightly lower level, to those at city-scale (Fig. 7 ). The results presented demonstrated that even on a small-scale, the use of passive samplers allows an easy and cost-effective detection of SARS-CoV-2 and is thus probably well suited for the detection of infections in a population. Thus, passive samplers can be considered as a simple tool for the identification of potential ‘hot spots’ on various scales.
Fig. 7.
Comparison of detected SARS-CoV-2 GC in passive samplers at city-scale level (green) and district-scale level (dark blue) in wastewater from March–August 2022, presented as aggregate data.
4. Conclusions
Our results show that passive samplers in wastewater at the city-scale level can provide an integrated signal of SARS-CoV-2 GC over several days indicating an accurate level of positive infections in the population. In addition, on a small-scale level, the use of passive samplers was also feasible and effective to detect SARS-CoV-2 easily and cost-effectively, mirroring a similar trend to that at a city-scale level. Thus, we demonstrated that passive samplers can be considered as a simple monitoring tool for identifying potential ‘hot spots’ during low incidence levels at various scales where the detection of SARS-CoV-2 GC can be considered as a signal of a possible (re)occurrence.
Passive samplers provide an integrated signal of SARS-CoV-2 GCover several days and are therefore ideal for routine monitoring. This is particularly relevant in the German context, where polyethylene-based passive samplers are already sampled across cities, albeit only to identify illegal wastewater discharges containing heavy metals. Passive samplers have the advantage that they can be used at different locations in settlement areas without using complex and cost intensive monitoring equipment (pumps, samplers). They do not need a power supply; sampling is not time consuming (<1 h) and can be done without significant disturbance to road traffic. To conclude, we have shown that passive samplers can provide reproducible SARS-CoV-2 signals from wastewater. We recommend that passive samplers could be used as an alternative method for WBE for SARS-CoV-2 and can be considered for estimating the level of infection in the population, because the calculation is not based on individual testing, which we could show does not accurately represent a realistic infection level of SARS-CoV-2 in the population (7-day incidence). This is particularly relevant for resource-limited communities.
Limitation of the study were that we did not know, whether the 13 sub-catchments represent a relatively uniform percentage of the population or if some catchments represent a greater proportion of the population than others. Furthermore, clinical data on sub-catchment level was not available for the study. Including this kind of detailed data in future work will help to get an even better insight into the use of passive samplers in wastewater. Furthermore, more research is needed since it is still difficult to quantify SARS-CoV-2 with confidence. In general, some uncertainties concerning the collection efficiency of passive samplers still exist and sorption, desorption, and potential degradation of viral RNA over a time-interval are still not fully understood. Finally, the use of passive samplers in WBE should not be limited to the detection of SARS-CoV-2, but also rather also include other pathogens of concern.
CRediT authorship contribution statement
Marc Breulmann: Conceptualization, Methodology, Validation, Investigation, Visualization, Writing – original draft. René Kallies: Methodology, Validation, Writing – review & editing. Katy Bernhard: Methodology, Investigation. Andrea Gasch: Methodology. Roland Arno Müller: Supervision, Funding acquisition. Hauke Harms: Funding acquisition. Antonis Chatzinotas: Methodology, Validation, Writing – review & editing, Project administration, Funding acquisition. Manfred van Afferden: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Sächsische Aufbaubank (SAB), grant no. 100535971 and is part of the project “Abwasser-CoV-2-Tracking: Abwassermonitoring zur Bestimmung des SARS-CoV-2-Infektionsgrads der Bevölkerung: Aufbau eines flächendeckenden Warn- und Trackingsystems für Pandemien”. We thank Grit Weichert for assistance during sampling and laboratory work, Franziska Beier and Jeremy Knespel for technical assistance in the laboratory and Jörg Berbig from the municipal waterworks (Kommunale Wasserwerke Leipzig - KWL).
Editor: Warish Ahmed
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2023.164143.
Appendix A. Supplementary data
Supplementary material
Data availability
The data that has been used is confidential.
References
- Acer P.T., Kelly L.M., Lover A.A., Butler C.S. Quantifying the relationship between SARS-CoV-2 wastewater concentrations and building-level COVID-19 prevalence at an isolation residence: a passive sampling approach. Int. J. Environ. Res. Public Health. 2022;19 doi: 10.3390/ijerph191811245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Travel Med. 2020;27 doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Simpson S.L., Bertsch P.M., Bibby K., Bivins A., Blackall L.L., et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022;805 doi: 10.1016/j.scitotenv.2021.149877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martinez-Lamas L., Vasallo F.J., Regueiro B., et al. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Sci. Total Environ. 2021;772 doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., et al. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Lott M., Shaffer M., Wu Z.Y., North D., Lipp E.K., et al. Building-level wastewater surveillance using tampon swabs and RT-LAMP for rapid SARS-CoV-2 RNA detection. Environ. Sci. Water Res. Technol. 2021;8:173–183. [Google Scholar]
- Bivins A., Kaya D., Ahmed W., Brown J., Butler C., Greaves J., et al. Passive sampling to scale wastewater surveillance of infectious disease: lessons learned from COVID-19. Sci. Total Environ. 2022;835 doi: 10.1016/j.scitotenv.2022.155347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boës M., Caspary H. Sielhautuntersuchungen - Ein erfolgversprechende Methode zum Auffinden von Schwermetallemittenten im Kanalnetz. Korresp. Abwasser. 1987;34:23–128. [Google Scholar]
- Braunstein G.D., Schwartz L., Hymel P., Fielding J. False positive results with SARS-CoV-2 RT-PCR tests and how to evaluate a RT-PCR-positive test for the possibility of a false positive result. J. Occup. Environ. Med. 2021;63:e159–e162. doi: 10.1097/JOM.0000000000002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchis-Scott R., Geng Q., Seth R., Ray R., Beg M., Biswas N., et al. Averting an outbreak of SARS-CoV-2 in a university residence hall through wastewater surveillance. Microbiol. Spectr. 2021;9 doi: 10.1128/Spectrum.00792-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:23. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crank K., Chen W., Bivins A., Lowry S., Bibby K. Contribution of SARS-CoV-2 RNA shedding routes to RNA loads in wastewater. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis K., Keeling D., Yetka K., Larson A., Gonzalez R. 2021. Wastewater SARS-CoV-2 RNA concentration and loading variability from grab and 24-hour composite samples. Preeprint. medRxiv (2020.07.10.20150607) [Google Scholar]
- Dumke R., de la Cruz Barron M., Oertel R., Helm B., Kallies R., Berendonk T.U., et al. Evaluation of two methods to concentrate SARS-CoV-2 from untreated wastewater. Pathogens. 2021:10. doi: 10.3390/pathogens10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvallet C., Wu F., McElroy K.A., Imakaev M., Endo N., Xiao A., et al. Nationwide trends in COVID-19 cases and SARS-CoV-2 RNA wastewater concentrations in the United States. ACS ES T Water. 2022;2:1899–1909. doi: 10.1021/acsestwater.1c00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik B., Tavazzi S., Mariani G., Skejo H., Sponar M., Higgins T., et al. EUR 30684 EN, Publications Office of the European Union; Luxembourg: 2021. SARS-CoV-2 Surveillance Employing Sewage: Towards a Sentinel System. [Google Scholar]
- George A.D., Kaya D., Layton B.A., Bailey K., Mansell S., Kelly C., et al. Impact of sampling type, frequency, and scale of the collection system on SARS-CoV-2 quantification fidelity. Environ. Sci. Technol. Lett. 2022;9:160–165. doi: 10.1021/acs.estlett.1c00882. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Collado N., Abasolo N., Anzaldi G., Bofill-Mas S., Bosch A., et al. The Catalan Surveillance Network of SARS-CoV-2 in sewage: design, implementation, and performance. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-20957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Parker J., Smits S., Underwood J., Dolwani S. Persistent viral shedding of SARS-CoV-2 in faeces - a rapid review. Color. Dis. 2020;22:611–620. doi: 10.1111/codi.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtewold J., McCarthy D., McBean E., Law I., Goodridge L., Habash M., et al. Passive sampling, a practical method for wastewater-based surveillance of SARS-CoV-2. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E.K., Sweeney C.L., Anderson L.E., Li B., Erjavec G.B., Gouthro M.T., et al. A novel passive sampling approach for SARS-CoV-2 in wastewater in a Canadian province with low prevalence of COVID-19. Environ. Sci. Water Res. Technol. 2021;7:1576–1586. [Google Scholar]
- Hayes E.K., Stoddart A.K., Gagnon G.A. Adsorption of SARS-CoV-2 onto granular activated carbon (GAC) in wastewater: implications for improvements in passive sampling. Sci. Total Environ. 2022;847 doi: 10.1016/j.scitotenv.2022.157548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Stange C., Suhrborg R., Wurzbacher C., Drewes J.E., Tiehm A. SARS-CoV-2 wastewater surveillance in Germany: long-term RT-digital droplet PCR monitoring, suitability of primer/probe combinations and biomarker stability. Water Res. 2022;210 doi: 10.1016/j.watres.2021.117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning U., Sbieschni C. Schwermetallproblematik in Klärschlämmen - Lokalisierung von Emittenten mit Hilfe von Sielhautanalytik. Wasserwirtsch. Wassertech. 1991:2. [Google Scholar]
- Jones D.L., Grimsley J.M.S., Kevill J.L., Williams R., Pellett C., Lambert-Slosarska K., et al. Critical evaluation of different passive sampler materials and approaches for the recovery of SARS-CoV-2, faecal-indicator viruses and bacteria from wastewater. Water. 2022;14:3568. [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., et al. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Sait A., Kadi H., Sarac F., Aydın I., et al. Nationwide SARS-CoV-2 surveillance study for sewage and sludges of wastewater treatment plants in Turkey. Preprint. medRxiv. 2020 (2020.11.29.20240549) [Google Scholar]
- Lee S., Kim T., Lee E., Lee C., Kim H., Rhee H., et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment Center in the Republic of Korea. JAMA Intern. Med. 2020;180:1447–1452. doi: 10.1001/jamainternmed.2020.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ahmed W., Metcalfe S., Smith W.J.M., Tscharke B., Lynch P., et al. Monitoring of SARS-CoV-2 in sewersheds with low COVID-19 cases using a passive sampling technique. Water Res. 2022;218 doi: 10.1016/j.watres.2022.118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Ibaraki M., VanTassell J., Geith K., Cavallo M., Kann R., et al. A sensitive, simple, and low-cost method for COVID-19 wastewater surveillance at an institutional level. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.151047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mlejnkova H., Sovova K., Vasickova P., Ocenaskova V., Jasikova L., Juranova E. Preliminary study of Sars-Cov-2 occurrence in wastewater in the Czech Republic. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17155508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori R., Miura F., Kitajima M. Age-dependent association between SARS-CoV-2 cases reported by passive surveillance and viral load in wastewater. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton R.S., Overton C.E., Ward T. The rapid replacement of the SARS-CoV-2 Delta variant by Omicron (B.1.1.529) in England. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abo5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rafiee M., Isazadeh S., Mohseni-Bandpei A., Mohebbi S.R., Jahangiri-Rad M., Eslami A., et al. Moore swab performs equal to composite and outperforms grab sampling for SARS-CoV-2 monitoring in wastewater. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann K., Clasen R., Munch M., Wurzbacher C., Tiehm A., Drewes J.E. SARS-CoV-2 crisis management with a wastewater early-warning system in the Bavarian District of Berchtesgadener Land, Germany. Dtsch. Arztebl. Int. 2021;118:479–480. doi: 10.3238/arztebl.m2021.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann K., Grossmann G., Frangoulidis D., Clasen R., Munch M., Hasenknopf M., et al. Innovative SARS-CoV-2 crisis management in the public health sector: Corona dashboard and wastewater surveillance using the example of Berchtesgadener Land, Germany. Bundesgesundheitsbl. Gesundheitsforsch. Gesundheitsschutz. 2022;65:367–377. doi: 10.1007/s00103-021-03425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang C., Crosbie N.D., Nolan M., Poon R., Wang M., Jex A., et al. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ. Sci. Technol. 2021;55:10432–10441. doi: 10.1021/acs.est.1c01530. [DOI] [PubMed] [Google Scholar]
- Silverman A.I., Boehm A.B. Systematic review and meta-analysis of the persistence and disinfection of human coronaviruses and their viral surrogates in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:544–553. doi: 10.1021/acs.estlett.0c00313. [DOI] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Hubert F., Morga B., Renault T., Le Guyader F.S. Adsorption of norovirus and ostreid herpesvirus type 1 to polymer membranes for the development of passive samplers. J. Appl. Microbiol. 2017;122:1039–1047. doi: 10.1111/jam.13394. [DOI] [PubMed] [Google Scholar]
- Vincent-Hubert F., Wacrenier C., Desdouits M., Jousse S., Schaeffer J., Le Mehaute P., et al. Development of passive samplers for the detection of SARS-CoV-2 in sewage and seawater: application for the monitoring of sewage. Sci. Total Environ. 2022;833 doi: 10.1016/j.scitotenv.2022.155139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M., Qiu Y., Yu J., Lee B.E., McCarthy D.T., Pang X. Comparison of auto sampling and passive sampling methods for SARS-CoV-2 detection in wastewater. Pathogens. 2022:11. doi: 10.3390/pathogens11030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020:5. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(1831–1833) doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that has been used is confidential.