Abstract
Physician-led perioperative surgical home models are developing as a method for improving the American health care system. These models are novel, team-based approaches that help to provide continuity of care throughout the perioperative period. Another avenue for improving care for surgical patients is the use of enhanced recovery after surgery pathways. These are well-described methods that have shown to improve perioperative outcomes. An established perioperative surgical home model can help implementation, efficiency, and adherence to enhanced recovery after surgery pathways. For these reasons, the Tennessee Valley Healthcare System, Nashville Veterans Affairs Medical Center created an Anesthesiology Perioperative Care Service that provides comprehensive care to surgical patients from their preoperative period through the continuum of their hospital course and postdischarge follow-up. In this brief report, we describe the development, implementation, and preliminary outcomes of the service.
Health care in the United States is more expensive, less efficient, and more fragmented than in many comparable Western countries.1 The United States spends nearly 18% of its gross domestic product on health care but has fewer licensed physicians and even fewer hospital beds per capita than other Western countries.2 In response to the gap between spending and quality in the American health care system, the Institute for Healthcare Improvement issued a “triple aim.” This triple aim was to improve the health of a defined population, improve the individual experience of patients, and reduce the cost of care.3 The American Society of Anesthesiologists has endorsed the physician-led perioperative surgical home (PSH) model as a vehicle for achieving these aims in the perioperative setting.1
The PSH model, led by a Director of Perioperative Services, is a team-based approach that involves shared decision making between patients and providers that aims to provide continuity of care throughout the perioperative period. Possible benefits of this streamlined care model include reduced preoperative testing, fewer surgery cancellations, improved clinical outcomes, reduced perioperative complications, reduced cost, and improved coordination of care, including discharge planning and follow-up care.4–6
Another well-described approach to improving perioperative outcomes is the use of Enhanced Recovery After Surgery (ERAS) pathways. These pathways aim to reimagine each phase of perioperative care. Attention is paid to preoperative fasting, multimodal analgesia including regional anesthesia use, fluid management, early refeeding, and early patient mobilization. These pathways have been shown to decrease the incidence of postoperative complications and length of stay but have not yet been studied within the Veterans Affairs (VA) Health System.7–9 ERAS pathways, however, have important differences from the PSH model. ERAS pathways are well-defined clinical protocols that utilize particular items to be implemented for specific surgeries.7 The PSH model, on the other hand, is a much larger system that includes coordination of care from the decision to operate until follow-up visits after hospital discharge.7 The fusion of these 2 models may serve as the best solution to maximize patient care and achieve the triple aim. In this article, we describe the creation and execution of a Perioperative Care Service (PCS) at a VA Medical Center (the VA-PCS) that combines elements of the PSH model and ERAS pathways, and we provide preliminary outcome data from its implementation.
METHODS
At the Tennessee Valley Healthcare System (TVHS), Nashville VA Medical Center, the Department of Anesthesiology, Pain Management & Perioperative Medicine developed a VA-PCS to provide 24 hours a day, 7 days a week involvement for our surgical patients. The PCS team executes the PSH model within our VA Medical Center, ensures appropriate preoperative assessment and execution of ERAS pathways, medically manages the patient alongside the surgical services throughout the perioperative period, and performs postdischarge follow-up. The service’s data collection was approved by the TVHS IRB, and the requirement for written informed consent was waived by the IRB.
SERVICE DESIGN
Staffing
Beginning in January 2016, a subset of board-certified critical care anesthesiologists (CCA) were appointed to provide staffing for this new assignment—led by a CCA as the Medical Director. One CCA serves as the VA-PCS attending each day, including daytime in-hospital coverage and overnight home call. To date, the CCA receives an average of 1 to 2 phone calls daily between the hours of 6:00 PM and 10:00 PM and then 2 to 3 phone calls per week between 10:00 PM and 6:00 AM. On average, each CCA commits 5 to 7 continuous days to the service. Only 1 VA full-time equivalent position has been required to fill the staffing needs. This has allowed multiple CCAs to help with this novel service by only requiring a small percentage of their full-time effort. Eight nurse practitioners (NPs) were hired to provide continuous in-hospital coverage to all patients managed by the VA-PCS.10
ERAS Pathways and Service Lines
In order for the VA-PCS to initiate this PSH model, 5 service lines were chosen for the development of ERAS pathways, focusing initially on the most frequent and major surgical procedures of each service line. Pathways were created with the involvement of VA-PCS attendings and NPs, anesthesiologists, surgeons, physical therapists, pharmacists, and social workers. To date, ERAS pathways have been created for total knee arthroplasty (TKA), total hip arthroplasty (THA), spine surgery, open cystectomy, and radical nephrectomies. Similar to traditional ERAS pathways, these pathways focus not only on multimodal perioperative pain management, but also on preoperative nutrition, physical therapy, smoking cessation, perioperative anticoagulation, perioperative antibiotics, intraoperative fluid management with balanced solutions, early ambulation, early refeeding, removal of indwelling catheters, and other important perioperative initiatives. The VA-PCS team was also available for consult on surgical patients not within the dedicated ERAS service lines.
There were some initial challenges with the development of the ERAS pathways. The time requirements put forth by the Anesthesiology Chief, Critical Care Chief, the VA-PCS Medical Director, the surgeons, and various clinicians to develop the ERAS pathways were significant. A majority of this effort was put forth in addition to their clinical workload at either the VA or the corresponding academic institution. These pathways and eventual VA-PCS workflow took approximately 4 months to develop. After their completion, educational sessions led by the VA-PCS Medical Director occurred over a 1-month period to ensure all parties (eg, holding room nursing, operating room nursing, certified nurse anesthetists, anesthesiologists, postanesthesia care unit nursing, floor nursing, surgeons) were educated to the ERAS pathway and the new VA-PCS team.
Workflow
In January 2016, the VA-PCS started to execute the PSH model and supervise the adherence to the developed ERAS pathways. Patient care through the PSH is summarized in Figure 1, and the daily workflow for the VA-PCS team is outlined in Figure 2. On average, 2 NPs arrive in the morning to receive patient sign-out from the night NP. Once sign-out is complete, 1 NP will preround on the service patients and communicate with the surgical teams. The other NP will evaluate the preoperative patients for that day and work with the attending operating room anesthesiologists to meet the preoperative needs of all new admissions. One important aspect of preoperative evaluation involves ensuring coordination of postoperative rehabilitation to prevent delays in discharge due to lack of rehabilitation referral. After surgical rounds and after surgery begins in the operating theater, the NPs prepare for multidisciplinary rounds that take place at the same time every day.11,12 The daily rounds include the attending, 2 NPs, a dedicated PCS social worker, and a surgical clinical pharmacist (PharmD). They take place at the patient’s bedside and include any family members present. The bedside nurse is not always present; they are welcomed into rounds when present and are immediately communicated with once available if not present for rounds.
Figure 1.
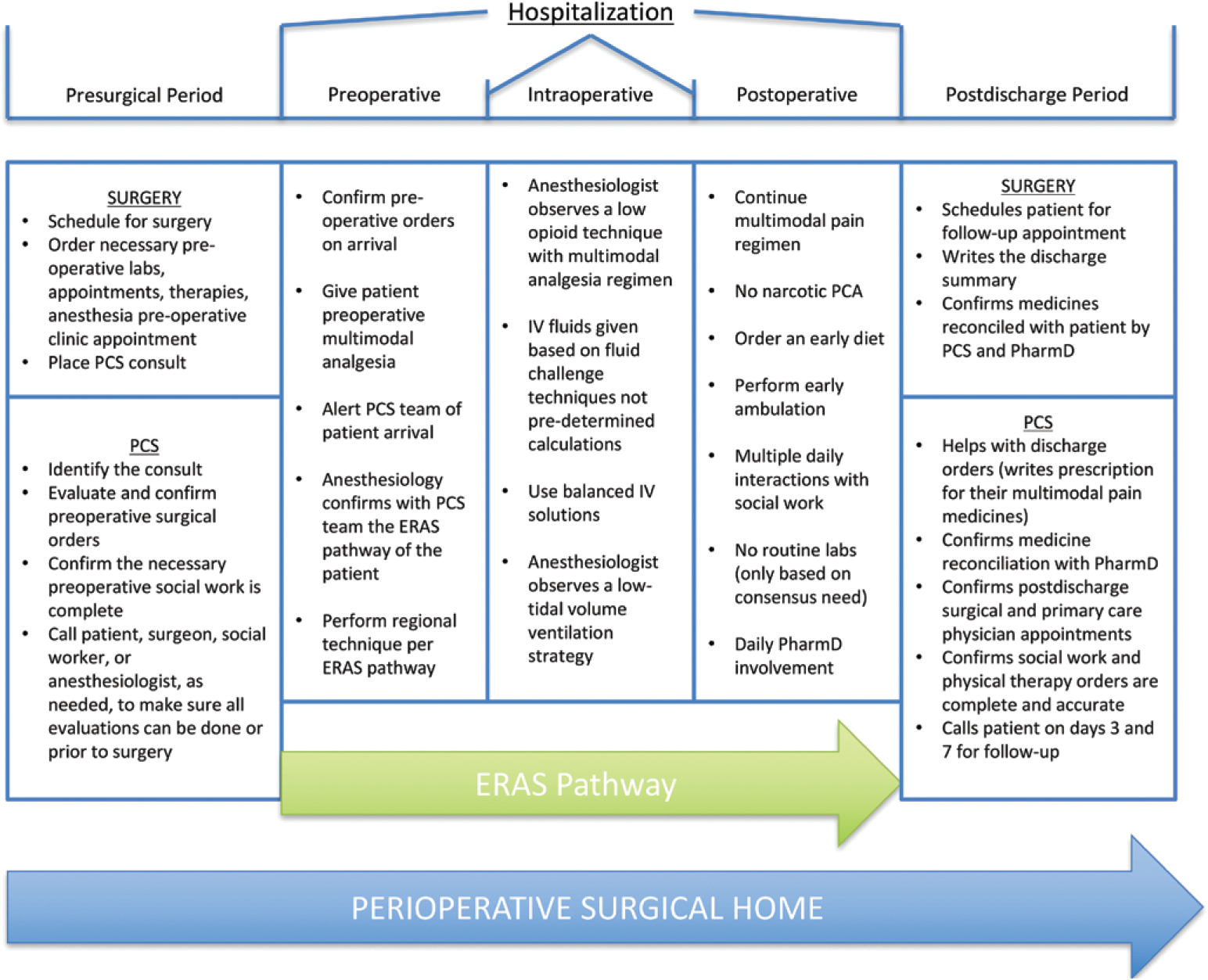
Patient flow through the perioperative surgical home. This figure represents the patient flow and important tasks within the perioperative surgical home of the VA-PCS team. ERAS indicates enhanced recovery after surgery; IV, intravenous; PCA, patient-controlled analgesia; PCS, perioperative care service; PharmD, pharmacist.
Figure 2.
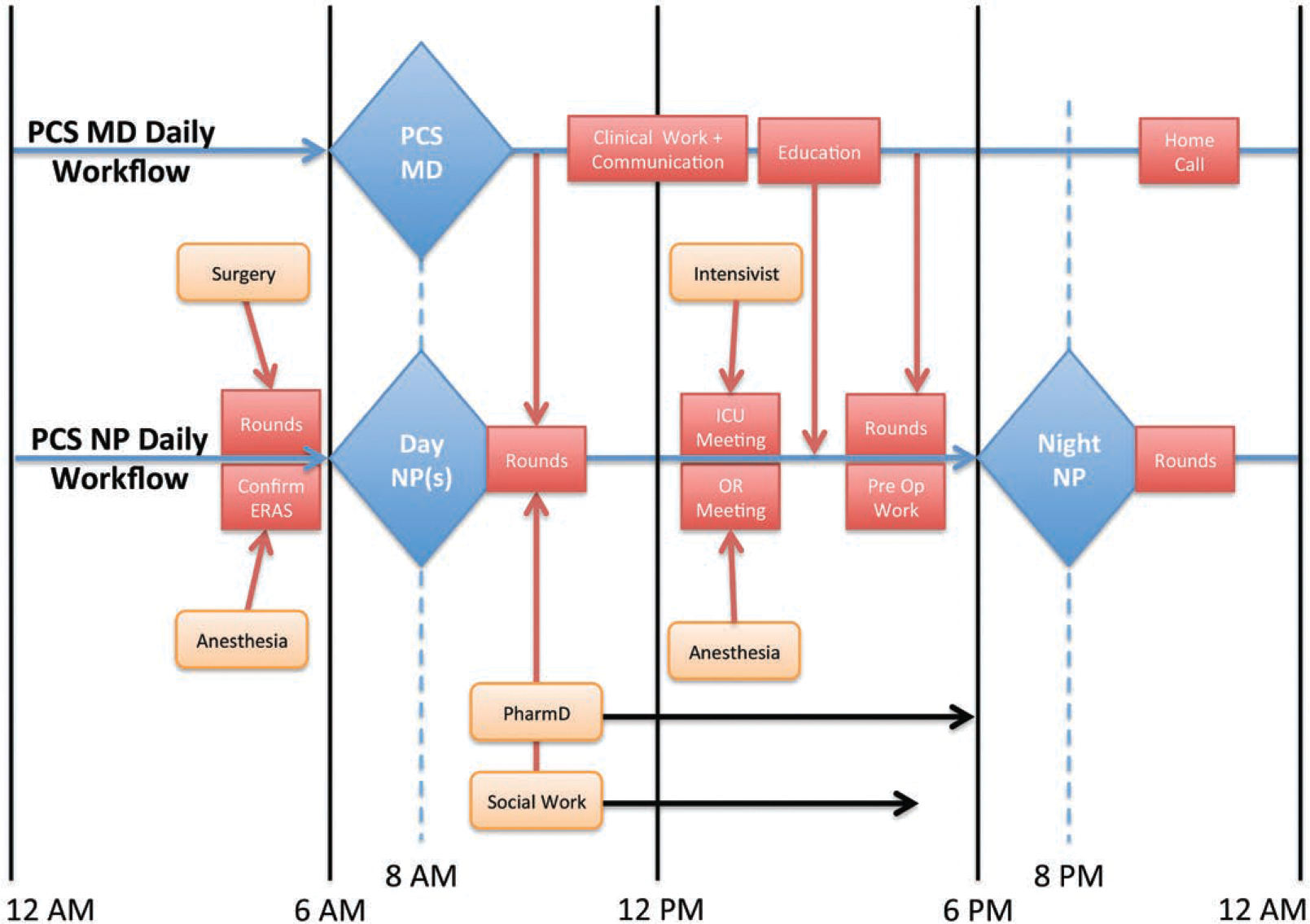
Daily workflow of the PCS. This figure summarizes the typical daily workflow of the Perioperative Care Service Physician (PCS MD) and Nurse Practitioner (NP). Labeled boxes along their respective timelines represent an activity and the approximate time they are performed. The shift change for the NP takes place at approximately 8:00 am and 8:00 pm. The PCS MD provides continuous 24-hour coverage. ERAS indicates enhanced recovery after surgery; ICU, intensive care unit; MD, medical doctor; OR, operating room; PCS, perioperative care service; PharmD, pharmacist; preop, preoperative.
The VA-PCS attending is available by phone at all times and is physically present for multidisciplinary rounds. After rounding, the workload is triaged accordingly with the team, social worker, and PharmD. For the remainder of the day, the NPs under the guidance of the VA-PCS attending are responsible for the necessary clinical follow-up, updating surgical teams, clinical notes, communication, preoperative evaluations, postdischarge follow-up via phone calls, data entry, and needed physical therapy that was not able to be performed that day. In addition, they work in concert with the social worker and PharmD to coordinate discharge planning. Throughout the day they are in communication with the VA-PCS attending. The workload of the VA-PCS attending includes, but is not limited to, rounding, documentation, education, leadership positions, committee meetings, necessary follow-up, bed triage, surgeon communication, and preoperative evaluation of the next day cases.
The night NP arrives in the evening to receive sign-out and then communicates with the on-call VA-PCS attending to discuss the remaining patients and any input or feedback from the day. Through the remainder of the night, the night NP rounds on patients, executes necessary clinical follow-up, updates clinical notes and communication, performs postdischarge follow-up via phone calls that remain, and completes data entry.
The VA surgeon is an extremely important provider to the success of the VA-PCS. Once they schedule a patient for surgery, it is their responsibility to place the consult for the VA-PCS. Upon patient arrival to the hospital, the surgical team will communicate their plans to the VA-PCS team and then help ensure adherence to the developed ERAS pathway preoperatively and intraoperatively. After surgery, the surgical team continues to communicate with the VA-PCS throughout the hospital stay. This communication typically takes place after morning rounds and then, again, in the afternoon. An important note is this communication includes, but is not limited to, discharge planning for each patient every day. This constant discussion regarding discharge has proven valuable and has allowed the discharge to be an agreement between the VA-PCS and the surgical team.
Database Creation and Utilization
After obtaining local IRB exemption as a quality improvement project, the VA-PCS Medical Director and a research assistant with expertise in database creation built an online REDCap13 database for prospective data collection on every patient the VA-PCS follows. Data include, but are not limited to, patient name, surgery, surgeon, demographic data, Critical Care Pain Observation Tool scores,14 Richmond Agitation-Sedation Scale,15 Confusion Assessment Method for the intensive care unit,16 adherence to ERAS pathways, medication administration, Surgical Care Improvement Project outcomes, medication reconciliation, postoperative follow-up appointments, and patient satisfaction. In addition, we collect the date at which patients meet discharge criteria and the time of actual discharge. The daytime NP spends an average of 10–20 minutes entering each patient’s daily data into the database after morning rounds. Once they input the daily data on the inpatients, the patients who are postdischarge day 3 and 7 are contacted via telephone. Their pain scores, activity levels, understanding of their medication prescriptions, adherence to their multimodal pain regimens, adherence to all medications that were reconciled before discharge, follow-up appointments (both primary care provider and surgery), transportation to follow-up appointments and physical therapy (if applicable), and any complications or readmissions are documented in the database. This prospective collection of data allows for ongoing quality improvement, ERAS compliance, and identification of areas requiring attention, such as early physical therapy, prompt urinary catheter removal, early diet advancement, and daily medication reconciliation. Examples of process improvement projects performed by the VA-PCS are listed in Table 1.
Table 1.
Examples of Process Improvements by the PCS
| VA Process Improvements | |
|---|---|
| Preoperative joint class | All scheduled TKA and THA patients are followed from the time they are scheduled. The PCS team ensures that all necessary social work, physical therapy, and surgical appointments are made by the patient. |
| Laboratory measurements | Laboratory orders are reviewed by the PCS for necessity, and timeliness is confirmed through direct RN interaction. |
| Nursing education | Multiple education sessions are given on various topics developed by the floor nurses and by the PCS. Importance of intake and output documentation and why vital signs are vital are examples of topics taught to date. |
| Overnight availability Weekend physical therapy | PCS team is available in-hospital 24 hours per day and 7 days per week, providing increased availability and level of patient evaluation and care. Attempts to have physical therapist availability over the weekend are made. When unavailable, PCS assures physical therapy evaluation before weekend and performs approved exercises. |
| Weekend discharge | The presence of the PCS team has improved the logistic processes during the week that have allowed improved weekend discharge. |
This table summarizes a few of the process improvements that have taken place since implementation of the VA-PCS.
Abbreviations: PCS, perioperative care service; RN, registered nurse; THA, total hip arthroplasty; TKA, total knee arthroplasty; VA, Veterans Affairs.
Preliminary Outcomes
Baseline (before VA-PCS implementation) outcome data for patients undergoing TKA, THR, and spine surgeries were obtained from January 1, 2015, to December 31, 2015, and used as historical controls. This included length of stay and opioid administration. Post VA-PCS implementation data were collected prospectively from the study database as described above. Given the preliminary nature of the study, descriptive statistics were used and not univariate or multivariable analyses. We show percentages (%) for categorical variables and median (interquartile range [IQR]) for continuous outcomes.
PRELIMINARY RESULTS TO DATE
From January 2016 to July 2016, 136 patients underwent surgical procedures and were followed by the VA-PCS. Demographic data are summarized in Table 2. Of these patients, 55 (40%) had TKA, 23 (17%) had THA, 7 (5%) had spine surgery, and 51 (38%) were consults with non-ERAS surgeries. Complete ERAS compliance was 87% for the TKA patients, 87% for THA patients, and 86% for spine surgery patients. The preoperative pain medication administration was the most common deviation from the ERAS pathways for all service lines. The use of Ringer′s lactate solution and/or PlasmaLyte crystalloid solutions had the highest compliance, with no normal saline used for any patient to date. Once the patient was out of the postanesthesia care unit, adherence was 100% to each service line’s ERAS pathway. The median length of stay (LOS) for TKA patients in the implementation phase was 2 days (IQR 1–3 days), compared with 3 days (IQR 2–4 days) in the 2015 historical control (Figure 3). Similarly, the median LOS for THA patients was 2 days (IQR 1–3 days), compared with 3 days (IQR 1–5 days). The median LOS for spine patients was 1 day (IQR 0–2 days), compared with 2 days (IQR 0–4 days). The median LOS for additional consult patients was 3 days (IQR 0–8 days), with comparison data for 2015 not available. The median total intravenous (IV) hydromorphone given to TKA patients in the implementation phase was 0 mg (IQR 0 mg) for their entire postoperative hospitalization (postanesthesia care unit and ward) compared with 2.5 mg (IQR 0–6 mg) in the 2015 historical control. The median total IV hydromorphone given to THA patients was 0 mg (IQR 0 mg) compared with 1.5 mg (IQR 0–4 mg). The median total IV hydromorphone given to spine patients was 0 mg (IQR 0 mg) compared with 0.5 mg (IQR 0–2 mg).
Table 2.
Demographic Data
| Characteristic | Pre-PCS (January–December 2015; n = 98) | Post-PCS (January–July 2016; n = 136) |
|---|---|---|
| Age (y) | 62 (10.5) | 61 (12.4) |
| Sex | ||
| Male | 91 (93%) | 128 (94%) |
| Female | 9 (13%) | 8 (5%) |
| BMI (kg/m2) | 30 (5.2) | 31 (5.5) |
| ASA classification | ||
| 1 | 1 (1%) | 1 (1%) |
| 2 | 21 (21%) | 31 (23%) |
| 3 | 70 (71%) | 97 (71%) |
| 4 | 6 (6%) | 7 (5%) |
| Surgical operation | ||
| TKA | 51 (52%) | 55 (40%) |
| THA | 32 (33%) | 23 (17%) |
| Spine | 15 (15%) | 7 (5%) |
| Consults | N/A | 51 (38%) |
| Protocol compliance | ||
| TKA | N/A | 48/55 (87%) |
| THA | N/A | 20/23 (87%) |
| Spine | N/A | 6/7 (86%) |
Demographic information for all 70 patients in 2015 (historical controls) and the 136 patients cared for by the PCS from January 11, 2016, to July 25, 2016. Values presented as averages (SD) or n (%).
Abbreviations: BMI, body mass index; N/A, not available; PCS, perioperative care service; THA, total hip arthroplasty; TKA, total knee arthroplasty.
Figure 3.
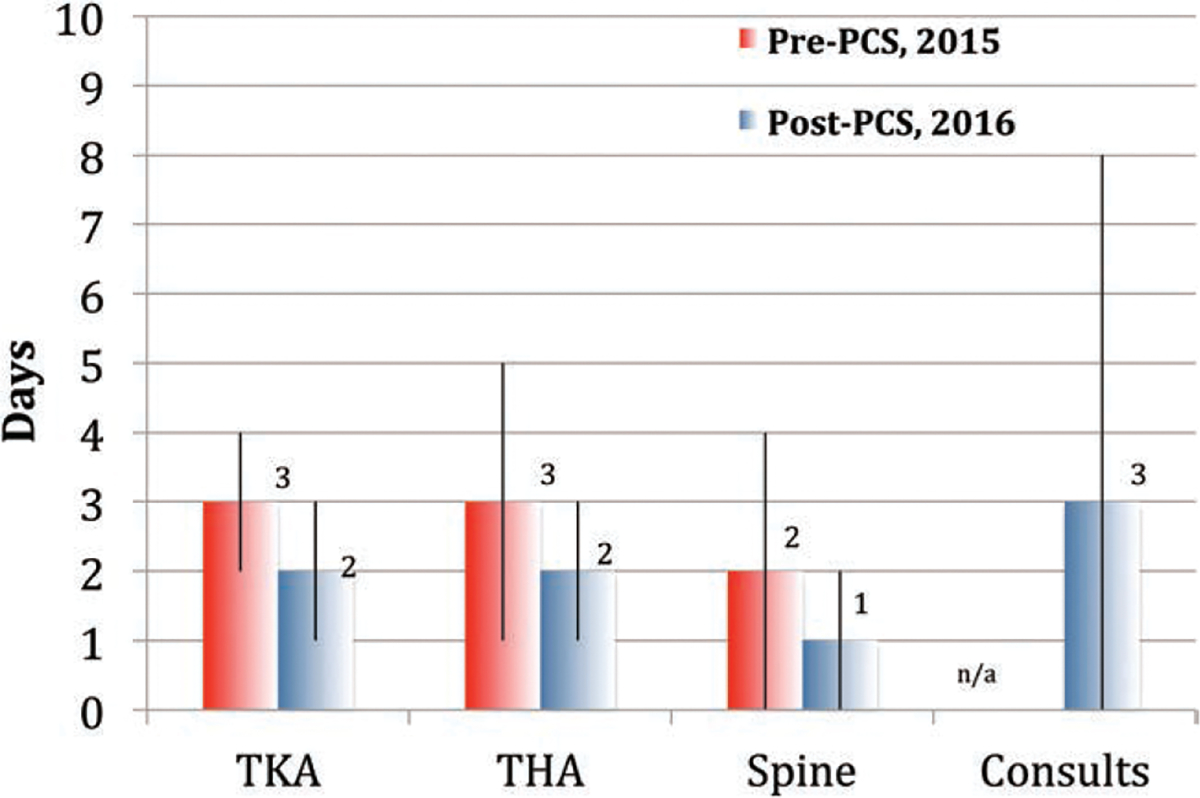
Length of stay before and after PCS implementation. This figure displays the median (interquartile range) LOS (days) in 2015 before PCS implementation versus 2016 after PCS implementation for patients undergoing TKA, THA, and spine surgery. The LOS data for our general consults are not available for 2015. LOS indicates length of stay; PCS, perioperative care service; THA, total hip arthroplasty; TKA, total knee arthroplasty.
DISCUSSION AND FUTURE DIRECTIONS
Development of the VA-PCS has provided a multidisciplinary framework to improve perioperative patient care at TVHS. The VA-PCS provides continuous coverage by NPs with a supervising CCA attending and collaborates with surgical teams, social workers, pharmacists, and physical therapists. To date, the VA-PCS has shortened the average length of hospital stay by over 1 day for TKA, THA, and spine surgery patients. This has been accomplished by creating a PSH that ensures adherence to the established ERAS pathways and coordinates the comprehensive medical care of each patient in the perioperative period. Using the Health Economics Resource Center inpatient data on the 2014 average VA surgical inpatient daily cost ($5121 per day),17 an estimated $435,285 has been saved based reduced LOS within 6 months of initiation of this service. There have also been significant cost savings with the ability to reduce utilization of the surgical intensive care unit (SICU) by decreasing overall admissions and allowing faster transition of complex patients from the SICU to the ward. We have estimated 83 SICU days saved since implementation. The exact savings from this is difficult to estimate because the evidence for mean daily intensive care unit cost estimates range from $1436 to $12,931.18–20 With these figures, an additional $119,188–$1,073,273 of savings may exist. These figures serve only as estimates, and we did not include additional potential savings from decreases in case cancellations, increases in surgical volume secondary to our involvement, reduction in workload burden of other services, decreases in laboratory costs, reductions in medication costs, or decreasing readmissions. More robust analyses examining the role of the VA-PCS in outcome improvement and cost reduction will be performed after increased patient and data accrual.
The VA-PCS team assists with social work, case management, physical therapy, and surgical concerns. They also continuously communicate with the patient from the scheduling of their case to phone calls on day 3 and 7 after discharge. These actions have contributed to the patient’s decreased length of hospital stay. A multidisciplinary team approach that includes both anesthesiologists and surgeons will help improve the care of surgical patients. As surgical patients in the United States become older, sicker, and more complex, the expertise of perioperative care teams will be needed. It is our expectation that our VA patients will benefit from the knowledge, dedication, and proactive stewardship that our VA-PCS team offers. It is the goal of the service to extend the PSH model with the VA-PCS team throughout all the surgical services at the TVHS-VA.
Funding:
The author(s) received no financial support for the research, authorship, and/or publication of this article. We used REDCap, a secure online database, supported in part by the National Institutes of Health TR000445.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES
Name: Bret D. Alvis, MD.
Contribution: This author is the VA-PCS Medical Director and participated in the study design, conducted the study, performed data analysis and its interpretation, and was involved in manuscript preparation.
Name:Adam B. King, MD.
Contribution: This author participated in the study design, conducted the study, performed data analysis and its interpretation, and was involved in manuscript preparation.
Name: Pratik P. Pandharipande, MD, MSCI.
Contribution: This author participated in the study design, conducted the study, interpreted the data, and was involved in manuscript preparation.
Name: Liza M. Weavind, MD.
Contribution: This author participated in the study design, conducted the study, interpreted the data, and was involved in manuscript preparation.
Name: Katelin Avila, NP.
Contribution: This author participated in the study design, conducted the study, performed data analysis and its interpretation, and was involved in manuscript preparation.
Name: Philip J. Leisy, MD.
Contribution: This author performed data collection, analysis and edited the manuscript for content.
Name: Muhammad Ajmal, MD.
Contribution: This author participated in the study design, interpreted the data, and was involved in manuscript preparation.
Name: Michael McHugh, MD.
Contribution: This author participated in the study design, interpreted the data, and was involved in manuscript preparation.
Name: Kirk A. Keegan, MD.
Contribution: This author participated in the study design, interpreted the data, and was involved in manuscript preparation.
Name: David A. Baker, MD, MBA.
Contribution: This author participated in the study design, interpreted the data, and was involved in manuscript preparation.
Name: Ann Walia, MD.
Contribution: This author participated in the study design, performed data analysis and its interpretation, and was involved in manuscript preparation.
Name: Christopher G. Hughes, MD.
Contribution: This author participated in the study design, conducted the study, performed data analysis and its interpretation, and was involved in manuscript preparation.
This manuscript was handled by: Avery Tung, MD, FCCM.
REFERENCES
- 1.Butterworth JFt Green JA. The anesthesiologist-directed perioperative surgical home: a great idea that will succeed only if it is embraced by hospital administrators and surgeons. Anesth Analg. 2014;118:896–897. [DOI] [PubMed] [Google Scholar]
- 2.Bank TW. 2014. Indicators Available at: http://dataworldbankorg/indicator/SHXPDTOTLZS.
- 3.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27:759–769. [DOI] [PubMed] [Google Scholar]
- 4.Holt NF. Trends in healthcare and the role of the anesthesiologist in the perioperative surgical home—the US perspective. Curr Opin Anaesthesiol. 2014;27:371–376. [DOI] [PubMed] [Google Scholar]
- 5.Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118:1126–1130. [DOI] [PubMed] [Google Scholar]
- 6.Vetter TR, Goeddel LA, Boudreaux AM, Hunt TR, Jones KA, Pittet JF. The Perioperative Surgical Home: how can it make the case so everyone wins? BMC Anesthesiol. 2013;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannesson M, Kain Z. Enhanced recovery after surgery versus perioperative surgical home: is it all in the name? Anesth Analg. 2014;118:901–902. [DOI] [PubMed] [Google Scholar]
- 8.King PM, Blazeby JM, Ewings P, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis. 2006;8:506–513. [DOI] [PubMed] [Google Scholar]
- 9.Varadhan KK, Lobo DN, Ljungqvist O. Enhanced recovery after surgery: the future of improving surgical care. Crit Care Clin. 2010;26:527–547. [DOI] [PubMed] [Google Scholar]
- 10.Haan JM, Dutton RP, Willis M, Leone S, Kramer ME, Scalea TM. Discharge rounds in the 80-hour workweek: importance of the trauma nurse practitioner. J Trauma. 2007;63:339–343. [DOI] [PubMed] [Google Scholar]
- 11.Dutton RP, Cooper C, Jones A, Leone S, Kramer ME, Scalea TM. Daily multidisciplinary rounds shorten length of stay for trauma patients. J Trauma. 2003;55:913–919. [DOI] [PubMed] [Google Scholar]
- 12.Sen A, Xiao Y, Lee SA, et al. Daily multidisciplinary discharge rounds in a trauma center: a little time, well spent. J Trauma. 2009;66:880–887. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–427. [PubMed] [Google Scholar]
- 15.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–2710. [DOI] [PubMed] [Google Scholar]
- 17.Wagner T, Chow A, Su P, Barnett PG. HERC’s average cost datasets for VA inpatient care, FY1998-FY2015. Menlo Park, CA: VA Palo Alto, Health Economics Resource Center; 2016. [Google Scholar]
- 18.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. [DOI] [PubMed] [Google Scholar]
- 19.Dahl D, Wojtal GG, Breslow MJ, et al. The high cost of low-acuity ICU outliers. J Healthc Manag. 2012;57:421–433. [PubMed] [Google Scholar]
- 20.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. [DOI] [PubMed] [Google Scholar]


