Abstract
Cancer is an unprecedented proliferation of cells leading to abnormalities in differentiation and maturation. Treatment of primary and metastatic cancer is challenging. In addition to surgery, chemotherapy and radiation therapies have been conventionally used; however, they suffer from severe toxicity and non-specificity. Immunotherapy, the science of programming the body’s own defence system against cancer has gained tremendous attention in the last few decades. However, partial immunogenic stimulation, premature degradation and inability to activate dendritic and helper T cells has resulted in limited clinical success. The era of nanomedicine has brought about several breakthroughs in various pharmaceutical and biomedical fields. Hereby, we review and discuss the interplay of tumor microenvironment (TME) and the immunological cascade and how they can be employed to develop nanoparticle-based cancer vaccines and immunotherapies. Nanoparticles composed of lipids, polymers and inorganic materials contain useful properties suitable for vaccine development. Proteinaceous vaccines derived from mammalian viruses, bacteriophages and plant viruses also have unique advantages due to their immunomodulation capabilities. This review accounts for all such considerations. Additionally, we explore how attributes of nanotechnology can be utilized to develop successful nanomedicine-based vaccines for cancer therapy.
Keywords: Cancer, Dendritic cells, Immunotherapy, Nanovaccine
Graphical/Visual Abstract and Caption
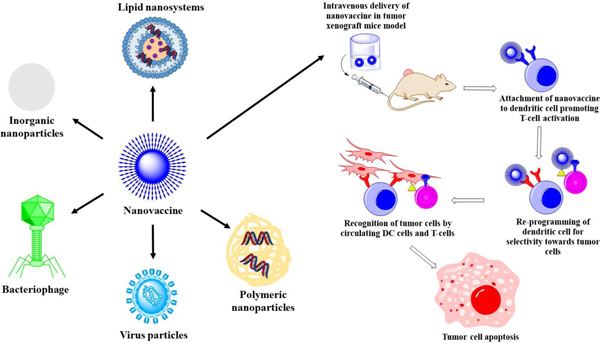
Emergence of the epoch of nanovaccines in reprogramming the body’s immune system for prophylactic and therapeutic potential against cancer.
1. INTRODUCTION
Cancer is a result of abnormalities in the proliferation, differentiation, maturation and migration of cells (Kurmi, Patel, Paliwal, & Paliwal, 2020). The prevalence of cancer in inflicting mortality amongst humans is increasing. Throughout the world, the incidences of various cancers have surged up to 19.3 million annual cases with a substantial mortality of 10 million in 2020 (Union for International Cancer Control., 2020). Certain challenges in cancer management include the lack of early diagnosis, metastasis, multi-drug resistance, inefficient targeting, adverse effects, organ toxicities from treatment, etc., which contribute to the failure of chemotherapy in treating various cancers (Alshehri et al., 2021). The most common cancers in men include lung and prostate cancer, whereas breast, colorectal, cervical, pulmonary and thyroid cancers are common among women (Mattiuzzi & Lippi, 2019). The occurrence of cancer depends on various factors like age, gender, socio-economic disparity, epidemiological and genotypic factors, etc. (Mbemi, Khanna, Njiki, Yedjou, & Tchounwou, 2020). Figure 1 demonstrates the incidences and mortality of patients across the continents along with age and cancer dependent classification of the incidences (Sung et al., 2021).
Figure 1.
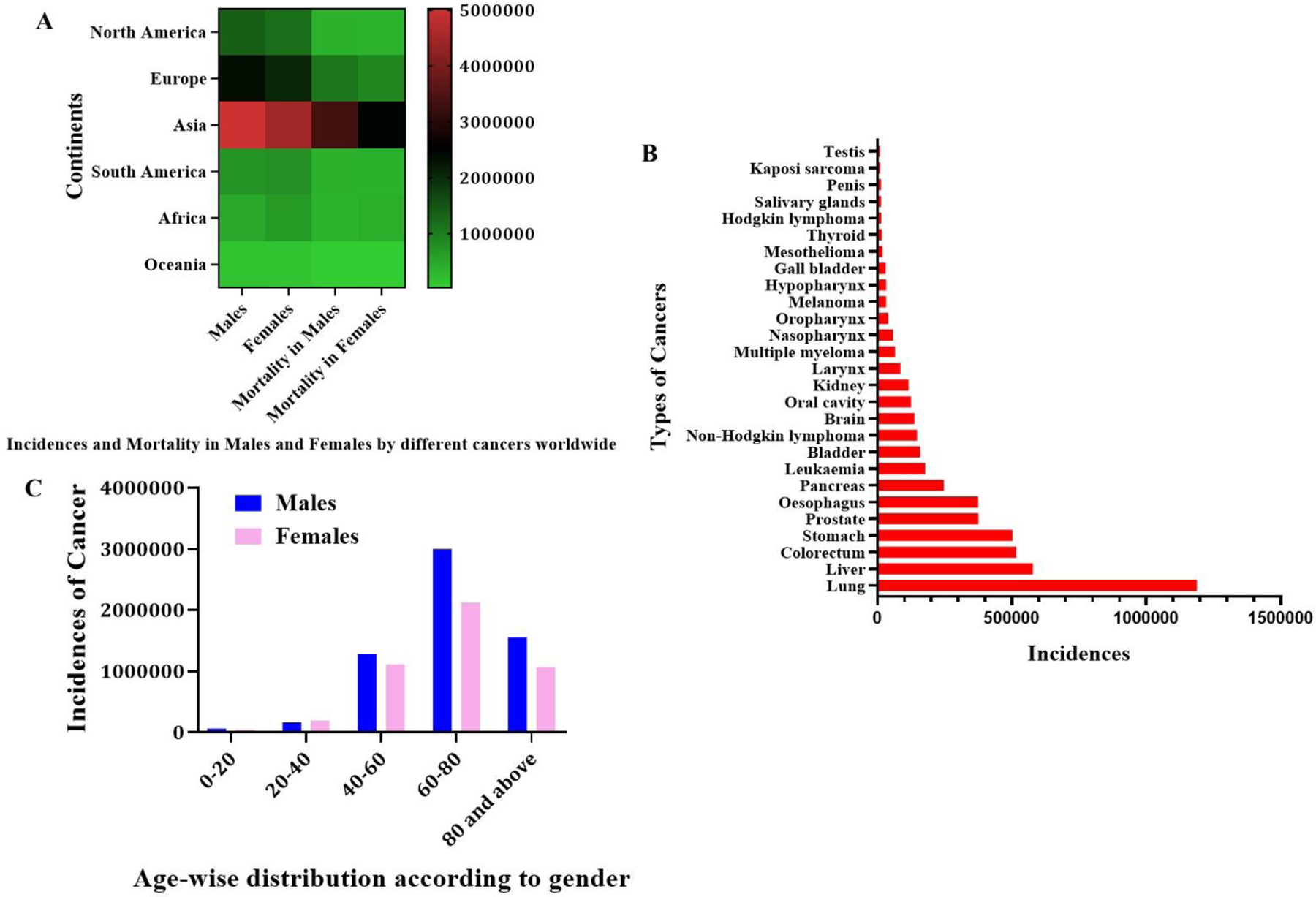
A) Continent-wise distribution of incidence and mortality in males and females by different types of cancers, B) Types of cancers versus the number of incidences and C) Age-wise distribution of cancer patients according to gender.
Nanomedicine has emerged as a versatile platform in circumventing the drawbacks associated with conventional anticancer therapies. Nanomedicine based approaches have been successful in alleviating adverse reactions owing to dosage and dosing frequency reduction, enhancement in bioavailability, improvement in cell internalization and targeting abilities, etc. (A. P. Singh, Biswas, Shukla, & Maiti, 2019). Nanomedicine has proven to be a vital tool in drug, vaccine and peptide delivery, organelle and cellular targeting. However, feasibility towards scalability and efficacy post pre-clinical assessment pose as challenges hindering its clinical translation (Shah et al., 2020). Several active targeting approaches utilize the immobilization of a ligand over the nanoparticulate surface which impart specificity towards selective cancer cells overexpressing the receptor to which the ligand binds. This harnesses the targeting ability and attenuates unwanted cytotoxicity. The major drawback associated with the clinical translation of ligand functionalized nanoparticles is the clinical reproducibility (due to heterogeneity of the disease amongst patients, between the primary and metastatic sites, and within the tumor itself). However, other limitations include low coating efficiency over the nanoparticle surface, and scalable manufacturing are considerable hurdles (Adityan, Tran, Bhavsar, & Wu, 2020). Stimuli responsive systems also known as smart systems, which respond to physiological differences within the TME, have also been widely explored. They have the potential to diminish unsolicited toxicities allied with the drug and have greater probability towards preclinical to clinical translation. However, their inability to reach distant tumors via the enhanced permeation and retention effect and relative insufficiency against metastatic and non-solid tumors make them less effective (Golombek et al., 2018). Therefore, there is an urgent need to advance the current nanotherapeutic approaches to combat the high mortality rate against various cancers. This review emphasizes the interplay between the TME and the immune system and the programming of the immune system against cancer cells along with a summary of the nanovaccines being developed against cancer today.
2. TUMOR MICROENVIRONMENT AND IMMUNE SYSTEM
Since the advent of Dr. William Coley’s idea on using one’s own immunity against cancer cells in the 1800’s, cancer immunotherapy has reached monumental milestones and success and has gained tremendous attention from researchers today (Abbott & Ustoyev, 2019). Immunity consists of innate and adaptive immunity. The innate and adaptive immunity contribute towards immediate and long-term protection against various antigens throughout the life time. Innate immunity is conferred by APC like monocytes, dendritic cells (DC) and natural killer (NK) cells (Germic, Frangez, Yousefi, & Simon, 2019). The cells interact with and are stimulated by foreign substances via toll-like receptors (TLRs), C-type receptors, cytoplasmic nucleotide-binding oligomerization domain (NOD) like receptors, and Retinoic acid-inducible gene (RIG-I) like receptors. When foreign materials or cancer antigens are engulfed by innate immune cells, they are digested to small peptide chains (epitopes) which are then displayed on major histocompatibility complexes type 1 or 2 (MHC I or II) (Gautam, Chauhan, Srivastava, Jadon, & Rathi, 2019). The MHC acts as a billboard to present these epitopes to specialized cells of the adaptive arm of the immune system. Epitopes on MHC I are presented to cytotoxic T lymphocytes and epitopes on MHC II are presented to helper T cells which memorize the signalling cascade and the mechanism of opsonisation required to inactivate the antigens during future exposure. Also, NK cells render cytotoxicity specifically towards the recognized antigen via binding to Fc receptors with diminished expression of MHC I by infected cell (Jia et al., 2017). The NK cells possess semi-invariant receptors which help in distinguishing lipid antigens mediating immune responses via pro-inflammatory cytokines. On the other hand, adaptive immunity is mainly regulated via B and T cells. Stimulation of T cells is followed by their maturation into cytotoxic T lymphocytes (CTL). However, their activation is hindered unless stimulated by helper T cells or via MHC signals (Del Vecchio et al., 2021). Additionally, B cells play a major role in releasing antibodies for destruction of pathogens, cancer cells, and infected cells.
Normal cells become cancerous owing to mutations in oncogenes and tumor suppressor genes which promote unrestrained cell division, angiogenesis, and metastasis and demote regulatory cell apoptosis. Dying tumor cells release several mediators like calreticulin, filamentous actin, etc. leading to cancer cell identification and stimulation of antigen presenting cells (APC). Once the APC are immobilized, clonal selection is initiated, where CD8+ T cells divide into effector T cells which hunt down the tumor cells bearing MHC I across the body (Park et al., 2017). The effector T cells selectively bind and kill the tumor cells by releasing perforins and granzyme which compromise tumor cell membrane integrity. Furthermore, stimulated CD4+ T cells secrete pro-inflammatory mediators like IFN-γ, IL-12 and TNF-α kill the tumor cells by aiding in MHC I upregulation and identification by CD8+ T cells.
3. BARRIERS TO IMMUNE RECOGNITION
The presence of tumor promoting immune cells like tumor associated macrophages (TAM), myeloid derived suppressor cells (MDSC) and regulatory T cells (Treg) in the tumor micro environment (TME) prevent tumor cell recognition and promote their growth with distinct mechanisms (Lindau, Gielen, Kroesen, Wesseling, & Adema, 2013). Along with their immunosuppressive functions, TAMs promote metastasis, angiogenesis and chemo-resistance in tumor cells (Famta et al., 2021; Haist, Stege, Grabbe, & Bros, 2021; L. Li et al., 2020). Matrix metalloproteinase (MMP) released by TAMs increase VEGF levels to implement angiogenesis (J. Wang, Li, Cang, & Guo, 2019). TAMs facilitate metastasis by stimulating colony-stimulating factor 1 (CSF-1) and epidermal growth factors (EGF) release. They modulate the structural framework of the extracellular matrix (ECM) by monitoring collagen accumulation and protease release (Raskov, Orhan, Gaggar, & Gögenur, 2021). The epithelial myo-fibroblast trans-differentiation (EMT) process promotes metastasis, and TAMs play a vital role in this transition through the secretion of TGF-β (Gulei et al., 2017). The secretion of immunosuppressive factors like IL-10, PGE2, arginase, etc. diminishes tumor cell recognition, which prevents the binding and destruction of cancer cells by downregulating MHC II (Armitage, Newnes, McDonnell, Bosco, & Waithman, 2021). TAMs have the ability to release a plethora of chemokines which diminish CD4+ and CD8+ T cell levels in the TME and promote the conversion of CD4+ T cells to immunosuppressive regulatory T cells (Quaranta & Schmid, 2019). TAM expression of cytotoxic T lymphocyte-associated antigen 4 leads to suppression of CD8+ T cells and induction of inhibitory dendritic cell phenotypes (Cassetta & Kitamura, 2018). TAMs release programmed cell death 1 (PD-1), which bind to programmed cell death protein-1 on T cells promoting T cell dysfunction and ultimately lowering cytotoxic action against tumor cells (L. Chen et al., 2022; Finbloom, Sousa, Stevens, & Desai, 2020; Xiuting Liu, Hogg, & Denardo, 2021). MDSC release immuno-suppressive cytokines like IL-10 prevent T cell infiltration and deprive the infiltrated T cells through amino acid starvation, cell death and abolition of intracellular signalling pathways (C. Li, Jiang, Wei, Xu, & Wang, 2020). Peroxy nitrite released by MDSC diminishes the identification of MHC I by CD8+ T cells (Colligan, Tzetzo, & Abrams, 2020). MDSC facilitate macrophage maturation towards the immunosuppressive M2 phenotype thereby prohibiting DC antigen presentation (Salminen, Kaarniranta, & Kauppinen, 2019). Immuno-suppressive cytokines have also been found to suppress the killing roles of NK cells via membrane-bound TGF-β (Suszczyk, Skiba, Jakubowicz-Gil, Kotarski, & Wertel, 2021). Another group of cells which help to camouflage tumor cells include the FOXP3 expressing Tregs, which diminish the adaptive immune systems capacity in killing cancer cells. Greater infiltration of these cells is attributed to release of immunosuppressive cytokines in the TME followed by increased expression of anti-apoptotic and suppression of pro-apoptotic genes (Nishikawa & Sakaguchi, 2014).
4. VACCINE DEVELOPMENT AGAINST CANCERS
The research conducted by Paul Ehrlich and William Coley increased the awareness for vaccines in cancer therapy across the globe. Ehrlich endeavoured to create an immune response against tumor cells using attenuated tumor cells. However, no therapeutic response was demonstrated. Meanwhile, Coley prepared an admixture of heat killed S. pyogenes and S. marcescens which obtained efficacious results in cancer patients owing to the generation of an immune reaction (Waldmann, 2003). Successful tumor vaccine development mainly consists of three major components which influence the sustenance of immune response: the antigen, adjuvant and the delivery system (Ge Zhu, Yang, & Sun, 2022). Antigens consist of proteins, peptides, lipids, etc. which are the components of the target cell against which the vaccine is to be created to render immunity (Schijns et al., 2020). Antigens impart target specificity and provoke an adaptive response (Saeed, Gao, Shi, Lammers, & Yu, 2019). Adjuvants are used to amplify and sustain the response and activate the innate immune system. In order to develop a successful vaccine, antigens and adjuvants should stimulate APC by inducing helper T cells (Th1 or Th2 cells) or CTL. Furthermore, to develop effective vaccines, employing peptides with affinity towards MHC I, which are specific to tumor cells and easily identified by T cells, promotes CTL activation (Campillo-Davo, Flumens, & Lion, 2020). These peptides play an important role as tumor associated antigens (TAA) in the design of tumor vaccines. Three types of antigens exist which have been classified in Table 1. The reports on universal TAA reveal that such antigens are often expressed in several distinct tumor types and are easily discernible targets for vaccine development (W. H. Li & Li, 2020). The determination of specific TAA which generate superior immune responses specific towards cancer cells in a large population compared to other TAA still remains a challenge (Lohmueller, Ham, Kvorjak, & Finn, 2018). Personalized vaccination manages to circumvent the challenges associated with universal TAA based vaccines by diminishing the phenotypic and genotypic diversity and considering the immune miscellany by estimating CTL precursor frequency. TAA are cellular proteins formed by class I MHC molecules for immune response generation while, adequate activation promotes CD8+ T cells differentiation into CTL (Snell et al., 2018). Stimulated CTL further promote cytotoxicity and secrete cytokines resulting in local inflammation. One of the most indispensable functions of any effective vaccine is instituting CD8+ T cell memory. Such memory must be maintained for a prolonged duration without stimulation. Traditionally, effector CD4+ cells discern into T-helper 1 (Th1) and T-helper 2 (Th2) cells. Of the two types of helper T cells, Th1 cells usually possess greater anticancer activity owing to enhanced IFN-γ secretion (L. Liu et al., 2020). The pooled action of CD8+ and IFN-γ-secreting Th1 CD4+ T cells lead to tumor cell apoptosis and NK cell activation which are important factors for potential cancer vaccine development.
Table 1.
Types of antigens explored for tumor vaccine development
| Antigen types | Attributes | Reference |
|---|---|---|
| Shared antigens | They are observed in different tumors; however, normal tissues express them differently, e.g. antigens like MAGE, NY-ESO-1, etc. are considered shared antigens. | (Raza et al., 2020) |
| Unique antigens | They are expressed specifically by individual tumors & are expressed by point mutations or splicing alterations; e.g. cancer testis antigen. | (Gordeeva, 2018) |
| Indispensable antigens | They are necessary for cancer progression and are usually overexpressed by totipotent neoplastic and pluripotent foetal cells, but are rarely seen in normal cells; e.g. HER2, claudin 18.2. Targeting such antigens could be highly beneficial for successful and efficacious vaccine development. | (Barati et al., 2021) |
| Neoantigens | They are tumor-specific in nature, produced by mutations in tumor cells. High specificity at the cellular level for targeting primary tumor cells as well as circulating tumor cell clusters. | (Duarte & Jill M.Lenardo, 2020) |
5. DENDRITIC CELLS AND IMMUNITY
DC have been well recognized for their enhanced antigen presenting capabilities to T cells resulting in stimulation of the adaptive immune response. They are found in tissues like skin, mucosal surfaces, etc. and migrate to T cell depots, further activating both innate and adaptive immunity via upregulation of MHC complexes and cytokines like IL-12 and type I IFN (Leifer, 2017). Therefore, DC are established as a favoured vaccine target owing to the preferential control of both innate and adaptive immunity. Immature DC have the ability to function as sentinel cells in detecting pathogens and activating pro-inflammatory cytokine cascades (Z. J. Yang et al., 2021). Once DC incorporate antigens, they proliferate and express CCR7 receptor which promote the expression of CCL19 and CCL21 ligands leading to their migration into lymphatic vessels. Post migration, DC activate MHC class I, MHC class II, or lipid antigen-presenting cluster of differentiation 1 (CD1) moieties (Hampton & Chtanova, 2019). Another approach for antigen presentation comprises of cross-presentation or cross-priming, whereby myeloid DC procure exogenous soluble antigens from tumor cells and display them to MHC class I. The process occurs via retro-translocation of antigens across endosomal components within the cytosol followed by proteosome processing. The antigens re-enter the exosomal vesicles through transporters for antigen presentation, where peptide charging of reprocessed MHC class I molecules takes place (Colbert, Cruz, & Rock, 2020). In order to be successfully stimulated by DC, T cells should distinguish the processed antigens from MHC class I or MHC class II for CD8+ and CD4+ T cells, respectively. For cancer applications, effective vaccination especially requires the induction of CTL responses thereby necessitating cross-presentation of MHC class I molecules to CD8+ T cells. Additionally, each T cell possesses an inimitable T cell receptor which imparts specificity towards antigen presentation by DC and recognition through the MHC-antigen complex. Hence, loading antigens into DC through different approaches like recombinant vectors, liposomes, nanoparticles, etc. are being explored (Bhardwaj, Bhatia, Sharma, Ahamad, & Banerjee, 2020). Figure 2 depicts the role of DC in immune stimulation via nanovaccines.
Figure 2.
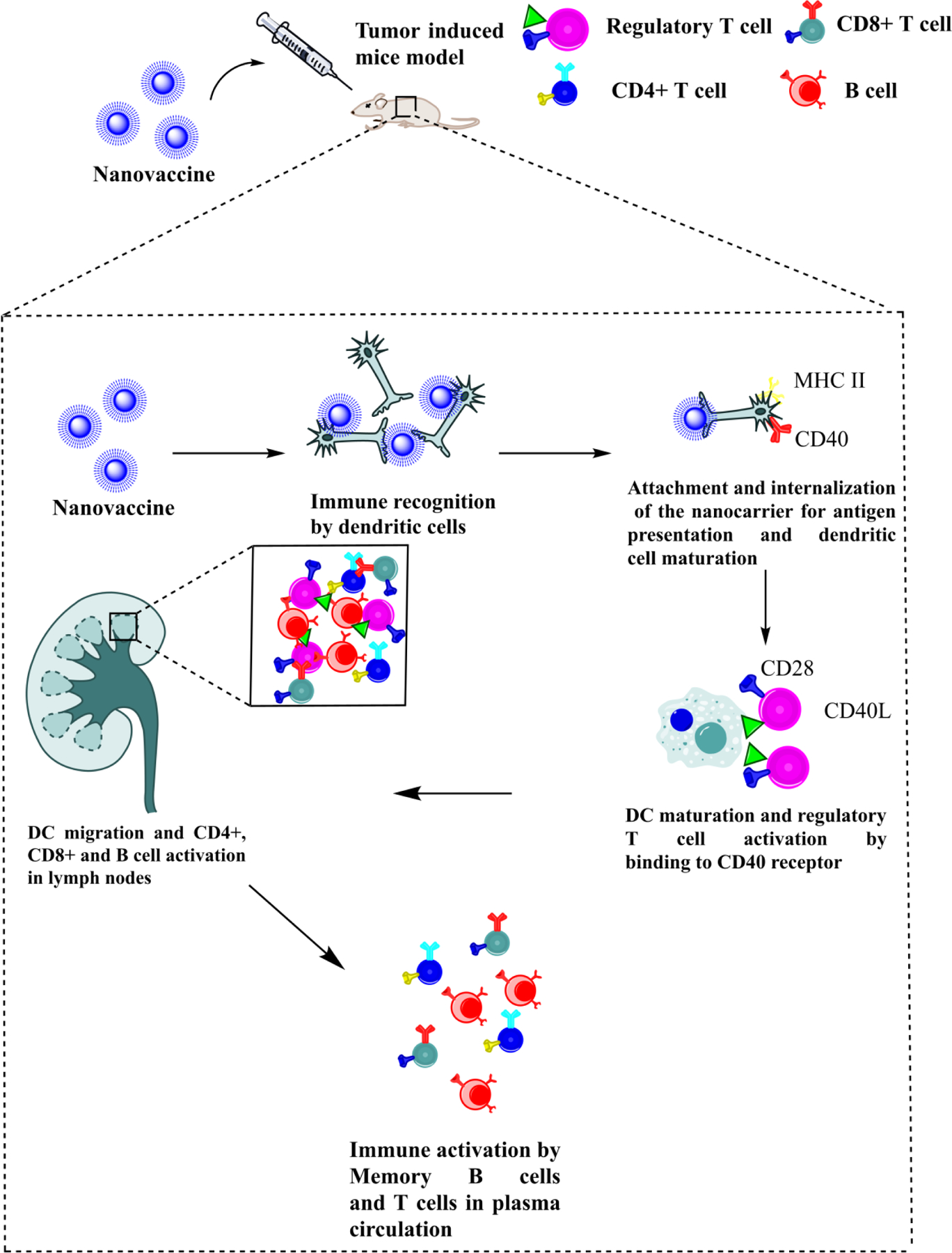
DC interact with the nanovaccine followed by the process of antigen presentation through CD40 and MHC II. Post attachment and antigen presentation of nanocarrier, maturation and migration of DC cell results in regulatory T cell activation in lymph nodes. This further results in immune memory activation via T and B cells.
6. TUMOR ANTIGENS AS VACCINE TARGETS
In brief, anti-tumor vaccine development could be carried out with the help of six categories of antigens which include TAA, tumor lysates, DNA, RNA, subunit and neoantigen based vaccines.
6.1. Recognized TAA
Recognized TAA are specifically overexpressed by tumor cells of a particular origin. Some examples include tyrosinase related proteins (Trp1/Trp2) overexpressed in melanoma, carcinoembryonic antigen in colorectal cancers, prostate specific membrane antigen in prostate cancer, and HER2/neu in breast cancer (Jingjing Liu, Miao, Sui, Hao, & Huang, 2020). Preclinical efficacy has been demonstrated by targeting these antigens (Heck et al., 2017; Murgas et al., 2018; Rahimmanesh & Khanahmad, 2021; Ziogas, Konstantinou, Bouros, Theochari, & Gogas, 2021). However, their clinical efficacy with respect to their applications in vaccine development are yet to be uncovered. The identification of such antigens is difficult and further studies are required to justify the antigen selection, mechanism and efficacy associated with immune response generation and expression levels in different global populations.
6.2. Whole tumor cells
Whole tumor cells or lysates contain TAA and mutations associated with the tumor tissue along with MHC class I and II entities. Loading of tumor lysates with DC prior to vaccination improves immune activation efficacy. The major advantage of using tumor lysates over other classes is its application in personalized medicine. The patients’ own tumor cells can be isolated and coupled with DC in an attempt to reprogram the body’s immune system for personalized cancer cell recognition and apoptosis (Schaller & Sampson, 2017). The dilemma of selecting a suitable TAA amidst a plethora of TAA is resolved when using tumor lysate vaccines. However, clinical success has been dampened due to prospective tumorigenic toxicity pitfalls and inadequate antigen concentrations leading to T cell tolerance (Jianping Liu, Zhang, & Xu, 2019; Lollini, Cavallo, Nanni, & Forni, 2006).
6.3. DNA based vaccines
DNA based vaccines consist of plasmids engineered to code for diverse tumor specific antigens within healthy host cells with other immunomodulatory mediators to influence the subsequent immunogenic reaction against the tumor. With several virtues like scalability, marketability and safety, a DNA vaccine coding for prostatic acid phosphatase along with GM-CSF as an adjuvant were verified in a phase II clinical trial against prostate cancer (Colluru, Johnson, Olson, & McNeel, 2016). In spite of the encouraging clinical trial results, the efficiency of bare DNA vaccines is restricted by diminished transfection in vivo, necessitating optimal delivery conditions or systems (J. Li & Kataoka, 2021).
6.4. mRNA vaccines
In recent times, mRNA vaccines have captivated remarkable attention attributed to their rapid production, target specificity and safety. They are produced by transcription carried out with the help of a bacteriophage RNA polymerase and template DNA that translates the antigen(s) of interest and upregulates proteins in the cytoplasm to provoke immune reactions (Blakney, Ip, & Geall, 2021). mRNA vaccines are being emphasized as a favourable vaccine approach in the near future, and numerous mRNA-based vaccines have entered clinical phase of development. mRNA vaccines possess quite a few advantages over contemporary vaccines. mRNA vaccines promote the distribution of specific TAAs, and the immune response is predictable with relatively diminished risk of infection and mutagenesis. Additionally, they are amenable to rapid, inexpensive and scalable manufacturing (Gómez-Aguado et al., 2020). Conversely, the instability and inefficiency of mRNA could be circumvented with the help of nanotechnology approaches to render stability and targeting efficiency.
6.5. Subunit peptide vaccine
Out of all the vaccine strategies available, subunit peptide vaccines are the most frequently employed strategy in vaccine development. Sipuleucel-T was the first peptide based vaccine for prostatic tumors which was ratified by the US Food and Drug Administration (FDA) along with various subunit vaccines for colorectal, lung, pancreatic, gastric and breast cancer (A.E., D.H., B.J., & P., 2011). Despite their successes in the clinic, subunit vaccines suffer from major pitfalls like weak immunogenicity, antigen escape and short duration immune responses (Moyle, 2017).
6.6. Personalized vaccines and neoantigens
The development of new gene sequencing techniques has paved the way for personalized vaccines targeting neoantigens. Neoantigens are defined by somatic gene mutations in tumor cells which are acknowledged as foreign antigens by the immune system. Targeting neoantigens reduces immune tolerance and tissue toxicity thereby improving selective antitumor immune responses. To date, there are primarily two categories of personalized vaccines, i.e. RNA vaccines and synthetic long peptide vaccines (X. Chen, Yang, Wang, & Liu, 2020). Tailored RNA mutanome vaccines established by Sahin et al. and peptide vaccines prepared by Ott and his colleagues show encouraging phase I trial outcomes (Shetty & Ott, 2020; Vormehr, Diken, Türeci, Sahin, & Kreiter, 2020). Figure 3 describes the various types of nanovaccines for cancer immunotherapy.
Figure 3.
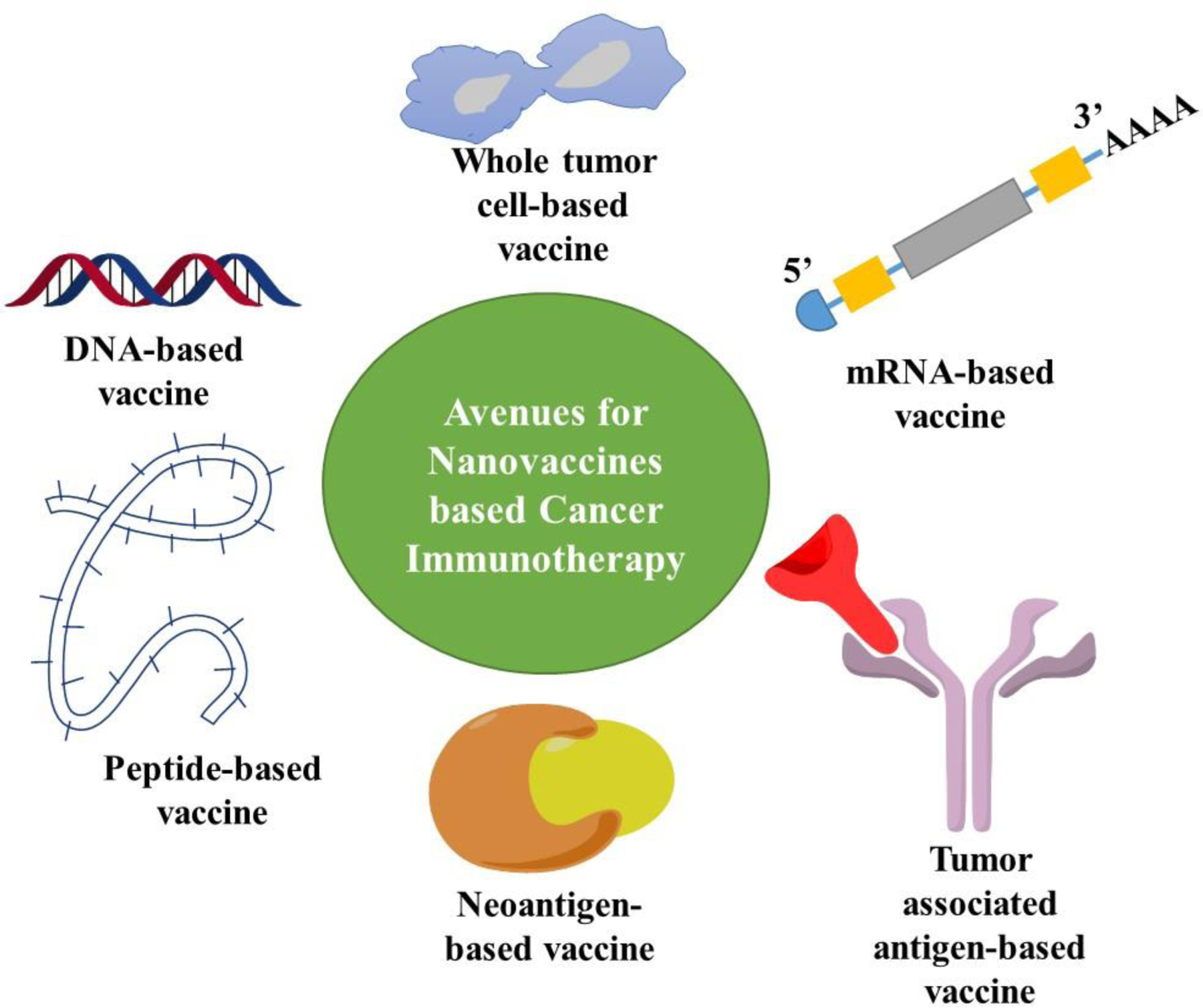
Avenues for nanovaccine-based cancer immunotherapy.
7. ADVENT OF NANOMEDICINE IN CANCER VACCINE DEVELOPMENT
The commencement of the nanomedicine era has brought exhilarating opportunities in the fields of biotechnology and vaccine delivery. DNA and mRNA-based vaccines, which primarily suffered from poor immunological responses due to inadequate internalization and rapid degradation, have been successfully incorporated into the nanoparticulate matrix and circumvented said drawbacks. Protein subunit vaccines suffer from diminished immunogenic activity and quick plasma degradation. These problems can be ameliorated with the help of nanomaterial carriers which can be tuned to protect the vaccines from degradation and enhance the duration of response. As a general rule, nanocarriers with size less than 10 nm are leaked into the systemic circulation, while nanocarriers lying between the size range of 10–100 nm drain into the lymph nodes and remain localized within the lymph nodes for prolonged periods of time. Nanocarriers greater than 100 nm are taken up by APC which then migrate to the lymph nodes for further activation (Guizhi Zhu, Zhang, Ni, Niu, & Chen, 2017). Advantages of nanoscale materials lie in their enhanced surface area, ease of internalization and antigen presentation, and their ability to escape renal filtration. Additionally, employment of immune-adjuvants, multi-epitope antigens decorated over the nanocarrier surface could help boost immunity during circulation. Nanovaccines differ from immune-adjuvants e.g. toll-like receptor agonists wherein, nanovaccines, themselves are the ones which initiate highly specific immune responses towards targeted cells (Huang, Ge, Liu, Li, & Zhang, 2022). TLR agonists merely help in the amplification of the immune responses against target cells (Van den Boorn & Hartmann, 2013). New horizons in cancer vaccine development are opening due to factors such as the incorporation of antigens within targeted nanomaterials allowing for site-specific immunization, smart release profiles/sustained release, and protection from enzymatic degradation during circulation. For instance, hydroxy propyl methacryl amide based nanoparticles loaded with TLR7/8a improved the immunogenicity by approximately 400-fold compared to unformulated small molecule 7/8a (Lynn et al., 2015). Various nanocarriers utilized to enhance the efficacy of tumor vaccines are described as follows:
7.1. Liposomes
Liposomes are multi-lamellar vesicular structures made up of physiologically derived phospholipids and cholesterol. The composition and type of phospholipids used, vesicular size, surface charge, surface functionalization, etc. influence their efficiency as vaccine subunit carriers. Bilayers consisting of immunogenic phospholipids like cardiolipin can enhance the immunogenic potential of the cancer vaccinse (Sprott, Dicaire, Gurnani, Deschatelets, & Krishnan, 2004). Phospholipids with cationic surface charge present exciting opportunities in the field of vaccine delivery. Dileep and co-workers studied the impact of cationic liposomes on DC immuno-stimulation for delivering human papilloma virus antigens. They found that positively charged lipids with short saturated hydrophobic chains exhibited enhanced immunostimulatory activity (Vangasseri et al., 2006). Varypataki and colleagues performed a comparative study between the efficiency of cationic liposomes and poly (lactic-co-glycolic acid) (PLGA) nanoparticles for long peptide-based vaccine delivery and DC stimulation in vivo. They demonstrated that long chain peptide enriched cationic liposomes were the most proficient carriers for T cell stimulation in vivo in comparison to PLGA nanoparticles (Varypataki et al., 2016). Liposomes are susceptible to enzymatic degradation in the liver which diminishes the sustained effect and therapeutic efficacy of nanovaccines (Mahjub et al., 2018). In such cases, employment of lipid polymer hybrids could impart stability, controlled release and sustained immunostimulation circumventing the drawbacks of conventional liposomes (Raemdonck, Braeckmans, Demeester, & De Smedt, 2014; Shah, Rangaraj, Singh, & Srivastava, 2021). Recently, Su et al. prepared cationic polymer–lipid hybrid liposomes as carriers for delivering anionic antigen epitopes such as toll-like receptor-9 agonist (TLR9), CpG (AE/CpG), indoleamine-2,3-dioxygenase (IDO) inhibitor, and 1-methyl-tryptophan (1-MT), to enhance their immunogenicity potential. The primed liposomes boosted vaccine uptake by DC and remarkably increased the amount of CD86+MHC-I+DC, ensuing in a powerful CTL reaction to counter B16F10-OVA tumor cells in vitro. In vivo efficacy was improved with the combinatorial immunotherapy in comparison with monotherapy leading to a resilient tumor specific T cell reaction depicting the potential of cationic liposomes in cancer immunotherapy (Su et al., 2021). Liposomes have the potential to form complexes known as lipoplexes for stabilization and loading of nucleic acids via electrostatic interactions which could help promote endosomal uptake and decrease premature degradation (Z. Liu et al., 2021). A study performed by Loira-Pastoriza and co-workers investigated the antitumor activity of CpG motifs and polyinosinic - polycytidylic acid dsRNA loaded liposomes against metastatic lung cancer. Cationic liposomal incorporation of nucleic acids enhanced lung phagocytic uptake leading to TLR stimulation within endosomes. The liposomes could proficiently entrap polyinosinic - polycytidylic acid dsRNA while effectively loaded CpG enhanced IFN-γ levels. Pulmonary administration of CpG diminished the tumor progression by promoting the secretion of granzyme B, IFN-γ, MIG (monokine induced by interferon-γ also known as chemokine ligand CXCL9) and RANTES (chemokine receptor type 5), and increase T helper type 1 cells in the lungs (Loira-Pastoriza et al., 2021).
Coupling of liposomes to ligands for active targeting could improve anti-tumor efficacy by redirecting the immunostimulation directly into the TME (Gao, Yang, Xu, Qiu, & Zhai, 2021). Yang and colleagues employed the principles of active targeting by employing cRGD peptide anchored liposomes for targeting αvβ3 integrin receptor overexpressed in cancer cells. With the help of thymidine conjugate after activation by ultra-violet radiation in the form of cancer vaccine, liposomes and cRGD targeted liposomes enhanced intracellular accumulation resulting in superior cytotoxic potency post UV activation. This approach further helped in the initial detection of immunogenic cell death markers including ATP, HMGB1 and calreticulin. In vivo vaccination challenge revealed active tumor growth inhibition implying the safety and efficacy in generating an immunogenic response selectively against tumor cells (R. Yang, Wang, Yuan, Qian, & Zhou, 2019).
Liposomes possess the intrinsic ability to encapsulate and protect nucleic acids for increased antigen generation and an elongated therapeutic profile. For instance, Mai et al. prepared cationic liposome-protamine mRNA vaccine complexes and evaluated their antitumor activity in a Lewis lung cancer model. They showed that stable ionic complexes could be formed from binding of cationic protamine with the anionic mRNA, and that nasal incorporation of the vaccines which displayed significantly improved tumor cell apoptotic efficiency in vitro, stimulating DC maturation, enhanced CD86+, CD8+, and CD4+ T cells and increasing IL-2 and TNF-α levels. Intra-nasal administration with spherical cationic liposomes consisting of mRNA expressing cytokeratin 19 triggered strong immune reaction and diminished tumor progression in an aggravated Lewis lung cancer model (Figure 4 A-F) (Mai et al., 2020). Zhang and colleagues incorporated mRNA into liposomes with cholesterol functionalized with cationic peptide DP7 (VQWRIRVAVIRK). The DP7-C-functionalized dioleoyl-3-trimethylammonium propane (DOTAP) liposomes could transfer mRNA efficiently into DC in vitro. As an immune-adjuvant, DOTAP/DP7-C liposomes showed greater efficacy in stimulating DC maturation, CD103+ DC antigen presentation and pro-inflammatory cytokine secretion than DOTAP liposomes in vitro and in vivo. In vivo studies demonstrated tremendous tumor growth inhibition with antigen-specific lymphocyte reactions (R. Zhang et al., 2020). Yang et al. devised a DNA-based neoantigen vaccine using whole-exome sequencing with bio-informatics based prediction of neo-epitopes and fabrication of liposome-encapsulated multi-epitope DNA vaccine. Efficient uptake by DC promoted significant melanoma growth and lung metastasis inhibition (p < 0.0001) in vivo. Intra-tumoral infiltration of CD8+ T cells with melanoma-specific apoptosis proved the efficiency of multiepitope neoantigen DNA liposome vaccines as a promising approach for personalized cancer immunotherapy (X. Yang et al., 2021).
Figure 4.
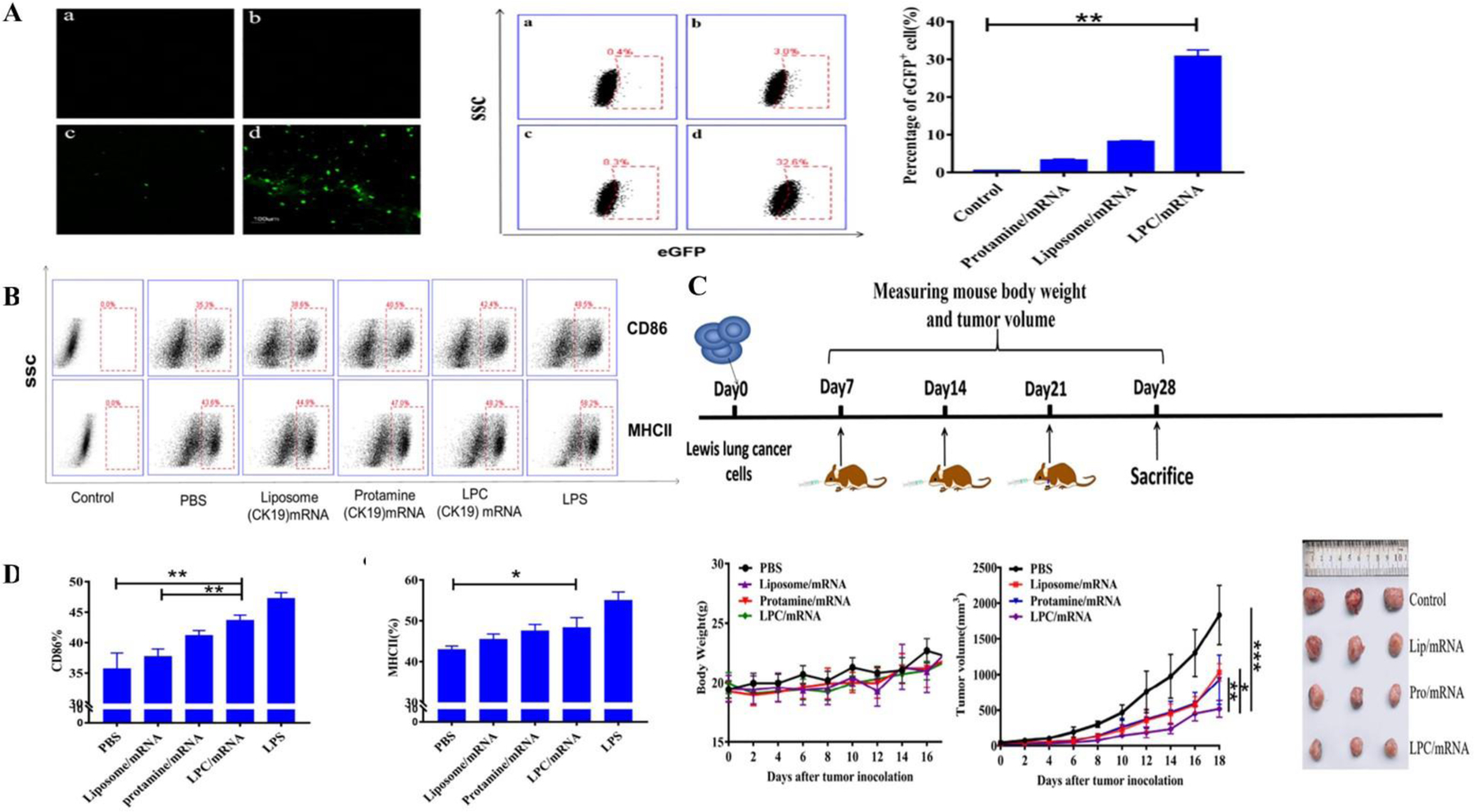
A – Qualitative and quantitative eGFP expression using confocal microscopy and flow cytometry indicating enhanced expression using liposome-protamine complex in DC2.4 cells (p < 0.01); B, D) Flow cytometry for estimation of enhanced CD86+ and MHC II in bone marrow derived dendritic cells; C) Antitumor efficacy in terms of change in tumor volume, body weight and tumor size in a Lewis lung cancer mice model indicating significant tumor volume reduction in the cationic liposome protamine group compared to liposome mRNA (p < 0.01), protamine mRNA (p < 0.05) and control group (p < 0.001). Reprinted with permission from Mai et al. (Mai et al., 2020). Copyright © 2020. Elsevier Ltd.
7.2. Lipid nanoparticles
Lipid based nanoparticles are composed of solid lipid (s) and/or an amalgamation of solid and liquid lipids stabilized with the help of surfactants in an aqueous dispersion. During the last few years, lipid nanoparticles have sparked remarkable interest in immunotherapy owing to improved biocompatibility, sustained release and prolonged circulation time. The use of cationic lipids in the lipid matrix substantially increases their immunogenicity and antigen presentation, but subsequently increases nanoparticle toxicity. Enhancing the surface hydrophilicity via PEGylation can further prolong the systemic circulation; however, this may diminish the immunogenic potency (Kolate et al., 2014). It may also result in the development of anti-PEG antibodies which further attack the nanocarriers and attenuate vaccine efficiency in repeat dosing (P. Zhang, Sun, Liu, & Jiang, 2016). Therefore, a balance must be maintained between the safety and efficacy of the prepared nanoparticles. Sayour and co-workers demonstrated that tumor RNA incorporated within a cationic charged DOTAP-lipid nanoparticulate matrix activated the majority of systemic and tumor-specific myeloid cells (categorized by co-expression of PD-L1 and CD86). Immune checkpoint inhibitors enhanced the intra-tumoral PD-L1+ CD8+ cells and facilitated synergistic anti-tumor efficacy. Clinical translational studies displayed the efficiency of personalized mRNA incorporated nanoparticles which were found to be safe and effective in malignant glioma. The pervasive immune stimulation from nanoparticles with inducible PD-L1 expression could be explored in the near future for potent and safe personalized nanomedicines (Sayour et al., 2018). Persano and co-workers developed a lipopolyplex mRNA-based vaccine containing a poly-(β-amino ester) polymer mRNA core incorporated within a 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine/1,2-dioleoyl-sn-glycero3-phosphatidyl-ethanolamine/1,2-distearoyl-sn-glycero-3-phosphoethanolamine -N- [amino (polyethylene glycol)-2000 (EDOPC/DOPE/DSPE-PEG) lipid shell. This type of core-shell morphology enabled easy entrance into DC via macropinocytosis. Intrinsic adjuvant potency through interferon and IL-12 countenance in DC was mediated with the help of TLR 7/8 signalling. DC subjected to mRNA vaccine showed greater antigen presentation competence. Mice induced with lung metastatic B16-OVA tumors showed greater than 90% reduction of tumor nodules when immunized with an ovalbumin antigen (Persano et al., 2017).
Chen et al. designed a lipidoid nanoparticulate system to accomplish concurrent cross-presentation and STING activation resulting in superior immune activation. From a combinatorial library screen, they recognized 93-O17S-F, a cationic lipidoid promoted both cross-presentation of tumoral antigens and intracellular cGAMP delivery, a STING agonist. Intra-tumoral injection along with pre-treatment of doxorubicin exhibited excellent antitumor efficacy, with 35% of mice demonstrating total recovery from a primary B16-F10 tumor challenge with 71% of those mice showing complete recovery from concurrent challenge indicating immune memory (J. Chen et al., 2021). Cancer stem cells (CSC) are recognized to drive tumor metastasis and recurrence. Aldehyde dehydrogenase was used as a marker by Najafabadi and colleagues for CSC isolation. ALDH1-A1 and ALDH1-A3 epitopes from CSC were used to develop synthetic high-density lipoprotein nanodiscs for vaccination. Nanodiscs promoted antigen trafficking to lymph nodes thereby producing robust T cell responses and exerted potent antitumor efficacy and prolonged animal survival in murine models (Hassani Najafabadi et al., 2020). Similarly, Scheetz et al. fabricated high-density lipoproteins loaded with CpG, a TLR 9 agonist, and tumor-specific neoantigens for glioma treatment. In combination with a PDL1 immune checkpoint blocker, the nanodiscs eliminated orthotopic GL261 glioma in 33% of mice. Complete tumor remission with the presence of immunological memory during re-challenge experimentation in the contralateral hemisphere indicated the development of immune memory (Scheetz et al., 2020). Munakata and co-workers prepared lipid nanoparticles functionalized with type-A CpG oligonucleotides. Either intratumoral or intravenous administration of the lipid nanoparticles repressed tumor development in a CD8+ T cell-dependent manner while plain oligonucleotides displayed no effectiveness. Tumor suppression was found to be linked with Th-1 gene induction and stimulation of CD8+ T cells. Co-delivery of lipid nanoparticles with anti-PD-1 antibodies enhanced the therapeutic efficiency. Furthermore, the therapeutic dose failed to produce any incidences of apparent liver toxicity indicating the safety of the formulation (Munakata et al., 2019). Yu et al. prepared self-assembling melittin loaded lipid nanoparticles promoting whole tumor antigen release and activation of APC in lymph nodes. This led to increased dendritic and macrophage cell proliferation as shown in Figure 5H. In comparison with plain melittin, the lipid nanoparticles significantly improved lymph node accumulation and APC stimulation resulting in 3.6-fold greater CD8+ T cell activation with enhanced immunostimulatory cytokine/chemokine levels as shown in figure 5I. In a bilateral B16F10 tumor model in C57BL/6 mice, primary and distant tumor growth were suppressed with 95% and 92% inhibition rates, respectively (Figure 5 A-J) (Yu et al., 2020).
Figure 5.
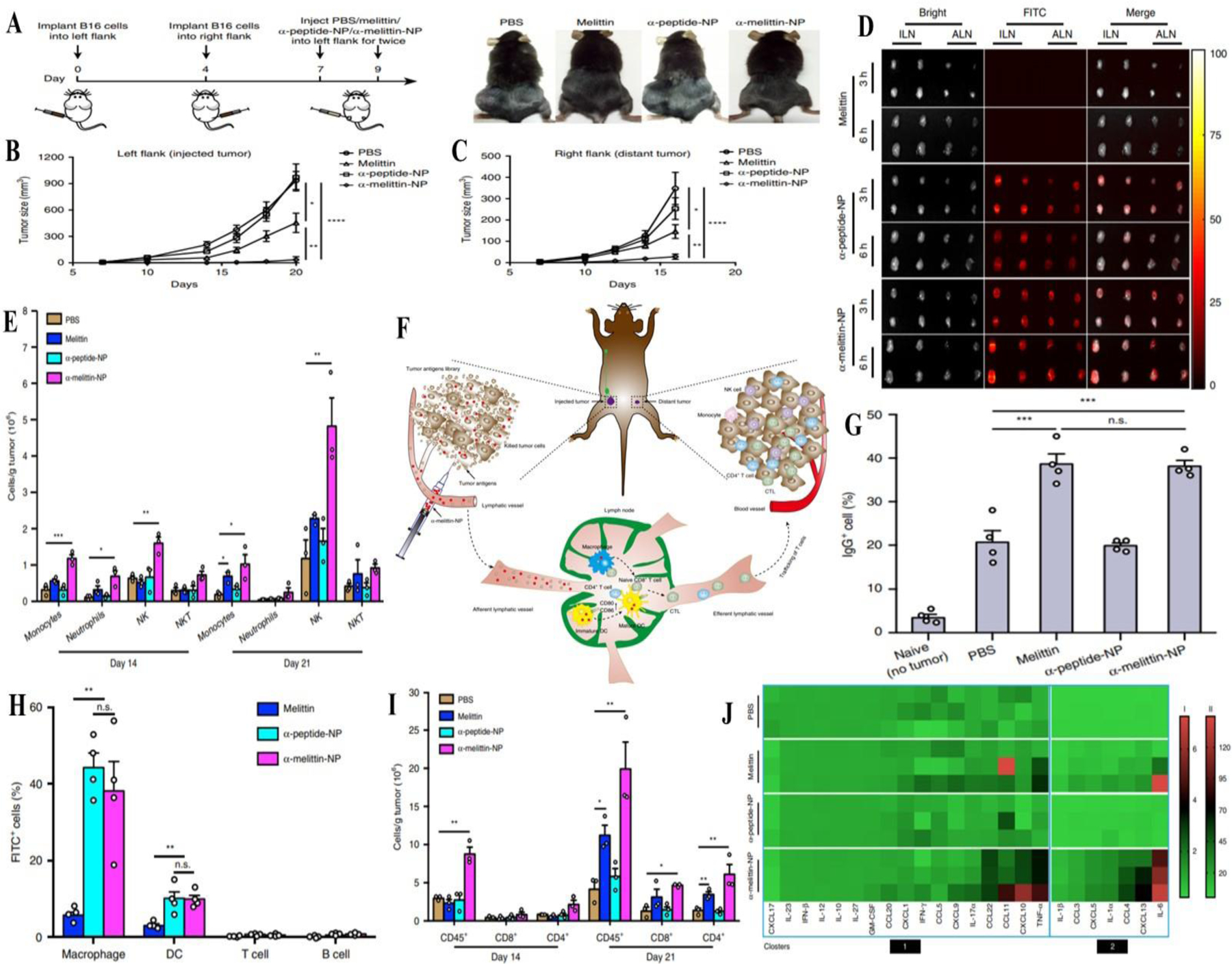
A) Schematic representation of treatment of B16F10 melanoma tumors in mice inoculated in the left and right flanks (n=10 mice per group); B) Tumor size distribution in left flank for PBS, melittin, α-peptide and α-melittin nanoparticles; C) Tumor size distribution in right flank for PBS, melittin, α-peptide and α-melittin nanoparticles; D) Fluorescence images of excised lymph nodes of C57BL/6 mice post subcutaneous injection; E) Immune cell count at the 14th and 21st day following immunization; F) Schematic representation of vaccine effect in situ; G) IgG antibody count in different treatment groups; H) Flow cytometry assisted immune cell count; I) Cluster of differentiation cell count at 14th and 21st day following immunization; J) Cytokine/chemokine levels in the TME. Reprinted from Yu et al. (Yu et al., 2020) licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/legalcode). Copyright © 2020.
7.3. Polymeric nanoparticles
Polymeric nanoparticles serve as versatile vaccine delivery systems. Ease of controlling surface attributes, enhanced stability and protection from systemic degradation, prolonged systemic circulation along with extended release resulting in sustained immunogenic response, etc. bestow polymeric nanoparticles with the attributes for an ideal vaccine delivery system. The FDA has approved polymer nanoparticle formulations containing polyethylene glycol (PEG) and PLGA as they contain these desired characteristics (Wen, Umeano, Kou, Xu, & Farooqi, 2019). Kim and colleagues introduced unique TLR 7/8 bi-specific agonists that considerably boosted cytokine release in comparison with TLR7 mono-selective compounds. Entrapment in PLGA nanoparticles enhanced the co-stimulatory molecular upregulation and antigen demonstration via MHC I by DC in comparison with the soluble agonist. Following subcutaneous administration, the nanoparticles travelled to the draining lymph nodes and elicited DC stimulation and development. This resulted in the proliferation of antigen-specific CD8+ T cells and improved CTL response, which led to substantial therapeutic efficacy in melanoma, bladder and renal carcinoma models as shown in Figure 6E. Notably, their experiments demonstrated successful rejection of metastasis showcasing the therapeutic efficacy of their polymeric nanovaccines (Figure 6 A-G) (H. Kim et al., 2018). Lou and co-workers prepared ligands for TLR 7/8 with antigen decorated virus mimicking nanoparticles. Cationic polymers with azide/bicycle nonyne (BCN) groups were prepared which successfully stabilized anionic ssRNA via electrostatic stabilization. The model antigen ovalbumin and a mannosylated or galactosylated BCN-decorated HPMA-based co-polymer were linked with the RNA core via disulfide linkages utilizing the principles of copper free click chemistry. The surface mannosylated nanoparticles demonstrated 5-fold greater DC uptake and activation in comparison to the galactosylated nanoparticles. Vaccination of mice with mannosylated nanoparticles provoked resilient CTL and humoral responses towards the ovalbumin antigen (Lou et al., 2019). Luo et al. reported a simple admixture of an antigen and synthetic polymeric nanoparticle (PC7A) was successful in generating a robust CTL response with effective cytosolic delivery of tumor antigens to the lymph nodes. This resulted in enhanced surface presentation leading to concomitant stimulation of type I interferon activated genes and STING, which is independent of TLR or the mitochondrial antiviral-signalling protein (MAVS) pathway. The nanovaccine exhibited effective tumor growth reduction in melanoma, colonic cancer and HPV-E6/E7 induced tumor models. The synergy between PC7A and anti-PD-1 antibody displayed 100% survival during the 60-day duration of the experiment. Re-challenging tumor-free animals with TC-1 cells led to complete tumor growth inhibition indicating the emergence of prolonged anti-tumor memory. STING-activating nanovaccines may be an easy, safe and effective tactic in enhancing immunity with potential implications in cancer immunotherapy (Luo et al., 2017).
Figure 6.
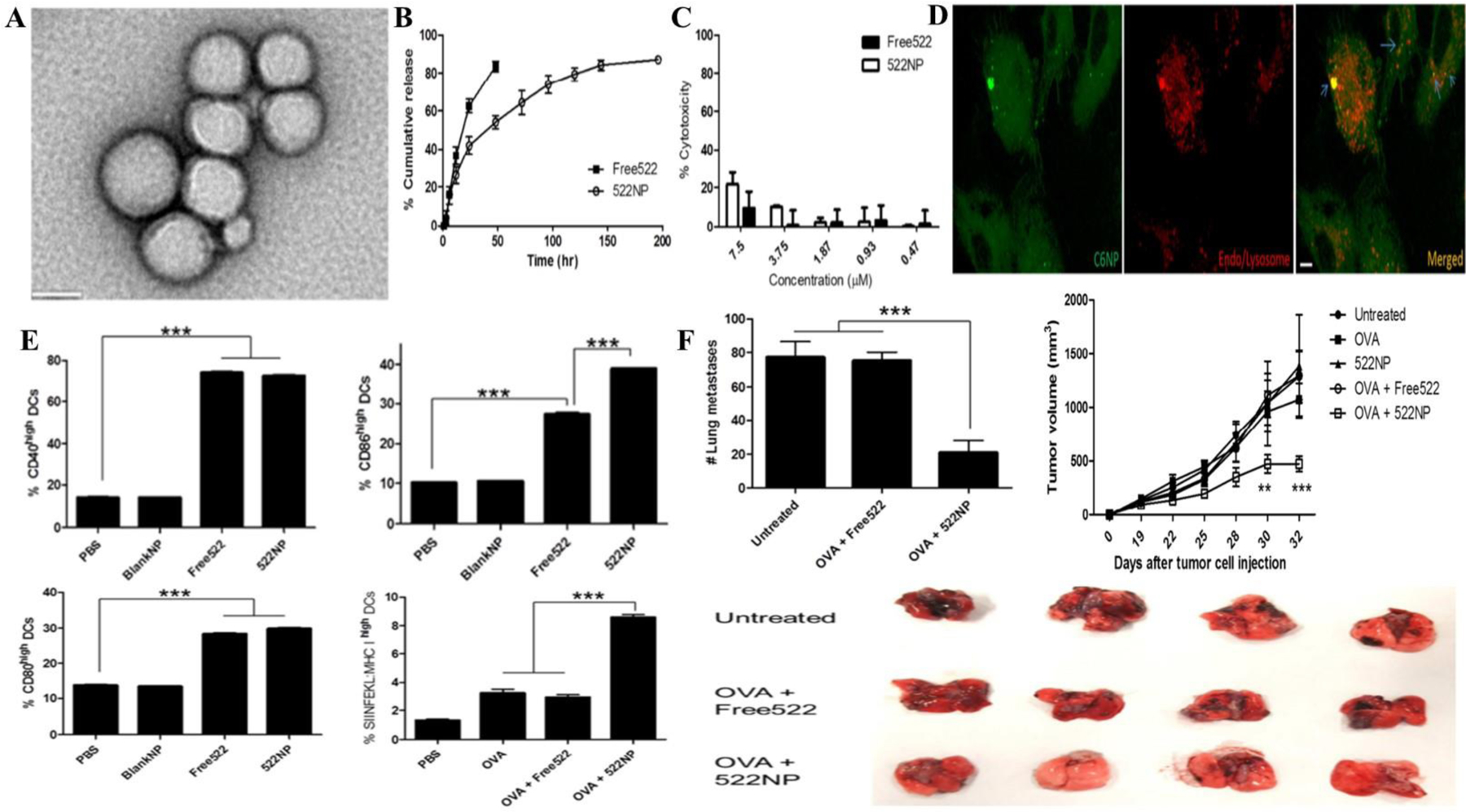
A) TEM image of TLR agonist loaded PLGA nanoparticles; B) Sustained drug release imparted by polymeric nanoparticles for 200 h compared to free drug; C, D) Enhanced cytotoxicity of loaded nanoparticles compared to free drug against peripheral blood mononuclear cells at 48 h due to enhanced intracellular accumulation; E) Flow cytometry studies of CD40, CD86, SIINFEKL peptide and CD80 on bone marrow derived dendritic cells by ovalbumin and combination with 522 loaded nanoparticles (p < 0.001) compared to control group; F) Lung metastasis and tumor inhibition on B16F10 tumor mice model. Reprinted with permission from Kim et al. (H. Kim et al., 2018). Copyright © 2018. Elsevier Ltd.
A novel approach adopted by Min and co-workers included the utilization of antigen capturing nanoparticles for tumor-specific delivery. They fabricated antigen-capturing nanoparticles with successful delivery of tumor-specific proteins to APC and substantially improving αPD-1 targeting efficiency. In a B16F10 melanoma model, survival was increased by 20% compared to the control group (p < 0.005). Antigen-capturing nanoparticles promoted robust activation of CD8+ CTL with elevated CD4+ T/Treg and CD8+ T/Treg ratios. Substantial abscopal effect was observed when the immunotherapeutic intervention was combined with radiotherapy (Min et al., 2017). Ni and colleagues fabricated a bi-adjuvant neoantigen nanovaccine made up of a PEG-PLA micellar core and a CpG decorated shell that co-delivered a peptide neoantigen (adpgk) with TLR - 7/8 agonist R848 for cancer immunotherapy. The immunogenicity of the neoantigen was potentiated by the co-delivery of the neoantigen and dual adjuvants. Co-administration with anti-immune checkpoint PD-1 resulted in complete regression in 70% of neoantigen-specific tumors without recurrence (Ni et al., 2020). Another innovative strategy adopted by Xiao et al. combines personalized immunization through neoantigen-loaded nanovaccines with adoptive DC transfer. The prepared nanovaccines elicited chemokine release of CCL2, CCL3, and C-X-C motif ligand 10 from macrophages and improved macrophage lymph node infiltration. Coordinated neoantigen and autologous tumor lysate-derived antigen delivery resulted in antitumor patient-specific T cell immunity. Significant inhibition of tumor growth in prophylactic and established mouse tumor models could pave the way as a proof of concept for personalized immunotherapeutic alternative treatments (P. Xiao et al., 2021).
7.4. Inorganic nanoparticles
Inorganic nanoparticles are element-based nanostructures lying within the range of 1–100 nm. Ease of surface functionalization, tenability with respect to size, shape and charge, etc. render them suitable drug delivery agents. The predominant difference between inorganic nanoparticles and other delivery systems lies in the foundation of the protein corona around the nanoparticle interface (Shah et al., 2021). Fogli and colleagues employed the formation of cancer cell lysate corona over the nanoparticulate surface to boost the immunogenic potential. They produced gold and silica nanoparticles which were further exposed to two whole cancer cell lysates, i.e. hepatic and ovarian cancer cell lines as shown in figure 7 E. They found that the nanoparticles incubated with the cancer cell lysates formed a spontaneous protein corona around their surface which promoted DC-mediated lymphocyte proliferation (Figure 7 A-F) (Fogli et al., 2017). Zhao and co-workers employed superparamagnetic iron oxide nanoparticles (SPION) as a platform for vaccine delivery and immunogenicity induction. Interaction with cytokine secretion in macrophages and DC in vitro as well as tumor growth in vivo was observed. Co-delivery of ovalbumin and the iron oxide nanoparticles led to increased immune response and CT26 tumor inhibition in comparison to the individually-injected controls (p < 0.05). Iron oxide nanoparticles prominently promoted the stimulation of immune cells and cytokine production, promoting strong humoral and cellular reactions. These results implicate the attributes of inorganic nanoparticles with promising potential in the field of tumor immunotherapy (Y. Zhao, Zhao, Cheng, Guo, & Yuan, 2018). Nanomaterial-based tumor photo-thermal therapy (PTT) has emerged as an attractive therapeutic alternative owing to its site-specific cytotoxicity and non-invasiveness. However, thermotherapy solely cannot prevent tumor progression, metastasis and recurrence. Therefore, Wang and colleagues employed surface-functionalized and tumor antigen adsorbed copper sulfide nanoparticles with hyperthermia by thermal mediation. Modifying CuS nanoparticles with maleimide-PEG strengthened antigen adsorption, and combination therapy with an immune checkpoint blocker and hyperthermia elevated the inflammatory cytokines levels with increased mobilization of tumor-infiltrating CD8+ T cells and inhibition of primary and secondary tumors in a 4T1 breast cancer tumor model (R. Wang et al., 2019). Zhou et al. fabricated ovalbumin (OVA)-decorated PEGylated MnFe2O4 nanoparticles loaded with R837 immunoadjuvant to synergize photo-thermal and immunotherapy for the management of breast cancer. The prepared inorganic nanoparticles elicited substantial immune responses in vitro as well as in vivo. Reduction of systemic immunosuppression through downregulation of M2-associated cytokines accompanied with laser irradiation inhibited tumor growth and lung metastases compared to saline control (p < 0.0001) improving survival (B. Zhou et al., 2020). Table 2 displays the summary of recently reported nanovaccines in cancer immunotherapy.
Figure 7.
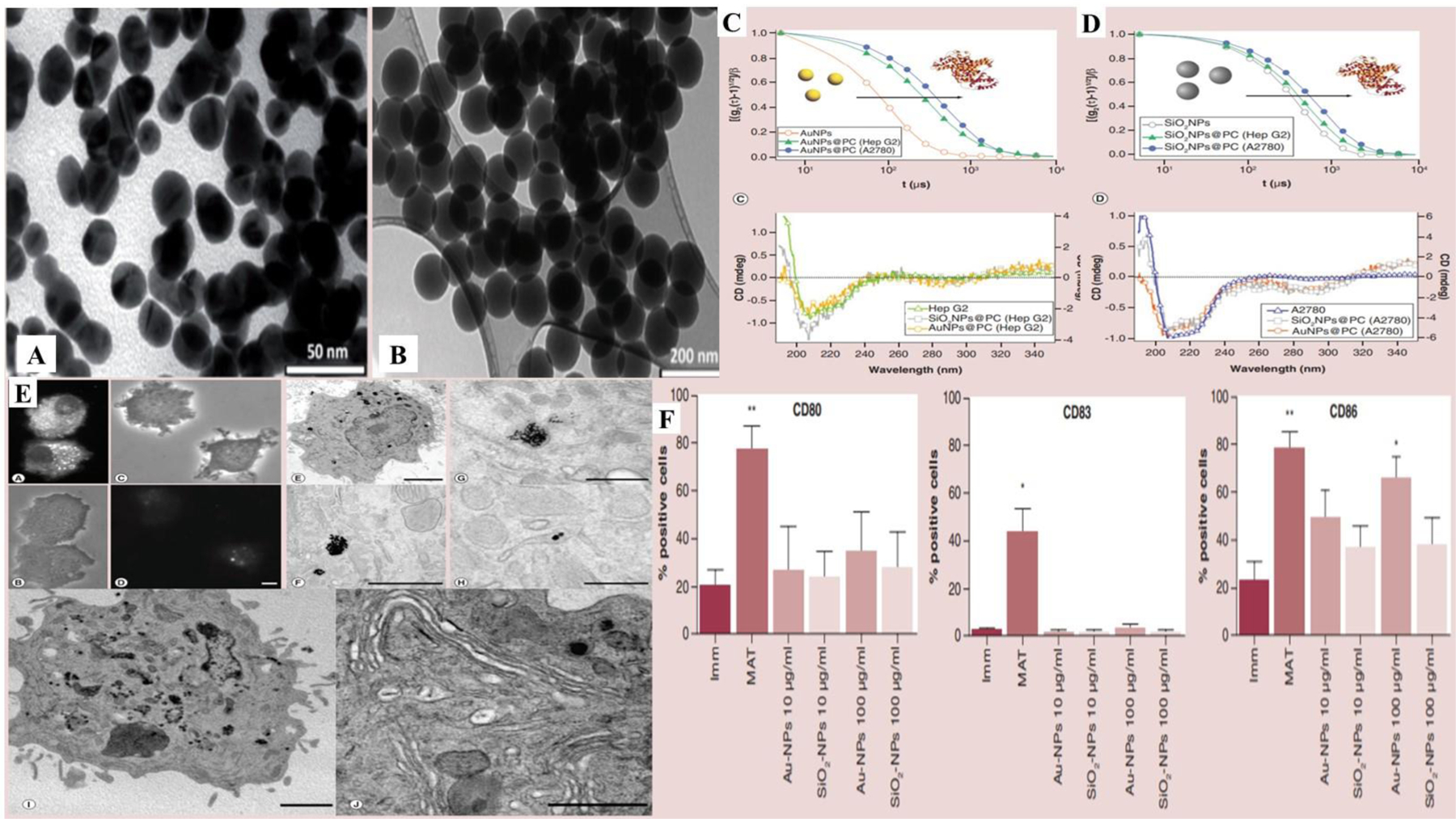
A), B) TEM images of gold and silica nanoparticles, respectively; C) Protein corona characterization via dynamic light scattering and circular dichroism spectra of gold nanoparticles on HepG2 and A2780 cells; D) Protein corona characterization via DLS and circular dichroism spectra of silica nanoparticles on HepG2 and A2780 cells; E) Interaction of protein corona of nanoparticles with DC indicated by fluorescence microscopy (A and B – Rhodamine B loaded nanoparticles without protein corona) and (C and D – Rhodamine B loaded nanoparticles with protein corona) after 24 h; (E and F - gold nanoparticles) and (G and H – gold nanoparticles with protein corona) at 2 μm (E), 1 μm (F), 500 nm (G), 100 nm (H), 2 μm (I) and 1 μm (J). Vesicular endocytic uptake in Golgi area with delayed endosome formation. F) Maturation of DC leading to enhanced CD80+, CD83+ and CD86+ at 10 and 100 ug/mL concentration of gold and silica nanoparticles versus control group (p < 0.01). Reprinted with permission from (Fogli et al., 2017). Copyright © 2017.
Table 2.
Summary of recent advancements in nanovaccines for cancer immunotherapy
| Incorporated moiety | Nanocarrier system | Mechanistic insights | Reference |
|---|---|---|---|
| Chicken ovalbumin (OVA241–270) | pH-sensitive proton-driven polymeric peptide nano-transformer based vaccine | In the presence of an acidic endosomal microenvironment, the nanotransformer vaccine alters its morphology from nanospheres (100 nm diameter wide) into sheets (few μm in length) disturbing the endosomal membrane and delivering the antigenic peptide into the cytoplasm thereby boosting immunity. It proficiently inhibited tumor growth in the B16F10-OVA and human papilloma virus-E6/E7 induced tumor models in mice. Merging the nano-transformer attributes with anti-PD-L1 antibodies resulted in survival greater than 83 days with complete tumor regression in half of the mice. | (Gong et al., 2020) |
| Peptide based neoantigen (adpgk) | PEG-PLA nanoparticles | The immunogenic potential of the neoantigen was substantially enhanced by proficient co-delivery of neoantigen and two adjuvants. Immune checkpoint pathways with PD-1 on T cells were sensitized leading to 70% neoantigen specific tumor regression without recurrence. | (Ni et al., 2020) |
| Curcumin and CpG-ODN | Thermo-sensitive polymeric nanoparticles | Thermo-sensitive curcumin containing polymer nanoparticles incorporated within the hydrogel matrix promoted immunogenic cell death and subsequently improved tumor immunogenicity in vivo. The cancer nanovaccine consisted of CpG-ODN and cationic polymer which activated DC and provoked strong vaccine-mediated T cell immune reactions. In the presence of malignant breast carcinoma 4T1 models, the combination immunotherapy augmented the host T cell immunity, encouraged the accumulation of CTL within the tumor, and diminished tumor recurrence and pulmonary metastasis. | (Xiang Liu et al., 2020) |
| Ovalbumin | Mesoporous silica-polyethyleneimine (PEI) nanoparticles coated with a metal phenolic network | The pH and reduction dual-sensitive nanovaccine core contained PEI-amended mesoporous silica nanoparticles encapsulated with ovalbumin and the shell was composed of a disulfide bond-involved metal-phenolic network. Smart release in the presence of glutathione and not in neutral phosphate buffer along with in vitro cellular assays indicated the increase in ovalbumin uptake by DC along with lysosomal escape attributed to the proton sponge effect. In vivo studies revealed that the prepared nanoparticles prompted a substantial tumor-specific immune response. | (X. Zhou et al., 2020) |
| Ovalbumin | Cationic fluoropolymer nanoparticles | Nanoparticles prepared by an admixture of the cationic fluoropolymer with ovalbumin caused DC maturation via TLR 4-mediated signalling and encouraged antigen translocation within the cytosol of DC leading to effective antigen cross-presentation. Mixture of the fluoropolymer and cell membranes from primary tumors injected alongside checkpoint inhibitors inhibited post-surgical tumor recurrence and metastasis in two skin cancer models and an orthotopic breast cancer model. | (Xu et al., 2020) |
| CpG oligonucleotide | Lipid nanoparticles | CpG-embedded lipid nanoparticles demonstrated potent antitumor efficiency in prophylactic as well as therapeutic E.G7 tumor models. The vaccine promoted T cell exhaustion by enriching PD-1 expression resulting in tumor recurrence. Combination with anti-PD-1 antibody led to similar therapeutic efficacy compared to the nanovaccine administered individually. Adequate therapeutic efficacy after first cycle of immunization with the nanovaccine resulted in tumor relapse suppression indicating checkpoint blockade therapy. | (Y. Kim et al., 2020) |
| Ovalbumin | MnO2 and polydopamine nanoparticles | The prepared nanoparticles demonstrated outstanding anticancer activity against orthotopic melanoma and could additionally prevent liver metastasis in a tumor re-challenge mouse model. The relocation of DC in the inguinal lymph node was observed by magnetic resonance imaging implying successful DC initiation and immunity generation. | (B. Xiao et al., 2021) |
| Ovalbumin and CpG ODN | Graphene oxide and PEI | The graphene oxide-PEI nanocomposite vaccine increased DC induction and maturation, antigen cross presentation and cytokine responses against B16F10 melanoma tumors demonstrating prolonged survival time. | (L. Zhang et al., 2022) |
| Ovalbumin | Mn2+ ions and meso-2,6-diaminopimelic acid (DAP) | Stimulation of DC by Nod1 pathway with migration into lymph nodes was detected by MRI and fluorescence imaging in vivo. Substantial prophylactic as well as anti-tumor activity against B16F10 tumors was observed. | (H. Zhao et al., 2019) |
| Ovalbumin and CpG | Polyamidoamine dendrimer modified with guanidine-benzoic acid | The dendrimer based nanovaccines showed outstanding prophylactic activity with excellent therapeutic potential indicated by 40% survival up to 60 days compared to control group which showed complete mortality within 30 days. | (Xu et al., 2019) |
7.5. Virus and protein-based vaccines
Viruses and protein-based nanoparticles represent another class of cancer nanovaccines. Virus-based nanoparticles range from mammalian viruses (e.g. gene delivery vectors and mammalian oncolytic viruses) to bacteriophages and plant viruses (Figure 8) (Chung, Cai, & Steinmetz, 2020). They can be viruses engineered with specific functions, native viruses (also termed viral nanoparticles or VNPs) or virus-like particles (VLPs), which are genome-free. Protein nanoparticles (PNPs) and protein cages are comparable to VLPs in their multivalent protein organization. Prominent examples of PNPs include ferritin, E2, heat shock proteins (HSPs), and protein vaults (Figure 8) (Neek, Kim, & Wang, 2019).
Figure 8. Biological unit structures of different protein-based nanovaccines.
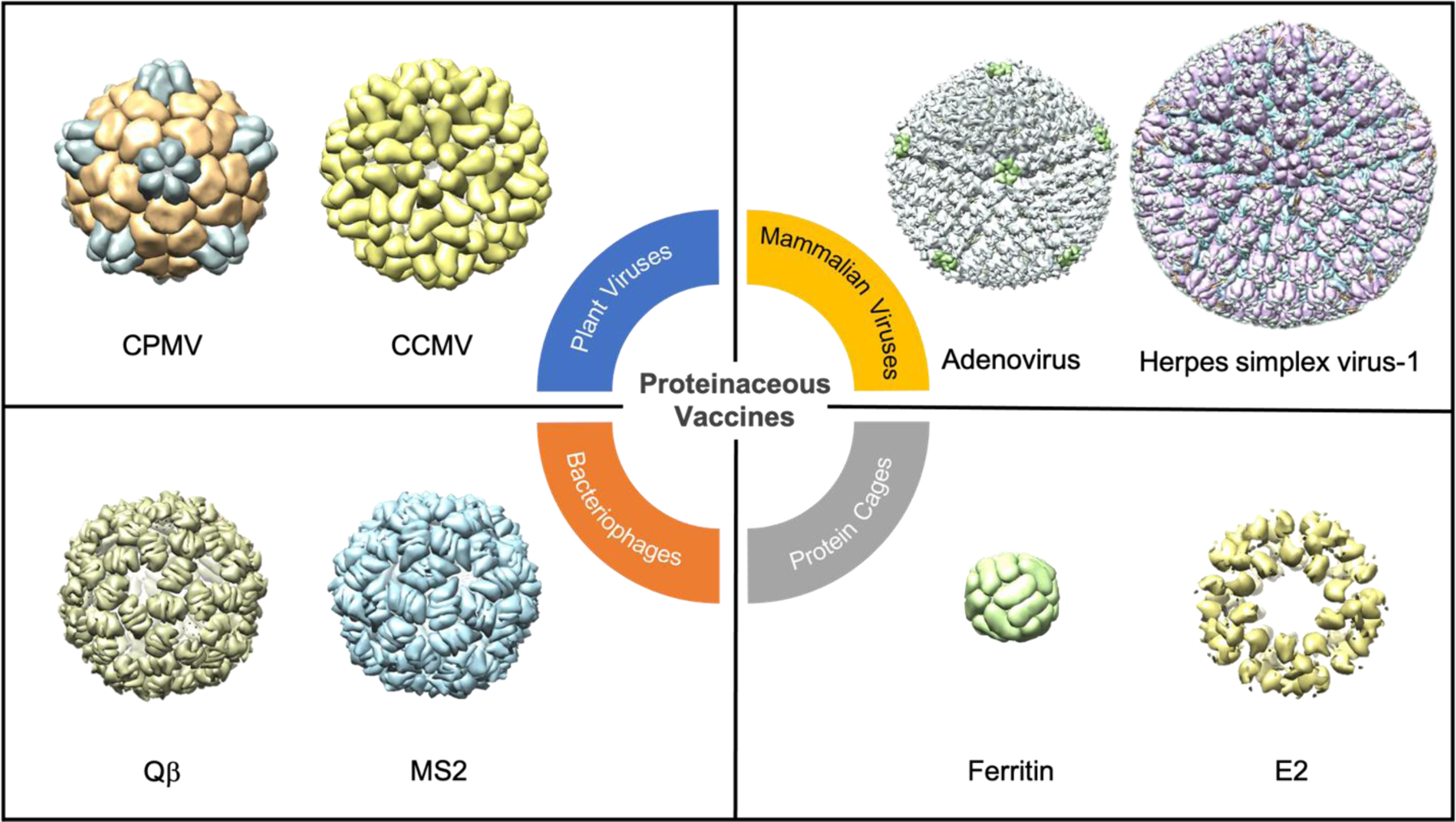
Proteinaceous vaccines can range from plant viruses (top left), mammalian viruses (top right), bacteriophages (bottom left), to protein cages (bottom right). All the viruses other than the mammalian viruses are drawn to scale with one another while the two mammalian viruses are drawn to scale to each other. All biological units were drawn on Chimera. CPMV = cowpea mosaic virus (PDB ID: 1NY7), CCMV = cowpea chlorotic mottle virus (PDB ID: 1ZA7), adenovirus (PDB ID: 6CGV), herpes simplex virus-1 (PDB ID: 6CGR), Qβ (PDB ID: 1QBE), MS2 (PDB ID: 2MS2), ferritin (PDB ID: 2HFA), E2 (PDB ID: 6H5).
VNPs and PNPs can be functionalized through genetic engineering, bio-conjugation or self-assembly and can be produced at scale through fermentation, molecular farming, and cell culture. For immunotherapy and vaccines, VNPs and PNPs are advantageous due to their inherent immunogenicity/adjuvanticity, biocompatibility, biodegradability, and direct uptake and processing by APC thereby stimulating innate and adaptive immune responses. Their repetitive, multivalent structures are recognized as pathogen-associated molecular patterns (PAMPs), and binding to pattern recognition receptors (PRRs) on immune cells instigates the innate immune response providing immunostimulatory adjuvant capabilities (Chung et al., 2020; Chung, Church, et al., 2021; Warnock, Merten, & Al-Rubeai, 2006).
An emerging direction of virus-based cancer vaccines is the direct in situ injection of the immunostimulatory agent into the tumor (Figure 9 A). In situ vaccination leads to reprogramming of the TME from a “cold” to a “hot” tumor leading to better recruitment of innate immune cells to kill tumor cells. The TAA and neoantigens which become released are then processed by these innate immune cells, which then activates the adaptive arm of the immune system and potentiates systemic protection. With the approval of talimogene laherparepvec (T-VEC), being sold under the brand name Imlygic, in situ viral vaccine therapy is now used clinically leading the way for potentially a multitude of other VNPs such as vaccinia, measles, and polio-based viruses to be approved in the future (Lawler, Speranza, Cho, & Chiocca, 2017). TVEC is an oncolytic herpes simplex virus type I which also encodes for GM-CSF and is sanctioned for the treatment of advanced melanoma (Bommareddy, Patel, Hossain, & Kaufman, 2017). However, with oncolytic therapies, there is the possibility of reversion to a virulent form and antibodies can hamper the effectiveness of future doses (Aurelian, 2013). As an alternative, plant viruses have been proposed for in situ vaccination approaches; while they are non-infectious in mammals, thereby adding an extra layer of safety, they still present PAMPs and are thus potent immune stimulators. Cow pea mosaic virus (CPMV) has been extensively studied in the context of in situ vaccination, and the drug candidate was shown to be effective against murine models of melanoma, ovarian, glioma, colon, and breast cancer (Cai, Wang, Shukla, & Steinmetz, 2019; Kerstetter-Fogle et al., 2019; Lizotte et al., 2016; Shukla et al., 2020; C. Wang, Fiering, & Steinmetz, 2019) as well as in canine patients with spontaneous melanoma (Hoopes et al., 2018). CPMV is also not hampered by previous antibody production, and in fact, antibodies against the virus may improve in situ immunotherapy (Shukla et al., 2020). While previous efforts utilize CPMV as an in situ vaccine; recently, CPMV has also been targeted to the lungs to treat and prevent metastatic breast cancer and melanoma (Chung, Park, Cai, & Steinmetz, 2021). Other plant viruses such as the alfalfa mosaic virus as well as the papaya mosaic virus are also effective as in situ vaccines (Lebel et al., 2016; Shahgolzari, Dianat-Moghadam, & Fiering, 2021).
Figure 9.
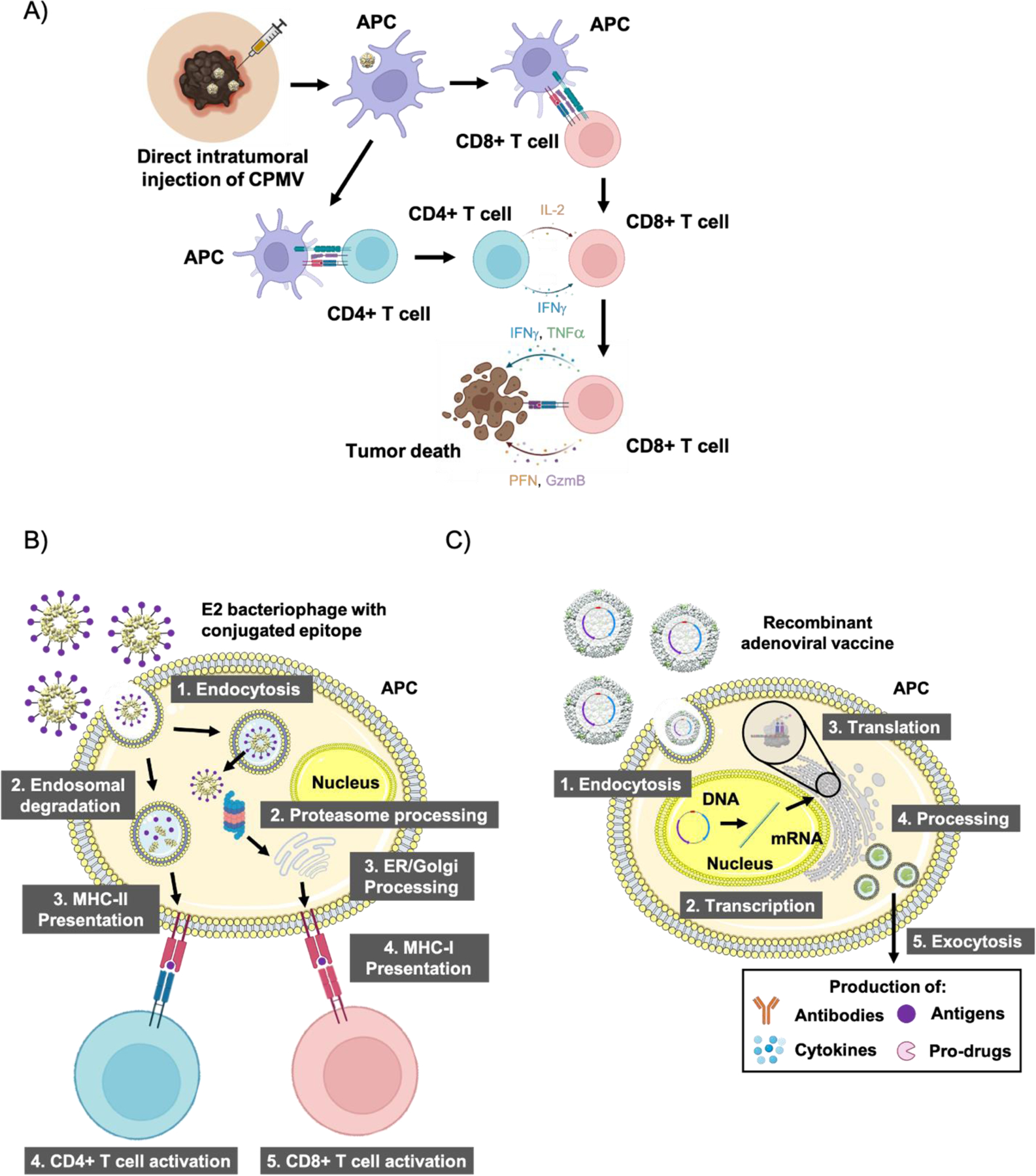
Different strategies to promote tumor killing using proteinaceous nanovaccines. A) Direct in situ injection of CPMV initiates an innate immune response, which goes on to potentiate a systemic adaptive response against the tumor causing tumor death. B) TAA can be conjugated to the outer capsid of bacteriophages such as E2, which can initiate both CD4+ and CD8+ T cell reactions through both MHC-II and MHC-I epitope presentation, respectively. C) Viral vectors can carry plasmids which instruct cells to produce a range of proteins such as antibodies, TAA, cytokines/chemokines, and enzymes that go on to promote tumor cell death with varying mechanisms. Abbreviations: CPMV = cowpea mosaic virus, APC = antigen presenting cell, CD4+ = cluster of differentiation 4, CD8+ = cluster of differentiation 8, IL-2 = interleukin-2, IFNγ = interferon-γ, TNFα = tissue necrosis factor α, PFN = perforin, GzmB = granzyme B, MHC-I = major histocompatibility complex class I, MHC-II = major histocompatibility complex class II, ER = endoplasmic reticulum.
Lastly, the bacteriophage Qβ with a CpG TLR9 agonist and nivolumab has completed phase II clinical trials for in situ vaccination against Stage IIIB/C/D melanoma patients and is currently being explored in other combination therapies against various cancers. Similarly, to plant viruses, Qβ also benefits from previous antibody formation against the viral capsid. In fact, the treatment protocol and publication indicate that presence of antibodies is required to enable efficient immune cell uptake and activation. In the clinic, patients are pre-immunized against Qβ to prime their immune systems to recognize and target the Qβ. Another example of a VLP packaging and protecting a TLR agonist is the plant virus cowpea chlorotic mottle virus (CCMV) loaded with CpG; this formulation used as an in situ vaccine demonstrated efficacy against murine models of colon cancer and melanoma (Cai, Shukla, & Steinmetz, 2020).
As mentioned earlier, TAA can be co-delivered with protein nanovaccines through multiple mechanisms; antigens can be conjugated to the interior or exterior, encapsulated within, or genetically conjugated to the capsid (Figure 9 B) (Chung et al., 2020). This diversity in antigen functionalization capabilities promotes the use of protein nanovaccines in a multitude of different applications. One such example is the use of bacteriophages, specifically AP205 displaying the human epidermal growth factor receptor-2 (HER2), which is expressed in 20–30% of invasive breast cancers (Palladini et al., 2018). Here the VLP acts as a display, delivery, and adjuvant platform. Multivalent display allows high payload delivery while the VLP carrier acts as an adjuvant due to PRR recognition and presence of T helper epitopes – these features hold true for many VLPs (K. L. Lee, Twyman, Fiering, & Steinmetz, 2016). Others have utilized plant viruses such as CPMV and the Physalis mottle virus to display HER2 epitopes (Hu & Steinmetz, 2021; Shukla et al., 2017). In other approaches, bacteriophage Qβ has also been utilized to deliver tumor-associated carbohydrate antigens such as Tn (GalNAcα1-Ser/Thr) (Yin et al., 2015). Similarly, PNPs such as ferritin, E2, and protein vaults have also been explored as cancer vaccines (Kar et al., 2012; B. R. Lee et al., 2016; Molino, Anderson, Nelson, & Wang, 2013). One example utilizes E2 nanoparticles to deliver both NY-ESO-1 and MAGE-A3 epitopes with synergistic effect (Neek et al., 2018).
A class of PNPs called the heat shock proteins (HSPs) naturally bind to endogenous tumor antigens and can be extracted from tumor lysates for cancer vaccination (Y. Zhang & Zheng, 2013). While non-immunogenic themselves, HSPs act as chaperone proteins that transport polypeptides and aid in the folding of these polypeptides within cells. Therefore, in tumor cells, the HSPs form non-covalent complexes with TAA polypeptides, and these peptides become recognized by immune cells to instigate tumor-specific immune responses. Multiple clinical trials utilizing such HSPs are currently underway (Co., 2018, 2019). Similarly, patient’s tumors following surgery can be irradiated and used as the antigen source and be combined with an appropriate adjuvant. Recently it was shown that the plant virus CPMV could be utilized for this purpose as CPMV mixed with irradiated ovarian cancers cells caused 72% rejection of murine ovarian cancer challenge (Stump et al., 2021).
Instead of directly delivering protein antigens or peptide epitopes thereof, VNPs can be engineered to deliver genes encoding TAA, cytokines/chemokines, pro-drug activators, immunotherapies, and others (Figure 9 C) (Wilson, 2005). Recombinant adenoviruses (rAds) have especially been investigated for this role with many in clinical trials. rAds delivering prostate-specific antigens against prostate cancer (Lubaroff, 2019a, 2019b), human papilloma virus (HPV) against HPV-induced cancers (Çuburu et al., 2018), and melanoma-associated antigen 3 against melanoma (Biologics, 2021) have all been explored. Alternatively, VNPs delivering cytokines have advanced into the clinic. For instance, T-VEC encodes for GM-CSF which helps to augment the T cell response. An engineered Wyeth strain vaccinia virus developed by Jennerex Inc. called JX-594 also encodes for GM-CSF (Bommareddy et al., 2017) and is injected intratumorally against hepatocellular carcinoma, and an orphan drug designation has been conferred by the US FDA (Merrick, Ilett, & Melcher, 2009). Outside of cytokine expression, a Measles virus was engineered to express antibodies against checkpoint proteins like cytotoxic T-lymphocyte-associated protein 4 and PD-L1, which showed strong efficacy against human melanoma xenografts (Engeland et al., 2014). A rapidly expanding field, an entire generation of engineered mammalian oncolytic viruses have entered clinical testing and comes with it hopes for new viral therapeutic nanovaccines (Galanis, Kirn, & Liu, 2007).
While mammalian viral therapies have advanced further into clinical testing, plant viruses are also being explored as gene-delivery vectors. CCMV has been used to deliver self-amplifying mRNA encoding an ovalbumin epitope, which demonstrated a significant increase in ovalbumin-specific T cell activation (Biddlecome et al., 2019). Similarly, the tobacco mosaic virus (TMV) coat protein can encapsidate foreign RNA as long as it contains the TMV origin of assembly sequence. A Semliki Forest virus vector carrying the gene for the β-galactosidase antigen was encapsulated within TMV, which showed improved humoral and cellular responses compared to controls (Smith et al., 2007). The same group utilized TMV to encapsidate Flock House virus (FHV) RNA encoding eGFP in planta – the vaccine was able to produce significantly greater titres against eGFP compared to negative controls and in vitro produced TMV-FHV-eGFP constructs (Y. Zhou, Maharaj, Mallajosyula, McCormick, & Kearney, 2015).
8. ROLE OF ADJUVANTS IN NANOVACCINES
The aim of vaccination is to induce defensive immunity, and adjuvants are used in most vaccines to optimize the potent and protective immune responses. Vaccines consist of an antigenic component that mimics the pathogen to trigger immunity and an adjuvant component that dictates the efficacy and amount of immune reaction to the antigen (Awate, Babiuk, & Mutwiri, 2013). There are several proposed mechanisms by which adjuvants act to enhance immunogenicity of vaccines: (1) adjuvant act as a depot for extended release termed the depot effect, (2) adjuvants initiate proliferation of cytokines and chemokines, (3) adjuvants cause cellular employment at the site of injection, (4) adjuvants enhance antigen uptake and presentation to APC (5) adjuvants initiate activation and maturation of APC via increasing MHC class II and co-stimulatory molecule expression and (6) adjuvants activate inflammasomes (Awate et al., 2013). Although commonly used with vaccines to increase efficiency, only a few adjuvants have been developed and approved as a marketed component in vaccines in the US. Different types of compounds can be utilized as adjuvants that includes mineral salts, microbial products, emulsions, saponins, cytokines, polymers, microparticles, nanoparticles, and liposomes (Guy, 2007). Depending on the mechanism of action, vaccine adjuvants are classified into two types - delivery systems and immune-stimulatory adjuvants (M. Singh & O’Hagan, 2003). Delivery system adjuvants accumulate and reveal antigens in monotonous sequences, target vaccine antigens to APC and promote localization of antigens and immune potentiators. Cationic microparticles are an example of a delivery system adjuvant. On the other hand, immune potentiator adjuvants stimulate innate immunity directly using cytokines (e.g. IL-2) or through PRR activation (e.g. CPMV) (O’Hagan, 1998).
In nanovaccine formulations, the adjuvant can be extraneously added to enhance immunogenicity or the nanoparticulate formulation itself can act as the adjuvant. Nanoparticles have enormous potential in vaccine development since they have the potential to not only act as an antigen carrier and presenter, but also as an adjuvant allowing for delivery into the same cell and addressing the issues raised by conventional vaccines (Uddin, Kouzi, & Hussain, 2015). Nanoparticulate vaccine formulations offer a number of advantages. Nanoparticles act as effective delivery system adjuvants with enhanced uptake of antigens by APC like DC or macrophages (Walter et al., 2001). Also, nanoparticle based antigen carriers can act as adjuvants by attracting immune cells like macrophages and modulating their size and charge can help in antigen presentation (S. Wang, Sun, & Hou, 2021). Controlled antigen release from their depot not only the augments the duration of the immune response but also improves its quality (Rice-Ficht, Arenas-Gamboa, Kahl-McDonagh, & Ficht, 2010; Thomasin, Corradin, Men, Merkle, & Gander, 1996). In addition, nanoparticulate vaccines protect antigens against environmental and enzymatic degradation (Slütter et al., 2010). This is of profound importance in orally administered vaccine formulations where antigens are required to be protected from the harsh acidic gastrointestinal conditions (O’Hagan, 1998).
Nanoparticles acting as self-adjuvants cross-present the antigen generating CD8+ T cells and eliminate the use of external adjuvants, a clear advantage in terms of safety, cost, and scalability (Jain, Yap, & Irvine, 2005). Nanoparticles can act as antigen carrier and adjuvant (and/or adjuvant carrier). Its ability to carry cargo through chemical conjugation while activating TLR receptors allows for co-delivery of the antigen and adjuvant to the same APC. Therefore, nanoparticle vaccines are advantageous to conventional vaccines, which require external adjuvants such as alum, the most common adjuvant. Alum has been used as an adjuvant for many years; however, recently it has been found that the compound has several adverse effects. Alum carries the risk of autoimmunity, prolonged brain accumulation and inflammation along with neurological complications which could result in profound and widespread adverse effects (Tomljenovic & A. Shaw, 2011). Thus, a nanoparticulate adjuvant vaccine can be less expensive, more efficient, and safe.
9. CONCLUSION
The advent of the era of nanomedicine in immunotherapy has brought about several breakthroughs in the fields of vaccinology and cancer immunotherapy. Clinical outcome of cancer patients is poor, and current therapy suffers from specificity pitfalls, severe adverse effects and high mortality. The interplay of the TME and the immune system with nanomedicine vaccines along with adjuvants plays an important role in how the immune system could be programmed to achieve immunomodulation. Nanovaccines derived from liposomes, lipid nanoparticles, polymeric nanoparticle, inorganic nanoparticles, protein nanoparticles and viruses have showed promising results. The advent of nanomedicines in developing clinically translatable vaccines for potential immunotherapy against cancer is essential. The combined venture of nanotechnology, and vaccine delivery, could inspire new therapeutic product developments in cancer immunotherapy in the near future.
Acknowledgement
The authors would like to acknowledge the research funding support by Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India to National Institute of Pharmaceutical Education and Research (NIPER) Hyderabad.
Funding information
This work was partially supported by National Heart, Lung, and Blood Institute of the National Institutes of Health award, R15HL138718. This work was also funded in part by NIH grants U01CA218292, R01CA274640, R01CA253615 and R01CA224605 as well as CDMRP award W81XWH2010742 (to NFS).
Abbreviations Description
- DC
Dendritic cells
- NK
Natural killer cells
- TLRs
Toll-like receptors
- NOD
Nucleotide-binding oligomerization domain
- RIG-I
Retinoic acid-inducible gene
- MHC
Histocompatibility complexes
- APC
Antigen presenting cell
- TAM
Tumor associated macrophages
- MDSC
Myeloid derived suppressor cells
- Treg
Regulatory T cells
- MMP
Matrix metalloproteinase
- CSF-1
Colony-stimulating factor 1
- EGF
Epidermal growth factors
- VEGF
Vascular endothelial growth factor
- ECM
Extracellular matrix
- EMT
Epithelial myo-fibroblast trans-differentiation
- PDL-1
Expressed programmed cell death ligand 1
- TAA
Tumor associated antigens
- TLR-9
Toll-like receptor-9 agonist
- IDO
Indoleamine-2,3-dioxygenase
- 1-MT
1-methyl-tryptophan
- CTL
Cytotoxic T-lymphocyte
- SPION
Superparamagnetic iron oxide nanoparticles
- PD-1
Programmed death receptor 1
- PLGA
Poly (lactide-co-glycolide)
- PEG
Polyethylene glycol
- PBS
Phosphate buffered saline
- TEM
Transmission electron microscopy
Footnotes
Conflict of Interest
Dr. Steinmetz is a co-founder of, has equity in, and has a financial interest with Mosaic ImmunoEngineering Inc. Dr. Steinmetz serves as Director, Board Member, and Acting Chief Scientific Officer, and paid consultant to Mosaic. The other authors declare no potential conflicts of interest.
Contributor Information
Saurabh Shah, Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, INDIA..
Paras Famta, Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, INDIA..
Vinod Tiwari, Department of Pharmaceutical Engineering, & Technology, Indian Institute of Technology, Banaras Hindu University, Varanasi, INDIA.
Arun K Kotha, Department of Pharmaceutical Sciences, College of Pharmacy, Mercer University, Atlanta, GA, USA.
Rama Kashikar, Department of Pharmaceutical Sciences, College of Pharmacy, Mercer University, Atlanta, GA, USA.
Mahavir Bhupal Chougule, Department of Pharmaceutical Sciences, College of Pharmacy, Mercer University, Atlanta, GA, USA.
Young Hun Chung, Departments of Bioengineering, University of California, San Diego, La Jolla, CA 92093, USA..
Nicole F. Steinmetz, Departments of Bioengineering, NanoEngineering, Radiology, Moores Cancer Center, Center for Nano-ImmunoEngineering, Institute for Materials Discovery and Design, University of California, San Diego, La Jolla, CA 92093, USA
Mohammad Uddin, Department of Pharmaceutical Sciences, College of Pharmacy, Mercer University, Atlanta, GA, USA.
Shashi Bala Singh, Department of Biological Sciences, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, INDIA.
Saurabh Srivastava, Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, INDIA..
References
- A.E., H., D.H., C., B.J., A., & P., S. (2011). Cancer immunotherapy: Sipuleucel-T and beyond. Pharmacotherapy, 31(8), 813–828. Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L362273685%5Cnhttp://pharmacotherapyjournal.org/doi/pdfplus/10.1592/phco.31.8.813%5Cnhttp://dx.doi.org/10.1592/phco.31.8.813%5Cnhttp://bj7rx7bn7b.search.serialssolutions.com?sid=EMBA [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott M, & Ustoyev Y (2019). Cancer and the Immune System: The History and Background of Immunotherapy. Seminars in Oncology Nursing, 35(5). 10.1016/j.soncn.2019.08.002 [DOI] [PubMed] [Google Scholar]
- Adityan S, Tran M, Bhavsar C, & Wu SY (2020). Nano-therapeutics for modulating the tumour microenvironment: Design, development, and clinical translation. Journal of Controlled Release, 327, 512–532. 10.1016/j.jconrel.2020.08.016 [DOI] [PubMed] [Google Scholar]
- Alshehri S, Imam SS, Rizwanullah M, Akhter S, Mahdi W, Kazi M, & Ahmad J (2021). Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: Preclinical promise and translational challenges. Pharmaceutics, 13(1), 1–35. 10.3390/pharmaceutics13010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JD, Newnes HV, McDonnell A, Bosco A, & Waithman J (2021). Fine-Tuning the Tumour Microenvironment: Current Perspectives on the Mechanisms of Tumour Immunosuppression. Cells, 10(1). 10.3390/cells10010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelian L (2013). Oncolytic virotherapy: the questions and the promise. Oncolytic Virotherapy, 19. 10.2147/ov.s39609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awate S, Babiuk LA, & Mutwiri G (2013). Mechanisms of action of adjuvants. Frontiers in Immunology, 4(MAY). 10.3389/fimmu.2013.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barati M, Akhondi M, Mousavi NS, Haghparast N, Ghodsi A, Baharvand H, … Hassani SN (2021). Pluripotent Stem Cells: Cancer Study, Therapy, and Vaccination. Stem Cell Reviews and Reports 10.1007/s12015-021-10199-7 [DOI] [PMC free article] [PubMed]
- Bhardwaj P, Bhatia E, Sharma S, Ahamad N, & Banerjee R (2020). Advancements in prophylactic and therapeutic nanovaccines. Acta Biomaterialia, 108, 1–21. 10.1016/j.actbio.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddlecome A, Habte HH, McGrath KM, Sambanthamoorthy S, Wurm M, Sykora MM, … Gelbart WM (2019). Delivery of self-amplifying RNA vaccines in in vitro reconstituted virus-like particles. PLoS ONE, 14(6). 10.1371/journal.pone.0215031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biologics T (2021). MG1-MAGEA3 With Ad-MAGEA3 and Pembrolizumab in Patients With Previously Treated Metastatic Melanoma or Cutaneous Squamous Cell Carcinoma
- Blakney AK, Ip S, & Geall AJ (2021). An update on self-amplifying mRNA vaccine development. Vaccines, 9(2), 1–26. 10.3390/vaccines9020097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommareddy PK, Patel A, Hossain S, & Kaufman HL (2017). Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. American Journal of Clinical Dermatology, 18(1), 1–15. 10.1007/s40257-016-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Shukla S, & Steinmetz NF (2020). The Antitumor Efficacy of CpG Oligonucleotides is Improved by Encapsulation in Plant Virus-Like Particles. Advanced Functional Materials, 30(15). 10.1002/adfm.201908743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wang C, Shukla S, & Steinmetz NF (2019). Cowpea Mosaic Virus Immunotherapy Combined with Cyclophosphamide Reduces Breast Cancer Tumor Burden and Inhibits Lung Metastasis. Advanced Science, 6(16). 10.1002/advs.201802281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campillo-Davo D, Flumens D, & Lion E (2020). The Quest for the Best: How TCR Affinity, Avidity, and Functional Avidity Affect TCR-Engineered T-Cell Antitumor Responses. Cells, 9(7). 10.3390/cells9071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L, & Kitamura T (2018). Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology, 155(3), 285–293. 10.1111/imm.12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Qiu M, Ye Z, Nyalile T, Li Y, Glass Z, … Xu Q (2021). In situ cancer vaccination using lipidoid nanoparticles. Science Advances, 7(19). 10.1126/sciadv.abf1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jiang X, Zhang Q, Li Q, Zhang X, Zhang M, … Gao D (2022). How to overcome tumor resistance to anti-PD-1/PD-L1 therapy by immunotherapy modifying the tumor microenvironment in MSS CRC. Clinical Immunology, 108962. 10.1016/j.clim.2022.108962 [DOI] [PubMed] [Google Scholar]
- Chen X, Yang J, Wang L, & Liu B (2020). Personalized neoantigen vaccination with synthetic long peptides: recent advances and future perspectives. Theranostics, 10(13), 6011–6023. 10.7150/thno.38742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Cai H, & Steinmetz NF (2020). Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Advanced Drug Delivery Reviews, 156, 214–235. 10.1016/j.addr.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Church D, Koellhoffer EC, Osota E, Shukla S, Rybicki EP, … Steinmetz NF (2021). Integrating plant molecular farming and materials research for next-generation vaccines. Nature Reviews Materials 10.1038/s41578-021-00399-5 [DOI] [PMC free article] [PubMed]
- Chung YH, Park J, Cai H, & Steinmetz NF (2021). S100A9-Targeted Cowpea Mosaic Virus as a Prophylactic and Therapeutic Immunotherapy against Metastatic Breast Cancer and Melanoma. Advanced Science, 8(21). 10.1002/advs.202101796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co., C. and S. B. (2018). A Large-scale Research for Immunotherapy of Glioblastoma With Autologous Heat Shock Protein gp96 Https://Clinicaltrials.Gov/Show/NCT03650257. Retrieved from https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01662728/full
- Co., C. and S. B. (2019). GP96 Heat Shock Protein-Peptide Complex Vaccine in Treating Patients With Liver Cancer. Case Medical Research https://doi.org/10.31525/ct1-nct04206254
- Colbert JD, Cruz FM, & Rock KL (2020). Cross-presentation of exogenous antigens on MHC I molecules. Current Opinion in Immunology, 64, 1–8. 10.1016/j.coi.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colligan SH, Tzetzo SL, & Abrams SI (2020). Myeloid-driven mechanisms as barriers to antitumor CD8+ T cell activity. Molecular Immunology, 118, 165–173. 10.1016/j.molimm.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colluru VT, Johnson LE, Olson BM, & McNeel DG (2016). Preclinical and clinical development of DNA vaccines for prostate cancer. Urologic Oncology: Seminars and Original Investigations, 34(4), 193–204. 10.1016/j.urolonc.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çuburu N, Khan S, Thompson CD, Kim R, Vellinga J, Zahn R, … Schiller JT (2018). Adenovirus vector-based prime-boost vaccination via heterologous routes induces cervicovaginal CD8+ T cell responses against HPV16 oncoproteins. International Journal of Cancer, 142(7), 1467–1479. 10.1002/ijc.31166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio F, Martinez‐Rodriguez V, Schukking M, Cocks A, Broseghini E, & Fabbri M (2021). Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T‐cells and tumour cells. Journal of Extracellular Vesicles, 10(6). 10.1002/jev2.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JH, & Jill M Lenardo MJF (2020). Individualized neoantigen vaccines. Nature Reviews Immunology, 20(11), 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK, … Ungerechts G (2014). CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Molecular Therapy, 22(11), 1949–1959. 10.1038/mt.2014.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famta P, Shah S, Chatterjee E, Singh H, Dey B, Guru SK, … Srivastava S (2021). Exploring new Horizons in overcoming P-glycoprotein-mediated multidrug-resistant breast cancer via nanoscale drug delivery platforms. Current Research in Pharmacology and Drug Discovery, 2, 100054. 10.1016/j.crphar.2021.100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbloom JA, Sousa F, Stevens MM, & Desai TA (2020). Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Advanced Drug Delivery Reviews, Vol. 167, pp. 89–108. 10.1016/j.addr.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogli S, Montis C, Paccosi S, Silvano A, Michelucci E, Berti D, … Romagnoli P (2017). Inorganic nanoparticles as potential regulators of immune response in dendritic cells. Nanomedicine, 12(14), 1647–1660. 10.2217/nnm-2017-0061 [DOI] [PubMed] [Google Scholar]
- Galanis E, Kirn D, & Liu T-C (2007). Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature Clinical Practice Oncology, 4(2), 101. [DOI] [PubMed] [Google Scholar]
- Gao S, Yang X, Xu J, Qiu N, & Zhai G (2021). Nanotechnology for Boosting Cancer Immunotherapy and Remodeling Tumor Microenvironment: The Horizons in Cancer Treatment. ACS Nano, 15(8), 12567–12603. 10.1021/acsnano.1c02103 [DOI] [PubMed] [Google Scholar]
- Gautam A, Chauhan AS, Srivastava A, Jadon C, & Rathi M (2019). Major Histocompatibility Complex Binding and Various Health Parameters Analysis. Smart Healthcare Systems, 151–164. 10.1201/9780429020575-10 [DOI]
- Germic N, Frangez Z, Yousefi S, & Simon HU (2019). Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death and Differentiation, 26(4), 715–727. 10.1038/s41418-019-0297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F, & Lammers T (2018). Tumor targeting via EPR: Strategies to enhance patient responses. Advanced Drug Delivery Reviews, 130, 17–38. 10.1016/j.addr.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Aguado I, Rodríguez-Castejón J, Vicente-Pascual M, Rodríguez-Gascón A, Solinís MÁ, & Del Pozo-Rodríguez A (2020). Nanomedicines to deliver mRNA: State of the art and future perspectives. Nanomaterials, 10(2). 10.3390/nano10020364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, Zhang Y, Teng X, Wang Y, Huo S, Qing G, … Liang XJ (2020). Proton-driven transformable nanovaccine for cancer immunotherapy. Nature Nanotechnology, 15(12), 1053–1064. 10.1038/s41565-020-00782-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeeva O (2018). Cancer-testis antigens: Unique cancer stem cell biomarkers and targets for cancer therapy. Seminars in Cancer Biology, 53, 75–89. 10.1016/j.semcancer.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Gulei D, Mehterov N, Ling H, Stanta G, Braicu C, & Berindan-Neagoe I (2017). The “good-cop bad-cop” TGF-beta role in breast cancer modulated by non-coding RNAs. Biochimica et Biophysica Acta - General Subjects, 1861(7), 1661–1675. 10.1016/j.bbagen.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Guy B (2007). The perfect mix: Recent progress in adjuvant research. Nature Reviews Microbiology, 5(7), 505–517. 10.1038/nrmicro1681 [DOI] [PubMed] [Google Scholar]
- Haist M, Stege H, Grabbe S, & Bros M (2021). The functional crosstalk between myeloid-derived suppressor cells and regulatory t cells within the immunosuppressive tumor microenvironment. Cancers, 13(2), 1–34. 10.3390/cancers13020210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton HR, & Chtanova T (2019). Lymphatic migration of immune cells. Frontiers in Immunology, 10(MAY). 10.3389/fimmu.2019.01168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani Najafabadi A, Zhang J, Aikins ME, Najaf Abadi ZI, Liao F, Qin Y, … Moon JJ (2020). Cancer Immunotherapy via Targeting Cancer Stem Cells Using Vaccine Nanodiscs. Nano Letters, 20(10), 7783–7792. 10.1021/acs.nanolett.0c03414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck MM, Retz M, Tauber R, Knorr K, Kratochwil C, & Eiber M (2017). PSMA-targeted radioligand therapy in prostate cancer. Urologe, 56(1). 10.1007/s00120-016-0274-3 [DOI] [PubMed] [Google Scholar]
- Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, … Fiering SN (2018). Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Molecular Pharmaceutics, 15(9), 3717–3722. 10.1021/acs.molpharmaceut.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, & Steinmetz NF (2021). Development of a virus-like particle-based Anti-HER2 breast cancer vaccine. Cancers, 13(12). 10.3390/cancers13122909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ge X, Liu Y, Li H, & Zhang Z (2022). The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics, 14(6), 1228. 10.3390/pharmaceutics14061228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Yap WT, & Irvine DJ (2005). Synthesis of protein-loaded hydrogel particles in an aqueous two-phase system for coincident antigen and CpG oligonucleotide delivery to antigen-presenting cells. Biomacromolecules, 6(5), 2590–2600. 10.1021/bm0503221 [DOI] [PubMed] [Google Scholar]
- Jia H, Truica CI, Wang B, Wang Y, Ren X, Harvey HA, … Yang JM (2017). Immunotherapy for triple-negative breast cancer: Existing challenges and exciting prospects. Drug Resistance Updates, 32, 1–15. 10.1016/j.drup.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Kar UK, Jiang J, Champion CI, Salehi S, Srivastava M, Sharma S, … Kelly KA (2012). Vault nanocapsules as adjuvants favor cell-mediated over antibody-mediated immune responses following immunization of mice. PLoS ONE, 7(7). 10.1371/journal.pone.0038553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PLR, Sloan AE, & Steinmetz NF (2019). Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers, 11(4). 10.3390/cancers11040515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Niu L, Larson P, Kucaba TA, Murphy KA, James BR, … Panyam J (2018). Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials, 164, 38–53. 10.1016/j.biomaterials.2018.02.034 [DOI] [PubMed] [Google Scholar]
- Kim Y, Kang S, Shin H, Kim T, Yu B, Kim J, … Jon S (2020). Sequential and Timely Combination of a Cancer Nanovaccine with Immune Checkpoint Blockade Effectively Inhibits Tumor Growth and Relapse. Angewandte Chemie, 132(34), 14736–14746. 10.1002/ange.202006117 [DOI] [PubMed] [Google Scholar]
- Kolate A, Baradia D, Patil S, Vhora I, Kore G, & Misra A (2014). PEG - A versatile conjugating ligand for drugs and drug delivery systems. Journal of Controlled Release, 192, 67–81. 10.1016/j.jconrel.2014.06.046 [DOI] [PubMed] [Google Scholar]
- Kurmi B. Das, Patel P, Paliwal R, & Paliwal SR (2020, June 1). Molecular approaches for targeted drug delivery towards cancer: A concise review with respect to nanotechnology. Journal of Drug Delivery Science and Technology, Vol. 57, p. 101682. 10.1016/j.jddst.2020.101682 [DOI] [Google Scholar]
- Lawler SE, Speranza MC, Cho CF, & Chiocca EA (2017). Oncolytic viruses in cancer treatment a review. JAMA Oncology, 3(6), 841–849. 10.1001/jamaoncol.2016.2064 [DOI] [PubMed] [Google Scholar]
- Lebel MÈ, Chartrand K, Tarrab E, Savard P, Leclerc D, & Lamarre A (2016). Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Letters, 16(3), 1826–1832. 10.1021/acs.nanolett.5b04877 [DOI] [PubMed] [Google Scholar]
- Lee BR, Ko HK, Ryu JH, Ahn KY, Lee YH, Oh SJ, … Lee J (2016). Engineered Human Ferritin Nanoparticles for Direct Delivery of Tumor Antigens to Lymph Node and Cancer Immunotherapy. Scientific Reports, 6. 10.1038/srep35182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Twyman RM, Fiering S, & Steinmetz NF (2016). Virus-based nanoparticles as platform technologies for modern vaccines. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 8(4), 554–578. 10.1002/wnan.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer CA (2017). Dendritic cells in host response to biologic scaffolds. Seminars in Immunology, 29, 41–48. 10.1016/j.smim.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Li C, Jiang P, Wei S, Xu X, & Wang J (2020). Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer, 19(1). 10.1186/s12943-020-01234-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, & Kataoka K (2021). Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. Journal of the American Chemical Society, 143(2), 538–559. 10.1021/jacs.0c09029 [DOI] [PubMed] [Google Scholar]
- Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, … Cai Y (2020). Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. International Immunopharmacology, 88. 10.1016/j.intimp.2020.106939 [DOI] [PubMed] [Google Scholar]
- Li WH, & Li YM (2020). Chemical strategies to boost cancer vaccines. Chemical Reviews, 120(20), 11420–11478. 10.1021/acs.chemrev.9b00833 [DOI] [PubMed] [Google Scholar]
- Lindau D, Gielen P, Kroesen M, Wesseling P, & Adema GJ (2013). The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology, 138(2), 105–115. 10.1111/imm.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Jianping, Zhang R, & Xu ZP (2019). Nanoparticle-Based Nanomedicines to Promote Cancer Immunotherapy: Recent Advances and Future Directions. Small, 15(32). 10.1002/smll.201900262 [DOI] [PubMed] [Google Scholar]
- Liu Jingjing, Miao L, Sui J, Hao Y, & Huang G (2020). Nanoparticle cancer vaccines: Design considerations and recent advances. Asian Journal of Pharmaceutical Sciences, 15(5), 576–590. 10.1016/j.ajps.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Bi E, Ma X, Xiong W, Qian J, Ye L, … Yi Q (2020). Enhanced CAR-T activity against established tumors by polarizing human T cells to secrete interleukin-9. Nature Communications, 11(1). 10.1038/s41467-020-19672-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Xiang, Feng Z, Wang C, Su Q, Song H, Zhang C, … Wang W (2020). Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials, 230. 10.1016/j.biomaterials.2019.119649 [DOI] [PubMed] [Google Scholar]
- Liu Xiuting, Hogg GD, & Denardo DG (2021). Rethinking immune checkpoint blockade: Beyond the T cell. Journal for ImmunoTherapy of Cancer, 9(1). 10.1136/jitc-2020-001460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang S, Tapeinos C, Torrieri G, Känkänen V, El-Sayed N, … Santos HA (2021). Non-viral nanoparticles for RNA interference: Principles of design and practical guidelines. Advanced Drug Delivery Reviews, 174, 576–612. 10.1016/j.addr.2021.05.018 [DOI] [PubMed] [Google Scholar]
- Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, & Fiering S (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature Nanotechnology, 11(3), 295–303. 10.1038/nnano.2015.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmueller JJ, Ham JD, Kvorjak M, & Finn OJ (2018). mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. OncoImmunology, 7(1). 10.1080/2162402X.2017.1368604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loira-Pastoriza C, Vanvarenberg K, Ucakar B, Machado Franco M, Staub A, Lemaire M, … Vanbever R (2021). Encapsulation of a CpG oligonucleotide in cationic liposomes enhances its local antitumor activity following pulmonary delivery in a murine model of metastatic lung cancer. International Journal of Pharmaceutics, 600. 10.1016/j.ijpharm.2021.120504 [DOI] [PubMed] [Google Scholar]
- Lollini PL, Cavallo F, Nanni P, & Forni G (2006). Vaccines for tumour prevention. Nature Reviews Cancer, 6(3), 204–216. 10.1038/nrc1815 [DOI] [PubMed] [Google Scholar]
- Lou B, De Beuckelaer A, Boonstra E, Li D, De Geest BG, De Koker S, … Hennink WE (2019). Modular core-shell polymeric nanoparticles mimicking viral structures for vaccination. Journal of Controlled Release, 293, 48–62. 10.1016/j.jconrel.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Lubaroff DM (2019a). Phase II Study of Adenovirus/PSA Vaccine in Men With Hormone - Refractory Prostate Cancer - Full Text View - ClinicalTrials.gov. Retrieved from https://clinicaltrials.gov/show/NCT00583024
- Lubaroff DM (2019b). Phase II Study of Adenovirus/PSA Vaccine in Men With Hormone - Refractory Prostate Cancer (APP22) Retrieved from https://clinicaltrials.gov/ct2/show/NCT00583024
- Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, … Gao J (2017). A STING-activating nanovaccine for cancer immunotherapy. Nature Nanotechnology, 12(7), 648–654. 10.1038/nnano.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, … Seder RA (2015). In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nature Biotechnology, 33(11), 1201–1210. 10.1038/nbt.3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjub R, Jatana S, Lee SE, Qin Z, Pauli G, Soleimani M, … Li SD (2018). Recent advances in applying nanotechnologies for cancer immunotherapy. Journal of Controlled Release, 288, 239–263. 10.1016/j.jconrel.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Mai Y, Guo J, Zhao Y, Ma S, Hou Y, & Yang J (2020). Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cellular Immunology, 354. 10.1016/j.cellimm.2020.104143 [DOI] [PubMed] [Google Scholar]
- Mattiuzzi C, & Lippi G (2019). Current cancer epidemiology. Journal of Epidemiology and Global Health, 9(4), 217–222. 10.2991/jegh.k.191008.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbemi A, Khanna S, Njiki S, Yedjou CG, & Tchounwou PB (2020). Impact of gene–environment interactions on cancer development. International Journal of Environmental Research and Public Health, 17(21), 1–15. 10.3390/ijerph17218089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick AE, Ilett EJ, & Melcher AA (2009). JX-594, a targeted oncolytic poxvirus for the treatment of cancer. Current Opinion in Investigational Drugs, 10(12), 1372–1382. [PubMed] [Google Scholar]
- Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, … Wang AZ (2017). Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nature Nanotechnology, 12(9), 877–882. 10.1038/nnano.2017.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino NM, Anderson AKL, Nelson EL, & Wang SW (2013). Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano, 7(11), 9743–9752. 10.1021/nn403085w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle PM (2017). Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnology Advances, 35(3), 375–389. 10.1016/j.biotechadv.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Munakata L, Tanimoto Y, Osa A, Meng J, Haseda Y, Naito Y, … Aoshi T (2019). Lipid nanoparticles of Type-A CpG D35 suppress tumor growth by changing tumor immune-microenvironment and activate CD8 T cells in mice. Journal of Controlled Release, 313, 106–119. 10.1016/j.jconrel.2019.09.011 [DOI] [PubMed] [Google Scholar]
- Murgas P, Bustamante N, Araya N, Cruz-Gómez S, Durán E, Gaete D, … Lladser A (2018). A filamentous bacteriophage targeted to carcinoembryonic antigen induces tumor regression in mouse models of colorectal cancer. Cancer Immunology, Immunotherapy, 67(2), 183–193. 10.1007/s00262-017-2076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neek M, Kim T Il, & Wang SW (2019). Protein-based nanoparticles in cancer vaccine development. Nanomedicine: Nanotechnology, Biology, and Medicine, 15(1), 164–174. 10.1016/j.nano.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neek M, Tucker JA, Kim T Il, Molino NM, Nelson EL, & Wang SW (2018). Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials, 156, 194–203. 10.1016/j.biomaterials.2017.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, … Chen X (2020). A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Science Advances, 6(12). 10.1126/sciadv.aaw6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H, & Sakaguchi S (2014). Regulatory T cells in cancer immunotherapy. Current Opinion in Immunology, 27(1), 1–7. 10.1016/j.coi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- O’Hagan DT (1998). Microparticles and polymers for the mucosal delivery of vaccines. Advanced Drug Delivery Reviews, 34(2–3), 305–320. 10.1016/S0169-409X(98)00045-3 [DOI] [PubMed] [Google Scholar]
- Palladini A, Thrane S, Janitzek CM, Pihl J, Clemmensen SB, de Jongh WA, … Sander AF (2018). Virus-like particle display of HER2 induces potent anti-cancer responses. OncoImmunology, 7(3). 10.1080/2162402X.2017.1408749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Shevlin E, Vedvyas Y, Zaman M, Park S, Hsu YMS, … Jin MM (2017). Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Scientific Reports, 7(1). 10.1038/s41598-017-14749-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persano S, Guevara ML, Li Z, Mai J, Ferrari M, Pompa PP, & Shen H (2017). Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials, 125, 81–89. 10.1016/j.biomaterials.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V, & Schmid MC (2019). Macrophage-Mediated Subversion of Anti-Tumour Immunity. Cells, 8(7), 747. 10.3390/cells8070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raemdonck K, Braeckmans K, Demeester J, & De Smedt SC (2014). Merging the best of both worlds: Hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chemical Society Reviews, 43(1), 444–472. 10.1039/c3cs60299k [DOI] [PubMed] [Google Scholar]
- Rahimmanesh I, & Khanahmad H (2021). Chimeric antigen receptor-T cells immunotherapy for targeting breast cancer. Research in Pharmaceutical Sciences, 16(5), 447–454. 10.4103/1735-5362.323911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskov H, Orhan A, Gaggar S, & Gögenur I (2021). Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Frontiers in Oncology, 11. 10.3389/fonc.2021.668731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Merhi M, Inchakalody VP, Krishnankutty R, Relecom A, Uddin S, & Dermime S (2020). Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy. Journal of Translational Medicine, 18(1). 10.1186/s12967-020-02306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Ficht AC, Arenas-Gamboa AM, Kahl-McDonagh MM, & Ficht TA (2010). Polymeric particles in vaccine delivery. Current Opinion in Microbiology, 13(1), 106–112. 10.1016/j.mib.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Saeed M, Gao J, Shi Y, Lammers T, & Yu H (2019). Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics, 9(26), 7981–8000. 10.7150/thno.37568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, & Kauppinen A (2019). Immunosenescence: the potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cellular and Molecular Life Sciences, 76(10), 1901–1918. 10.1007/s00018-019-03048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayour EJ, Grippin A, De Leon G, Stover B, Rahman M, Karachi A, … Mitchell DA (2018). Personalized Tumor RNA Loaded Lipid-Nanoparticles Prime the Systemic and Intratumoral Milieu for Response to Cancer Immunotherapy. Nano Letters, 18(10), 6195–6206. 10.1021/ACS.NANOLETT.8B02179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller TH, & Sampson JH (2017). Advances and challenges: dendritic cell vaccination strategies for glioblastoma. Expert Review of Vaccines, 16(1), 27–36. 10.1080/14760584.2016.1218762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz L, Kadiyala P, Sun X, Son S, Najafabadi AH, Aikins M, … Moon JJ (2020). Synthetic High-density Lipoprotein Nanodiscs for Personalized Immunotherapy against Gliomas. Clinical Cancer Research, 26(16), 4369–4380. 10.1158/1078-0432.CCR-20-0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijns V, Fernández-Tejada A, Barjaktarović Ž, Bouzalas I, Brimnes J, Chernysh S, … Lavelle EC (2020). Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunological Reviews, 296(1), 169–190. 10.1111/imr.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Nene S, Rangaraj N, Raghuvanshi RS, Singh SB, & Srivastava S (2020, November 1). Bridging the gap: academia, industry and FDA convergence for nanomaterials. Drug Development and Industrial Pharmacy, Vol. 46, pp. 1735–1746. 10.1080/03639045.2020.1821055 [DOI] [PubMed] [Google Scholar]
- Shah S, Rangaraj N, Singh SB, & Srivastava S (2021). Exploring the unexplored avenues of surface charge in nano-medicine. Colloid and Interface Science Communications, 42, 100406. 10.1016/j.colcom.2021.100406 [DOI] [Google Scholar]
- Shahgolzari M, Dianat-Moghadam H, & Fiering S (2021). Multifunctional plant virus nanoparticles in the next generation of cancer immunotherapies. Seminars in Cancer Biology 10.1016/j.semcancer.2021.07.018 [DOI] [PMC free article] [PubMed]
- Shetty K, & Ott PA (2020). Personal Neoantigen Vaccines for the Treatment of Cancer. Annual Review of Cancer Biology, 5, 259–276. 10.1146/annurev-cancerbio-060820-111701 [DOI] [Google Scholar]
- Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, … Steinmetz NF (2017). Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials, 121, 15–27. 10.1016/j.biomaterials.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Shukla S, Wang C, Beiss V, Cai H, Washington T, Murray AA, … Steinmetz NF (2020). The unique potency of Cowpea mosaic virus (CPMV): In situ cancer vaccine. Biomaterials Science, 8(19), 5489–5503. 10.1039/d0bm01219j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Biswas A, Shukla A, & Maiti P (2019). Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduction and Targeted Therapy, 4(1). 10.1038/s41392-019-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, & O’Hagan DT (2003). Recent advances in veterinary vaccine adjuvants. International Journal for Parasitology, 33(5–6), 469–478. 10.1016/S0020-7519(03)00053-5 [DOI] [PubMed] [Google Scholar]
- Slütter B, Soema PC, Ding Z, Verheul R, Hennink W, & Jiskoot W (2010). Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. Journal of Controlled Release, 143(2), 207–214. 10.1016/j.jconrel.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Smith ML, Corbo T, Bernales J, Lindbo JA, Pogue GP, Palmer KE, & McCormick AA (2007). Assembly of trans-encapsidated recombinant viral vectors engineered from Tobacco mosaic virus and Semliki Forest virus and their evaluation as immunogens. Virology, 358(2), 321–333. 10.1016/j.virol.2006.08.040 [DOI] [PubMed] [Google Scholar]
- Snell LM, MacLeod BL, Law JC, Osokine I, Elsaesser HJ, Hezaveh K, … Brooks DG (2018). CD8+ T Cell Priming in Established Chronic Viral Infection Preferentially Directs Differentiation of Memory-like Cells for Sustained Immunity. Immunity, 49(4), 678–694.e5. 10.1016/j.immuni.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott GD, Dicaire CJ, Gurnani K, Deschatelets LA, & Krishnan L (2004). Liposome adjuvants prepared from the total polar lipids of Haloferax volcanii, Planococcus spp. and Bacillus firmus differ in ability to elicit and sustain immune responses. Vaccine, 22(17–18), 2154–2162. 10.1016/j.vaccine.2003.11.054 [DOI] [PubMed] [Google Scholar]
- Stump CT, Ho G, Mao C, Veliz FA, Beiss V, Fields J, … Fiering S (2021). Remission-stage ovarian cancer cell vaccine with cowpea mosaic virus adjuvant prevents tumor growth. Cancers, 13(4), 1–16. 10.3390/cancers13040627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Wang C, Song H, Zhang C, Liu J, Huang P, … Wang W (2021). Co-delivery of anionic epitope/CpG vaccine and IDO inhibitor by self-assembled cationic liposomes for combination melanoma immunotherapy. Journal of Materials Chemistry B, 9(18), 3892–3899. 10.1039/d1tb00256b [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, & Bray F (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Suszczyk D, Skiba W, Jakubowicz-Gil J, Kotarski J, & Wertel I (2021). The Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Development and/or Progression of Endometriosis-State of the Art. Cells, 10(3). 10.3390/cells10030677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasin C, Corradin G, Men Y, Merkle HP, & Gander B (1996). Tetanus toxoid and synthetic malaria antigen containing poly(lactide)/poly(lactide-co-glycolide) microspheres: Importance of polymer degradation and antigen release for immune response. Journal of Controlled Release, 41(1–2), 131–145. 10.1016/0168-3659(96)01363-6 [DOI] [Google Scholar]
- Tomljenovic L, & Shaw A, C. (2011). Aluminum Vaccine Adjuvants: Are they Safe? Current Medicinal Chemistry, 18(17), 2630–2637. 10.2174/092986711795933740 [DOI] [PubMed] [Google Scholar]
- Uddin MN, Kouzi SA, & Hussain MD (2015). Strategies for developing oral vaccines for human papillomavirus (HPV) induced cancer using nanoparticle mediated delivery system. Journal of Pharmacy and Pharmaceutical Sciences, 18(2), 220–234. 10.18433/j3rs3v [DOI] [PubMed] [Google Scholar]
- Union for International Cancer Control. (2020). GLOBOCAN 2020: New Global Cancer Data | UICC. Retrieved from https://www.uicc.org/news/globocan-2020-new-global-cancer-data [Google Scholar]
- Van den Boorn JG, & Hartmann G (2013). Turning Tumors into Vaccines: Co-opting the Innate Immune System. Immunity, 39(1), 27–37. 10.1016/j.immuni.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, & Huang L (2006). Immunostimulation of dendritic cells by cationic liposomes. Molecular Membrane Biology, 23(5), 385–395. 10.1080/09687860600790537 [DOI] [PubMed] [Google Scholar]
- Varypataki EM, Silva AL, Barnier-Quer C, Collin N, Ossendorp F, & Jiskoot W (2016). Synthetic long peptide-based vaccine formulations for induction of cell mediated immunity: A comparative study of cationic liposomes and PLGA nanoparticles. Journal of Controlled Release, 226, 98–106. 10.1016/j.jconrel.2016.02.018 [DOI] [PubMed] [Google Scholar]
- Vormehr M, Diken M, Türeci Ö, Sahin U, & Kreiter S (2020). Personalized neo-epitope vaccines for cancer treatment. Recent Results in Cancer Research, 214, 153–167. 10.1007/978-3-030-23765-3_5 [DOI] [PubMed] [Google Scholar]
- Waldmann TA (2003, March 1). Immunotherapy: Past, present and future. Nature Medicine, Vol. 9, pp. 269–277. 10.1038/nm0303-269 [DOI] [PubMed] [Google Scholar]
- Walter E, Dreher D, Kok M, Thiele L, Kiama SG, Gehr P, & Merkle HP (2001). Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells. Journal of Controlled Release, 76(1–2), 149–168. 10.1016/S0168-3659(01)00413-8 [DOI] [PubMed] [Google Scholar]
- Wang C, Fiering SN, & Steinmetz NF (2019). Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model. Advanced Therapeutics, 2(5). 10.1002/adtp.201900003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li D, Cang H, & Guo B (2019). Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Medicine, 8(10), 4709–4721. 10.1002/cam4.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, He Z, Cai P, Zhao Y, Gao L, Yang W, … Gao F (2019). Surface-Functionalized Modified Copper Sulfide Nanoparticles Enhance Checkpoint Blockade Tumor Immunotherapy by Photothermal Therapy and Antigen Capturing. ACS Applied Materials and Interfaces, 11(15), 13964–13972. 10.1021/acsami.9b01107 [DOI] [PubMed] [Google Scholar]
- Wang S, Sun Z, & Hou Y (2021). Engineering Nanoparticles toward the Modulation of Emerging Cancer Immunotherapy. Advanced Healthcare Materials, 10(5). 10.1002/adhm.202000845 [DOI] [PubMed] [Google Scholar]
- Warnock JN, Merten OW, & Al-Rubeai M (2006). Cell culture processes for the production of viral vectors for gene therapy purposes. Cytotechnology, 50(1–3), 141–162. 10.1007/s10616-005-5507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R, Umeano AC, Kou Y, Xu J, & Farooqi AA (2019). Nanoparticle systems for cancer vaccine. Nanomedicine, 8(1), 627–648. 10.2217/nnm-2018-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D (2005). Viral-Mediated Gene Transfer for Cancer Treatment. Current Pharmaceutical Biotechnology, 3(2), 151–164. 10.2174/1389201023378445 [DOI] [PubMed] [Google Scholar]
- Xiao B, Li D, Xu H, Zhou X, Xu X, Qian Y, … Tang J (2021). An MRI-trackable therapeutic nanovaccine preventing cancer liver metastasis. Biomaterials, 274. 10.1016/j.biomaterials.2021.120893 [DOI] [PubMed] [Google Scholar]
- Xiao P, Wang J, Fang L, Zhao Z, Sun X, Liu X, … Li Y (2021). Nanovaccine-Mediated Cell Selective Delivery of Neoantigens Potentiating Adoptive Dendritic Cell Transfer for Personalized Immunization. Advanced Functional Materials, 31(36). 10.1002/adfm.202104068 [DOI] [Google Scholar]
- Xu J, Lv J, Zhuang Q, Yang Z, Cao Z, Xu L, … Liu Z (2020). A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nature Nanotechnology, 15(12), 1043–1052. 10.1038/s41565-020-00781-4 [DOI] [PubMed] [Google Scholar]
- Xu J, Wang H, Xu L, Chao Y, Wang C, Han X, … Liu Z (2019). Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials, 207, 1–9. 10.1016/j.biomaterials.2019.03.037 [DOI] [PubMed] [Google Scholar]
- Yang R, Wang Z, Yuan Y, Qian T, & Zhou Q (2019). cRGD target liposome delivery system promoted immunogenic cell death through enhanced anticancer potency of a thymidine conjugate under UVA activation as a cancer vaccine. European Journal of Medicinal Chemistry, 167, 499–509. 10.1016/j.ejmech.2019.02.031 [DOI] [PubMed] [Google Scholar]
- Yang X, Fan J, Wu Y, Ma Z, Huang J, Zhang Y, … Chen S (2021). Synthetic multiepitope neoantigen DNA vaccine for personalized cancer immunotherapy. Nanomedicine: Nanotechnology, Biology, and Medicine, 37. 10.1016/j.nano.2021.102443 [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Wang BY, Wang TT, Wang FF, Guo YX, Hua RX, … Xu JD (2021). Functions of Dendritic Cells and Its Association with Intestinal Diseases. Cells, 10(3). 10.3390/cells10030583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Chowdhury S, McKay C, Baniel C, Wright WS, Bentley P, … Huang X (2015). Significant Impact of Immunogen Design on the Diversity of Antibodies Generated by Carbohydrate-Based Anticancer Vaccine. ACS Chemical Biology, 10(10), 2364–2372. 10.1021/acschembio.5b00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Dai Y, Zhao Y, Qi S, Liu L, Lu L, … Zhang Z (2020). Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nature Communications, 11(1110). 10.1038/s41467-020-14906-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xu L, Wang Y, Liu J, Tan G, Huang F, … Lu Z (2022). A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy. Chinese Chemical Letters 10.1016/j.cclet.2022.01.071 [DOI]
- Zhang P, Sun F, Liu S, & Jiang S (2016). Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. Journal of Controlled Release, 244, 184–193. 10.1016/j.jconrel.2016.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Tang L, Tian Y, Ji X, Hu Q, Zhou B, … Yang L (2020). DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. Journal of Controlled Release, 328, 210–221. 10.1016/j.jconrel.2020.08.023 [DOI] [PubMed] [Google Scholar]
- Zhang Y, & Zheng L (2013). Tumor immunotherapy based on tumor-derived heat shock proteins (review). Oncology Letters, 6(6), 1543–1549. 10.3892/ol.2013.1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu J, Li Y, Guan X, Han X, Xu Y, … Liu Z (2019). Nanoscale Coordination Polymer Based Nanovaccine for Tumor Immunotherapy. ACS Nano, 13(11), 13127–13135. 10.1021/acsnano.9b05974 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao X, Cheng Y, Guo X, & Yuan W (2018). Iron Oxide Nanoparticles-Based Vaccine Delivery for Cancer Treatment. Molecular Pharmaceutics, 15(5), 1791–1799. 10.1021/acs.molpharmaceut.7b01103 [DOI] [PubMed] [Google Scholar]
- Zhou B, Wu Q, Wang M, Hoover A, Wang X, Zhou F, … Chen WR (2020). Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chemical Engineering Journal, 396. 10.1016/j.cej.2020.125239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Su Q, Zhao H, Cao X, Yang Y, & Xue W (2020). Metal-Phenolic Network-Encapsulated Nanovaccine with pH and Reduction Dual Responsiveness for Enhanced Cancer Immunotherapy. Molecular Pharmaceutics, 17(12), 4603–4615. 10.1021/acs.molpharmaceut.0c00802 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Maharaj PD, Mallajosyula JK, McCormick AA, & Kearney CM (2015). In planta Production of Flock House Virus Transencapsidated RNA and Its Potential Use as a Vaccine. Molecular Biotechnology, 57(4), 325–336. 10.1007/s12033-014-9826-1 [DOI] [PubMed] [Google Scholar]
- Zhu Ge, Yang Y-G, & Sun T (2022). Engineering optimal vaccination strategies: effects of physical properties of the delivery system on functions. Biomaterials Science, 10(6), 1408–1422. 10.1039/d2bm00011c [DOI] [PubMed] [Google Scholar]
- Zhu Guizhi, Zhang F, Ni Q, Niu G, & Chen X (2017). Efficient Nanovaccine Delivery in Cancer Immunotherapy. ACS Nano, 11(3), 2387–2392. 10.1021/acsnano.7b00978 [DOI] [PubMed] [Google Scholar]
- Ziogas DC, Konstantinou F, Bouros S, Theochari M, & Gogas H (2021). Combining BRAF/MEK Inhibitors with Immunotherapy in the Treatment of Metastatic Melanoma. American Journal of Clinical Dermatology, 22(3), 301–314. 10.1007/s40257-021-00593-9 [DOI] [PubMed] [Google Scholar]


