Abstract
Background
The resources for critical care are limited in many settings, exacerbating the significant morbidity and mortality associated with critical illness. Budget constraints can lead to choices between investing in advanced critical care (e.g. mechanical ventilators in intensive care units) or more basic critical care such as Essential Emergency and Critical Care (EECC; e.g. vital signs monitoring, oxygen therapy, and intravenous fluids).
Methods
We investigated the cost effectiveness of providing EECC and advanced critical care in Tanzania in comparison with providing ‘no critical care’ or ‘district hospital-level critical care’ using coronavirus disease 2019 (COVID-19) as a tracer condition. We developed an open-source Markov model (https://github.com/EECCnetwork/POETIC_CEA) to estimate costs and disability-adjusted life-years (DALYs) averted, using a provider perspective, a 28-day time horizon, patient outcomes obtained from an elicitation method involving a seven-member expert group, a normative costing study, and published literature. We performed a univariate and probabilistic sensitivity analysis to assess the robustness of our results.,
Results
EECC is cost effective 94% and 99% of the time when compared with no critical care (incremental cost-effectiveness ratio [ICER] $37 [−$9 to $790] per DALY averted) and district hospital-level critical care (ICER $14 [−$200 to $263] per DALY averted), respectively, relative to the lowest identified estimate of the willingness-to-pay threshold for Tanzania ($101 per DALY averted). Advanced critical care is cost effective 27% and 40% of the time, when compared with the no critical care or district hospital-level critical care scenarios, respectively.
Conclusion
For settings where there is limited or no critical care delivery, implementation of EECC could be a highly cost-effective investment. It could reduce mortality and morbidity for critically ill COVID-19 patients, and its cost effectiveness falls within the range considered ‘highly cost effective’. Further research is needed to explore the potential of EECC to generate even greater benefits and value for money when patients with diagnoses other than COVID-19 are accounted for.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-023-00418-x.
Key Points for Decision Makers
| Essential Emergency and Critical Care (EECC) is defined as the care that should be provided to all critically ill patients in all hospitals and includes such care as vital signs monitoring, oxygen therapy, and intravenous fluids. |
| The probability of EECC being cost effective in the low-resource setting of a Tanzanian district hospital was over 90%, relative to a willingness-to-pay threshold of $101 per disability-adjusted life-year. |
| Implementation research is needed around the effectiveness and costs of strategies for scaling up critical care in low-resource settings. |
Introduction
Since detection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, large pandemic waves of coronavirus disease 2019 (COVID-19) have occurred throughout the world [1, 2]. These waves have resulted in significant pressure on national health systems due to the rapid influx of critically ill patients who require inpatient care [3]. Demand for critical care has often outstripped existing supply, especially in lower-resource settings, resulting in increased mortality and morbidity [4] The COVID-19 response has drawn attention to the large pre-pandemic gap between critical care needs and availability in low-resource settings [5].
Before the pandemic, increasing critical care capacity (e.g., improving oxygen supply, procuring pulse oximeters, or training healthcare workers), especially in lower-resource settings, was largely neglected. This may be due to a perception that investment in critical care may not be cost effective, the significant complexity of scaling up health systems, large upfront costs and lengthy time periods for returns on investment [6–9]. Where critical care was documented, it was generally found in intensive care units (ICUs) in referral hospitals, and was either not available or under-resourced in primary and secondary facilities [8]. During the pandemic, critical care received greater attention, with a scale-up that initially focused on procurement of expensive, resource-intensive and hi-tech equipment such as mechanical ventilators [6].
Research is now showing that increasing critical care capacity through the expansion of ICU bed capacity may not be a cost-effective use of scarce resources [10]. Whether to invest limited resources in basic or advanced critical care (ACC) is a question of clinical, economic and ethical dimensions [7, 11, 12]. Allocating limited resources towards lower-cost essential care that can be delivered across all hospitals as well as throughout each hospital could be a cost-effective solution [6, 13, 14]. This is especially relevant in a lower-middle income country such as Tanzania with under-resourced district hospitals and a majority rural population [15].
To improve outcomes for critically ill patients by means that are feasible and low-cost, the Essential Emergency and Critical Care (EECC) concept was devised [16, 17]. EECC is defined as the care that should be provided to all critically ill patients of all ages in all hospitals in the world. It is distinguished by three principles. First, priority to those with the most urgent clinical need, including both early identification and timely care. Second, provision of the life-saving treatments that support failing vital organ functions. Finally, third, a focus on effective care of low cost and low complexity. EECC consists of 40 clinical processes with examples, including vital signs monitoring, oxygen therapy, intravenous fluids and patient positioning, plus the 66 requirements for hospitals to be ready to provide that care, agreed in a large global consensus [6, 16, 17]. However, although basic, the coverage of EECC is often low [18–24].
EECC is unlikely to be sufficient for a subset of critically ill patients with extremely severe disease who will need more ACC in combination with EECC. However, budgets for ACC are likely to be limited, emphasising the need for evidence that demonstrates the economic implications of different approaches to critical care provision [6, 16, 18].
We aimed to quantify the cost effectiveness of EECC and ACC from a health provider perspective by developing a de novo four-state Markov model. We considered typical district hospital care in Tanzania as a case study of a low-resource setting with observed gaps in critical care services, and used COVID-19 as a tracer condition for critical illness [13, 15, 25, 26]. Our results can contribute towards evidence informed policy making and policy planning of critical care services not only during the COVID-19 pandemic but also in providing critical illness care across all conditions [15, 26].
Methods
Study Design
To determine the cost effectiveness of EECC and ACC for treating critically ill patients with COVID-19 in Tanzania, we developed a Markov model for a hypothetical cohort of 10,000 hospitalised critically ill adult patients (aged >18 years) with COVID-19 in Tanzania. EECC was defined as using the 40 processes that include monitoring basic vital signs, provision of oxygen therapy and intravenous fluids, and positioning of unconscious patients to maintain a free airway [17]. ACC was defined as EECC in combination with the more advanced support of vital organs, such as mechanical ventilation, that is usually provided in intensive care units (ICUs) with specialised staff, facilities and technologies [27], as described by two critical care specialists in Tanzania (See electronic supplementary material [ESM] Note S1) [16]. The interventions are compared with two alternative baseline scenarios. First, we assumed that no specific critical care services were available, although general hospital care is in place. Second, informed by a systematic review of critical care in Tanzania, we developed a scenario to reflect critical care delivered in a district hospital with limited critical care resources, typical of a low-resource sub-Saharan hospital such as Tanzania [15, 26]. Such settings do not have an ICU, and non-ICU critical care is also limited (e.g. oxygen might or not might be available when needed) [Table 1; ESM Note S1] [15, 16, 18, 26]. A healthcare provider perspective was selected to reflect decision making at the hospital level and all direct costs incurred for the inpatient episode were captured. Outcomes were measured in terms of disability-adjusted life-year (DALY) averted, as recommended, and to facilitate comparison with willingness-to-pay thresholds [28].
Table 1.
Comparator definition and assumptions
| Name | Description | Comparator scenario assumptions | EECC intervention effect assumptions | ACC intervention effect assumptions |
|---|---|---|---|---|
| No critical care | No critical care is provided at all to severe or critically ill COVID-19 patients | Other care is provided, as in a typical Tanzanian district hospital |
EECC reduces the probability of severe patients becoming critical and increases the probability of critical patients becoming severe. EECC does not directly reduce the probability of mortality but does so indirectly Clinical effectiveness of EECC is determined from the nominal group exercise |
ACC reduces the probability of critical patients dying Clinical effectiveness of advanced critical care is determined from the literature |
| District hospital-level critical care | Severe patients and critical patients have access to only limited non-ICU critical care, as per a typical district hospital in Tanzania |
We assumed district hospitals offer some non-ICU critical care (e.g. some therapeutics, oxygen therapy, pulse oximeters, etc.) and refer the patient to higher-level hospitals for advanced ICU-based critical care [14] Other care is provided, as in a typical Tanzanian district hospital |
EECC reduces the probability of severe patients becoming critical and increases the probability of critical patients becoming severe. EECC does not directly reduce the probability of mortality but does so indirectly Clinical effectiveness of EECC is determined from the nominal group exercise |
ACC reduces the probability of critical patients dying Clinical effectiveness of advanced critical care is determined from the literature |
ICU intensive care unit, EECC Essential Emergency and Critical Care, ACC advanced critical care, COVID-19 coronavirus disease 2019
ACC constitutes EECC in combination with more advanced organ support. EECC is delivered to both severe and critical COVID-19 patients, whereas advanced critical care is only delivered to critical COVID-19 patients
We developed a four-state Markov model based on clinical severity of critically ill COVID-19 patients. The four mutually exclusive severity states were defined as ‘severe’, ‘critical’, ‘death’ and ‘recover’ (Fig. 1), where severe and critical COVID states are defined in line with World Health Organisation classifications (see ESM Note S2) [29, 30]. A Markov model allows for a hypothetical cohort of patients to move in and out of, or stay within, each state over time, thereby reflecting the course of critical illness [31]. In the model, patients with ‘severe’ COVID-19 can deteriorate and develop ‘critical’ COVID-19, and ‘critical’ COVID-19 patients can improve to ‘severe’ COVID-19. In this analysis, we assumed that severe patients will always deteriorate and become critical before they die. We also assumed that moderate and mild patients are not hospitalised and do not incur a critical care cost or health impact. In addition, we assumed that patients who recover accrue minimal morbidity impacts within the time horizon and cannot become severe or critical again. Hence, death and recover become absorbing states.
Fig. 1.
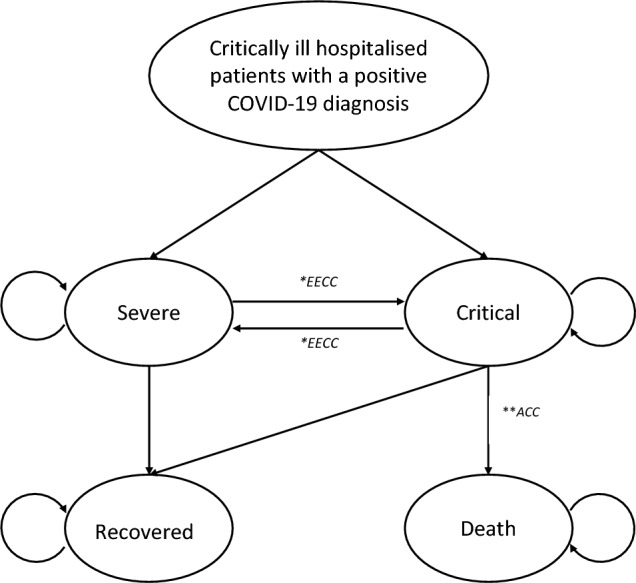
Markov model structure. All admitted and hospitalised critically ill adult patients (aged >18 years) with COVID-19 in Tanzania are triaged as severe or critical as per World Health Organisation classifications. Circular arrows represent the construct that a patient can remain in the same health state for more than one cycle. *EECC is an intervention that reduces clinical severity between severe and critical patients. ACC constitutes EECC in combination with more advanced organ support. bACC only reduces mortality in critically ill patients. EECC is delivered to both severe and critical COVID-19 patients, whereas ACC is only delivered to critical COVID-19 patients. Severe patients will, by definition, progress to the critical state before dying. COVID-19 coronavirus disease 2019, EECC Essential Emergency and Critical Care, ACC advanced critical care
The intervention, EECC, is provided to both ‘severe’ and ‘critical’ COVID-19 patients, whereas ACC is only provided to ‘critical’ COVID-19 patients. Patients with severe COVID-19, in-line with WHO classifications, are those in need of life-sustaining oxygen therapy and other non-advanced clinical care, while those with critical COVID-19 are those requiring “life-sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy”. Therefore, a patient who requires ACC, by definition, has critical COVID-19, and those with severe COVID-19 do not require ACC.
The clinical effectiveness of EECC and ACC has not been established in clinical trials. We assumed EECC reduces clinical severity in severe or critical patients by reducing the probability of severe patients becoming critical and increasing the probability of critical patients improving and becoming severe. On the other hand, the incremental effect of ACC is assumed to only reduce mortality in patients with ‘critical’ COVID-19 (Fig. 1).
We evaluated the health impacts of COVID-19 in terms of DALYs over a time horizon equivalent to 28 days, to capture an inpatient episode, with time cycles being a 24-h period. Any longer-term health effects beyond hospital discharge are not captured in the model; however, the majority of clinical benefits were found to accrue within the in-patient episode [10, 27]. In addition, the clinical epidemiology of long COVID-19 is still uncertain and heterogenous, thereby limiting application to this analysis [32]. No discount rate is used considering the short time horizon chosen. DALYs were calculated as the sum of years of life lost (YLLs) and years of life with disability (YLDs) as per the standardised methods [33]. YLL were computed using age-standardised mortality rates from critically ill COVID-19 patients in African high-care units or ICUs relative to a healthy life expectancy for Tanzania [35, 36]. YLDs were computed by applying a disability weight for hospitalised severe and critical COVID-19 inpatient episodes. In the absence of current data on disability weights or quality of life for COVID-19 disease, we used disability weights for severe respiratory infection from the Global Disease Burden study (2013) for severe inpatient episodes, and the disability weight for ICU admission for critical inpatient episodes, as applied by Kairu et al. (see Table 2) [27, 34]. We reviewed different willingness-to-pay thresholds for Tanzania and chose to use the most conservative value of $101 per DALY as a low side estimate [35, 36]. Any intervention considered cost effective at this value will be cost effective at higher thresholds. Details of the intervention and comparators are reported in Table 1. All analyses were conducted using R. [37]
Table 2.
Model parameters
| Scenario | Parameter type | Parameter | Lower bound | Valuea | Upper bound | Source |
|---|---|---|---|---|---|---|
| All | Model population (triage) | Proportion of hospitalised population with severe COVID-19 | 0.76 | 0.86 | 0.96 | [29] |
| Proportion of hospitalised population with critical COVID-19 | 0.24 | 0.14 | 0.04 | |||
| No critical care | Transition probabilities b | Probability of a patient in the severe state progressing to the critical state | 0.10 | 0.40 | 0.70 | Nominal group exercise |
| Probability of a patient in the severe state progressing to the recover state | 0.10 | 0.20 | 0.25 | |||
| Probability of a patient in the critical state returning to the severe state | 0.00 | 0.05 | 0.15 | |||
| Probability of a patient in the critical state progressing to the death state | 0.50 | 0.70 | 0.80 | |||
| Probability of a patient in the critical state progressing to the recover state | 0.00 | 0.00 | 0.00 | |||
| Intervention effectiveness | Effectiveness of EECC in comparison with ‘no critical care’ in reducing the probability of progressing from the severe state to the critical state | 0.00 | 0.38 | 1.00 | ||
| Effectiveness of EECC in comparison with ‘no critical care’ in increasing the probability of progressing from the critical state to the severe state | 0.00 | 0.50 | 1.00 | |||
| Effectiveness of ACC in comparison with ‘no critical care’ in reducing the probability of progressing from the critical state to death | 0.00 | 0.34 | 1.00 | [10] | ||
| Costs (US$, 2020 prices) | Unit cost of treating a severe patient in a ‘no critical care’ scenario (baseline) | 2.45 | 25.57 | 37.68 | [42] | |
| Unit cost of treating a critical patient in a ‘no critical care’ scenario (baseline) | 2.45 | 25.57 | 37.68 | |||
| Unit cost of treating a severe patient with EECC in a ‘no critical care’ scenario | 8.74 | 10.83 | 22.12 | [44] | ||
| Unit cost of treating a critical patient with EECC in a ‘no critical care’ scenario | 27.92 | 32.84 | 73.92 | |||
| Unit cost of treating a severe patient with ACC in a ‘no critical care’ scenario | 10.82 | 13.11 | 25.65 | |||
| Unit cost of treating a critical patient with ACC in a ‘no critical care’ scenario | 224.65 | 297.30 | 372.12 | |||
| District hospital Level of critical care | Transition probabilitiesb | Probability of a patient in the severe state progressing to the critical state | 0.25 | 0.30 | 0.38 | Nominal group exercise |
| Probability of a patient in the severe state progressing to the recover state | 0.12 | 0.25 | 0.30 | |||
| Probability of a patient in the critical state returning to the severe state | 0.05 | 0.07 | 0.10 | |||
| Probability of a patient in the critical state progressing to the death state | 0.50 | 0.53 | 0.70 | |||
| Probability of a patient in the critical state progressing to the recover state | 0.00 | 0.00 | 0.00 | |||
| Intervention effectiveness | Effectiveness of EECC in comparison with district hospital-level critical care in reducing the probability of progressing from the severe state to the critical state | 0.00 | 0.38 | 1.00 | ||
| Effectiveness of EECC in comparison with district hospital-level critical care in increasing the probability of progressing from the critical state to the severe state | 0.00 | 0.50 | 1.00 | |||
| Effectiveness of ACC in comparison with district hospital-level critical care in reducing the probability of progressing from the critical state to death | 0.00 | 0.34 | 1.00 | [10] | ||
| Costs (US$, 2020 prices) | Unit cost of treating a severe patient in a ‘district hospital-level critical care’ scenario (baseline) | 3.68 | 26.78 | 56.65 | [42] | |
| Unit cost of treating a critical patient in a ‘district hospital-level critical care’ scenario (baseline) | 3.68 | 26.78 | 56.65 | |||
| Unit cost of treating a severe patient with EECC in a ‘district hospital-level critical care’ scenario | 0.26 | 1.87 | 3.95 | [44] | ||
| Unit cost of treating a critical patient with EECC in a ‘district hospital-level critical care’ scenario | 3.28 | 23.88 | 50.51 | |||
| Unit cost of treating a severe patient with ACC in a ‘district hospital-level critical care’ scenario | 0.57 | 4.15 | 8.77 | |||
| Unit cost of treating a critical patient with ACC in a ‘district hospital-level critical care’ scenario | 39.61 | 288.34 | 610.01 | |||
| Health-related quality of life | Disability weight for a severe care episode | 0.09 | 0.13 | 0.19 | [29] | |
| Disability weight for a critical care episode | 0.58 | 0.66 | 0.73 | [29] | ||
| Life expectancy | – | 70.00 | – | Assumption | ||
| Proportion of COVID-19 deaths in patients aged between 18 and 45 years | 0.13 | 0.23 | 0.33 | [35] | ||
| Proportion of COVID-19 deaths in patients aged between 46 and 56 years | 0.14 | 0.24 | 0.34 | |||
| Proportion of COVID-19 deaths in patients aged between 57 and 67 years | 0.22 | 0.32 | 0.42 | |||
| Proportion of COVID-19 deaths in patients aged between 68 and 100 years | 0.11 | 0.21 | 0.31 | |||
All parameters within our analysis were assigned triangular distributions due to a lack of variability around point estimates and an absence of sample data. ACC constitutes EECC in combination with more advanced organ support. EECC is delivered to both severe and critical COVID-19 patients, whereas ACC is only delivered to critical COVID-19 patients. Unit costs are estimated as the incremental cost of providing EECC or ACC relative to the comparator scenario. Severe patients will, by definition, progress to the critical state before dying. Effectiveness values reflect the nominal group exercise where clinicians’ estimated that the probability of patients transitioning from severe to critical is reduced by a factor of 38% and the probability of progressing from the critical state to the severe state is increased by a factor of 50% when EECC is administered, in comparison with the no critical care scenario and the district hospital care scenario
COVID-19 coronavirus disease 2019, EECC Essential Emergency and Critical Care, ACC advanced critical care
aThe value is a measure of central tendency
bSevere patients will, by definition, progress to the critical state before dying
Clinical and Epidemiological Data
A comprehensive review of existing databases on critical and COVID-19 care was carried out to identify clinical and epidemiological parameters for the model. However, while there are studies describing the outcomes of critically ill patients, it is not known whether those patients received EECC, and hence the effectiveness of EECC cannot by elicited from any of these studies and valid empirical data are lacking for critical care effectiveness in the study setting. We therefore used expert elicitation in the form of a nominal group to quantify the probability of patients moving between each of the states for each comparator scenario. The nominal group technique is a structured small-group discussion for reaching consensus [38]. We invited seven experts to the nominal group, all with expertise in the care of critically ill patients with COVID-19 from settings of differing resources, including those with a detailed understanding of the EECC concept, ACC and Tanzanian district hospital care (see ESM Note S3). The nominal group was convened and asked by a moderator to discuss and reach consensus around the expected outcomes of patients with severe and critical COVID-19 under our predefined scenarios (‘no critical care’, ‘district hospital-level critical care’, ‘EECC’ and ‘ACC [including EECC]’). Table 2 provides a detailed list of economic and epidemiological parameters, including those generated by the nominal group.
Costing Methodology
A systematic review of resource use and costs of critical care was carried out to inform the costing [15, 26]. Baseline critical care costs were zero under the ‘no critical care’ scenario. The baseline costs for treating COVID-19 patients for the ‘district hospital-level critical care’ comparator were derived from the literature, with the costs of inpatient pneumonia treatment used as a proxy [39].
To calculate the incremental costs of delivering EECC and ACC to severe and critical COVID-19 patients in Tanzania, we employed a normative costing approach, and the methods are reported elsewhere [15, 26, 40, 41]. All costs were inflated from their valuations in earlier years to 2020 Tanzania Shillings (TzSh) and then converted to 2020 US dollars (US$) at the 2020 exchange rate of TzSh2309 to US$1 (https://www.bot.go.tz/).
Cost-Effectiveness Analysis
Cost-Effectiveness Acceptability Curves
Incremental cost-effectiveness ratios (ICERs) were simulated with probabilistic sensitivity analyses using a Monte Carlo procedure [42]. A total of 10,000 parameter sets were drawn from each parameter’s individual distribution to assess the probability of EECC or ACC being cost effective. As most estimates were not derived from sampled observations, triangular distributions were used for which the central, minimum and maximum values were used to characterise the distribution. In the case of the transition probabilities, the central value was taken from the nominal group’s consensus estimate and the minimum and maximum values were taken from the lowest and highest estimates of individual experts in the nominal group [43]. In addition, in recognition that no clinical effectiveness data exist on EECC or ACC, all clinical effectiveness parameters were given an uncertainty range between 0 and 100, thereby capturing all possible uncertainty in clinical effectiveness.
We present mean, lower, and higher range ICER values to show the spread of cost-effectiveness results. We then constructed cost-effectiveness acceptability curves (CEACs) to represent the probability that EECC or ACC is cost effective relative to different threshold values, which we set at $US101 per DALY averted [35, 36]. For each threshold value, the probability was calculated as the proportion of each simulated sample of cost and effect pairs that represents a net health benefit (for example, see Stinnett et al., Wilson, and McCabe et al. [44–46]).
Probabilistic One-Way Sensitivity Analysis
In addition to generating CEACs, we explored the sensitivity of the results to each individual model parameter using a conditional net health benefit approach. For each parameter, we systematically selected a set of values from its distribution, covering the full range of possible values and using equal intervals between values. A full probabilistic analysis of the model using a Monte Carlo simulation was then run for each of the selected values, holding the parameter of interest constant, and the expected costs and outcomes were recorded. The process was repeated for all parameters, for both interventions and both scenarios. The conditional expected cost and outcome data were then used to generate the conditional expected net health benefit curves, relative to the selected values, from the distribution of the given parameter [45, 46]. Finally, a sensitivity analysis was performed where uniform distributions were applied instead of triangular distributions, to see the impact of the type of distribution on the range of cost effectiveness.
Results
Cost Effectiveness
The model outputs and results of the cost-effectiveness analysis are presented in Table 3. EECC in comparison with no critical care resulted in a mean ICER value of $37, with a range of −$9.46 to $791 per DALY averted. Comparing ACC with no critical care resulted in an ICER value of $186 (range $8.61–$3085) per DALY averted. ICER values for EECC and ACC relative to district hospital-level critical care were $14 (range −$200 to $263) and $144 (range −$20 to $1294) per DALY averted, respectively. The mean ICER value suggests EECC is cost effective in both scenarios when compared with the willingness-to-pay threshold (i.e., $101 per DALY averted). As the ranges of these ICERs extend outside the threshold value, the CEACs provide a more intuitive interpretation by providing the probability of the ICER being below the threshold value.
Table 3.
Cost-effectiveness results
| Comparator | Intervention | Incremental DALYs averted | Incremental costs (US$, 2020 prices) | ICER (US$/DALY averted) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Lower | Upper | Mean | Lower | Upper | Mean | Lower | Upper | ||
| No critical care | EECC | 3.45 | 0.11 | 14.09 | 85.63 | −17.02 | 284.20 | 36.69 | −9.46 | 790.53 |
| ACC | 4.32 | 0.23 | 17.25 | 581.89 | 109.62 | 4404.91 | 186.26 | 8.61 | 3084.61 | |
| District hospital-level critical care | EECC | 3.58 | 0.20 | 13.22 | 38.57 | −98.30 | 207.83 | 14.45 | −200.25 | 262.56 |
| ACC | 4.61 | 0.42 | 15.70 | 532.11 | −41.01 | 3932.27 | 144.48 | −19.86 | 1294.19 | |
All results are presented at the individual level; ‘lower’ denotes the lower range and ‘upper’ denotes the upper range
Costs are reported in US$
All incremental results are relative to a ‘no intervention’ base-case within each comparator. ACC constitutes EECC in combination with more advanced organ support. EECC is delivered to both severe and critical COVID-19 patients, whereas ACC is only delivered to critical COVID-19 patients
DALYs disability-adjusted life-year, ICER incremental cost-effectiveness ratio, EECC Essential Emergency and Critical Care, ACC advanced critical care, COVID-19 coronavirus disease 2019
The incremental costs and health outcomes generated by the model in a ‘no critical care’ scenario were 3.45 (range 0.11–14.09) DALYs being averted per person at an incremental individual cost of $86 (range −$17 to $284) for EECC. In the same scenario, ACC resulted in 4.32 (range 0.23–17.25) DALYs being averted per person at an incremental individual cost of $582 (range $110–$4405) (Table 3).
In the Tanzanian district hospital scenario, the model estimates implementing EECC may avert an estimated 3.58 (range 0.20–13.22) DALYs at an incremental cost of $39 (range −$98 to $208) per person, and ACC can avert 4.61 (range 0.42–15.70) DALYs at an incremental cost of $532 (range −$41 to $3932) (Table 3). Further results are reported in ESM Note S4.
Sensitivity and Uncertainty Analysis
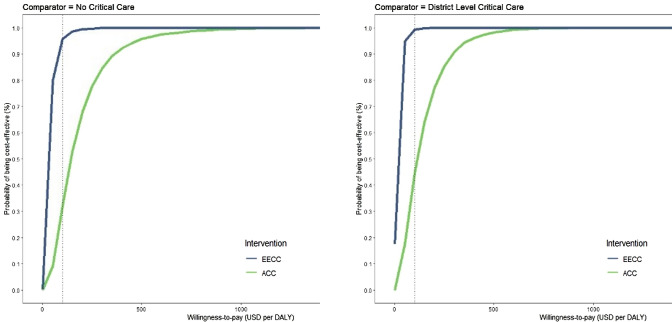
The CEACs show that at a willingness-to-pay threshold of $101, the probability of EECC being cost effective is estimated to be 95.7% and 99.1% when compared with ‘no critical care’ and ‘district hospital-level critical care’, respectively (Fig. 2). The probability of ACC being cost effective in the ‘no critical care’ or ‘district hospital-level critical care’ scenarios was 31.9% and 44.6%, respectively. The cost-effectiveness planes can be viewed in ESM Note S5.
Fig. 2.
Cost-effectiveness acceptability curves. Acceptability curves delineating the probability that either EECC or ACC is cost effective in comparison with either ‘no critical care’ or ‘district hospital-level critical care’ scenarios. Each curve depicts the probability that an intervention, in comparison with a comparator, would present the greatest net health benefits across a range of cost-effectiveness thresholds, estimated by the proportion of simulations in which that intervention was cost effective at each threshold level. Black dotted lines depict a conservative willingness-to-pay threshold range for Tanzania ($101). ACC constitutes EECC in combination with more advanced organ support. EECC is delivered to both severe and critical COVID-19 patients, whereas ACC is only delivered to critical COVID-19 patients. COVID-19 coronavirus disease 2019, EECC Essential Emergency and Critical Care, ACC advanced critical care
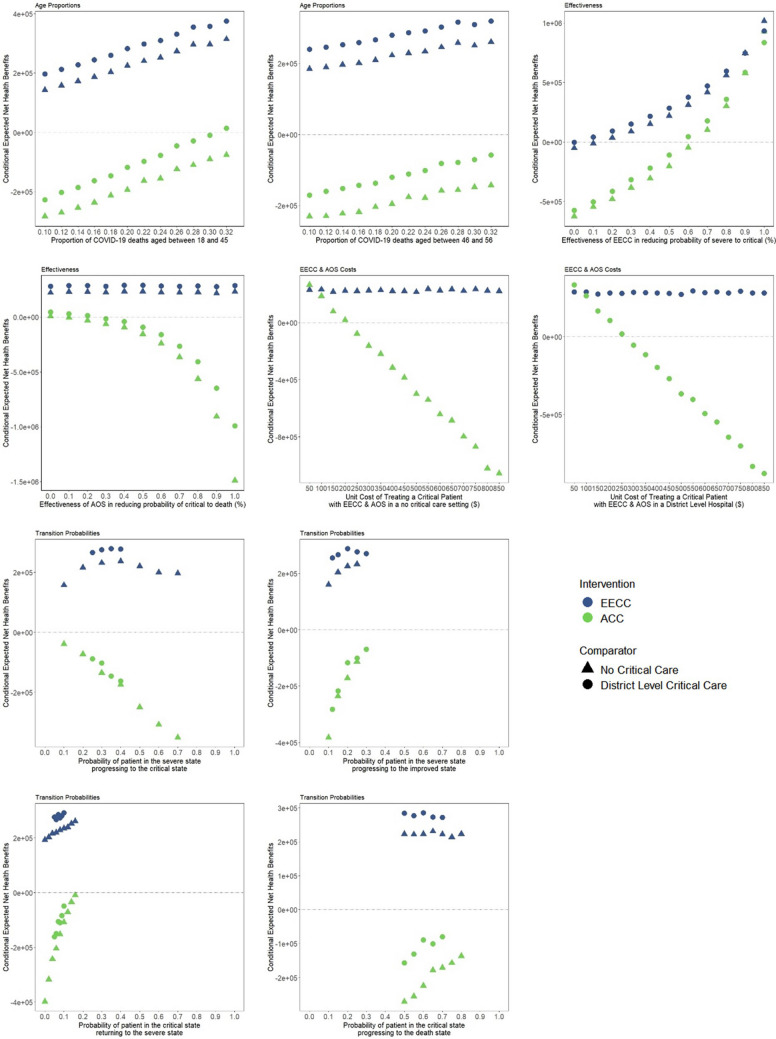
The sensitivity of the net health benefit of EECC or ACC to each model parameter for each comparator is shown in Figs. 3 and 4. Net health benefit values were most sensitive to changes in the following parameters: the clinical effectiveness of EECC in reducing the probability of a severe patient becoming critical, and the unit cost of treating a critical patient with ACC in a ‘no critical care’ or ‘district hospital-level critical care’ setting (Fig. 3). The cost effectiveness of EECC and/or ACC changed as each parameter was increased.
Fig. 3.
Conditional net benefit curves for the most sensitive parameters. Each conditional net benefit graph shows how sensitive the conditional net benefit is to the value of the respective parameter. The lower the gradient of each curve for each intervention vis-a-vis comparator, the less sensitive the conditional net benefit is to that parameter. ACC constitutes EECC in combination with more advanced organ support. EECC is delivered to both severe and critical COVID-19 patients, whereas advanced critical care is only delivered to critical COVID-19 patients. COVID-19 coronavirus disease 2019, EECC Essential Emergency and Critical Care, ACC advanced critical care
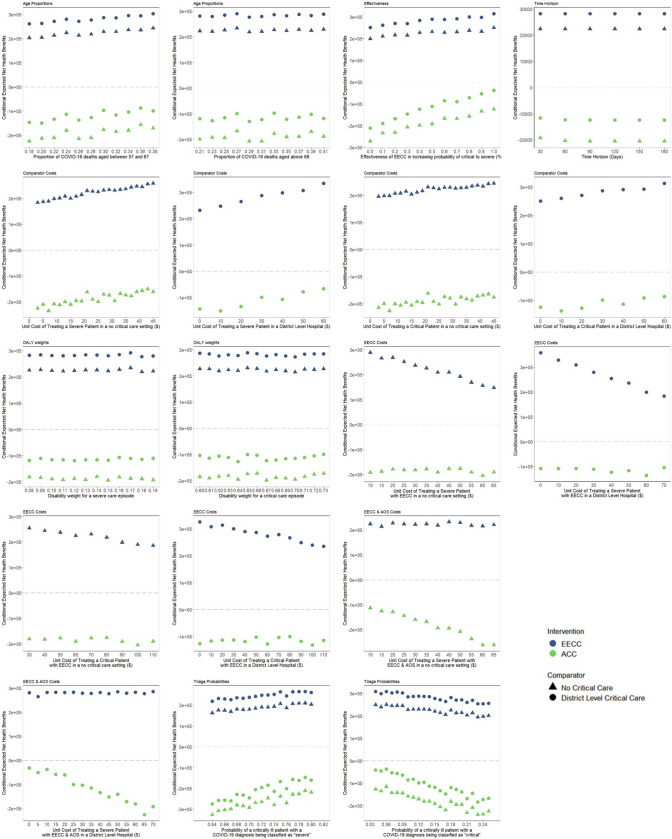
Fig. 4.
Conditional net benefit curves for all insensitive parameters. Each conditional net benefit graph shows how sensitive the conditional net benefit is to the value of the respective parameter. The lower the gradient of each curve for each intervention vis-a-vis comparator, the less sensitive the conditional net benefit is to that parameter. ACC constitutes EECC in combination with more advanced organ support. EECC is delivered to both severe and critical COVID-19 patients, whereas advanced critical care is only delivered to critical COVID-19 patients
Net health benefit values were slightly sensitive to the effectiveness of ACC in reducing the probability of progressing from the critical state to death, effectiveness of EECC in increasing the probability of progressing from the critical state to the severe state, proportion of COVID-19 deaths in patients aged between 18 and 56 years, probability of being diagnosed as critical or severe, and all four transition probabilities. However, the net health benefit values did not shift from positive to negative or vice versa when increasing each of these parameters, and the overall cost effectiveness remained constant. The net health benefit of either intervention was not sensitive to any other parameters in a ‘no critical care’ or ‘district hospital-level critical care’ comparator scenario (Fig. 4). Finally, when assessing the cost effectiveness of EECC or ACC compared with ‘no critical care’ using uniform distributions as opposed to triangular distributions, we found that using a uniform distribution lowers the probability of cost effectiveness for EECC and ACC compared with ‘no critical care’ by approximately 10% (ESM Note S7).
Discussion
Our analysis indicates that EECC, including simple physiological monitoring and timely life-saving interventions, is likely to be highly cost effective in low-resource settings, and is comparable with other highly cost-effective interventions identified by the Disease Control Priorities Programme (2018), such as treating malaria with artesunate, or emergency obstetric care [47].
Data Sparsity
Systems for care of critically ill patients, at advanced and essential levels, are set up to cater for severely ill patients regardless of their underlying diagnosis. This heterogeneity is an in-built challenge to studying critical care. For pragmatic reasons, we used COVID-19 as a tracer condition for this first economic evaluation of EECC. At the time of analysis, COVID-19 clinical data from low-resourced hospitals, such as district hospitals in low- and middle-income settings, appropriate for the model were sparse. In Tanzania, there was only one study reporting mortality rates from a private not-for-profit hospital in Tanzania and not reflective of all healthcare providers in Tanzania [48]. Initiatives such as the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) partnership and the African COVID-19 Critical Care Outcomes Study (ACCCOS) have resulted in large, standardised collections of comprehensive individual-level clinical data from hundreds of ICU and other critical-care facilities across dozens of countries [49–51]. However, there is still under-representation of data from low-income countries and lower-resourced facilities, such as general wards and district hospitals, that represent a major share of health care utilisation in sub-Saharan Africa. The gap in COVID-19 clinical data from Tanzania and sub-Saharan Africa has led to a cautious approach in this paper in the identification of parameters and the uncertainty ranges used in our three baseline scenarios.
Information on clinical effectiveness of critical care interventions is even more sparse. For example, in the case of oxygen, some studies compared the relative effectiveness or cost effectiveness of different modes of oxygen therapy in substitute scenarios (e.g., children with pneumonia), while other studies focused on oxygen therapy in comparison with no oxygen therapy [52–54]. Any measure of clinical effectiveness likely includes substantial heterogeneity as oxygen delivery is in combination with other treatments, processes, and procedures, depending on a range of factors (i.e., differing modalities of oxygen therapy, constant supply, back-up electricity, trained staff, observation frequency, treatment modifications and, notably, all other care delivered). Due to this lack of evidence, the heterogeneity and the fact that EECC is more than just the effective delivery of oxygen, we estimated the likely clinical effectiveness of EECC using a nominal group technique [55].
Cost Effectiveness of Essential Emergency and Critical Care
In a typical ICU of a high-income country, critical care is conducted with expensive equipment, high-quality laboratory support and numerous highly trained staff [6]. Throughout the pandemic, low- and middle-income countries considered investment in such high-resource care models. However, when critical care capacity is low, an ACC model is usually impractical as the required resources are unavailable, too expensive, or not clinically effective [10, 56]. Our results support this finding and suggest that investing in EECC could be a highly cost-effective solution.
Financing
Prioritising EECC in settings where there are currently little or no critical care services has a very high probability of being cost effective (> 90%). We used a conservative threshold of $101 per DALY averted, which was estimated while Tanzania was still a low-income country, and therefore this threshold is likely to have increased, implying that EECC will have a greater probability of being cost effective. In addition, this threshold may vary depending on the provider. Financing of Tanzania’s network of health facilities, including non-governmental organisation (NGO) providers, is derived from a mix of sources, including government, donors and prepayment schemes such as the National Health Insurance Fund [56, 57]. The resulting heterogeneity in critical care funding and healthcare priorities can result in variations in the willingness-to-pay threshold across different purchasers of healthcare. Specifically, public providers will likely have low willingness-to-pay thresholds, whereas more autonomous institutions may have significantly higher willingness-to-pay thresholds.
For providers who have a higher willingness-to-pay threshold (i.e. >$1000 per DALY averted), ACC has a higher probability of being cost effective. However, as the findings show that the probability of ACC being cost effective is substantially less than that for EECC, starting such a scale-up by ensuring high hospital-wide effective coverage of EECC could result in a substantially better return of investment and lives saved.
However, financing is not enough. EECC is designed to be simple, but implementation research on horizontal approaches to care, such as EECC, ACC and integrated management of childhood illness (IMCI), suggest that changes to health system processes, additional staff, training, stakeholder engagement and political buy-in would be needed to achieve the human resources and institutional change required. [58–60]
Limitations
There were several challenges and limitations in performing this analysis. First, the novel nature of EECC, the urgency to inform decision making, and restrictions on primary data collection necessitated a reliance on scarce secondary data and nominal group methods as well as flexibility in model building. To address these concerns, a comprehensive review was carried out to ensure that all the available information was used and probabilistic analysis was performed to allow full exploration of uncertainty [15, 26, 45, 46].
Second, our analysis did not include moderate patients or the limits in critical care capacity and barriers to healthcare access. While, EECC has a significant role in identifying which moderate patients become critically ill, we focused on severe and critical patients, as EECC treatments are only provided to these populations. In addition, the findings are not generalisable to situations in which critical care capacity (e.g., number of beds or oxygen masks being used) is constrained, such as during surges in COVID-19, as we assumed all patients receive either care regardless of capacity constraints. However, our model is fully adaptable and capacity constraints, once measured, can be included in the form of costs, while equity parameters can be added prior to hospitalisation to account for barriers to access.
Third, critical care infrastructure, such as human resources, consumables, equipment, training, and laboratory support, is highly variable across the healthcare system. District-level facilities deliver only non-ICU-based critical care, if at all, whereas referral or regional hospitals are more likely to provide some ICU and non-ICU-based critical care [15, 26]. This analysis did not focus on care provided in regional or referral hospitals. Further research on the costs and cost effectiveness of critical care delivery in regional or referral hospitals could provide greater insight into the optimal allocation of new investments in critical care.
Fourth, one of the limitations of our approach is that we opted to employ a Markov cohort approach over a 24-h cycle and a 28-day time horizon due to severe data constraints. Based on expert consultation from the nominal group, if patients die as severe, they have been critical between 1 min and 23 h 59 min (i.e., within a 24-h period). During this time, the severe patients may have received different levels of care and the level of care delivered will affect their chance of survival. To capture this, an alternative modelling methodology would be required, such as individual-based modelling or discrete event simulation, which would have different data requirements.
Finally, we employed the use of triangular distributions to perform our probabilistic sensitivity analysis. Traditionally, cost data are given gamma distributions, and probabilities are given beta or log-normal distributions. However, as many parameters were based on expert opinion and others were point estimates, the use of triangular distributions was considered appropriate for this analysis [43]. Although we did perform a sensitivity analysis using uniform distributions, future research should aim to comprehensively assess the impact of alternative distributions on the cost effectiveness of EECC and ACC once more improved clinical efficacy or effectiveness data are available.
Despite these challenges, our model structure provides a first step in generating evidence on the value of different models of critical care delivery for lower-resource settings using real-world data [48, 49, 61–67]. Our model structure can be adapted with further country-specific real-world evidence to parameterise early health technology assessments of critical care interventions.
Future primary research on the cost effectiveness of EECC and critical care strategies more generally must explore and implement pragmatic or step wedge intervention trials focusing on different strategies for the expansion, scaling up or improving of critical care services to generate data that can combat the limitations in the analysis. This should include ensuring the consistent reporting of mortality rates for critically ill patients across various geographies, using standardised clinical categorisation and controlling for intervention delivery. In addition, we recommend carrying out primary data collection on EECC, including the effectiveness of and resource use required for EECC and ACC, exploring methods for improving nominal group techniques to parameterise economic evaluations, and the use of Bayesian approaches that could fit Markov models to reported mortality rates, improve model calibration to quantify unknown parameter values, and potentially conduct Bayesian meta analyses of elicitation data [67].
Conclusion
EECC could be a highly cost-effective investment for providing care to critically ill patients with COVID-19 in settings where little or no critical care is available. The findings indicate that EECC is more cost effective than ACC. While investing in EECC will benefit all critically ill patients, further data are required to provide more robust findings, and further development of the model will be required to explore this beyond the treatment of COVID-19 patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the wider Provision of Essential Treatment in Critical Illness (POETIC) project team for their expertise on EECC and their contribution to the intervention definitions.
Declarations
Funding
This work was supported by the Wellcome Trust [221571/Z/20/Z], as part of the 'Innovation in Low- and Middle-Income Countries' Flagship, and by the International Decision Support Initiative, funded by the Bill & Melinda Gates Foundation.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have provided consent for publication.
Competing financial interests
Tim Baker declares personal fees from UNICEF, the World Bank, USAID and the Wellcome Trust, all outside the submitted work. Lorna Guinness declares fees from the Bill & Melinda Gates Foundation and the London School of Hygiene and Tropical Medicine, both outside the scope of this work. Hiral Anil Shah contributed to this study while employed by the Center for Global Development, however he is now an employee for GSK and holds shares in the GSK group of companies. Carl Otto Schell, August Kuwawenaruwa, Khamis Awadh, Karima Khalid, Angela Kairu, Vincent Were, Edwine Barasa, and Peter Baker declare no competing interests.
Author contributions
Concept and design: HAS, TB, COS, EB, LG, PB. Acquisition of data: HAS, TB, COS, AKa, AKu, KA, KK, LG. Analysis and interpretation of data: HAS, TB, COS, PB, LG. Drafting of the manuscript: HAS, LG. Critical revision of the paper for important intellectual content: HAS, TB, COS, AKa, Aku, VW, EB, KK, PB, LG. Statistical analysis: HAS, LG. Obtaining funding: TB, COS, PB, LG. Supervision: LG
Data availability
All data are available from the authors upon request.
Code availability
The R code that supports the findings of this study is available on github (https://github.com/EECCnetwork/POETIC_CEA).
References
- 1.Flaxman S, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 2.Walker PGT, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;1979(369):413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCabe R, et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020;18:1–12. doi: 10.1186/s12916-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig J, Kalanxhi E, Hauck S. National estimates of critical care capacity in 54 African countries. medRxiv. 2020;46:e41. [Google Scholar]
- 5.World Health Organisation Strategic Advisory Group of Experts (SAGE). WHO Strategic Advisory Group of Experts (SAGE) on Immunization Working Group on COVID-19 Vaccines: Prioritized Infectious Disease and Economic Modelling Questions. WHO, Geneva; 2020.
- 6.Baker T, et al. Essential care of critical illness must not be forgotten in the COVID-19 pandemic. The Lancet. 2020;395:1253–1254. doi: 10.1016/S0140-6736(20)30793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz MJ, et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med. 2017;43:612–624. doi: 10.1007/s00134-017-4750-z. [DOI] [PubMed] [Google Scholar]
- 8.Vukoja M, et al. A survey on critical care resources and practices in low- and middle-income countries. Glob Heart. 2014;9:337–342.E5. doi: 10.1016/j.gheart.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Usher AD. Health systems neglected by COVID-19 donors. Lancet. 2021;397:83. doi: 10.1016/S0140-6736(21)00029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleary SM, Wilkinson T, TamandjouTchuem CR, Docrat S, Solanki GC. Cost-effectiveness of intensive care for hospitalized Covid-19 patients: experience from South Africa. BMC Health Serv Res. 2020;4:1–10. doi: 10.1186/s12913-021-06081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valley TS, Noritomi DT. ICU beds: less is more? Yes. Intensive Care Med. 2020;46:1594–1596. doi: 10.1007/s00134-020-06042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manda-Taylor L, Mndolo S, Baker T. Critical care in Malawi: the ethics of beneficence and justice. Malawi Med J. 2017;29:268–271. doi: 10.4314/mmj.v29i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker T, et al. Emergency and critical care services in Tanzania: a survey of ten hospitals. BMC Health Serv Res. 2013;13:140. doi: 10.1186/1472-6963-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy S, Leligdowicz A, Adhikari NKJ. Intensive care unit capacity in low-income countries: a systematic review. PLoS ONE. 2015;10:1–12. doi: 10.1371/journal.pone.0116949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazibwe J, et al. Resource availability, utilisation and cost in the provision of critical care in Tanzania: a protocol for a systematic review. BMJ Open. 2021;11:1–5. doi: 10.1136/bmjopen-2021-050881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schell CO, et al. The global need for essential emergency and critical care. Crit Care. 2018;22:1–5. doi: 10.1186/s13054-018-2219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schell CO, et al. Essential Emergency and Critical Care: a consensus among global clinical experts. BMJ Glob Health. 2021;6:e006585. doi: 10.1136/bmjgh-2021-006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayambankadzanja RK, et al. Unmet need of essential treatments for critical illness in Malawi. PLoS ONE. 2021;16:9749. doi: 10.1371/journal.pone.0256361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dart PJ, et al. An evaluation of inpatient morbidity and critical care provision in Zambia. Anaesthesia. 2017;72:172–180. doi: 10.1111/anae.13709. [DOI] [PubMed] [Google Scholar]
- 20.Graham HR, et al. Oxygen systems and quality of care for children with pneumonia, malaria and diarrhoea: analysis of a stepped-wedge trial in Nigeria. PLoS ONE. 2021;16:e0254229. doi: 10.1371/journal.pone.0254229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker T, et al. Vital Signs directed therapy: improving care in an intensive care unit in a low-income country. PLoS ONE. 2015;10(12):e0144801. doi: 10.1371/journal.pone.0144801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdu M, et al. Resource availability for the management of maternal sepsis in Malawi, other low-income countries, and lower-middle-income countries. Int J Gynecol Obstet. 2018;140:175–183. doi: 10.1002/ijgo.12350. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds TA, et al. Strengthening Health Systems to Provide Emergency Care. In: Disease Control Priorities: Improving Health and Reducing Poverty. 3rd edition (Vol 9). Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2017. pp. 247–265. 10.1596/978-1-4648-0527-1_CH13. [PubMed]
- 24.Biccard BM, et al. Perioperative patient outcomes in the African Surgical Outcomes Study: a 7-day prospective observational cohort study. The Lancet. 2018;391:1589–1598. doi: 10.1016/S0140-6736(18)30001-1. [DOI] [PubMed] [Google Scholar]
- 25.Murthy S, Adhikari NK. Global health care of the critically ill in low-resource settings. Ann Am Thorac Soc. 2013;10:509–513. doi: 10.1513/AnnalsATS.201307-246OT. [DOI] [PubMed] [Google Scholar]
- 26.Kazibwe J, et al. Resource use, availability and cost in the provision of critical care in Tanzania: a systematic review. BMJ Open. 2022;12:e060422. doi: 10.1136/bmjopen-2021-060422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kairu A, et al. Modelling the cost-effectiveness of essential and advanced critical care for COVID-19 patients in Kenya. BMJ Glob Health. 2021;6:185. doi: 10.1136/bmjgh-2021-007168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson T, et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value in Health. 2016;19:921–928. doi: 10.1016/j.jval.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organisation. COVID-19 Clinical management: living guidance. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- 30.World Health Organization . Guideline Clinical management of COVID-19 patients: living guideline. Geneva: World Health Organization; 2021. [Google Scholar]
- 31.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 32.Desforges M, Gurdasani D, Hamdy A, Leonardi AJ. Uncertainty around the long-term implications of COVID-19. Pathogens. 2021;10(10):1267. doi: 10.3390/pathogens10101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16:326–331. doi: 10.1093/heapol/16.3.326. [DOI] [PubMed] [Google Scholar]
- 34.Salomon JA, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 35.Ochalek J, Lomas J, Claxton K. Cost per DALY averted thresholds for low- and middle-income countries: evidence from cross country data. Center Health Econ. 2015 doi: 10.1128/MCB.00849-10. [DOI] [Google Scholar]
- 36.Revill P, et al. Cost-effectiveness thresholds: guiding health care spending for population health improvement. A report by the Centre for Health Economics, University of York, for the International Decision Support Initiative (iDSI). Centre of Health Economics, University of York; 2015. pp. 1–24.
- 37.RStudio Team; R Studio: Integrated Development for R. 2015. http://www.rstudio.com/.
- 38.Makundi EA, et al. The use of nominal group technique in identifying community health priorities in Moshi rural district, northern Tanzania. Tanzan Health Res Bull. 2005;7:133–141. doi: 10.4314/thrb.v7i3.14250. [DOI] [PubMed] [Google Scholar]
- 39.James C, Bura M, Ensor T. Cost of Delivering Health Services in Tanzania: Findings from a comprehensive costing analysis. Oxford Policy Management; 2013. pp. 52.
- 40.PATH. Quantification and Costing Tools | PATH. 2020. https://www.path.org/resources/quantification-and-costing-tools/.
- 41.Guinness L, et al. Essential Emergency and Critical Care as a health system response to critical illness and the COVID19 pandemic: what does it cost? Cost Effective Resour Alloc. 2023;21(1):15. doi: 10.21203/rs.3.rs-1628806/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford handbook in health economics. Oxford: University of Oxford; 2006. p. 237. [Google Scholar]
- 43.Armstrong N, et al. A systematic review and cost-effectiveness analysis of specialist services and adrenaline auto-injectors in anaphylaxis. Health Technol Assess. 2013;17(17):1–117. doi: 10.3310/hta17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decision Mak. 1998;18(2 Suppl):S68–80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 45.Wilson ECF. Methodological note: reporting deterministic versus probabilistic results of Markov, partitioned survival and other non-linear models. Appl Health Econ Health Policy. 2021;19(6):789–795. doi: 10.1007/S40258-021-00664-2. [DOI] [PubMed] [Google Scholar]
- 46.McCabe C, Tramonti G, Sutton A, Hall P, Paulden M. Probabilistic one-way sensitivity analysis with multiple comparators: the conditional net benefit frontier. Pharmacoeconomics. 2021;39:19–24. doi: 10.1007/s40273-020-00983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horton S. Economic Evaluation Results from Disease Control Priorities, Third Edition. Disease Control Priorities: Improving Health and Reducing Poverty. 3rd edition. International Bank for Reconstruction and Development/The World Bank; 2018; pp. 147–156. [PubMed]
- 48.Kassam N, et al. Factors associated with mortality among hospitalized adults with COVID-19 pneumonia at a private tertiary hospital in Tanzania: a retrospective cohort study. Int J Gen Med. 2021;14:5431–5440. doi: 10.2147/IJGM.S330580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biccard BM, et al. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. Lancet. 2021;397:1885. doi: 10.1016/S0140-6736(21)00441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdukahil SA, et al. COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection. 2021;49:889–905. doi: 10.1007/s15010-021-01599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbas A, et al. The value of open-source clinical science in pandemic response: lessons from ISARIC. Lancet Infect Dis. 2021;21:1623–1624. doi: 10.1016/S1473-3099(21)00565-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duke T, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372:1328–1333. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 53.Oliaei S, SeyedAlinaghi S, Mehrtak M, et al. The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): a systematic review. Eur J Med Res. 2021;26:96. doi: 10.1186/s40001-021-00570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam F, Stegmuller A, Chou VB, Graham HR. Oxygen systems strengthening as an intervention to prevent childhood deaths due to pneumonia in low-resource settings: systematic review, meta-analysis and cost-effectiveness. BMJ Glob Health. 2021;6:e007468. doi: 10.1136/bmjgh-2021-007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rankin NM, et al. Adapting the nominal group technique for priority setting of evidence-practice gaps in implementation science. BMC Med Res Methodol. 2016;16:1–9. doi: 10.1186/s12874-016-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ally M, Piatti-Funfkirchen M. Tanzania health financing policy notes. Singapore: World Bank; 2020. [Google Scholar]
- 57.Kapologwe NA, et al. Understanding the implementation of Direct Health Facility Financing and its effect on health system performance in Tanzania: a non-controlled before and after mixed method study protocol. Health Res Policy Syst. 2019;17:1–13. doi: 10.1186/s12961-018-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.English M. Improving emergency and admission care in low-resource, high mortality hospital settings—not as easy as A, B and C. Health Policy Plan. 2022;37(6):808–810. doi: 10.1093/heapol/czab128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muinga N, et al. Using a human-centred design approach to develop a comprehensive newborn monitoring chart for inpatient care in Kenya. BMC Health Serv Res. 2021;21:1–14. doi: 10.1186/s12913-021-07030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayieko P, et al. A multifaceted intervention to implement guidelines and improve admission paediatric care in Kenyan district hospitals: a cluster randomised trial. PLoS Med. 2011;8:15. doi: 10.1371/journal.pmed.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nachega JB, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103:2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bahloul M, et al. Clinical characteristics and outcomes of critically ill COVID-19 patients in Sfax, Tunisia. Acute Crit Care. 2022;37:84–93. doi: 10.4266/acc.2021.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leulseged TW, et al. Characteristics and outcome profile of hospitalized African patients with COV-19: the Ethiopian context. PLoS ONE. 2021;16(1):e0259454. doi: 10.1371/journal.pone.0259454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Surendra H, et al. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: a hospital-based retrospective cohort study. Lancet Reg Health West Pac. 2021;9:100108. doi: 10.1016/j.lanwpc.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agrupis KA, et al. Epidemiological and clinical characteristics of the first 500 confirmed COVID-19 inpatients in a tertiary infectious disease referral hospital in Manila, Philippines. Trop Med Health. 2021;49(1):48. doi: 10.1186/s41182-021-00340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brizzi A, et al. Spatial and temporal fluctuations in COVID-19 fatality rates in Brazilian hospitals. Nat Med. 2022;28:1476–1485. doi: 10.1038/s41591-022-01807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menzies NA, Soeteman DI, Pandya A, Kim JJ. Bayesian methods for calibrating health policy models: a tutorial. Pharmacoeconomics. 2017;35:613–624. doi: 10.1007/s40273-017-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the authors upon request.