Abstract
Low HDL-cholesterol (HDL-C) concentrations are a typical trait of the dyslipidemia associated with chronic kidney disease (CKD). In this condition, plasma HDLs are characterized by alterations in structure and function, and these particles can lose their atheroprotective functions, e.g., the ability to promote cholesterol efflux from peripheral cells, anti-oxidant and anti-inflammatory proprieties and they can even become dysfunctional, i.e., exactly damaging. The reduction in plasma HDL-C levels appears to be the only lipid alteration clearly linked to the progression of renal disease in CKD patients. The association between the HDL system and CKD development and progression is also supported by the presence of genetic kidney alterations linked to HDL metabolism, including mutations in the APOA1, APOE, APOL and LCAT genes. Among these, renal disease associated with LCAT deficiency is well characterized and lipid abnormalities detected in LCAT deficiency carriers mirror the ones observed in CKD patients, being present also in acquired LCAT deficiency. This review summarizes the major alterations in HDL structure and function in CKD and how genetic alterations in HDL metabolism can be linked to kidney dysfunction. Finally, the possibility of targeting the HDL system as possible strategy to slow CKD progression is reviewed.
Keywords: HDL, Chronic kidney disease, LCAT
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death in chronic kidney disease (CKD) patients and represents one of the major adverse outcomes in this clinical population [1]. Data from the US Renal Data System (USRDS) 2020 revealed that the prevalence of CVD increases with the progression of CKD, doubling in advanced CKD compared to patients without CKD [1]. A metanalysis of 21 general population cohorts (over 100,000 participants) demonstrated that lower estimated glomerular filtration rate (eGFR) and higher urine albumin creatinine ratio (UACR) were independently associated with higher CVD mortality, after adjusting for potential confounders such as diabetes and hypertension [2]. The spectrum of CVDs associated with CKD includes atherosclerotic CVD (ischemic heart disease, stroke and peripheral artery disease), ventricular hypertrophy, heart failure, as well as non-atherosclerotic CVD [3]. Increased CV risk in these patients is multifactorial, including both traditional (e.g., dyslipidemia and hypertension), and specific risk factors related to kidney function, such as endothelial dysfunction, increased activity of renin–angiotensin system and hyperphosphatemia [4]. Among the major pathophysiological mechanisms linking CKD to CVD, alterations in lipid profile are shared risk factors [5,6].
Reduced HDL-cholesterol (HDL-C) concentrations are a typical trait of CKD dyslipidemia [5]. Quantitative and qualitative alterations of plasma lipids and lipoproteins are detected in patients with renal disorders, in particular CKD and end stage renal disease (ESRD). Beyond the reduction in HDL-C levels, renal disease influences HDL composition affecting their functions.
HDLs are a highly heterogeneous class of lipoproteins, varying in size, shape and protein/lipid composition. This heterogeneity is consequent of the complexity of their metabolism and interconversion within the plasma compartment, where different HDL subclasses on the basis of their density, size, shape and protein/lipid composition may be identified [7]. Small discoidal HDLs, called preβ-HDLs, are mainly generated by the lipidation of the main protein component, apolipoprotein A-I (apoA-I), through the interaction with the ATP-binding cassette transporter A1 (ABCA1) [8]. These particles are good acceptors of additional cholesterol from ABCA1 and become substrates of lecithin:cholesterol acyltransferase (LCAT), responsible for cholesterol esterification in the plasma compartment, which lead to the maturation of nascent discoidal HDLs into spherical particles with a core of cholesteryl esters (CE) [9]. Subsequently, the cholesteryl ester transfer protein (CETP) promotes the interchange of CE and triglycerides (TG) between HDLs and apoB-containing lipoproteins, thus modifying the lipid core of HDLs and generating larger and less dense particles. Finally, the action of the endothelial lipase (EL) and the phospholipid transfer protein (PLTP) leads to the hydrolysis of TG and phospholipids (PL), generating again small lipid poor particles [10]. The major site of apoA-I and small HDL particle disposition is the kidney. Indeed, HDLs can be removed from the circulation by glomerular filtration, as their size allows crossing of the glomerular barrier [11]. In proximal tubules, apoA-I is entirely reabsorbed by the action of the cubilin – megalin complex. Specifically, cubilin, a multiligand receptor expressed in apical membranes of kidney tissue, binds apoA-I with high affinity and mediates endocytosis [12]. Lipid and apolipoprotein composition of HDLs affects their surface charge and apoA-I conformation, influencing their plasma clearance; in fact, small, discoidal and positively charged HDL particles are filtered more quickly than mature HDLs [13].
HDLs are involved in different atheroprotective mechanisms, including their central role in reverse cholesterol transport (RCT) in which they act as extracellular acceptors of cholesterol from cells, thus mediating cholesterol efflux capacity, the first step of this process. Cholesterol within HDL is then esterified by LCAT and migrates to the HDL core, thus maintaining the cholesterol gradient between HDL surface and cell membranes. Thereafter, CE can be routed to the liver by two different pathways for elimination through the bile. The first pathway is mediated by the interaction of HDL with hepatic SR-BI and the selective uptake of CE from HDL without particle internalization. The second one is an indirect pathway, with CE transferred from HDL to apoB-containing lipoproteins by the action of CETP; CE are then delivered to the liver by apoB-containing particles through the interaction with their receptors. It has been estimated that in humans, the indirect pathway mediated by CETP is responsible for the hepatic uptake of 70% CE, while the direct interaction of HDL with SR-BI for the remaining 30% [14]. Besides this major activity, HDLs contribute to atheroprotection by exerting antioxidant and anti-inflammatory actions and preventing and correcting several features of the endothelial dysfunction typical of the atherosclerotic process [15].
This review addresses the current knowledge on the three aspects: (i) how CKD affects HDL structure, composition, and functionality; (ii) how genetic and acquired HDL defects affect renal function; and (iii) how targeting the HDL system can benefit renal disease.
2. HDL structure and function in chronic kidney disease
The link between plasma HDLs and the kidney is bidirectional. On one side, CKD affects plasma HDL-C levels, HDL structure and subclass distribution, as well as HDL functionality; on the other end, inherited HDL disorders can lead to kidney dysfunction.
2.1. Structural changes
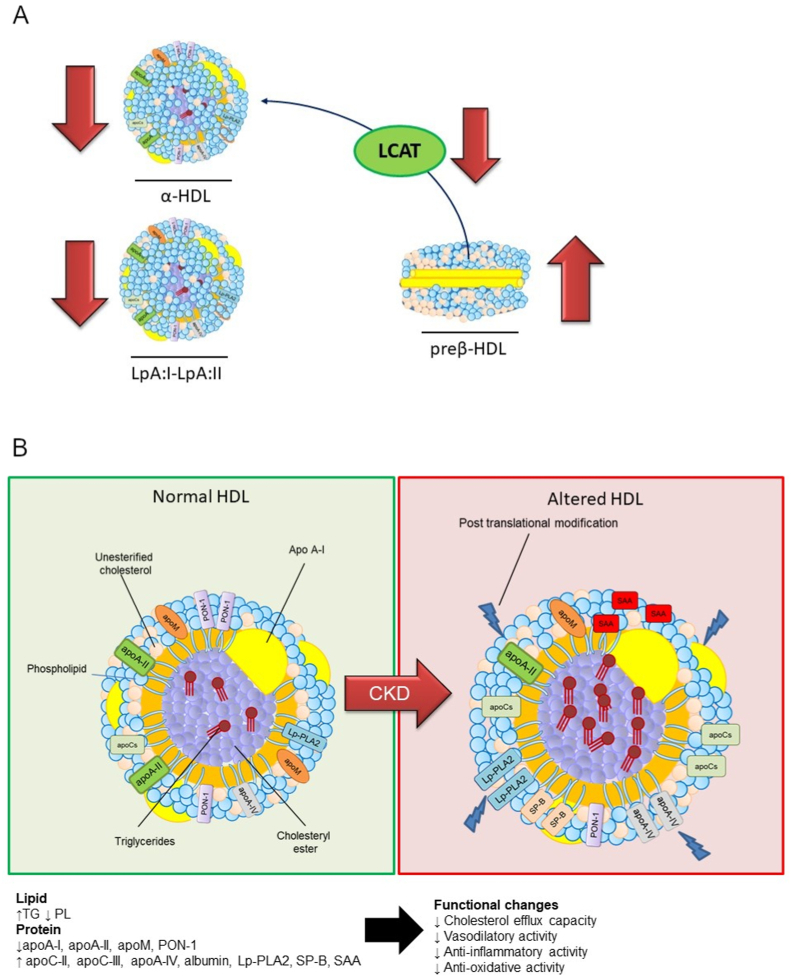
During CKD, both ABCA1 and LCAT are reduced, thus resulting in poor lipidation of apoA-I and in an impaired HDL maturation process. This leads to an alteration of HDL subclass distribution, with the accumulation of preβ-HDLs, as a consequence of reduced LCAT activity and selective reduction of particles containing both apoA-I and apoA-II (LpA-I:A-II). With the progression of the disease, a reduction of the particles containing only apoA-I (LpA-I) is also observed [5,16]. Besides alterations in HDL subclass distribution, HDL protein and lipid content are modified in CKD. HDLs become enriched in TG and depleted of PL, consistent with reduced activity of hepatic lipase and enhanced activity of PLTP [17,18]. Also the HDL proteome is markedly modified: apoA-I, apoA-II, apoM levels are reduced, whereas particles are enriched in serum amyloid A (SAA), apoC-II, apoC-III, apoA-IV, albumin, lipoprotein-associated phospholipase A2 (Lp-PLA2),surfactant protein B (SP–B), and a-1-microglobulin/bikunin precursor [19]. Furthermore, a significant reduction in HDL-associated antioxidant paroxonase (PON-1) activity is described in patients with stable mild to moderate CKD [20].
Several post-translational modifications occur in the HDL of CKD patients, among these carbamylation and glycation are implicated in alterations of HDL functionality. The reactive oxygen/nitrogen species increase myeloperoxidase (MPO) levels, resulting in increased apolipoprotein carbamylation induced by urea-derived cyanate, generally elevated in patients with CKD [21]. Hyperglycaemia in ESRD patients generates highly reactive α-oxoaldehydes that covalently modify plasma proteins, including apoA-I, resulting in alterations of the conformation of specific domains of apoA-I crucial for LCAT activation, consequently to the ability of glycated apoA-I to activate LCAT is reduced [22]. Moreover, the progressive decline in renal function increases reactive lipid peroxidation with a consequent raise of plasma levels of malondialdehyde (MDA) able to modify and cross-link specific residues in apoA-I [23]. Some of the structural modifications observed in HDLs are implicated in the impairment of fundamental HDL functions. The main structural and functional alterations of HDLs in CKD are summarized in Fig. 1.
Fig. 1.
Summary of the main changes in HDL subclasses (A) and structural and compositional changes (B) in chronic kidney disease.
ApoA-I, apolipoprotein A-I; apoA-II, apolipoprotein A-II; apoM, apolipoprotein M; apoA-IV, apolipoprotein A-IV; apoC-II, apolipoprotein C-II; apoC-III, apolipoprotein C-III; LCAT, lecithin:cholesterol acyltransferase. Lp-PLA2, lipoprotein-associated phospholipase A2; PL, phospholipids; PON-1, paraoxonase-1; SAA, serum amyloid A; SP-B, surfactant protein B; TG, triglycerides.
2.2. Functional alterations
2.2.1. Cholesterol efflux capacity
CKD affects RCT at different levels including the HDL ability to promote cell cholesterol efflux and HDL maturation. Several studies have highlighted the impaired capacity of HDL from uremic patients, especially patients under dialytic treatments, to promote cell cholesterol efflux [[24], [25], [26], [27]] and impairment is likely related to changes in lipid and protein composition. Changes in the PL and TG content of HDL are shown to correlate with impairment in cell cholesterol efflux, in particular the depletion of PL associated to the reduced content of apoA-I and apoA-II, due to increased content of SAA, is linked to impaired cholesterol efflux capability [24]. Also, post-translational modifications are implicated in this process. Indeed the myeloperoxidase binding to apoA-I inhibits the ABCA1-dependent cholesterol efflux activity of the modified lipoprotein [28]. Furthermore, a recent study has shown that monocytes from CKD patients display a lower expression of ABCA1 and, consequently, the ABCA1-mediated cholesterol efflux is impaired [29], confirming that ABCA1 and ABCG1 are down-regulated also in endothelial cells exposed to uremic plasma [30]. On the contrary, the impairment in efflux capacity is not observed in children with CKD and ESRD that show only a non-significant trend for reduced efflux [31].
2.2.2. Vasodilatory activity
The presence of CKD abolishes the vasoprotective properties of HDLs and drives the transformation of HDLs into harmful particles able to inhibit endothelial NO production [32]. Addressing the mechanism of the impaired vasoprotective properties of HDLs only in the presence of CKD is a complex task. However the direct effect of renal disease on HDL-mediated NO production was also evident in a study carried out in children with CKD, thus allowing the exclusion of confounding factors due to the presence of concomitant diseases in adults such as diabetes, coronary artery disease and smoking [33].HDL loses its endothelial protective properties even in early CKD, with a graded change with the degree of renal impairment, the most profound changes occurring in dialysis patients, partly recovered after transplantation [33]. The structural alterations that change the HDL physiological ability to promote NO production can be multifaceted: HDLs from CKD patients are enriched in symmetric dimethylarginine (SDMA), an isomer of asymmetric dimethylarginine (ADMA), an endogenous endothelial nitric oxide synthase (eNOS) inhibitor [32]; moreover, in uremic patients the increased level of cyanate, promoting protein carbamylation, reduces the eNOS expression [34]. Furthermore, the depletion of LpA-I particles in patients under dialytic treatment [5] can contribute to the impaired activation of eNOS, since this specific HDL subclass is particularly efficient in promoting NO production [35].
2.2.3. Anti-inflammatory activity
HDLs from ESRD patients show impaired anti-inflammatory capacities, indeed the ability to inhibit the expression of interleukin-1 β (IL-1-β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) is significantly reduced in macrophage incubated with these particles compared to control HDL. It may thus enhance the recruitment of monocytes into the subendothelial space [25]. According with the studies carried out in adults, also HDLs from children with CKD and ESRD show an impaired ability to inhibit the macrophage expression of IL-1β, TNF-α and MCP-1 [31]. Moreover, HDLs from both adults and children with CKD and ESRD are not able to reduce MCP-1-induced chemotaxis [25,31].
Post-translational modifications have a primary role in altering the anti-inflammatory activity of HDL of CKD patients. MPO-modified HDLs increase the expression of the cell adhesion molecule VCAM-1 [36], and the enrichment in SDMA leads to the activation of endothelial TLR-2 via TLR-1- and TLR-6-coreceptor-independent alternative pathways impairing endothelial repair and enhancing endothelial pro-inflammatory activation [32]. Furthermore, evidence in rabbits show that glycated apoA-I is not able to prevent both endothelial adhesion molecule expression and infiltration of neutrophils into the inflamed carotid arteries [37]. Also, the proteome composition of HDLs from uremic patients can be implicated in the impaired anti-inflammatory activity, mainly because of alterations in protein composition, such as the enrichment in SAA raising the production of IL-12, IL-10, TNF-a, and IL-6 from human monocytes [26].
2.2.4. Anti-oxidative activity
Oxidative stress is directly involved in the pathogenesis and progression of renal disease, and structural characteristics in CKD, loss of renal energy, and uremia result in an imbalance between free radical production and antioxidant defenses. In physiological conditions, HDL acts as an antioxidant defense and HDL-associated antioxidant enzymes such as PON-1 and glutathione peroxidase attenuate oxidative stress and inflammation by production of oxidized LDL and hydroperoxides. HDLs from CKD patients lose their ability to prevent LDL oxidation and show reduced levels of PON-1, LCAT, glutathione peroxidase and apoA-I [[38], [39], [40]]. Glycation, especially in patients with CKD and diabetes, may affect the HDL anti-oxidant activity [19]. In addition myeloperoxidase binding to apoA-I facilitates the selective targeting of apoA-I for site-specific chlorination and nitration by MPO-generated reactive oxidants [41]. Besides the reduction in PON-1 concentrations also PON arylesterase activity is decreased in CKD patients [20].
3. HDL and CKD progression in the general population
The alterations in the HDL system described above worsen the progression of CKD [42]. Reduction of HDL-C concentrations is the only lipid alteration associated with the progression of renal disease in hemodialysis patients [5] as well as in mild-to-moderate CKD patients, resulting in earlier entry into a dialysis program or in doubling of the plasma creatinine levels, independent of the classical risk factors such as diabetes and hypertension [43]. These findings are supported by the results of a large cohort of 2 million American veterans with a median follow-up of 9 years, in which individuals with HDL-C concentrations <30 mg/dL showed a 10%–20% higher risk for CKD and/or progression of CKD compared to subjects with HDL-C levels ≥40 mg/dL with a U-shaped relationship between HDL-C levels and incidence of CKD [44]. This and other studies noted a paradoxical association between elevated HDL-C levels and cardiovascular events or mortality in patients undergoing hemodialysis with chronic inflammation [45,46].The negative effects of high HDL-C levels on CKD progression can be partly explained by structural and functional alterations paradoxically exerting damaging effects, leading to adverse outcomes, including the deterioration of kidney function [47,48].
However, the relationship between HDL-C and CKD progression is not always observed; in the CRIC study carried out in 3939 CKD patients, HDL-C levels were not independently associated with the composite endpoint of ESRD or a 50% reduction in eGFR [49]. It is also important to underline that the epidemiologic studies on HDL-C levels and CKD progression cannot establish causality or consequentiality [50].
Overall, preclinical and clinical evidence support the association between HDL-C levels and CKD progression. However, this relationship leaves some open questions and controversial aspects that need to be further investigated.
4. HDL and CKD in the presence of genetic HDL defects
The association between altered lipid metabolism and CKD development and progression is supported by the presence of kidney alterations in genetic defects linked to HDL metabolism, such as in familial LCAT deficiency (FLD) [51].
Mutations in genes coding for key structural HDL proteins (i.e., APOA1, APOE and APOL) or HDL-related enzymes (i.e., LCAT) can cause diseases characterized by renal disorders.
The major HDL protein component is represented by apoA-I. More than 60 variants of the APOA1 gene have been reported, but only half of them associate with low HDL-C [52], and more than 20 have been associated with an hereditary form of amyloidosis (OMIM #105200) [53]. The amyloidogenic effect is caused by single amino acid substitutions that increase the propensity of the apoA-I protein to form insoluble fibrils, mainly constituted by N-terminal polypeptide fragments [54]. These fibrils deposit in different organs, including the kidney as the primary target [55].Kidney apoA-I fibril deposition can lead to tubulointerstitial nephritis initially associated with mild renal dysfunction, and slowly progressing to end-stage renal disease [55].
APOA2 gene mutations leading to similar hereditary amyloidosis have been also reported, although less frequently [56].
ApoE is a structural regulatory protein constituent of chylomicrons, VLDL, LDL and HDL and mutations in the APOE gene have been associated with glomerulopathy (apoE2 homozygote glomerulopathy and lipoprotein glomerulopathy, LPG, OMIM#611771) [57]. ApoE2 homozygote glomerulopathy is characterized by glomerulosclerosis with foam cells infiltration and found in homozygous carriers of the apoE2 isoform [58]. LPG is characterized by dilation of capillaries in the glomerulus in presence of lamellated lipoprotein thrombi, due to abnormal lipoproteins composed of instable and aggregation-prone apoE mutants [59]. The first identified mutations in the APOE gene responsible for LPG were Arg25→Cys (apoEKyoto) and Arg145→Pro (apoESendai) [60,61]. LPG-related proteinuria is sometimes mild but progresses to nephrotic syndrome in most cases [62].
Common APOE polymorphisms can affect renal function in several ways. For instance, a higher frequency of the ε2 allele and a reduced frequency of the ε4 is found in patients with end-stage renal disease [63] and other studies confirmed this observation also in the general population [64] and in type 2 diabetics [65,66]. Lastly, APOE expression may be dysregulated at the glomerular level in focal and segmental glomerulosclerosis, independent of the APOE genotype [67].
Apolipoprotein L-I (apoL-I) is a key component of the trypanolytic factor of human serum associated with HDLs [68], involved in the defense against pathogens, specifically in the adaptation to parasitic diseases. Two common risk alleles (G1 and G2) in the APOL1 gene have been identified in individuals with sub-Saharan African ancestry and found to be associated with an increased risk of non-diabetic kidney disease, particularly focal segmental glomerulosclerosis (OMIM #612551) and HIV-associated nephropathy [[69], [70], [71]]. These variants presumably resulting from genetic protection against sleeping sickness, being absent in non-African populations, likely explain the higher prevalence of CKD in African Americans [71,72]. A focal and segmental glomerulosclerosis phenotype is associated to the above cited variants and linked to increased APOL1 expression in glomeruli [73]. In the Atherosclerosis Risk in Communities (ARIC) study, black participants carrying the APOL1 high-risk genotype had a significantly higher incidence of kidney failure and a more rapid eGFR decline compared to those carrying the low-risk allele [74].Since the role of apoL-I in kidney development or function is not known, it is not clear how it may be involved in CKD pathogenesis. Several mechanisms have been proposed. One of the hypotheses is that APOL1 risk variants may create pores in membranes of kidney cells, acting as in trypanosomal organelles [75]. Expression of G1 or G2 is associated with endolysosomal and mitochondrial dysfunction [76], altered ion channel activity [77], induction of stress-activated protein kinase p38 MAPK and JNK [78] and autophagy [79,80], all contributing to renal damage.
LCAT is the enzyme responsible of cholesterol esterification in plasma, primarily in HDLs (α-activity) but also in apoB-containing particles (β-activity). Loss-of-function mutations in the LCAT gene are responsible for two syndromes: familial LCAT deficiency (FLD, OMIM#245900) and fish-eye disease (FED, OMIM#136120), two rare autosomal recessive disorders, characterized by extremely low HDL-C levels and the presence of corneal opacity, i.e., the hallmark of the diseases. FLD-causing mutations lead to complete LCAT deficiency, since LCAT lacks bothα and β activity, whereas FED-causing mutations lead to partial LCAT deficiency, in which LCAT retains its capacity to esterify cholesterol on apoB-containing lipoproteins [81]. While FED mutations lead to a relatively more benign phenotype [82], FLD carriers can develop proteinuria as early as in the second decade of life [83] usually progressing to CKD by the fourth decade of life [84]. Lipid and lipoprotein abnormalities are present in FLD patients. Consistent with the lack of esterification, plasma unesterified cholesterol is raised and the unesterified to total cholesterol ratio is above 75%. Plasma HDLs do not only shown reduced cholesterol content, but also altered size and shape, with a preponderance of small discoidal pre-β HDL particles, indicative of incomplete HDL maturation [35].
In addition, due to the excessive amount of unesterified cholesterol an abnormal lipoprotein, called lipoprotein X (LpX), may be present [82]. LpX is composed by more than a 60%of phospholipids and 30% unesterified cholesterol [85]. LpX appears as a multilamellar vesicle with bi or multilayer phospholipids by electron microscopy size ranging from 30 to 70 nm in diameter [85]. Renal disease is the main cause of mortality and morbidity in FLD patients. Development and progression of renal damage in FLD patients is unpredictable and variable, also within the same family members, suggesting a role of genetics and environment on kidney decline [86]. Kidney biopsies of FLD show peculiar histopathologic changes, including mesangial expansion, irregular thickening of the glomerular capillary walls and increased mesangial cellularity [87]. In addition, unesterified cholesterol and phospholipids can deposit in the glomeruli, inducing vacuolization of the basement membrane and conferring a “foamy” appearance [87]. Alterations of podocyte structure with fused endothelial processes can be observed on electron microscopy [88], while immunofluorescence microscopy reveals the presence of IgM deposit and C3 in the capillary loops, mesangium and arteriolar walls [89,90].
Although the exact pathogenesis of renal disease in FLD is not fully elucidated, a direct role of LpX has been demonstrated in a mouse study. After intravenous administration of synthetic LpX, Lcat-/-mice develop proteinuria and FLD renal histological hallmarks [91].
In humans, LpX is found in the capillary loops of the glomerulus, where it induces vascular and endothelial damage [90]. Some years ago, in an observational study, it has been shown that plasma unesterified cholesterol levels at diagnosis were able to predict the rate of progression of any renal events (i.e., dialysis, kidney transplantation or death for renal complications) in the larger cohort of FLD carriers described so far [84]. Moreover, unesterified cholesterol levels above the median were associated with lower event-free survival [84]. Excess unesterified cholesterol in these patients accumulates in LpX.
Lipoprotein remodeling with consequent reduction of plasma LpX, operated by HDL mimetic administration, can lead to partial amelioration of renal disease, and decreased lipid deposition in the kidneys [92]. In addition, the absence of renal disease in FED suggests that the residual LCAT activity is sufficient to prevent the formation of a sufficient amount of LpX to induce renal damage [81].
No cure is available so far for FLD patients. Available pharmacological approaches are meant to correct dyslipidemia and to delay renal failure. Renal transplantation represents an option in case of end-stage renal disease; however, it does not correct the enzymatic defect and renal disease can reoccur [92].
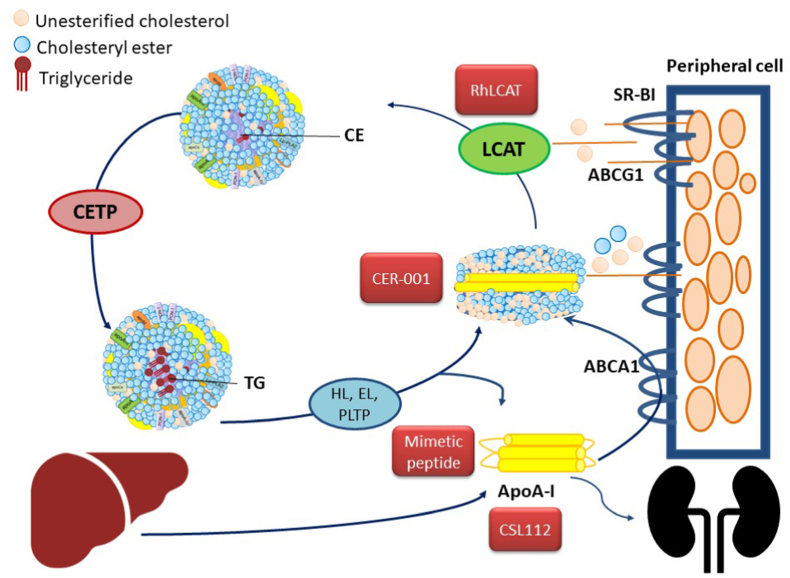
Recombinant human LCAT is currently in clinical development [93,94]. Small molecule LCAT activators have been tested in vitro and able to activate some mutant LCAT, but no further update is available [95,96]. Very recently, the HDL mimetic CER-001 proved its efficacy in reducing albuminuria and increasing podocyte in a mouse model of FLD [97]. Pharmacological approaches will be discussed further below.
5. Targeting HDL system to benefit renal disease
The presence of reduced plasma levels of HDL-C and impaired HDL functionality in CKD suggest the potential efficacy of pharmacological strategies acting on HDL metabolism to prevent and revert CKD, both in primary and secondary renal diseases.
5.1. Preclinical studies
Animal studies demonstrate that targeting apoA-I/HDL can ameliorate kidney injury; mice overexpressing apoA-I show indeed lower serum creatinine following lipopolysaccharide-induced kidney damage compared to control wild-type mice [98]. As a supporting evidence, infusion of an apoA-I mimetic peptide improved glomerular filtration rate, decreased tubular injury and tubulointerstitial fibrosis [99,100].Additionally, studies have shown that supplementation with an apoA-I mimetic peptide reduced proteinuria, glomerular, and tubulointerstitial injury in proteinuric apoE−/− mice [101].
In a mouse model of renal disease due to genetic LCAT deficiency, treatment with the synthetic HDL, CER-001, limited albuminuria and podocyte dysfunction [90]. In the same mouse model, treatment with recombinant human LCAT (rhLCAT) led to a normalization of the lipid profile, including maturation of HDL, elimination of LpX in plasma and kidneys, and markedly reduced proteinuria [102]. In vitro studies support the evidence that supplementation with rhLCAT restore HDL-antioxidant activity by reducing the formation of ROS in cultured renal cells [103].
Targeting the HDL system by administration of fenofibrate, a drug increasing plasma levels of HDL-C, showed cardiorenal protective effects. In a rat model of cardiac hypertrophy and nephropathy treatment with fenofibrate can indeed attenuate cardiac hypertrophy, and protect the kidneys, preventing morphological alterations [104].
Taken together this data support the clinical development of treatments targeting the HDL system to slow CKD progression.
5.2. Clinical studies
Most clinical studies on HDL-related drugs in CKD aimed to reduce the atherosclerotic burden observed in CKD have not shown any effect on renal disease progression. Approaches are in clinical development for genetically-determine CKD and in the future can be potentially apply to other renal disease.
CSL-112, a human plasma-derived apoA-I, was tested in post-acute myocardial infarction (AMI) patients with the aim of increasing cholesterol efflux capacity, which is substantially impaired following AMI [105]. The phase 2 trial CSL112_2001 enrolled AMI patients with moderate chronic kidney disease (eGFR <60 mL/min/1.73 m2) to evaluate the safety of infusions in this subgroup [106]. Whether CSL112 protects from kidney injury in this cohort deserves further study.
Hung and colleagues demonstrate in a post-hoc analysis that IL-1 inhibition (canakinumab) improves HDL functionality in patients with stage 3–5 CKD, including those in maintenance hemodialysis, however whether these changes produced a delay in CKD progression is not proven [107].
A safe profile of a single infusion of rhLCAT was assessed by Shamburek et al. in patients with stable coronary heart disease and low HDL-C [94]. RhLCAT was next tested in a single FLD patient where it corrected lipid abnormalities by esterifying the excess of unesterified cholesterol, thus preventing LpX formation. Consequently, renal function shows modest improvement during treatment, despite the presence of an advanced disease. Interestingly, anemia is also improved [93].
Recently, the HDL mimetic CER-001 has been tested through a compassionate program in two FLD patients with a very progressive renal disease [92,108]. CER-001 is a novel engineered negatively charged HDL mimetic previously tested for atherosclerosis regression by virtue of its capacity to mobilize cholesterol from peripheral cells [109]. CER-001 normalizes the lipoprotein profile, decreasing LpX in favour of normal-sized LDL [92] and ameliorates or at least slows renal function decline by the reduction of nephrotoxic LpX deposition in the kidney and by removing LpX-induced lipid deposit [92,108]. CER-001 has the potential to be tested in more common kidney diseases characterized by kidney lipid deposits.
The potential targets in HDL metabolism and pharmacological approaches in clinical development are summarized in Fig. 2.
Fig. 2.
Potential therapeutic approaches for CKD targeting HDL metabolism. ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; apoA-I, apolipoprotein A-I; CE, cholesteryl esters; CETP, cholesteryl ester transfer protein; LCAT, lecithin:cholesterol acyltransferase; SR-BI, scavenger receptor BI; TG, triglycerides.
6. Conclusion
During CKD the reduction of HDL-C levels are followed through significant alterations in HDL structure and function, which worsen with the progression of renal impairment. The link between HDL system and kidney is clearly emerged in general population as well in genetic disorder of HDL system, which are characterized by impairment in renal function. In familial LCAT deficiency, leading to dramatic HDL defects and serious kidney disease, the LCAT enzyme, crucial player in HDL metabolism, is reduced and plasma concentrations are associated with CKD progression. Targeting the HDL system, including LCAT enzyme, can be a possible therapeutic strategy to restore plasma HDL-C levels and functionality of HDL particles, potentially slowing CKD progression.
Author contributions
All authors wrote the first draft and revised version of the manuscript. All authors have contributed to the content and have approved the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Professor Cesare Sirtori for its help in proofreading the manuscript.
References
- 1.Matsushita K., Ballew S.H., Wang A.Y., Kalyesubula R., Schaeffner E., Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18:696–707. doi: 10.1038/s41581-022-00616-6. [DOI] [PubMed] [Google Scholar]
- 2.Chronic Kidney Disease Prognosis. Matsushita C.K., van der Velde M., Astor B.C., Woodward M., Levey A.S., de Jong P.E., Coresh J., Gansevoort R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarnak M.J., Levey A.S., Schoolwerth A.C., Coresh J., Culleton B., Hamm L.L., McCullough P.A., Kasiske B.L., Kelepouris E., Klag M.J., Parfrey P., Pfeffer M., Raij L., Spinosa D.J., Wilson P.W., H. B. P. R. C. C. American Heart Association Councils on Kidney in Cardiovascular Disease, Epidemiology, and Prevention Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 4.Rysz J., Gluba-Brzozka A., Rysz-Gorzynska M., Franczyk B. the role and function of HDL in patients with chronic kidney disease and the risk of cardiovascular disease. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabresi L., Simonelli S., Conca P., Busnach G., Cabibbe M., Gesualdo L., Gigante M., Penco S., Veglia F., Franceschini G. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J Intern Med. 2015;277:552–561. doi: 10.1111/joim.12290. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri N.D. Causes of dysregulation of lipid metabolism in chronic renal failure. Semin Dial. 2009;22:644–651. doi: 10.1111/j.1525-139X.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabresi L., Gomaraschi M., Franceschini G. High-density lipoprotein quantity or quality for cardiovascular prevention? Curr Pharmaceut Des. 2010;16:1494–1503. doi: 10.2174/138161210791050960. [DOI] [PubMed] [Google Scholar]
- 8.Basso F., Freeman L., Knapper C.L., Remaley A., Stonik J., Neufeld E.B., Tansey T., Amar M.J., Fruchart-Najib J., Duverger N., Santamarina-Fojo S., Brewer H.B., Jr. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi L., Gomaraschi M., Simonelli S., Bernini F., Franceschini G. HDL and atherosclerosis: insights from inherited HDL disorders. Biochim Biophys Acta. 2015;1851:13–18. doi: 10.1016/j.bbalip.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Rye K.A., Clay M.A., Barter P.J. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis. 1999;145:227–238. doi: 10.1016/s0021-9150(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 11.Kozyraki R., Fyfe J., Kristiansen M., Gerdes C., Jacobsen C., Cui S., Christensen E.I., Aminoff M., de la Chapelle A., Krahe R., Verroust P.J., Moestrup S.K. The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med. 1999;5:656–661. doi: 10.1038/9504. [DOI] [PubMed] [Google Scholar]
- 12.Hammad S.M., Barth J.L., Knaak C., Argraves W.S. Megalin acts in concert with cubilin to mediate endocytosis of high density lipoproteins. J Biol Chem. 2000;275:12003–12008. doi: 10.1074/jbc.275.16.12003. [DOI] [PubMed] [Google Scholar]
- 13.Aseem O., Smith B.T., Cooley M.A., Wilkerson B.A., Argraves K.M., Remaley A.T., Argraves W.S. Cubilin maintains blood levels of HDL and albumin. J Am Soc Nephrol. 2014;25:1028–1036. doi: 10.1681/ASN.2013060671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz C.C., VandenBroek J.M., Cooper P.S. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Calabresi L., Gomaraschi M., Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003;23:1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 16.Kuchta A., Cwiklinska A., Czaplinska M., Wieczorek E., Kortas-Stempak B., Gliwinska A., Dabkowski K., Salaga-Zaleska K., Mickiewicz A., Debska-Slizien A., Krol E., Jankowski M. Plasma levels of prebeta1-HDL are significantly elevated in non-dialyzed patients with advanced stages of chronic kidney disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20051202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang K., Vaziri N.D. Down-regulation of hepatic lipase expression in experimental nephrotic syndrome. Kidney Int. 1997;51:1933–1937. doi: 10.1038/ki.1997.263. [DOI] [PubMed] [Google Scholar]
- 18.Holzer M., Schilcher G., Curcic S., Trieb M., Ljubojevic S., Stojakovic T., Scharnagl H., Kopecky C.M., Rosenkranz A.R., Heinemann A., Marsche G. Dialysis modalities and HDL composition and function. J Am Soc Nephrol. 2015;26:2267–2276. doi: 10.1681/ASN.2014030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon V., Yang H., Fazio S. Residual cardiovascular risk in chronic kidney disease: role of high-density lipoprotein. Arch Med Res. 2015;46:379–391. doi: 10.1016/j.arcmed.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy D.J., Tang W.H., Fan Y., Wu Y., Mann S., Pepoy M., Hazen S.L. Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L., Nukuna B., Brennan M.L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P.L., Ischiropoulos H., Smith J.D., Kinter M., Hazen S.L. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rye K.A. Biomarkers associated with high-density lipoproteins in atherosclerotic kidney disease. Clin Exp Nephrol. 2014;18:247–250. doi: 10.1007/s10157-013-0865-x. [DOI] [PubMed] [Google Scholar]
- 23.Shao B., Pennathur S., Pagani I., Oda M.N., Witztum J.L., Oram J.F., Heinecke J.W. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem. 2010;285:18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzer M., Birner-Gruenberger R., Stojakovic T., El-Gamal D., Binder V., Wadsack C., Heinemann A., Marsche G. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22:1631–1641. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto S., Yancey P.G., Ikizler T.A., Jerome W.G., Kaseda R., Cox B., Bian A., Shintani A., Fogo A.B., Linton M.F., Fazio S., Kon V. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weichhart T., Kopecky C., Kubicek M., Haidinger M., Doller D., Katholnig K., Suarna C., Eller P., Tolle M., Gerner C., Zlabinger G.J., van der Giet M., Horl W.H., Stocker R., Saemann M.D. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012;23:934–947. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier S.M., Wultsch A., Hollaus M., Ammann M., Pemberger E., Liebscher F., Lambers B., Fruhwurth S., Stojakovic T., Scharnagl H., Schmidt A., Springer A., Becker J., Aufricht C., Handisurya A., Kapeller S., Rohrl C., Stangl H., Strobl W. Effect of chronic kidney disease on macrophage cholesterol efflux. Life Sci. 2015;136:1–6. doi: 10.1016/j.lfs.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Holzer M., Gauster M., Pfeifer T., Wadsack C., Fauler G., Stiegler P., Koefeler H., Beubler E., Schuligoi R., Heinemann A., Marsche G. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxidants Redox Signal. 2011;14:2337–2346. doi: 10.1089/ars.2010.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganda A., Yvan-Charvet L., Zhang Y., Lai E.J., Regunathan-Shenk R., Hussain F.N., Avasare R., Chakraborty B., Febus A.J., Vernocchi L., Lantigua R., Wang Y., Shi X., Hsieh J., Murphy A.J., Wang N., Bijl N., Gordon K.M., de Miguel M.H., Singer J.R., Hogan J., Cremers S., Magnusson M., Melander O., Gerszten R.E., Tall A.R. Plasma metabolite profiles, cellular cholesterol efflux, and non-traditional cardiovascular risk in patients with CKD. J Mol Cell Cardiol. 2017;112:114–122. doi: 10.1016/j.yjmcc.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardinal H., Raymond M.A., Hebert M.J., Madore F. Uraemic plasma decreases the expression of ABCA1, ABCG1 and cell-cycle genes in human coronary arterial endothelial cells. Nephrol Dial Transplant. 2007;22:409–416. doi: 10.1093/ndt/gfl619. [DOI] [PubMed] [Google Scholar]
- 31.Kaseda R., Jabs K., Hunley T.E., Jones D., Bian A., Allen R.M., Vickers K.C., Yancey P.G., Linton M.F., Fazio S., Kon V. Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism. 2015;64:263–273. doi: 10.1016/j.metabol.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speer T., Rohrer L., Blyszczuk P., Shroff R., Kuschnerus K., Krankel N., Kania G., Zewinger S., Akhmedov A., Shi Y., Martin T., Perisa D., Winnik S., Muller M.F., Sester U., Wernicke G., Jung A., Gutteck U., Eriksson U., Geisel J., Deanfield J., von Eckardstein A., Luscher T.F., Fliser D., Bahlmann F.H., Landmesser U. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 2013;38:754–768. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Shroff R., Speer T., Colin S., Charakida M., Zewinger S., Staels B., Chinetti-Gbaguidi G., Hettrich I., Rohrer L., O'Neill F., McLoughlin E., Long D., Shanahan C.M., Landmesser U., Fliser D., Deanfield J.E. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol. 2014;25:2658–2668. doi: 10.1681/ASN.2013111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Gamal D., Rao S.P., Holzer M., Hallstrom S., Haybaeck J., Gauster M., Wadsack C., Kozina A., Frank S., Schicho R., Schuligoi R., Heinemann A., Marsche G. The urea decomposition product cyanate promotes endothelial dysfunction. Kidney Int. 2014;86:923–931. doi: 10.1038/ki.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomaraschi M., Ossoli A., Castelnuovo S., Simonelli S., Pavanello C., Balzarotti G., Arca M., Di Costanzo A., Sampietro T., Vaudo G., Baldassarre D., Veglia F., Franceschini G., Calabresi L. Depletion in LpA-I: A-II particles enhances HDL-mediated endothelial protection in familial LCAT deficiency. J Lipid Res. 2017;58:994–1001. doi: 10.1194/jlr.P072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Undurti A., Huang Y., Lupica J.A., Smith J.D., DiDonato J.A., Hazen S.L. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nobecourt E., Tabet F., Lambert G., Puranik R., Bao S., Yan L., Davies M.J., Brown B.E., Jenkins A.J., Dusting G.J., Bonnet D.J., Curtiss L.K., Barter P.J., Rye K.A. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2010;30:766–772. doi: 10.1161/ATVBAHA.109.201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moradi H., Pahl M.V., Elahimehr R., Vaziri N.D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res. 2009;153:77–85. doi: 10.1016/j.trsl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Tolle M., Pawlak A., Schuchardt M., Kawamura A., Tietge U.J., Lorkowski S., Keul P., Assmann G., Chun J., Levkau B., van der Giet M., Nofer J.R. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol. 2008;28:1542–1548. doi: 10.1161/ATVBAHA.107.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morena M., Cristol J.P., Dantoine T., Carbonneau M.A., Descomps B., Canaud B. Protective effects of high-density lipoprotein against oxidative stress are impaired in haemodialysis patients. Nephrol Dial Transplant. 2000;15:389–395. doi: 10.1093/ndt/15.3.389. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls S.J., Zheng L., Hazen S.L. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med. 2005;15:212–219. doi: 10.1016/j.tcm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Ossoli A., Pavanello C., Giorgio E., Calabresi L., Gomaraschi M. Dysfunctional HDL as a therapeutic target for atherosclerosis prevention. Curr Med Chem. 2019;26:1610–1630. doi: 10.2174/0929867325666180316115726. [DOI] [PubMed] [Google Scholar]
- 43.Baragetti A., Norata G.D., Sarcina C., Rastelli F., Grigore L., Garlaschelli K., Uboldi P., Baragetti I., Pozzi C., Catapano A.L. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Intern Med. 2013;274:252–262. doi: 10.1111/joim.12081. [DOI] [PubMed] [Google Scholar]
- 44.Bowe B., Xie Y., Xian H., Balasubramanian S., Al-Aly Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 2016;89:886–896. doi: 10.1016/j.kint.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 45.Chang T.I., Streja E., Soohoo M., Ko G.J., Rhee C.M., Kovesdy C.P., Kashyap M.L., Vaziri N.D., Kalantar-Zadeh K., Moradi H. Increments in serum high-density lipoprotein cholesterol over time are not associated with improved outcomes in incident hemodialysis patients. J Clin Lipidol. 2018;12:488–497. doi: 10.1016/j.jacl.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Moradi H., Streja E., Kashyap M.L., Vaziri N.D., Fonarow G.C., Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. 2014;29:1554–1562. doi: 10.1093/ndt/gfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam K.H., Chang T.I., Joo Y.S., Kim J., Lee S., Lee C., Yun H.R., Park J.T., Yoo T.H., Sung S.A., Lee K.B., Oh K.H., Kim S.W., Lee J., Kang S.W., Choi K.H., Ahn C., Han S.H., Investigators K.-C. Association between serum high-density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW-CKD. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C.Y., Lin F.Y., Shih C.M., Au H.K., Chang Y.J., Nakagami H., Morishita R., Chang N.C., Shyu K.G., Chen J.W. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating Rho-associated kinase pathways. Arterioscler Thromb Vasc Biol. 2012;32:2405–2417. doi: 10.1161/ATVBAHA.112.248617. [DOI] [PubMed] [Google Scholar]
- 49.Rahman M., Yang W., Akkina S., Alper A., Anderson A.H., Appel L.J., He J., Raj D.S., Schelling J., Strauss L., Teal V., Rader D.J., C. S. Investigators Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2014;9:1190–1198. doi: 10.2215/CJN.09320913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronenberg F. HDL in CKD-the devil is in the detail. J Am Soc Nephrol. 2018;29:1356–1371. doi: 10.1681/ASN.2017070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strazzella A., Ossoli A., Calabresi L. High-density lipoproteins and the kidney. Cells. 2021;10 doi: 10.3390/cells10040764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oldoni F., Sinke R.J., Kuivenhoven J.A. Mendelian disorders of high-density lipoprotein metabolism. Circ Res. 2014;114:124–142. doi: 10.1161/CIRCRESAHA.113.300634. [DOI] [PubMed] [Google Scholar]
- 53.Arciello A., Piccoli R., Monti D.M. Apolipoprotein A-I: the dual face of a protein. FEBS Lett. 2016;590:4171–4179. doi: 10.1002/1873-3468.12468. [DOI] [PubMed] [Google Scholar]
- 54.Obici L., Franceschini G., Calabresi L., Giorgetti S., Stoppini M., Merlini G., Bellotti V. Structure, function and amyloidogenic propensity of apolipoprotein A-I. Amyloid. 2006;13:191–205. doi: 10.1080/13506120600960288. [DOI] [PubMed] [Google Scholar]
- 55.Gregorini G., Izzi C., Ravani P., Obici L., Dallera N., Del Barba A., Negrinelli A., Tardanico R., Nardi M., Biasi L., Scalvini T., Merlini G., Scolari F. Tubulointerstitial nephritis is a dominant feature of hereditary apolipoprotein A-I amyloidosis. Kidney Int. 2015;87:1223–1229. doi: 10.1038/ki.2014.389. [DOI] [PubMed] [Google Scholar]
- 56.Benson M.D., Liepnieks J.J., Yazaki M., Yamashita T., Hamidi Asl K., Guenther B., Kluve-Beckerman B. A new human hereditary amyloidosis: the result of a stop-codon mutation in the apolipoprotein AII gene. Genomics. 2001;72:272–277. doi: 10.1006/geno.2000.6499. [DOI] [PubMed] [Google Scholar]
- 57.Saito T., Matsunaga A. Lipoprotein glomerulopathy may provide a key to unlock the puzzles of renal lipidosis. Kidney Int. 2014;85:243–245. doi: 10.1038/ki.2013.404. [DOI] [PubMed] [Google Scholar]
- 58.Saito T., Matsunaga A., Fukunaga M., Nagahama K., Hara S., Muso E. Apolipoprotein E-related glomerular disorders. Kidney Int. 2020;97:279–288. doi: 10.1016/j.kint.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 59.Katsarou M., Stratikos E., Chroni A. Thermodynamic destabilization and aggregation propensity as the mechanism behind the association of apoE3 mutants and lipoprotein glomerulopathy. J Lipid Res. 2018;59:2339–2348. doi: 10.1194/jlr.M088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oikawa S., Matsunaga A., Saito T., Sato H., Seki T., Hoshi K., Hayasaka K., Kotake H., Midorikawa H., Sekikawa A., Hara S., Abe K., Toyota T., Jingami H., Nakamura H., Sasaki J. Apolipoprotein E Sendai (arginine 145-->proline): a new variant associated with lipoprotein glomerulopathy. J Am Soc Nephrol. 1997;8:820–823. doi: 10.1681/ASN.V85820. [DOI] [PubMed] [Google Scholar]
- 61.Matsunaga A., Sasaki J., Komatsu T., Kanatsu K., Tsuji E., Moriyama K., Koga T., Arakawa K., Oikawa S., Saito T., Kita T., Doi T. A novel apolipoprotein E mutation, E2 (Arg25Cys), in lipoprotein glomerulopathy. Kidney Int. 1999;56:421–427. doi: 10.1046/j.1523-1755.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 62.Saito T., Matsunaga A., Oikawa S. Impact of lipoprotein glomerulopathy on the relationship between lipids and renal diseases. Am J Kidney Dis. 2006;47:199–211. doi: 10.1053/j.ajkd.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Oda H., Yorioka N., Ueda C., Kushihata S., Yamakido M. Apolipoprotein E polymorphism and renal disease. Kidney Int Suppl. 1999;71:S25–S27. doi: 10.1046/j.1523-1755.1999.07107.x. [DOI] [PubMed] [Google Scholar]
- 64.Hsu C.C., Kao W.H., Coresh J., Pankow J.S., Marsh-Manzi J., Boerwinkle E., Bray M.S. Apolipoprotein E and progression of chronic kidney disease. JAMA. 2005;293:2892–2899. doi: 10.1001/jama.293.23.2892. [DOI] [PubMed] [Google Scholar]
- 65.Xue C., Nie W., Tang D., Yi L., Mei C. Apolipoprotein E gene variants on the risk of end stage renal disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi J., Cheng Z., Qiu S., Cui H., Gu Y., Zhao Q., Ren Y., Zhang H., Sun H., Liu Y., Li Y., Qiao Y., Hu Y., Liu Y., Cheng Y. epsilon2 allele and epsilon2-involved genotypes (epsilon2/epsilon2, epsilon2/epsilon3, and epsilon2/epsilon4) may confer the association of APOE genetic polymorphism with risks of nephropathy in type 2 diabetes: a meta-analysis. Lipids Health Dis. 2020;19:136. doi: 10.1186/s12944-020-01307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruschi M., Catarsi P., Candiano G., Rastaldi M.P., Musante L., Scolari F., Artero M., Carraro M., Carrea A., Caridi G., Zennaro C., Sanna-Cherchi S., Viola F.B., Ferrario F., Perfumo F., Ghiggeri G.M. Apolipoprotein E in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Kidney Int. 2003;63:686–695. doi: 10.1046/j.1523-1755.2003.00777.x. [DOI] [PubMed] [Google Scholar]
- 68.Duchateau P.N., Pullinger C.R., Orellana R.E., Kunitake S.T., Naya-Vigne J., O'Connor P.M., Malloy M.J., Kane J.P. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272:25576–25582. doi: 10.1074/jbc.272.41.25576. [DOI] [PubMed] [Google Scholar]
- 69.Genovese G., Friedman D.J., Ross M.D., Lecordier L., Uzureau P., Freedman B.I., Bowden D.W., Langefeld C.D., Oleksyk T.K., Uscinski Knob A.L., Bernhardy A.J., Hicks P.J., Nelson G.W., Vanhollebeke B., Winkler C.A., Kopp J.B., Pays E., Pollak M.R. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parsa A., Kao W.H., Xie D., Astor B.C., Li M., Hsu C.Y., Feldman H.I., Parekh R.S., Kusek J.W., Greene T.H., Fink J.C., Anderson A.H., Choi M.J., Wright J.T., Jr., Lash J.P., Freedman B.I., Ojo A., Winkler C.A., Raj D.S., Kopp J.B., He J., Jensvold N.G., Tao K., Lipkowitz M.S., Appel L.J., Investigators A.S., Investigators C.S. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daneshpajouhnejad P., Kopp J.B., Winkler C.A., Rosenberg A.Z. The evolving story of apolipoprotein L1 nephropathy: the end of the beginning. Nat Rev Nephrol. 2022;18:307–320. doi: 10.1038/s41581-022-00538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nadkarni G.N., Gignoux C.R., Sorokin E.P., Daya M., Rahman R., Barnes K.C., Wassel C.L., Kenny E.E. Worldwide frequencies of APOL1 renal risk variants. N Engl J Med. 2018;379:2571–2572. doi: 10.1056/NEJMc1800748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNulty M.T., Fermin D., Eichinger F., Jang D., Kretzler M., Burtt N.P., Pollak M.R., Flannick J., Weins A., Friedman D.J., Nephrotic Syndrome Study N., Sampson M.G. A glomerular transcriptomic landscape of apolipoprotein L1 in Black patients with focal segmental glomerulosclerosis. Kidney Int. 2022;102:136–148. doi: 10.1016/j.kint.2021.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grams M.E., Rebholz C.M., Chen Y., Rawlings A.M., Estrella M.M., Selvin E., Appel L.J., Tin A., Coresh J. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27:2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomson R., Finkelstein A. Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: relevance to trypanosome lysis. Proc Natl Acad Sci U S A. 2015;112:2894–2899. doi: 10.1073/pnas.1421953112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah S.S., Lannon H., Dias L., Zhang J.Y., Alper S.L., Pollak M.R., Friedman D.J. APOL1 kidney risk variants induce cell death via mitochondrial translocation and opening of the mitochondrial permeability transition pore. J Am Soc Nephrol. 2019;30:2355–2368. doi: 10.1681/ASN.2019020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruno J., Edwards J.C. Kidney-disease-associated variants of Apolipoprotein L1 show gain of function in cation channel activity. J Biol Chem. 2021;296 doi: 10.1074/jbc.RA120.013943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olabisi O.A., Zhang J.Y., VerPlank L., Zahler N., DiBartolo S., 3rd, Heneghan J.F., Schlondorff J.S., Suh J.H., Yan P., Alper S.L., Friedman D.J., Pollak M.R. APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A. 2016;113:830–837. doi: 10.1073/pnas.1522913113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng D., Weckerle A., Yu Y., Ma L., Zhu X., Murea M., Freedman B.I., Parks J.S., Shelness G.S. Biogenesis and cytotoxicity of APOL1 renal risk variant proteins in hepatocytes and hepatoma cells. J Lipid Res. 2015;56:1583–1593. doi: 10.1194/jlr.M059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan G., Zhaorigetu S., Liu Z., Kaini R., Jiang Z., Hu C.A. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pavanello C., Calabresi L. Genetic, biochemical, and clinical features of LCAT deficiency: update for 2020. Curr Opin Lipidol. 2020;31:232–237. doi: 10.1097/MOL.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 82.Santamarina-Fojo S., Hoeg J.M., Assmann G., Bryan Brewer H. In: The online metabolic and molecular bases of inherited disease. Valle D.L., Antonarakis S., Ballabio A., Beaudet A.L., Mitchell G.A., editors. McGraw-Hill Education; New York, NY: 2019. Lecithin cholesterol acyltransferase deficiency and fish eye disease. [Google Scholar]
- 83.Holleboom A.G., Kuivenhoven J.A., van Olden C.C., Peter J., Schimmel A.W., Levels J.H., Valentijn R.M., Vos P., Defesche J.C., Kastelein J.J., Hovingh G.K., Stroes E.S., Hollak C.E. Proteinuria in early childhood due to familial LCAT deficiency caused by loss of a disulfide bond in lecithin:cholesterol acyl transferase. Atherosclerosis. 2011;216:161–165. doi: 10.1016/j.atherosclerosis.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 84.Pavanello C., Ossoli A., Arca M., D'Erasmo L., Boscutti G., Gesualdo L., Lucchi T., Sampietro T., Veglia F., Calabresi L. Progression of chronic kidney disease in familial LCAT deficiency: a follow-up of the Italian cohort. J Lipid Res. 2020;61:1784–1788. doi: 10.1194/jlr.P120000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Narayanan S. Biochemistry and clinical relevance of lipoprotein X. Ann Clin Lab Sci. 1984;14:371–374. [PubMed] [Google Scholar]
- 86.Calabresi L., Pisciotta L., Costantin A., Frigerio I., Eberini I., Alessandrini P., Arca M., Bon G.B., Boscutti G., Busnach G., Frasca G., Gesualdo L., Gigante M., Lupattelli G., Montali A., Pizzolitto S., Rabbone I., Rolleri M., Ruotolo G., Sampietro T., Sessa A., Vaudo G., Cantafora A., Veglia F., Calandra S., Bertolini S., Franceschini G. The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler Thromb Vasc Biol. 2005;25:1972–1978. doi: 10.1161/01.ATV.0000175751.30616.13. [DOI] [PubMed] [Google Scholar]
- 87.Boscutti G., Calabresi L., Pizzolitto S., Boer E., Bosco M., Mattei P.L., Martone M., Milutinovic N., Berbecar D., Beltram E., Franceschini G. [LCAT deficiency: a nephrological diagnosis] G Ital Nefrol. 2011;28:369–382. [PubMed] [Google Scholar]
- 88.Sessa A., Battini G., Meroni M., Daidone G., Carnera I., Brambilla P.L., Vigano G., Giordano F., Pallotti F., Torri Tarelli L., Calabresi L., Rolleri M., Bertolini S. Hypocomplementemic type II membranoproliferative glomerulonephritis in a male patient with familial lecithin-cholesterol acyltransferase deficiency due to two different allelic mutations. Nephron. 2001;88:268–272. doi: 10.1159/000046001. [DOI] [PubMed] [Google Scholar]
- 89.Borysiewicz L.K., Soutar A.K., Evans D.J., Thompson G.R., Rees A.J. Renal failure in familial lecithin: cholesterol acyltransferase deficiency. Q J Med. 1982;51:411–426. [PubMed] [Google Scholar]
- 90.Imbasciati E., Paties C., Scarpioni L., Mihatsch M.J. Renal lesions in familial lecithin-cholesterol acyltransferase deficiency. Ultrastructural heterogeneity of glomerular changes. Am J Nephrol. 1986;6:66–70. doi: 10.1159/000167056. [DOI] [PubMed] [Google Scholar]
- 91.Ossoli A., Neufeld E.B., Thacker S.G., Vaisman B., Pryor M., Freeman L.A., Brantner C.A., Baranova I., Francone N.O., Demosky S.J., Jr., Vitali C., Locatelli M., Abbate M., Zoja C., Franceschini G., Calabresi L., Remaley A.T. Lipoprotein X causes renal disease in LCAT deficiency. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavanello C., Turri M., Strazzella A., Tulissi P., Pizzolitto S., De Maglio G., Nappi R., Calabresi L., Boscutti G. The HDL mimetic CER-001 remodels plasma lipoproteins and reduces kidney lipid deposits in inherited lecithin:cholesterol acyltransferase deficiency. J Intern Med. 2022;291:364–370. doi: 10.1111/joim.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shamburek R.D., Bakker-Arkema R., Auerbach B.J., Krause B.R., Homan R., Amar M.J., Freeman L.A., Remaley A.T. Familial lecithin:cholesterol acyltransferase deficiency: first-in-human treatment with enzyme replacement. J Clin Lipidol. 2016;10:356–367. doi: 10.1016/j.jacl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shamburek R.D., Bakker-Arkema R., Shamburek A.M., Freeman L.A., Amar M.J., Auerbach B., Krause B.R., Homan R., Adelman S.J., Collins H.L., Sampson M., Wolska A., Remaley A.T. Safety and tolerability of ACP-501, a recombinant human lecithin:cholesterol acyltransferase, in a phase 1 single-dose escalation study. Circ Res. 2016;118:73–82. doi: 10.1161/CIRCRESAHA.115.306223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pavanello C., Ossoli A., Turri M., Strazzella A., Simonelli S., Laurenzi T., Kono K., Yamada K., Kiyosawa N., Eberini I., Calabresi L. Activation of naturally occurring lecithin:cholesterol acyltransferase mutants by a novel activator compound. J Pharmacol Exp Therapeut. 2020;375:463–468. doi: 10.1124/jpet.120.000159. [DOI] [PubMed] [Google Scholar]
- 96.Freeman L.A., Demosky S.J., Jr., Konaklieva M., Kuskovsky R., Aponte A., Ossoli A.F., Gordon S.M., Koby R.F., Manthei K.A., Shen M., Vaisman B.L., Shamburek R.D., Jadhav A., Calabresi L., Gucek M., Tesmer J.J.G., Levine R.L., Remaley A.T. Lecithin:Cholesterol acyltransferase activation by sulfhydryl-reactive small molecules: role of cysteine-31. J Pharmacol Exp Therapeut. 2017;362:306–318. doi: 10.1124/jpet.117.240457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ossoli A., Strazzella A., Rottoli D., Zanchi C., Locatelli M., Zoja C., Simonelli S., Veglia F., Barbaras R., Tupin C., Dasseux J.L., Calabresi L. CER-001 ameliorates lipid profile and kidney disease in a mouse model of familial LCAT deficiency. Metabolism. 2021;116 doi: 10.1016/j.metabol.2020.154464. [DOI] [PubMed] [Google Scholar]
- 98.Li Y., Dong J.B., Wu M.P. Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur J Pharmacol. 2008;590:417–422. doi: 10.1016/j.ejphar.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 99.Moreira R.S., Irigoyen M., Sanches T.R., Volpini R.A., Camara N.O., Malheiros D.M., Shimizu M.H., Seguro A.C., Andrade L. Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol Regul Integr Comp Physiol. 2014;307:R514–R524. doi: 10.1152/ajpregu.00445.2013. [DOI] [PubMed] [Google Scholar]
- 100.Moreira R.S., Irigoyen M.C., Capcha J.M.C., Sanches T.R., Gutierrez P.S., Garnica M.R., Noronha I.L., Andrade L. Synthetic apolipoprotein A-I mimetic peptide 4F protects hearts and kidneys after myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2020;318:R529–R544. doi: 10.1152/ajpregu.00185.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsuchida Y., Zhong J., Otsuka T., Dikalova A., Pastan I., Anantharamaiah G.M., Linton M.F., Yancey P.G., Ikizler T.A., Fogo A.B., Yang H., Kon V. Lipoprotein modulation of proteinuric renal injury. Lab Invest. 2019;99:1107–1116. doi: 10.1038/s41374-019-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vaisman B.L., Neufeld E.B., Freeman L.A., Gordon S.M., Sampson M.L., Pryor M., Hillman E., Axley M.J., Karathanasis S.K., Remaley A.T. LCAT enzyme replacement therapy reduces LpX and improves kidney function in a mouse model of familial LCAT deficiency. J Pharmacol Exp Therapeut. 2019;368:423–434. doi: 10.1124/jpet.118.251876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baragetti A., Ossoli A., Strazzella A., Simonelli S., Baragetti I., Grigore L., Pellegatta F., Catapano A.L., Norata G.D., Calabresi L. Low plasma lecithin: cholesterol acyltransferase (LCAT) concentration predicts chronic kidney disease. J Clin Med. 2020;9 doi: 10.3390/jcm9072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castiglioni L., Pignieri A., Fiasche M., Giudici M., Crestani M., Mitro N., Abbate M., Zoja C., Rottoli D., Foray C., Fiordaliso F., Guerrini U., Tremoli E., Sironi L., Gelosa P. Fenofibrate attenuates cardiac and renal alterations in young salt-loaded spontaneously hypertensive stroke-prone rats through mitochondrial protection. J Hypertens. 2018;36:1129–1146. doi: 10.1097/HJH.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 105.Bounafaa A., Berrougui H., Ikhlef S., Essamadi A., Nasser B., Bennis A., Yamoul N., Ghalim N., Khalil A. Alteration of HDL functionality and PON1 activities in acute coronary syndrome patients. Clin Biochem. 2014;47:318–325. doi: 10.1016/j.clinbiochem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 106.Gibson C.M., Kerneis M., Yee M.K., Daaboul Y., Korjian S., Mehr A.P., Tricoci P., Alexander J.H., Kastelein J.J.P., Mehran R., Bode C., Lewis B.S., Mehta R., Duffy D., Feaster J., Halabi M., Angiolillo D.J., Duerschmied D., Ophuis T.O., Merkely B. The CSL112-2001 trial: safety and tolerability of multiple doses of CSL112 (apolipoprotein A-I [human]), an intravenous formulation of plasma-derived apolipoprotein A-I, among subjects with moderate renal impairment after acute myocardial infarction. Am Heart J. 2019;208:81–90. doi: 10.1016/j.ahj.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Hung A.M., Tsuchida Y., Nowak K.L., Sarkar S., Chonchol M., Whitfield V., Salas N., Dikalova A., Yancey P.G., Huang J., Linton M.F., Ikizler T.A., Kon V. IL-1 inhibition and function of the HDL-containing fraction of plasma in patients with stages 3 to 5 CKD. Clin J Am Soc Nephrol. 2019;14:702–711. doi: 10.2215/CJN.04360418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Faguer S., Colombat M., Chauveau D., Bernadet-Monrozies P., Beq A., Delas A., Soler V., Labadens I., Huart A., Benlian P., Schanstra J.P. Administration of the high-density lipoprotein mimetic CER-001 for inherited lecithin-cholesterol acyltransferase deficiency. Ann Intern Med. 2021;174:1022–1025. doi: 10.7326/L20-1300. [DOI] [PubMed] [Google Scholar]
- 109.Tardy C., Goffinet M., Boubekeur N., Ackermann R., Sy G., Bluteau A., Cholez G., Keyserling C., Lalwani N., Paolini J.F., Dasseux J.L., Barbaras R., Baron R. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 2014;232:110–118. doi: 10.1016/j.atherosclerosis.2013.10.018. [DOI] [PubMed] [Google Scholar]