Postpartum hemorrhage continues to be the leading prevent-able cause of maternal illness and death globally.1,2 Worldwide, postpartum hemorrhage accounts for 8% of maternal deaths in developed regions of the world and 20% of maternal deaths in developing regions.2 The United States has one of the highest maternal mortality rates among developed countries, with approximately 11% of all maternal deaths associated with postpartum hemorrhage.3 During the period from 1993 through 2014, the rate of postpartum hemorrhage (which was defined as blood loss >1000 ml after vaginal or cesarean delivery) requiring a blood transfusion4 increased from approximately 8 cases per 10,000 deliveries to 40 per 10,000 deliveries in the United States.5
With the increasing prevalence of postpartum hemorrhage, well-designed cohort studies and randomized clinical trials that evaluate interventions that are critical for predicting, preventing, and managing postpartum hemorrhage remain a high priority.6 However, as a result of challenges in the quantification of blood loss, various definitions of postpartum hemorrhage, and differences in outcome reporting, data from relevant randomized clinical trials are difficult to interpret and compare across studies.6 In addition, guidelines for preventing and managing postpartum hemorrhage vary substantially among major national obstetrics and gynecology organizations, including the American College of Obstetricians and Gynecologists,7 the Society of Obstetricians and Gynaecologists of Canada, the French College of Gynecologists and Obstetricians, the Royal College of Obstetricians and Gynaecologists (United Kingdom), and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists.8 This review discusses the causes, identification, management, prevention, and prediction of postpartum hemorrhage.
DEFINITION, CAUSES, AND RISK FACTORS
The normal rate of blood flow to the uterus at full term is approximately 600 ml per minute, in contrast to approximately 60 ml per minute in the nonpregnant state.9 The control of postpartum blood loss depends primarily on uterine contractions and, to a lesser degree, on activation of the coagulation cascade.
The traditional definition of postpartum hemorrhage is blood loss of more than 500 ml after a vaginal delivery or more than 1000 ml after a cesarean delivery.10 More recently, postpartum hemorrhage has been redefined as a cumulative blood loss of 1000 ml or more or blood loss associated with signs or symptoms of hypovolemia, irrespective of the route of delivery.10 Typical clinical signs and symptoms of hypovolemia (e.g., hypotension and tachycardia) due to postpartum hemorrhage may not appear until blood loss exceeds 25% of total blood volume (>1500 ml during late pregnancy).11
Postpartum hemorrhage is considered to be primary when it occurs within the first 24 hours after delivery and secondary when it occurs between 24 hours and up to 12 weeks after delivery.10,12 The causes of postpartum hemorrhage can be summarized by the four “T’s”: tone (uterine atony), trauma (lacerations or uterine rupture), tissue (retained placenta or clots), and thrombin (clotting-factor deficiency).10 The most common cause is uterine atony (accounting for approximately 70% of cases), followed by obstetrical lacerations (approximately 20%), retained placental tissue (approximately 10%), and clotting-factor deficiencies (<1%).10 Postpartum hemorrhage can lead to severe anemia requiring blood transfusion, disseminated intravascular coagulopathy, hysterectomy, multisystem organ failure, and death.10
Postpartum hemorrhage due to uterine atony is often preceded by chorioamnionitis, therapeutic use of magnesium sulfate, prolonged labor or precipitous delivery, labor induction or augmentation, uterine fibroids, or uterine overdistention as a result of multiple gestation, fetal macrosomia, or polyhydramnios. Cesarean delivery is associated with a higher risk of postpartum hemorrhage than vaginal delivery. Advanced maternal age and extremes of parity (0 and >4) are additional risk factors.
Other risk factors for postpartum hemorrhage are closely linked to the type of hemorrhage that develops. For example, obstetrical lacerations can be caused by operative vaginal delivery, precipitous delivery, or episiotomy, whereas retained placental tissue can be caused by placenta accreta spectrum (PAS; a spectrum of abnormal placentation disorders, including placenta accreta, placenta increta, and placenta percreta), which is associated with prior uterine surgery. Retained placental tissue can also be the result of incomplete delivery of the placental tissue and membranes. Maternal coagulopathy that leads to postpartum hemorrhage can be a complication of severe preeclampsia and eclampsia, HELLP (hemolysis, elevated liver-enzyme level, and low platelet count) syndrome, intrauterine fetal death, placental abruption, or a coagulation disorder that is acquired (e.g., amniotic fluid embolism) or inherited.
Despite efforts to identify patients who are at increased risk for postpartum hemorrhage, this life-threatening complication often occurs in women who have no identifiable risk factors.10 Therefore, vigilance is crucial after all deliveries.
MANAGEMENT OF POSTPARTUM HEMORRHAGE
GENERAL APPROACH
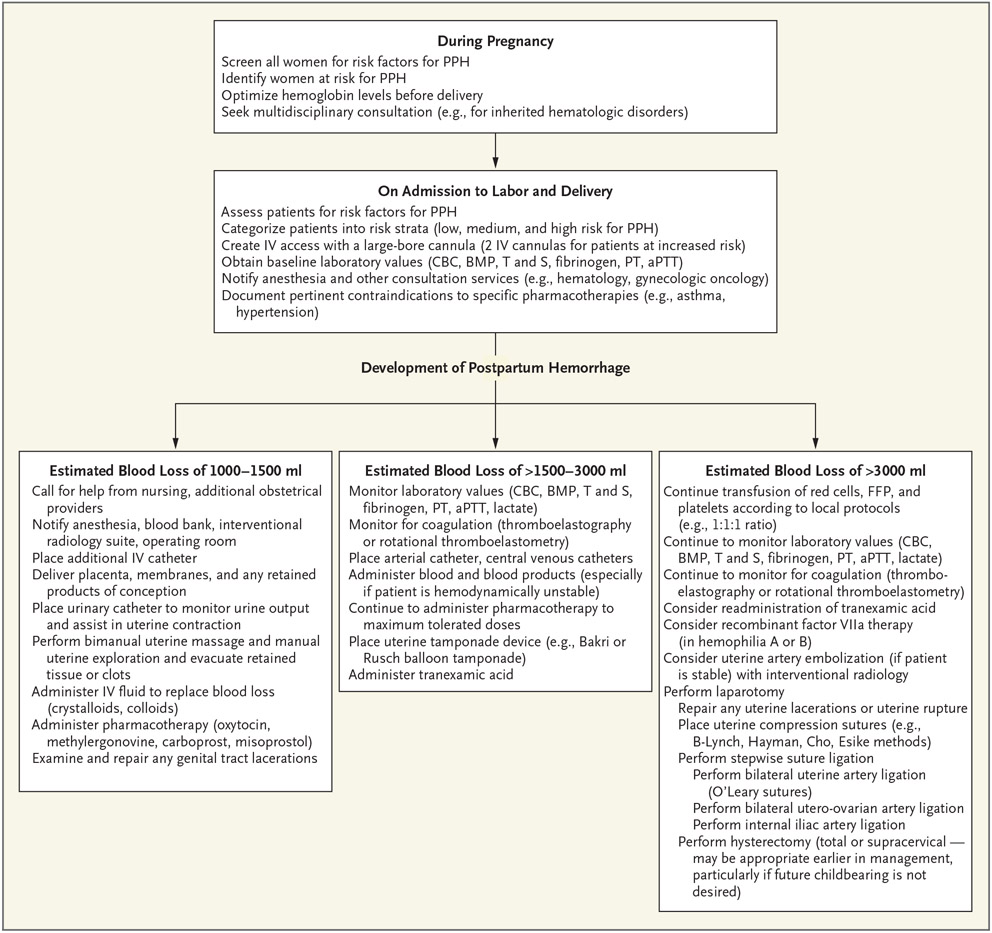
Management of postpartum hemorrhage necessitates a coordinated multidisciplinary approach, which involves good communication, accurate assessment of blood loss, monitoring of maternal vital signs and symptoms, fluid replacement, and arrest of the source of hemorrhage, all occurring concurrently (Fig. 1).10,13 Assessment of ongoing blood loss is a critical step in the management of postpartum hemorrhage. Blood loss can be assessed on the basis of visual estimation or weighing of materials, including blood and amniotic fluid–soaked surgical sponges and drapes.7 Although there is no strong evidence that one method of assessing blood loss is better than the other, quantification provides a more accurate estimation of blood loss, as compared with subjective assessment.7,14 Morbidity among women with severe postpartum hemorrhage may be reduced when quantitative estimation of blood loss is used as a component of maternal safety protocols,7 but this method has not consistently been found to improve clinical outcomes. A recent Cochrane review and meta-analysis showed no evidence that quantitative estimation of blood loss reduces the need for uterotonic agents, blood transfusion, or volume expanders during postpartum hemorrhage.15 Despite these limitations, some obstetrics and gynecology societies7 favor quantification of blood loss by weighing of blood-soaked materials (lap sponges and pads), monitoring of fluid used during irrigation, and use of underbuttock, graduated cylinder drapes during postpartum hemorrhage. More recently, the use of colorimetric techniques, which involve electronic artificial intelligence (e.g., smartphone applications), to estimate blood loss in real time seems encouraging.16
Figure 1. Postpartum Hemorrhage (PPH) Screening, Evaluation, and Management.
The abbreviation aPTT denotes activated partial-thromboplastin time, BMP basic metabolic panel, CBC complete blood count, FFP fresh-frozen plasma, IV intravenous, PT prothrombin time, and T and S type and screen.
Once a woman is admitted for delivery, if there is a high index of suspicion for the development of postpartum hemorrhage (e.g., placenta previa, PAS, or active vaginal bleeding), two large-bore intravenous cannulas should be inserted, a complete blood count should be obtained, and a specimen should be sent to the blood bank. The blood bank should be notified, and at least 2 units of blood should be typed and cross-matched for the patient. Additional maternal monitoring should be tailored to the cause and degree of increased risk of postpartum hemorrhage and can include the use of continuous pulse oximetry, assessment of urine output with the use of an indwelling bladder catheter, continuous cardiac monitoring, assessment of coagulation status (on the basis of prothrombin time, fibrinogen level, and activated thromboplastin time), and a comprehensive metabolic panel.10 If the patient is at very high risk for postpartum hemorrhage, central venous and arterial catheters should be placed. A heating–cooling circulating water pad or a forced-air warming system may be used to help mitigate the hypothermia that is often associated with massive fluid resuscitation and prolonged surgery. Although both crystalloids and colloids can be used as intravenous fluids,17 crystalloids are slightly favored over colloids.18
MANAGEMENT OF RETAINED PLACENTAL TISSUE
Inspection of the placenta after delivery is important to rule out retained placental tissue or a retained succenturiate lobe of the placenta (an abnormality in the placental structure in which one or more accessory lobes is connected to the main part of the placenta by blood vessels). When retained placental tissue is suspected, evacuation with manual exploration or a banjo (blunt) curette under ultrasonographic guidance is recommended; the positive and negative predictive values of ultrasonography in detecting retained placental tissue are approximately 58% and 87%, respectively.19 Abnormal uterine bleeding that warrants manual removal of the placenta increases the likelihood that the bleeding is due to a PAS disorder.20
MANAGEMENT OF GENITAL TRACT LACERATIONS
Careful inspection of the lower genital tract for cervical, vaginal, perineal, or rectovaginal lacerations is important. Lacerations should be promptly repaired with absorbable sutures. There is insufficient evidence in support of routine antibiotic prophylaxis after uterine evacuation for postpartum hemorrhage or repair of a perineal laceration.21
MANAGEMENT OF UTERINE ATONY
Bimanual uterine massage is usually the first step in the management of postpartum hemorrhage due to uterine atony. Massage is performed in an attempt to induce uterine contractions by stimulating endogenous prostaglandins.10,22 Oxytocin (administered intravenously or intramuscularly) is the mainstay of treatment for controlling postpartum hemorrhage due to uterine atony; administration of oxytocin is usually begun simultaneously with uterine massage, if the agent has not already been administered prophylactically. The uterine response after the administration of intravenous oxytocin is usually immediate (oxytocin half-life, 1 to 6 minutes in plasma).23
Additional agents (e.g., methylergonovine maleate, a semisynthetic ergot alkaloid) and intramuscular prostaglandins (e.g., carboprost tromethamine, a 15-methyl analogue of prostaglandin F2α) can be used as second-line pharmacotherapy to control postpartum hemorrhage. A Cochrane review and meta-analysis have questioned the usefulness of misoprostol, a prostaglandin E1 analogue.24,25 Although oxytocin causes rhythmic contractions of the uterus, methylergonovine maleate stimulates uterine smooth muscle and uterine vascular α1-adrenergic receptors in a sustained manner, causing vasoconstriction and cessation of bleeding. Methylergonovine maleate is often considered the next agent to be administered after oxytocin.23 The indications and contraindications for pharmacotherapy in postpartum hemorrhage are listed in Table 1.
Table 1.
Medical Therapy for Postpartum Hemorrhage.*
| Medication | Mechanism of Action | Route of Administration and Dose | Concerns and Contraindications | Adverse Effects |
|---|---|---|---|---|
| First-line therapy: oxytocin | Stimulates oxytocin receptors in the uterus | IV route, 10–40 IU/500–1000 ml of lactated Ringer’s solution; IM or IMM route, 5–10 IU for up to 4 doses | SIADH, hypotension | Rapid bolus administration may cause hyponatremia, hypotension, tachycardia, and arrhythmia |
| Second-line therapy | ||||
| Methylergonovine maleate (ergot alkaloid) | Partial agonist or antagonist at serotoninergic, dopaminergic, α1-adrenergic receptors in the uterus | IM or IMM route, 0.2 mg every 2–4 hr, for a maximum of 5 doses; oral route, 0.2 mg every 6–8 hr for 2–7 days | Hypertension, cardiovascular disease (stroke, Renaud’s disease) | Elevated blood pressure, nausea, vomiting, myocardial infarction |
| Carboprost tromethamine (PGF2α) | PGF2α agonist in uterine myometrium | IM or IMM route, 250 μg every 15–90 min for a maximum of 8 doses | Asthma, cardiovascular disease, hepatic disease, renal disease | Nausea, vomiting, and diarrhea |
| Adjunctive agents | ||||
| Tranexamic acid | Diminishes the dissolution of hemostatic fibrin by plasmin, stabilizing clot in uterine vessels | IV route, 1 g (100 mg/ml) over a 10-min period; if bleeding persists after 30 min or stops and restarts within 24 hr after the first dose, a second dose may be administered | Contraindicated if known hypersensitivity to tranexamic acid, thromboembolic event during pregnancy, history of hypercoagulopathy | Headache, musculoskeletal pain, nausea, diarrhea |
| Recombinant factor VIIa | Activates clotting cascade by cleaving factor IX and factor X, which activates these factors and leads to activation of thrombin and fibrin | IV route, 50–100 μg/kg (single dose) | Severe anemia, severe thrombocytopenia, hyperfibrinogenemia, allergy to mouse, hamster, or bovine proteins | Thromboembolic events, cerebrovascular infarcts, myocardial infarction |
| Treatment of uncertain usefulness: misoprostol | PGE1 agonist in the uterine myometrium | Sublingual, oral, or rectal route (sublingual route preferred), 600–1000 μg in single dose; repeat doses not recommended | Sepsis, allergy to misoprostol, concurrent anticoagulant therapy, cardiovascular disease; efficacy is disputed | Nausea, vomiting, fever, diarrhea |
IM denotes intramuscular, IMM intramyometrial, IV intravascular, PGE1 prostaglandin E1, PGF2α 15-methyl prostaglandin F2α, and SIADH syndrome of inappropriate antidiuretic hormone secretion.
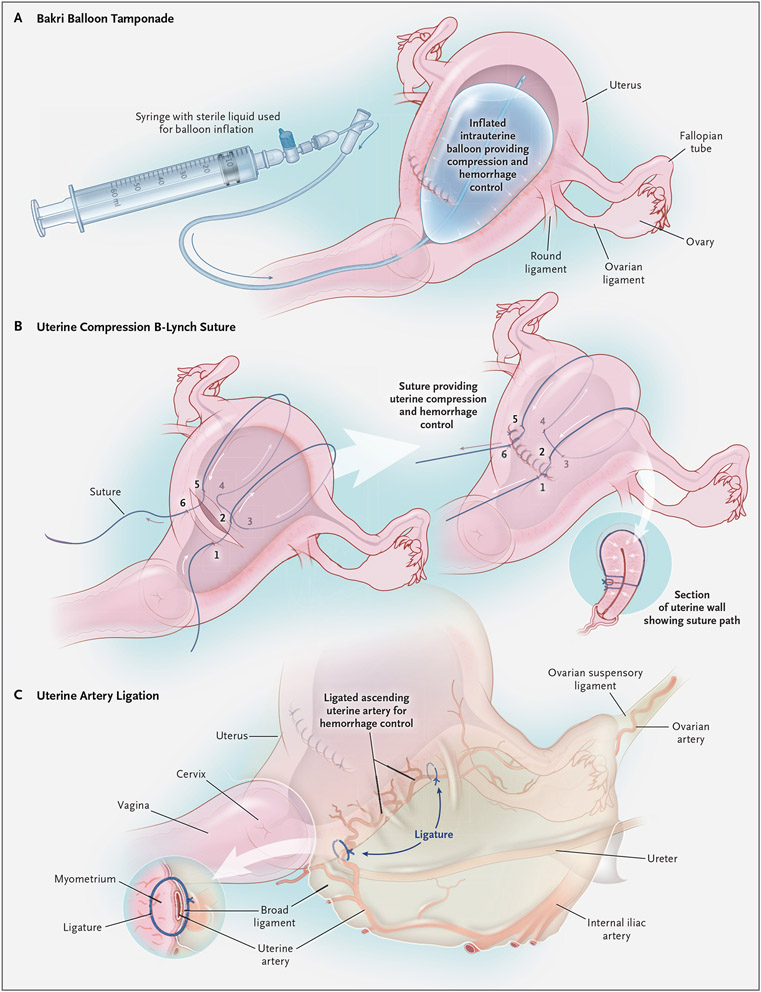
If pharmacotherapy fails in the management of uterine atony, mechanical methods, including balloon tamponade (Fig. 2A) and uterine compression sutures (Fig. 2B), can be lifesaving. Balloon tamponade systems, such as the Bakri balloon, first described in 2001,26 involve instilling fluid (to a maximum volume of approximately 500 ml) into an intrauterine balloon, with removal of the balloon up to 24 hours after insertion; the tamponade effect of the filled balloon is intended to stop or reduce intrauterine bleeding.27 A 2020 systematic review and meta-analysis concluded that uterine balloon tamponade systems appear to be safe,28 with a success rate of more than 85% in the management of postpartum hemorrhage.
Figure 2 (facing page). Mechanical Methods for Managing Uterine Atony.
Panel A shows balloon tamponade (with a Bakri balloon), Panel B uterine compression sutures (B-Lynch sutures, placed according to the numbers, from 1 to 6), and Panel C uterine artery ligation.
Uterine compression sutures, also known as “brace sutures,” were first described in 1997 by B-Lynch and colleagues and were shown to be highly effective in controlling postpartum hemorrhage.29 Since 1997, several other compression suture techniques have been described.30-36 Some techniques involve sutures that enter the uterine cavity34 and abut the anterior and posterior walls of the uterus, with a potential to increase the risk of uterine synechiae, but other techniques do not.29,36 Several systematic reviews of case series have shown a combined success rate of more than 90% with the use of brace sutures in managing postpartum hemorrhage.37,38 Uterine necrosis and intrauterine synechiae are possible complications of uterine compression procedures.39,40 The frequency of successful pregnancy after management of postpartum hemorrhage with uterine compression sutures has ranged from 11 to 75%.37
Uterine and vaginal packing has been used successfully in cases of postpartum hemorrhage, but it is not routinely recommended because of the potential for intrauterine infection.41 Although a positive tamponade test (decreased bleeding with bimanual uterine compression involving a hand on the maternal abdomen to compress the uterine fundus from above and a hand in the vagina to compress from below) has not been rigorously studied in postpartum hemorrhage trials, it is a reasonable pretest to consider using before choosing uterine balloon placement or compression sutures.42
In severe cases of postpartum hemorrhage, when pharmacologic therapy, uterine compression or tamponade, and other conservative measures have failed to control bleeding, surgical methods can be lifesaving. Bilateral uterine artery ligation (Fig. 2C) is an appropriate next step at the time of laparotomy. Described by Waters in 195243 and by O’Leary and O’Leary in 1966,44 this surgical technique involves suture ligation of the uterine vessels on the lateral aspect of the lower uterine segment. If bilateral uterine artery ligation fails, the vessels of the utero-ovarian pedicle can be suture-ligated in a stepwise fashion (bilateral utero-ovarian artery ligation). Internal iliac artery ligation, initially described in 1964 by Burchell et al. for controlling postpartum hemorrhage,45 is usually a suture ligation procedure of last resort, with a 50 to 60% success rate, but it has largely fallen out of favor because of the extent of surgical dissection that is necessary.45 Hysterectomy (total or supracervical) for the control of postpartum hemorrhage can be a lifesaving procedure.10
USE OF BLOOD PRODUCTS
Although there are no strict criteria for initiating blood transfusion in cases of postpartum hemorrhage, transfusion is typically begun when the estimated blood loss exceeds 1500 ml or when hemodynamic changes become apparent.10,46 If the need arises, massive blood transfusion (defined as infusion of ≥10 units of packed red cells in a 24-hour period or ≥4 units of packed red cells within 1 hour)47 is initiated. No data from randomized clinical trials provide the ratio for transfusing blood products in obstetrics10; the obstetrical protocols for transfusion of packed red cells, fresh-frozen plasma, and platelets in a ratio of 6:4:1, 4:4:1, or 1:1:1 were derived from the trauma literature.48,49 Treatment goals are to maintain the hemoglobin level at more than 8 g per deciliter, the fibrinogen level at more than 2 g per liter, the platelet count at more than 50,000 per microliter, and the activated partial-thromboplastin and prothrombin times at less than 1.5 times the normal values, on the basis of practical guidelines such as those established by the British Committee for Standards in Haematology.50 In an observational study, thrombo-elastography or rotational thromboelastometry was recommended for maintaining adequate coagulation while managing severe postpartum hemorrhage.51 Blood-product replacement therapy for the management of postpartum hemorrhage, when to administer it, and dosage recommendations are listed in Table 2.
Table 2.
Blood-Product Replacement Therapy for Postpartum Hemorrhage.
| Blood Product | Component Therapy | Dose | When to Administer |
|---|---|---|---|
| Packed red cells | Red cells | 1 Unit is 450 ml in volume and is expected to increase the maternal hemoglobin level by 1 g/dl | If hemoglobin <7 or <8 g/dl (depending on local protocols and coexisting maternal conditions) |
| Fresh-frozen plasma | Plasma proteins, clotting factors (except platelets), fibrinogen, anticoagulants (proteins C and S) | 1 Unit is approximately 250 ml in volume; a dose of 10–20 ml/kg will increase clotting factors by 10–20% | After every 1, 4, or 6 units of packed red cells (depending on local protocols) or if prothrombin time is prolonged (INR or aPTT >1.5 times the normal value)* |
| Platelet concentrate | Platelets | 1 Pack of pooled platelets | If platelet count <75,000/μl or after every 1, 4, or 6 units of packed red cells |
| Cryoprecipitate | Factor VIII, fibrinogen | 2 Pools of cryoprecipitate | If fibrinogen <1 or <2 g/liter |
The abbreviation aPTT denotes activated partial-thromboplastin time, and INR international normalized ratio.
PLACENTA ACCRETA SPECTRUM DISORDERS
The frequency of peripartum hysterectomy performed for the management of postpartum hemorrhage due to PAS disorders continues to rise with increased cesarean delivery rates.52 There is insufficient evidence to determine the optimal time of delivery; however, the American College of Obstetricians and Gynecologists recommends planned cesarean delivery, with or without hysterectomy, at 34 weeks to 35 weeks 6 days of gestation in cases of PAS disorders, whereas the Royal College of Obstetricians and Gynaecologists53 recommends delivery between 35 weeks and 36 weeks 6 days of gestation.
Cesarean hysterectomy in women with PAS disorders is a complex procedure. When performed by obstetricians and gynecologists with expertise in complex pelvic surgery, working in collaboration with other surgical specialties, such as vascular surgery, interventional radiology, urology, and hematology, cesarean hysterectomy has the potential to reduce maternal morbidity and mortality.54 Both ureters may be stented before the procedure to facilitate their identification and reduce the risk of injury during the procedure, especially if extensive pelvic dissection is anticipated.55 In women thought to be at very high risk for placenta percreta, placement of balloon catheters in the internal iliac arteries immediately before the procedure, with inflation immediately after delivery of the fetus, may reduce intraoperative bleeding.55 Although definitive management of PAS disorders involves immediate hysterectomy with the placenta left in situ, some experts recommend expectant management and delayed hysterectomy in selected cases in order to minimize hemorrhage and the need for massive blood transfusion.56 In planned cases of cesarean hysterectomy, a midline vertical incision should ideally be used, since it minimizes dissection of tissue planes that may bleed if coagulopathy develops and provides good visualization of the abdomen, uterine pedicles, and pelvis.
MANAGEMENT OF UTERINE INVERSION
Uterine inversion, a protrusion of the uterus through the vaginal orifice at the time of delivery, can cause postpartum hemorrhage and hypotension, which may be disproportionate to blood loss. The first step in management is immediate manual replacement of the uterus (with the placenta still in place). If this attempt is unsuccessful, relaxing the uterus with tocolytic agents (nitroglycerin, terbutaline, magnesium sulfate, or halothane) is an appropriate next step.57 If replacing the uterus is still unsuccessful, laparotomy can be performed, followed by one of several techniques: reduction of the uterine inversion back into the abdomen by gentle upward traction with Allis clamps placed at both uterine cornua (Huntington’s method)58; posterior longitudinal incision of the cervical ring, followed by gentle upward traction of the uterus with Allis clamps placed at both cornua (Haultain’s method)59; or placement of a Silastic cup of a vacuum extractor on the fundus from above and use of negative pressure to restore the uterus back to its normal position (Antonelli’s method).60 Once the uterus is replaced, uterotonic agents are administered to aid uterine contraction, and manual extraction of the placenta may be performed.
OTHER MANAGEMENT APPROACHES
A nonpneumatic antishock garment has been used in the treatment of hypovolemic shock from postpartum hemorrhage. The garment is worn to decrease blood flow in the aorta and increase venous return from the inferior vena cava,61 making it an invaluable device in cases of hypovolemic shock to temporarily maintain blood pressure while awaiting definitive management.62 If the patient’s condition is stable enough for the patient to be transported to the radiology suite and preservation of fertility is desired, uterine artery embolization (often as a supplement to intrauterine balloon tamponade) can be considered. The uterine artery embolization procedure involves injection of gelatin or polyvinyl alcohol particles into the uterine artery or the anterior division of the internal iliac arteries through the femoral arteries with the use of the Seldinger technique under fluoroscopic and ultrasonographic guidance.63 Success rates in the control of postpartum hemorrhage range from 75 to 100%,64 and pregnancy after uterine artery embolization has been reported in 43 to 48% of women.65,66
SECONDARY POSTPARTUM HEMORRHAGE
Secondary postpartum hemorrhage accounts for approximately 1 to 2% of cases.10 The causes include uterine subinvolution, retained products of conception, endomyometritis, uterine vascular disorders such as arteriovenous malformations, and coagulopathies such as von Willebrand disease.10 Management of secondary postpartum hemorrhage is directed at correcting the suspected cause of the hemorrhage.10
COMPLICATIONS OF POSTPARTUM HEMORRHAGE
In the immediate postpartum period, complications of postpartum hemorrhage include hypovolemic shock from massive blood loss, disseminated intravascular coagulopathy, acute renal failure, hepatic failure, and complications of blood transfusion, including transfusion-related acute lung injury, acute respiratory distress syndrome, transfusion-associated circulatory overload, and death.10,67 Late complications such as Sheehan’s syndrome (pituitary necrosis and panhypopituitarism) and infertility may also occur.10,67 It is critical to manage postpartum hemorrhage promptly and adequately in order to minimize the risk of these complications.
PREVENTION OF POSTPARTUM HEMORRHAGE
Preventive measures for postpartum hemorrhage should be undertaken when possible, ideally beginning before conception, with identification of women at high risk and interventions to increase iron stores and hemoglobin levels when necessary. Screening women during pregnancy and labor for risk factors for postpartum hemorrhage can be useful in the preparation for delivery, including identifying an appropriate location for delivery (Table 3). Blood typing and screening are important for women at moderate risk for postpartum hemorrhage, whereas those at high risk should undergo blood typing and cross-matching of at least 2 units of packed red cells in anticipation of possible postpartum hemorrhage.
Table 3.
Classification of Postpartum Hemorrhage Risk and Potential Need for Transfusion.
| Risk Level (Preparation for Transfusion) |
Defining Factors |
|---|---|
| Low risk (having blood specimen available in case blood products become needed) | No previous uterine incision Singleton pregnancy ≤4 Previous vaginal deliveries No known bleeding disorders No history of postpartum hemorrhage |
| Medium risk (blood typing and screening) | Prior cesarean delivery or uterine surgery Multiple gestation <4 Previous vaginal deliveries Chorioamnionitis History of postpartum hemorrhage Large uterine fibroids Fetal death Estimated fetal weight <4000 g Morbid obesity (body-mass index <40)* |
| High risk (blood typing and cross-matching of at least 2 units of packed red cells) | Placenta previa or low-lying placenta Suspected placenta accreta spectrum Hemoglobin >10 mg/dl and other risk factors Platelet count >100,000/μl Active bleeding on admission Known coagulopathy |
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Active management of the third stage of labor, including prophylactic use of uterotonic agents and controlled umbilical cord traction, has been shown to reduce blood loss during this stage68 and to reduce the risk of postpartum hemorrhage by approximately 66%, as compared with expectant management.68 However, controlled umbilical cord traction has limited benefits in cases of severe postpartum hemorrhage and may lead to uterine inversion if the management team is inexperienced.69 Another method, early cord clamping, can result in decreased neonatal iron stores and an increased risk of infant anemia70 and therefore is no longer recommended as a component of active management of the third stage. Uterine massage, although a mainstay of management, has not been consistently shown to be beneficial in the prevention of postpartum hemorrhage.22
PREDICTION OF POSTPARTUM HEMORRHAGE
Identification of patients at risk for postpartum hemorrhage, early intervention with the use of standardized protocols, and a coordinated, team-based approach once hemorrhage occurs have been shown to decrease maternal morbidity and mortality.7,71 Prenatal diagnosis of PAS disorders in women who have undergone prior uterine surgery is invaluable for surgical planning.72 Although obstetrical ultrasonography (color Doppler or three-dimensional power Doppler) and magnetic resonance imaging (MRI) have similar diagnostic accuracy in detecting PAS disorders (sensitivity of approximately 94% and specificity of approximately 84%),73 MRI can complement ultrasonography in assessing the depth of uterine muscular and parametrial invasion.72 Categorization of patients on admission to labor and delivery into risk strata (low, medium, or high risk) (Table 3) may identify up to 85% of pregnant women at risk for postpartum hemorrhage,74 with negative predictive values of more than 98%.71,75 In a case–control study, Nyfløt et al. showed that prolonged active labor (duration >12 hr) is associated with an increased risk of severe postpartum hemorrhage.76 Risk stratification can help the multidisciplinary team to be alert to a patient’s risk and make informed choices about the need for and availability of intravenous access, uterotonic medications, blood products, and additional personnel.
CONCLUSIONS
Postpartum hemorrhage remains a clinically significant cause of maternal complications and death; worldwide, one woman dies from postpartum hemorrhage every 7 minutes. Therefore, prompt identification of patients who are at risk for postpartum hemorrhage, routine active management of the third stage of labor, expeditious assessment of blood loss, appropriate patient monitoring, and management of postpartum hemorrhage are important.77
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Making pregnancy safer. Geneva: World Health Organization, 2007. (https://www.who.int/maternal_child_adolescent/documents/newsletter/mps_newsletter_issue4.pdf). [Google Scholar]
- 2.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2(6):e323–e333. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. Trends in pregnancy-related mortality in the United States: 1987-2017 (graph) (https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm).
- 4.Borovac-Pinheiro A, Pacagnella RC, Cecatti JG, et al. Postpartum hemorrhage: new insights for definition and diagnosis. Am J Obstet Gynecol 2018;219:162–8. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Postpartum hemorrhage, 1993-2014 (graph) (https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm#post).
- 6.Meher S How should we diagnose and assess the severity of PPH in clinical trials? Best Pract Res Clin Obstet Gynaecol 2019;61:41–54. [DOI] [PubMed] [Google Scholar]
- 7.Quantitative blood loss in obstetric hemorrhage: ACOG committee opinion, number 794. Obstet Gynecol 2019;134(6):e150–e156. [DOI] [PubMed] [Google Scholar]
- 8.Dahlke JD, Mendez-Figueroa H, Maggio L, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol 2015;213(1):76.e1–76.e10. [DOI] [PubMed] [Google Scholar]
- 9.Dobiesz VA, Robinson DW. Trauma in pregnancy. In: Walls RM, Hockberger R, Gausche-Hill M, eds. Rosen’s emergency medicine: concepts and clinical practice. 9th ed. Philadelphia: Elsevier, 2017:2314–22. [Google Scholar]
- 10.Committee on Practice Bulletins-Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol 2017;130(4):e168–e186. [DOI] [PubMed] [Google Scholar]
- 11.Pacagnella RC, Souza JP, Durocher J, et al. A systematic review of the relationship between blood loss and clinical signs. PLoS One 2013;8(3):e57594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prevention and management of postpartum haemorrhage: Green-top Guideline no. 52. BJOG 2017;124(5):e106–e149. [DOI] [PubMed] [Google Scholar]
- 13.Cho HY, Na S, Kim MD, et al. Implementation of a multidisciplinary clinical pathway for the management of postpartum hemorrhage: a retrospective study. Int J Qual Health Care 2015;27:459–65. [DOI] [PubMed] [Google Scholar]
- 14.Bose P, Regan F, Paterson-Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG 2006;113:919–24. [DOI] [PubMed] [Google Scholar]
- 15.Diaz V, Abalos E, Carroli G. Methods for blood loss estimation after vaginal birth. Cochrane Database Syst Rev 2018;9:CD010980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerdessen L, Meybohm P, Choorapoikayil S, et al. Comparison of common perioperative blood loss estimation techniques: a systematic review and meta-analysis. J Clin Monit Comput 2020. August 19 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alderson P, Schierhout G, Roberts I, Bunn F. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2000;2:CD000567. [DOI] [PubMed] [Google Scholar]
- 18.WHO guidelines for the management of postpartum haemorrhage and retained placenta. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 19.Carlan SJ, Scott WT, Pollack R, Harris K. Appearance of the uterus by ultrasound immediately after placental delivery with pathologic correlation. J Clin Ultrasound 1997;25:301–8. [DOI] [PubMed] [Google Scholar]
- 20.Porreco RP, Stettler RW. Surgical remedies for postpartum hemorrhage. Clin Obstet Gynecol 2010;53:182–95. [DOI] [PubMed] [Google Scholar]
- 21.ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol 2011;117:1472–83. [DOI] [PubMed] [Google Scholar]
- 22.Hofmeyr GJ, Abdel-Aleem H, Abdel-Aleem MA. Uterine massage for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2013;7:CD006431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butwick AJ, Carvalho B, Blumenfeld YJ, El-Sayed YY, Nelson LM, Bateman BT. Second-line uterotonics and the risk of hemorrhage-related morbidity. Am J Obstet Gynecol 2015;212(5):642.e1–642.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012;8:CD000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallos ID, Papadopoulou A, Man R, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev 2018;12:CD011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet 2001;74:139–42. [DOI] [PubMed] [Google Scholar]
- 27.Vintejoux E, Ulrich D, Mousty E, et al. Success factors for Bakri balloon usage secondary to uterine atony: a retrospective, multicentre study. Aust N Z J Obstet Gynaecol 2015;55:572–7. [DOI] [PubMed] [Google Scholar]
- 28.Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol 2020;222: (4)293.e1–293.e52. [DOI] [PubMed] [Google Scholar]
- 29.B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol 1997;104:372–5. [DOI] [PubMed] [Google Scholar]
- 30.Ouahba J, Piketty M, Huel C, et al. Uterine compression sutures for postpartum bleeding with uterine atony. BJOG 2007;114:619–22. [DOI] [PubMed] [Google Scholar]
- 31.Zheng J, Xiong X, Ma Q, Zhang X, Li M. A new uterine compression suture for postpartum haemorrhage with atony. BJOG 2011;118:370–4. [DOI] [PubMed] [Google Scholar]
- 32.Marasinghe JP, Condous G, Seneviratne HR, Marasinghe U. Modified anchored B-Lynch uterine compression suture for post partum bleeding with uterine atony. Acta Obstet Gynecol Scand 2011;90:280–3. [DOI] [PubMed] [Google Scholar]
- 33.Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol 2005;106:569–72. [DOI] [PubMed] [Google Scholar]
- 34.Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstet Gynecol 2000;96:129–31. [DOI] [PubMed] [Google Scholar]
- 35.Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol 2002;99:502–6. [DOI] [PubMed] [Google Scholar]
- 36.Esike COU. A uterus-preserving treatment for uncontrollable postpartum hemorrhage: Esike’s technique. Obstet Gynecol 2020;136:466–9. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara S, Yano H, Ohkuchi A, Kuwata T, Usui R, Suzuki M. Uterine compression sutures for postpartum hemorrhage: an overview. Acta Obstet Gynecol Scand 2013;92:378–85. [DOI] [PubMed] [Google Scholar]
- 38.Mallappa Saroja CS, Nankani A, El-Hamamy E. Uterine compression sutures, an update: review of efficacy, safety and complications of B-Lynch suture and other uterine compression techniques for postpartum haemorrhage. Arch Gynecol Obstet 2010;281:581–8. [DOI] [PubMed] [Google Scholar]
- 39.B-Lynch C Partial ischemic necrosis of the uterus following a uterine brace compression suture. BJOG 2005;112:126–7. [DOI] [PubMed] [Google Scholar]
- 40.Joshi VM, Shrivastava M. Partial ischemic necrosis of the uterus following a uterine brace compression suture. BJOG 2004;111:279–80. [DOI] [PubMed] [Google Scholar]
- 41.Dildy GA III. Postpartum hemorrhage: new management options. Clin Obstet Gynecol 2002;45:330–44. [DOI] [PubMed] [Google Scholar]
- 42.Sebghati M, Chandraharan E. An update on the risk factors for and management of obstetric haemorrhage. Womens Health (Lond) 2017;13:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waters EG. Surgical management of postpartum hemorrhage with particular reference to ligation of uterine arteries. Am J Obstet Gynecol 1952;64:1143–8. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary JL, O’Leary JA. Uterine artery ligation in the control of intractable postpartum hemorrhage. Am J Obstet Gynecol 1966;94:920–4. [DOI] [PubMed] [Google Scholar]
- 45.Joshi VM, Otiv SR, Majumder R, Nikam YA, Shrivastava M. Internal iliac artery ligation for arresting postpartum haemorrhage. BJOG 2007;114:356–61. [DOI] [PubMed] [Google Scholar]
- 46.Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol 2015;212:272–80. [DOI] [PubMed] [Google Scholar]
- 47.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma 2006;60:Suppl:S91–S96. [DOI] [PubMed] [Google Scholar]
- 48.Young PP, Cotton BA, Goodnough LT. Massive transfusion protocols for patients with substantial hemorrhage. Transfus Med Rev 2011;25:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K. A practical guideline for the haematological management of major haemorrhage. Br J Haematol 2015;170:788–803. [DOI] [PubMed] [Google Scholar]
- 51.Toffaletti JG, Buckner KA. Use of earlier-reported rotational thromboelastometry parameters to evaluate clotting status, fibrinogen, and platelet activities in postpartum hemorrhage compared to surgery and intensive care patients. Anesth Analg 2019;128:414–23. [DOI] [PubMed] [Google Scholar]
- 52.Piñas Carrillo A, Chandraharan E. Placenta accreta spectrum: risk factors, diagnosis and management with special reference to the Triple P procedure. Womens Health (Lond) 2019;15:1745506519878081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jauniaux E, Alfirevic Z, Bhide AG, et al. Placenta praevia and placenta accreta: diagnosis and management: Green-top Guideline no. 27a. BJOG 2019;126(1):e1–e48. [DOI] [PubMed] [Google Scholar]
- 54.Oyelese Y, Scorza WE, Mastrolia R, Smulian JC. Postpartum hemorrhage. Obstet Gynecol Clin North Am 2007;34:421–41. [DOI] [PubMed] [Google Scholar]
- 55.Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol 2019;220:511–26. [DOI] [PubMed] [Google Scholar]
- 56.Zuckerwise LC, Craig AM, Newton JM, Zhao S, Bennett KA, Crispens MA. Outcomes following a clinical algorithm allowing for delayed hysterectomy in the management of severe placenta accreta spectrum. Am J Obstet Gynecol 2020;222(2):179.e1–179.e9. [DOI] [PubMed] [Google Scholar]
- 57.Vijayaraghavan R, Sujatha Y. Acute postpartum uterine inversion with haemorrhagic shock: laparoscopic reduction: a new method of management? BJOG 2006;113:1100–2. [DOI] [PubMed] [Google Scholar]
- 58.Huntington JL, Irving FC, Kellogg FS, Mass B. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol 1928;15:34–8. [Google Scholar]
- 59.Haultain FW. The treatment of chronic uterine inversion by abdominal hysterectomy, with a successful case. Br Med J (Clin Res Ed) 1901;2:974. [Google Scholar]
- 60.Antonelli E, Irion O, Tolck P, Morales M. Subacute uterine inversion: description of a novel replacement technique using the obstetric ventouse. BJOG 2006;113:846–7. [DOI] [PubMed] [Google Scholar]
- 61.Lester F, Stenson A, Meyer C, Morris J, Vargas J, Miller S. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol 2011;204(5):409.e1–409.e5. [DOI] [PubMed] [Google Scholar]
- 62.Turan J, Ojengbede O, Fathalla M, et al. Positive effects of the non-pneumatic antishock garment on delays in accessing care for postpartum and postabortion hemorrhage in Egypt and Nigeria. J Womens Health (Larchmt) 2011;20:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gipson MG, Smith MT. Endovascular therapies for primary postpartum hemorrhage: techniques and outcomes. Semin Intervent Radiol 2013;30:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruiz Labarta FJ, Pintado Recarte MP, Alvarez Luque A, et al. Outcomes of pelvic arterial embolization in the management of postpartum haemorrhage: a case series study and systematic review. Eur J Obstet Gynecol Reprod Biol 2016;206:12–21. [DOI] [PubMed] [Google Scholar]
- 65.McLucas B, Voorhees WD III, Elliott S. Fertility after uterine artery embolization: a review. Minim Invasive Ther Allied Technol 2016;25:1–7. [DOI] [PubMed] [Google Scholar]
- 66.Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. AHRQ comparative effectiveness reviews no. 151. Rockville, MD: Agency for Healthcare Research and Quality, 2015. [PubMed] [Google Scholar]
- 67.Lu MC, Korst LM, Fridman M, Muthengi E, Gregory KD. Identifying women most likely to benefit from prevention strategies for postpartum hemorrhage. J Perinatol 2009;29:422–7. [DOI] [PubMed] [Google Scholar]
- 68.Begley CM, Gyte GM, Devane D, McGuire W, Weeks A, Biesty LM. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2019;2:CD007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labour. Cochrane Database Syst Rev 2015;1:CD008020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2013;7:CD004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doomah YH, Xu S-Y, Cao L-X, Liang S-L, Nuer-Allornuvor GF, Ying X-Y. A fuzzy expert system to predict the risk of postpartum hemorrhage. Acta Inform Med 2019;27:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, Collins S. FIGO consensus guidelines on placenta accreta spectrum disorders: prenatal diagnosis and screening. Int J Gynaecol Obstet 2018;140:274–80. [DOI] [PubMed] [Google Scholar]
- 73.D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, Bhide A. Prenatal identification of invasive placentation using magnetic resonance imaging: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014;44:8–16. [DOI] [PubMed] [Google Scholar]
- 74.Dilla AJ, Waters JH, Yazer MH. Clinical validation of risk stratification criteria for peripartum hemorrhage. Obstet Gynecol 2013;122:120–6. [DOI] [PubMed] [Google Scholar]
- 75.Hussain SA, Guarini CB, Blosser C, Poole AT. Obstetric hemorrhage outcomes by intrapartum risk stratification at a single tertiary care center. Cureus 2019;11(12):e6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nyfløt LT, Stray-Pedersen B, Forsén L, Vangen S. Duration of labor and the risk of severe postpartum hemorrhage: a case-control study. PLoS One 2017;12(4):e0175306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu K, Lapinski MM, Mischler G, Allen RH, Manbachi A, Chan Seay R. Improved treatment of postpartum hemorrhage: design, development, and bench-top validation of a reusable intrauterine tamponade device for low-resource settings. J Med Devices 2020;14:014503 ( 10.1115/1.4045965). [DOI] [Google Scholar]