Key Points
-
•
The median OS of patients with MCL was 1.6 years; a diagnosis of MCL-AHN and an abnormal karyotype were each associated with inferior outcomes.
-
•
Midostaurin was the most commonly used agent in MCL and was associated with improved OS in a multivariate analysis.
Visual Abstract
Abstract
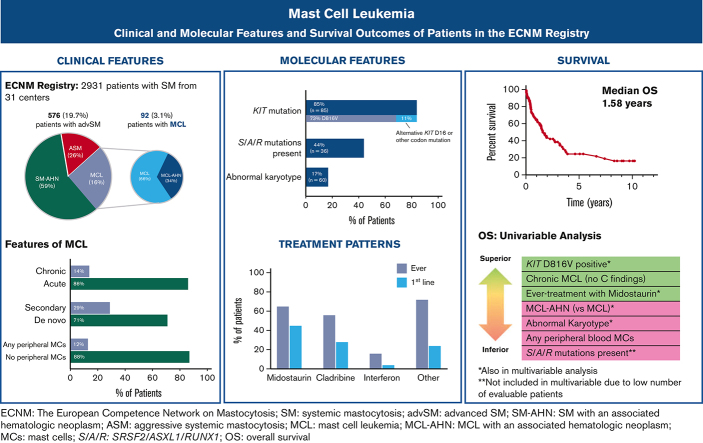
Mast cell leukemia (MCL) is a rare subtype of systemic mastocytosis defined by ≥20% mast cells (MC) on a bone marrow aspirate. We evaluated 92 patients with MCL from the European Competence Network on Mastocytosis registry. Thirty-one (34%) patients had a diagnosis of MCL with an associated hematologic neoplasm (MCL-AHN). Chronic MCL (lack of C-findings) comprised 14% of patients, and only 4.5% had “leukemic MCL” (≥10% circulating MCs). KIT D816V was found in 62/85 (73%) evaluable patients; 9 (11%) individuals exhibited alternative KIT mutations, and no KIT variants were detected in 14 (17%) subjects. Ten evaluable patients (17%) had an abnormal karyotype and the poor-risk SRSF2, ASXL1, and RUNX1 (S/A/R) mutations were identified in 16/36 (44%) patients who underwent next-generation sequencing. Midostaurin was the most common therapy administered to 65% of patients and 45% as first-line therapy. The median overall survival (OS) was 1.6 years. In multivariate analysis (S/A/R mutations excluded owing to low event rates), a diagnosis of MCL-AHN (hazard ratio [HR], 4.7; 95% confidence interval [CI], 1.7-13.0; P = .001) and abnormal karyotype (HR, 5.6; 95% CI, 1.4-13.3; P = .02) were associated with inferior OS; KIT D816V positivity (HR, 0.33; 95% CI, 0.11-0.98; P = .04) and midostaurin treatment (HR, 0.32; 95% CI, 0.08-0.72; P = .008) were associated with superior OS. These data provide the most comprehensive snapshot of the clinicopathologic, molecular, and treatment landscape of MCL to date, and should help further inform subtyping and prognostication of MCL.
Introduction
Systemic mastocytosis (SM) is a myeloid neoplasm characterized by the expansion and accumulation of clonal mast cells (MC) in the bone marrow (BM) and other organs. Nonadvanced subtypes of SM include indolent SM (ISM) and smoldering SM (SSM); advanced SM (AdvSM) subtypes include aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).1, 2, 3, 4 MCL is rare, comprising <5% of all SM cases, and is defined by World Health Organization (WHO) diagnostic criteria for SM plus the criterion of ≥20% MCs on a BM aspirate smear.5 Although 1 or more C-findings (SM-related organ damage) is a prerequisite for a diagnosis of ASM, it is not required for a diagnosis of SM-AHN or MCL, despite being commonly encountered in these diseases. The prognosis of MCL is often grim, with a median overall survival (OS) of <2 years.5, 6, 7
MCL can be further subdivided into variants based on clinicopathologic features. These subtypes include: primary (de novo) vs secondary MCL (arising from another SM variant), acute (with C-findings) vs chronic (without C-findings) MCL, MCL with or without an AHN, and leukemic (≥10% circulating MC) vs aleukemic MCL (<10% circulating MC).4,5,8, 9, 10, 11, 12
More recently, cytogenetic and molecular data have provided additional insight into the biology and prognosis of MCL. As with other AdvSM subtypes, patients with MCL frequently exhibit the KIT D816V driver mutation, albeit at a generally lower frequency than ASM and SM-AHN.13, 14, 15 Other commonly mutated genes include TET2, SRSF2, ASXL1, and RUNX1.6,16,17 The presence of SRSF2, ASXL1,and/or RUNX1 (S/A/R) mutations predicts inferior survival, and S/A/R mutations (in addition to other mutations such as NRAS and DNMT3A) have been incorporated into prognostic scoring systems for patients with AdvSM.6,17, 18, 19, 20, 21
Historically, MCL has been treated with cytoreductive chemotherapy, most commonly single-agent cladribine (2-CdA), and in some cases, multiagent acute myeloid leukemia (AML)–type induction regimens.5 A possible salvage treatment option for these patients is allogeneic hematopoietic cell transplantation (allo-HCT), especially when a response to 2-CdA and/or chemotherapy is obtained. However, a multicenter, retrospective series reported that patients with MCL (n = 12) exhibited worse OS among all AdvSM patients undergoing allo-HCT, with a 3-year OS of only 17%.22
More recently, KIT-targeting drugs have demonstrated encouraging activity in patients with AdvSM, including MCL. Reduction in objective measures of MC burden (percentage of BM MC, serum tryptase level, splenomegaly, KIT D816V variant allele frequency), as well as reversion of C-findings and symptoms, have been consistent findings. Based on a global, nonrandomized phase 2 trial, the multikinase/KIT inhibitor midostaurin was approved by the Food and Drug Administration and European Medicines Agency in 2017 for patients with AdvSM.17 More recently, the selective KIT D816V inhibitor, avapritinib, was also approved by the Food and Drug Administration in 2021 as first-line therapy and by the European Medicines Agency in 2022 as second-line therapy based on safety and efficacy results from the phase 1 EXPLORER trial and an interim analysis of the phase 2 PATHFINDER trial in patients with AdvSM.23,24
To date, MCL has been systematically characterized in a few reports, including a review of 51 patients from various centers, a series of 28 cases from Germany, and a series of 13 patients from the United States.5, 6, 7 However, many features of this rare disorder remain incompletely understood. In this study, we describe the clinical characteristics, molecular features, current treatment patterns, and survival outcomes of a well-characterized, multi-institutional cohort of patients with MCL from the European Competence Network on Mastocytosis (ECNM) registry. To our knowledge, this is the largest cohort of patients with this rare advanced myeloid neoplasm.
Methods
ECNM Registry database and patients
Data were abstracted from the fifth data wave of the ECNM Registry, which contains clinical, laboratory, pathologic, and molecular information on patients with SM from 30 European centers and 1 center in the United States (Stanford Cancer Institute).25 All patients with a diagnosis of MCL were included in this analysis. All diagnoses of MCL were made between 1994 and 2019, and data were abstracted through 1 July 2020. The diagnosis of MCL was established according to diagnostic criteria provided by the WHO and the consensus group.1, 2, 3, 4,12 The study design adhered to the tenets of the Declaration of Helsinki and was approved by the participating centers’ institutional review boards. Before inclusion in the ECNM Registry, all patients provided written informed consent or, if deceased or inactive at the treating center, were included according to IRB standards at the treating center.
The following parameters were captured for this study: age, sex, date of diagnosis (histology based), diagnosis according to the WHO classification,26 any SM diagnoses before diagnosis of MCL, laboratory values at time of MCL diagnosis, percentage of MC in BM aspirate smears and blood films, molecular and cytogenetic data, presence of hepatosplenomegaly, weight loss (defined as >10% loss during the last 12 months before diagnosis), skeletal involvement of SM (defined as an osteolytic lesion(s) ≥2 cm), treatment courses, and death or last follow-up. The mutation-adjusted risk score (MARS), the global prognostic score for mastocytosis (GPSM), and the international prognostic scoring system for mastocytosis (IPSM) for AdvSM prognosis were each calculated from the above relevant variables.19,21,27
All cytogenetic analyses were performed at local laboratories using conventional methods. KIT D816V mutation status was measured at local laboratories using either single-gene assays or next-generation sequencing multigene panels on BM or peripheral blood (PB), following ECNM recommendations.14 If a KIT D816V mutation was absent, laboratories were encouraged to search for alternative KIT mutations whenever feasible.
Statistical analysis
Continuous variables were summarized by median and range, and binary outcomes were summarized by proportion. For secondary MCL, progression was defined as a transformation from 1 WHO SM category (ISM, SSM, ASM, or SM-AHN) to MCL. OS was defined as time from MCL diagnosis to death from any cause. For patients who underwent allo-HCT, progression-free survival was defined as time from allo-HCT to disease progression or death from any cause. OS was estimated using the Kaplan-Meier method and compared using log-rank tests. Univariate covariate effects on OS were evaluated using linear or logistic regression. Covariates with a P value of < .05 were included in a multivariate Cox proportional hazard model. P values < .05 were considered significant. Analyses were performed using R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical and laboratory characteristics
At the time of data abstraction, the ECNM contained 2931 patients with SM, of which 576 (19.7%) had AdvSM and 92 (3.1%) had MCL and were included in this analysis. Fifty-seven (62%) patients with MCL had follow-up records available; of those, the median follow-up time was 1.1 years (range 0.1-10.2).
Clinical and laboratory characteristics at the time of MCL diagnosis are described in Table 1 (stratification by MCL vs MCL-AHN in supplemental Table 1; stratification by acute vs chronic MCL in supplemental Table 2). The median age at diagnosis was 60.4 years (range 25.4-90.8). Most patients (70.7%) had de novo MCL, whereas 29.3% had secondary MCL. Sixty-one (66.3%) patients had a diagnosis of MCL only and 31 (33.7%) had a diagnosis of MCL-AHN; of those, the most common AHN was myelodysplastic syndrome/myeloproliferative neoplasm-unclassifiable (45.2%), followed by chronic myelomonocytic leukemia (25.8%), chronic eosinophilic leukemia (9.7%), and AML (9.7%). Concurrently identified lymphoid neoplasms included multiple myeloma and non-Hodgkin lymphoma (both 3.2%).
Table 1.
Clinical and laboratory characteristics of patients with MCL at diagnosis
| Variable | All patients (n = 92) | De novo MCL (n = 65) | Secondary MCL (n = 27) |
|---|---|---|---|
| Age at MCL diagnosis (y), median (range) | 60.4 (25.4-90.8) | 60.4 (27.1-90.8) | 60.0 (25.4-73.4) |
| Males, n (%) | 59 (64.1) | 43 (66.2) | 16 (59.3) |
| Diagnosis, n (%) | |||
| MCL | 61 (66.3) | 46 (70.7) | 15 (55.6) |
| MCL-AHN | 31 (33.7) | 19 (29.2) | 12 (44.4) |
| MDS/MPN-U | 14 (45.2) | 12 (18.5) | 2 (7.4) |
| CMML | 8 (25.8) | 2 (3.1) | 6 (22.2) |
| CEL/eosinophilia | 3 (9.7) | 2 (3.1) | 1 (3.7) |
| AML | 3 (9.7) | 1 (1.5) | 2 (7.4) |
| Multiple myeloma | 1 (3.2) | 1 (1.5) | 0 |
| NHL | 1 (3.2) | 1 (1.5) | 0 |
| Not specified | 1 (3.2) | 0 | 1 (3.7) |
| C-findings, n/N (%)∗ | |||
| No C-findings present | 13 (14) | 8 (12.3) | 5 (18.5) |
| Hemoglobin < 10 g/dL | 43 (46.7) | 31 (47.7) | 12 (44.4) |
| Platelets < 100 × 109/L | 46 (50.0) | 30 (46.2) | 16 (59.3) |
| Absolute neutrophil count < 1 × 109/L | 2 (2.2) | 2 (3.1) | 0 |
| Weight loss (> 10% in 6 mo) | 41/85 (41.8) | 37/61 (60.7) | 4/24 (16.7) |
| Albumin < 3.5 g/dL | 26/83 (31.3) | 21/57 (36.8) | 5/26 (19.2) |
| Hepatomegaly with ascites or portal hypertension | 19/84 (22.6) | 13/59 (22.0) | 6/25 (24.0) |
| Alkaline phosphatase > 150 U/L | 47/87 (54.0) | 31/60 (51.7) | 16/27 (59.3) |
| Osteolytic lesion(s) ≥ 2 cm | 4/70 (5.7) | 2/51 (3.9) | 2/19 (10.5) |
| Other relevant findings, n/N (%)∗ | |||
| Serum tryptase, μg/L median (range) | 333.5 (50.9-7490) | 308 (57.2-7490) | 396 (50.9-1820) |
| ≥200 μg/L, n (%) | 75 (81.5) | 53 (81.5) | 22 (81.5) |
| Any PB MCs detectable, n/N (%) | 11/89 (12.4) | 8/63 (12.7) | 3/26 (11.5) |
| ≥3% PB MCs | 7/89 (7.9) | 6/63 (9.5) | 1/26 (3.8) |
| ≥10% PB MCs | 4/89 (4.5) | 4/63 (6.3) | 0/26 (0) |
| MC infiltration in BM aspirate smear, %; median (range)† | 30 (20-100) | 30 (20-100) | 30 (20-88) |
| Abnormal karyotype, n/N (%) | 10/60 (17) | 7/38 (18.4) | 3/22 (13.6) |
| S/A/R mutations present, n/N (%) | 16/36 (44) | 9/24 (37.5) | 7/12 (58.3) |
CEL, chronic eosinophilic leukemia; CMML, chronic myelomonocytic leukemia; MDS/MPN-U, myelodysplastic syndrome/myeloproliferative neoplasm-unclassifiable; NHL, non-Hodgkin lymphoma.
Denominator is the total number of patients (92, 65, or 27 for full cohort, de novo, or secondary MCL, respectively) unless otherwise specified.
One patient did not have a BM biopsy but had >10% MCs in the PB.
The median serum tryptase at diagnosis was 333.5 μg/L (range 50.9-7490; normal level <11). Most of the patients (81.5%) had a tryptase of ≥ 200 μg/L. Of the 89 patients with a complete blood count with differential available, 11 (12.4%) had circulating MCs, whereas only 4 (4.5%) met the criteria for “leukemic MCL” with ≥ 10% circulating MCs. Of the 60 patients with karyotype information available, 10 (17%) had an abnormal karyotype.
Most of the patients (86%) had acute MCL, with at least 1 C-finding present. Among them, 68% had both hematologic and nonhematologic C-findings, 25% of patients had nonhematologic C-findings only, and only 5 patients (6%) had hematologic C-findings only (Table 1 for listing of C-findings). Of the 13 patients with chronic MCL, 8/13 (62%) had de novo MCL, 10/13 (77%) had MCL without an AHN, and 10/13 (77%) were KIT D816V positive; no chronic patients with MCL had circulating PB MCs (0/13) or an abnormal karyotype (0/9 evaluable). Follow-up data was available for 7 patients with chronic MCL; of these, 3 progressed to acute MCL (median time to progression 5 months, range 4.7-27).
Progression to secondary MCL
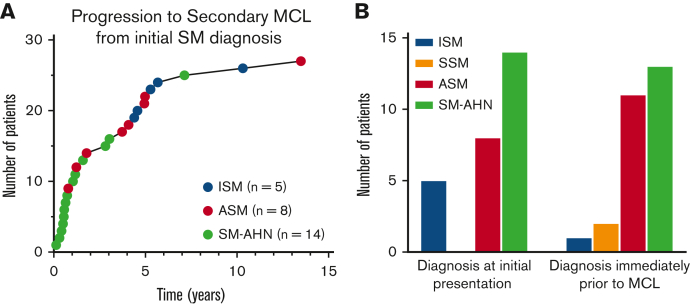
Of the 576 patients with AdvSM, 24 (4.2%) progressed to secondary MCL, including 11/147 (7.5%) patients with ASM and 13/337 (3.9%) with SM-AHN. Of the patients with SM-AHN, 11/13 progressed to MCL-AHN and 2/13 progressed to MCL. By contrast, in patients with non-AdvSM (n = 2208), only 5 (0.2%) ultimately progressed to secondary MCL, including 5/2132 (0.2%) patients with ISM and 2/76 (2.6%) with SSM (both of which had proceeding ISM). Two patients progressed from ISM to ASM to MCL, 1 patient progressed from ASM to SM-AHN to MCL, and the 2 patients progressed from ISM to SSM to MCL (these individuals were counted among the aforementioned patients who progressed to MCL) (Table 2).
Table 2.
Progression to secondary MCL by preceding diagnosis
| Diagnosis before MCL | Number of patients, n/N (%) |
Time to MCL progression from initial diagnosis at presentation (y), median (range) | |
|---|---|---|---|
| Initial diagnosis at presentation | Diagnosis immediately before MCL | ||
| ISM | 6/27 (22) | 1/27 (4) | 5.3 (4.4-10.3) |
| SSM | 0/27 (0) | 2/27 (7) | Not applicable |
| ASM | 7/27 (26) | 11/27 (41) | 3.9 (0.8-13.5) |
| SM-AHN | 14/27 (52) | 13/27 (48) | 0.7 (0.1-7.1) |
Of the 27 patients with secondary MCL, the most common diagnosis at initial presentation was SM-AHN (14 patients, 52%), ASM (7 patients; 26%), and ISM (6 patients; 22%); no patients with secondary MCL were diagnosed with SSM at initial presentation. The median time to progression from initial SM diagnosis was 1.8 years (range 0.1-13.5) (Figure 1A). Progression to MCL occurred most quickly in patients with an initial diagnosis of SM-AHN, with a median time to progression of 0.7 years (range 0.1-7.1 years), followed by ASM (3.9 years; range 0.8-13.5 years), and ISM (5.3 years; range 4.4-10.3 years). The most common diagnosis immediately before MCL progression was SM-AHN (13 patients; 48%), followed by ASM (11 patients; 41%), SSM (2 patients; 7%), and ISM (1 patient; 4%) (Figure 1B). Three patients did not progress to MCL until ≥5 years. Of those 3 patients, 2 had therapy information available and both were treated with interferon alfa.
Figure 1.
Progression to secondary MCL. Progression to secondary MCL (n = 27). (A) Time to progression to secondary MCL from initial SM diagnosis. (B) Diagnoses immediately before secondary MCL (right bar graph) and at initial presentation (left bar graph).
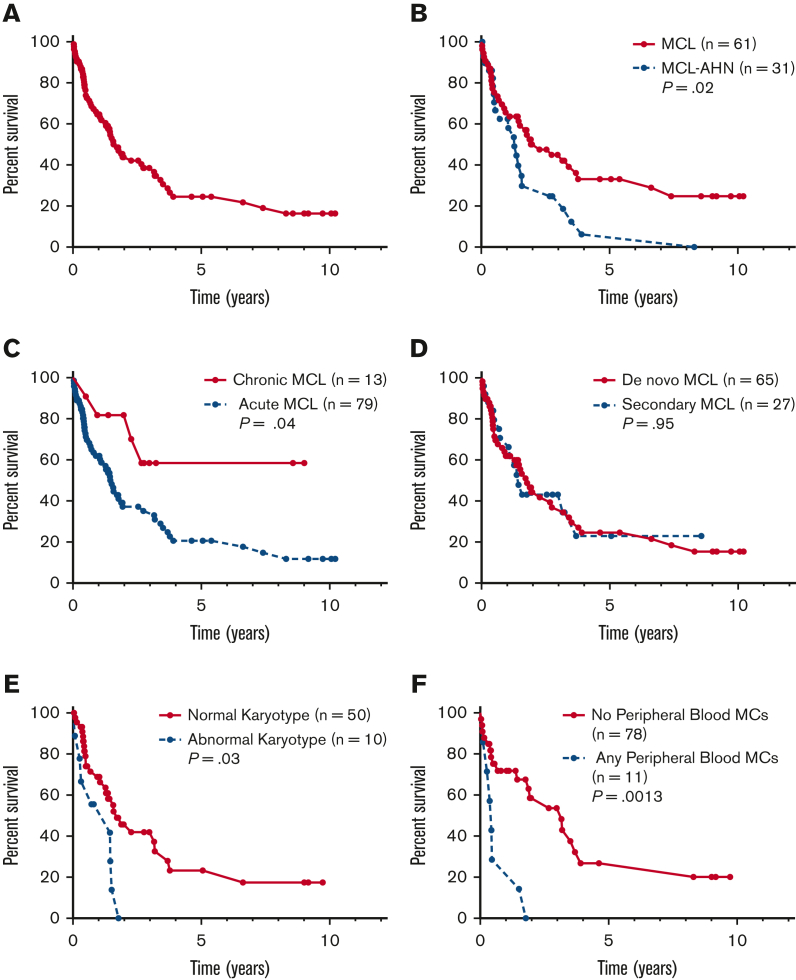
OS in subgroups of patients with MCL
The median OS of the entire cohort was 1.6 years (95% confidence interval [CI], 1.27-3.16) (Figure 2A). Notably, select patients in this cohort demonstrated long-term survival, with 11 patients alive ≥5 years from MCL diagnosis and 2 patients alive ≥10 years. Of the 11 patients alive ≥5 years from MCL diagnosis, 10 patients (91%) had MCL (vs MCL-AHN), all patients were KIT D816V positive, 5 patients had karyotype information available (all of whom had normal cytogenetics), and only 2 (18%) patients had chronic MCL (supplemental Table 3). Of the 54 patients with cause of death data available, 41 (76%) died from their disease, 3 (6%) died from treatment complications, and 10 (18%) had other causes of death.
Figure 2.
OS of patients by subtype of MCL. Kaplan-Meier estimates of OS for (A) the full cohort of patients with MCL (n = 92) and stratified by (B) MCL vs MCL-AHN; (C) chronic vs acute MCL; (D) de novo vs secondary MCL; (E) normal vs abnormal karyotype; and (F) any vs no PB MCs.
Compared with patients with MCL alone (n = 61), patients with MCL-AHN (n = 31) had an inferior OS (median OS 1.3 vs 2.3 years, P = .02) (Figure 2B). Patients with acute MCL (n = 79) also had inferior OS compared with patients with chronic MCL (n = 13) (median OS 1.5 years vs not reached, P = .04) (Figure 2C). There was no difference in OS for patients with de novo (n = 65) vs secondary MCL (n = 27) (median OS 1.8 vs 1.4 years, P = .95) (Figure 2D). An abnormal karyotype (n = 10) was associated with inferior OS compared with MCL cases with a normal karyotype (n = 50) (1.4 vs 1.72 years, P = .025) (Figure 2E). Compared with patients with aleukemic MCL (n = 85), patients with leukemic MCL (n = 4) exhibited inferior OS (0.4 vs 1.9 years, P = .0064). Similarly, patients with any circulating MCs (n = 11) had inferior OS compared with patients with no circulating MCs (n = 78) (0.5 vs 3.2 years, P = .0013) (Figure 2F).
Comparison of OS in patients with MCL vs other subtypes of AdvSM
Of the 576 patients with AdvSM, patients with MCL (n = 92) had significantly inferior OS compared with patients with ASM (n = 147) and SM-AHN (n = 337) (1.6 vs 6.2 vs 2.8 years, respectively, P < .001) (supplemental Figure 1A). This relationship was preserved when the patients with SM-AHN were restricted to patients with ASM-AHN (n = 197) (1.6 vs 6.2 vs 2.1 years, respectively, P < .001) (supplemental Figure 1B). Similarly, when compared with patients with ASM-AHN (n = 197), patients with MCL-AHN (n = 31) had inferior OS (2.1 vs 1.3 years, respectively, P = .03) (supplemental Figure 1C).
Impact of mutational profiles in patients with MCL
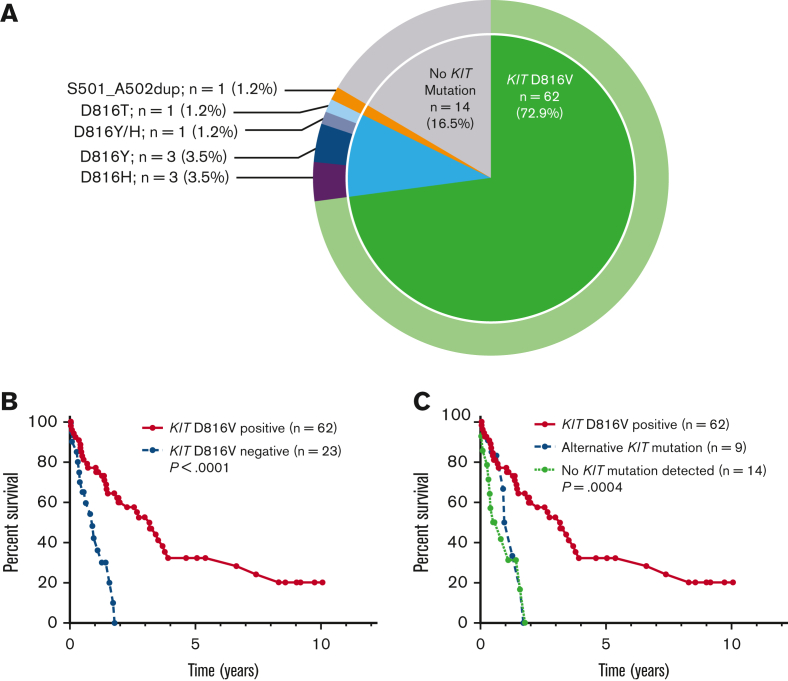
Of the 85 patients with KIT mutational status available, 71 (84%) had a KIT mutation (Figure 3A). The most common KIT mutation was D816V (n = 62, 72.9%), whereas 9 patients (10%) had an alternative KIT mutation. Of the 9 patients with an alternative KIT mutation, 8 patients had an alternative substitution at the D816 locus (eg, D816Y, D816H, D816T), whereas 1 patient had a KIT S501_A502 duplication, which has been reported as an activating mutation.28 Fourteen patients (16.5%) had no KIT mutation detected, although it is unknown whether full KIT sequencing was obtained in these patients.
Figure 3.
KIT mutations in patients with MCL. (A) Relative frequency distribution of KIT mutations in 85 patients with MCL. (B) Kaplan-Meier estimates of OS stratified by KIT D816V mutation status; and (C) stratified by KIT D816V positivity vs alternative KIT mutation positivity vs no detectable KIT mutations.
Compared with KIT D816V-negative patients (n = 23), KIT D816V-positive patients (n = 62) had superior OS (median OS 3.2 vs 0.9 years, P < .001) (Figure 3B). Patients with alternative KIT mutations, however, had inferior OS compared with KIT D816V-positive patients, with OS resembling that of patients without KIT mutations detected, with median OS 3.2 vs 1.1 vs 0.65 years for KIT D816V-positive (n = 71) vs alternative KIT mutation (n = 9) vs no detectable KIT mutation (n = 14) (P = .004) (Figure 3C).
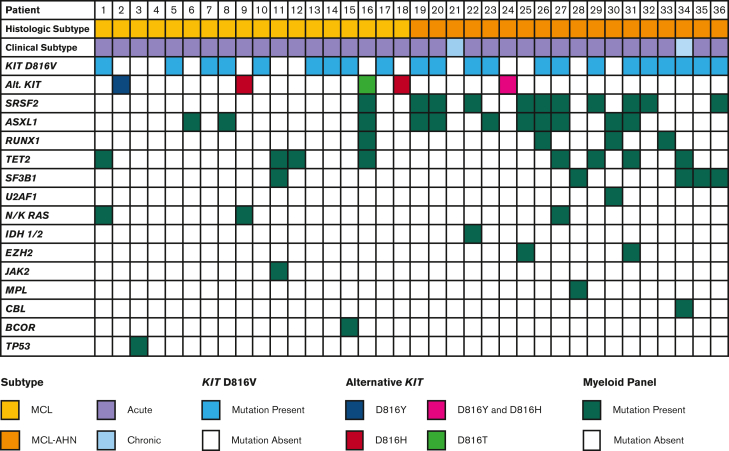
Data from multigene myeloid panels was available for 36 patients (Figure 4). The most commonly mutated genes aside from KIT were SRSF2 and ASXL1 (11 patients each, 30.6%), followed by TET2 (7 patients, 19.4%), SF3B1 (5 patients, 13.9%), RUNX1 (4 patients, 11.1%), and N/KRAS (3 patients, 8.3%). The number of mutations besides KIT was higher in patients with MCL-AHN, with a median of 2 mutations per patient compared with 0.5 mutations per patient in patients with MCL alone (P = .005). Of the 36 patients with multigene myeloid panels available, 16 (44.4%) had mutations in SRSF2, ASXL1, or RUNX1 (S/A/R). Patients with S/A/R mutations had inferior OS compared with patients without S/A/R mutations (median OS 0.5 years vs not reached, P = .005) (supplemental Figure 2A).
Figure 4.
Mutational profile of patients with MCL. Mutational profiles for 36 patients with MCL, stratified by diagnosis of MCL vs MCL-AHN (2nd row). Each column represents an individual patient.
Prognostic scores
MARS and GPSM prognostic scores were calculated for the 36 patients with molecular data available. Patients with low MARS had superior OS compared with patients with intermediate and high MARS (median OS NR vs 1.3 vs 0.7 years, P = .022) (supplemental Figure 2B). Similarly, patients with low or intermediate GPSM had superior OS compared with patients with high GPSM (median OS 0.7 vs 1.7 years, P = .04) (supplemental Figure 2C). All patients had IPSM scores calculated with lower scores associated with significantly improved OS (OS for AdvSM-1 vs AdvSM-2 vs AdvSM-3 vs AdvSM-4: 6.6 vs 7.4 vs 1.4 vs 1.3 years, P = .004) (supplemental Figure 2D).
Treatment outcomes in patients with MCL
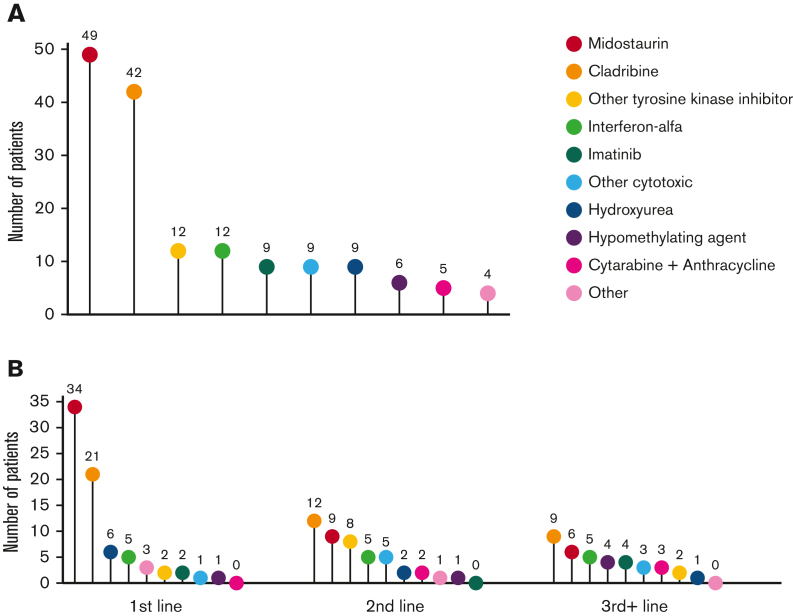
Treatment data following MCL diagnosis was available for 75 patients, including 75 patients receiving first-line treatment, 46 second-line, and 28 third-line or greater (Figure 5A-B). Midostaurin was the most common therapy, administered to 49 patients (65.3%) at any point during their treatment course and to 34 patients (45.3%) as first-line therapy. The next most common therapy was cladribine, administered to 42 patients (56%) at some point during their treatment course and to 21 patients (28%) as first-line therapy. Cladribine was also the most common therapy given in the second- and third-line or greater settings, administered to 12 (26%) and 9 (32%) patients, respectively.
Figure 5.
Treatment modalities in patients with MCL. Treatment modalities administered to patients with MCL in (A) at any time during the treatment course and (B) stratified by therapeutic line. The numbers above each data point indicate the number of patients who received each treatment.
Patients who received midostaurin at any point during their treatment course had superior OS compared with patients who did not (median OS 2.3 vs 1.1 years, P = .01) (supplemental Figure 3A). In the first-line setting, patients who received midostaurin had a median OS of 3.2 years vs 1.3 years for patients who received a different first-line therapy (P = .08) (supplemental Figure 3B).
Of the 92 patients in our cohort, 8 patients (4 with MCL and 4 with MCL-AHN) received an allo-HCT, which occurred at a median of 6.8 months following MCL diagnosis. Following allo-HCT, the median progression-free survival was 0.4 years and the median OS was 0.8 years (supplemental Figure 4). The median duration of follow-up was 0.7 years (range 0-2.3). At the time of last follow-up, 3 of the 8 patients who received allo-HCT were alive; 4 patients died from relapsed/progressive disease and 1 from treatment complications.
Comparative treatment patterns between MCL and other subtypes of AdvSM
Compared with patients with MCL, a significantly smaller proportion of patients with ASM received treatment with midostaurin at any point during their treatment course (35% vs 53%, P = .006) or as first-line therapy (23% vs 37%, P = .02). Similarly, compared with patients with MCL, a smaller proportion of patients with SM-AHN received midostaurin at any point during their treatment course (32% vs 53%, P = .0002) or as first-line therapy (24% vs 37%, P = .02) (supplemental Figure 5A).
There was no difference in the proportion of patients with ASM vs those with MCL who received treatment with cladribine at any point during their treatment course (35% vs 46%, P = .1) or as first-line therapy (18% vs 23%, P = .41). By contrast, a smaller proportion of patients with SM-AHN received treatment with cladribine at any point during their treatment course (16% vs 46%, <0.0001) or as first-line therapy (9% vs 23%, P = .0008) (supplemental Figure 5B).
Factors influencing survival in patients with MCL
Univariate and multivariate analyses of the association between baseline patient, disease, and treatment characteristics on OS are shown in Table 3. In univariate analysis, final diagnosis of MCL-AHN (hazard ratio [HR], 1.9; 95% CI, 1.1-3.2; P = .02), the presence of any circulating MCs (HR, 3.9; 95% CI, 1.6-9.4; P = .003), an abnormal karyotype (HR, 2.5; 95% CI, 1.1-5.7; P = .03), and the presence of S/A/R mutations (HR, 5.8; 95% CI, 1.9-17.0; P = .002) were associated with inferior OS; chronic MCL (HR, 0.3; 95% CI, 0.1-0.9; P = .04), KIT D816V positivity (HR, 0.3; 95% CI, 0.1-0.5; P < .001) and treatment with midostaurin at any point following MCL diagnosis (HR, 0.5; 95% CI, 0.3-0.9; P = .01) were associated with superior OS.
Table 3.
Univariate and multivariate analyses of factors associated with OS in MCL
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Patient characteristics | ||||
| Male sex | 0.92 (0.52-1.6) | .77 | ||
| Age ≥ 60 | 1.5 (0.87-2.5) | .15 | ||
| Disease characteristics | ||||
| Chronic MCL (no C-findings) | 0.34 (0.12-0.94) | .04 | 0.91 (0.19-4.3) | .90 |
| Secondary MCL | 1.0 (0.57-1.8) | .95 | ||
| Diagnosis of MCL-AHN | 1.9 (1.1-3.2) | .02 | 5.22 (1.9-14.2) | .001 |
| Tryptase ≥ 200 ug/L | 1.1 (0.45-2.5) | .88 | ||
| Any PB MCs | 3.9 (1.6-9.4) | .003 | 1.71 (0.42-7.0) | .46 |
| Percent MCs on BM aspirate smear (continuous variable) | 1.0 (0.99-1.0) | .93 | ||
| Abnormal karyotype | 2.5 (1.1-5.7) | .03 | 3.56 (1.21-14.9) | .02 |
| KIT D816V-positive∗ | 0.27 (0.13-0.53) | <.001 | 0.43 (0.16-0.96) | .04 |
| S/A/R mutations present† | 5.8 (1.9-17.0) | .002 | ||
| Treatment characteristics | ||||
| First-line midostaurin | 0.61 (0.34-1.1) | .09 | ||
| Ever-treatment with midostaurin | 0.49 (0.28-0.86) | .01 | 0.45 (0.07-0.63) | .002 |
Bolded patient characteristics indicate statistical significance in either univariate or multivariate analysis.
From PB or BM.
Not included in multivariate analysis because of low number of evaluable patients.
The aforementioned variables were entered into a multivariate analysis. Final diagnosis of MCL-AHN (HR, 4.7; 95% CI, 1.7-13.0; P = .001) and abnormal karyotype (HR, 5.6; 95% CI, 1.4-13.3; P = .02) were associated with inferior OS; KIT D816V positivity (HR, 0.3; 95% CI, 0.1-0.98; P = .04) and midostaurin treatment (HR, 0.3; 95% CI, 0.08-0.7; P = .008) were associated with superior OS. S/A/R mutations were not included in the multivariate analysis owing to their low event rate.
Discussion
Here, we describe the clinical features, molecular characteristics, treatment patterns, and survival outcomes in a cohort of 92 patients with MCL collected in the ECNM Registry. We confirm that leukemic and chronic MCL are rare subtypes, comprising 4.5% and 14% of our cohort, respectively, consistent with historical reports.6
In our cohort, the median OS of patients with MCL was 1.6 years, although a few patients survived ≥5 or even ≥10 years following their MCL diagnosis. This is similar to previously published reports, in which OS ranges from 0.5 to 2.6 years (supplemental Table 4).5, 6, 7 Patients with MCL exhibited the worst survival of all patients with AdvSM in the registry, including individuals with ASM as well as SM-AHN (irrespective of whether all patients with SM-AHN were analyzed [OS = 2.8 years] or just those with ASM-AHN [OS = 2.1 years]).
We demonstrate that a diagnosis of MCL-AHN, abnormal karyotype, any circulating MCs, KIT D816V negativity, and treatment status (not receiving midostaurin) were all significantly associated with inferior OS in a multivariate analysis. The observation that any number of circulating MCs found in a PB smear is associated with inferior OS should prompt reconsideration of the current threshold of ≥10% as the definition of “leukemic” MCL.2 In addition, no difference in survival outcomes was observed between de novo and secondary MCL, consistent with prior reports.6 Given this, the prognostic value of subdividing MCL into de novo vs secondary variants may be limited. Indeed, the similar survival curves between the 2 groups may suggest that “de novo” MCL could reflect some patients who lacked a prior BM biopsy demonstrating an antecedent variant of SM. By contrast, the presence vs absence of C-findings, which respectively define acute and chronic MCL, demonstrates a statistically significant difference in OS between these 2 groups. The use of prognostic scoring systems such as MARS or GPSM, which incorporate the S/A/R panel, or the IPSM, provides complementary methods for risk stratifying patients with MCL, as demonstrated by the differences in OS in our cohort.
The KIT D816V mutation drives the proliferation of neoplastic MC. In patients with AdvSM, KIT D816V positivity ranges from 84% to 95%.19,23,29,30 Alternative KIT mutations are less common at ≤3%.19,30 In our cohort, KIT D816V positivity was lower (73%) than that described in the broader AdvSM population. This has also been described in other MCL studies, where KIT D816V positivity ranged from 23% to 68%.5, 6, 7 In addition, we found that alternative KIT mutations were more common than in the AdvSM population, at 10%, consistent with prior reports of 15% to 21%,5, 6, 7 and that alternate or lack of KIT mutations were associated with less favorable outcomes compared with cases with KIT D816V. Because not all patients in our cohort received sequencing for alternate KIT mutations, it is possible patients with “no” KIT mutation in fact had an alternative KIT mutation, either within or outside of exon 17. The true prevalence of alternate KIT mutations and their impact on clinical outcomes should be further evaluated in a larger cohort of KIT D816V-negative patients using uniform sequencing techniques.
In addition to KIT, MCL cases demonstrated a variety of myeloid-associated gene mutations, with SRSF2, ASXL1, TET2, SF3B1, and RUNX1 being the most common. Nearly half (44%) of the patients had S/A/R mutations, and these were associated with inferior prognosis, consistent with previous studies of AdvSM.6,30 These additional alterations were more common in patients with MCL-AHN compared with those with MCL alone, suggesting that the presence of additional somatic myeloid mutations should prompt evaluation for coexisting AHN. As these alterations were detected via bulk sequencing, it is unclear whether the increased mutational burden in patients with MCL-AHN was because of increased mutations in the MCL clone, in the AHN clone, or in both, reflecting a common myeloid progenitor affecting both populations. Previous studies have indicated that the KIT D816V mutation can be identified in cells derived from the AHN clone as well as in MCL.15,31 Additional studies, including the use of single-cell sequencing, should help delineate the clonal landscape of SM-AHN.
In our cohort, midostaurin was the most common treatment, administered to over half of patients at some point following MCL diagnosis and largely in the frontline setting. Patients who received midostaurin had superior OS compared with those who did not. Avapritinib was the second KIT-targeting agent approved for AdvSM in 2021 and 2022. However, only 4 patients in our cohort received avapritinib, and the impact of this KIT-targeting agent on MCL-specific outcomes requires further evaluation. Although no head-to-head comparison has been undertaken, the 24-month OS rates of patients with MCL treated with midostaurin and avapritinib were 26% and 92%, respectively, from registrational trials of these drugs.23,29 In the current series, 8 patients received allo-HCT but OS was not improved in these individuals, consistent with a prior retrospective report of patients who received transplantation with AdvSM, where patients with MCL had the worst outcomes, with a 3-year OS of only 17%.22
As expected from the nature of a registry analysis, our study has several limitations. Although the ECNM cohort is well characterized, patients were treated across multiple sites, and data for all pertinent variables for the 92-patient cohort was not always available. For example, assays undertaken for alternative KIT mutations were only available for a subset of patients, as was the use of multigene next-generation sequencing panels. Nevertheless, our study represents the largest described cohort of patients with MCL and provides valuable insight into both disease histopathology, molecular genetics, and clinical outcomes. These data should help inform subtyping of MCL in the context of new updates to the classification of SM. Further evaluation regarding the prognostic significance of KIT D816V vs alternative or no KIT mutation is warranted, as is further investigation of long-term disease outcomes following treatment with midostaurin and avapritinib.
Conflict-of-interest disclosure: A.R. received honoraria from Novartis, Blueprint Medicines, Incyte, Celgene/Bristol Myers Squibb (BMS), AOP Orphan Pharmaceuticals, GlaxoSmithKline, and AbbVie and serves in a consulting or advisory role at Novartis, Blueprint Medicines, Incyte, Celgene/Bristol Myers Squibb, AOP Orphan Pharmaceuticals, and AbbVie. M.J. serves as a consultant on the advisory board of Novartis. H.C.K.-N. is a nonpaid independent monitoring committee member for avapritinib study. W.S. received research support for the conduct of clinical trials from Blueprint Medicines and serves on the advisory board of Blueprint Medicines. C.L. serves in a central review committee data management role for studies of avapritinib and bezuclastinib in advanced SM. M.T. serves on the advisory board of and received honoraria from Blueprint Medicines and Novartis. J.P. serves on the advisory board of and received honoraria from Blueprint Medicines, Novartis, and Deciphera. D.C. serves on the advisory board of and received honoraria from Blueprint Medicines. M.H. serves on the advisory board and received consulting honoraria from Novartis Pharma Schweiz AG. K.S. serves on the advisory board of and received honoraria from Blueprint Medicines and Novartis. C.E. serves on the advisory board of and received honoraria from Blueprint Medicines and Gilead. K.B. serves on the advisory board of and received honoraria from Blueprint Medicines. S.G.P. reports consultancy with Amgen, Astellas, Genesis Pharma, and Sanofi; serves on scientific advisory boards with AbbVie, Amgen, BMS, Genesis Pharma, Novartis Sandoz, Pfizer, Janssen, Roche, and Takeda; and reports honoraria from AbbVie, Amgen, BMS, Genesis Pharma, Gilead Sciences, Janssen, Innovis, Novartis, Pfizer, Roche, Sandoz, and Winmedica. M.D. reports consultations and research support from AbbVie, Amgen, AOP Orphan, Novartis, and Janssen. V.S. serves on the advisory board of Blueprint Medicines and Novartis. J.S. serves on the advisory board of and received honoraria from Novartis and Blueprint Medicines. M.A. received research grants from Blueprint Medicines and received honoraria from AB Science, Blueprint Medicines, and Novartis. P.V. serves on the advisory board of and received honoraria from Novartis, Blueprint, Deciphera, Celgene, and Incyte. W.R.S. received honoraria from Novartis, Pfizer, AbbVie, Daiichi Sankyo, Stemline, Thermo Fisher, Deciphera, Celgene, and Jazz Pharmaceuticals. J.G. received research grants (funds for administration of clinical trials) from Novartis, Blueprint Medicines, Deciphera, and Cogent Biosciences; serves on the advisory board of and received honoraria from Blueprint Medicines, Novartis, Deciphera, and Cogent Biosciences; and received reimbursement of travel expenses from Novartis and Blueprint Medicines. The remaining authors declare no competing financial interests.
Acknowledgments
J.G. expresses gratitude to the Charles and Ann Johnson Foundation and the Stanford Cancer Institute Clinical Innovation Fund for their support of MC disease research, and the investigators of the ECNM who contributed patients to the registry.
J.L., J.S., A.R., and M.J. were supported by Deutsche José Carreras Leukämie-Stiftung grant DJCLS 08R/2020. P.V. was supported by the Austrian Science Fund grants F4704-B20 and P32470-B. L.M. was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (Investigator grant #20125; AIRC 5x1000 project #21267), Cancer Research UK, FC AECC, and AIRC under the International Accelerator Award Program (project #C355/A26819 and #22796). V.S. is a senior clinical researcher of the Research Foundation Flanders (FWO: 1804518N).
Authorship
Contribution: V.E.K., C.P., and J.G. designed the study and conducted the statistical analyses of patient outcomes; W.R.S. and P.V. designed the ECNM patient registry; all investigators contributed patients to the registry for this analysis; and all authors contributed substantially to writing and/or reviewing/editing parts of the manuscript and approval of the final version of the document.
Footnotes
Data are available on request from the author, Vanessa E. Kennedy (vanessa.kennedy@ucsf.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017;77(6):1261–1270. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25(7):603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 6.Jawhar M, Schwaab J, Meggendorfer M, et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017;102(6):1035–1043. doi: 10.3324/haematol.2017.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain P, Wang S, Patel KP, et al. Mast cell leukemia (MCL): clinico-pathologic and molecular features and survival outcome. Leuk Res. 2017;59:105–109. doi: 10.1016/j.leukres.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim KH, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 9.Valent P, Akin C, Sperr WR, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122(5):695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Sotlar K, Sperr WR, Reiter A, Arock M, Horny HP. Chronic mast cell leukemia: a novel leukemia-variant with distinct morphological and clinical features. Leuk Res. 2015;39(1):1–5. doi: 10.1016/j.leukres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardanani A. Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification and management. Am J Hematol. 2016;91(11):1146–1159. doi: 10.1002/ajh.24553. [DOI] [PubMed] [Google Scholar]
- 12.Valent P, Sotlar K, Sperr WR, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25(9):1691–1700. doi: 10.1093/annonc/mdu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 14.Arock M, Sotlar K, Akin C, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29(6):1223–1232. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter A, George TI, Gotlib J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood. 2020;135(16):1365–1376. doi: 10.1182/blood.2019000932. [DOI] [PubMed] [Google Scholar]
- 16.Pardanani AD, Lasho TL, Finke C, et al. ASXL1 and CBL mutations are independently predictive of inferior survival in advanced systemic mastocytosis. Br J Haematol. 2016;175(3):534–536. doi: 10.1111/bjh.13865. [DOI] [PubMed] [Google Scholar]
- 17.Jawhar M, Schwaab J, Hausmann D, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30(12):2342–2350. doi: 10.1038/leu.2016.190. [DOI] [PubMed] [Google Scholar]
- 18.Damaj G, Joris M, Chandesris O, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9(1):e85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jawhar M, Schwaab J, Alvarez-Twose I, et al. MARS: mutation-adjusted risk score for advanced systemic mastocytosis. J Clin Oncol. 2019;37(31):2846–2856. doi: 10.1200/JCO.19.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardanani A, Shah S, Mannelli F, et al. Mayo alliance prognostic system for mastocytosis: clinical and hybrid clinical-molecular models. Blood Adv. 2018;2(21):2964–2972. doi: 10.1182/bloodadvances.2018026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz-Gonzalez JI, Alvarez-Twose I, Jara-Acevedo M, et al. Proposed global prognostic score for systemic mastocytosis: a retrospective prognostic modelling study. Lancet Haematol. 2021;8(3):e194–e204. doi: 10.1016/S2352-3026(20)30400-2. [DOI] [PubMed] [Google Scholar]
- 22.Ustun C, Reiter A, Scott BL, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32(29):3264–3274. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotlib J, Reiter A, Radia DH, et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: interim analysis of the phase 2 PATHFINDER trial. Nat Med. 2021;27(12):2192–2199. doi: 10.1038/s41591-021-01539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeAngelo DJ, Radia DH, George TI, et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat Med. 2021;27(12):2183–2191. doi: 10.1038/s41591-021-01538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valent P, Oude Elberink JNG, Gorska A, et al. The data registry of the European Competence Network on Mastocytosis (ECNM): set up, projects, and perspectives. J Allergy Clin Immunol Pract. 2019;7(1):81–87. doi: 10.1016/j.jaip.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horny H, Akin C, Arber DA, Peterson LC, Tefferi A, Metcalf CC. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 4th ed. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, editors. International Agency for Research on Cancer; 2017. Mastocytosis; pp. 62–69. [Google Scholar]
- 27.Sperr WR, Kundi M, Alvarez-Twose I, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. 2019;6(12):e638–e649. doi: 10.1016/S2352-3026(19)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Georgin-Lavialle S, Lhermitte L, Suarez F, et al. Mast cell leukemia: identification of a new c-Kit mutation, dup(501-502), and response to masitinib, a c-Kit tyrosine kinase inhibitor. Eur J Haematol. 2012;89(1):47–52. doi: 10.1111/j.1600-0609.2012.01761.x. [DOI] [PubMed] [Google Scholar]
- 29.Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 30.Jawhar M, Schwaab J, Naumann N, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130(2):137–145. doi: 10.1182/blood-2017-01-764423. [DOI] [PubMed] [Google Scholar]
- 31.Jawhar M, Schwaab J, Schnittger S, et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia. 2015;29(5):1115–1122. doi: 10.1038/leu.2015.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.