Abstract
To advance care for persons with Alzheimer’s disease and related dementias (ADRD), real-world health system effectiveness research must actively engage those affected to understand what works, for whom, in what setting, and for how long – an agenda central to Learning Health System principles. This perspective discusses how emerging payment models, quality improvement initiatives, and population health strategies present opportunities to embed best practice principles of ADRD care within the Learning Health System. We discuss how stakeholder engagement in an ADRD Learning Health System when embedding, adapting, and refining prototypes can ensure that products are viable when implemented. Finally, we highlight the promise of consumer-oriented health information technologies in supporting persons living with ADRD and their care partners and delivering embedded ADRD interventions at scale. We aim to stimulate progress toward sustainable infrastructure paired with person- and family-facing innovations that catalyze broader transformation of ADRD care.
Keywords: dementia, learning health system, patient portal, health equity, implementation science
1. Introduction
More than 6 million Americans are living with Alzheimer’s Disease and Related Dementias (ADRD).1 Systems of care are not well aligned to meet the needs of those who are affected, and care quality is poor and costly.2 Half of persons with ADRD do not receive a diagnosis,3 leading to missed opportunities to proactively address safety concerns and plan for future care needs.4 Family and unpaid caregivers and care partners (“care partners” hereafter) are at the forefront of helping persons with ADRD manage their care.5 However, care partners are not routinely identified, and their needs for information, training, and support too often go unmet.6, 7 Gaps in care delivery systems and the lack of attention to care partners exacerbate ADRD impacts and impose unnecessary burden on individuals, families, and society. 8, 9
A 2021 AHRQ systematic review of hundreds of randomized clinical trials of ADRD care and services interventions found that the overwhelming majority were disconnected from systems of care delivery; none met criteria of high or moderate evidence of benefit.10 Two 2021 reports by NASEM Consensus Committees commissioned to evaluate findings from the AHRQ review and survey the research landscape found limited evidence to guide the implementation of interventions at the organizational and community levels.2, 11 This body of work makes a compelling case for the need for real-world effectiveness research that actively engages those living with ADRD and their care partners to understand what works, for whom, in what setting, and for how long – an agenda that intersects with principles of the Learning Health System.12
Given this background, this article summarizes opportunities and strategies to sustain innovation and improvement in care of persons with ADRD and their care partners through consumer-oriented health information in Learning Health Systems. We begin by defining the Learning Health System, including why this concept merits special consideration for driving improvements in ADRD care. We then summarize how emerging payment and quality improvement initiatives align with principles of best practices in ADRD care and opportunities for embedding these principles within the Learning Health System. Finally, we put forward a case for consumer-oriented health information technologies as an especially promising strategy for engaging patients and care partners and delivering embedded ADRD interventions at scale. Our goal is to stimulate conversation and progress toward developing sustainable infrastructure paired with person- and family-facing innovations that are poised for broader transformation of ADRD care.
2. Potential of The Learning Health System to Improve Dementia Care
The Learning Health System (LHS) has been defined as “one in which science, informatics, incentives, and culture are aligned for continuous improvement and innovation, with best practices seamlessly embedded in the delivery process, patients and families active participants in all elements, and new knowledge captured as an integral by-product of the delivery experience”.12 This aspirational vision involves harnessing data and analytics to drive clinical transformation by learning from patients and feeding knowledge of what works best back to clinicians, public health professionals, health system leaders, patients and their care partners, and other stakeholders in cycles of continuous improvement.12 Motivation for the LHS lies in inefficiencies, waste, and burden arising from complexity and fragmentation in current systems of care, uncertainty surrounding best practices, and a translation gap between scientific discovery and clinical practice12 - factors that are especially salient in the context of ADRD.2, 11 The LHS aligns with implementation science and improvement science in health care: we contend that it poses special opportunities for accelerating efforts to address real-world challenges in ADRD care, as follows (Table 1).
Table 1.
The Learning Health System and Systems-Level Challenges in ADRD Care
| Issue | Current State | Learning Health System |
|---|---|---|
| Overcoming surveillance challenges | Reliance on curated data sources from national surveys and administrative claims | Clinical data is systematically gathered at the point of care in rapid cycles of continuous quality improvement.1 |
| Attenuating disparities | Poor representation and reporting on minority subgroups in randomized clinical trials | Routine collection of clinical data affords opportunities to understand and mitigate inequities in care, such as enhanced detection through the Annual Wellness Visit2 and algorithms drawing from electronic health records.1 |
| Bridging clinicians, settings, silos of care | Siloed information across multiple providers and settings, including long-term services & supports | Internal data and experiences are integrated with external evidence to facilitate transparent data sharing,3 communication, and action,4 across clinicians, settings, and silos of care. |
| Engaging care partners | Care partners are not routinely identified, assessed, or supported | LHS are “inherently observant” and seek new information and data from multiple sources, and thus are poised to identify in-person and electronic workflows that leverage the input of care partners.5 |
| Driving care redesign | Comprehensive care models exist but have not been diffused | Internal surveillance and integration of technologies (including consumer-oriented technologies) facilitate identification & refinement of incremental solutions at scale.6 |
Barnes, D. E., et al. (2020). "Development and Validation of eRADAR: A Tool Using EHR Data to Detect Unrecognized Dementia." J Am Geriatr Soc 68(1): 103-111.
Lind, K. E., et al. (2021). "The effect of direct cognitive assessment in the Medicare annual wellness visit on dementia diagnosis rates." Health Serv Res 56(2): 193-203.
Norton, J. M., et al. (2022). "Assessing Progress Toward the Vision of a Comprehensive, Shared Electronic Care Plan: Scoping Review." J Med Internet Res 24(6): e36569.
Reuben, D., et al. (2009). "Closing the dementia care gap: Can referral to Alzheimer's Association chapters help?" Alzheimers Dement 5(6): 498-502.
Fortinsky, R. H., et al. (2020). "Effectiveness of the Care of Persons With Dementia in Their Environments Intervention When Embedded in a Publicly Funded Home- and Community-Based Service Program." Innov Aging 4(6): igaa053.
Tuzzio, L., et al. (2020). "Transforming Dementia Care Through Pragmatic Clinical Trials Embedded in Learning Healthcare Systems." J Am Geriatr Soc 68 Suppl 2: S43-S48
2.1. Overcoming real-time surveillance challenges.
Because of challenges in ADRD detection and diagnosis, our knowledge of the prevalence, services use, and experiences of persons living with ADRD and their care partners overwhelmingly originates from curated data collected in national surveys or analyses of deidentified administrative claims with linked data sources.11 These data provide insight regarding care gaps and potential targets of intervention, but are disconnected from systems that diagnose and care for individuals. Existing data are not positioned to produce real-time benefit or new knowledge regarding the effects of care redesign, as envisioned by the LHS.
2.2. Attenuating disparities.
There are profound inequities in ADRD care: racial and ethnic minorities are at greater risk for ADRD, yet less likely to receive an accurate diagnosis or benefit from the care of a specialist.11 The growing diversity of aging populations and disproportionate impacts of ADRD on racial and ethnic minority subgroups pose a risk that ADRD may exacerbate health and economic disparities. Racial and ethnic minority subgroups have been poorly represented in randomized efficacy trials of ADRD prevention and care.13 The LHS draws on real-world data collected at the point of care without exclusion criteria, creating opportunities to understand and mitigate health disparities through data that include populations who have traditionally been underrepresented in research.
2.3. Bridging clinicians, settings, and silos of care.
Persons with ADRD have a high burden of chronic medical conditions and are heavy users of health services while functional impairments impose extensive long-term services and supports needs. ADRD care spans health care and long-term services and supports settings.14 Data integration across settings is critical for effective care planning and coordination, a requirement well-aligned with “bottom-up” LHS initiatives to transform clinical care in partnership with community stakeholders. For example, ambulatory clinics with integrated referrals to community-based organizations can facilitate timely care coordination and access to services.15 The fragmentation and high costs of ADRD care make it a model condition for organizations operating within value-based payment models that encourage investments leading to improved care quality and health.9
2.4. Engaging care partners.
Care partners have a foundational role in all aspects of ADRD care - from initial detection, managing treatment and support, through end-of-life care. Given the ramifications of ADRD on memory, judgment, and autonomy, care partners serve as a critical source of information about a patient’s health history – and they coordinate treatments, facilitate care planning, and participate in routine and high-stakes decision-making. Importantly, with rare exception, care partners are not routinely identified or supported in care.16-18 The competence and capacity of care partners affect the care quality, outcomes, and well-being of both the person they assist,19-21 as well as themselves.6 Support of care partners has been identified as a key opportunity for achieving savings in care delivery redesign.9 The LHS has been described as “inherently observant – seeking new information and data from many sources.”22 Scaling strategies to learn from and better support care partners could help close ADRD care gaps to improve care experiences, quality, and efficiency.
2.5. Driving care redesign.
Comprehensive, multi-component ADRD care models demonstrate the ability to achieve better care, improved outcomes, and lower costs through combining therapeutic elements such as comprehensive assessments, care planning, medication management, and care partner support.10 However, these models are complex, costly, and challenging to implement and deliver, and existing reimbursement models do not support diffusion into clinical practice at scale.2, 11 Pockets of excellence in ADRD care have been achieved at select institutions,8, 23 and geographies,24 but mainstream care is too often reactive and unsystematic. Investigator-initiated interventional studies are typically limited to examining the effects of a specific program or model.10 In contrast, the LHS is directed at identifying, tailoring, acting on, and learning about incremental efforts to address specific problems12 and therefore holds promise for operationalizing best practices to achieve quality and efficiencies with broader reach and sustainability.
3. Existing Building Blocks to Support Development of an ADRD Learning Health System
We are unaware of existing LHSs that specifically focus on ADRD, but emerging policy and payment initiatives offer building blocks that support the feasibility and potential of ADRD-focused LHS applications (Table 2). Tuzzio recently described how features of the LHS support embedded pragmatic clinical trials to advance the evidence base for testing interventions for persons living with ADRD and their care partners.25 Here, we focus on features that can motivate longer-term, sustained institutional investments.
Table 2.
Modality and Elements of Recent ADRD Payment & Quality Reporting Initiatives
| Medicare Annual Wellness Visit 1,2 |
Medicare ADRD Care Planning 2,3 |
ADRD Management Quality Measurement Set 4 |
|
|---|---|---|---|
| Modality | Self-reported health risk assessment | Detailed history, with care partner report, evaluation, & creation of written care plan; ~50 minutes face-to-face | Documentation of processes from electronic medical record or clinical data registry (numerator, denominator & reference period) |
| Element | |||
| Detection of cognitive impairment | Report of subjective cognitive change with clinician direct observation | Cognition-focused history and examination; Staging of dementia | Not included |
| Diagnosis | Not included | Establish or confirm diagnosis, etiology, severity | Disclosure of diagnosis (y/n) |
| Care partner identification & needs assessment | Not specified (social support and living arrangement is assessed) | Identify social supports, including how much care partners know and able/willing to care | Provision of education to care partner on dementia and referrals to resources for support (last 12 months) |
| Functional status assessment | Difficulty with daily activities; sensory impairment; fall risk | Basic and instrumental activities of daily living & decision-making capacity | Functional status assessment (last 12 months) |
| Behavioral and psychiatric symptom assessment | Depression screening at initial (but not subsequent) Annual Wellness Visits | Evaluate symptoms using standardized screening tool | Screening and recommendations for management (last 12 months) |
| Safety screen | Safety at home & abuse | Evaluation for home and driving safety | Safety screen & follow up Driving screening & follow up/ referral (last 12 months) |
| Advance care planning (ACP) | Not required, but may be discussed at beneficiary discretion | Address ACP or updates and palliative care needs | Advance directive & counseling on palliative care (within 2 years of diagnosis) |
| Pain assessment | Recent increase | Not included | Screening & follow up (each visit) |
| Medication review & reconciliation | Not required beyond review of current opioid prescriptions. | Reconcile & review for high-risk medication | Discussion of treatment options (last 12 months) |
Cordell, C. B., et al. (2013). "Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting." Alzheimers Dement 9(2): 141-150, and https://www.ecfr.gov/current/title-42/chapter-IV/subchapter-B/part-410/subpart-B/section-410.15
Appropriately trained members of the clinical team working with the eligible provider may provide this service.
Borson, S., et al. (2017). "Innovation in care for individuals with cognitive impairment: Can reimbursement policy spread best practices?" Alzheimers Dement 13(10): 1168-1173 & Alzheimer’s Association. (2017) Medicare's cognitive impairment assessment and care planning; https://www.alz.org/professionals/health-systems-clinicians/care-planning ; https://www.cms.gov/files/document/cognitive-assessment-care-plan-services-cpt-code-99483.pdf
Schultz, S. K., et al. (2020). "Quality Improvement in Dementia Care: Dementia Management Quality Measurement Set 2018 Implementation Update." Am J Psychiatry 177(2): 175-181.
3.1 New payment mechanisms launched by the Centers for Medicare and Medicaid Services provide reimbursement for detection, diagnosis, and care planning, contributing to the financial feasibility of an ADRD-focused LHS. The Medicare Annual Wellness Visit, introduced in 2011, involves a health risk assessment that includes reporting on subjective cognitive impairment and direct observation of cognitive function as a condition of reimbursement, thus providing information from cognitive screening at the point of care. Cognitive assessment and care planning visits, introduced in 2017, include billing codes for multidimensional assessment and creation of a written care plan that reinforce actions linked to best practices in ADRD care, including caregiver identification and support. 2, 26 A recent analysis found 1.5% of eligible beneficiaries incurred a claim for billed ADRD care planning visits, with foregone revenues estimated to be as much as $12,891 per provider per year27 suggesting that greater attention to such codes represent opportunities for ADRD LHS sustainability.
3.2 Quality metrics, such as the Dementia Management Quality Measurement Set, 28 provide a practical starting point for linking evidence to practice in ADRD care. These metrics draw on data collected in routine care to quantify algorithms for process measures in clearly specified populations and time frames, focusing on outcomes that demonstrate cost efficiency and principles of high-quality care. Examples of ADRD quality metrics include disclosure of a dementia diagnosis, providing educational resources to ADRD care partners, and conducting screening for home safety, driving, and pain. The use of benchmark performance rates and trends by organizations enable comparisons with peer institutions and monitoring of progress over time. Measures from the Dementia Management Quality Measurement Set have been included in recent value-based payment schemes, such as the Merit-Based Incentive Payment System (MIPS) and set the stage for financially compensating higher-performing providers.
3.3 Population health initiatives and disease-specific registries enable the implementation and iterative refinement and evaluation of targeted interventions and quality improvement initiatives, key components of an ADRD-focused LHS.11 As a starting place, algorithms may be applied to electronic health records to identify individuals who may have ADRD based on structured data such as diagnosis codes, problem lists, medical history, medications, cognitive screens, or clinical notes. For example, because underdiagnosis of ADRD is common, Barnes et al. describe eRADAR, a high-performing algorithm that uses common EHR data to identify patients with undiagnosed ADRD.29 Novel population health-oriented approaches that draw on natural language processing to mine clinician notes may further broaden systems-level identification of persons with ADRD. 30 Once a registry is established and validated, quality metrics can be routinely applied to identify individuals who may benefit from targeted initiatives to improve diagnosis and care quality, including strategies to identify and attenuate disparities.
3.4 Increasing availability of information about cognitive function in the clinical setting (e.g., data collected during dementia care planning visits, in patient/care partner questionnaires, or aggregated within an ADRD registry), sets the stage for an LHS to pursue rapid-cycle initiatives to improve detection, diagnosis, and treatment and attenuate identified health disparities and other gaps (Table 1). For example, patient-reported outcome measures that capture the patient voice may be routinely collected to understand experiences of care. A recent review found seven ADRD-specific patient- and care partner-reported outcome measures were suitable for widespread use, yet none were found to have been deployed in an ADRD registry.31 Such data could be harnessed to support the LHS transition, such as in risk stratification and population-based monitoring to evaluate quality improvement activities that link to appropriate interventions.11, 26 A rapid and iterative ADRD LHS would expand the pace and type of interventions and implementation strategies, adaptation or fit to clinical and community context, and ultimately the magnitude of evidence generation by bridging best practices in comprehensive care models toward processes and workflows that support systems-level implementation and learning.
4. Care Partners and ADRD Care
Scaling strategies to better support ADRD care partners is among the most promising of value-based systems-level ADRD interventions9 and aligns with tenets of the LHS to identify and address gaps in knowledge and skills that could lead to better care quality and outcomes. 12, 22 However, systems-level strategies to identify and support care partners are not well developed.2, 11, 32 In-person interactions between care partners and clinicians have been described as tense, and can be adversarial.33 Doctors may, for example, question the reliability of information reported by persons with ADRD34 but hesitate to direct questions to the care partner for fear of undermining patient autonomy.35 Care partner efforts to support, protect, and respect the person living with ADRD may mask clinician understanding of cognitive, functional, and behavioral changes and challenges, thereby unintentionally eroding care quality and outcomes.36 Caring for a person with ADRD may confer physical, emotional, and financial hardship, but the needs of care partners are typically not asked considered in the clinical context7, 32 and often remain unmet.37 A recent physician survey found fewer than 1 in 10 internists to report conducting formal assessments with ADRD care partners.32
Because the demands of ADRD care are so highly interwoven with disease stage and symptoms, interventions to support patients and care partners share commonalities in being guided by individual assessment and tailored to meet situational challenges.11 Common therapeutic elements of care partner support address psychosocial and emotional needs to attenuate guilt, frustration, grief, and depression; skill building to increase knowledge and capacity to manage symptoms such as repetitive questions or behavior changes; and strategies to improve communication and develop meaningful connection with the person living with ADRD.10 Multicomponent, tailored interventions attenuate the demands of ADRD caregiving,10 but their complexity and costs have inhibited widespread scaling.2, 11
The LHS provides a model platform for identifying and testing innovative discrete strategies to identify, engage, and support care partner involvement in ADRD care, with opportunities to refine, combine, and scale those that prove meritorious. In the context of an ADRD registry, forward-thinking organizations could pilot strategies to leverage reimbursement and quality improvement structures while engaging and supporting ADRD care partners. For example, an ADRD LHS could test supplemental questions that are fielded at the time of the Annual Wellness Visit to determine the identity and contact information of involved care partners of patients with identified or suspected ADRD as a foundational step in facilitating proactive partnerships. Similarly, an ADRD LHS could test workflows, modalities of outreach, and impacts of fielding screening surveys to better understand gaps in care partner capacity before or during ADRD Care Planning Visits, and opportunities for addressing them.
5. Consumer-Oriented Health Information Technology and the ADRD LHS
Consumer-oriented health information technologies are electronic applications and systems that enable patients and care partners to access health information, undertake health management tasks, and engage in bidirectional interaction with clinicians and other members of the care team. These technologies meet the expressed desire of patients and families for timely, accurate, comprehensive information and contextualized medical advice.38, 39 The rapid evolution of such technologies and their ability to diffuse innovative strategies to engage, educate, and support patients and care partners at scale16, 40 make them highly relevant to LHS applications.
The patient portal, which connects to a care delivery organization’s electronic medical record, is among the most widely available consumer health information technologies. The patient portal may be used to conduct telehealth visits, collect patient-reported health information, and complete legal documents such as advance directives. 41, 42 Although the patient portal is a powerful tool for cultivating and sustaining partnerships with engaged and empowered patients and care partners (a key element of the LHS),12 use in ADRD care has been limited. Privacy rules intended to safeguard personal health information are especially problematic in the context of ADRD, as they may inhibit care partner access to information that is vital when executing the patient’s treatment plan.40
Notable human factors considerations associated with emerging technologies have important benefits - and drawbacks that must be addressed to achieve their full potential. For example, the time demands of electronic messaging pose a risk for compounding burnout among front-line clinicians, while tempering enthusiasm for widespread use and innovation. Similarly, persons with ADRD are typically unable to navigate the patient portal, but little attention has been directed at proactively engaging with care partners who commonly do so on their behalf: a recent scoping review found less than 3% of registered portal users to have “shared access” with care partners, who more often engaged in portal use with patient identity credentials.43
6. ADRD Care Partners and Consumer-Oriented Health Information Technology
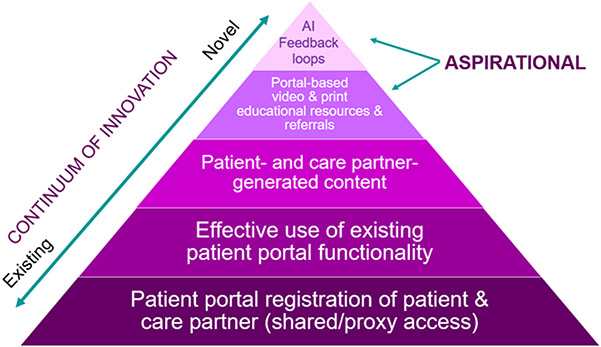
Greater attention to systematic engagement of care partners through consumer-oriented health information technologies holds promise for overcoming systems-level challenges in ADRD care. At a most basic level, simplifying registration processes and workflows for shared portal access (also termed, “family”, or “proxy” access), such as at the time of diagnosis, explicitly documents who the person living with ADRD has chosen to involve in their care, while facilitating transparent information sharing as a step toward longitudinal partnership (bottom panel of Figure 1).40 Engaging care partners through the patient portal opens new possibilities for data collection and practice by capturing the reality of “shared care” with multiple involved care partners that is common in ADRD.11 ADRD interventional research and clinical practice typically focuses on a single “primary” care partner, but the ability to engage multiple care partners through the patient portal gives rise to a host of novel opportunities that reflect the reality of ADRD caregiving.
Figure 1. Opportunity to Leverage Health System Patient Portals in an ADRD-focused Learning Health System.
AI – artificial intelligence
Once one or more care partners have been registered for the patient portal, a range of functionalities may be mobilized to facilitate greater awareness and knowledge of patient health and treatments (e.g., diagnoses, prescribed medications, test results, clinician notes), timely communication with clinicians about emerging health concerns, and coordination of care with primary and specialty clinicians (Table 3). Care partner screening and assessment – a structured process for identifying care partner needs and risks – is the foundation of evidence-based strategies to deliver tailored services and supports and the formulation of care plans that are tailored to care partner capabilities - but has not been widely deployed throughout care delivery.16, 32 Embedding standardized screening and assessment instruments within the patient portal represents an efficient way to identify care partners’ perspectives on the needs of the person living with ADRD, as well as address unmet needs of the care partner related to knowledge of ADRD symptoms, treatments, and support. ADRD print and video education, tools, and resources may be assembled and electronically disseminated (e.g., electronic health record vendor “illness journey” platforms such as Epic’s “Care Companion”) to address needs such as post-diagnosis anticipatory guidance; behavioral, safety, and communication challenges; and information about and referrals to legal and financial planning.44, 45
Table 3.
Patient Portal Innovations to Support Persons with ADRD and Their Care Partners
| Element | Description | Strategies (examples) |
|---|---|---|
| Detection of possible ADRD | Identify suspected cognitive impairment when there is a decline from previous function in daily activities, occupational ability, or social engagement; facilitate referrals for evaluation | Electronic completion of annual wellness visits via pre-screening surveys are delivered through the patient portal to identify subjective memory concerns or change in function.1 |
| Continuous monitoring & assessment | Monitor and assess cognitive, functional, behavioral, and psychological needs as well as safety and care partner stress | Care partner-responses to structured assessments2 are fielded to identify and proactively address emerging health issues. Care partners may inquire about cognitive changes and dementia progression. |
| Ongoing care planning | Develop and implement a care plan that is evaluated and modified as needed, including wishes about future and end-of-life care | Care partner-reported assessments2 and advance care planning documents (e.g, advance directives) completed outside the health care setting can be submitted through the patient portal.3 |
| Psychosocial interventions | Deliver interventions to prevent or reduce impacts of cognitive, functional, behavioral, and psychological symptoms and care partner stress | Educational materials and resources (e.g., vetted videos, websites, reading materials, referrals to community supports) to attenuate the demands of ADRD are disseminated through the patient portal. 4,5 |
| Self -management | Provide self-management tools to enhance skills of persons living with ADRD and care partners in managing ADRD, care navigation, and supporting person-centered goals. | Patients and care partners receive insufficient guidance about ADRD management.6 The patient portal affords opportunities for disseminating education and resources in response to seminal events (e.g., hospitalization, new diagnosis), or specific health management issues (e.g., polypharmacy). |
| Care partner support | Identify one or more care partners to include in evaluation, decision-making, and care planning; and provide support and assistance to help them. | Care partners may be registered with their own credentials for the patient portal through “proxy”/”shared,” 7,8 setting the stage for needs assessments to identify areas for tailored education and resources. |
| Medication management | Use evidence-based medication management including deprescribing medications with adverse cognitive effects and increasing medication adherence. | Care partners may interact with pharmacists and clinicians through secure messaging to clarify dosing instructions, report concerns (e.g. potential adverse effects), obtain accurate medication lists, request pharmacy referrals and refills, and electronic education may be disseminated.9,10 |
| Communication with clinicians | Facilitate timely information sharing between care partners and clinicians between medical visits. | Care partners may privately share concerns regarding potentially sensitive topics (e.g., driving or behaviors) in advance of a medical visit. |
| Treat related conditions | Take steps to prevent and treat conditions related to ADRD, such as depression, falls, and delirium. | Secure messaging allows for medication titration and proactive and efficient management of emerging health concerns between visits.11 |
| Coordination of care | Coordinate transitional and other healthcare services across hospitals, nursing homes, and ambulatory care and community-based settings, including long-term services and supports | Families are often the conduit of information between primary care and specialty clinicians and long-term services and supports providers. The patient portal facilitates asynchronous communication, transparent information exchange, and awareness of the nature and scope of treatments and care among the patient/care partner, primary care clinicians, and specialty clinicians.12 |
Adapted from Lees Haggerty, K., et al. (2020). "Recommendations to Improve Payment Policies for Comprehensive Dementia Care." J Am Geriatr Soc 68(11): 2478-2485 and Wiener, J. M., et al. (2016). Examining Models of Dementia Care: Final Report. Assistant Secretary of Planning and Evaluation, Division of Disability Aging and Long Term Care. Washington DC, U.S. Department of Health and Human Services.
Notes:
Sorondo, B., et al. (2016). "Using a Patient Portal to Transmit Patient Reported Health Information into the Electronic Record: Workflow Implications and User Experience." EGEMS (Wash DC) 4(3): 1237.
Ayton, D. R., et al. (2021). "Patient-Reported Outcome Measures to Inform Care of People With Dementia-A Systematic Scoping Review." Gerontologist 61(5): e185-e194.
Lum, H. D., et al. (2019). "Design and Implementation of Patient Portal-Based Advance Care Planning Tools." J Pain Symptom Manage 57(1): 112-117 e112.
Kales, H. C., et al. (2018). "Effect of the WeCareAdvisor on family caregiver outcomes in dementia: a pilot randomized controlled trial." BMC Geriatr 18(1): 113.
Moehead, A., et al. (2020). "A Web-Based Dementia Education Program and its Application to an Australian Web-Based Dementia Care Competency and Training Network: Integrative Systematic Review." J Med Internet Res 22(1): e16808.
Shafir, A., et al. (2022). ""Captive by the Uncertainty"-Experiences with Anticipatory Guidance for People Living with Dementia and Their Caregivers at a Specialty Dementia Clinic." J Alzheimers Dis 86(2): 787-800.
Wolff, J. L., et al. (2022). "Shared Access to Patient Portals for Older Adults: Implications for Privacy and Digital Health Equity." JMIR Aging 5(2).
Wolff, J. L., et al. (2019). "Sharing in Care: Engaging care partners in the care and communication of breast cancer patients." Breast Cancer Research and Treatment 177(1): 127-136.
Bayliss, E. A., et al. (2022). "Deprescribing Education vs Usual Care for Patients With Cognitive Impairment and Primary Care Clinicians: The OPTIMIZE Pragmatic Cluster Randomized Trial." JAMA Intern Med 182(5): 534-542.
Osborn, C. Y., et al. (2013). "Understanding patient portal use: implications for medication management." J Med Internet Res 15(7): e133.
Shimada, S. L., et al. (2017). "An analysis of patient-provider secure messaging at two Veterans Health Administration medical centers: message content and resolution through secure messaging." J Am Med Inform Assoc 24(5): 942-949.
Otte-Trojel, T., et al. (2014). "How outcomes are achieved through patient portals: a realist review." J Am Med Inform Assoc 21(4): 751-757
Any patient portal-based innovation must recognize the considerable time demands of ADRD care for ambulatory care clinicians, who are simultaneously experiencing overwhelming workload, burnout, and increasing numbers of direct messages that necessitate time outside of patient visits.46 Novel technologies, such as artificial intelligence, could efficiently take action to address non-medical care gaps. For example, structured care partner screening and assessment could be automatically deployed through a patient portal-messaged questionnaire at the time of diagnosis without the need for immediate clinician or staff involvement. Creating and testing structured messages with appropriate resources, (e.g., information about ADRD, ADRD care, and referral resources) and supportive workflows involving trained non-clinician team members has the potential to decrease clinician workload while improving ADRD care quality.
7. Conclusion
Emerging payment models, quality improvement initiatives, and consumer-oriented health information technologies provide important building blocks that could catalyze rapid and substantial improvements to close widely recognized ADRD care gaps. An ADRD LHS would afford opportunities of multiple, small, rapid trials through consumer health information technologies that enable clinicians and health system leaders to quickly discard strategies that prove to be infeasible or ineffective in real world systems, while refining and further enhancing the potency of innovations that show promise and high acceptability by patients and care partners. Our premise is that the ADRD LHS poses special opportunities for accelerating efforts to overcome real-time surveillance challenges, understand and attenuate disparities, bridge fragmented care, and more effectively engage and support care partners in the service of care delivery transformation.
The National Institute on Aging has made notable investments to build research capacity in implementation science. Less attention has been directed at developing longer-term sustained organizational investments in ADRD care transformation. For example, the multi-institutional NIA-funded IMPACT Collaboratory has created community, built research infrastructure, and expanded the pool of investigators with the necessary skills and training to lead embedded pragmatic trials in health care delivery.25 However, context matters in implementation science: it is not simply the features of an intervention, but characteristics of the setting, the involved individuals, available resources, organizational culture, and socio-technological environment that affect the relative success or failure of its implementation process and outcomes.2, 11 Thus, the seeding of short-term pilot studies and investigator-initiated pragmatic trials, most of which are constrained to a 5-year project period may yield very different outcomes than longer-term commitments undertaken by LHS with extended time horizons, supportive culture, and nimble informatics teams with a commitment to sustained long-term impact beyond a specific study.
An active and vibrant area of emerging scholarly work has triangulated the aspirational nature of the LHS concept with how it is unfolding in practice. This work suggests that rather than focusing on the “fully realized” LHS, that organizations may be thought as embarking a process of system-change directed at evidence-informed continuous learning. 47 This nascent body of work speaks to enabling conditions that support advancement along this path, most notably the availability of effective, committed leadership, supportive culture, and alignment across organizational missions and functions – manifested by LHS-dedicated resources, data systems and informatic infrastructure, and a prepared, skilled workforce.48 In the context of the high costs of ADRD care, shifts toward bundled payments and/or leveraging value-based payment programs to implement ADRD-specific models of care could prove a powerful incentive in garnering the attention of key organizational leaders to ADRD as an initial target of priority. 26
The applications of consumer-oriented health information technologies in ADRD care and support are wide ranging and evolving rapidly, making attention to strategies that bridge the “digital divide” especially timely and urgent. In the context of an ADRD LHS, organizations can engage patients and care partners, clinicians, informaticists, health system leaders, and researchers to collaboratively design, introduce, embed, adapt, and refine prototypes of consumer-oriented technologies to ensure that the products are viable in real-world settings. Co-developing, refining, and testing innovations in consumer-oriented technologies with patients and care partners overcomes important methodologic concerns in which prototype technologies fail to transfer to real-world settings.49
Early work from the NIH Common Fund’s Health Care Systems Research Collaboratory identified informatics challenges in successfully executing large-scale embedded pragmatic clinical trials, including: 1) using clinical data for research, 2) integrating data from heterogenous systems, 3) leveraging electronic health records to support intervention delivery or health system change, and 4) improving data capture to define study populations and outcomes.50, 51 Each of these challenges involves special considerations in successfully supporting an ADRD LHS to improve care and support for persons with ADRD and their care partners. The complex and costly profile of those affected makes the ADRD LHS the ideal test case for organizations seeking to invest in the LHS construct, as lessons learned are likely to be applicable to patients with other conditions or complex needs, and broader sets of clinicians involved in the care of comorbid chronic medical conditions.
Research in Context.
Systematic review:
The evidence supporting the content of this paper was identified using three main strategies: 1) The coauthors provided direct experience and specialized knowledge, 2) We performed focused searches of the peer-reviewed literature using PubMed, 3) We used forward and backward selection procedures of pertinent articles.
Interpretation:
We interpret the available evidence as supporting the Learning Health System as having special opportunities for accelerating efforts to overcome real-time surveillance challenges, understand and attenuate disparities, bridge fragmented care, and more effectively engage and support care partners in the service of care delivery transformation. These findings support importance and value of systems-level care delivery initiatives to identify and support persons with dementia and their care partners through consumer health information technology at scale.
Future directions.
Emerging payment models, quality improvement initiatives, and consumer-oriented health information technologies provide important building blocks that could catalyze rapid and substantial improvements to close widely recognized care gaps. Our goal is to stimulate conversation and progress toward developing sustainable infrastructure paired with person- and family-facing innovations that are poised for broader transformation of ADRD care.
Funding:
This work was supported by the National Institute on Aging R35AG072310 to JLW and K23AG064036 to HA, R01AG077011 to ARG, R03AG060170 and K23AG072037 to SKN, K01AG61275 to CAR and T32AG066576 which supported the effort of DSP. HDL and CDF were supported by the National Institute on Aging (NIA) of the National Institutes of Health under Award Number U54AG063546, which funds NIA Imbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials Collaboratory (NIA IMPACT Collaboratory). The content is solely the responsibility of the authors.
Footnotes
Disclosures: CT Lin reports serving as an unpaid advisory board member to Doximity Telehealth and Epic Physician Advisory Council. The remaining authors have nothing to disclose.
Contributor Information
Jennifer L. Wolff, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, 624 N. Broadway, Room 692, Baltimore, MD 21205.
Catherine M. DesRoches, OpenNotes/Beth Israel Deaconess Medical Center, Department of Medicine, Harvard Medical School, Boston, MA. 02215..
Halima Amjad, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, MD.
Julia G. Burgdorf, Center for Home Care Policy & Research, Visiting Nurse Service of New York, New York NY 10001.
Melanie Caffrey, Springer Science+Business Media LLC, Oracle Magazine, Former Adjunct Faculty, Computer Technology and Applications Program, Columbia University, New York, NY, Colorado Springs, CO 80903.
Chanee D. Fabius, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205.
Kelly T. Gleason, Johns Hopkins University School of Nursing, Baltimore, MD.
Ariel R. Green, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, MD.
Chen-Tan Lin, University of Colorado, Aurora, CO 80045.
Stephanie K. Nothelle, Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, MD.
Danielle Peereboom, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205.
Danielle S. Powell, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205.
Catherine A. Riffin, Division of Geriatrics and Palliative Medicine, Weill Cornell Medical Center, New York, NY 10021.
Hillary D Lum, Division of Geriatric Medicine, University of Colorado School of Medicine, Aurora, CO 80045.
REFERENCES
- 1.Association As. 2022 Alzheimer’s disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2022. doi: 10.1002/alz.12638 2022. https://alz-journals.onlinelibrary.wiley.com/doi/epdf/10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 2.NASEM. Meeting the challenge of caring for persons living with dementia and their care partners and caregivers: a way forward. National Academies Press; 2021. Accessed 5/1/2021. https://www.nap.edu/catalog/26026/meeting-the-challenge-of-caring-for-persons-living-with-dementia-and-their-care-partners-and-caregivers [PubMed] [Google Scholar]
- 3.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of Dementia: an Observational Study of Patterns in Diagnosis and Awareness in US Older Adults. Journal of general internal medicine. Mar 5 2018;doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amjad H, Roth DL, Samus QM, Yasar S, Wolff JL. Potentially Unsafe Activities and Living Conditions of Older Adults with Dementia. Journal of the American Geriatrics Society. Jun 2016;64(6):1223–32. doi: 10.1111/jgs.14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasper JD, Freedman VA, Spillman BC, Wolff JL. The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health affairs. 2015;34(10):1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NASEM. Families Caring for an Aging America. 2016. https://nam.edu/families-caring-for-an-aging-america/ [PubMed]
- 7.Wolff JL, Freedman VA, Mulcahy JF, Kasper JD. Family Caregivers' Experiences With Health Care Workers in the Care of Older Adults With Activity Limitations. JAMA Netw Open. Jan 3 2020;3(1):e1919866. doi: 10.1001/jamanetworkopen.2019.19866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan CM, Sachs GA, Lamantia MA, Unroe KT, Arling G, Boustani MA. Redesigning systems of care for older adults with Alzheimer's disease. Health affairs. Apr 2014;33(4):626–32. doi: 10.1377/hlthaff.2013.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bott NT, Sheckter CC, Yang D, et al. Systems Delivery Innovation for Alzheimer Disease. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. Feb 2019;27(2):149–161. doi: 10.1016/j.jagp.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler M, Gaugler JE. Care interventions for people with dementia (PWD) and their caregivers. 2020. [Google Scholar]
- 11.NASEM. Reducing the Impact of Dementia in America. National Academy Press; 2021:286. Accessed 8/15/2021. https://www.nap.edu/catalog/26175/reducing-the-impact-of-dementia-in-america-a-decadal-survey [Google Scholar]
- 12.IOM. Best care at lower cost: the path to continuously learning health care in America. 2013. Accessed 1/15/2022. https://nap.nationalacademies.org/catalog/13444/best-care-at-lower-cost-the-path-to-continuously-learning [PubMed]
- 13.Gilmore-Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: A systematic review. Alzheimers Dement (N Y). 2019;5:751–770. doi: 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NQF. Priority setting for health care performance measurement: Addressing performance measure gaps for dementia, including Alzheimer's Disease. 2014. October 15, 2014. http://www.qualityforum.org/priority_setting_for_healthcare_performance_measurement_alzheimers_disease.aspx
- 15.Reuben D, Levin J, Frank J, et al. Closing the dementia care gap: Can referral to Alzheimer's Association chapters help? Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review. Alzheimer's & dementia : the journal of the Alzheimer's Association. Nov 2009;5(6):498–502. doi: 10.1016/j.jalz.2009.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riffin C, Griffin JM, Brody L, et al. Engaging and Supporting Care Partners of Persons With Dementia in Health-Care Delivery: Results From a National Consensus Conference. Public Policy Aging Rep. 2022;32(2):58–65. doi: 10.1093/ppar/prac004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riffin C, Wolff JL, Estill M, Prabhu S, Pillemer KA. Caregiver Needs Assessment in Primary Care: Views of Clinicians, Staff, Patients, and Caregivers. Journal of the American Geriatrics Society. Mar 13 2020;doi: 10.1111/jgs.16401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgdorf J, Roth DL, Riffin C, Wolff JL. Factors Associated with Receipt of Training Among Caregivers of Older Adults. JAMA internal medicine. Apr 8 2019;179(6):833–835. doi: 10.1001/jamainternmed.2018.8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgdorf JG, Arbaje AI, Stuart EA, Wolff JL. Unmet family caregiver training needs associated with acute care utilization during home health care. Journal of the American Geriatrics Society. Jul 2021;69(7):1887–1895. doi: 10.1111/jgs.17138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amjad H, Mulcahy J, Kasper JD, et al. Do Caregiving Factors Affect Hospitalization Risk Among Disabled Older Adults? Journal of the American Geriatrics Society. Jan 2021;69(1):129–139. doi: 10.1111/jgs.16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgdorf JG, Fabius CD, Riffin C, Wolff JL. Receipt of Posthospitalization Care Training Among Medicare Beneficiaries' Family Caregivers. JAMA Netw Open. Mar 1 2021;4(3):e211806. doi: 10.1001/jamanetworkopen.2021.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene SM, Reid RJ, Larson EB. Implementing the learning health system: from concept to action. Research Support, Non-U.S. Gov't. Annals of internal medicine. Aug 7 2012;157(3):207–10. doi: 10.7326/0003-4819-157-3-201208070-00012 [DOI] [PubMed] [Google Scholar]
- 23.Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer's and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. Journal of the American Geriatrics Society. Dec 2013;61(12):2214–2218. doi: 10.1111/jgs.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollister BA, Yeh J, Ross L, Schlesinger J, Cherry D. Building an advocacy model to improve the dementia-capability of health plans in California. Journal of the American Geriatrics Society. Dec 2021;69(12):3641–3649. doi: 10.1111/jgs.17429 [DOI] [PubMed] [Google Scholar]
- 25.Tuzzio L, Hanson LR, Reuben DB, et al. Transforming Dementia Care Through Pragmatic Clinical Trials Embedded in Learning Healthcare Systems. Journal of the American Geriatrics Society. Jul 2020;68 Suppl 2:S43–S48. doi: 10.1111/jgs.16629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lees Haggerty K, Epstein-Lubow G, Spragens LH, et al. Recommendations to Improve Payment Policies for Comprehensive Dementia Care. Journal of the American Geriatrics Society. Nov 2020;68(11):2478–2485. doi: 10.1111/jgs.16807 [DOI] [PubMed] [Google Scholar]
- 27.Agarwal SD, Basu S, Landon BE. The Underuse of Medicare's Prevention and Coordination Codes in Primary Care : A Cross-Sectional and Modeling Study. Annals of internal medicine. Jun 28 2022;doi: 10.7326/M21-4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz SK, Llorente MD, Sanders AE, et al. Quality Improvement in Dementia Care: Dementia Management Quality Measurement Set 2018 Implementation Update. Am J Psychiatry. Feb 1 2020;177(2):175–181. doi: 10.1176/appi.ajp.2019.19121290 [DOI] [PubMed] [Google Scholar]
- 29.Barnes DE, Zhou J, Walker RL, et al. Development and Validation of eRADAR: A Tool Using EHR Data to Detect Unrecognized Dementia. Journal of the American Geriatrics Society. Jan 2020;68(1):103–111. doi: 10.1111/jgs.16182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuben DB, Hackbarth AS, Wenger NS, Tan ZS, Jennings LA. An Automated Approach to Identifying Patients with Dementia Using Electronic Medical Records. Journal of the American Geriatrics Society. Mar 2017;65(3):658–659. doi: 10.1111/jgs.14744 [DOI] [PubMed] [Google Scholar]
- 31.Ayton DR, Gardam ML, Pritchard EK, et al. Patient-Reported Outcome Measures to Inform Care of People With Dementia-A Systematic Scoping Review. The Gerontologist. Jul 13 2021;61(5):e185–e194. doi: 10.1093/geront/gnz179 [DOI] [PubMed] [Google Scholar]
- 32.Riffin C, Wolff JL, Pillemer KA. Assessing and Addressing Family Caregivers' Needs and Risks in Primary Care. Journal of the American Geriatrics Society. Nov 20 2020;doi: 10.1111/jgs.16945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vick J, Amjad H, Smith KC, et al. "Let him speak:" A descriptive qualitative study of the roles and behaviors of family companions in primary care visits among older adults with cognitive impairment. International journal of geriatric psychiatry. 2018;33(1):e103–e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunsaker AE, Schmidt K, Lingler JH. Discussing dementia-related behaviors during medical visits for people with Alzheimer's disease. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. American journal of Alzheimer's disease and other dementias. May 2010;25(3):248–54. doi: 10.1177/1533317509357734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradford A, Upchurch C, Bass D, et al. Knowledge of documented dementia diagnosis and treatment in veterans and their caregivers. Comparative Study Research Support, Non-U.S. Gov't. American journal of Alzheimer's disease and other dementias. Mar 2011;26(2):127–33. doi: 10.1177/1533317510394648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haikio K, Sagbakken M, Rugkasa J. Dementia and patient safety in the community: a qualitative study of family carers' protective practices and implications for services. BMC health services research. Sep 5 2019;19(1):635. doi: 10.1186/s12913-019-4478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennings LA, Reuben DB, Evertson LC, et al. Unmet needs of caregivers of individuals referred to a dementia care program. Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S. Journal of the American Geriatrics Society. Feb 2015;63(2):282–9. doi: 10.1111/jgs.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds TL, Ali N, Zheng K. What Do Patients and Caregivers Want? A Systematic Review of User Suggestions to Improve Patient Portals. AMIA Annu Symp Proc. 2020;2020:1070–1079. [PMC free article] [PubMed] [Google Scholar]
- 39.Antonio MG, Petrovskaya O, Lau F. The State of Evidence in Patient Portals: Umbrella Review. Journal of medical Internet research. Nov 11 2020;22(11):e23851. doi: 10.2196/23851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff JL, Dukhanin V, Burgdorf JG, DesRoches CM. Shared Access to Patient Portals for Older Adults: Implications for Privacy and Digital Health Equity. JMIR Aging. 2022;5(2)doi: 10.2196/34628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lum HD, Brungardt A, Jordan SR, et al. Design and Implementation of Patient Portal-Based Advance Care Planning Tools. Journal of pain and symptom management. Jan 2019;57(1):112–117 e2. doi: 10.1016/j.jpainsymman.2018.10.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nittas V, Lun P, Ehrler F, Puhan MA, Mutsch M. Electronic Patient-Generated Health Data to Facilitate Disease Prevention and Health Promotion: Scoping Review. Journal of medical Internet research. Oct 14 2019;21(10):e13320. doi: 10.2196/13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gleason KT, Peereboom D, Wec A, Wolff JL. Patient Portals to Support Care Partner Engagement in Adolescent and Adult Populations: Scoping Review JAMA Netw Open. 2022;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shafir A, Ritchie CS, Garrett SB, et al. "Captive by the Uncertainty"-Experiences with Anticipatory Guidance for People Living with Dementia and Their Caregivers at a Specialty Dementia Clinic. Journal of Alzheimer's disease : JAD. 2022;86(2):787–800. doi: 10.3233/JAD-215203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moehead A, DeSouza K, Walsh K, Pit SW. A Web-Based Dementia Education Program and its Application to an Australian Web-Based Dementia Care Competency and Training Network: Integrative Systematic Review. Journal of medical Internet research. Jan 22 2020;22(1):e16808. doi: 10.2196/16808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai-Seale M, Dillon EC, Yang Y, et al. Physicians' Well-Being Linked To In-Basket Messages Generated By Algorithms In Electronic Health Records. Health affairs. Jul 2019;38(7):1073–1078. doi: 10.1377/hlthaff.2018.05509 [DOI] [PubMed] [Google Scholar]
- 47.Davis FD, Williams MS, Stametz RA. Geisinger's effort to realize its potential as a learning health system: A progress report. Learn Health Syst. Apr 2021;5(2):e10221. doi: 10.1002/lrh2.10221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Easterling D, Perry AC, Woodside R, Patel T, Gesell SB. Clarifying the concept of a learning health system for healthcare delivery organizations: Implications from a qualitative analysis of the scientific literature. Learn Health Syst. Apr 2022;6(2):e10287. doi: 10.1002/lrh2.10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guisado-Fernandez E, Blake C, Mackey L, et al. A Smart Health Platform for Measuring Health and Well-Being Improvement in People With Dementia and Their Informal Caregivers: Usability Study. JMIR Aging. Jul 23 2020;3(2):e15600. doi: 10.2196/15600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richesson RL, Green BB, Laws R, et al. Pragmatic (trial) informatics: a perspective from the NIH Health Care Systems Research Collaboratory. Journal of the American Medical Informatics Association : JAMIA. Sep 1 2017;24(5):996–1001. doi: 10.1093/jamia/ocx016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richesson RL, Hammond WE, Nahm M, et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. Journal of the American Medical Informatics Association : JAMIA. Dec 2013;20(e2):e226–31. doi: 10.1136/amiajnl-2013-001926 [DOI] [PMC free article] [PubMed] [Google Scholar]