TO THE EDITOR:
This study aims to identify targeted agents designed for treating other malignancies that can be repurposed to treat acute leukemias. To identify potential therapeutic targets, we assembled a transcriptome data set from 1915 pediatric and adult leukemias. By comparing leukemia-expressed genes with a library of immunotherapy agents including antibody-drug conjugates (ADC) (www.clinicaltrials.gov and www.adcreview.com), we identified 141 targets for which ADCs are currently in clinical trials for a variety of cancers (see supplemental data). We then evaluated the transcript expression of the 141 targets in the pediatric acute myeloid leukemia (AML) cohort (supplemental Figure 1-2). Of these, CD74 was the most prevalent and highly transcribed (supplemental Figure 1-2). The expression of CD74 is variable among AML subtypes and is usually elevated in pediatric AML expressing RUNX1-CBFA2T3, NUP98-NSD1, and CBFB-MYH11 fusion proteins, AML with monosomy 7 or CBL deletion as well as immature adult AML (supplemental Figures 3 and 4).
CD74 is a type II transmembrane glycoprotein involved in antigen presentation, the formation of major histocompatibility complex class II proteins, and the regulation of B-cell maturation, proliferation, and survival (see supplemental data).1, 2, 3, 4, 5 It is expressed in a variety of immune cells, including B cells, monocytes, macrophages, dendritic cells, a subset of T cells and thymic epithelium.6 The expression of CD74 has been reported in several AML cell lines and patient samples that are sensitive to anti-CD74 induced cytotoxicity (milatuzumab).7,8 CD74 is an excellent target for ADC because the cell surface form is rapidly internalized through the endosomal pathway.9,10 CD74 can be targeted by STRO-001, an anti-CD74 ADC containing a tubulin inhibitor (maytansinoid) with efficacy against B-cell lymphomas and multiple myeloma in preclinical studies.11 Currently, STRO-001 is being evaluated in a phase 1 clinical trial for mature B-cell malignancies (NCT03424603).
We first demonstrated cell surface CD74 protein by flow cytometry of primary cells from pediatric patients with AML enrolled in Children’s Oncology Group (COG) trials. Pediatric AML biological samples were collected with informed consent (and in accordance with the Declaration of Helsinki) from patients enrolled on COG trials AAML0531 (NCT00372593), or AAML1031 (NCT01371981). Each protocol was approved by the National Cancer Institute’s central institutional review board (IRB) and the local IRB at Fred Hutchinson Cancer Center (Protocol 9950). CD74 was detected on AML blasts in 26.2% (297 of 1134) of the samples, with a median mean fluorescent intensity of 22.7 (range, 8.2-95.4). CD74 expression was not restricted to AML blasts but was also expressed in a subset of normal lymphocytes; mature myeloid cells did not express CD74 (supplemental Figure 1B). Studies of additional CD74+ AML samples (N = 17) revealed that CD74 is expressed more frequently and at higher levels on AML blasts than in lymphocytes, and with a rare dim expression on mature myeloid cells (supplemental Figure 1C-D).
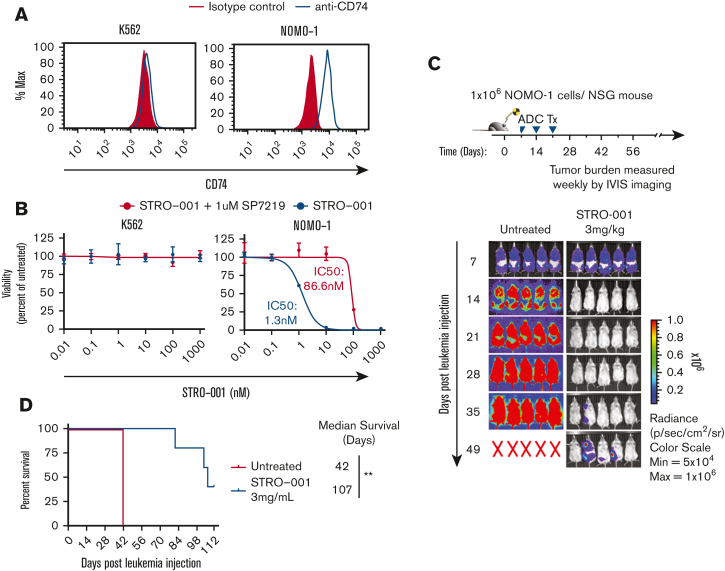
We then evaluated the cytotoxicity of STRO-001 in the CD74+ AML cell line (NOMO-1) and in a control CD74- line (K562; Figure 1A). Treatment with STRO-001 showed potent, target-dependent cytotoxicity in NOMO-1 cells with half maximal inhibitory concentration (IC-50) of 1.3 nm but had no significant effect on control K562 cells (Figure 1B; supplemental Figure 5). In addition, STRO-001 therapy (3 weekly doses with 3 mg/kg) significantly inhibited leukemia growth in NSG mice transplanted with NOMO-1 cells with 2 of 5 mice remaining disease free until the study endpoint of 112 days, with a median survival of 107 days after leukemia injection (Figure 1C-D). These results demonstrate that STRO-001 therapy effectively eradicates CD74+ NOMO-1 leukemia cells in culture and in xenograft models.
Figure 1.
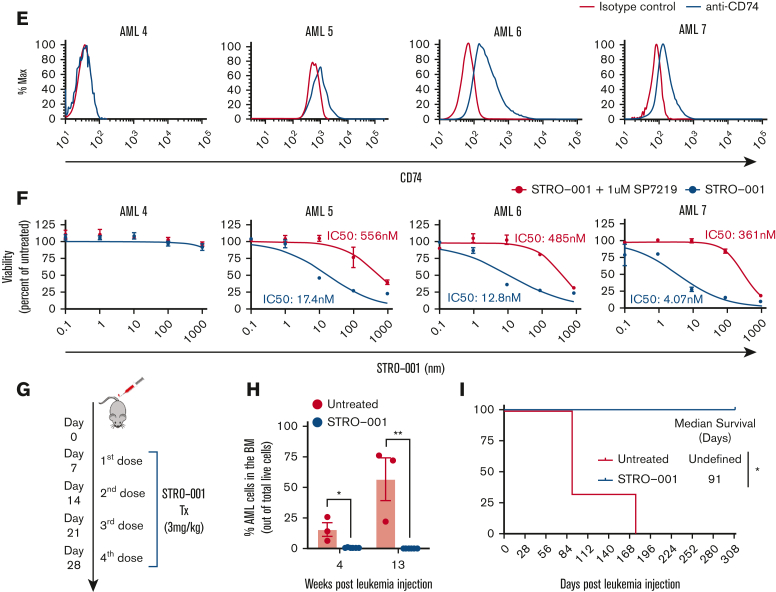
STRO-001 therapy demonstrates preclinical efficacy against CD74 expressing NOMO-1 AML cell line and primary AML cells. (A) Flow cytometric analysis of CD74 cell surface expression on K562 and NOMO-1 cell lines. (B) In vitro cytotoxicity of STRO-001 against K562 and NOMO-1 cells. Cells were treated with increasing doses of STRO-001 alone (blue) or excess of naked antibody SP7219 (1 uM, red). After 3 days of continuous exposure, viability was assessed by Cell Titer-Glo assay. Data are normalized to untreated controls. Error bars denote standard deviation from 2 technical replicates at each dose. Experiments were repeated at least twice (supplemental Figure 5). (C) Top, experimental schema evaluating STRO-001 in vivo efficacy in NOMO-1 xenograft model. Bottom, leukemia burden measured by bioluminescence (IVIS) imaging in NOMO-1 xenograft mice untreated (left) or treated with STRO-001 at 3 mg/kg weekly for 3 weeks (right). Shown are representative timepoints. N = 5 mice per group. X denotes death. (D) Kaplan-Meier survival curves of NOMO-1 xenografts untreated or treated with STRO-001. N = 5 per group. Statistical differences in survival were evaluated using Log-rank Mantel-Cox. (E) Flow cytometric analysis of CD74- (AML-4) and CD74+ AML patient specimens (AML-5-7). (F) In vitro cytotoxicity of STRO-001 primary AML specimens. Cells were treated as described above. Error bars denote standard deviation from 3 technical replicates at each dose. (G) Experimental design to assess in vivo activity of STRO-001 against a PDX model transplanted with a primary AML sample, AML-7. Peripheral blood was obtained every other week following the last dose of STRO-001, bone marrow aspirate was obtained 4 and 13 weeks after transplant. (H) Percent AML cells in the bone marrow at indicated weeks following leukemia injection determined by flow cytometry. (I) Kaplan-Meier survival curves of PDX mice untreated (n = 3) or treated with STRO-001 (n = 3). Statistical differences in survival were evaluated using Log-rank Mantel-Cox.
We also evaluated the in vitro efficacy of STRO-001 against primary AML patient samples with variable CD74 expression (AML4-7; Figure 1E). Primary cells were incubated for 3 days with increasing concentrations of STRO-001. No cytotoxicity was observed with the control CD74- patient sample, whereas STRO-001 exhibited potent, antileukemic activity against other samples (IC50 of 4.1, 12.8 and 17.4 nm). Target-specific cytotoxicity of STRO-001 was confirmed by incubation with excess anti-CD74 blocking antibody (SP7219) that inhibited the binding of STRO-001(Figure 1F).
To determine whether STRO-001 is active against primary AML cells in vivo, we generated a PDX model with an aggressive CD74+ primary AML sample (AML7) carrying the NUP98-NSD1 fusion, FLT3-ITD, and WT1 mutations.12 One week after transplant, PDX mice were treated with STRO-001 (3 mg/kg) weekly for 4 weeks (Figure 1G). The additional week of therapy for the AML PDX mice was added based on the lower efficacy of STRO-001 in the in vitro studies with primary samples. Bone marrow aspiration showed significant AML engraftment in untreated control mice, whereas AML engraftment was undetected in STRO-001–treated mice at the indicated timepoints (Figure 1H). Most significantly, STRO-001 monotherapy induced complete remission in the AML PDX model (>280 days after therapy; Figure 1I). In addition, STRO-001 therapy selectively eliminated the CD74+ AML population whereas normal engrafted myeloid cells (CD74-) were preserved (supplemental Figure 6A-D). Together, these results show potent and selective antileukemia activity of STRO-001 against primary AML cells.
Analysis of the publicly available B-cell acute lymphocytic leukemia (B-ALL) and T-cell ALL (T-ALL) data sets revealed significant CD74 expression in B-ALL whereas only a paucity of expression in T-ALL (supplemental Figure 7A). Furthermore, among the B-ALL cases, CD74 transcript levels are higher in Philadelphia chromosome (Ph+) B-ALL than in other B-ALL subtypes (supplemental Figure 7B). Flow cytometric analysis confirmed cell surface expression of CD74 in B-ALL cells (B-ALL1-3) with limited to no expression in T-ALL cells (T-ALL1-3; supplemental Figure 7C). Analysis of additional samples showed that CD74 cell surface expression was present in 16 out of 16 B-ALL samples but in only 1 of 6 T-ALL samples (supplemental Figure 7D-E).
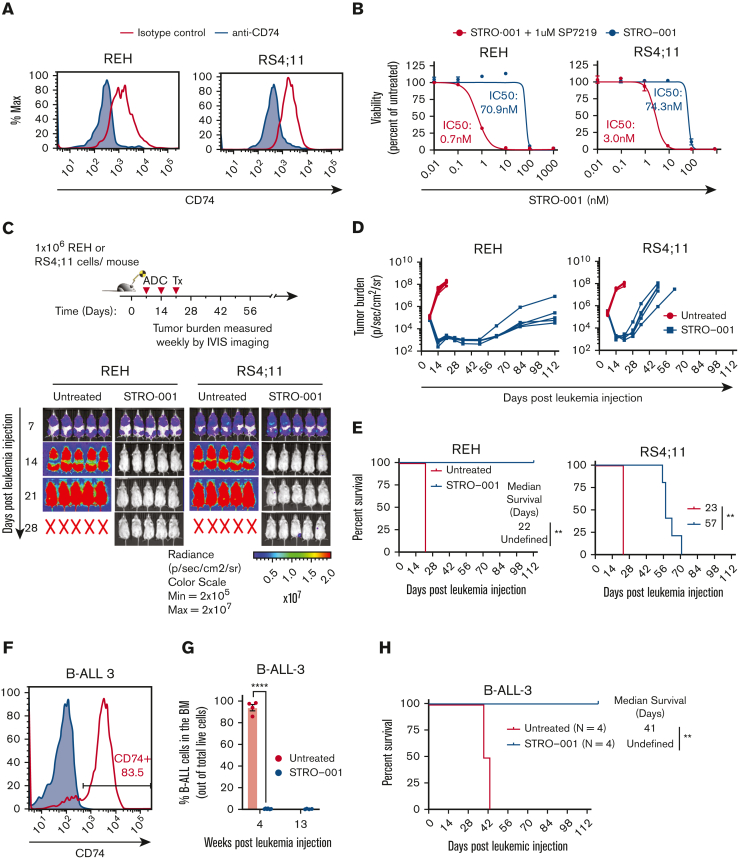
To determine whether STRO-001 demonstrates antileukemia activity against B-ALL cells, we evaluated in vitro and in vivo cytotoxicity of STRO-001 against REH and RS4;11 B-ALL cell lines that express cell surface CD74 (Figure 2A). Both cell lines were highly sensitive to STRO-001 (IC50 of 0.7 nm and 3 nm, respectively; Figure 2B, supplemental Figure 5). In addition, treatment of REH xenograft mice with STRO-001 targeted the tumor effectively, with complete survival throughout the study duration (112 days after leukemia injection) whereas the untreated mice succumbed to the disease 22 days after leukemia injection (P = .003; Figure 2C-E). STRO-001 was less effective in the RS4;11 xenograft mice extending survival from 23 to 57 days after leukemia injection (P = .003, Figure 2C-E).
Figure 2.
STRO-001 therapy demonstrates preclinical efficacy against CD74-positive B-ALL cell lines and primary B-ALL. (A) Flow cytometric analysis of CD74 cell surface expression on REH and RS4;11 B-ALL cell lines. (B) In vitro cytotoxicity of STRO-001 against REH and RS4;11 cells. Experiments were repeated twice (supplemental Figure 4). Data are normalized to untreated controls. Error bars denote standard deviation from 2 or 3 technical replicates at each dose. (C) Top, experimental schema evaluating STRO-001 in vivo efficacy in REH and RS4;11 xenograft models. Bottom, representative images of leukemia burden detected by IVIS imaging in REH and RS4;11 xenograft mice untreated or treated with STRO-001 at 3 mg/kg weekly for 3 weeks. N = 5 mice per group. X indicates death. (D) Quantification of radiance shown in C. N = 5 mice per group. (E) Kaplan-Meier survival curves of REH and RS4;11 xenograft mice untreated or treated with STRO-001. N = 5 per group. Statistical differences in survival were assessed by Log-rank Mantel-Cox test. (F) Flow cytometric analysis of CD74 expression in patient specimen B-ALL-3. (G) Percent B-ALL-3 cells (huCD19+) in the bone marrow at indicated weeks following leukemia injection (H) Kaplan-Meier survival curves of PDX B-ALL-3 mice untreated or treated with STRO-001. Data are presented as mean +/− SEM. Statistical differences were determined by unpaired, 2-tailed Student t test. Details of the B-ALL-3 PDX studies (8.5e6 cells/mouse; n = 4 for untreated group, n = 7 for STRO-001-treated group).
We generated PDX B-ALL models by transplanting NSG-SGM3 mice with a CD74+ primary B-ALL sample encoding a KMT2A/MLL fusion (B-ALL-3; Figure 2F). One week after transplant, PDX mice were treated with STRO-001 (3 mg/kg) weekly for 3 weeks. Bone marrow aspiration showed the marrow to be completely replaced by malignant cells (>90% marrow cellularity) in untreated mice, whereas B-ALL was undetected in STRO-001–treated mice (Figure 2G). Most significantly, STRO-001 therapy extended the survival of B-ALL PDX mice till the end of the study (>112 days after leukemic injection, Figure 2H). STRO-001 therapy also induced complete remission in a second high-risk B-ALL PDX model with a Ph-like immunophenotype (B-ALL-4) (supplemental Figure 8A-B). Together, these results demonstrate potent, selective antileukemia activity of STRO-001 against PDX models of primary B-ALL.
To accelerate identification of new antileukemic therapies, we have leveraged our large AML and ALL transcriptome data set to identify targets of ADCs in clinical trials for other malignancies. The highest expressed target was CD74 that is recognized by STRO-001, an anti-CD74 ADC. Here, we showed that primary AML and B-ALL express high levels of CD74. STRO-001 therapy induces target-dependent cytotoxicity against AML and B-ALL primary cells and cell lines in vitro, and in xenograft models with complete remissions in AML and B-ALL PDX models. These studies support STRO-001 as a promising therapeutic for CD74+ AML and B-ALL.
Conflict-of-interest disclosure: L. Pardo, L.E.B. and M.R.L. are employees of Hematologics Inc.; and M.R.L. has equity ownership in Hematologics Inc. C.A., K.B., and A.M. are employees of Sutro Biopharma. All other authors declare no competing financial interests.
Acknowledgments
Acknowledgments: This study was supported by Project Stella and COG Translation Pilot Studies for Hematopoietic Malignancies (K.R.L.).
Contribution: Q.L. designed the experiments; Q.L. and K.R.L. wrote the manuscript; Q.L., T.T., S.C., C.N.M., L. Perkins, L. Pardo, T.H., L.C., and M.M. performed the experiments and analyzed the data; D.K., C.A., K.B., A.M., L.E.B., M.R.L., K.T., K.R.L., and S.M. provided general scientific guidance and designed the experiments; all authors reviewed the manuscript before submission.
Footnotes
∗S.M. and K.R.L. are joint senior authors.
Data sets are publicly available as indicated in the article. Additional data are available on request from the corresponding author, Keith R. Loeb (kloeb@fredhutch.org).
The full-text version of this article contains a data supplement that includes additional details on identification of targeted therapies, background on CD74 and material and methods.
Supplementary Material
References
- 1.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell. 2005;16(11):5061–5069. doi: 10.1091/mbc.E05-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen S, Shachar I. Cytokines as regulators of proliferation and survival of healthy and malignant peripheral B cells. Cytokine. 2012;60(1):13–22. doi: 10.1016/j.cyto.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces b cell survival by activation of a CD74-CD44 receptor complex∗. J Biol Chem. 2008;283(5):2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 4.Matza D, Wolstein O, Dikstein R, Shachar I. Invariant chain induces B cell maturation by activating a TAF(II)105-NF-kappaB-dependent transcription program. J Biological Chem. 2001;276(29):27203–27206. doi: 10.1074/jbc.M104684200. [DOI] [PubMed] [Google Scholar]
- 5.Starlets D, Gore Y, Binsky I, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107(12):4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 6.Stein R, Mattes MJ, Cardillo TM, et al. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13(18):5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 7.Chandra AB, Burton J, Stein R, et al. CD74 expression by AML cell lines and bone marrow specimens, and augmented in vitro cytotoxicity of anti-CD74 antibody after interferon-gamma (IFN-γ) treatment. Blood. 2010;116(21):2888. [Google Scholar]
- 8.Burton JD, Stein R, Chandra A, et al. Expression of CD74 by AML blasts and cell lines, and enhanced in vitro cytotoxicity of anti-CD74 antibody after interferon-gamma (IFN-γ) treatment. J Clin Oncol. 2010;28(15_suppl):6576. [Google Scholar]
- 9.Freisewinkel IM, Schenck K, Koch N. The segment of invariant chain that is critical for association with major histocompatibility complex class II molecules contains the sequence of a peptide eluted from class II polypeptides. Proc National Acad Sci. 1993;90(20):9703–9706. doi: 10.1073/pnas.90.20.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc National Acad Sci. 1993;90(18):8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrahams CL, Li X, Embry M, et al. Targeting CD74 in multiple myeloma with the novel, site-specific antibody-drug conjugate STRO-001. Oncotarget. 2018;9(102):37700–37714. doi: 10.18632/oncotarget.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostronoff F, Othus M, Gerbing RB, et al. NUP98/NSD1 and FLT3/ITD coexpression is more prevalent in younger AML patients and leads to induction failure: a COG and SWOG report. Blood. 2014;124(15):2400–2407. doi: 10.1182/blood-2014-04-570929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.