Abstract
Myxozoan parasites pose huge threat to wild and cultured fishes and reported to cause heavy mortality, retarded growth and post-harvest quality degradation. It is one of the highly divergent groups of parasites which infects skin, gill, muscle, cartilage and internal organs of host fish and the severity of pathology varies depending on the water temperature, species of fish, site of tissue infection and immune resistance of the individual host. Most infections are difficult to treat, as they can easily evade host cellular and humoral defence mechanisms by proliferating or migrating through immune compromised sites of the host and forming large plasmodia encapsulated by the host cellular elements. This spore-forming parasite is harmless to humans but often detected in faecal samples of immunosuppressed humans. The incidences are mostly associated with the consumption of infected fish having a high concentration of spores which causes diarrhea and stomach pain. Currently, there are no immunostimulants or vaccines available for controlling these parasites, however, fumagillin is the drug of choice in fish for controlling this parasitic infection. Excessive usage of fumagillin causes tissue damage and retarded growth in fish, hence feed incorporation of this antibiotic in proper dose is essential for effective treatment. In this review detailed information on the diseases caused by myxozoan parasites in fishes and their zoonotic potential is discussed.
Keywords: Myxozoan parasites, Chemotherapy, Zoonoses
Introduction
Myxozoan is a common spore-forming parasite of aquatic animals. After many conflicts, based on the 18 s sequence pattern, it has been confirmed that myxozoans are metazoan parasites. The subphylum comprises two classes: myxosporea and malacosporea, which have typical spore-forming vegetative structures and actinospore as an intermediate stage. Sexual reproduction occurs in invertebrates (mostly annelids) as definitive hosts and actinospores infect mostly lower vertebrates like fish, or invertebrates such as turtle, reptile, and amphibians and remain as coelozoic or histozoic spores forming vegetative bodies in tissues. Over 2000 species of myxospore and 3 species of malacospore are so far known and few of them are highly infectious to fish and cause severe economic loss to aquaculture (Lom and Dykova 2013a, b). The old classification is based on valve number, spore structure, polar capsule number and its position as well as the folds of polar filament. However, phylogenetic classification (based on molecular evidence) has revolutionized myxozoan taxonomy and many new species have been described worldwide.
Some of the significant fish diseases caused by myxozoans (Table 1) are; proliferative kidney diseases in salmon, swim bladder inflammation in carps, hamburger gill/ proliferative gill disease in catfish, polycystic kidney or hofferellosis in goldfish, whirling swimming disease in salmon fish, enteronecrosis in carps, thelohanellosis in carps and muscle rmyoliquification in salmons. Many of these diseases are specific to a class of fish and the severity varies depending on the host, water quality parameters and the site of infection. Currently, there are no vaccine element identified for neutralizing these parasites however anti-proteases and serum protein inhibitors seem to be a promising treatment candidate for myxozoans. This article intends to discuss a group of myxozoan parasites causing severe diseased conditions in fish and their respective treatment methods. The zoonotic importance of this parasite which is being less discussed is also highlighted.
Table 1.
Myxozoan parasite causing serious diseases in fresh water and marine water fishes
| Name of parasite | Affected group | Geographical area | Stage affected | Affected organ | Disease | References |
|---|---|---|---|---|---|---|
| Tetracapsuloides bryosalmonae | Salmonid of genera Salmo,onchorhynchus, Thymallus and pikes | America and Europe | Fry to fingerling | kidney | Proliferative kidney disease | Oredalen et al. (2022) |
| Sphaerospora molnari/ S. dykovae | Common carp and gold fish | Central Europe | fingerling | gill | Swim bladder inflammation | Holzer et al. (2014) |
| Henneguya ictaluri | Channel catfish | Southern America | alevins | gill | Proloferative gill disease/ hamburger disease | Vieira et al. (2022) |
| Myxobolus cerebralis | Salmonids and rainbow trout | Northern Asia, Europe and America | fry | Cartilage of head, gill, vertebral colum | Whirling disease | El. Matbouli and Hoffman (1998) |
| Myxobolus encephalicus | Common carp | Europe | Fry and fingerlings | Brain blood vessels | Oedema, locomotory disorder | Antychowicz and Reichert (2005) |
| Sphaerospora renicola | Common carp | Europe and Israel | Fingerling | Renal tubule and swimbladder wall | Kidney disfunction and swimbladder inflamation | Dykovfi and Lom (1982) |
| Ceratomyxa Shasta | Salmonids | North America | Juvenile, pre-spawning, matured fish | Digestive tract | Ceratomyxosis/ enteronecrosis | Johnson et al. (1979) |
| Hofferelus carassi | goldfish | Asia, North America, Europe | 2 to 3 year old gold fish | Kidney tubules | Kidney enlargement disease/kidney bloater disease | Ahmed (1973) |
| Kudoa thyrsites and/or K. paniformis | Cultured salmo salar and several marine sp, | South Africa, North America, Australia and Asia | Salmon smolts, market size fish | Muscle fiber | Myoliquification milky flesh’, ‘soft flesh’ or ‘jelly flesh | Giulietti et al. (2022) |
| Thelohanellus hovorkai | Common carp | Japan, China | 2–3 year old fish | Subcutaneous and connective tissue | haemorrhagic skin lesions | Akhmerov (1960) |
Tetracapsuloides bryosalmonae
The parasite belongs to the class Malacosporea (Fig. 1) in the phylum Myxozoa and includes freshwater bryozoans in the lifecycle (Anderson et al. 1999; Canning et al. 2002). It is associated with a serious parasitic disease in freshwater cultured salmonid fish of Europe and North America which is known as Proliferative kidney disease (PKD). It infects primarily kidney interstitium of salmonid fingerlings (Onchorhynchus tschawytscha, O. kisutch) and Stealhead in North America, Salmo salar, S. trutta, Thymallus thymallus in Europe and Rainbow trout in both continents. In Rainbow trout the mortality usually varies 30–50% which is temperature dependent. The PKD is a proliferative extrasporogonic stage of the parasite which is a primary cell (15 µm in size) with one or more secondary and tertiary cells. PKD infiltrates kidney interstitium and develops inside and within host cells. It penetrates into the tubular lumen of kidney tissue and forms sporoblast which do not mature.
Fig. 1.
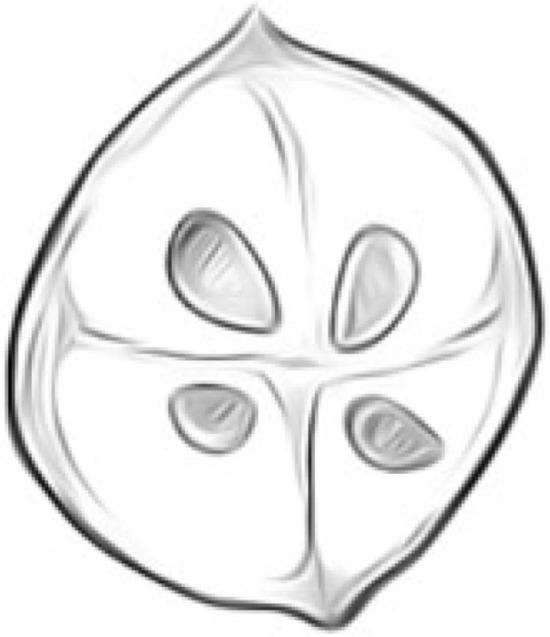
Tetracapsuloides bryosalmonae, subspherical shape (7.1–7.4 μm in diameter)
The infected fish's kidney was enlarged into swollen greyish bulbous ridges (Ferguson and Needham 1978). Gross clinical signs include a darkened body, pale gills, abdominal distension due to hypertrophic trunk kidney, exophthalmia, and anaemia (Hedrick et al. 1993). The host defense system intensely reacts to the PKD which elicits the disease. It causes interstitial hyperplasia, along with chronic granulomatous, interstitial nephritis, and tubular atrophy. The severity of the immune response is characterized by the infiltration of macrophages and lymphocytes. Most of the time high mortalities due to PKD in fish farms are typically associated with secondary infections (Feist and Bucke 1993).
Sphaerospora molnari
It is regarded as a serious pathogen of common carp fingerlings in Central Europe. It infects gill of Common carp fingerlings and Carassius carassius in Europe. The infective non-sporogonic stage (Fig. 2) multiplies in blood and liver and invades the epithelium of gill filaments, head skin, gill cavity lining, kidney and swim bladder to complete sporogony (Dykovfi and Lom 1982). Affected epithelia show marked dystrophic changes and necrosis, causing secondary bacterial infections (Sirri et al. 2020). In severe infection, the parasite replaces tissues and causes local circulatory disorder and dystrophic changes. The severity may range up to 100% mortality in carp fingerlings.
Fig. 2.
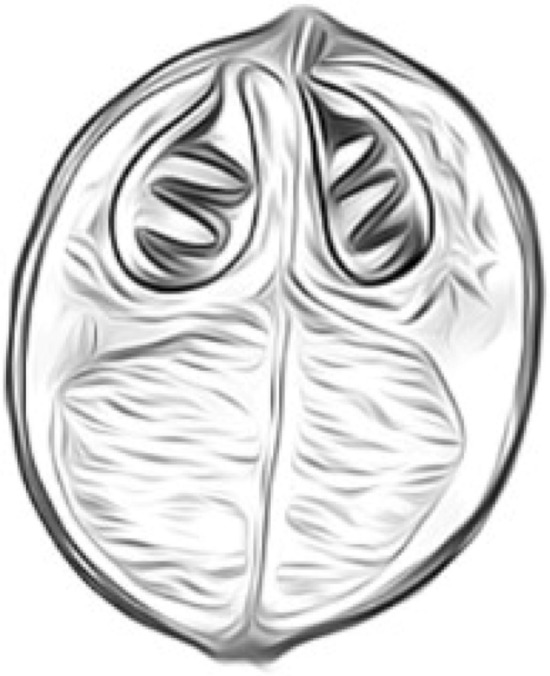
Sphaerospora renicola, spherical (1.7–2.3 μm diameter)
Henneguya ictaluri
The Henneguya ictaluri (Fig. 3) is the causative agent of one of the major parasitic diseases in Alevins of Channel Catfish in the southern USA called proliferative gill disease. The affected fish gill resembles raw hamburger meat. Hence the name of the disease called Hamburge/proliferative gill disease (PGD). The common symptoms noticed are reduction in feed intake, fish congregating at the outlet and sometimes gulping at the surface. The parasite causes heavy destruction of gill tissue therefore, fishes die due to respiratory stress. The clinical sign is most prominent in gill of affected fishes. Swelling of gill filaments and mottled red and white color in appearance. The gill filaments fuse together and filaments on oneside-gill arch are not distinct from filaments on other arches. The gills often look mashed and may bleed when touched or when the fish are simply lifted from the water. The skin of catfish affected with PGD appears healthy and PGD is occasionally found in internal organs (liver, kidney, spleen, and brain). It stimulates a strong granulomatous inflammation in the internal organs. The hyperplasia of the gill epithelium is associated with multifocal degeneration and necrosis of gill cartilage (Vieira et al. 2019; 2022).
Fig. 3.

Henneguya ictaluri, ellipsoid shape with caudal appendage (9.7 ± 0.5 μm spore length)
Myxobolus cerebralis
It is one of the most common salmonid fish infecting parasites (Fig. 4) in the USA. The severity of infection varies from species to species. The most affected stage is fry to fingerlings when the cartilage is not fully ossified. After 20 days of exposure the parasite concentrates in cartilage, primarily in the cranium and spine (El-Matbouli and Hoffmann 1998). It digests the cartilage tissue that causes lesions and inflammation, and potentially damaging the skeletal structure of the fish. Due to the typical whirling symptom of affected fish, the disease gets the name “whirling disease”. The fish shows an abnormal tail chasing movement. It is due to the damage of cartilage around the auditory organ. The prolonged whirling causes stress, malnutrition and death. The first visible sign of infection is the darkening of the caudal peduncle and fin which is due to the lesion in the posterior vertebral column. There is deformation noticed in the head and spines. In the case of rainbow trout, the cartilage lesion is found in the cranium and base of the brain but can be found throughout the cartilage in the body. The location of lesions may vary in different fish species.
Fig. 4.
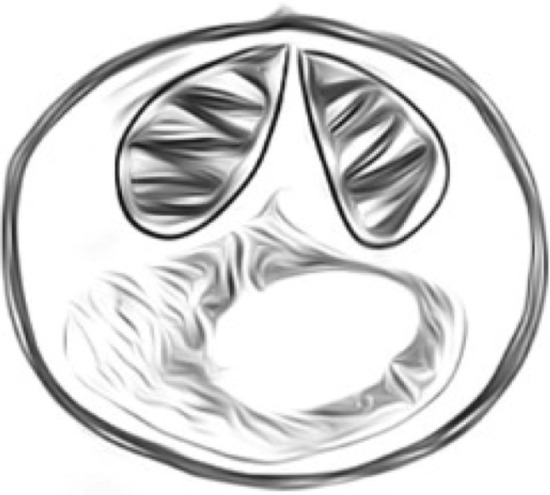
Myxobolus cerebralis, spherical shape (8–10 mm diameter)
Myxobolus encephalicus
It is a highly prevalent common carp fingerling infecting parasite in Europe. It is found in the brain and blood vessels of fish whose large plasmodia may block blood circulation. There is occasional severity due to this parasite but it impairs blood circulation and cause edema. Heavy infection in fry and fingerlings cause locomotory disorder.
Ceratomyxa Shasta
This parasite (Fig. 5) is a major threat to the cultured and wild population of salmonids in the Pacific Northwest in North America (Johnson et al. 1979). It infects pre-spawning adults in hatchery and in wild affects juvenile salmonids. The infection is fatal to juvenile fish. The disease is called “enteronecrosis”, a lethal disease characterized by intestinal haemorrhage and necrosis. Clinical signs include loss of appetite, a distended abdomen, exophthalmia, emaciation, swelling, and haemorrhages in the area of the anus. It also forms nodules in the gut of adult chinook salmon. The tissue pathology varies between susceptible and resistant salmon varieties. The trophozoite causes extensive inflammation, necrosis and sloughing of posterior intestinal layers in susceptible fish during 18 days post infection, whereas the trophozoite could not penetrate the intestinal lumen of resistant fish (Bartholomew et al.1989).
Fig. 5.
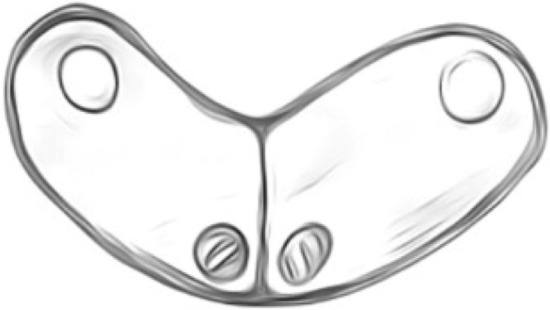
Ceratomyxa Shasta, crescent shaped spores (14–23 μm length)
Sphaerospora renicola
The prevalence of Sphaerospora renicola (Fig. 2) varies from 70–100% in common carp fingerling stocks of Central Europe. The pseudoplasmodia are found in the lumen of renal tubules and produce sub-spherical spores. But the severity is associated with extrasporogonic stage of the parasite which infects the swimbladder wall. It causes swimbladder inflammation in carps (Dykovfi and Lom 1982). The diseased fish shows distress due to locomotory disorder and circular movement. It is located intra and extravascular and elicits exudative-proliferative inflammation. Hyperplasia of the epithelium of lamina propria, the proliferation of connective tissue and changes in serosa result in a greatly thickened swim bladder wall and noted with severe haemorrhages (Sirri et al. 2020).
Hofferellus carassii
A disease named “Hoferellosis”, causing heavy losses in goldfish, was originally described in Prussian carp (C. gibelio, Bloch). It is a common parasite (Fig. 6) of Carassius sp. found in Asia, Europe and North America. In North America, Europe and Asia, it is called as kidney bloater disease, kidney enlargement disease and polycystic kidney disease of goldfish, respectively. There is 20% seasonal mortality during early spring of farmed goldfish due to this disease. Later it was characterized by Ahmed (1973) as a parasite causing kidney bloater in farmed C. auratus, causing a polycystic, swollen kidney and consequent abdominal distension. The massive enlargement of the kidney is recognized externally as swelling of one or both sides of the abdomen. The abdomen may become so enlarged that the fish can no longer swim or feed effectively. This abdominal swelling of infected fish due to kidney hyperplasia. The extrasporogonic stage proliferates intracellularly in epithelial cell lining of kidney tubules and cause papillary cystic hyperplasia. Then they migrate to the tubular lumen and collecting duct where it grows to polysporic plasmodia.
Fig. 6.
Hoferellus carassii, bullet shaped (10.3–14.3 μm length)
Kudoa thyrsites and K. paniformis
The Kudoa sp. (Fig. 7) are associated with postmortem myoliquification of fish flesh, commonly known as the causative agent of ‘milky flesh’. This parasite is so far reported infecting 27 fish species worldwide. It is of great economic concern in the Northwest Pacific of North America where it affects farmed Atlantic salmon and to a lesser extent, coho salmon (O. kisutch). The infection goes undetected, until several hours (38–56 h) after harvest, when myoliquifaction becomes apparent. It is due to the action of enzymes produced by parasites. The proteolytic enzyme produced by K. paniformis is both acidic and neutral whereas K. thyrsites produces acidic enzymes (Giulietti et al. 2022). The affected fish are anemic and have enlarged kidney. The parasite penetrates the core of muscle fiber and spreads along myocytes without damaging the sarcolemma, resulting in individual muscle fibers being filled with spores without involving the connective tissue.
Fig. 7.
Kudoa thyrsites, stellate shape, with 4 unequal pyriform PC (14.5–15.5 μm)
Thelohanellosis
Among the myxosporean genus, Thelohanellus kudo, 1933 is the sixth most abundant parasite among the 108 species reported so far (Zhang et al. 2013). These are mostly histozoic spore forming, single capsule bearing and pyriform shaped parasite of freshwater fish. Except few species i.e., T. wuhanensis, T. hovorkai, T. nikolskii, T. kitauei and T. wangi (Xiao and Chen 1993; Akhmerov 1960, 1955; Egusa and Nakajima 1981; Yuan et al. 2015) the genus does not cause severe infection. The species T. hovorkai infects subcutaneous and connective tissue of 2 to 3 years adults of C. carpio and causes mass mortality as case reports from Japan and China. An endoparasitic species T. kitauei causes “intestinal giant cystic disease” of common carp. These spores of 6-8 mm large blocks the intestine and fishes are often found emaciated and anorexic. The pathology includes petechial, scattered haemorrhages on the body surface and fishes are often emaciated and anorexic. The disease is also called as hemorrhagic thelohanellosis in carp.
Control of myxozoan disease
Good fish husbandry is necessary for all types of disease control. It includes proper feeding, biosecurity measures, quarantine measures for diseases fish, aeration and disinfection. The parasitic spores are highly tolerant to extreme weather conditions and can remain in pond bottom soil for years. Pond disinfection with calcium hydroxide, calcium oxide, calcium cyanimide should be used to eradicate spores. The most widely used chemotherapy for myxozoan diseases are malachite green and fumagillin. Different doses of fumagillin with respective diseases are mentioned in Table 2. The antibiotic fumagillin DCH (2,4,6,8-Decatetraenedioic acid mono-(5-methoxy-4- {2-methyl-3- (3-methyl-2-butenyl) oxiranyl)-1 oxaspirol {2.5}-ox-6-yl} ester) is originally a mycotoxin derived from detrivorous fungus (Aspergillus fumigates) and found effective on controlling cellular growth by inactivating enzyme methionine aminopeptidases-2 which is essential for cellular protein modelling and functioning (Sin et al. 1997). Though it is harmful for the host cell but it is found effective in controlling carcinoma, tumour, microsporidian infection in animal and human myxozoan diseases. It was first used by controlling microsporidian diseases such as that caused by Nosema apis in honey bees, Apis mellifica (Katznelson and Jamieson 1952; Bailey 1953). It is also detected that fumagillin preferentially affect DNA or RNA synthesis of the parasite (Hartwig and Przelecka 1971; Jaronski 1972) whereas according to Liu (1973), the antibiotic affects spore membranes. The usage of fumagillin is effective at the proper dose but at higher dose increases mortality and loss of appetite in fishes. Fish displayed severe hypertrophy in the spleen and the anterior kidney after the treatment for 8 weeks (Hedrick et al. 1993). Malachite green is an antifungal chemical and found to be effective against myxozoan diseases. However, its use is restricted in fish farming as it gets accumulated in muscle tissue and found to be carcinogenic, mutagenic and teratogenic in mammals (Sudova et al. 2007).
Table 2.
Dose of fumagillin for controlling Myxozoan parasitic infection in fish
| Parasite | Fish | Mode of application | Dose of fumagillin | References |
|---|---|---|---|---|
| Tetracapsuloides bryosalmonae | Rainbow trout | Feed | 0.13–0.25 g/kg feed | Hedrick et al. (1993) |
| Tetracapsuloides bryosalmonae | Chinook salmon | Feed | 0.5 g/kg feed | Hedrick et al. (1993) |
| Shaerospora renicola | Common carp | Feed | 1 g/kg feed | Molnar et al. (1987) |
| Myxobolus cerebralis | Rainbow trout | Feed | 1 g/kg feed for 1% body weight | EI-matbouli and Hoffman (1998) |
| Hoferellus carassii | Gold fish | Feed | 0.1% or 1.0% in diets | Yokowama et al. (1990) |
Proliferative kidney disease (PKD): There are many research carried out for controlling PKD due to its negative impact on rainbow trout cultivation. Hedrick et al. (1993) has reported that antibiotic Fumagillin DCH is effective in controlling experimental infections in Chinook salmon with PKD. It is effective at concentrations in the feed as low as 0.5 g/kg-when fed at approximately 1% body weight per day. Another report informs that fumagillin is safe to use at 3 mg /kg/BW/day for 10 days during the PKD incubation phase to control PKD in rainbow trout (Gouvello et al. 1999). It is experimentally proved that the resistance of more than one year or older fish is due to previous exposure and is not an age-related immunity. It is also concluded that delayed stocking reduces mortality in 0 + aged salmon fry (Ferguson et al. 1979). It is accidentally observed during the treatment of Ich infection of Rainbow trout with malachite green (1.0 ppm) and formalin (20 ppm) during flush treatment that PKD infection is delayed in the treated fish in comparison to control fishes (Clifton-Hadley and Alderman 1987).
Whirling disease: The best way to control this disease is to break the parasitic cycle. As the intermediate host of M. cerebralis triactinomyxon stage is tubifex, irradiation of worms reduces infection severity. It is experimentally observed that applying low voltage DC current for 1000 s kills the worm within 48 h. But it is not safe for field level application. The lampicide is found to be effective in killing Tubifex at 4.2–14.0 g/L concentration however, it has an untargeted effect on other animals. Therefore, it can be used in a tank after removing fishes. However, there are many trials to irradiate worms from pond by means of temperature, salinity and chemicals.
Pond disinfection with quick lime at 380 g/m2 treated for two weeks reduced infection (Hoffman and Putz 1969). However, the treatment of earthen ponds with 4,550 g/m2 of CaO or 1200 ppm chlorin for 18 to 24 h is not effective for killing all stages of the myxozoan parasite.The myxospore are killed by heating in 0.85% saline at 60, 80 and 100 °C. The parasite contaminated water can be sterilized with 2537 Angstrom units of ultraviolet light at dosages of 35,000, 43,000, and 112,000 microwatts sec/cm2 by which infection of fry was prevented but there was heavy mortality and growth retardation.
Ceratomyxosis: The occurrence of disease can be reduced by avoiding the use of infected water and fish. The parasite can transmit to naive fish within a short duration (30 min) of exposure to the contaminated water. Hence, it is must to filter and decontaminate every input water before entering the hatchery unit by using chlorination or UV irradiation (Sanders et al.1972). Treatment of infected water with a combination of filtration followed by chlorine or UV irradiation is effective for controlling this disease (Sanders et al. 1972). The fish from infected hatchery should not be transported. It was reported by Zinn et al. (1977) that stocking with resistant variety reduces infection rate in endemic areas of infection due to selective resistant gene transfer.
Swim bladder inflammation: Molnar et al. (1987) reported that fumagillin at a dose of 0.1% in the feed prevented the sporogonic stage of the parasite in the renal tubules but ineffective for early parasitic stages (k-protozoan). Furazolidone was effective against early blood stages of Sphaerospora, when supplied at a dose of 0.05–0.1% in feed for 4 to 6-day whereas toltrazuril failed to kill these parasites (Molnar et al. 1993). Other preventive measures are same as that of the basic good husbandry practices.
Proliferative gill disease—There are no treatment measures for this disease but prevention of high mortality can be reduced by maintaining the oxygen level through proper aeration as the parasite infect gill.
Kidney bloater disease—An experimental effective treatment procedure is the usage of fumagillin at 0.1% or 1.0% in diets. Yokowama et al. (1990) reported that there was no kidney enlargement observed after 1 month in treated fishes.
Kudoa infection—There is limited information regarding the treatment of Kudoa infection. For treatment of seawater reared Atlantic salmon infected with K. thrysis, application of Nicarbazin incorporated into diets at 2.5 g kg−1 is found effective. But there are problems like growth retardation due to unacceptance of medicated feed, residues of drugs remaining in skeletal muscle, liver and skin, as well as high mortality in comparison to control fish (Jones et al. 2012).
Zoonotic diseases associated with myxozoans
In few incidences, myxozoan spores were detected in fecal samples of patients with gastroenteritis (Boreham et al. 1998). The immunosuppressed patient suffering from diarrhea was also detected with this parasitic infestation in stool samples (Moncada et al. 2001). The symptoms of abdominal pain noticed for 4 weeks and hematological parameters show low hemoglobin and mild leukocytosis. Most of them had consumed fish captured from local ponds, reservoirs, and frozen fish fillets, which were contaminated with parasitic infection and undigested spores were released in stool samples. In many cases, fish with high concentrations of parasites are the cause of such incidences.
Conclusion
Myxozoans are highly diverse group of parasites, alternating between their invertebrate and vertebrate hosts. Despite their importance, the evolutionary history and phylogenetic relationship of myxozoan with respect to closely related host species remain unaddressed. The co-evolutionary analysis with respect to specific host lineages will provide more insights into parasite distribution patterns, biology and host specificity. Due to the lack of standardized, isolation and culture protocol, contamination is a major bottleneck behind genomic sequencing of myxozoan parasites. Future studies may target high throughput sequencing of myxozoan parasites, which will facilitate generating more database for co-evolutionary analysis. The treatment of myxozoan diseases is challenging due to lack of effective, safe drugs and sterilization protocol. Research may target investigating more immunostimulants and therapeutics which can provide sustainable protection against myxozoan infestation.
Funding
The authors received no specific funding for this work.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed ATA. Morphology and life history of Mitraspora cyprini Fujita, parasitic in the kidney of goldfish. Jpn J Med Sci Biol. 1973;26:87–101. doi: 10.7883/yoken1952.26.87. [DOI] [PubMed] [Google Scholar]
- Akhmerov AK. Ways of the origin of Myxosporidia species of the genus Thelohanellus Kudo from Amur wild carp. Dokl Akad Nauk SSSR. 1955;105:1129–2113. [Google Scholar]
- Akhmerov AK. Myxosporidia of fishes of the Amur River Basin. Rybnoe Khozyaistvo Vnutrikh Vodoemov Latviiskoi SSR. 1960;5:239–308. [Google Scholar]
- Anderson CL, Canning EU, Okamura B. Molecular data implicate bryozoans as hosts for PKX (Phylum Myxozoa) and identify a clade of bryozoan parasites within the Myxozoa. Parasitology. 1999;119:555–561. doi: 10.1017/s003118209900520x. [DOI] [PubMed] [Google Scholar]
- Antychowicz J, Reichert M. Occurrence of Myxobolus encephalicus [Muslow 1911] in Poland: possible relationship between the parasite infection and clinical symptoms in common carp [Cyprinus carpio] Bull Vet Inst Pulawy. 2005;49:35–39. [Google Scholar]
- Bailey L (1953) Effect of fumagillin upon Nosema apis (Zander). Nature 171(4344):212–213 [DOI] [PubMed]
- Bartholomew JL, Smith CE, Rohovec JS, Fryer JL. Characterization of a host response to the myxosporean parasite, Ceratomyxa shasta (Noble), by histology, scanning electron microscopy and immunological techniques. J Fish Dis. 1989;12:509–522. doi: 10.1111/j.1365-2761.1989.tb00561.x. [DOI] [Google Scholar]
- Boreham RE, Hendrick S, Donoghue PJ, Stenzel DJ. Incidental finding of Myxobolus spores (Protozoa: Myxozoa) in stool samples from patients with gastrointestinal symptoms. J Clin Microbiol. 1998;36:3728–3730. doi: 10.1128/JCM.36.12.3728-3730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning EU, Tops S, Curry A, Wood TS, Okamura B. Ecology, development and pathogenicity of Buddenbrockia plumatellae Schröder, 1910 (Myxozoa, Malacosporea)(syn. Tetracapsula bryozoides) and establishment of Tetracapsuloides n. gen. for Tetracapsula bryosalmonae. J Eukaryot Microbiol. 2002;49:280–295. doi: 10.1111/j.1550-7408.2002.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley RS, Alderman DJ. The effects of malachite green upon proliferative kidney disease. J Fish Dis. 1987;10:101–107. doi: 10.1111/j.1365-2761.1987.tb00725.x. [DOI] [Google Scholar]
- Dykova I, Lom J. Sphaerospora renicola n. sp., a myxosporean from carp kidney, and its pathogenicity. Z Parasitenkd. 1982;68:259–268. doi: 10.1007/BF00927404. [DOI] [PubMed] [Google Scholar]
- Egusa S, Nakajima K. A new myxozoa Thelohanellus kitauei, the cause of intestinal giant cystic disease of carp. Fish Pathol. 1981;15:213–218. doi: 10.3147/jsfp.15.213. [DOI] [Google Scholar]
- El-Matbouli M, Hoffmann RW. Light and electron microscopic studies on the chronological development of Myxobolus cerebralis to the actinosporean stage in Tubifex tubifex. Int J Parasitol. 1998;28:195–217. doi: 10.1016/s0020-7519(97)00176-8. [DOI] [PubMed] [Google Scholar]
- Feist SW, Bucke D. Proliferative kidney disease in wild salmonids. Fish Res. 1993;17:51–58. doi: 10.1016/0165-7836(93)90006-S. [DOI] [Google Scholar]
- Ferguson HW, Ball HJ (1979) Epidemiological aspects of proliferative kidney disease amongst rainbow trout Salmo gairdneri Richardson in Northern Ireland. J Fish Dis 2(3):219–225. 10.1111/j.1365-2761.1979.tb00161.x
- Ferguson HW, Needham EA. Proliferative kidney disease in rainbow trout Salmo gairdneri Richardson. J Fish Dis. 1978;1:91–108. doi: 10.1111/j.1365-2761.1978.tb00008.x. [DOI] [Google Scholar]
- Giulietti L, Karlsbakk E, Cipriani P, Bao M, Storesun JE, Marathe NP, Levsen A (2022) Long-term investigation of the ‘soft flesh’ condition in Northeast Atlantic mackerel induced by the myxosporean parasite Kudoa thyrsites (Cnidaria, Myxozoa): temporal trends and new molecular epidemiological observations. Fish Res 248:106221. 10.1016/j.fishres.2021.106221
- Hartwig A, Przełȩcka A. Nucleic acids in intestine of Apis mellifica infected with Nosema apis and treated with fumagillin DCH: cytochemical and autoradiographic studies. J Invertebr Pathol. 1971;18:331–336. doi: 10.1016/0022-2011(71)90034-6. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, MacConnell E, De Kinkelin P (1993) Proliferative kidney disease of salmonid fish. Ann Rev Fish Dis 3:277–290. 10.1016/0959-8030(93)90039-E
- Hoffman GL, Putz RE. Host susceptibility and the effect of aging, freezing, heat, and chemicals on spores of Myxosoma cerebralis. Prog Fish C. 1969;31:35–37. doi: 10.1577/1548-8640(1969)31[35:HSATEO]2.0.CO;2. [DOI] [Google Scholar]
- Holzer AS, Hartigan A, Patra S, Pecková H, Eszterbauer E. Molecular fingerprinting of the myxozoan community in common carp suffering Swim Bladder Inflammation (SBI) identifies multiple etiological agents. Parasit Vectors. 2014;7:1–9. doi: 10.1186/1756-3305-7-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaronski ST. Cytochemical evidence for RNA synthesis inhibition by fumagillin. J Antibiot (tokyo) 1972;25:327–331. doi: 10.7164/antibiotics.25.327. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Sanders JE, Fryer JL (1979) Ceratomyxa shasta in salmonids (No. 58). US Fish and Wildlife Service
- Jones SR, Forster I, Liao X, Ikonomou MG. Dietary nicarbazin reduces prevalence and severity of Kudoa thyrsites (Myxosporea: Multivalvulida) in Atlantic salmon Salmo salar post-smolts. Aquaculture. 2012;342:1–6. doi: 10.1016/j.aquaculture.2012.01.033. [DOI] [Google Scholar]
- Katznelson H, Jamieson CA (1952) Control of nosema disease of honeybees with fumagillin. Science 115(2977):70–71. 10.1126/science.115.2977.70 [DOI] [PubMed]
- le Gouvello R, Pobel T, Richards RH, Gould C. Field efficacy of a 10-day treatment of fumagillin against proliferative kidney disease in rainbow trout Oncorhynchus mykiss. Aquaculture. 1999;171:27–40. doi: 10.1016/S0044-8486(98)00430-X. [DOI] [Google Scholar]
- Liu TP. Effects of Fumidil B on the spore of Nosema apis and on lipids of the host cell as revealed by freeze-etching. J Invertebr Pathol. 1973;22:364–368. doi: 10.1016/0022-2011(73)90165-1. [DOI] [Google Scholar]
- Lom J, Dykova I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol (praha) 2013;53:1–36. doi: 10.14411/fp.2006.001. [DOI] [PubMed] [Google Scholar]
- Lom J, Dykova I. Myxozoan genera definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol (praha) 2013;53:1–36. doi: 10.14411/fp.2006.001. [DOI] [PubMed] [Google Scholar]
- Molnar K, Baska F, Szekely C. Fumagillin, an efficacious drug against renal sphaerosporosis of the common carp Cyprinus carpio. Dis Aquat Org. 1987;2:187–190. doi: 10.3354/dao015041. [DOI] [Google Scholar]
- Molnar K, Baska F, Csaba G, Glávits R, Székely C. Pathological and histopatnotogical studies of the swimbladder of eels Anguilla anguitla infected by Angutlticpta crassus (Nemafoda:Dracuncuioidea) Dis Aquat Org. 1993;15:41–50. doi: 10.3354/dao015041. [DOI] [Google Scholar]
- Moncada LI, López MC, Murcia MI, Nicholls S, León F, Guío OL, Corredor A. Myxobolus sp., another opportunistic parasite in immunosuppressed patients. J Clin Microbiol. 2001;39:1938–1940. doi: 10.1128/JCM.39.5.1938-1940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oredalen TJ, Sæbø M, Mo TA. Patterns of Tetracapsuloides bryosalmonae infection of three salmonid species in large, deep Norwegian lakes. J Fish Dis. 2022;45:185–202. doi: 10.1111/jfd.13548. [DOI] [PubMed] [Google Scholar]
- Sanders JE, Fryer JL, Leith DA, Moore KD. Control of the infectious protozoan Ceratomyxa shasta by treating hatchery water supplies. Prog Fish C. 1972;34:13–17. doi: 10.1577/1548-8640(1972)34[13:COTIPC]2.0.CO;2. [DOI] [Google Scholar]
- Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci. 1997;94:6099–6103. doi: 10.1073/pnas.94.12.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri R, Mandrioli L, Zamparo S, Errani F, Volpe E, Tura G, Ciulli S. Swim bladder disorders in Koi Carp (Cyprinus carpio) Animals. 2020;10:1974. doi: 10.3390/ani10111974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudova E, Machova J, Svobodova Z, Vesely T. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Vet Med. 2007;52:527. doi: 10.17221/2027-VETMED. [DOI] [Google Scholar]
- Vieira DHMD, Abdallah VD, da Silva RJ, de Azevedo RK. Skin nodules associated with parasitism with Henneguya sp. (Cnidaria: Myxosporea) in the neotropical fish Cyphocharax modestus. Microb Pathogen. 2019;128:294–300. doi: 10.1016/j.micpath.2019.01.027. [DOI] [PubMed] [Google Scholar]
- Vieira DHMD, Narciso RB, de Azevedo RK, da Silva RJ. Description of two novel Henneguya (Cnidaria: Myxosporea) infecting curimatid fish, using morphological, histological, and molecular analyses. Acta Parasitol. 2022;67:233–243. doi: 10.1007/s11686-021-00454-9. [DOI] [PubMed] [Google Scholar]
- Xiao CX, Chen QL. Description of two new species of Myxosporidia from Carassius auratus gibelio. Trans Res Fish Dis. 1993;1:83–86. doi: 10.1007/s00436-017-5545-4. [DOI] [Google Scholar]
- Yokoyama H, Ogawa K, Wakabayashi H. Chemotherapy with fumagillin and toltrazuril against kidney enlargement disease of goldfish caused by the myxosporean Hoferellus carassii. Fish Pathol. 1990;25:157–163. doi: 10.3147/jsfp.25.157. [DOI] [Google Scholar]
- Yuan S, Xi BW, Wang JG, Xie J, Zhang JY. Thelohanellus wangi n. sp. (Myxozoa, Myxosporea), a new gill parasite of allogynogenetic gibel carp (Carassius auratus gibelio Bloch) in China, causing severe gill myxosporidiosis. Parasitol Res. 2015;1143:7–45. doi: 10.1007/s00436-014-4157-5. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Gu ZM, Kalavati C, Costa EJ, Liu Y, Guo QY, Molnár K. Synopsis of the species of Thelohanellus Kudo, 1933 (Myxozoa: Myxosporea: Bivalvulida) Sys Parasitol. 2013;86:235–256. doi: 10.1007/s11230-013-9449-0. [DOI] [PubMed] [Google Scholar]
- Zinn JL, Johnson KA, Sanders JE, Fryer JL. Susceptibility of salmonid species and hatchery strains of Chinook salmon (Oncorhynchus tshawytscha) to infections by Ceratomyxa shasta. J Fish Res Board Can. 1977;34:933–936. doi: 10.1139/f77-145. [DOI] [Google Scholar]


